Abstract
Background:
Currently approved medications for opioid addiction have shown clinical efficacy, but undesired side effects, dependence induced by the medications themselves, and low treatment compliance necessitate the need for novel therapies.
Methods:
A novel morphine-keyhole limpet hemocyanin conjugate vaccine was synthesized with 6-glutarylmorphine as the hapten and a lengthened linker of 6 carbon atoms. The titer and specificity of the triggered antibody were assessed by enzyme-linked immunosorbent assay. The effects of the vaccine on the morphine-induced elevation of dopamine levels in the nucleus accumbens were determined by high-performance liquid chromatography. The effects of the vaccine on morphine-induced locomotor sensitization and heroin-primed reinstatement of heroin self-administration were also assessed.
Results:
After subcutaneous administration in rats, the vaccine triggered a high antibody titer, with comparable specificity for morphine, 6-acetylmorphine, and heroin, but no interaction with dissimilar therapeutic opioid compounds, including buprenorphine, naloxone, and nalorphine, was observed. The vaccine significantly prevented the elevation of dopamine levels in the nucleus accumbens induced by a single morphine challenge. Moreover, the vaccine prevented the expression of morphine-induced locomotor sensitization and heroin-primed reinstatement of heroin seeking, suggesting its potential for preventing relapse.
Conclusion:
These results demonstrate that active immunization with the present vaccine induces a robust morphine/heroin-specific antibody response in rats and attenuates the behavioral effects of morphine and heroin.
Keywords: drug addiction, immunization, morphine, heroin, vaccine
Introduction
Opioids are some of the most widely abused illicit drugs worldwide, resulting in health problems, criminal activity, and economic burdens (UNODC, 2014). The prevention of relapse is one of the most challenging problems in addiction treatment. Currently, some pharmaceutical agents are available for the maintenance of opiate abstinence. Opioid receptor agonists, such as methadone, and partial agonists, such as buprenorphine, are used as substitution therapies to reduce opioid craving and improve physical health and social functioning, whereas opioid antagonists, such as naloxone and naltrexone, are effective in detoxification and reversal of the acute adverse effects of opioid (Fareed et al., 2011). However, several disadvantages overshadow the benefits of these medications. For example, methadone and buprenorphine have abuse potential (Fareed et al., 2011), overdose risk (Bell et al., 2009; Megarbane et al., 2010), and sexual side effects (Nik Jaafar et al., 2013). Naltrexone causes side effects because of long-term opioid receptor blockade (Kosten et al., 1986; Ritter, 2002; Sauro and Greenberg, 2005). Therefore, ideal therapies with lasting treatment effects and few side effects are needed to enhance treatment adherence and prevent relapse (Fareed et al., 2011).
Immunotherapy has a mechanism of action that is different from the above therapeutic agents and is a promising alternative for relapse prevention (Anton et al., 2009; Kinsey et al., 2009). A drug-carrier protein conjugate vaccine stimulates the immune response to generate antibodies that are specific to the target drug. The antibodies restrict the abused drug to the periphery and thus prevent its entry into and actions in the central nervous system (Kosten and Owens, 2005; Anton et al., 2009). Several clinical trials have shown that higher antibody levels that are triggered by vaccines for cocaine (Martell et al., 2009; Haney et al., 2010) and nicotine (Hatsukami et al., 2005; Cornuz et al., 2008; Hatsukami et al., 2011) are predictive of higher abstinence rates. Studies of methamphetamine vaccines have focused mainly on hapten designs to trigger a sufficiently high antibody level (Duryee et al., 2009; Laurenzana et al., 2009).
To our knowledge, the published opioid vaccine studies are mainly preclinical research. Rabbits that were immunized with morphine-6-hemisuccinate conjugated to bovine serum albumin (BSA) produced antibodies 8 weeks later (Wainer et al., 1972). Monkeys that were immunized with morphine-6-hemisuccinate-BSA exhibited a reduction of heroin intake in a self-administration model (Bonese et al., 1974). A morphine/heroin vaccine with tetanus toxoid as the carrier bound to the hapten via a lengthened linker arm derived from N-(ε-trifluoroacetylcaproyloxy) succinimide ester (TFCS) resulted in robust antibody titers (Anton and Leff, 2006). Another morphine-keyhole limpet hemocyanin (KLH) vaccine with the same hapten triggered a sustained antibody response that lasted at least 8 weeks (Kosten et al., 2013). However, the hapten in these studies was the same as the very first morphine vaccine introduced in 1974. An M-KLH vaccine with a 12-atom linker altered the distribution of heroin and its metabolites and attenuated heroin-induced behavior in rats (Raleigh et al., 2013), but the antibody titers were relatively low. Another study presented morphine-KLH and heroin-KLH vaccines with a bridge-head nitrogen on the morphine/heroin structure as the hapten-carrier attachment point (Stowe et al., 2011). However, the acquisition of heroin self-administration was prevented only in rats that were vaccinated with heroin-KLH but not morphine-KLH. Our previous study presented a vaccine with a novel hapten, 6-glutarylmorphine, conjugated to KLH without a linker arm (Li et al., 2011), which stimulated a high antibody titer response and attenuated the behavioral effects of morphine and heroin in rats, but its inhibitory effects on heroin-primed reinstatement declined 25 days after vaccination. Higher antibody concentrations and affinity would result in better antagonism of abused drugs. Therefore, vaccines with a higher antibody response and specificity need to be developed.
