Abstract
Background:
Central serotonergic pathways influence brain areas involved in vagal cardiovascular regulation and, thereby, influence sympathetic efferent activity. Selective serotonin reuptake inhibitors (SSRIs) affect multiple serotonergic pathways, including central autonomic pathways. However, only a few studies have assessed SSRI-mediated effects on autonomic reactivity in healthy individuals using heart rate variability (HRV).
Methods:
The present study assessed the influence of long-term treatment with escitalopram (ESC) on autonomic reactivity to an intravenous application of 50 µg cholecystokinin tetrapeptide (CCK-4) in 30 healthy young men using a double-blind, placebo (PLA)-controlled, randomized, within-subject cross-over design. Main outcome measures were time- and frequency-domain HRV parameters, assessed at both baseline and immediately after CCK-4 application.
Results:
Results showed substantial effects for the treatment × CCK-4 challenge interaction with respect to heart rate (p < 0.001; pη2 = 0.499), SDNN (p < 0.001; pη2 = 576), RMSSD (p = 0.015; pη2 = 194), NN50% (p = 0.008; pη2 = 0.224), and LF% (p = 0.014; pη2 = 0.196), and moderate effects with respect HF% (p = 0.099; pη2 = 0.094), with PLA subjects showing a higher increase in HR and SDNN and a higher decrease in RMSSD, NN50, LF and HF than subjects in the ESC condition. Thus, ESC treatment significantly blunted the autonomic reactivity to CCK-4. Secondary analysis indicated no effect of the 5-HTTLPR polymorphism on CCK-4-induced autonomic response.
Conclusions:
Our results support findings suggesting an effect of SSRI treatment on autonomic regulation and provide evidence that ESC treatment is associated with blunted autonomic reactivity in healthy men.
Keywords: 5-HTTLPR, autonomic nervous system, CCK-4, cholecystokinin tetrapetide, escitalopram, heart rate variability, panic, serotonin-transporter-linked polymorphic region, SSRI
Introduction
The autonomic nervous system (ANS) regulates a plethora of physiological reactions and contributes to self-regulation and adaptability of the organism in order to meet the metabolic demands of ongoing physical, emotional, and cognitive challenges (Thayer and Lane, 2000; Critchley, 2005). On the other hand, autonomic imbalance is associated with decreased dynamic adaptability of the organism and increased morbidity and mortality (Thayer and Lane, 2000; Thayer and Sternberg, 2006).
Central autonomic control underlies the task- and division-specific influence of the brainstem and other cerebral and cerebellar structures of the central autonomic network (Beissner et al., 2013). The activity and functional connectivity of these brain regions is partly influenced by serotonergic signaling (Strawn et al., 2012; Fisher and Hariri, 2013). Central serotonin (5-hydroxytryptamine [5-HT]) transmission is thus not only involved in the modulation of emotional and cognitive behavior, but also in autonomic regulation (Ramage, 2001; Jordan, 2005; Youn et al., 2013). Consequently, altered serotonin regulation in the central nervous system (CNS) is associated with autonomic dysregulation, while reduced CNS serotonergic activity is linked to elevated autonomic responsiveness to stressors (Audero et al., 2008; Hildreth et al., 2008; Cummings et al., 2011).
The serotonergic activity of the CNS can be pharmacologically modulated. Selective serotonin reuptake inhibitors (SSRIs) have emerged as a major therapeutic advance in psychopharmacology and are currently the most frequently prescribed and best characterized therapeutic compounds affecting multiple central projection pathways in the topography of serotonin function (Vaswani et al., 2003; Sghendo and Mifsud, 2012). Binding of these drugs to the presynaptic serotonin transporter leads to its negative allosteric modulation, which effectively inhibits its ability to reuptake serotonin from the synaptic cleft (Goodnick and Goldstein, 1998). However, the acute SSRI action is associated with rather modest synaptic serotonin increases due to negative feedback loops through somatodendritic 5-HT1A autoreceptors. However, chronic SSRI administration leads to desensitization of 5-HT1A autoreceptors and downregulation of the negative feedback inhibition and, thus, results in increased serotonin release at postsynaptic heteroreceptor sites (Sghendo and Mifsud, 2012; Walker, 2013).
Since serotonergic pathways influence brain areas involved in vagal cardiovascular regulation and thereby modulate sympathetic efferent activity (Ramage, 2001; Jordan, 2005; Youn et al., 2013), SSRI administration affecting serotonergic transmission may also modulate autonomic reactivity. For example, there is an FDA safety warning on the SSRI Celexa (citalopram hydrobromide) for causing pathological cardiovascular symptoms at high doses (FDA, 2012; Castro et al., 2013). These symptoms can be mimicked in mice by strong activation of postsynaptic 5-HT1A receptors to which serotonin has a high affinity (Youn et al., 2013). On the other hand, SSRIs demonstrate the safest cardiovascular profile of all antidepressants and are considered the first choice of drugs in cardiovascular-risk patients (Roose and Miyazaki, 2005; Hamer et al., 2011; Chittaranjan et al., 2013; Hare et al., 2014).
Nevertheless, relatively few studies have assessed the acute and long-term effects of SSRIs on autonomic function. Most of those studies have investigated autonomic effects in either psychiatric patients or patients with cardiovascular disease. In healthy adults, the few studies suggest absent clinically-significant effects on baseline autonomic measures through single-dose (Ahrens et al., 2007), short-term (Penttila et al., 2001; Siepmann et al., 2003; Chappell et al., 2013), or long-term treatment with SSRIs (Pohl et al., 2003). Only two studies investigated the effects of acute SSRI treatment on autonomic stress reactivity in healthy adults, reporting beneficial effects of a single dose of escitalopram (ESC) through attenuated autonomic responses to social and physiological stress tasks in healthy females (Hanson et al., 2013; Kemp et al., 2014). However, to date, no study has assessed the effects of long-term SSRI treatment on ANS reactivity in healthy individuals using autonomic measures in the clinical dose range.
Thus, the main objective of our study was to assess the effects of long-term SSRI treatment on autonomic reactivity to a pharmacological panic challenge in healthy individuals. One of the best-established non-invasive methods to assess parasympathetic activity is the analysis of heart rate variability (HRV; Camm et al., 1996; Reyes del Paso et al., 2013). HRV results from heart rate (HR) oscillations within its physiological range (beat-to-beat variability), controlled by parasympathetic and sympathetic modulation of intrinsic cardiac pacemakers (Akselrod et al., 1981) and reflects the capacity of the organism for regulated physical and emotional responses. Higher HRV implicates parasympathetic dominance favoring energy conservation, while low HRV suggests attenuated cardiac parasympathetic modulation (Reyes del Paso et al., 2013). Low HRV is associated with higher overall mortality, specifically heart mortality, and is considered a valid marker of heart disease (Thayer and Sternberg, 2006; Thayer et al., 2012). Psychiatric research has repeatedly used HRV to investigate physiological alterations in psychiatric disorders (Gorman and Sloan, 2000; Kemp and Quintana, 2013), suggesting an association between psychopathology and reduced parasympathetic activity (Thayer and Sternberg, 2006; Kemp et al., 2010).
We therefore investigated autonomic reactivity measured by HRV to the pharmacological panic challenge by the cholecystokinin tetrapeptide (CCK-4) in a homogenous group of young, healthy men. Intravenous administration of CCK-4 reliably reproduces consistent, dose-dependent, short-lasting anxiety paroxysms via CCK B receptors in the CNS and constitutes a well-established model to investigate autonomic and neuroendocrine panic reactions in healthy volunteers (Bradwejn et al., 1991; Eser et al., 2007; Kellner, 2011). Based on previous findings (Golding et al., 2002; Hanson et al., 2013; Kemp et al., 2014), we hypothesized that chronic SSRI treatment would lower the autonomic reactivity elicited by CCK-4. To determine differences potentially resulting from altered neuroautonomic control depending on the serotonin transporter gene-linked polymorphic region (5-HTTLPR) genotype, as previously reported by assessing only the non-medicated subjects of our sample (Agorastos et al., 2014), we additionally analyzed differences between short/short (s/s) and long/long (l/l) carriers of the 5-HTTLPR genotype.
