Abstract
Background:
Polypharmacy for schizophrenia treatment is not justified by the available clinical evidence. We evaluated a treatment reduction approach that reduces the dose and number of antipsychotic medications simultaneously prescribed to patients.
Methods:
In a randomized open study of the Safe Correction of Antipsychotic Polypharmacy and High-Dose Prescriptions program funded by the Japanese Ministry of Health, Labour, and Welfare, we evaluated a drug reduction method consisting of a dose reduction intervention performed on 163 patients with schizophrenia for twelve or 24 weeks. One antipsychotic medication was removed each week from each patient’s treatment regimen by reducing the dose by 0 to 50 chlorpromazine equivalents. Data on health-related indices of quality of life, clinical symptoms, and risk of side effects were analyzed using a two-way repeated-measures mixed linear model.
Results:
Despite a 23% reduction in antipsychotic dose, no differences in outcomes were observed between the dose reduction and observation groups (effect size = 0.001 – 0.085, P = .24–.97), despite high statistical power (1-β = 0.48–0.97). The findings are limited by the nonuniformity of the participants’ treatment history, duration, and dose reduction amount. Dose reduction protocol patients exhibited no difference in psychotic symptoms or adverse events compared with the observation group.
Conclusions:
Importantly, the low dropout rate in our study (6.9% of participants withdrew because of patient factors and 23.8% for all secondary reasons) indicates that our “slowly” method is well tolerated. We hope that this approach will result in therapeutic improvements.
Keywords: psychopharmacology, clinical trial, schizophrenia, polypharmacy, antipsychotics
Introduction
Receptor occupancy at doses commonly used in the polypharmacy treatment of schizophrenia frequently surpasses the dose needed for effective therapy. Despite an increased risk of side effects (Farde et al., 1992), therapy with a combination of agents remains a part of the general practice (Barnes and Paton, 2011). In particular, polypharmacy is widely practiced in the treatment of schizophrenia in Japan. Between 26 and 34% of Japanese patients with schizophrenia are treated with a single agent, whereas between 32 and 42% of patients undergo therapy with more than 3 agents (Yoshio et al., 2012; Okumura et al., 2013). In a study comparing treatment practices in East Asian countries, the Japanese usage of more than 3 agents simultaneously was found to be high in comparison with treatment regimens commonly used in other countries in the region (Ito et al., 2012; Xiang, 2012). A review comparing monotherapy with treatment with a combination of multiple antipsychotic agents (Collell et al., 2009) evaluated 22 studies (n=1202) performed under a variety of clinical situations. The findings of the review indicate that combination therapy may be superior to monotherapy (confidence interval=0.63–0.90), although the results were very heterogeneous (evolution of heterogeneity, I2 =78.9%). To address this issue, the Ministry of Health, Labour, and Welfare proposed in 2009 “a study on understanding of the actual condition and correction of polypharmacy” and provided incentives to approximately one-half of psychiatric clinics and institutions for the use of 2 or less antipsychotic agents starting in 2010. Such a study would provide robust scientific data potentially supporting the general criticism of polypharmacy and justifying the government’s incentives for the reduction in doses of antipsychotic agents.
A recent review discussed the issues associated with antipsychotic polypharmacy and proposed potential approaches for reducing the number of agents concurrently used in the treatment of schizophrenia (Fleischhacker and Uchida, 2014). A number of interventions were proposed that could be implemented to modify the physicians’ prescribing habits regarding polypharmacy. Two clinical studies (Suzuki et al., 2004; Essock et al., 2011) presented the effects of dose reduction on patients who were previously treated by polypharmacy. An evaluation of the effectiveness of various interventions showed that approaches relying on education account for only a small effect in the reduction of polypharmacy. Overall, the results of clinical studies evaluating dose reduction implementations suggest that clinical symptoms do not change, whereas symptoms were reported to worsen in 20% of subjects and more subjects discontinued their treatment. Therefore, evidence supporting the safety and effectiveness of antipsychotic dose reduction is presently very limited, and not enough validated dose reduction approaches are currently available.
In our study, therefore, we aimed to provide evidence that would establish a novel, realistic approach to polypharmacy correction for use in Japanese psychiatric institutions. We conducted a realistic and fair correction of polypharmacy in Japanese psychiatric medical care environments comparing the consequences of reducing the various combined agents used. If no changes were observed in antipsychotic effectiveness between treatments with single and multiple agents, as suggested by a number of previous studies, it is feasible that the side effects and health-related quality-of-life (QOL) indices are not altered by a switch from multiple agents. We carefully considered biologic factors and psychological stress when modulating the speed of drug reduction, particularly in reducing agents that had been administered for a long time. The Study of the Safe Correction of Antipsychotic Polypharmacy and High-Dose Prescriptions (SCAP) was supported solely by scientific research funds from the Ministry of Health, Labour, and Welfare. We analyzed the safety and effectiveness of the correction of polypharmacy in Japan. Based on previous limited reports, we analyzed the protocol (SCAP method) in 2010 and conducted a randomized open study (Sukegawa et al., 2014) to develop a safe and effective correction method.