In the present study, we hypothesized that a novel opioid vaccine with 6-glutarylmorphine as the hapten conjugated to KLH via a linker arm of 6 carbon atoms leads to a stronger immune response, higher antibody affinity, and thus better opioid blockade.
Methods
Animals
Male Sprague-Dawley rats, weighing 220 to 240g upon arrival, were housed in groups of 5 per cage in a temperature- (23±2ºC) and humidity- (50±5%) controlled room and maintained on a reverse 12-h/12-h–light/dark cycle (lights on at 8:00 am) with food and water available ad libitum. The animals were obtained from the Laboratory Animal Center, Peking University Health Science Center. All of the procedures were approved by the local Committee of Animal Use and Protection and were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Preparation of Morphine-TFCS-KLH Vaccine
The hapten 6-glutarylmorphine (M-6-G) was synthesized similarly to our previous work (Li et al., 2011). Briefly, 1.3g morphine hydrochloride (Qinghai Pharmaceutical Co., Xining, Qinghai, China) was dissolved in 30mL distilled water, followed by the addition of NH3•H2O dropwise to adjust the pH to 8.5. After reacting at 37ºC for 10 minutes, the mixture was filtrated and vacuum-dried for 24 hours to yield morphine base. The resultant morphine base (1g) and excess glutaric anhydride (1.2g) were dispersed in 30mL benzene, with N-dimethylaminopyridine as the catalyst, followed by refluxing at 70 to 80ºC for 4 hours. The mixture then underwent reduced pressure distillation for solvent removal. The target product and excess morphine were separated by thin-layer chromatography on silica gel with ethanol, dioxane, benzene, and ammonia water (volume ratio 8:1:10:1) as the solvent system. The residues were then dissolved in 50mL distilled water and adjusted to pH 9.0 with NaOH solution (1M). After filtration, the filtrate of the mixture was adjusted to pH 6.5 with HCl solution (1M) and underwent filtration and vacuum drying to yield M-6-G.
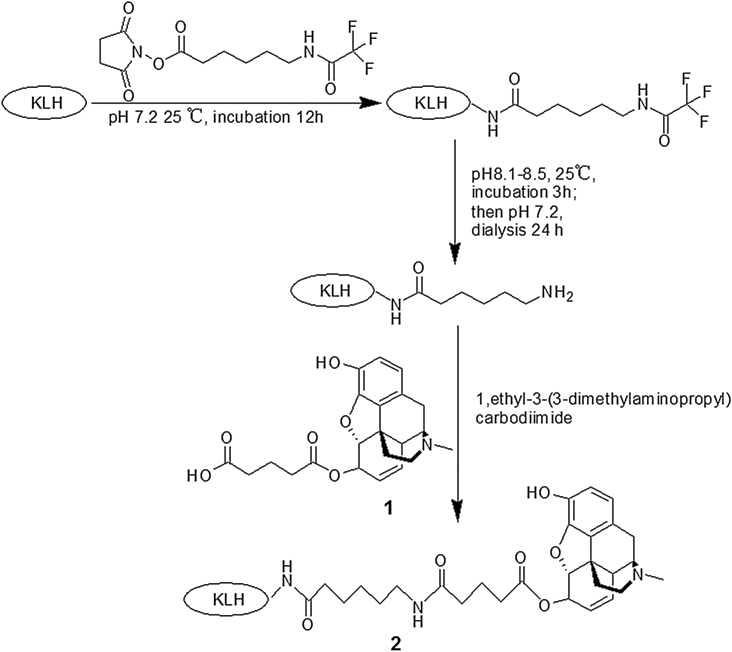
For the preparation of morphine-TFCS-KLH, KLH was dissolved in 0.01M phosphate-buffered saline (PBS), pH 7.2. TFCS (weight ratio 10:1 to KLH) was dissolved in 20% dimethylsulfoxide in distilled water and immediately added to the KLH solution, followed by incubation at 25ºC for 12 hours. NaOH solution (1M) was then added to adjust the pH to 8.1 to 8.5, and the solution was incubated at 25ºC for 3 hours. The solution was then dialyzed at 4ºC for 24 hours to yield KLH-TFCS moiety. Afterward, 100mg M-6-G and 100mg 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC; Sigma-Aldrich, St. Louis, MO) were dispersed in 100mL distilled water, pH 5.5, and stirred at 37ºC for 2 hours to yield EDC-M-6-H derivative (135mg). The EDC-M-6-H derivative (weight ratio 5:1 to KLH-TFCS moiety) was immediately mixed with KLH-TFCS moiety in 100mL PBS (pH 7.5) and stirred at 25ºC for 12 hours. The reaction mixture was purified by dialysis in PBS (pH 7.5) at 4ºC for 48 hours and then concentrated by pressure dialysis and freeze-drying for 24 to 48 hours to yield morphine-TFCS-KLH (Figure 1).