Methods and Materials
Subjects
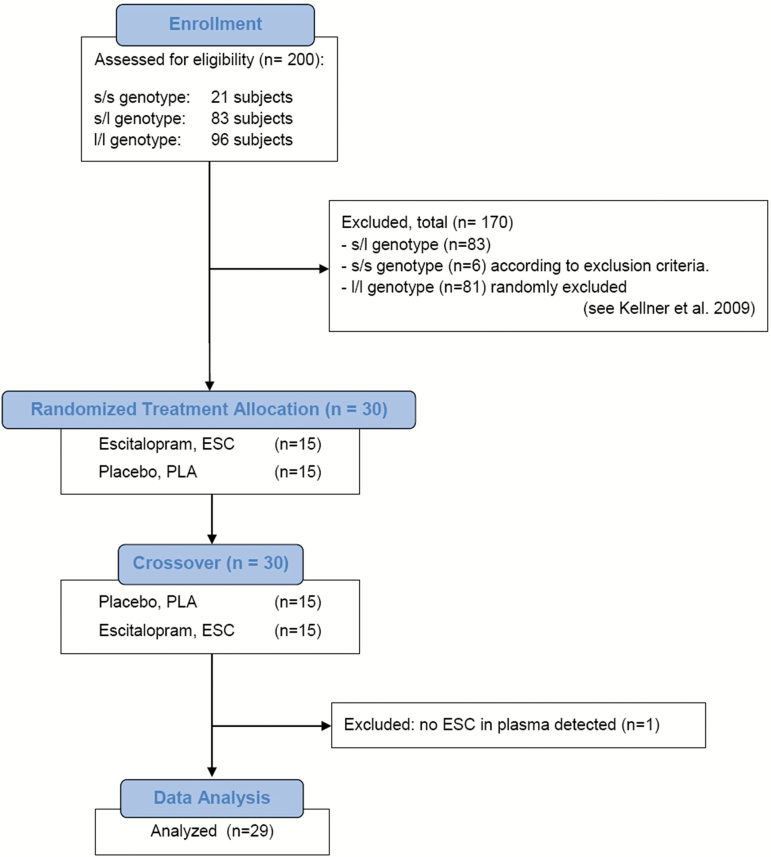
We collected data from 30 healthy young Caucasian male study volunteers (15 subjects with the s/s genotype of the 5-HTTLPR and 15 randomly-chosen eligible l/l genotype subjects from the screening sample), who participated in an experimental panic provocation study approved by the Ethics Committee of the Hamburg Medical Board. Screening procedure and study protocol have been described in detail elsewhere (Kellner et al., 2009; Hinkelmann, Dragoi, et al., 2010). Participant selection and attrition across the experiment, based on specific exclusion criteria, are provided in Figure 1. Exclusion criteria were: presence or history of any physical and Axes I and II mental co-morbidities, history of sporadic panic attacks, family history of Axis I mental disorders, frequent usage of any illicit or prescribed drugs or over-the-counter medications, current use of any medication, consumption of more than four cigarettes per day, drinking of more than four cups of coffee a day and more than 100g of alcohol per week, current adverse life events, night shifts or transcontinental flights across more than four time zones during the past four weeks, abnormal physical and neurological examinations, basic blood laboratory test values deviating from the normal range (including thyroid function tests, transaminases, electrolytes, CO2 anion gap, fasting glucose, basic blood and coagulation tests, blood lipids, hemoglobin A1c, C-reactive protein, creatinine, folic acid, and vitamin B12), positive urine toxicology screen, and pathological chest x-ray or initial electrocardiogram (ECG). Current or lifetime psychiatric disorders were excluded using the Structured Clinical Interview for the DSM-IV, Axes I and II, assessed by a trained physician. All other exclusion criteria were assessed in a clinical interview setting through study questionnaires. After full oral and written explanation of the purpose and procedures of the investigation, written informed consent was obtained from each subject.
Figure 1.
CONSORT Flow Diagram displaying the progress of all participants through the trial.
Procedures
Panic challenges were performed after 42 days of daily treatment with 10mg escitalopram in a double-blind, placebo (PLA)-controlled, randomized, within-subject, crossover design with a wash-out phase of at least three weeks in between pre-treatment periods. To check for compliance of intake of study medication, ESC was measured in plasma specimens taken the day after the study had been finished, as described in Greiner et al. (2007).
Venous blood samples were obtained for DNA extraction, and the 5-HTTLPR genotype was determined as described before (Maron et al., 2004; Kellner et al., 2009). After standardized lunch at 12:00 hours, subjects were studied from 13:00 to 17:00 hours, exclusively in a supine position in a soundproof experimental room. CCK-4 (Clinalfa) was stored at -80°C and freshly prepared for each injection. A total of 50 µg of CCK-4 were dissolved in 10ml sterile saline. At 15:00 hours, subjects received 50 µg CCK-4 as an intravenous bolus injection within 20 s. Subjects were closely monitored after the injection of CCK-4. ECG recordings were obtained throughout using a 5-lead holter recording system (Schiller medilog® AR12, Schiller Medizintechnik GmbH). Data were recorded at a 4096 Hz sampling rate in 16-bit resolution and were stored digitally on the recorder. ECG recording was performed by specially-trained study staff. ECG analyses were performed using specific software (Schiller medilog® DARWIN, Schiller Medizintechnik GmbH). Adverse side effects were assessed through a German version of the Udvalg for Kliniske Undersogelser (UKU) side effects rating scale (Lingjaerde et al., 1987).
ECG Analysis
Data from two 5-min segments (baseline and immediately after CCK-4 application) were used to determine differences between treatment conditions. Recording of CCK-4–mediated effects was initiated 45–60 s after the bolus injection at 15:01 hours, while baseline recording started 60min before CCK-4 injection at 14:00 hours. Instantaneous HR was calculated on the basis of the RR interval. HRV in the time domain was calculated by taking the standard deviation of the N-N intervals (SDNN), by calculating the root-mean-square of subsequent interval differences (RMSSD), and by the percentage of the number of pairs of adjacent NN intervals that differ in length by >50ms (NN50%). HRV in the frequency domain was calculated by analysis of two frequency components [low frequency (LF): 0.04–0.15 Hz; high frequency (HF): 0.15–0.4 Hz]. Results are presented as the percentage of each frequency component from the total power and the LF/HF ratio. The assessed HRV variables have been selected according to the guidelines for short-term recordings of the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology (Camm et al., 1996), which are also supported by new studies (Meyer and Stiedl, 2003; Jarrin et al., 2012). We used the percentage of the total power of each frequency component to compare the fractional energy (Camm et al., 1996), rather than the absolute energy per frequency band.
Statistical Analyses
Preliminary analyses were performed to ensure no violation of the assumptions of normality, linearity, and homoscedasticity. Parameters with skewed distribution, i.e., the LF/HF ratio, were log10 transformed for further parametric analysis. All data are given in mean values (standard error of the mean). CCK-4-induced changes have been calculated as ∆M = MCCK-4 - Mbaseline. Differences between treatment conditions were tested for significance by analyses of variance (ANOVAs). Additionally, mixed between-within subjects ANOVAs were used to assess the impact of CCK-4 application (baseline vs. CCK-4), CCK-4 × treatment, and then, additionally, 5-HTTLPR genotype (s/s vs. l/l; CCK-4 × treatment × genotype) on HR dynamics and the differences within groups (PLA vs. ESC). An error probability of p < 0.05 was accepted as statistically significant. Effect size is reported as eta squared (η 2 = 0.01: small effect size, η 2 = 0.06: medium effect size, η 2 = 0.14: large effect size). To correct for potentially inflated type I error because of multiple comparisons, we used the false discovery rate approach (Benjamini and Hochberg, 1995), as in our previous studies (Agorastos et al., 2013, 2014). Following a previously reported procedure (Verhoeven et al., 2005) p-values were corrected by the minimum positive false discovery rate with a threshold set at 5%. Statistical analyses were conducted using the Statistical Package for Social Sciences Version 20 (SPSS).
Results
All 30 volunteers completed the study. Enrolled subjects had a median age of 27 years (range 19–36) and BMI of 21.9kg/m2 (range 18.0–27.1) and there were no differences in these measures between the ESC and PLA conditions (data not shown). In one subject, no ESC could be detected in plasma after 42 days of verum treatment. Therefore, this subject was excluded from further analyses. Mean plasma ESC concentration was 15.5ng/ml on day 42, and mean plasma desmethyl-ESC level was 6.6ng/ml. No active drug was detected in any subject during PLA intake. Side effects, as per UKU ratings, did not differ significantly between treatments.