Methods
The SCAP trial is a study of antipsychotic agent dose reduction in patients with chronic schizophrenia who were previously treated with multiple antipsychotic agents in Japanese medical institutions. All procedures performed in this study were developed with input from epidemiologic statisticians and were reviewed by the Fujita Health University ethical review board and the ethical review boards of the participating institutions. The study is registered in the Japanese clinical research database, University Hospital Medical Information Network Clinical Trials Registry (https://upload.umin.ac.jp/cgi-open-bin/ctr/ctr.cgi?function=brows&action=brows&type=summary&recptno=R000005391&language=E; UMIN-CTR; ID: UMIN000004511), and meets the standards of the International Committee of Medical Journal Editors (http://www.icmje.org/faq_clinical.html). In addition, the study was reported in the annual report of the Ministry of Health, Labor, and Welfare for the 3-year period from 2011 to 2013.
Design
The subjects were recruited from outpatient clinics or from among hospitalized patients diagnosed with schizophrenia using the structured clinical interview for DSM-IV-TR module B (First et al., 1997; American Psychiatric Association, 2000). Recruited subjects were administered 2 or more antipsychotic agents on a regular basis (continuously for 2 months or more) with a total chlorpromazine (CP) dose equivalent of 500 to 1500mg/d. The subjects were selected according to the judgments of the participating institutions. To evaluate the patient population for any selection bias, we compared the initial drug administration regimen between the subjects and hospitalized patients with schizophrenia at the participating institutions. The subjects were given a thorough explanation of the study procedures based on an approved document and provided written consent. Subjects who met the inclusion/exclusion criteria and gave consent were assigned to the dose-reduction group or observation group using a simple randomization with a random number table prepared by the study group organization and an agency independent of the participating institutions. According to the SCAP protocol, the dosage of 1 antipsychotic agent was reduced every week for subjects in the dose reduction group. The choice of which agents’ dose was reduced was left to the discretion of the attending physician. High-potency antipsychotics are defined as agents for which a dose equivalent to 100mg of CP is achieved with 10mg or less, whereas agents for which the dose equivalent of CP exceeds 10mg are considered low potency. Agents with low potency were reduced by 0 to 25mg/wk of the Japanese version of their CP dose equivalents (Inagaki and Inada, 2008), whereas dosages of high-potency agents were reduced by 0 to 50mg/wk of their CP dose equivalents. Each week, the attending physician determined whether to reduce the dose or temporarily revert to a higher dose based on the patient’s condition. In addition to psychiatric symptoms and safety, health-related QOL indices were also evaluated. The reduction in antipsychotic dose was continued for 12 weeks. The dose reduction procedures were discontinued in subjects who met all of the following criteria: treated effectively with <2 agents, the total dose was reduced to <1000mg of CP equivalent and to 80% or less of the initial dose, and 1 of 2 agents were reduced to <50mg. The subjects were then followed-up with the same dose for 12 weeks. Subjects who did not meet the above criteria were given another 12-week dose reduction, followed by a 12-week follow-up with a constant final dose. Subjects in the observation group were followed up for 12 weeks without any change in treatment dose. All changes in drug administration based on the judgment of the attending physician were recorded.
The Manchester scale was used for the evaluation of age, the patient’s history of hospitalization, and the severity of psychiatric symptoms (Manchanda et al., 1986; Takekawa et al., 1994). The drug-induced extrapyramidal symptoms scale was used for the assessment of extrapyramidal system side effects (Inada, 2009), and the UKU side effects rating scale was used to rate autonomic nervous system side effects (Lingjaerede, 1987). Additionally, general condition was evaluated using the Global Assessment of Function (American Psychiatric Association, 2000) and Clinical Global Impression of Symptom scales (Guy, 2000). The assessments of psychiatric symptoms and adverse effects were not blinded. The Manchester scale evaluated 8 basic symptoms, including positive, negative, and depressive symptoms. It was selected for use in this study based on its practicality, facilitating easy use in a daily clinical setting for the assessment of patients with chronic schizophrenia. To detect adverse effects on general health, we evaluated body height and weight, performed general biochemical blood tests, and carried out a typical 12-lead electrocardiography examination. Additionally, the EQ-5D (Tsuchiya et al., 2002) scale was used for the evaluation of health-related QOL using a self-recorded survey. The EQ-5D is commonly used for the evaluation of healthy life expectancy in a wide range of illnesses. Functional disturbances in 5 distinct items (movement, personal management, daily activity, pain/discomfort, and anxiety/depression) were evaluated in 3 stages, with 243 indexes allocated for each rating, ranging from 0 (dead) to 1 (best possible health status) and using the time trade-off approach. Visual analog scales were also administered at the same time to evaluate health indices using a 0- to 100-point scale. Since there is no verification of the Japanese version of visual analog scales evaluations, only the time trade-off approach scale, which has established reliability and validity in a Japanese clinical context, was used in the analysis (Tsuchiya, 2002). These evaluations were carried out every 12 weeks, with the Manchester scale and EQ-5D considered the primary standard evaluations. To perform a detailed observation, evaluations were carried out every 4 weeks in the first 12 weeks of the drug-reduction period.
