Abstract
Background:
Ethanol exposure to rodents during postnatal day 7 (P7), which is comparable to the third trimester of human pregnancy, induces long-term potentiation and memory deficits. However, the molecular mechanisms underlying these deficits are still poorly understood.
Methods:
In the present study, we explored the potential role of epigenetic changes at cannabinoid type 1 (CB1R) exon1 and additional CB1R functions, which could promote memory deficits in animal models of fetal alcohol spectrum disorder.
Results:
We found that ethanol treatment of P7 mice enhances acetylation of H4 on lysine 8 (H4K8ace) at CB1R exon1, CB1R binding as well as the CB1R agonist-stimulated GTPγS binding in the hippocampus and neocortex, two brain regions that are vulnerable to ethanol at P7 and are important for memory formation and storage, respectively. We also found that ethanol inhibits cyclic adenosine monophosphate response element-binding protein (CREB) phosphorylation and activity-regulated cytoskeleton-associated protein (Arc) expression in neonatal and adult mice. The blockade or genetic deletion of CB1Rs prior to ethanol treatment at P7 rescued CREB phosphorylation and Arc expression. CB1R knockout mice exhibited neither ethanol-induced neurodegeneration nor inhibition of CREB phosphorylation or Arc expression. However, both neonatal and adult mice did exhibit enhanced CREB phosphorylation and Arc protein expression. P7 ethanol-treated adult mice exhibited impaired spatial and social recognition memory, which were prevented by the pharmacological blockade or deletion of CB1Rs at P7.
Conclusions:
Together, these findings suggest that P7 ethanol treatment induces CB1R expression through epigenetic modification of the CB1R gene, and that the enhanced CB1R function induces pCREB, Arc, spatial, and social memory deficits in adult mice.
Keywords: cannabinoid receptor system, epigenetics, FASD, memory loss, synaptic signaling
Introduction
Alcohol consumption during pregnancy exposes fetal brains to ethanol that causes various birth defects (Jones and Smith, 1973) in humans, collectively known as fetal alcohol spectrum disorders (FASDs; Streissguth et al., 1990). The consequential neurological abnormalities (Goodman et al., 1999; Mattson et al., 1999) are understood to be one of the major causes of intellectual disability in Western nations (Mattson et al., 2011). The studies using developmental animal models have long established that fetal ethanol exposure is associated with enormous reduction in the number of neurons in numerous brain regions, including the hippocampus (Olney, 2004), in addition to long-lasting synaptic and memory deficits in adult rodents (Izumi et al., 2005; Wilson et al., 2011; Sadrian et al., 2012; Subbanna et al., 2013a). Several pathways appear to activate neuronal death by ethanol; however, recent studies suggest that the endocannabinoid system not only contributes to neurodegeneration (Hansen et al., 2008; Subbanna et al., 2013a), but also plays a significant role in the development of synaptic and learning and memory deficits in adult mice (Subbanna et al., 2013a).
The endocannabinoid system includes endogenous ligands, cannabinoid type 1 (CB1R) and 2 receptors, and synthesizing and degrading enzymes (Piomelli, 2003; Basavarajappa, 2007; Subbanna et al., 2013a). A substantial amount of previous research has demonstrated multiple ways in which the endocan nabinoid system regulates synaptic events (Ohno-Shosaku et al., 2001; Wilson and Nicoll, 2001; Bacci et al., 2004) in the develop ing (Berghuis et al., 2007; Mulder et al., 2008; Subbanna et al., 2013a) and adult brain (see Basavarajappa et al., 2009). Research findings from animal and human studies imply that the endocannabinoid system is one of the most relevant biochemical systems mediating the action of ethanol in multiple brain regions (Basavarajappa et al., 1998, 2003, 2006, 2008; Basavarajappa and Hungund, 1999a, 1999b; Roberto et al., 2010; DePoy et al., 2013; Hirvonen et al., 2013; Subbanna et al., 2013a; Ceccarini et al., 2014).
The CB1R is one of the most abundant inhibitory G-protein-coupled receptors expressed in the brain (Howlett et al., 1986; Herkenham et al., 1990) and is primarily located on presynaptic terminals, where it controls neurotransmitter release (Matyas et al., 2007). The ability of the CB1R to suppress neurotransmission allows endogenous cannabinoids such as anandamide (AEA) and 2-arachidonylglycerol to prevent the recruitment of new synapses (Kim and Thayer, 2001), which has a profound negative impact on neuronal communication, learning, and memory (Castellano et al., 2003; Mechoulam and Parker, 2013; Subbanna et al., 2013a). Moreover, cannabis use during brain development induces several specific human developmental disorders (Stefanis et al., 2004), including fetal alcohol syndrome–like deficits (Wu et al., 2011), which are likely mediated through the activation of CB1Rs.
Recently, epigenetic alterations have been shown to play a role in both normal development and several human developmental disorders (Campuzano et al., 1996; Petronis, 2003; Makedonski et al., 2005; Ryu et al., 2006; Warren, 2007; Gavin and Sharma, 2010), and have been implicated in developmental ethanol effects (Kaminen-Ahola et al., 2010; Bekdash et al., 2013; Perkins et al., 2013), including neurodegeneration (Subbanna et al., 2013b; Subbanna et al., 2014). Although the detailed mechanisms are not yet clear, we have recently shown that P7 ethanol treatment increases AEA/CB1R signaling, results in neonatal neurodegeneration, and contributes to the development of synaptic and object recognition memory deficits relevant to FASD (Subbanna et al., 2013a). In the present study, we explored the epigenetic and CB1R-mediated signaling events that may directly cause the neurodegeneration in neonatal mice and spatial and social recognition memory deficits in adult mice.
Methods
Animals and Treatment
Male C57BL/6J, CB1R wild type (WT), and knock out (KO) mice (Subbanna et al., 2013a) on a C57BL/6J background were generated from a heterozygous breeding colony at NKI. C57BL/6J, CB1RWT, and KO mice were housed in groups under standard laboratory conditions (12h light/dark cycle) with food and water available ad libitum. Animal care and handling procedures followed institutional (NKI IACUC) and National Institutes of Health guidelines. Ethanol (2.5g/kg s. c. at 0h and again at 2h) and [N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4- methyl-1 H-pyrazole-3-carboxamidehydrochloride)] (SR141716A) (SR; 1mg/kg, 10 μl/g body weight) treatment and determination of ethanol levels were carried out as previously described by our laboratory (Subbanna et al., 2013a; Subbanna et al., 2013b). Three- to four-month-old mice were used for several analyses, as described below. For independent experiments, 5–8 animals were used.
ChIP Assay
Chromatin immunoprecipitation (ChIP) assay was performed as described before (Subbanna et al., 2014). For the ChIP assay, pups were sacrificed by decapitation and the hippocampus and neocortex were dissected 8h after the first saline or ethanol injection. Tissue (25mg) was fixed by 1% formaldehyde, homogenized, and subjected to DNA shearing; the amount of sample was normalized to contain equivalent protein amounts. Chromatin was immunoprecipitated with anti-acetyl histone H4K8 (# 07-328; Millipore) and anti-H3K9me2 (#4658; Cell Signaling) antibodies. As a negative control, samples were immunoprecipitated with rabbit IgG (Millipore). Immune-complexes were collected and processed as described before (Subbanna et al., 2014) using primers for mouse CNR1 (CB1R) exon I (mouse CNR1 219 F 5’-AGGAGACACAACCAACATTACA-3’, mouse CNR1 277 P 5’-AACGAGGACAACATCCAGTGTGGG-3’, and mouse CNR1 307 5’-TGAAGCACTCCATGTCCATAAA-3’). Relative quantification for acetylated and methylated histone-associated genes in saline and ethanol groups was calculated by the ∆∆Ct method (Schmittgen and Livak, 2008).
Protein extraction, Electrophoresis, and Immunoblotting
For Western blot analysis, homogenates from the flash frozen hippocampus (HP) and neocortex (NC) from neonates and adult (P 90) was processed to prepare nuclear and total extracts (Basavarajappa et al., 2014; Basavarajappa and Subbanna, 2014) as described previously (Lubin and Sweatt, 2007; Subbanna et al., 2013b). The samples were prepared in a sample buffer as described previously (Basavarajappa et al., 2008; Subbanna et al., 2013a). Membranes were Ponceau S stained to confirm equal loading in each lane and were incubated in a primary antibody: anti-rabbit-pCREB (Ser133; # 9198, 1:1 000) and anti-rabbit-CREB (# 9197, 1:1 000; Cell Signaling Technology), anti-mouse activity-regulated cytoskeleton-associated protein (Arc; #sc 17839, 1:1 000; Santa Cruz Biotechnology), anti-rabbit cleaved caspase-3 (CC3; # 9661, 1:1 000), or anti-mouse-β-actin (#3700, 1:1 000; Cell Signaling Technology) for 3h at room temperature or overnight at 4°C and processed as described previously (Basavarajappa et al., 2008). Incubation of blots with a secondary antibody (goat anti-mouse peroxidase conjugate, #AP 124P, 1:5 000; goat anti-rabbit, #AP132P, 1:5000; Millipore) alone did not produce any bands.
CB1R-Binding Assay
The CB1R-binding assay was performed with [3H] CP-55,940 as the labeled ligand. A rapid filtration–binding assay was followed by a procedure as described previously (Basavarajappa et al., 1998, 2006). HP and NC from P7 saline- and ethanol-treated mice were isolated and homogenized in an ice-cold homogenization buffer (Basavarajappa et al., 2014; Basavarajappa and Subbanna, 2014) containing a freshly added 1% protease inhibitor mixture (Roche). Plasma membranes (PMs) were prepared as described previously (Basavarajappa et al., 1998, 2006) and appropriate aliquots were stored at -80°C until use. Assay solutions were incubated in silicone-treated tubes for 60min at 30°C, with a final assay volume of 0.5ml containing 4nM [3H] CP-55,940 and a final PM concentration of 0.02mg protein/ml. Nonspecific binding was determined in the presence of 30 μM CP-55,940 and was subtracted to yield specific binding values. Bound [3H] CP-55,940 was harvested by vacuum filtration through Whatman GF/B filters with a Brandel Cell Harvester. Radioactivity was determined by liquid scintillation counting after overnight incubation of filters in 5ml of scintillation mixture UniverSol.
Agonist-Stimulated [35S] GTPγS Binding
CP-55,940-stimulated [35S] GTPγS binding was performed as described previously (Basavarajappa and Hungund, 1999a; Basavarajappa et al., 2006). PMs from HP and NC from P7 saline- and ethanol-treated mice were diluted in an assay buffer. Homogenates (20 μg of protein) were incubated with 0.1nM [35S] GTPγS, appropriate concentrations of CP55,940 in an assay buffer containing 100 μM GDP, and 0.1% BSA in a final volume of 0.5ml for 60min at 30°C. Nonspecific binding was determined in the presence of 30 μM GTPγS and was subtracted to yield specific binding values. Bound [35S] GTPγS was determined by liquid scintillation counting after overnight incubation of filters in 5ml of UniverSol scintillation fluid.