CCK-4 Challenge x Treatment Interaction Effects
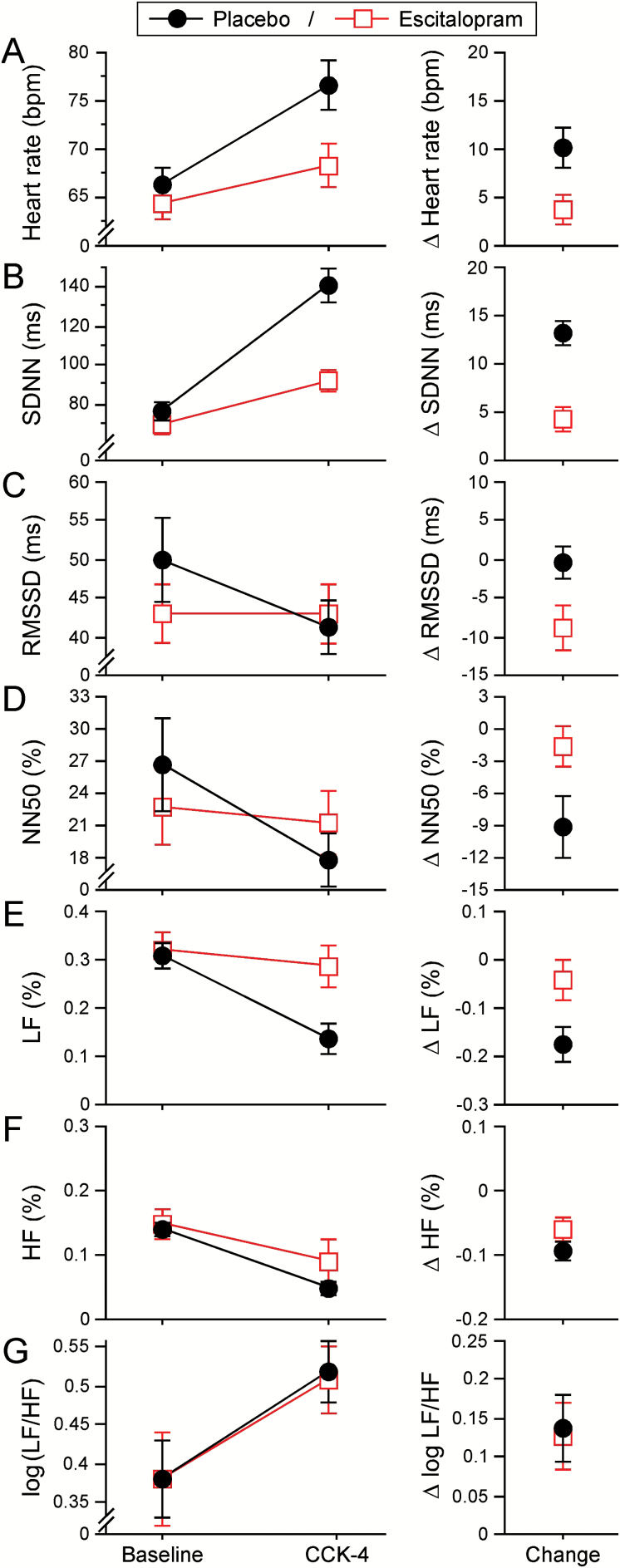
Mixed ANOVAs were conducted to assess the impact of treatment (ESC vs. PLA) on dependent variables across the two time periods (baseline, post-CCK-4). The results showed substantial effects for the treatment × CCK-4 challenge interaction with respect to HR (Wilks λ = 0.501; F(1,28) = 27.853; p < 0.001, pη2 = 0.499), SDNN (Wilks λ = 0.424; F(1,28) = 38.051; p < 0.001; pη2 = 576), RMSSD (Wilks λ = 0.806; F(1,28) = 6.723; p = 0.015, pη2 = 194), NN50% (Wilks λ = 0.776; F(1,28) = 8.066; p = 0.008, pη2 = 0.224), and LF% (Wilks λ = 0.804; F(1,28) = 6.845; p = 0.014, pη2 = 0.196) and moderate, but not statistically significant, effects with respect to HF% (Wilks λ = 0.906; F(1,28) = 2.908; p = 0.099, pη2 = 0.094). Subjects showed an significantly higher increase in HR and SDNN and a higher decrease in RMSSD, NN50, and LF in the PLA than in the ESC condition (Figure 2; Table 1).
Figure 2.
Effects of placebo and long-term escitalopram treatment on a range of cardiovascular time domain (A–D) and frequency domain measures (E–G).
Values are presented as means ± standard error of the mean. Measures were determined at baseline and after pharmacological panic challenge by cholecystokinin tetrapeptide (CCK-4; left panels). CCK-4-induced changes (right panels) have been calculated as ∆M = Mpost CCK-4 - Mbaseline. SDNN: standard deviation of the N-N intervals; RMSSD: root mean square of subsequent differences; NN50%: percentage of the number of pairs of adjacent NN intervals that differ in length by >50ms; LF: low frequency 0.04–0.15 Hz; HF: high frequency 0.15–0.4 Hz; LF% and HF%: percentage of each frequency component from the total power.
Table 1.
Effects of CCK-4 challenge and treatment x CCK-4 challenge on heart dynamics
| Domain | Measure | rmANOVA | Mixed ANOVA | ||
|---|---|---|---|---|---|
| CCK-4 challenge | Treatment x CCK-4 challenge | ||||
| p | p η 2 | p | p η 2 | ||
| Time | HR (bpm) | <0.001 | 0.548 ++ | <0.001 | 0.499 ++ |
| SDNN (ms) | <0.001 | 0.753 ++ | <0.001 | 0.576 ++ | |
| RMSSD (ms) | <0.001 | 0.303 ++ | 0.015 | 0.194 ++ | |
| NN50% | <0.001 | 0.349 ++ | 0.008 | 0.224 ++ | |
| Frequency | LF% | <0.001 | 0.672 ++ | 0.014 | 0.196 ++ |
| HF% | <0.001 | 0.651 ++ | 0.099 | 0.094+ | |
| LF/HFlog | <0.001 | 0.240 ++ | 0.888 | 0.001 | |
Repeated-measures ANOVAs and between- and within-subjects ANOVAs were used to assess the impact of the cholecystokinin tetrapeptide (CCK-4) challenge and the differences within treatment groups (placebo [PLA] vs. escitalopram [ESC]). HR: heart rate; SDNN: standard deviation of the N-N intervals; RMSSD: root mean square of subsequent differences; NN50%: percentage of the number of pairs of adjacent NN intervals that differ in length by >50ms; LF: low frequency 0.04–0.15 Hz; HF: high frequency 0.15–0.4 Hz; LF% and HF%: percentage of each frequency component from the total power. P-values denoting statistically significant differences are shown in bold. False discovery rate analysis revealed no potential type I errors.
+moderate effect size; ++large effect size.
Main Effects of CCK-4 Challenge
When investigating the effect of CCK-4 challenge in the total population, repeated-measures ANOVAs indicated significant to highly significant differences between baseline and post-CCK-4 measures with very large effect sizes in all assessed measures, leading to higher HR (Wilks λ = 0.453; F(1,58) = 69.081; p < 0.001; pη2 = 0.548), SDNN (Wilks λ = 0.247; F(1,58) = 173.605; p < 0.001; pη2 = 0.753), and LF/HFlog (Wilks λ = 0.760; F(1,58) = 17.976; p < 0.001; pη2 = 0.240), and lower RMSSD (Wilks λ = 0.697; F(1,58) = 24.808; p < 0.001; pη2 = 0.303), NN50% (Wilks λ = 0.651; F(1,58) = 30.578; p < 0.001; pη2 = 0.349), LF% (Wilks λ = 0.328; F(1,58) = 116.745; p < 0.001; pη2 = 0.672), and HF% (Wilks λ = 0.349; F(1,58) = 106.489; p < 0.001; pη2 = 0.651) values (Figure 2; Table 1).
Treatment Differences
Baseline
A group comparison revealed only differences with moderate effect sizes but no statistical significance between the two groups with respect to baseline HR measures (Figure 2; Table 2).
Table 2.