Data Management and Statistical Analysis
Study progress data were collected and stored using an electronic data capture input to a secured cloud database. Analysts independent of the study group performed monitoring and management of the data input. Additionally, data were encoded in a way that prevented any possibility of identifying the individuals in the database.
The χ2 and Mann-Whitney tests were used for statistical comparisons of the characteristics of both groups, the drug administration dose (expressed in CP dose equivalents), and the dropout rate in both groups. Taking into consideration the effect of time, we tested group interactions in a comparison of the evaluation scales in both groups using the “no-response binary set-up repeated measurement linear mixed model” by setting the groups and period as fixed effects. The time period was unevenly distributed among the cases (ie, 12 weeks in the observation group and either a 12- or 24-week program, depending on progress, in the dose reduction group). Additionally, a number of institutions provided incomplete clinical study data, resulting in complicating effects that needed to be considered. The period of 4 weeks was considered as 1 unit, and 0, 1, 2, 3, 6, and 9 time points were used corresponding to 0, 4, 8, 12, 24, and 36 weeks. In addition, each evaluation scale was independently analyzed. Statistical analyses were performed using the Japanese version of SPSS 21.0 (IBM, Tokyo, Japan). Calculation of the statistical power was carried out using G*power 3.1.5 (Dusseldorf University, Dusseldorf, Germany).
Sample sizes were estimated for the clinical study to allow an evaluation of the noninferiority of the drug reduction group compared with the observation group on the primary evaluation scale based on a binary set-up repeated measurement linear mixed model. Parameters were set according to the customary practice used for repeated-measures analysis of variance analysis (Mizumoto and Takeuchi, 2010) with an α error of 0.025 (to allow for the analysis of multiple scales), a statistical power of 0.8, a noninferiority margin of Cohen’s D of 0.2 (the difference was predicted to be smaller than moderate), a correlation among repeated measures of 0.5, and the number of measurements was 6 times the calculated n, or n=142. Based on these parameters, the target number of cases for each group was 72.
Results
A total of 50 psychiatric medical institutions participated in the SCAP study (Acknowledgements) from November 2010 to March 2012. Each medical institution participating in the study was assessed by the Fujita Health University ethical review board and the ethical review board of the participating institution, with the evaluation reported in the annual report of Ministry of Health, Labor, and Welfare. A total of 6786 hospitalized patients with schizophrenia were undergoing treatment at the participating medical institutions (0–308 patients per institution, mean=138.5, SD=69.6). The patients participating in the study were receiving between 0 and 8 antipsychotic agents at the time of study commencement (mean=1.9, SD=1.1) with dosages ranging from 0 to 5309 CP equivalent mg/d (mean=774.5, SD=615.4). From November 2010 to March 2012, an additional 1 to 12 patients (mean=3.4, SD=2.7) were enrolled based on the judgments of the medical institutions. In total, 169 subjects were included in the clinical study.
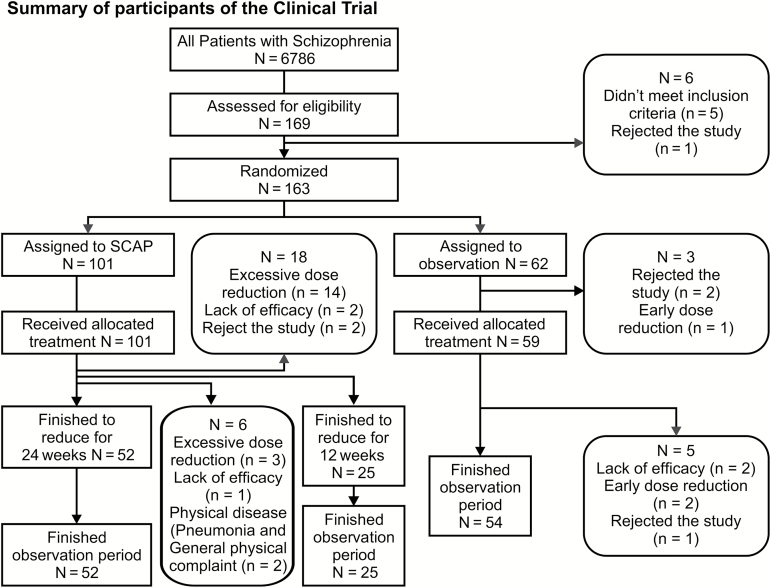
As shown in Figure 1, among the 169 study participants, 6 subjects withdrew consent or did not meet the inclusion criteria. Among the remaining 163 subjects, 101 subjects were assigned using a simple randomization to the dose reduction group, and 62 subjects were assigned to the observation group. The demographics of both groups are provided in Table 1. The randomized subjects were undergoing treatment with 2 to 5 antipsychotic agents (median, 2; interquartile range, 2–3) in accordance with the inclusion criteria at a dosage of 500 to 1499.9 CP equivalent mg/d (median, 1000; IQR, 800–1200). A comparison with the total patient population of the participating institutions showed a higher use of multiple agents (Z=9.0, 8.9, p=.000, .000, Mann-Whitney U test) among the study participants. The length of the intervention period in the dose reduction group was 24 or 36 weeks, depending on the effectiveness of the antipsychotic treatment. A total of 24 subjects (23.8%) dropped out of the SCAP group; 18 subjects