Behavioral Testing
Spontaneous Alternation Y Maze Task
Spontaneous alternation (Dember and Fowler, 1958) was tested as described previously (Holcomb et al., 1998) using the symmetrical Y maze exactly as described previously (Basavarajappa et al., 2014; Basavarajappa and Subbanna, 2014). Briefly, this experiment included three- to four-month-old male C57BL/6J mice treated at P7 with saline, ethanol, SR, or ethanol + SR, as well as CB1RWT and KO mice treated with saline or ethanol (n = 8/group). Percentage alternation is the number of trials containing entries into all three arms divided by the maximum possible alternations (the total number of arms entered minus 2) x 100.
Spatial Recognition Memory Using the Y Maze
Spatial recognition memory (Dellu et al., 1992) was tested as described previously (Sarnyai et al., 2000) using the symmetrical Y maze exactly as described previously (Basavarajappa et al., 2014; Basavarajappa and Subbanna, 2014). Briefly, this experiment included three- to four-month-old male C57BL/6J mice treated at P7 with saline, ethanol, SR, or ethanol + SR as well as CB1RWT and KO mice treated with saline or ethanol (n = 8/group). The number of entries and the time spent in each arm, as well as the first choice of entry, were registered manually and from video recordings by an observer blind to the treatment or genotype of the mice.
SRM
A social recognition memory (SRM) test (Thor et al., 1982) was performed exactly as previously described (Subbanna and Basavarajappa, 2014). In brief, three- to four-month-old male C57BL/6J mice treated at P7 with saline, ethanol, SR, or ethanol + SR as well as CB1RWT and KO mice treated with saline or ethanol (n = 8/group) were used. Each mouse was placed into an individual cage in an observation room under dim light and was allowed to habituate to the new environment for 15min immediately prior to the experimental sessions. A male juvenile mouse (3–4 weeks old) was placed into a cage with an adult for an initial interaction trial of 2min. Behaviors that were scored as social investigation were previously described (Subbanna and Basavarajappa, 2014). The percentage of social investigation was calculated by dividing the investigation time during the second exposure by the initial investigation time × 100.
Statistics
A statistical comparison of the data was performed by either a student’s t-test, one-way analysis of variance (ANOVA), or two-way ANOVA with Bonferroni’s post hoc test. In all of the comparisons, p < 0.05 was considered to indicate statistical significance. The statistical analyses were performed using Prism software (GraphPad).
Results
Neonatal Exposure to Ethanol Enhances H4K8 Acetylation and Demethylates H3K9 at CB1R Exon1
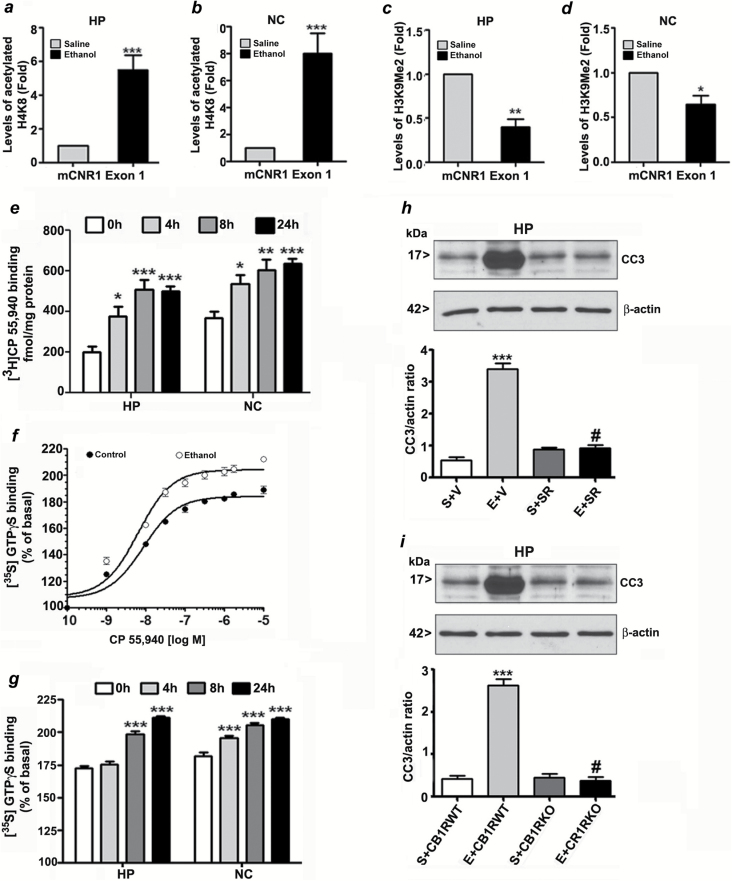
We used ChIP assay to determine whether CB1R transcriptional activation involves specific epigenetic modification of histone proteins in exon1 of the CB1R gene. The results indicated that ethanol treatment increased acetylated H4K8 levels (Figure 1a and b; p < 0.001) and reduced dimethylated H3K9 (Figure 1c and d) at CB1R exon I in the HP (p < 0.01) and NC (p < 0.05), which is correlated with increased CB1R transcription (Subbanna et al., 2013a).
Figure 1.
Enhanced H4K8 acetylation and reduced H3K9 dimethylation at exon1 of the CB1R gene regulate postnatal ethanol-induced expression of CB1R. ChIP analysis of the CB1R exon1 gene in hippocampal (HP) and neocortical (NC) tissues from the saline and ethanol groups (n = 8 pups/group) with anti-acetylated H4K8 (a and b) or anti-H3K9me2 (c and d) antibodies and levels of CB1R exon 1 chromatin enrichment in the IPs were measured by quantitative PCR. *p < 0.05,**p < 0.01,***p < 0.001; compared with respective saline group; student’s t-test. Error bars = SEM. (e) CB1R binding was performed with PM (20mg protein/ml) at 30°C for 60min using [3H] CP-55,940 as the labeled ligand and 30μM unlabeled CP-55,940 to define non-specific binding. Each point is the mean ± SEM (n = 12/group). [35S] GTPγS binding was performed with various concentrations (f) or 2 μM (g) of CP-55, 940. Non-specific binding was determined in the presence of unlabeled GTPγS (30 μM). Data are expressed as the percentage of basal [35S] GTPγS binding. Basal [35S] GTPγS binding in the absence of CP-55,940 was ranged between 4.4 ± 0.50 (NC) and 5.0 ± 0.2 (HP) pmol/mg protein. Error bars = SEM (n = 12/group). (h) Mice pre-treated for 30min with SR141716A (SR; 1mg/kg) or vehicle were exposed to ethanol, and CC3 levels were determined by a Western blot analysis (***p < 0.001 vs. S+V; #p < 0.001 vs. E+V). (i) CB1R WT and KO mice were exposed to ethanol, and CC3 levels were determined by a Western blot analysis. β-actin was used as a loading control. Each point is the mean ± SEM. ***p < 0.001 vs S+CB1RWT; #p < 0.001 vs E+CB1RWT.
Enhanced CB1R Binding and CB1R Agonist-Stimulated GTPγS Binding During Ethanol-Induced Neurodegeneration in the Developing Brain
Administration of ethanol to mouse pups at P7 resulted in an ethanol level of ~0.47 ± 0.25g/dL at 3h that was gradually reduced to 0.27 ± 0.07g/dL at 9h following injection. This ethanol paradigm has been shown to produce a widespread pattern of neurodegeneration throughout the forebrain, including the HP and NC, as indicated by the formation of CC3 in ethanol-exposed brains.
Our current results demonstrated that P7 ethanol treatment significantly enhanced the specific binding of CP-55,940 in a time-dependent manner both in the HP and NC (p < 0.05; Figure 1e). Furthermore, to examine the function of CB1Rs after P7 ethanol treatment, we examined CP-55,940-stimulated [35S] GTPγS binding in PMs (Figure 1f). The increase in [35S] GTPγS binding over basal levels stimulated by CP-55,940 was enhanced in a time-dependent manner in the HP and NC (p < 0.05) of the ethanol-treated groups compared to the saline groups (Figure 1g). To further evaluate the involvement of CB1R activity in ethanol-induced neurodegeneration, we used CB1RKO mice or a specific CB1R antagonist (SR) in WT mice and evaluated the ability of CB1R inhibition to prevent ethanol-induced CB1R-mediated neurodegeneration. The administration of SR (1mg/kg) 30min prior to ethanol treatment did not alter BALs (BAL peaked at 3h at 0.47 ± 0.08g/dL and was gradually reduced to 0.26 ± 0.07g/dL at 9h). In addition, the CB1RKO mice did not display altered BALs (BAL peaked at 3h at 0.48 ± 0.09g/dL and was gradually reduced to 0.28 ± 0.06g/dL at 9h). Two-way ANOVA analysis of CC3 levels suggested that the effects of ethanol (vs. saline F1, 20 = 135, p < 0.001) and SR (vs. saline, F1, 20 = 69, p < 0.001) were significant and that a significant interaction existed between ethanol and SR (F1, 20 = 65, p < 0.001; Figure 1h). Neither SR nor vehicle alone had any significant effects on CC3 levels in the absence of subsequent ethanol treatment. In addition, one-way ANOVA suggested that CB1RKO provided protection against P7 ethanol-induced neurodegeneration in the HP when compared with the WT mice (CC3: F3, 20 = 220, p < 0.001; Figure 1i). CB1RKO mice treated with saline showed normal CC3 levels, similar to observations in WT mice treated with SR alone.