Differences Between Treatment Conditions in HRV measures
| Domain | Measure | PLA vs. ESC | Baseline vs. CCK-4 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | CCK-4 | PLA | ESC | ||||||
| p | p η 2 | p | p η 2 | p | p η 2 | P | p η 2 | ||
| Time | HR (bpm) | 0.129 | 0.080 | <0.001 | 0.591 ++ | <0.001 | 0.541 ++ | 0.007 | 0.233 ++ |
| SDNN (ms) | 0.119 | 0.085 | <0.001 | 0.609 ++ | <0.001 | 0.780 ++ | <0.001 | 0.438 ++ | |
| RMSSD (ms) | 0.044 § | 0.137 + | 0.483 | 0.018 | 0.007 | 0.230 ++ | 0.886 | 0.001 | |
| NN50% | 0.072 | 0.111 + | 0.088 | 0.100 + | 0.002 | 0.301 ++ | 0.369 | 0.029 | |
| Frequency | LF% | 0.690 | 0.006 | <0.001 | 0.435 ++ | <0.001 | 0.591 ++ | 0.372 | 0.029 |
| HF% | 0.557 | 0.012 | <0.001 | 0.472 ++ | <0.001 | 0.670 ++ | 0.001 | 0.307 ++ | |
| LF/HFlog | 0.930 | 0.000 | 0.930 | 0.000 | 0.003 | 0.271 ++ | 0.001 | 0.318 ++ | |
HR: heart rate; SDNN: standard deviation of the N-N intervals; RMSSD: root mean square of subsequent differences; NN50%: percentage of the number of pairs of adjacent NN intervals that differ in length by >50ms; LF: low frequency 0.04–0.15 Hz; HF: high frequency 0.15–0.4 Hz; LF% and HF%: percentage of each frequency component from the total power; PLA: placebo; ESC: escitalopram; CCK-4: cholecystokinin tetrapeptide. P-values denoting statistically significant differences or a trend are shown in bold.
+moderate effect size; ++large effect size; §potential type I error based on false discovery rate analysis.
CCK-4 Challenge
Post-CCK-4-injection HR measures indicated statistically significant differences with large effect sizes between the two treatment conditions (Figure 2; Table 2). Subjects in the ESC condition showed overall significantly lower HR increases (Wilks λ = 0.409, F(1,28) = 40.450, p < 0.001, pη2 = 0.591) and SDNN (Wilks λ = 0.391, F(1,28) = 43.642; p < 0.001, pη2 = 0.609), and higher NN50% (Wilks λ = 0.900, F(1,28) = 3.119; p = 0.088, pη2 = 0.100), LF% (Wilks λ = 0.565, F(1,28) = 21.537; p < 0.001, pη2 = 0.435), and HF% (Wilks λ = 0.528, F(1,28) = 25.026; p < 0.001, pη2 = 0.472) values.
Differences Depending on the 5-HTTLPR Genotype
Since we previously reported significantly lower autonomic reactivity to CCK-4 in s/s versus l/l 5-HTTLPR carriers without treatment (Agorastos et al., 2014) we also conducted an additional analysis to investigate the genotype effects. Mixed models using repeated-measures ANOVA were used to assess the impact of the 5-HTTLPR genotype, i.e., s/s vs. l/l, on HRV measures, including both treatment conditions (CCK-4 × treatment × genotype). There was no statistically significant effect of genotype and no significant interaction between genotype and treatment condition (data not shown). When subjects in the ESC condition were analyzed separately, s/s carriers displayed reduced autonomic reactivity in comparison to the l/l carriers, with a similar trend to our previous findings (Agorastos et al., 2014). However, these differences were not statistically significant.
Discussion
This study assessed the effects of long-term SSRI treatment on acute CCK-4–mediated effects on HRV in healthy men, using the most selective SSRI available (Burke, 2002; Sanchez et al., 2003) as a potential modulator of autonomic control. Although not the first study to examine the impact of SSRI treatment on stress responsiveness, to the best of our knowledge, our study is the first one analyzing autonomic responses following long-term SSRI treatment related to pharmacological panic challenge by CCK-4 based on HR measures in healthy young men.
The main findings of this study include: (1) no statistically significant evidence for ESC-associated effects on baseline vagal activity; (2) attenuated cardiac vagal modulation in both treatment conditions by CCK-4; (3) significantly lower vagal tone and increased autonomic reactivity upon CCK-4 challenge in the PLA vs. ESC group; and (4) no 5-HTTLPR genotype effect on HRV measures and their changes after CCK-4 application over both treatment conditions. Jointly, these findings indicate blunted autonomic reactivity to CCK-4 in the ESC treatment condition, in comparison to PLA.
CCK-4 application has been repeatedly shown to lead to a robust increase of HR and systolic blood pressure (Benkelfat et al., 1995; Jerabek et al., 1999; Eser et al., 2007). However, so far only two previous studies from our group reported autonomic effects of CCK-4 on HRV (Wiedemann et al., 2001; Agorastos et al., 2014). Thus, our findings of enhanced sympathetic and/or attenuated vagal cardiac modulation of HRV by CCK-4 in both PLA and ESC treatment conditions replicate and extend our previous findings.
The present study investigated resting autonomic measures as a function of central serotonergic activity in both treatment conditions. HRV effects of long-term SSRI administration have been investigated in various categories of patients. In depression, in contrast to other antidepressant categories (e.g., tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors; Siepmann et al., 2007; Kemp et al., 2010; Chang et al., 2012), SSRI treatment has been reported not to have a significant impact on resting HRV (Rechlin, 1994; Straneva-Meuse et al., 2004; Koschke et al., 2009; Kemp et al., 2010, 2011, 2014; Brunoni et al., 2013; Hanson et al., 2013), although contradictory results have also been reported (Licht et al., 2010). In patients with panic disorder or post-traumatic stress disorder, SSRI treatment has been mainly associated with positive effects on HRV measures, i.e., reduced sympathetic activity/normalized ANS activity and baroreflex response (Tucker et al., 1997; Cohen et al., 2000). SSRI treatment in patients with cardiovascular diseases has been also associated with an improvement of HRV indices independent of the improvement of depressive symptoms, suggesting a clear benefit for the prognosis of the cardiovascular disease (Gorman and Sloan, 2000; Leftheriotis et al., 2010; Mazza et al., 2010; Pizzi et al., 2011). However, only a few studies have investigated single-dose or long-term SSRI treatment effects on HRV or other autonomic measures in healthy adults at rest. These studies suggest no clinically significant autonomic SSRI effects in either males or females in resting states (Penttila et al., 2001; Pohl et al., 2003; Siepmann et al., 2003; Ahrens et al., 2007; Chappell et al., 2013; Hanson et al., 2013; Kemp et al., 2014). Thus, our results in males support these previous studies.
Our study also assessed the modulating effect of long-term ESC treatment on autonomic reactivity in response to a pharmacological panic challenge in healthy subjects. To date, effects of SSRI treatment on autonomic reactivity have been only sparsely investigated, predominantly in psychiatric patients using various stressor models. Effects of SSRI treatment on autonomic reactivity have been reported in patients with panic disorder and post-traumatic stress disorder, suggesting a positive effect of SSRIs on HRV reactivity to physiological (orthostatic) or psychological challenges, towards normalizing autonomic indices (Tucker et al., 1997, 2000).
In healthy male and female individuals, Takata et al. (2002) reported decreased orthostatic baroreflex sensitivity after long-term paroxetine treatment, while Golding et al. (2002) observed decreased autonomic reactivity to a psychological stressor, which is also supported by our results. However, both studies used only HR and blood pressure measures to assess autonomic activity. Two recent studies investigated ESC treatment effects on autonomic stress reactivity in healthy females, reporting attenuated autonomic responses in social and physiological stress tasks (Hanson et al., 2013; Kemp et al., 2014). Both studies, however, only investigated effects of a single-dose of ESC. Besides a previous report from our group (Kellner et al., 2009) on the same sample group, only one additional study investigated long-term effects of ESC on CCK-4 challenge in both male and female healthy volunteers (Toru et al., 2013). In contrast to results in patients with panic disorder (Shlik et al., 1997; van Megen et al., 1997), no inhibitory effect of ESC was observed upon panic symptoms elicited by CCK-4 in healthy subjects. However, neither study reported any ESC effects on autonomic measures. Thus, this study is the first one to report long-term effects of the SSRI ESC on ANS reactivity to a pharmacological panic challenge by CCK-4 in healthy subjects, indicating reduced autonomic reactivity following long-term treatment by ESC in comparison to the PLA control in healthy subjects.