dropped out before 12 weeks and 6 subjects dropped out prior to the study conclusion; 17 subjects (16.8%) were withdrawn because of protocol violations (excessive dose reduction due to a mistake by the treating physician) and 7 subjects (6.9%) because of patient factors (3 subjects withdrew because of a lack of efficacy, 2 because of physical disease; and 2 refused to continue participation in the study). In the observation group, 8 subjects (12.9%) dropped out prior to the study conclusion: 3 subjects because of protocol violations within the 12-week program (the dose was mistakenly reduced by the treating physician, as in the dose reduction group) and 5 subjects because of patient factors (3 subjects with worsening psychiatric symptoms and 2 subjects that voluntarily withdrew from the study). Among the subjects that experienced a worsening of psychiatric symptoms and physical complications, no serious health problems were observed, according to the Ministry of Health, Labor, and Welfare report. The results from the chi-squared tests did not show any difference in the total dropout rate between the groups (χ2=2.87, p=.090) or in the dropout rate, except from physician mistakes (χ2=0.00, p=.976). In total, 75 subjects in the dose reduction group and 54 subjects in the observation group concluded the study, but the data from all randomized subjects were analyzed.
Figure 1.
Summary of the participants in the clinical trial.
Table 1.
Demographics of the Dose Reduction and Observation Groups
| Dose reduction group, % | Observation group,% | Statistics | P | ||||
|---|---|---|---|---|---|---|---|
| N | 101 | 62 | |||||
| Male | 58 (57.4) | 38 (61.2) | χ2 | 0.237 | 0.63 | ||
| Diagnosis | |||||||
| Paranoid type | 49 (48.5) | 30 (48.4) | χ2 | 1.92 | 0.75 | ||
| Disorganized type | 25 (24.8) | 11 (17.7) | |||||
| Catatonic type | 3 (3.0) | 2 (3.2) | |||||
| Residual type | 13 (12.9) | 12 (19.4) | |||||
| Undifferentiated type | 11 (10.9) | 7 (11.3) | |||||
| Hospitalized patients | 81 (80.2) | 49 (79.0) | χ2 | 0.032 | 0.86 | ||
| med | IQR | med | IQR | ||||
| Age, y | 60 | 51 to 64 | 59 | 50 to 64 | Ua | 3055 | 0.80 |
| Disease duration, y | 32 | 19 to 40 | 31.5 | 3 to 19.3 | U | 2405 | 0.92 |
| Total hospitalization duration, y | 9.3 | 4.5 to 17 | 10.1 | 3 to 19.3 | U | 2481 | 0.88 |
| Body weight, kg | 62 | 52 to 68 | 60 | 52 to 70 | U | 2983 | 0.95 |
| Number of administered antipsychotic agents | 2 | 2 to 3 | 2 | 2 to 3 | U | 3088 | 0.96 |
| Dose of administered antipsychotic agents, CP mg/mL | 1012.3 | 800 to 1212 | 1000 | 800 to 1174.8 | U | 2876 | 0.44 |
Abbreviations: CP, chlorpromazine; IQR,. interquartile range
aThe Mann-Whitney test was used because the data were not normally distributed.
The changes in the evaluation scales in both groups over time are shown in Table 2. Limitations in the interpretation of the study results are explained in the Discussion regarding the differences in the number of subjects at each time point and with each scale. No adverse effect of the intervention was observed in the blood biochemical analysis or electrocardiography results. Drug administration in the dose reduction group was as follows: 2 (IQR, 2–3) agents per patient with a 1012.3 (799.9–1212.3) CP equivalent mg/d dose at the initial time (n=101) to 2 (1–2) agents per patient, with a 762.5 (600–950) CP equivalent mg/d dose at 24 weeks (n=77), corresponding to a 24.8% median dose reduction. At the study onset, 59% of the subjects used 2 agents, 35% used 3 agents, 6% used 4 agents, and 1% of the subjects used 5 agents (n=101). At the 24-week time point, 38%, 48%, 12%, and 3% of the participants used 1, 2, 3, and 4 agents, respectively (n=77). Therefore, 29 subjects switched to using a single agent. The changes between agent types are shown in Table 3. A comparison of the evaluation scales between the 2 groups using a linear mixed model analysis is shown in Table 4. No interaction was found (p≥.05) when considering all the scales administered. The effect size was <0.1, and the 95% confidence interval of the estimated difference was 0 in the evaluations of all the scales used. There were no significant differences between the 2 groups. Effect sizes in the EQ-5D, UKU, drug-induced extrapyramidal symptoms scale, and Global Assessment of Function were all >0.8, suggesting high statistical power in the analysis. These results support the noninferiority of the dose reduction group compared with the observation group.