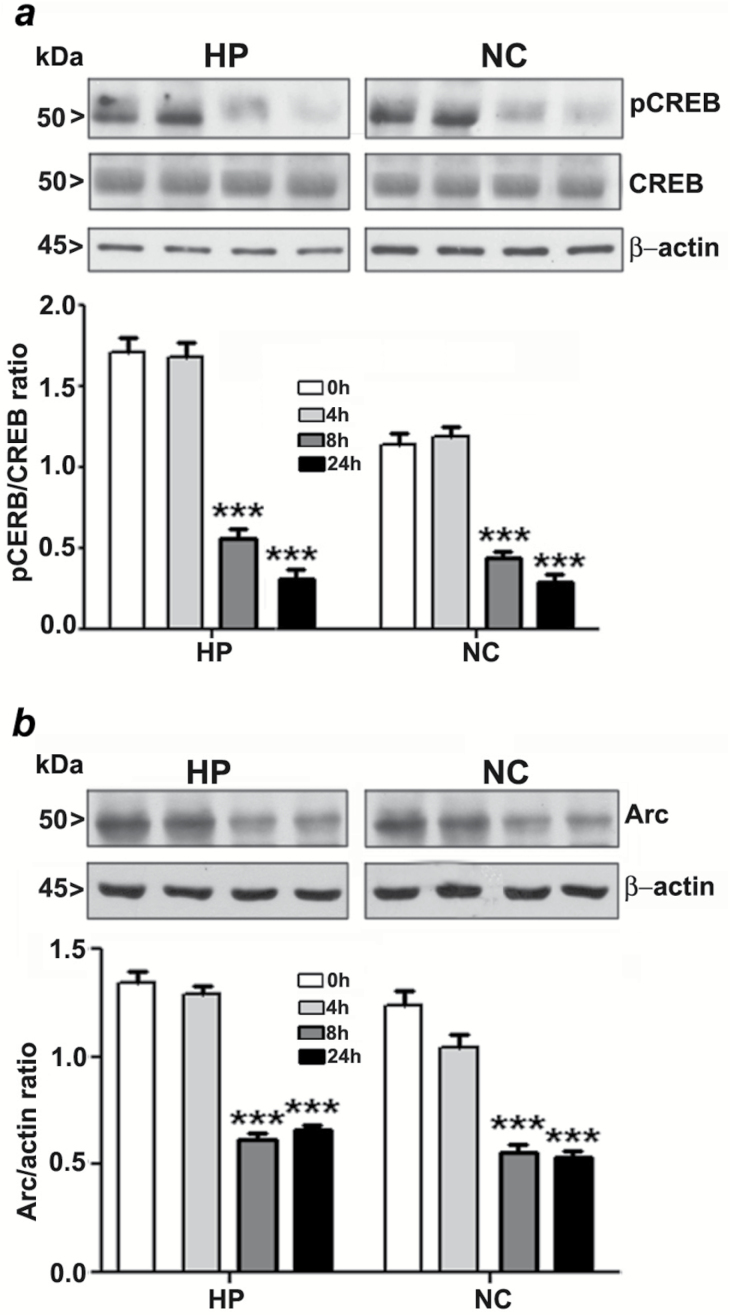
Phosphorylation of CREB was Inhibited After Ethanol Exposure in Neonatal Mice
Our previous studies demonstrate that P7 ethanol treatment significantly reduces ERK1/2 phosphorylation (Subbanna et al., 2013a). To further assess the contribution of intracellular signaling events to the action of ethanol on the developing brain, we determined the levels of CREB phosphorylation by Western blot analysis using specific phospho-CREB antibodies. P7 ethanol treatment significantly reduced the pCREB but not the total CREB protein levels in the HP (F3, 28 = 56, p < 0.001) or the NC (F3, 28 = 60, p < 0.001; Figure 2a) at the 8h and 24h intervals.
Figure 2.
Ethanol inhibits CREB phosphorylation and Arc protein expression in the neonatal brain. (a) A Western blot analysis of pCREB and CREB in hippocampal and cortical nuclear extracts from the saline or ethanol-treated groups (***p < 0.001 vs. 0h). (b) A Western blot analysis of Arc protein levels in hippocampal and neocortical total extracts from the saline- or ethanol-treated groups. β-actin was used as a loading control (n = 10 pups/group; ***p < 0.001 vs. 0h). Statistical analysis was done using one-way ANOVA with Bonferroni’s post hoc tests. Each point is the mean ± SEM. HP, hippocampus; NC, neocortex.
Ethanol Exposure in Neonatal Mice Reduces Arc Protein Expression
To further assess the contribution of intracellular signaling events to the action of ethanol on the developing brain, we determined the levels of Arc by Western blot analysis using specific Arc antibodies. P7 ethanol treatment significantly reduced the Arc protein levels in the HP (F3, 28 = 70, p < 0.001) and NC (F3, 28 = 55, p < 0.001; Figure 2b) at 8h and 24h time points.
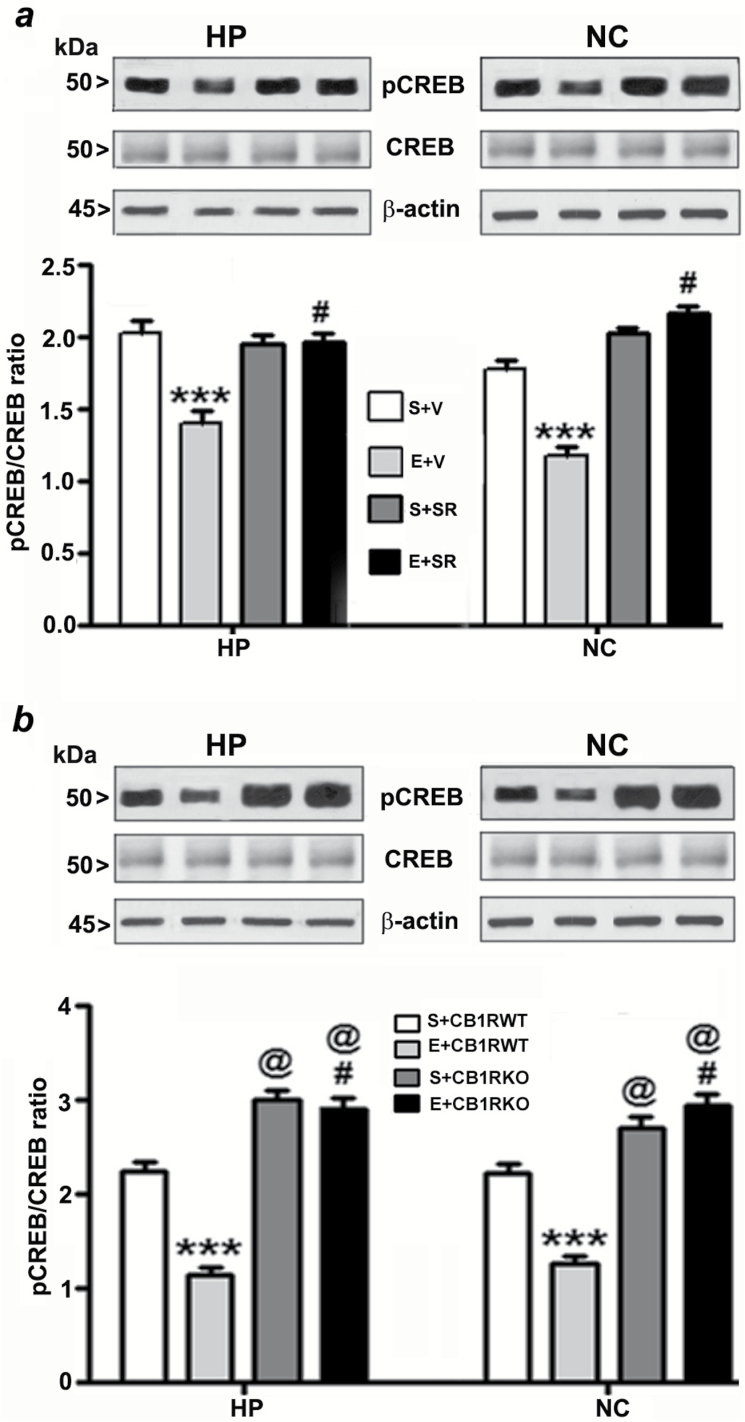
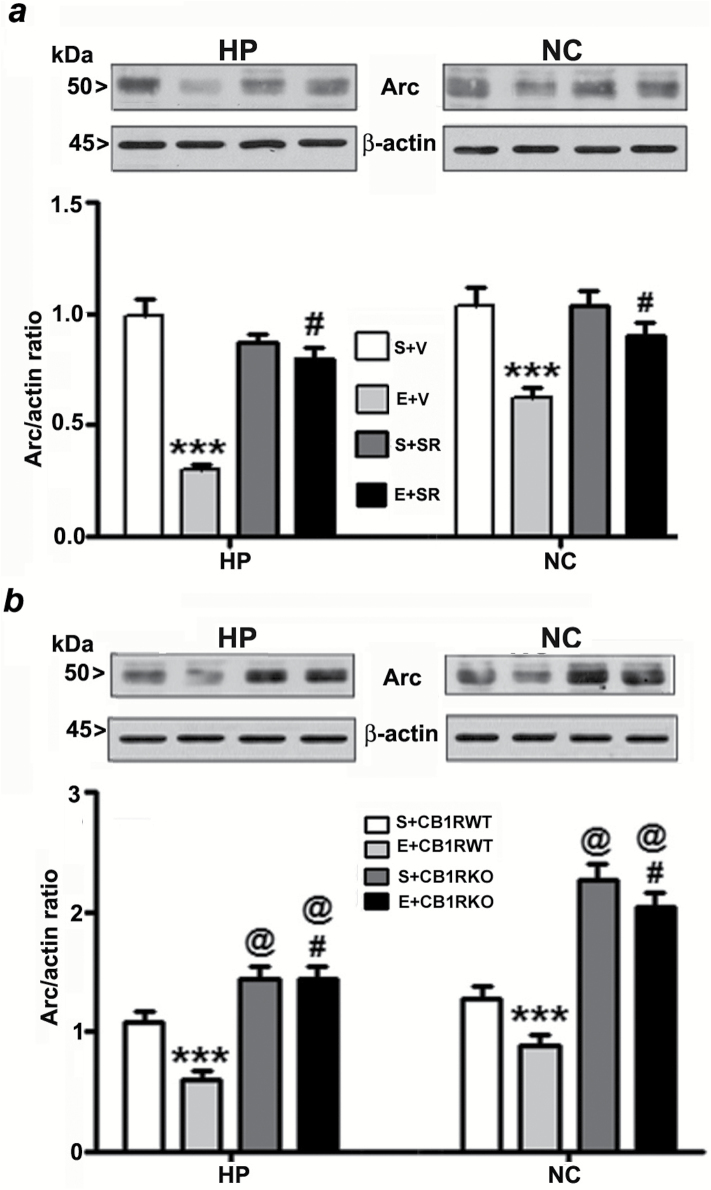
Impaired pCREB and Arc are Long-Lasting to Adulthood and Neuroprotective Effects of CB1R Blockade Involves pCREB and Arc Pathway
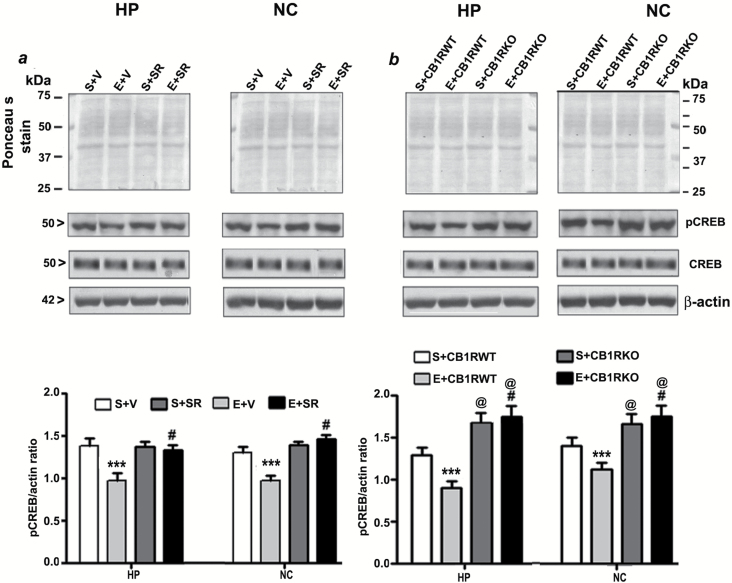
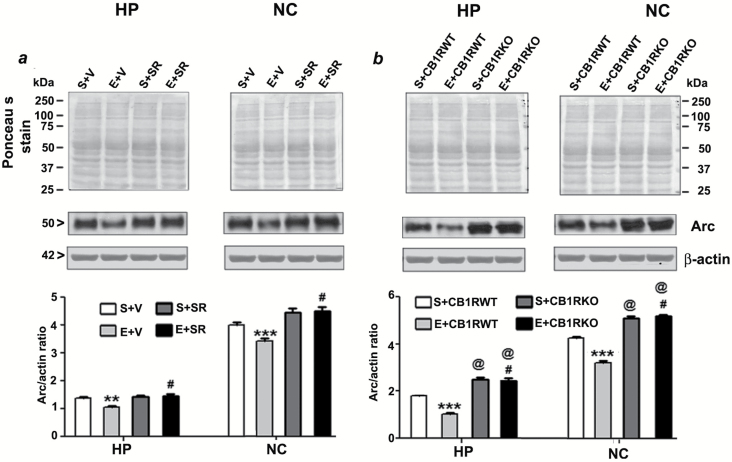
To elucidate the downstream intracellular pathways involved in the protective effects of the CB1R blockade, we studied the involvement of the pCREB and Arc pathway, a key regulator of cell survival (Luikart et al., 2008) and synaptic plasticity (Caroni et al., 2012). We investigated whether pre-treatment of SR, which prevents ethanol-induced neurodegeneration, could rescue these ethanol-induced pCREB and Arc deficits. Our results suggest that CREB phosphorylation, as well as Arc protein expression, were rescued by SR pre-treatment (compared with the ethanol group) in neonatal (HP: pCREB, F3, 20 = 55, p > 0.01, Arc, F3, 20 = 30, p > 0.01; NC: pCREB, F3, 20 = 35, p > 0.01, Arc, F3, 20 = 45, p > 0.01, two-way ANOVA; Figure 3a and 4a) and adult mice tissues (HP: pCREB, F3, 20 = 40, p > 0.01, Arc, F3, 20 = 25, p > 0.01; NC: pCREB, F3, 20 = 45, p > 0.01, Arc, F3, 20 = 55, p > 0.01, two-way ANOVA; Figure 5a and 6a). We found that the total CREB protein levels were not altered in the ethanol-treated samples compared with the saline samples. In addition, SR did not alter the CREB protein levels in either the ethanol or saline samples of neonatal (Figure 3a) and adult (Figure 5a) rats. Similarly, CB1RKO mice, which do not exhibit ethanol-induced neurodegeneration, provided protection against P7 ethanol-induced inhibition of CREB phosphorylation and Arc expression in the neonatal (HP: pCREB, F3, 20 = 42, p < 0.001, Arc, F3, 20 = 52, p < 0.001; NC: pCREB, F3, 20 = 58, p < 0.001, Arc, F3, 20 = 62, p < 0.001, one-way ANOVA; Figure 3b and 4b) and adult mice tissues (HP: pCREB, F3, 20 = 22, p < 0.01, Arc, F3, 20 = 32, p < 0.01; NC: pCREB, F3, 20 = 38, p < 0.01, Arc, F3, 20 = 32, p < 0.01, one-way ANOVA; Figure 5b and 6b). In addition, neonatal and adult CB1RKO mice also exhibited enhanced CREB phosphorylation and Arc levels compared to their WT littermates (p < 0.01; Figure 5b and 6b).
Figure 3.
Pharmacological blockade or genetic ablation of CB1Rs provides protection against ethanol-induced inhibition of CREB phosphorylation in the neonatal mouse brain. (a) Hippocampal and neocortical nuclear extracts from the four treatment groups (S+V, E+V, S+SR, and E+ SR) were subjected to Western blot to analyze the levels of pCREB and CREB (n = 10 pups/group; ***p < 0.001 vs. S+V; #p < 0.001 vs. E+V). (b) Additional Western blot analyses were performed to determine the levels of pCREB and CREB in the hippocampal and cortical nuclear extracts obtained from the saline and ethanol-treated P7 CB1RWT and KO mice. The representative blots are shown for the hippocampal and cortical nuclear extracts (n = 10 pups/group; ***p < 0.001 vs. S+CB1RWT; #p < 0.001 vs. E+ CB1RWT; @p < 0.001 vs. S+CB1RWT). β-actin was used as a loading control. Two-way ANOVA with Bonferroni’s post hoc tests was used for statistical analysis. Each point is the mean ± SEM. HP, hippocampus; NC, neocortex.