The CCK-4-induced increase in HR, together with a decrease in SDNN, RMSSD, NN50%, LF%, and HF%, is interpreted as increased sympathetic and/or attenuated parasympathetic (vagal) cardiac modulation, consistent with previous claims (Camm et al., 1996; Lombardi and Stein, 2011). The initial reactivity hypothesis proposed that enhanced cardiovascular autonomic reactivity is associated with increased cardiovascular risk as a mediator of psychosocial and behavioral risk factors (Phillips, 2011; Phillips and Hughes, 2011). Following this hypothesis, our data suggest that the reduced autonomic reactivity to the CCK-4 challenge observed in the ESC group may represent a marker of reduced cardiovascular risk, as postulated in a prior study (Brummett et al., 2011). This is also supported by the statistically absent differences in baseline HR between the two treatment conditions, as resting HR is considered an independent predictor of cardiovascular risk (Fox et al., 2007). SSRI treatment has shown beneficial cardiovascular effects in cardiovascular disease, which are associated with reduced autonomic reactivity. Similarly, advantageous cardiovascular effects of SSRIs leading to normalization of autonomic measures have been also reported in psychiatric disorders (see above), which have been associated with increased (Monk et al., 2001; Blechert et al., 2010; Felmingham et al., 2012) or reduced (Kikuchi et al., 2009; McTeague et al., 2010; Shinba, 2013) autonomic reactivity. In healthy females, comparable moderating effects on HR and HRV have been observed by acute SSRI treatment and regular high-intensity exercise to a physical stress challenge (Hanson et al., 2013). In contrast, robust evidence has been provided that reduced cardiovascular reactivity and slower recovery are associated with overall cardiovascular risk (Heponiemi et al., 2007; Salomon et al., 2009), which should be discussed here. Unfortunately, our study did not assess data on cardiac recovery after the stress challenge, which is also a major indicator of cardiac reactivity. Thus, on the basis of the currently used measures we cannot unambiguously resolve whether the reduced stress responsiveness reflects a beneficial (‘anxiolytic-like’) or a maladaptive state (‘hypo-responsiveness’). Nonlinear measures (Meyer and Stiedl, 2003) may provide for an unambiguous interpretation of physiological versus pathological change, but cannot be applied with short ECG recordings as used here.
The existence of functional subpopulations of serotonergic neurons acting at numerous sites of the CNS and the evidence for their tight control by stress hormones (Chaouloff, 1993; Johnson et al., 2004) suggest a complex interplay of central serotonergic activity with ANS and cardiac stress responsiveness. SSRI treatment may, thus, both influence supra-ordinate mechanisms (e.g., the central autonomic network) and/or affect hormone secretion patterns (Shores et al., 2001; Agelink et al., 2004). With respect to cardiovascular disease patients specifically, additional pleiotropic SSRI effects may also be responsible for the beneficial effects reported (Escolar et al., 2005; Paraskevaidis et al., 2006). Our study also investigated the potential contribution of the 5-HTTLPR polymorphism on PLA versus ESC treatment towards affecting CCK-4–mediated HR responses. Prior studies have reported a moderating role of 5-HTTLPR polymorphisms on the brain-heart interaction (Kauppila et al., 2013; Mueller et al., 2013). However, to date, our prior study assessing 5-HTTLPR genotype effects exclusively in healthy subjects receiving PLA was the first one investigating the association of 5-HTTLPR with ANS function using HRV in healthy individuals. We reported enhanced sympathetic and/or diminished baseline cardiac vagal activity and blunted autonomic reactivity in s/s versus l/l carriers without drug treatment (Agorastos et al., 2014). Our current analyses revealed no overall effect of the 5-HTTLPR polymorphism on autonomic responses to CCK-4 challenge. When ESC condition was investigated separately, analyses suggested a similar 5-HTTLPR polymorphism effect as to our previous study on PLA condition, but without statistical significance.
Limitations
Some limitations have to be taken into account for the presented study. To avoid putative confounding effects of the menstrual cycle phase on response to CCK-4 (Le Melledo et al., 1999) and to avoid problems associated with contraception, only male subjects were studied. Thus, long-term HRV effects still need to be investigated in women, as gender differences cannot be ruled out. Similarly, our results should be replicated in individuals of older age, since age-related changes in 5-HT transmission and SSRI effects have been reported (Olivier et al., 2011; Kemp et al., 2014). Effects of SSRIs after longer treatment (>4 weeks, as commonly used in psychiatric practice) still need to be investigated.
Since ESC is considered the most selective SSRI (Burke, 2002; Sanchez et al., 2003), our results may not be generalizable to other substances of the SSRI group. Despite sharing the same principal mechanism of action, recent reviews investigating SSRIs effects in healthy persons suggest various inconsistencies dues to differences in their pharmacodynamic and pharmacokinetic profiles (Goodnick and Goldstein, 1998; Knorr and Kessing, 2010). In addition, several long-term SSRI effects (e.g., neuroplastic and neurotrophic changes, effects on gene expression, anti-inflammatory properties) have been postulated, opposing the initially-assumed simplistic, monoaminergic pharmacological properties of these drugs (Kroeze et al., 2012; Walker, 2013), so that different pathways of action may be responsible for the observed effects. Another limitation is the use of one standardized dosage of ESC only. However, the ESC plasma levels measured were within the range recommended for treatment of affective disorders.
Furthermore, as disturbed sleep is also associated with autonomic alterations (Nielsen et al., 2010), it is important to note that we did not determine sleep quality in our initial assessments and can herewith not exclude sleep quality–related bias, although we did not encounter complaints about altered sleep patterns. In order to rule out conditioning effects of repeated CCK-4 administration (Hinkelmann, Yassouridis, et al., 2010), we controlled for the randomized treatment order, which did not affect our results. In addition, our study did not include subjects with the s/l genotype. Since some studies suggest a nonlinear gene-dose effect of the 5-HTTLPR (Neumeister et al., 2006), inclusion of s/l subjects in further studies with a considerably larger sample size is necessary to precisely characterize genotype differences. Regarding functional variants of the long allele, posterior analyses of our DNA specimens revealed that all long alleles in this study were of the LA subtype (Lesch, personal communication).
Finally, it is important to note that across all parameters investigated, no subject had a cardiovascular history and deviating laboratory or physical tests. We particularly accounted for several laboratory markers (e.g., fasting glucose, hemoglobin A1c levels, cholesterol/lipoproteins, pro-inflammatory cytokines, acute-phase proteins) and certain lifestyle habits (e.g., drug, alcohol, or tobacco intake) that have been shown to be associated with ANS dysregulation, altering cardiovascular measures, including HRV (Thayer and Sternberg, 2006; Dinas et al., 2013).
Conclusions
Studies about differential long-term effects of SSRIs on HRV reactivity using pharmacological stress challenges have not been reported so far in healthy subjects. By assessing autonomic responses to a defined pharmacological panic challenge, we provide the first data that chronic SSRI treatment is associated with reduced autonomic reactivity in healthy subjects. Our study supports an important role of central serotonergic activity in the modulation of the magnitude of acute cardiovascular responsiveness, which may affect susceptibility to stress-related disorders. Future studies are needed to replicate this finding and to further explore the functional contribution of effects through and regulation of different receptor subtypes of the serotonergic system to mechanistically understand the role of SSRI on ANS function in both health and disease.
Statement of Interest
Dr Kellner received funding as a PI through an investigation grant by H Lundbeck A/S, Denmark. All authors reported no biomedical financial interests or potential conflicts of interest and none of the other authors received funding for this article.
Acknowledgments
We gratefully acknowledge the fiscal contribution of H Lundbeck A/S, Denmark, through an investigation grant to Dr Kellner (PI). Clinical Trials Registration Number: ISRCTN04339282, http://www.controlled-trials.com/ISRCTN04559282/kellner.
Dr Kellner designed the study and wrote the protocol. Drs Demiralay and Muhtz collected the data. Dr Agorastos managed the literature searches. Drs Agorastos and Stiedl had access to the raw data and performed all statistical analyses and data interpretation. Drs Agorastos and Demiralay wrote the first draft of the paper. Drs Kellner, Wiedemann, and Stiedl revised the draft for important intellectual content. All authors have contributed to, read, and approved the final version of the manuscript.