Table 2.
Temporal Changes in the Evaluation Scales of the Dose Reduction and Observation Groups
| Scale | Dose reduction group | observationgroup | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Week | Week | ||||||||
| 0 | 4 | 8 | 12 | 24 | 36 | 0 | 12 | ||
| n | 101 | 94 | 85 | 83 | 77 | 52 | 62 | 54 | |
| Manchester scalea | Mean | 12.7 | 12.3 | 11.7 | 12.1 | 11.7 | 12.1 | 13.2 | 12.5 |
| SD | 5.2 | 5.5 | 5.6 | 5.6 | 6.0 | 6.1 | 5.6 | 5.7 | |
| EQ-5D TTOb | Mean | 0.83 | 0.75 | 0.79 | 0.85 | 0.85 | 0.83* | 0.79** | 0.8 |
| SD | 0.71 | 0.23 | 0.23 | 0.53 | 0.55 | 0.65 | 0.17 | 0.19 | |
| UKUc | Mean | 3.5 | 2.7 | 2.6 | 2.3 | 3.6 | 3.2 | ||
| SD | 2.7 | 2.6 | 2.2 | 2 | 3.3 | 3.5 | |||
| DIEPSSd | Mean | 5 | 4.3 | 3.9 | 3.8 | 5 | 4.2 | ||
| SD | 4.4 | 4.4 | 3.9 | 4 | 4.1 | 4.2 | |||
| CGI-Se | Mean | 4.5 | 4.5 | 4.5 | 4.4 | 4.6 | 4.5 | ||
| SD | 1.02 | 1.08 | 1.1 | 1.16 | 0.88 | 0.9 | |||
| GAFf | Mean | 45.8 | 46.6 | 47.7 | 48.1 | 47.7 | 49.8 | ||
| SD | 17.6 | 17.6 | 18.5 | 17.2 | 16.3 | 17.9 | |||
Abbreviations: CGI-S, Clinical Global Impression of Symptom; DIEPSS, drug-induced extrapyramidal symptoms scale; GAF, Global Assessment of Function; TTO, time trade-off approach.
* n = 51, **n = 61; both because 1 subject rejected the evaluation.
aManchester Scale: Psychiatric symptoms, 8–32 points (milder cases show lower values).
bEQ-5D TTO: 0.111–1.000 points (healthy participants show higher values)
cUKU-11: Autonomic nervous system side effects, 0–33 points (milder cases show lower values).
dDIEPSS: Motor system side effects, 0–36 points (milder cases show lower values)
eCGI-S: General impression of the clinician, 1–7 points (milder cases show lower values).
fGAF: Overall function evaluation, 0–100 points (higher functionality shows a higher value).
Table 3.
Changes in the Number of Subjects Administered Antipsychotic Agents in the Dose Reduction Group
| Agent | Before dose reduction | After dose reduction | Reduction rate, % |
|---|---|---|---|
| Risperidone | 63 | 47 | 25 |
| Olanzapine | 34 | 25 | 26 |
| Chlorpromazine | 31 | 11 | 65 |
| Levomepromazine | 27 | 8 | 70 |
| Zotepine | 19 | 10 | 47 |
| Haloperidol | 19 | 13 | 32 |
| Quetiapine | 17 | 14 | 18 |
| Aripiprazole | 16 | 10 | 37 |
| Blonanserin | 9 | 6 | 33 |
| Perospirone | 8 | 5 | 37 |
Before dose reduction, n = 101; after dose reduction, n = 77. Only agents administered to more than 5 subjects are included.
Table 4.
Analysis of the Evaluation Scales in the Dose Reduction Group and Observation Group Based on an LMM Analysis
| Dependent variables | n | Type III test of fixed effect | Estimated difference (Bonferroni correction) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| F intercept | F fixed factor | P value | Effect size | Power | Estimated meana | 95% CI | P value | ||
| Mann | 163 | 864.2 | 0.25 | 0.62 | 0.017 | 0.65 | –0.83 | (–2.56–0.9) | 0.35 |
| EQ-5D | 162 | 1002.7 | 0.0013 | 0.97 | 0.001 | 0.97 | 0.02 | (–0.08–0.13) | 0.68 |
| UKU-11 | 163 | 194.5 | 1.41 | 0.24 | 0.085 | 0.84 | –0.64 | (–1.48–0.2) | 0.14 |
| DIEPSS | 163 | 192.8 | 1.39 | 0.24 | 0.085 | 0.84 | –0.55 | (–1.85–0.75) | 0.4 |
| CGI-S | 163 | 3381.4 | 0.56 | 0.45 | 0.013 | 0.48 | –0.082 | (–0.39–0.23) | 0.6 |
| GAF | 163 | 1283.7 | 0.041 | 0.84 | 0.0057 | 0.84 | –1.4 | (–6.73–3.97) | 0.61 |
Abbreviations: CGI-S, Clinical Global Impression of Symptom; DIEPSS, drug-induced extrapyramidal symptoms scale; GAF, Global Assessment of Function.
aComparison of values between the dose reduction group and observation group.