Figure 4.
Pharmacological inhibition or genetic deletion of CB1Rs provides protection against ethanol-induced inhibition of Arc expression in the neonatal mouse brain. (a) Hippocampal and neocortical total extracts from the four treatment groups (S+V, E+V, S+SR, and E+ SR) were processed for Western blot to analyze the levels of Arc protein (n = 10 pups/group; ***p < 0.001 vs. S+V; #p < 0.001 vs. E+V). (b) To determine the Arc protein levels in saline and ethanol-treated CB1RWT and KO P7 mice samples, the hippocampal and neocortical total extracts were subjected to Western blot analyses. The representative blots are shown for the hippocampal and cortical cytosolic extracts (n = 10 pups/group; ***p < 0.001 vs. S+CB1RWT; #p < 0.001 vs. E+CB1RWT; @p < 0.001 vs. S+CB1RWT). Two-way ANOVA with Bonferroni’s post hoc tests was used for statistical analysis. Each point is the mean ± SEM. HP, hippocampus; NC, neocortex.
Figure 5.
Pre-administration of SR141716A or genetic ablation of CB1Rs provides protection against P7 ethanol-induced inhibition of CREB phosphorylation in the adult mouse brain. (a) Hippocampal and neocortical nuclear extracts from the four treatment groups (S+V, E+V, S+SR and E+ SR) were subjected to Western blot to analyze the levels of pCREB and CREB (n = 8 mice/group; ***p < 0.001 vs. S+V; #p < 0.001 vs. E+V). (b) Additional Western blot analyses were performed to determine the levels of pCREB and CREB in the hippocampal and cortical nuclear extracts obtained from the P7 saline and ethanol-treated adult CB1RWT and KO mice. The representative blots are shown for the hippocampal and cortical nuclear extracts (n = 5 mice/group; ***p < 0.001 vs. S+CB1RWT; #p < 0.001 vs. E+ CB1RWT; @p < 0.001 vs. S+CB1RWT). Ponceau S staining and β-actin was used as a loading control. Two-way ANOVA with Bonferroni’s post hoc tests was used for statistical analysis. Each point is the mean ± SEM. HP, hippocampus; NC, neocortex.
Figure 6.
Pre-administration of SR141716A or genetic deletion of CB1Rs provides protection against ethanol-induced inhibition of Arc expression in the adult mouse brain. (a) Hippocampal and neocortical total extracts from the four treatment groups (S+V, E+V, S+SR, and E+ SR) were processed for Western blot to analyze the levels of Arc protein (n = 8 mice/group; **p < 0.01; ***p < 0.001 vs. S+V; #p < 0.001 vs. E+V). (b) To determine the Arc protein levels in P7 saline and ethanol-treated adult CB1RWT and KO mice samples, the hippocampal and neocortical total extracts were subjected to Western blot analyses. The representative blots are shown for the hippocampal and cortical cytosolic extracts (n = 5 mice/group; ***p < 0.001 vs. S+CB1RWT; #p < 0.001 vs. E+CB1RWT; @p < 0.001 vs. S+CB1RWT). Ponceau S staining and β-actin was used as a loading control. Two-way ANOVA with Bonferroni’s post hoc tests was used for statistical analysis. Each point is the mean ± SEM. HP, hippocampus; NC, neocortex.
Pharmacological Blockade or Genetic Deletion of CB1Rs Before P7 Ethanol Treatment Rescues Memory Loss in Adult Mice
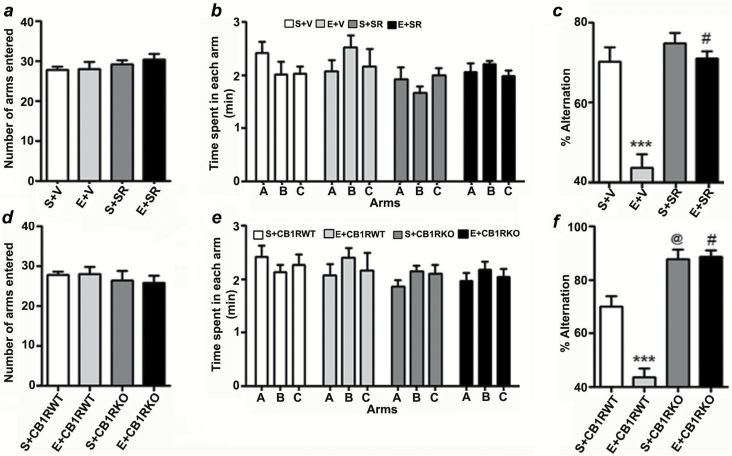
In our first behavioral test, adult mice treated with saline, ethanol (with vehicle), SR, or ethanol + SR at P7 were tested using spontaneous alternation in the Y maze. P7 ethanol, SR, or ethanol + SR treatment had no significant effect on exploratory activities assessed by the number of arm entries (Figure 7a) and time spent (Figure 7b) in each arm during Y-maze testing. Two-way ANOVA revealed that the ethanol-treated mice exhibited significantly reduced spontaneous alternation performance compared to saline-treated mice and that SR rescued these deficits (F3,21 = 10, p < 0.001; Figure 7c). Treatment with SR alone at P7 had no significant effect on spontaneous alternation performance. P7 ethanol treatment had no significant effect on the number of arm entries (Figure 7d) or the time spent in each arm (Figure 7e; exploratory activity) in either CB1RWT or KO mice. CB1RKO mice exhibited significantly enhanced spontaneous alternation behavior (p < 0.01) compared to WT mice. Notably, ethanol treatment at P7 failed to induce a spatial working memory deficit in the Y-maze test in adult CB1RKO mice (p > 0.05; Figure 7f).