References
- Agelink MW, Klimke A, Cordes J, Sanner D, Kavuk I, Malessa R, Klieser E, Baumann B. (2004). A functional-structural model to understand cardiac autonomic nervous system (ANS) dysregulation in affective illness and to elucidate the ANS effects of antidepressive treatment. Eur J Med Res 9:37–50. [PubMed] [Google Scholar]
- Agorastos A, Boel JA, Heppner PS, Hager T, Moeller-Bertram T, Haji U, Motazedi A, Yanagi MA, Baker DG, Stiedl O. (2013). Diminished vagal activity and blunted diurnal variation of heart rate dynamics in posttraumatic stress disorder. Stress 16:300–310. [DOI] [PubMed] [Google Scholar]
- Agorastos A, Kellner M, Stiedl O, Muhtz C, Becktepe JS, Wiedemann K, Demiralay C. (2014). The 5-HTTLPR genotype modulates heart rate variability and its adjustment by pharmacological panic challenge in healthy men. J Psychiatr Res 50:51–58. [DOI] [PubMed] [Google Scholar]
- Ahrens T, Frankhauser P, Lederbogen F, Deuschle M. (2007). Effect of single-dose sertraline on the hypothalamus-pituitary-adrenal system, autonomic nervous system, and platelet function. J Clin Psychopharmacol 27:602–606. [DOI] [PubMed] [Google Scholar]
- Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ. (1981). Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science 213:220–222. [DOI] [PubMed] [Google Scholar]
- Audero E, Coppi E, Mlinar B, Rossetti T, Caprioli A, Banchaabouchi MA, Corradetti R, Gross C. (2008). Sporadic autonomic dysregulation and death associated with excessive serotonin autoinhibition. Science 321:130–133. [DOI] [PubMed] [Google Scholar]
- Beissner F, Meissner K, Bar KJ, Napadow V. (2013). The autonomic brain: an activation likelihood estimation meta-analysis for central processing of autonomic function. J Neurosci 33:10503–10511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg J. (1995). Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Statist Soc B 57:289–300. [Google Scholar]
- Benkelfat C, Bradwejn J, Meyer E, Ellenbogen M, Milot S, Gjedde A, Evans A. (1995). Functional neuroanatomy of CCK4-induced anxiety in normal healthy volunteers. Am J Psych 152:1180–1184. [DOI] [PubMed] [Google Scholar]
- Blechert J, Wilhelm FH, Meuret AE, Wilhelm EM, Roth WT. (2010). Respiratory, autonomic, and experiential responses to repeated inhalations of 20% CO(2) enriched air in panic disorder, social phobia, and healthy controls. Biol Psychology 84:104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradwejn J, Koszycki D, Bourin M. (1991). Dose ranging study of the effects of cholecystokinin in healthy volunteers. J Psychiatry Neurosci 16:91–95. [PMC free article] [PubMed] [Google Scholar]
- Brummett BH, Siegler IC, Ashley-Koch A, Williams RB. (2011). Effects of 5HTTLPR on cardiovascular response to an emotional stressor. Psychosom Med 73:318–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunoni AR, Kemp AH, Dantas EM, Goulart AC, Nunes MA, Boggio PS, Mill JG, Lotufo PA, Fregni F, Bensenor IM. (2013). Heart rate variability is a trait marker of major depressive disorder: evidence from the sertraline vs. electric current therapy to treat depression clinical study. Int J Neuropsychop 16:1937–1949. [DOI] [PubMed] [Google Scholar]
- Burke WJ. (2002). Escitalopram. Expert Opin Investig Drugs 11:1477–1486. [DOI] [PubMed] [Google Scholar]
- Camm AJ, Malik M, Bigger JT, Breithardt G, Cerutti S, Cohen RJ, Coumel P, Fallen EL, Kennedy HL, Kleiger RE, Lombardi F, Malliani A, Moss AJ, Rottman JN, Schmidt G, Schwartz PJ, Singer D. (1996). Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation 93:1043–1065. [PubMed] [Google Scholar]
- Castro VM, Clements CC, Murphy SN, Gainer VS, Fava M, Weilburg JB, Erb JL, Churchill SE, Kohane IS, Iosifescu DV, Smoller JW, Perlis RH. (2013). QT interval and antidepressant use: a cross sectional study of electronic health records. The BMJ 346:f288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JS, Ha K, Yoon IY, Yoo CS, Yi SH, Her JY, Ha TH, Park T. (2012). Patterns of cardiorespiratory coordination in young women with recurrent major depressive disorder treated with escitalopram or venlafaxine. Prog Neuropsychopharmacol Biol Psychiatry 39:136–142. [DOI] [PubMed] [Google Scholar]
- Chaouloff F. (1993). Physiopharmacological interactions between stress hormones and central serotonergic systems. Brain Res Brain Res Rev 18:1–32. [DOI] [PubMed] [Google Scholar]
- Chappell JC, Kovacs R, Haber H, Wright R, Mitchell MI, Detke M, Pangallo B. (2013). Evaluation of the effects of duloxetine and escitalopram on 24-hour heart rate variability: a mechanistic study using heart rate variability as a pharmacodynamic measure. J Clin Psychopharmacol 33:236–239. [DOI] [PubMed] [Google Scholar]
- Chittaranjan A, Chethan KB, Sandarsh S. (2013). Cardiovascular mechanisms of SSRI drugs and their benefits and risks in ischemic heart disease and heart failure. Int Clin Psychopharmacol 28:145–155. [DOI] [PubMed] [Google Scholar]
- Cohen H, Kotler M, Matar M, Kaplan Z. (2000). Normalization of heart rate variability in post-traumatic stress disorder patients following fluoxetine treatment: preliminary results. Isr Med Assoc J 2:296–301. [PubMed] [Google Scholar]
- Critchley HD. (2005). Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol 493:154–166. [DOI] [PubMed] [Google Scholar]
- Cummings KJ, Li A, Nattie EE. (2011). Brainstem serotonin deficiency in the neonatal period: autonomic dysregulation during mild cold stress. J Physiol 589:2055–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinas PC, Koutedakis Y, Flouris AD. (2013). Effects of active and passive tobacco cigarette smoking on heart rate variability. Int J Cardiol 163:109–115. [DOI] [PubMed] [Google Scholar]
- Escolar G, Diaz-Ricart M, Gomez-Gil E, Serra M, Gasto C, Bozzo J, Galan AM. (2005). Serotonergic mechanisms: a potential link between affective disorders and cardiovascular risk. Drugs Today (Barc) 41:721–743. [DOI] [PubMed] [Google Scholar]
- Eser D, Schule C, Baghai T, Floesser A, Krebs-Brown A, Enunwa M, de la Motte S, Engel R, Kucher K, Rupprecht R. (2007). Evaluation of the CCK-4 model as a challenge paradigm in a population of healthy volunteers within a proof-of-concept study. Psychopharmacology (Berl) 192:479–487. [DOI] [PubMed] [Google Scholar]
- FDA (2012). Drug Safety Communication: Revised recommendations for Celexa (citalopram hydrobromide) related to a potential risk of abnormal heart rhythms with high doses. In: U.S. Food and Drug Administration.
- Felmingham KL, Rennie C, Gordon E, Bryant RA. (2012). Autonomic and cortical reactivity in acute and chronic posttraumatic stress. Biol Psychology 90:224–227. [DOI] [PubMed] [Google Scholar]
- Fisher PM, Hariri AR. (2013). Identifying serotonergic mechanisms underlying the corticolimbic response to threat in humans. Phil Trans R Soc B 368:20120192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K, Borer JS, Camm AJ, Danchin N, Ferrari R, Lopez Sendon JL, Steg PG, Tardif JC, Tavazzi L, Tendera M. (2007). Resting heart rate in cardiovascular disease. J Am Coll Cardiol 50:823–830. [DOI] [PubMed] [Google Scholar]
- Golding M, Kotlyar M, Garbutt JC, Guzzo J, Sontz E, Hinderliter A, Carson SW. (2002). Paroxetine modulates psychological and sympathetic responses during public speaking. J Clin Psychopharmacol 22:98–99. [DOI] [PubMed] [Google Scholar]
- Goodnick PJ, Goldstein BJ. (1998). Selective serotonin reuptake inhibitors in affective disorders--I. Basic pharmacology. J Psychopharmacol 12:S5–20. [DOI] [PubMed] [Google Scholar]
- Gorman JM, Sloan RP. (2000). Heart rate variability in depressive and anxiety disorders. Am Heart J 140:77–83. [DOI] [PubMed] [Google Scholar]
- Greiner C, Hiemke C, Bader W, Haen E. (2007). Determination of citalopram and escitalopram together with their active main metabolites desmethyl(es-)citalopram in human serum by column-switching high performance liquid chromatography (HPLC) and spectrophotometric detection. J Chromatogr B 848:391–394. [DOI] [PubMed] [Google Scholar]