Discussion
The practicality of reducing the antipsychotic agent dose in an individualized manner was verified in a randomized controlled trial evaluating 163 patients with schizophrenia enrolled from 50 Japanese psychiatric medical institutions. The aim of the trial was to propose and validate a safe, effective, and realistic method of correcting the high rate of polypharmacy in the Japanese medical therapy of schizophrenia, which is considered unnecessarily high by international standards. The enrollment was focused on chronic patients and included patients that had a significantly higher use of multiple agents compared with the general patient populations of the participating institutions. Regardless of the 23% drug reduction, the clinical study results using the SCAP method of dose reduction demonstrated that clinical symptoms, risk of side effects, and health-related QOL were not significantly different in the dose reduction group compared with the observation group at 12 and 24 weeks following the beginning of the dose reduction regimen. These observations support the hypothesized noninferiority of the dose reduction group to the observational group and suggest that the findings were obtained with high statistical power. In the current study, the dose was reduced by 9.7mg/wk, which is slower than the proposed dose reduction limit for a low-potency agent, that is, 25mg/wk. Additionally, no significant worsening of symptoms was observed at the follow-up 3 months after the dose reduction, suggesting that the SCAP method is a safe and useful method that can be applied in a clinical setting.
In previously reported approaches, Essock et al. (2011) reduced the agents over 30 days, whereas Suzuki et al. (2004) replaced polypharmacy with another agent of equal power. The approach used by Essock et al. (2011) may have been excessively fast, preventing them from arriving at a single dosage. We focused on optimizing the speed of dose reduction and obtained a dropout rate that was lower than that in their study for a similar period (24% and 31%, respectively). Dopamine hypersensitivity has been reported as a possible consequence of the long-term use of antipsychotic agents (Samaha, 2013). An abrupt reduction in antipsychotic dose is believed to severely disturb the homeostasis of patients receiving polypharmacy. The SCAP method adopts a gradual dose reduction, which can minimize the disruption to the patient’s neurophysiology, resulting in the demonstrated maintenance of effective treatment with no difference in adverse effects.
In the SCAP method, the choice of which agents to reduce is left to the discretion of the attending physician. Before the reduction in dose, 1 main agent is selected and the doses of the other agents are sequentially reduced. The agents selected for dose reduction in this study are presented in the Results, with the first generation of low-potency agents such as CP and levomepromazine generally chosen. In a Japanese clinical setting, in addition to the main agent, it is common to prescribe a small amount of CP (and combined agent) and levomepromazine for sedation. A dose reduction of the secondary agents in the SCAP method is achievable, because the selection of primary agent is generally clear.
On the other hand, the intervention methods varied in terms of differences in the dose reduction amounts and time period. Additionally, differences in the initial combinations and doses of antipsychotic agents at the onset of the study represent a limitation of the present study. To address this issue, a repeated measurement linear mixed model was used in the statistical analysis, taking into consideration the variability in the period between the subjects as well as missing values.
Medical therapy for schizophrenia is usually administered over a prolonged period of time. Several important aspects need to be considered when administering antipsychotic therapy, such as the effects of the antipsychotic agent on extrapyramidal motor function, autonomic visceral function, and the maintenance of the patient’s QOL. In this study, because changes in treatments other than the antipsychotic agents such as anti-Parkinsonian therapy, sedatives prescribed for sleep regulation, and laxatives used to treat constipation were not restricted, the effects of the changes on the primary outcomes are not known. However, based on the clinical judgment of each participating institution and considering that the above agents were adjusted in accordance with the dose reduction of the antipsychotic agents, no worsening of motor function or autonomic nervous system function was observed. This finding suggests that a gradual reduction in doses of antipsychotic agents according to the SCAP method can avoid the harmful effects of rapid treatment discontinuation. Additionally, no major health problems were reported during the study period, providing additional support for the safety of the proposed dose reduction protocol. There were also no significant changes in health-related QOL. Because most subjects in this study were long-term hospitalized patients, daily living activities were limited to their experiences inside the medical institution. Additionally, because of social cognitive function deficits common in patients with psychiatric disorders, a concern remains regarding whether health-related QOL can be compared with the impact of other diseases. Finally, the fact that the psychiatric patients’ health-related QOL was assessed using the EQ-5D self-reported survey completed by the patients is considered to be a limitation (Atkinson et al., 1997).