Figure 7.
Prior blockade or genetic deletion of CB1Rs prevents P7 ethanol-induced spontaneous alternation performance deficits in adult mice. (a) Total number of arm entries reflecting exploratory activities of mice in the Y-maze does not differ between S+V, E+V, S+SR, and E+SR-treated mice. (b) The time spent in each arm was not different between the four groups (p > 0.05). (c) Spatial working memory of S+V, E+V, S+SR, and E+SR-treated mice were tested by spontaneous alternation performance in the Y-maze. Note that E+V-treated mice perform poorly compared to S+V-treated controls (***p < 0.001), while E+SR treatment restores E+V levels of alternation performance (#p < 0.001 versus E+V). n = 8 mice per group. One-way ANOVA with Bonferroni’s post hoc test was used to analyze significant differences. (d) Total number of arm entries reflecting exploratory activities of mice in the Y-maze does not differ between the CB1RWT and KO with or without ethanol. (e) The time spent in each arm was not different between S+CB1RWT, E+CB1RWT, S+CB1RKO, and E+CB1RKO treated mice (p > 0.05). (f) Spontaneous alternation performance of CB1RWT and CB1RKO mice treated with or without saline or ethanol were tested in the Y-maze. Note that the performance of P7 saline-treated CB1RKO mice was significantly enhanced compared to P7 saline-treated CB1RWT mice (@p < 0.001), while ethanol failed to impair alternation performance in CB1RKO mice (#p < 0.001 vs. E + CB1RWT). n = 8 mice per group. One-way ANOVA with Bonferroni’s post hoc test was used to analyze significant differences. Each point is the mean ± SEM.
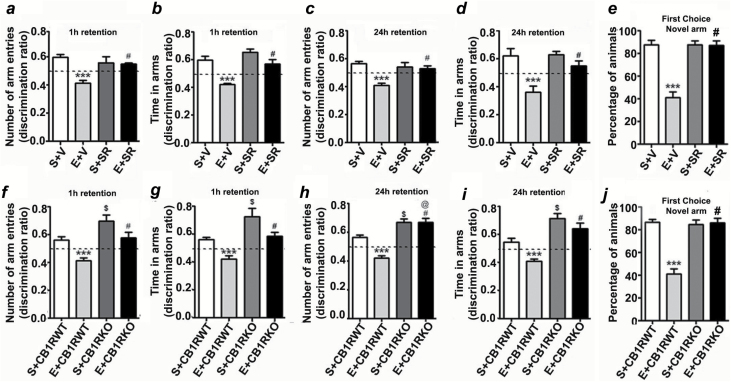
In our second behavioral test, we examined spatial recognition memory using the Y-maze. Two-way ANOVA revealed that saline- and SR-treated mice entered more frequently into (Arm Entry: 1h, F3,21 = 21, p < 0.01; 24h, F3,21 = 26, p < 0.01) and spent more time in (Dwell Time: 1h, F3,21 = 61, p < 0.01; 24h, F3,21 = 22, p < 0.01) the novel, previously unvisited arm of the maze. In contrast, P7 ethanol-treated mice showed a reduced preference toward the novel arm (p < 0.01) and spent less time (Dwell Time: p < 0.01) in the novel arm compared to P7 saline-treated mice in both the 1h (Figure 8a and b) and 24h (Figure 8c and d) retention trials. SR pre-treatment rescued ethanol-induced impairments in the preference for the novel arm (p < 0.01) and time spent (p < 0.01) in the novel arm in both the 1 and 24h retention trials. Although all saline- and SR-treated mice (combined 1 and 24h) selected the novel arm as the first choice, ethanol-treated animals showed a reduced preference for the novel arm (Figure 8e), which this was prevented by SR pretreatment (F3,45 = 50, p < 0.01). while CB1RKO mice showed an enhanced preference for the novel arm (Arm Entry, p < 0.001) and spent more time in the novel arm (Dwell Time, p < 0.001) compared to WT mice in both the 1h (Figure 8f and g) and 24h retention trials (Figure 8h and i). In addition, all saline- and ethanol-treated CB1RKO mice (combined 1 and 24h) selected the novel arm as the first choice (Figure 8j).
Figure 8.
P7 ethanol treatment impairs and SR pretreatment rescues impaired spatial memory performance as measured by the Y maze. Discrimination ratio (preference for the novel arm over the familiar or other arm: Novel/Novel + Other) for arm entries (a and c) and dwell time (b and d) of S+V- and E+V-treated mice with or without SR in the Y maze at 1h and 24h after the first encounter with the partially-opened maze. The dashed line indicates chance performance (0.5). (e) The percentage of animals selecting the novel arm as the first choice is shown for S+V- and E+V-treated mice with or without SR at 1h and 24h after the first encounter with the partially-opened maze. Each point is the mean ± SEM (n = 8 mice/group). Two-way ANOVA with Bonferroni’s post hoc test: ***p < 0.001 vs. S + V; #p < 0.05 vs. E + V. Discrimination ratio (preference for the novel arm over the familiar or other arm: Novel/Novel + Other) for arm entries (f and h) and dwell time (g and i) of S+CB1RWT, E+CB1RWT, S+CB1RKO, and E+CB1RKO mice in the Y maze at 1h and 24h after the first encounter with the partially-opened maze. The dashed line indicates chance performance (0.5). (j) The percentage of animals selecting the novel arm as the first choice is shown for S+CB1RWT, E+CB1RWT, S+CB1RKO, and E+CB1RKO mice at 1h and 24h after the first encounter with the partially-opened maze. Each point is the mean ± SEM (n = 8 mice/group). Two-way ANOVA with Bonferroni’s post hoc test: ***p < 0.001 vs. S + V; #p ≤ 0.01 vs. E ± V; $ p ≤ 0.01 vs. S ± CB1RWT; @ p ≤ 0.01 vs. S ± CB1RWT.
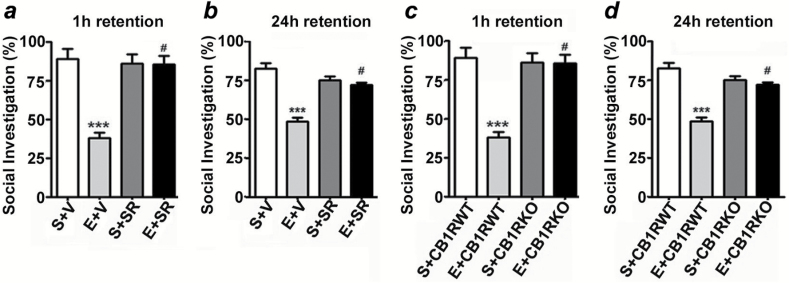
The social investigation results revealed that ethanol-treated mice exhibited significantly-reduced short-term (Figure 9a) and long-term (Figure 9b) SRM performance compared to saline-treated mice. Two-way ANOVA revealed that SR pretreatment rescued ethanol-induced short-term (F3,21 = 18, p < 0.01) and long-term (F3,21 = 14, p < 0.01) SRM deficits compared to ethanol-treated mice. In addition, SR alone had no significant effects (p > 0.05) on SRM, and these mice exhibited normal SRM. Ethanol failed to impair SRM in CB1RKO mice, and KO mice exhibited normal SRM (Figure 9c and d).
Figure 9.
SR pretreatment rescues and CB1RKO provides protection against P7 ethanol-induced social recognition memory loss in adult mice. The percentage of social investigation is shown for S+V-, E+V-, S+SR-, and E+ SR-treated mice at 1h (a) and 24h (b) after the first encounter with the same juvenile mouse. ***p < 0.001 vs. S + V; # p < 0.05 vs. E + V. The percentage of social investigation is shown for S+CB1RWT, E+CB1RWT, S+CB1RKO, and E+CB1RKO mice at 1h (c) and 24h (d) after the first encounter with the same juvenile mouse. Each point is the mean ± SEM (n = 8 mice/group). Two-way ANOVA with Bonferroni’s post hoc test: ***p < 0.001 vs. CB1RWT + S; #p < 0.05 vs. CB1RWT + E.
Discussion
In this study, we demonstrate for the first time that transcriptional activation of CB1R followed by widespread neurodegeneration in the neonatal brain (Subbanna et al., 2013a) involves specific increases in H4K8 acetylation and demethylation of H3K9 at exon 1 in the CB1R gene. Dimethylation of histone H3K9 correlates with transcriptional silencing, whereas the acetylation of histone H4 at lysine 8 (H4K8ace) is linked to active transcription (Jenuwein and Allis, 2001). Our findings are consistent with postnatal ethanol-induced enhancement of active transcription of G9a gene (Subbanna et al., 2014). Demethylation of H3K9 found at the CB1R gene may be due to the global loss of the H3K9me2 mark secondary to postnatal-ethanol-induced caspase-3 mediated H3K9me2 degradation (Subbanna et al., 2013b). While studies related to epigenetic changes at the CB1R gene are either primitive (Wang et al., 2008) or have not yet been conducted, our unprecedented initial studies suggest that the epigenetic mark which regulates active gene transcription (Subbanna et al., 2013a; Subbanna et al., 2013b; Subbanna et al., 2014) also regulates the CB1R gene expression (Subbanna et al., 2013a) triggered by neonatal ethanol neurotoxicity. Ethanol-induced CB1R protein expression (Subbanna et al., 2013a) also reflected increases in CB1R levels in the PM preparations as shown by specific binding of CP55,940. In addition, our studies suggest that ethanol-enhanced CB1R proteins are functionally active at the PM because CP55,940-stimulated GTPγS binding also enhanced in parallel with CB1R levels. Although the studies on the consequences of enhanced CB1R function in neonatal mice are limited (Hansen et al., 2008; Subbanna et al., 2013a), our previous studies suggest that postnatal ethanol-enhanced CB1R function induces neurodegeneration in neonatal mice that leads to long-lasting deficits in long-term potentiation and object recognition test (ORT) in adult mice (Subbanna et al., 2013a). Consistent with this observation, elevation of endogenous AEA through inhibition of fatty acid amide hydrolase during postnatal development, as observed with postnatal ethanol (Subbanna et al., 2013a), leads to impaired working memory in adult mice (Wu et al., 2014). CB1R has been shown to regulate the generation and maturation of excitatory and inhibitory neurons during brain development (Keimpema et al., 2013) and has also been shown to inhibit the release of glutamate and gamma aminobutyric acid (GABA) in matured neurons (Wilson and Nicoll, 2002). However, in neonatal mice, it appears that the ethanol blocking action at NMDARs and its stimulatory action at GABAARs are mainly responsible for its neurodegenerative responses (Ikonomidou et al., 2000). While more research is warranted to understand the specific effects of ethanol-activated CB1R on NMDA- and GABA-mediated neurotransmission in neonatal mice, it appears that GABA is excitatory during the early stages of brain development and becomes inhibitory later on as adulthood approaches (Ben-Ari, 2002). Therefore, dysregulation of the CB1R pathway which regulates NMDA and GABA neurotransmission may have long-lasting consequences on synaptic function.