- Hamer M, Batty GD, Seldenrijk A, Kivimaki M. (2011). Antidepressant medication use and future risk of cardiovascular disease: the Scottish Health Survey. Eur Heart J 32:437–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson CS, Outhred T, Brunoni AR, Malhi GS, Kemp AH. (2013). The impact of escitalopram on vagally mediated cardiovascular function to stress and the moderating effects of vigorous physical activity: a randomized controlled treatment study in healthy participants. Front Physiol 4:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare DL, Toukhsati SR, Johansson P, Jaarsma T. (2014). Depression and cardiovascular disease: a clinical review. Eur Heart J 35:1365–1372. [DOI] [PubMed] [Google Scholar]
- Heponiemi T, Elovainio M, Pulkki L, Puttonen S, Raitakari O, Keltikangas-Jarvinen L. (2007). Cardiac autonomic reactivity and recovery in predicting carotid atherosclerosis: the cardiovascular risk in young Finns study. Health Psychol 26:13–21. [DOI] [PubMed] [Google Scholar]
- Hildreth CM, Padley JR, Pilowsky PM, Goodchild AK. (2008). Impaired serotonergic regulation of heart rate may underlie reduced baroreflex sensitivity in an animal model of depression. Am J Physiol Heart Circ Physiol 294:H474–480. [DOI] [PubMed] [Google Scholar]
- Hinkelmann K, Dragoi L, Gompf J, Muhtz C, Demiralay C, Yassouridis A, Wiedemann K, Kellner M. (2010). Decreased recognition of negative affect after selective serotonin reuptake inhibition is dependent on genotype. Psychiatr Res 177:354–357. [DOI] [PubMed] [Google Scholar]
- Hinkelmann K, Yassouridis A, Mass R, Tenge H, Kellner M, Jahn H, Wiedemann K, Wolf K. (2010). CCK-4: Psychophysiological conditioning elicits features of spontaneous panic attacks. J Psychiatr Res 44:1148–1153. [DOI] [PubMed] [Google Scholar]
- Jarrin DC, McGrath JJ, Giovanniello S, Poirier P, Lambert M. (2012). Measurement fidelity of heart rate variability signal processing: the devil is in the details. Int J Psychophysiol 86:88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerabek I, Boulenger JP, Bradwejn J, Lavallee YJ, Jolicoeur FB. (1999). CCK4-induced panic in healthy subjects I: psychological and cardiovascular effects. Eur Neuropsychopharmacol 9:149–155. [DOI] [PubMed] [Google Scholar]
- Johnson PL, Lightman SL, Lowry CA. (2004). A functional subset of serotonergic neurons in the rat ventrolateral periaqueductal gray implicated in the inhibition of sympathoexcitation and panic. Ann NY Acad Sci 1018:58–64. [DOI] [PubMed] [Google Scholar]
- Jordan D. (2005). Vagal control of the heart: central serotonergic (5-HT) mechanisms. Exp Physiol 90:175–181. [DOI] [PubMed] [Google Scholar]
- Kauppila E, Vanninen E, Kaurijoki S, Karhunen L, Pietilainen KH, Rissanen A, Tiihonen J, Pesonen U, Kaprio J. (2013). Influence of serotonin transporter gene polymorphism (5-HTTLPR polymorphism) on the relation between brain 5-HT transporter binding and heart rate corrected cardiac repolarization interval. PLOS ONE 8:e50303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellner M. (2011). Experimental panic provocation in healthy man-a translational role in anti-panic drug development? Dialogues Clin Neurosci 13:485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellner M, Muhtz C, Demiralay C, Husemann J, Koelsch W, Yassouridis A, Wiedemann K. (2009). The selective serotonin re-uptake inhibitor escitalopram modulates the panic response to cholecystokinin tetrapeptide in healthy men depending on 5-HTTLPR genotype. J Psychiatr Res 43:642–648. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Quintana DS. (2013). The relationship between mental and physical health: insights from the study of heart rate variability. Int J Psychophysiol 89:288–296. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, Gatt JM. (2010). Impact of depression and antidepressant treatment on heart rate variability: a review and meta-analysis. Biol Psychiatry 67:1067–1074. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Quintana DS, Malhi GS. (2011). Effects of serotonin reuptake inhibitors on heart rate variability: methodological issues, medical comorbidity, and clinical relevance. Biological psychiatry 69:e25–26. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Outhred T, Saunders S, Brunoni AR, Nathan PJ, Malhi GS. (2014). Impact of escitalopram on vagally mediated cardiovascular function in healthy participants: implications for understanding differential age-related, treatment emergent effects. Psychopharmacology (Berl) 231:2281–2290. [DOI] [PubMed] [Google Scholar]
- Kikuchi M, Hanaoka A, Kidani T, Remijn GB, Minabe Y, Munesue T, Koshino Y. (2009). Heart rate variability in drug-naive patients with panic disorder and major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 33:1474–1478. [DOI] [PubMed] [Google Scholar]
- Knorr U, Kessing LV. (2010). The effect of selective serotonin reuptake inhibitors in healthy subjects. A systematic review. Nord J Psychiatry 64:153–163. [DOI] [PubMed] [Google Scholar]
- Koschke M, Boettger MK, Schulz S, Berger S, Terhaar J, Voss A, Yeragani VK, Bar KJ. (2009). Autonomy of autonomic dysfunction in major depression. Psychosom Med 71:852–860. [DOI] [PubMed] [Google Scholar]
- Kroeze Y, Zhou H, Homberg JR. (2012). The genetics of selective serotonin reuptake inhibitors. Pharmacol Ther 136:375–400. [DOI] [PubMed] [Google Scholar]
- Le Melledo JM, Merani S, Koszycki D, Bellavance F, Palmour R, Gutkowska J, Steinberg S, Bichet DG, Bradwejn J. (1999). Sensitivity to CCK-4 in women with and without premenstrual dysphoric disorder (PMDD) during their follicular and luteal phases. Neuropsychopharmacology 20:81–91. [DOI] [PubMed] [Google Scholar]
- Leftheriotis D, Flevari P, Ikonomidis I, Douzenis A, Liapis C, Paraskevaidis I, Iliodromitis E, Lykouras L, Kremastinos DT. (2010). The role of the selective serotonin re-uptake inhibitor sertraline in nondepressive patients with chronic ischemic heart failure: a preliminary study. Pacing Clin Electrophysiol 33:1217–1223. [DOI] [PubMed] [Google Scholar]
- Licht CM, de Geus EJ, van Dyck R, Penninx BW. (2010). Longitudinal evidence for unfavorable effects of antidepressants on heart rate variability. Biol Psychiatry 68:861–868. [DOI] [PubMed] [Google Scholar]
- Lingjaerde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K. (1987). The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand Suppl 334:1–100. [DOI] [PubMed] [Google Scholar]
- Lombardi F, Stein PK. (2011). Origin of heart rate variability and turbulence: an appraisal of autonomic modulation of cardiovascular function. Front Physiol 2:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maron E, Tasa G, Toru I, Lang A, Vasar V, Shlik J. (2004). Association between serotonin-related genetic polymorphisms and CCK-4-induced panic attacks with or without 5-hydroxytryptophan pretreatment in healthy volunteers. World J Biol Psychiatry 5:149–154. [DOI] [PubMed] [Google Scholar]
- Mazza M, Lotrionte M, Biondi-Zoccai G, Abbate A, Sheiban I, Romagnoli E. (2010). Selective serotonin reuptake inhibitors provide significant lower re-hospitalization rates in patients recovering from acute coronary syndromes: evidence from a meta-analysis. J Psychopharmacol 24:1785–1792. [DOI] [PubMed] [Google Scholar]
- McTeague LM, Lang PJ, Laplante MC, Cuthbert BN, Shumen JR, Bradley MM. (2010). Aversive imagery in posttraumatic stress disorder: trauma recurrence, comorbidity, and physiological reactivity. Biol Psychiatry 67:346–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M, Stiedl O. (2003). Self-affine fractal variability of human heartbeat interval dynamics in health and disease. Eur J Appl Physiol 90:305–316. [DOI] [PubMed] [Google Scholar]
- Monk C, Kovelenko P, Ellman LM, Sloan RP, Bagiella E, Gorman JM, Pine DS. (2001). Enhanced stress reactivity in paediatric anxiety disorders: implications for future cardiovascular health. Int J Neuropsychop 4:199–206. [DOI] [PubMed] [Google Scholar]
- Mueller EM, Stemmler G, Hennig J, Wacker J. (2013). 5-HTTLPR and anxiety modulate brain-heart covariation. Psychophysiology 50:441–453. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Hu XZ, Luckenbaugh DA, Schwarz M, Nugent AC, Bonne O, Herscovitch P, Goldman D, Drevets WC, Charney DS. (2006). Differential effects of 5-HTTLPR genotypes on the behavioral and neural responses to tryptophan depletion in patients with major depression and controls. Arch Gen Psychiatry 63:978–986. [DOI] [PubMed] [Google Scholar]