There was no stratification of participants based on age or sex in this study. The subjects were assigned to either the dose reduction group or the control group by a simple randomization using a random sampling table. As an outcome of the randomization, 101 subjects completed the protocol in the dose reduction group compared with 62 subjects in the observation group. Simple randomization is thought to keep the quality of both groups even, but the chance exists that the number of participants in the groups will become uneven with a total enrollment of <100 subjects (Kang et al., 2008). The target number of subjects for both groups was a total of 144 subjects in this study. Despite the prediction that the group numbers would be even in the study planning stage, the end result was an imbalance of 5:3, suggesting a limitation in the results because there were more subjects than targeted in the dose reduction group but 10 fewer subjects than planned in the observation group. Although options for a change in plans and to conduct stratification were available, we opted for a simple randomization for the study period. Additionally, if Cohen’s D was set at a moderate level of 0.25, the required number of subjects in each group would have been 38, which would conceivably show that dose reduction-related worsening was not higher than a moderate effect.
Conclusion
We conducted a verification of a realistic dose reduction method (the SCAP method) to minimize the risk of adverse effects caused by the abrupt discontinuation of long-term exposure to high doses of antipsychotic agents in schizophrenia treatment. The standard of care for the management of schizophrenia in Japan commonly involves the concurrent use of more than 3 antipsychotic agents, which is considered high by international standards. When interpreting the outcomes of this study, one should consider several limitations, including variability in the subjects’ antipsychotic treatment history, the magnitude of dose reduction, heterogeneity in the agents selected for reduction, the possibility of rater bias, the lack of consideration of other agents (ie, anti-Parkinsonian drugs), the difference in the numbers between the 2 groups because of the simple randomization method used, and the time period during which the dose reduction was implemented as well as the use of self-reported evaluation scales in patients with cognitive dysfunction. Patients who underwent the SCAP dose reduction method were observed not to exhibit any significant difference in psychiatric symptoms or nonpsychiatric side effects compared with participants in the observation group. Additionally, our study observed a low dropout rate and high tolerability of the SCAP dose reduction protocol. The distribution of an appropriate and safe dose reduction method with a suitable remuneration incentive using the SCAP method is thought to have the potential to positively influence decision-making towards reduced polypharmacy in medical therapy (Goh et al., 2011). This method could become one of the steps towards the reasonable optimization of polypharmacy.
Statement of Interest
None
Acknowledgments
The authors thank all those who collaborated with us in this study, namely the SCAP study cooperation group, everyone at the Research Office, as well as the Japanese Ministry of Health, Labor, and Welfare. Editage, an editing agency, assisted in the preparation of this manuscript.
References
- American Psychiatric Association (2000). Diagnostic and statistical manual of mental disorders. 4th ed text rev (DSM-IV-TR). Washington: American Psychiatric Association. [Google Scholar]
- Atkinson M, Zibin S, Chuang H. (1997). Characterizing quality of life among patients with chronic mental illness: a critical examination of the self-report methodology. Am J Psychiatry 154:99–105. [DOI] [PubMed] [Google Scholar]
- Barnes TRE, Paton C. (2011). Antipsychotic polypharmacy in schizophrenia: benefits and risks. CNS Drugs 25:383–399. [DOI] [PubMed] [Google Scholar]
- Collell CU, Rummel-Kluge C, Corves C, Kane JM, Leucht S. (2009). Antipsychotic combinations vs monotherapy in schizophrenia: a meta-analysis of randomized controlled trials. Schizophr Bull 35:443–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essock SM, Schooler NR, Stroup TS, McEvoy JP, Rojas I, Jackson C, Covell NH, Schizophrenia Trials Network (2011). Effectiveness of switching from antipsychotic polypharmacy to monotherapy. Am J Psychiatry 168:702–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farde L, Nordstrom AL, Wiesel FA, Pauli S, Halldin C, Sedvall G. (1992). Positron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine relation to extrapyramidal side effects. Arch Gen Psychiatry 49:538–544. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. (1997). Structured clinical interview for DSM-IV axis I disorders. Biometrics Research Department, Colombia University, NY [Japanese translation/rights arranged with First M.B. though Japan UNI Agency, Inc., Tokyo].