Our previous studies suggested the presence of a remarkable specificity involving the AEA/CB1R/ERK pathway, but not the AKT pathway (Young et al., 2008), in the regulation of ethanol-induced neonatal neurodegeneration (Subbanna et al., 2013a). Our current findings suggest that reduced CREB phosphorylation could be rescued by blockade or genetic deletion of CB1R in neonatal mice exposed to ethanol because the protein kinase A/cAMP/ERK pathway has been shown to phosphorylate CREB on Ser133; ethanol-induced inhibition of this pathway may be responsible for the observed deficits in CREB phosphorylation. It should be noted that CREB has been shown to mediate adaptive responses of neurons to several stimuli to regulate neuronal survival in the developing brain (Bonni et al., 1999). Interestingly, CB1R neonatal KO mice exhibit high levels of CREB phosphorylation similar to adult CB1RKO mice compared to their WT littermates (Basavarajappa et al., 2014; Basavarajappa and Subbanna, 2014). Activated CREB has been shown to regulate the expression of many genes involved in numerous cellular functions, including neuronal survival, synaptic plasticity, and learning and memory (Nonaka, 2009; Benito and Barco, 2010; Sakamoto et al., 2011). Therefore, ethanol-caused dysregulation of this pathway during postnatal development to adulthood may significantly contribute to long-term neurobehavioral deficits commonly associated with FASD (Izumi et al., 2005; Wilson et al., 2011; Sadrian et al., 2012; Subbanna et al., 2013a).
While the precise signaling cascades involved in Arc transcription are not well defined, one study has shown that PKA/MAPK cascades regulate Arc expression (Waltereit et al., 2001) and that the MAPK/CREB pathway is also essential for Arc expression (Ying et al., 2002; Nonaka et al., 2014). Given the impaired ERK1/2 and pCREB in postnatal ethanol-exposed neonatal mice, Arc protein levels were also significantly reduced in ethanol-exposed neonatal as well as adult mice and blockade or genetic deletion of CB1R prior to P7 ethanol treatment–restored Arc protein levels in neonatal and adult mice. Interestingly, both neonatal and adult CB1R KO mice expressed high levels of Arc compared to their WT littermates. It is therefore possible that the neuroprotective role of the CB1R antagonist in neonatal mice may be due to the activation of Arc expression through CB1R-mediated CREB pathway. Arc expression is very tightly controlled by neuronal activity downstream of multiple signaling pathways (Bramham et al., 2008; Shepherd and Bear, 2011). Therefore, several molecules responsible for mediating neural activity, such as NMDA and the P/Q-type voltage-dependent Ca2+ channel, also regulate Arc expression (Kakizawa et al., 2000; Hashimoto et al., 2011). To our knowledge, this is the first study to suggest that the regulation of Arc expression through CB1R activity in neonatal and adult mice. Arc transcription is also regulated by voltage-sensitive calcium channels (Adams et al., 2009), which are negatively regulated by CB1R (Basavarajappa and Arancio, 2008). Our findings suggest that the ERK1/2-pCREB-Arc pathway is involved in neuronal survival downstream of the CB1Rs in the developing brain and is compromised by ethanol treatment. It is possible that ethanol-induced suppression of the ERK1/2-pCREB-Arc signaling pathway might disrupt the fine-tuning of neuronal circuits, leading to persistent synaptic and memory dysfunction (Subbanna et al., 2013a). Consistent with this notion, CB1RKO mice do not exhibit P7 ethanol-induced neurodegeneration during the neonatal period (Hansen et al., 2008; Subbanna et al., 2013a) or deficits in long-term potentiation and ORT during adulthood (Subbanna et al., 2013a).
The current findings also revealed significant deficits in learning and memory in adult mice exposed to ethanol at P7 compared to controls, as tested in several domains (spontaneous alternation, spatial, and social recognition). These findings are in general agreement with previous reports showing that an acute dose of ethanol at P7 impairs synaptic plasticity in the HP (Izumi et al., 2005; Sadrian et al., 2012; Subbanna et al., 2013a) and olfacto-HP (Wilson et al., 2011; Sadrian et al., 2012) circuits as well as ORT (Subbanna et al., 2013a) in adult mice. Most importantly, we have shown that the blockade of CB1Rs before ethanol exposure is sufficient enough to rescue ethanol-induced neuronal deficits in every paradigm we have examined. Several other rodent models of FASD also show impaired learning and memory in adult rodents exposed to acute or chronic ethanol at pre- or postnatal stages of development (Girard et al., 2000; Savage et al., 2002; Christie et al., 2005; Iqbal et al., 2006; Thomas et al., 2008). There is also growing evidence that heavy prenatal alcohol exposure leads to widespread cognitive deficits in children across several domains, including memory and social and adaptive functioning (Norman et al., 2013).
The findings from SRM studies are in general agreement with other studies in which acute exposure to ethanol on P12 caused pronounced and permanent deficits in social behavior throughout ontogeny (Mooney and Varlinskaya, 2011). Similar SRM deficits were also found in another animal model of FASD (Shirasaka et al., 2012), as well as in the CD38 KO model of autism (Jin et al., 2007). Most importantly, SR pretreatment and genetic deletion of CB1R provided protection against ethanol-induced deficits in SRM in adult mice. It has been suggested that retaining normal SRMs throughout ontogeny would help to establish relationships within a group or between partners, besides developing the ability to recognize families (Cushing and Kramer, 2005). Evidence suggests that the two brain regions, the olfactory system (Sanchez-Andrade and Kendrick, 2009) and the limbic system (Brothers et al., 1990; Baron-Cohen et al., 1994) regulate social behavior. In ethanol-treated P7 mice, improper processing of socially relevant olfactory stimuli might produce the observed deficit in SRM in adult mice. Early ethanol exposure damages olfactory neuroanatomy and physiology in both humans and rodents (Wilson et al., 2011). The olfactory system provides a major input to the HP formation (Wilson and Sullivan, 2011), and this structure is involved in integrating the complex stimuli necessary for the recognition process (Alvarez et al., 2002; Ross and Eichenbaum, 2006), therefore the SRM might be regulated also by HP structure (Kogan et al., 2000). Consistent with this notion, our previous findings suggest that ethanol treatment of P7 mice significantly impairs olfacto-HP system function in adult mice (Wilson et al., 2011; Sadrian et al., 2012).
In conclusion, CB1R gene transcription is regulated by specific epigenetic modification, associated with active transcription leading to enhanced CB1R function. Our current findings directly pinpoint the participation of CB1R signaling during early brain development (Figure 10) leading to long-lasting pCREB-Arc impairments and neurobehavioral abnormalities. Currently, effective treatment for individuals suffering from FASD is not available. The CB1R-pCREB-Arc mediated molecular mechanisms in the effect of postnatal ethanol on abnormalities in neuronal survival and its long-lasting influence on synaptic plasticity, learning, and memory, including SRM, may eventually lead to the development of drugs to improve specific aspects of the symptomatology of ethanol-induced neurobehavioral teratogenicity.
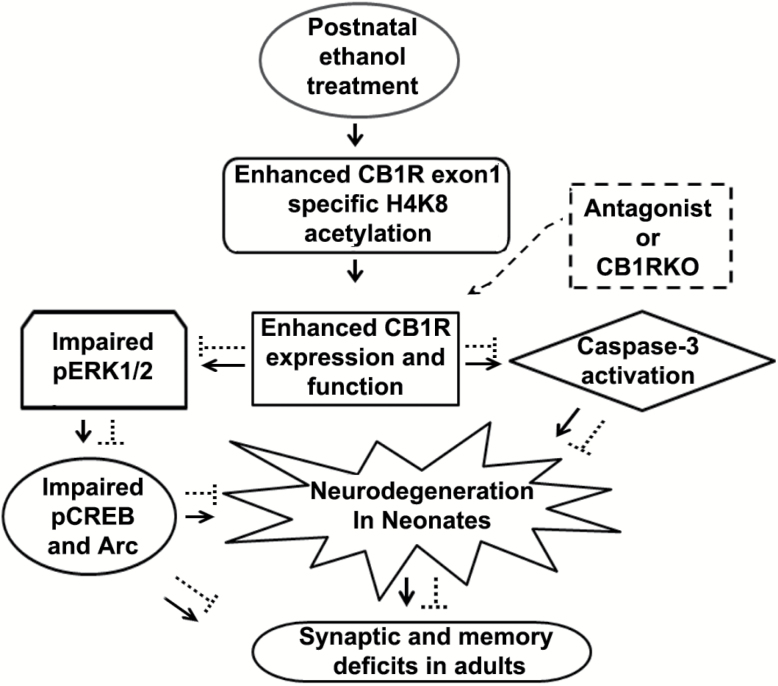
Figure 10.
A schematic diagram of the proposed mechanism of action by which epigenetically enhanced CB1R function in neonatal mice regulates ethanol-induced neurodegeneration, leading to neurobehavioral abnormalities in adult mice. P7 ethanol enhances specific H4K8ace of CB1Rs promoter to enhance CB1R expression and function, which causes caspase-3 activation as well as pERK1/2 (Subbanna et al., 2013a), pCREB and Arc deficits and leads to neonatal neurodegeneration (—>). These mechanisms during postnatal development may disrupt the refinement of neuronal circuits (Wilson et al., 2011; Sadrian et al., 2012) and lead to long-lasting deficits in synaptic plasticity (Subbanna et al., 2013a), learning, and memory, including social recognition memory in adult animals. The inhibition of CB1Rs (-----) rescue pERK1/2, pCREB, and Arc deficits as well as neonatal neurodegeneration (caspase-3 cleavage), which results in normal neurobehavioral function in adult mice. The genetic ablation of CB1Rs (-----) provides protection against ethanol-induced pCREB, Arc deficits, neonatal neurodegeneration, synaptic (Subbanna et al., 2013a), learning, and memory, including social recognition memory deficits in adult mice. Hence, the putative CB1R/pERK1/2/pCREB/Arc signaling mechanism may have a potential regulatory role in neuronal function during brain development and may be a valuable therapeutic target for FASD.
Statement of Interest
The authors declare no conflicts of interest.