- Nielsen T, Paquette T, Solomonova E, Lara-Carrasco J, Colombo R, Lanfranchi P. (2010). Changes in cardiac variability after REM sleep deprivation in recurrent nightmares. Sleep 33:113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier JD, Blom T, Arentsen T, Homberg JR. (2011). The age-dependent effects of selective serotonin reuptake inhibitors in humans and rodents: a review. Prog Neuropsychopharmacol Biol Psychiatry 35:1400–1408. [DOI] [PubMed] [Google Scholar]
- Paraskevaidis I, Parissis JT, Fountoulaki K, Filippatos G, Kremastinos D. (2006). Selective serotonin re-uptake inhibitors for the treatment of depression in coronary artery disease and chronic heart failure: evidence for pleiotropic effects. Cardiovasc Hematol Agents Med Chem 4:361–367. [DOI] [PubMed] [Google Scholar]
- Penttila J, Syvalahti E, Hinkka S, Kuusela T, Scheinin H. (2001). The effects of amitriptyline, citalopram and reboxetine on autonomic nervous system. A randomised placebo-controlled study on healthy volunteers. Psychopharmacology (Berl) 154:343–349. [DOI] [PubMed] [Google Scholar]
- Phillips AC. (2011). Blunted cardiovascular reactivity relates to depression, obesity, and self-reported health. Biol Psychology 86:106–113. [DOI] [PubMed] [Google Scholar]
- Phillips AC, Hughes BM. (2011). Introductory paper: Cardiovascular reactivity at a crossroads: where are we now? Biol Psychology 86:95–97. [DOI] [PubMed] [Google Scholar]
- Pizzi C, Rutjes AW, Costa GM, Fontana F, Mezzetti A, Manzoli L. (2011). Meta-analysis of selective serotonin reuptake inhibitors in patients with depression and coronary heart disease. Am J Cardiol 107:972–979. [DOI] [PubMed] [Google Scholar]
- Pohl R, Balon R, Jayaraman A, Doll RG, Yeragani V. (2003). Effect of fluoxetine, pemoline and placebo on heart period and QT variability in normal humans. J Psychosom Res 55:247–251. [DOI] [PubMed] [Google Scholar]
- Ramage AG. (2001). Central cardiovascular regulation and 5-hydroxytryptamine receptors. Brain Res Bull 56:425–439. [DOI] [PubMed] [Google Scholar]
- Rechlin T. (1994). The effect of amitriptyline, doxepin, fluvoxamine, and paroxetine treatment on heart rate variability. J Clin Psychopharmacol 14:392–395. [PubMed] [Google Scholar]
- Reyes del Paso GA, Langewitz W, Mulder LJ, van Roon A, Duschek S. (2013). The utility of low frequency heart rate variability as an index of sympathetic cardiac tone: a review with emphasis on a reanalysis of previous studies. Psychophysiology 50:477–487. [DOI] [PubMed] [Google Scholar]
- Roose SP, Miyazaki M. (2005). Pharmacologic treatment of depression in patients with heart disease. Psychosom Med 67(Suppl 1):S54–57. [DOI] [PubMed] [Google Scholar]
- Salomon K, Clift A, Karlsdottir M, Rottenberg J. (2009). Major depressive disorder is associated with attenuated cardiovascular reactivity and impaired recovery among those free of cardiovascular disease. Health Psychol 28:157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez C, Bergqvist PB, Brennum LT, Gupta S, Hogg S, Larsen A, Wiborg O. (2003). Escitalopram, the S-(+)-enantiomer of citalopram, is a selective serotonin reuptake inhibitor with potent effects in animal models predictive of antidepressant and anxiolytic activities. Psychopharmacology (Berl) 167:353–362. [DOI] [PubMed] [Google Scholar]
- Sghendo L, Mifsud J. (2012). Understanding the molecular pharmacology of the serotonergic system: using fluoxetine as a model. J Pharm Pharmacol 64:317–325. [DOI] [PubMed] [Google Scholar]
- Shinba T. (2013). Altered autonomic activity and reactivity in depression revealed by heart-rate variability measurement during rest and task conditions. Psychiatry Clin Neurosci 68:225–233. [DOI] [PubMed] [Google Scholar]
- Shlik J, Aluoja A, Vasar V, Vasar E, Podar T, Bradwejn J. (1997). Effects of citalopram treatment on behavioural, cardiovascular and neuroendocrine response to cholecystokinin tetrapeptide challenge in patients with panic disorder. J Psychiatry Neurosci 22:332–340. [PMC free article] [PubMed] [Google Scholar]
- Shores MM, Pascualy M, Lewis NL, Flatness D, Veith RC. (2001). Short-term sertraline treatment suppresses sympathetic nervous system activity in healthy human subjects. Psychoneuroendocrinology 26:433–439. [DOI] [PubMed] [Google Scholar]
- Siepmann M, Grossmann J, Muck-Weymann M, Kirch W. (2003). Effects of sertraline on autonomic and cognitive functions in healthy volunteers. Psychopharmacology (Berl) 168:293–298. [DOI] [PubMed] [Google Scholar]
- Siepmann T, Ziemssen T, Mueck-Weymann M, Kirch W, Siepmann M. (2007). The effects of venlafaxine on autonomic functions in healthy volunteers. J Clin Psychopharmacol 27:687–691. [DOI] [PubMed] [Google Scholar]
- Straneva-Meuse PA, Light KC, Allen MT, Golding M, Girdler SS. (2004). Bupropion and paroxetine differentially influence cardiovascular and neuroendocrine responses to stress in depressed patients. J Affect Disord 79:51–61. [DOI] [PubMed] [Google Scholar]
- Strawn JR, Wehry AM, DelBello MP, Rynn MA, Strakowski S. (2012). Establishing the neurobiologic basis of treatment in children and adolescents with generalized anxiety disorder. Depress Anxiety 29:328–339. [DOI] [PubMed] [Google Scholar]
- Takata TS, Wasmund SL, Smith ML, Li JM, Joglar JA, Banks K, Kowal RC, Page RL, Hamdan MH. (2002). Serotonin reuptake inhibitor (Paxil) does not prevent the vasovagal reaction associated with carotid sinus massage and/or lower body negative pressure in healthy volunteers. Circulation 106:1500–1504. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Ahs F, Fredrikson M, Sollers JJ, 3rd, Wager TD. (2012). A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neurosci Biobehav Rev 36:747–756. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. (2000). A model of neurovisceral integration in emotion regulation and dysregulation. J Affect Disord 61:201–216. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Sternberg E. (2006). Beyond heart rate variability: vagal regulation of allostatic systems. Ann NY Acad Sci 1088:361–372. [DOI] [PubMed] [Google Scholar]
- Toru I, Maron E, Raag M, Vasar V, Nutt DJ, Shlik J. (2013). The effect of 6-week treatment with escitalopram on CCK-4 challenge: a placebo-controlled study in CCK-4-sensitive healthy volunteers. Eur Neuropsychopharmacol 23:645–652. [DOI] [PubMed] [Google Scholar]
- Tucker P, Adamson P, Miranda R, Jr., Scarborough A, Williams D, Groff J, McLean H. (1997). Paroxetine increases heart rate variability in panic disorder. J Clin Psychopharmacol 17:370–376. [DOI] [PubMed] [Google Scholar]
- Tucker P, Smith KL, Marx B, Jones D, Miranda R, Lensgraf J. (2000). Fluvoxamine reduces physiologic reactivity to trauma scripts in posttraumatic stress disorder. J Clin Psychopharmacol 20:367–372. [DOI] [PubMed] [Google Scholar]
- van Megen HJ, Westenberg HG, den Boer JA, Slaap B, Scheepmakers A. (1997). Effect of the selective serotonin reuptake inhibitor fluvoxamine on CCK-4 induced panic attacks. Psychopharmacology (Berl) 129:357–364. [DOI] [PubMed] [Google Scholar]
- Vaswani M, Linda FK, Ramesh S. (2003). Role of selective serotonin reuptake inhibitors in psychiatric disorders: a comprehensive review. Prog Neuropsychopharmacol Biol Psychiatry 27:85–102. [DOI] [PubMed] [Google Scholar]
- Verhoeven KJF, Simonsen KL, McIntyre LM. (2005). Implementing false discovery rate control: increasing your power. Oikos 108:643–647. [Google Scholar]
- Walker FR. (2013). A critical review of the mechanism of action for the selective serotonin reuptake inhibitors: do these drugs possess anti-inflammatory properties and how relevant is this in the treatment of depression? Neuropharmacology 67:304–317. [DOI] [PubMed] [Google Scholar]
- Wiedemann K, Jahn H, Yassouridis A, Kellner M. (2001). Anxiolyticlike effects of atrial natriuretic peptide on cholecystokinin tetrapeptide-induced panic attacks: preliminary findings. Arch Gen Psychiatry 58:371–377. [DOI] [PubMed] [Google Scholar]
- Youn J, Hager T, Misane I, Pieneman AW, Jansen RF, Ogren SO, Meyer M, Stiedl O. (2013). Central 5-HT receptor-mediated modulation of heart rate dynamics and its adjustment by conditioned and unconditioned fear in mice. Br J Pharmacol 170:859–870. [DOI] [PMC free article] [PubMed] [Google Scholar]