- Fleischhacker WW, Uchida H. (2014). Critical review of antipsychotic polypharmacy in the treatment of schizophrenia. Int J Neuropsychopharmacol 17:1083–1093. [DOI] [PubMed] [Google Scholar]
- Goh YL, Seng KH, Chuan ASH, Chua HC. (2011). Reducing antipsychotic polypharmacy among psychogeriatric and adult patients with chronic schizophrenia. Perm J 15:52–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy W. (2000). Clinical Global Impressions (CGI) Scale. Modified from: Rush J, et al.: Psychiatric Measures. Washington DC: American Psychiatric Association. [Google Scholar]
- Inada T. (2009). DIEPSS: a second-generation rating scale for antipsychotic-induced extrapyramidal symptoms: drug-induced Extrapyramidal Symptoms Scale [in Japanese]. Tokyo: Seiwa Shoten Publishers, Inc. [Google Scholar]
- Inagaki A, Inada T. (2008). Calculation of the equivalent doses of new antipsychotics (V) [in Japanese]. Blonanserin Jpn J Clin Psychopharmacol 5:887–890. [Google Scholar]
- Ito H, Okumura Y, Higuchi T, Tan CH, Shinfuku N. (2012). International variation in antipsychotic prescribing for schizophrenia: pooled results from the research on East Asia psychotropic prescription (reap) studies. Open J Psychiatr 2:340–346. [Google Scholar]
- Kang M, Ragan BG, Park JH. (2008). Issues in outcomes research: an overview of randomization techniques for clinical trials. J Athl Train 43:215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingjaerede O, Ahlfors UG, Bech P, Dencker SJ, Elgen K. (1987). The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand Suppl 334:11–100. [DOI] [PubMed] [Google Scholar]
- Manchanda R, Saupe R, Hirsch SR. (1986). Comparison between the Brief Psychiatric Rating Scale and the Manchester Scale for the rating of schizophrenic symptoms. Acta Psychiatr Scand 74:563–568. [DOI] [PubMed] [Google Scholar]
- Miodownik C, Lerner V, Kibari A, Toder D, Cohen H. (2006). The effect of sudden clozapine discontinuation of management of schizophrenic patients: a retrospective controlled study. J Clin Psychiatry 67:1204–1208. [DOI] [PubMed] [Google Scholar]
- Mizumoto A, Takeuchi O. (2010). Primer of effect size and statistical power [in Japanese]. LET Kansai Chapter Collected Papers. 47–73. [Google Scholar]
- Murasugi K, Hagiwara T, Shouda S. (2004). Attempt to correct antipsychotic administration to monotherapy and dose reduction for chronic schizophrenia patients. Development of the treatment and rehabilitation guideline for schizophrenia and the demonstration study of its effectiveness: Final Report of the National Project Team, supported by the research grant for nervous and mental disorders from the Ministry of Health, Labour and Welfare, Japan in 2001, pp67–73. Tokyo: Ministry Health, Labour and Welfare in Japan. [in Japanese].
- Okumura Y, Noda T, Ito H. (2013). Antipsychotics prescribing patterns of with schizophrenia in Japan: using the national database of health insurance claim information and specified medical checkups [in Japanese]. Jpn J Clin Psychopharmacol 16:1201–1215. [Google Scholar]
- Samaha AN. (2013). Can antipsychotic treatment contribute to drug addiction in schizophrenia? Prog Neuropsychopharmacol Biol Psychiatry 52:9–16. [DOI] [PubMed] [Google Scholar]
- Sukegawa T1, Inagaki A, Yamanouchi Y, Inada T, Yoshio T, Yoshimura R, Iwata N. (2014). Study protocol: safety correction of high dose antipsychotic polypharmacy in Japan. BMC Psychiatry 14:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Uchida H, Tanaka KF, Nomura K, Takano H, Tanabe A, Watanabe K, Yagi G, Kashima H. (2004). Revising polypharmacy to a single antipsychotic regimen for patients with chronic schizophrenia. Int J Neuropsychopharmacol 7:133–142. [DOI] [PubMed] [Google Scholar]
- Takekawa Y, Hori A, Tsunashima K. (1994). Reliability and validity of Japanese version of Manchester Scale [in Japanese]. Seishinigaku 36:389–394. [Google Scholar]
- Tani H, Uchida H, Suzuki T, Fujii Y, Mimura M. (2012). Interventions to reduce antipsychotic polypharmacy: a systematic review. Schizophr Res 143:215–220. [DOI] [PubMed] [Google Scholar]
- Thompson A, Sullivan SA, Barley M, Strange SO, Moore L, Rogers P, Sipos A, Harrison G. (2008). The DEBIT trial: an intervention to reduce antipsychotic polypharmacy prescribing in adult psychiatry wards: a cluster randomized controlled trial. Psychol Med 38:705–715. [DOI] [PubMed] [Google Scholar]
- Tsuchiya A, Ikeda S, Ikegami N, Nishimura S, Sakai I, Fukuda T, Hamashima C, Hisashige A, Tamura M. (2002). Estimating an EQ-5D population value set: the case of Japan. Health Econ 11:341–353. [DOI] [PubMed] [Google Scholar]
- Xiang YT, Wang CY, Si TM, Lee EH, He YL, Ungvari GS, Chiu HF, Yang SY, Chong MY, Tan CH, Kua EH, Fujii S, Sim K, Yong KH, Trivedi JK, Chung EK, Udomratn P, Chee KY, Sartorius N, Shinfuku N. (2012). Antipsychotic polypharmacy in inpatients with schizophrenia in Asia (2001–2009). Pharmacopsychiatry 45:7–12. [DOI] [PubMed] [Google Scholar]
- Yoshio T, Inada T, Uno J, Miwa T, Kitagawa K, Miyahara Y, Umeda K, Kato T, Inagaki A, Nabeshima T. (2012). Prescription profiles for pharmacological treatment of Japanese inpatients with schizophrenia: comparison between 2007 and 2009. Hum Psychopharmacol 27:70–75. [DOI] [PubMed] [Google Scholar]