Acknowledgments
This work in part was supported by NIH/NIAAA grant AA019443 (Dr Basavarajappa). We thank Neha Balapal for editing the final version of the paper.
References
- Adams JP, Robinson RA, Hudgins ED, Wissink EM, Dudek SM. (2009). NMDA receptor-independent control of transcription factors and gene expression. Neuroreport 20:1429–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez P, Wendelken L, Eichenbaum H. (2002). Hippocampal formation lesions impair performance in an odor-odor association task independently of spatial context. Neurobiol Learn Mem 78:470–476. [DOI] [PubMed] [Google Scholar]
- Bacci A, Huguenard JR, Prince DA. (2004). Long-lasting self-inhibition of neocortical interneurons mediated by endocannabinoids. Nature 431:312–316. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring H, Moriarty J, Schmitz B, Costa D, Ell P. (1994). Recognition of mental state terms. Clinical findings in children with autism and a functional neuroimaging study of normal adults. Br J Psychiatry 165:640–649. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS. (2007). Neuropharmacology of the endocannabinoid signaling system-Molecular mechanisms, biological actions and synaptic plasticity. Current Neuropharmacol 5:81–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basavarajappa BS, Arancio O. (2008). Synaptic plasticity: emerging role for dndocannabinoid system. In: Synaptic Plasticity: New Research (Kaiser TF, Peters FJ, eds), pp77–112. New York, NY: Nova Science Publishers, Inc. [Google Scholar]
- Basavarajappa BS, Hungund BL. (1999a) Down-regulation of cannabinoid receptor agonist-stimulated [35S] GTPγS binding in synaptic plasma membrane from chronic ethanol exposed mouse. Brain Res 815:89–97. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Hungund BL. (1999b) Chronic Ethanol Increases the Cannabinoid Receptor Agonist, Anandamide and its Precursor N-Arachidonyl phosphatidyl ethanolamine in SK-N-SH Cells. J Neurochem 72:522–528. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Subbanna S. (2014). CB1 Receptor-Mediated Signaling Underlies the Hippocampal Synaptic, Learning and Memory Deficits Following Treatment with JWH-081, a New Component of Spice/K2 Preparations. Hippocampus 24:178–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basavarajappa BS, Cooper TB, Hungund BL. (1998). Chronic ethanol administration down-regulates cannabinoid receptors in mouse brain synaptic plasma membrane. Brain Res 793:212–218. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Ninan I, Arancio O. (2008). Acute ethanol suppresses glutamatergic neurotransmission through endocannabinoids in hippocampal neurons. J Neurochem 107:1001–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basavarajappa BS, Nixon RA, Arancio O. (2009). Endocannabinoid system: emerging role from neurodevelopment to neurodegeneration. Mini Rev Med Chem 9:448–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basavarajappa BS, Saito M, Cooper TB, Hungund BL. (2003). Chronic ethanol inhibits the anandamide transport and increases extracellular anandamide levels in cerebellar granule neurons. Eur J Pharmacol 466:73–83. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Nagre NN, Xie S, Subbanna S. (2014). Elevation of endogenous anandamide impairs LTP, learning, and memory through CB1 receptor signaling in mice. Hippocampus 24:808–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basavarajappa BS, Yalamanchili R, Cravatt BF, Cooper TB, Hungund BL. (2006). Increased ethanol consumption and preference and decreased ethanol sensitivity in female FAAH knockout mice. Neuropharmacology 50:834–844. [DOI] [PubMed] [Google Scholar]
- Bekdash RA, Zhang C, Sarkar DK. (2013). Gestational Choline Supplementation Normalized Fetal Alcohol-Induced Alterations in Histone Modifications, DNA Methylation, and Proopiomelanocortin (POMC) Gene Expression in beta-Endorphin-Producing POMC Neurons of the Hypothalamus. Alcohol Clin Exp Res 37:1133–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y. (2002). Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci 3:728–739. [DOI] [PubMed] [Google Scholar]
- Benito E, Barco A. (2010). CREB’s control of intrinsic and synaptic plasticity: implications for CREB-dependent memory models. Trends Neurosci 33:230–240. [DOI] [PubMed] [Google Scholar]
- Berghuis P, Rajnicek AM, Morozov YM, Ross RA, Mulder J, Urban GM, Monory K, Marsicano G, Matteoli M, Canty A, Irving AJ, Katona I, Yanagawa Y, Rakic P, Lutz B, Mackie K, Harkany T. (2007). Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science 316:1212–1216. [DOI] [PubMed] [Google Scholar]
- Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME. (1999). Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science 286:1358–1362. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Worley PF, Moore MJ, Guzowski JF. (2008). The immediate early gene arc/arg3.1: regulation, mechanisms, and function. J Neurosci 28:11760–11767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothers L, Ring B, Kling A. (1990). Response of neurons in the macaque amygdala to complex social stimuli. Behav Brain Res 41:199–213. [DOI] [PubMed] [Google Scholar]
- Campuzano V, et al. (1996). Friedreich’s ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science 271:1423–1427. [DOI] [PubMed] [Google Scholar]
- Caroni P, Donato F, Muller D. (2012). Structural plasticity upon learning: regulation and functions. Nat Rev Neurosci 13:478–490. [DOI] [PubMed] [Google Scholar]
- Castellano C, Rossi-Arnaud C, Cestari V, Costanzi M. (2003). Cannabinoids and memory: animal studies. Curr Drug Targets CNS Neurol Disord 2:389–402. [DOI] [PubMed] [Google Scholar]
- Ceccarini J, Hompes T, Verhaeghen A, Casteels C, Peuskens H, Bormans G, Claes S, Van Laere K. (2014). Changes in cerebral CB1 receptor availability after acute and chronic alcohol abuse and monitored abstinence. J Neurosci 34:2822–2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie BR, Swann SE, Fox CJ, Froc D, Lieblich SE, Redila V, Webber A. (2005). Voluntary exercise rescues deficits in spatial memory and long-term potentiation in prenatal ethanol-exposed male rats. Eur J Neurosci 21:1719–1726. [DOI] [PubMed] [Google Scholar]
- Cushing BS, Kramer KM. (2005). Mechanisms underlying epigenetic effects of early social experience: the role of neuropeptides and steroids. Neurosci Biobehav Rev 29:1089–1105. [DOI] [PubMed] [Google Scholar]
- Dellu F, Mayo W, Cherkaoui J, Le Moal M, Simon H. (1992). A two-trial memory task with automated recording: study in young and aged rats. Brain Res 588:132–139. [DOI] [PubMed] [Google Scholar]
- Dember WN, Fowler H. (1958). Spontaneous alternation behavior. Psychol Bull 55:412–428. [DOI] [PubMed] [Google Scholar]
- DePoy L, Daut R, Brigman JL, MacPherson K, Crowley N, Gunduz-Cinar O, Pickens CL, Cinar R, Saksida LM, Kunos G, Lovinger DM, Bussey TJ, Camp MC, Holmes A. (2013). Chronic alcohol produces neuroadaptations to prime dorsal striatal learning. Proc Natl Acad Sci USA 110:14783–14788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin DP, Sharma RP. (2010). Histone modifications, DNA methylation, and schizophrenia. Neurosci Biobehav Rev 34:882–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard TA, Xing HC, Ward GR, Wainwright PE. (2000). Early postnatal ethanol exposure has long-term effects on the performance of male rats in a delayed matching-to-place task in the Morris water maze. Alcohol Clin Exp Res 24:300–306. [PubMed] [Google Scholar]
- Goodman AM, Delis DC, Mattson SN. (1999). Normative data for 4-year-old children on the California Verbal Learning Test-Children’s Version. Clin Neuropsychol 13:274–282. [DOI] [PubMed] [Google Scholar]
- Hansen HH, Krutz B, Sifringer M, Stefovska V, Bittigau P, Pragst F, Marsicano G, Lutz B, Ikonomidou C. (2008). Cannabinoids enhance susceptibility of immature brain to ethanol neurotoxicity. Ann Neurol 64:42–52. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Tsujita M, Miyazaki T, Kitamura K, Yamazaki M, Shin HS, Watanabe M, Sakimura K, Kano M. (2011). Postsynaptic P/Q-type Ca2+ channel in Purkinje cell mediates synaptic competition and elimination in developing cerebellum. Proc Natl Acad Sci USA 108:9987–9992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, A.B. L, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC. (1990). Cannabinoid receptor localization in brain. Proc Natl Acad Sci USA 87:1932–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirvonen J, Zanotti-Fregonara P, Umhau JC, George DT, Rallis-Frutos D, Lyoo CH, Li CT, Hines CS, Sun H, Terry GE, Morse C, Zoghbi SS, Pike VW, Innis RB, Heilig M. (2013). Reduced cannabinoid CB1 receptor binding in alcohol dependence measured with positron emission tomography. Mol Psychiatry 18:916–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcomb L, Gordon MN, McGowan E, Yu X, Benkovic S, Jantzen P, Wright K, Saad I, Mueller R, Morgan D, Sanders S, Zehr C, O’Campo K, Hardy J, Prada CM, Eckman C, Younkin S, Hsiao K, Duff K. (1998). Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat Med 4:97–100. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Qualy JM, Khachatrian LL. (1986). Involvement of Gi in the inhibition of adenylate cyclase by cannabimimetic drugs. Mol Pharmacol 29:307–313. [PubMed] [Google Scholar]
- Ikonomidou C, Bittigau P, Ishimaru MJ, Wozniak DF, Koch C, Genz K, Price MT, Stefovska V, Horster F, Tenkova T, Dikranian K, Olney JW. (2000). Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science 287:1056–1060. [DOI] [PubMed] [Google Scholar]
- Iqbal U, Rikhy S, Dringenberg HC, Brien JF, Reynolds JN. (2006). Spatial learning deficits induced by chronic prenatal ethanol exposure can be overcome by non-spatial pre-training. Neurotoxicol Teratol 28:333–341. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Kitabayashi R, Funatsu M, Izumi M, Yuede C, Hartman RE, Wozniak DF, Zorumski CF. (2005). A single day of ethanol exposure during development has persistent effects on bi-directional plasticity, N-methyl-D-aspartate receptor function and ethanol sensitivity. Neuroscience 136:269–279. [DOI] [PubMed] [Google Scholar]
- Jenuwein T, Allis CD. (2001). Translating the histone code. Science 293:1074–1080. [DOI] [PubMed] [Google Scholar]
- Jin D, et al. (2007). CD38 is critical for social behaviour by regulating oxytocin secretion. Nature 446:41–45. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW. (1973). Recognition of the fetal alcohol syndrome in early infancy. Lancet 2:999–1001. [DOI] [PubMed] [Google Scholar]
- Kakizawa S, Yamasaki M, Watanabe M, Kano M. (2000). Critical period for activity-dependent synapse elimination in developing cerebellum. J Neurosci 20:4954–4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminen-Ahola N, Ahola A, Maga M, Mallitt KA, Fahey P, Cox TC, Whitelaw E, Chong S. (2010). Maternal ethanol consumption alters the epigenotype and the phenotype of offspring in a mouse model. PLOS Genet 6:e1000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keimpema E, Calvigioni D, Harkany T. (2013). Endocannabinoid signals in the developmental programming of delayed-onset neuropsychiatric and metabolic illnesses. Biochem Soc Trans 41:1569–1576. [DOI] [PubMed] [Google Scholar]
- Kim D, Thayer SA. (2001). Cannabinoids inhibit the formation of new synapses between hippocampal neurons in culture. J Neurosci 21:RC146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan JH, Frankland PW, Silva AJ. (2000). Long-term memory underlying hippocampus-dependent social recognition in mice. Hippocampus 10:47–56. [DOI] [PubMed] [Google Scholar]
- Lubin FD, Sweatt JD. (2007). The IkappaB kinase regulates chromatin structure during reconsolidation of conditioned fear memories. Neuron 55:942–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luikart BW, Zhang W, Wayman GA, Kwon CH, Westbrook GL, Parada LF. (2008). Neurotrophin-dependent dendritic filopodial motility: a convergence on PI3K signaling. J Neurosci 28:7006–7012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makedonski K, Abuhatzira L, Kaufman Y, Razin A, Shemer R. (2005). MeCP2 deficiency in Rett syndrome causes epigenetic aberrations at the PWS/AS imprinting center that affects UBE3A expression. Hum Mol Gen 14:1049–1058. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Crocker N, Nguyen TT. (2011). Fetal alcohol spectrum disorders: neuropsychological and behavioral features. Neuropsychol Rev 21:81–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Goodman AM, Caine C, Delis DC, Riley EP. (1999). Executive functioning in children with heavy prenatal alcohol exposure. Alcohol Clin Exp Res 23:1808–1815. [PubMed] [Google Scholar]
- Matyas F, Watanabe M, Mackie K, Katona I, Freund TF. (2007). Molecular architecture of the cannabinoid signaling system in the core of the nucleus accumbens. Ideggyogy Sz 60:187–191. [PubMed] [Google Scholar]
- Mechoulam R, Parker LA. (2013). The endocannabinoid system and the brain. Annu Rev Psychol 64:21–47. [DOI] [PubMed] [Google Scholar]
- Mooney SM, Varlinskaya EI. (2011). Acute prenatal exposure to ethanol and social behavior: effects of age, sex, and timing of exposure. Behav Brain Res 216:358–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder J, Aguado T, Keimpema E, Barabas K, Ballester Rosado CJ, Nguyen L, Monory K, Marsicano G, Di Marzo V, Hurd YL, Guillemot F, Mackie K, Lutz B, Guzman M, Lu HC, Galve-Roperh I, Harkany T. (2008). Endocannabinoid signaling controls pyramidal cell specification and long-range axon patterning. Proc Natl Acad Sci USA 105:8760–8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka M. (2009). A Janus-like role of CREB protein: enhancement of synaptic property in mature neurons and suppression of synaptogenesis and reduced network synchrony in early development. J Neurosci 29:6389–6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka M, Fujii H, Kim R, Kawashima T, Okuno H, Bito H. (2014). Untangling the two-way signalling route from synapses to the nucleus, and from the nucleus back to the synapses. Phil Trans R Soc B 369:1471–2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman AL, O’Brien JW, Spadoni AD, Tapert SF, Jones KL, Riley EP, Mattson SN. (2013). A functional magnetic resonance imaging study of spatial working memory in children with prenatal alcohol exposure: contribution of familial history of alcohol use disorders. Alcohol Clin Exp Res 37:132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Maejima T, Kano M. (2001). Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminal. Neuron 29:729–738. [DOI] [PubMed] [Google Scholar]
- Olney JW. (2004). Fetal alcohol syndrome at the cellular level. Addict Biol 9:137–149, 151. [DOI] [PubMed] [Google Scholar]
- Perkins A, Lehmann C, Lawrence RC, Kelly SJ. (2013). Alcohol exposure during development: impact on the epigenome. Int J Dev Neurosci 31:391–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petronis A. (2003). Epigenetics and bipolar disorder: new opportunities and challenges. Am J Med Genet C Semin Med Genet 123C:65–75. [DOI] [PubMed] [Google Scholar]
- Piomelli D. (2003). The molecular logic of endocannabinoid signalling. Nat Rev Neurosci 4:873–884. [DOI] [PubMed] [Google Scholar]
- Roberto M, Cruz M, Bajo M, Siggins GR, Parsons LH, Schweitzer P. (2010). The endocannabinoid system tonically regulates inhibitory transmission and depresses the effect of ethanol in central amygdala. Neuropsychopharmacology 35:1962–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RS, Eichenbaum H. (2006). Dynamics of hippocampal and cortical activation during consolidation of a nonspatial memory. J Neurosci 26:4852–4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu H, Lee J, Hagerty SW, Soh BY, McAlpin SE, Cormier KA, Smith KM, Ferrante RJ. (2006). ESET/SETDB1 gene expression and histone H3 (K9) trimethylation in Huntington’s disease. Proc Natl Acad Sci USA 103:19176–19181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadrian B, Subbanna S, Wilson DA, Basavarajappa BS, Saito M. (2012). Lithium prevents long-term neural and behavioral pathology induced by early alcohol exposure. Neuroscience 206:122–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K, Karelina K, Obrietan K. (2011). CREB: a multifaceted regulator of neuronal plasticity and protection. J Neurochem 116:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Andrade G, Kendrick KM. (2009). The main olfactory system and social learning in mammals. Behav Brain Res 200:323–335. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Sibille EL, Pavlides C, Fenster RJ, McEwen BS, Toth M. (2000). Impaired hippocampal-dependent learning and functional abnormalities in the hippocampus in mice lacking serotonin(1A) receptors. Proc Natl Acad Sci USA 97:14731–14736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage DD, Becher M, de la Torre AJ, Sutherland RJ. (2002). Dose-dependent effects of prenatal ethanol exposure on synaptic plasticity and learning in mature offspring. Alcohol Clin Exp Res 26:1752–1758. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. (2008). Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3:1101–1108. [DOI] [PubMed] [Google Scholar]
- Shepherd JD, Bear MF. (2011). New views of Arc, a master regulator of synaptic plasticity. Nat Neurosci 14:279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasaka T, Hashimoto E, Ukai W, Yoshinaga T, Ishii T, Tateno M, Saito T. (2012). Stem cell therapy: social recognition recovery in a FASD model. Transl Psychiatry 2:e188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanis NC, Delespaul P, Henquet C, Bakoula C, Stefanis CN, Van Os J. (2004). Early adolescent cannabis exposure and positive and negative dimensions of psychosis. Addiction 99:1333–1341. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Barr HM, Sampson PD. (1990). Moderate prenatal alcohol exposure: effects on child IQ and learning problems at age 7 1/2 years. Alcohol Clin Exp Res 14:662–669. [DOI] [PubMed] [Google Scholar]
- Subbanna S, Basavarajappa BS. (2014). Pre-administration of G9a/GLP inhibitor during synaptogenesis prevents postnatal ethanol-induced LTP deficits and neurobehavioral abnormalities in adult mice. Exp Neurol 261:34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbanna S, Nagre NN, Shivakumar M, Umapathy NS, Psychoyos D, Basavarajappa BS. (2014). Ethanol induced acetylation of histone at G9a Exon1 and G9a-mediated histone H3 dimethylation leads to neurodegeneration in neonatal mice. Neuroscience 258:422–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbanna S, Shivakumar M, Psychoyos D, Xie S, Basavarajappa BS. (2013a) Anandamide-CB1 receptor signaling contributes to postnatal ethanol-induced neonatal neurodegeneration, adult synaptic and memory deficits. J Neurosci 33:6350–6366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbanna S, Shivakumar M, Umapathy NS, Saito M, Mohan PS, Kumar A, Nixonc RA, Verin AD, Psychoyos D, Basavarajappa BS. (2013b) G9a-mediated histone methylation regulates ethanol-induced neurodegeneration in the neonatal mouse brain. Neurobiol Dis 54:475–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JD, Sather TM, Whinery LA. (2008). Voluntary exercise influences behavioral development in rats exposed to alcohol during the neonatal brain growth spurt. Behav Neurosci 122:1264–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thor DH, Wainwright KL, Holloway WR. (1982). Persistence of attention to a novel conspecific: some developmental variables in laboratory rats. Dev Psychobiol 15:1–8. [DOI] [PubMed] [Google Scholar]
- Waltereit R, Dammermann B, Wulff P, Scafidi J, Staubli U, Kauselmann G, Bundman M, Kuhl D. (2001). Arg3.1/Arc mRNA induction by Ca2+ and cAMP requires protein kinase A and mitogen-activated protein kinase/extracellular regulated kinase activation. J Neurosci 21:5484–5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Wang H, Ning W, Backlund MG, Dey SK, DuBois RN. (2008). Loss of cannabinoid receptor 1 accelerates intestinal tumor growth. Cancer Res 68:6468–6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren ST. (2007). The epigenetics of fragile X syndrome. Cell Stem Cell 1:488–489. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Sullivan RM. (2011). Cortical processing of odor objects. Neuron 72:506–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. (2001). Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature 410:588–592. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. (2002). Endocannabinoid signaling in the brain. Science 296:678–682. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Peterson J, Basavaraj BS, Saito M. (2011). Local and regional network function in behaviorally relevant cortical circuits of adult mice following postnatal alcohol exposure. Alcohol Clin Exp Res 35:1974–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CS, Jew CP, Lu HC. (2011). Lasting impacts of prenatal cannabis exposure and the role of endogenous cannabinoids in the developing brain. Future Neurol 6:459–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CS, Morgan D, Jew CP, Andrews MJ, Leishman E, Spencer CM, Czyzyk T, Bradshaw H, Mackie K, Lu HC. (2014). Long-term consequences of perinatal fatty acid amino hydrolase inhibition. Br J Pharmacol 171:1420–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying SW, Futter M, Rosenblum K, Webber MJ, Hunt SP, Bliss TV, Bramham CR. (2002). Brain-derived neurotrophic factor induces long-term potentiation in intact adult hippocampus: requirement for ERK activation coupled to CREB and upregulation of Arc synthesis. J Neurosci 22:1532–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young C, Straiko MM, Johnson SA, Creeley C, Olney JW. (2008). Ethanol causes and lithium prevents neuroapoptosis and suppression of pERK in the infant mouse brain. Neurobiol Dis 31:355–360. [DOI] [PMC free article] [PubMed] [Google Scholar]