Figure 1.
Preparation of morphine-N-(ε-trifluoroacetylcaproyloxy) succinimide ester (TFCS)-keyhole limpet hemocyanin (KLH), showing conjugation of 6-glutarylmorphine (M-6-G) to KLH via a TFCS-derived linker composition. Structures 1 and 2 represent M-6-G and morphine-TFCS-KLH, respectively.
Active Immunization
During the active immunization procedure, the rats received vaccine administrations 4 times. On day 0, each rat was injected subcutaneously (s.c.) with 100 μg morphine-TFCS-KLH emulsified with 0.4mL Complete Freund’s Adjuvant (Sigma-Aldrich, St. Louis, MO) and 0.4mL water for injection (Shijiazhuang No. 4 Pharmaceutical Co., Shijiazhuang, Hebei, China). Rats that received KLH emulsified with the same solvent were used as controls. Subsequently, 3 booster injections were s.c. administered on days 14, 28, and 42, when each rat received 100 μg morphine-TFCS-KLH or KLH emulsified with 0.3mL Incomplete Freund’s Adjuvant (Sigma-Aldrich) and 0.3mL water for injection.
Antibody Titers and Specificities
For the detection of antibody accumulation, lingual vein blood was collected on day 10 after each immunization. To assess the stability of the antibodies, blood was collected every 10 days until 2 months after the last vaccination. All of the blood samples were centrifuged at 4500 rotations per minute for 15 minutes, and the sera were stored at −80ºC until evaluated by an enzyme-linked immunosorbent assay (ELISA).
Total antibody titers were evaluated by indirect ELISA. The primary antigen, morphine-BSA conjugate, for plate coating was synthesized similarly to a previous report (Li et al., 2011). After being coated with 2mg/mL of the primary antigen overnight at 4ºC, the plates (Corning Costar, Corning, NY) were extensively washed with washing buffer (0.05% Tween 20-PBS, pH 7.4) 5 times for 5 minutes each time. A series of serum dilutions (1:10, 1:100, 1:1000, 1:10000, 1:100000, and 1:200000) were added and incubated at 37ºC for 1 hour. After an extensive wash, the secondary antibody (horseradish peroxidase goat anti-rat immunoglobulin G [IgG]; 1:2000; Santa Cruz Biotechnology, Santa Cruz, CA) was added and incubated at 37ºC for 30 minutes. 3,3’,5,5’-Tetramethylbenzidine (Sigma-Aldrich) was then added as the enzyme substrate, and the reactions were stopped approximately 10 minutes later by the addition of 1M sulphuric acid. Absorbance was spectrophotometrically read at 450nm and is expressed as optical density (OD; Keyler et al., 1994). The ODs on day 0 were used as controls. Antibody titers were calculated by interpolating the log(OD)−log(dilution) with a cutoff value equal to 2-fold the absorbance of the negative controls (Hicks et al., 2011).
Isotype-specific antibodies were determined by indirect ELISA. Briefly, sera were in a dilution of 1:1000. The steps were similar to those for total antibody titer detection, with the exception that anti-IgM antibody (Sigma-Aldrich), anti-IgG1 antibody (Abcam, Cambridge, UK), anti-IgG2a antibody (Sigma-Aldrich), and anti-IgG2b antibody (Sigma-Aldrich) were added to each independent well, incubated at 37ºC for 1 hour, and extensively washed before the addition of horseradish peroxidase goat anti-rat IgG (Hicks et al., 2011).
Antibody specificity was determined by competitive ELISA (Anton and Leff, 2006). Serum samples were collected on day 10 after the fourth vaccination. The competitive ELISA process was similar to indirect ELISA, with the exception that the competitor compounds were concurrently added with serum dilutions prior to plate incubation. The competitor compounds included morphine, heroin, buprenorphine, naloxone, nalorphine (all from Qinghai Pharmaceutical Co., Xining, Qinghai, China), and 6-acetylmorphine. 6-Acetylmorhine was extracted from 6-acetylmorphine acetonitrile solution (Sigma-Aldrich) via freeze-drying for 24 hours and identified by mass spectrography before use (data not shown). The 50% inhibition concentration (IC50) was calculated by probit regression with absorbance normalized to the concentrations of the competitors.
Dopamine Levels in the Nucleus Accumbens
Dopamine levels in the nucleus accumbens (NAc) induced by morphine were measured by high-performance liquid chromatography (HPLC) with electrochemical detection. Rats were divided into 4 groups. Two of the groups received standard morphine-TFCS-KLH immunization, and the other 2 groups received KLH immunization. Ten days after the fourth immunization, the rats received either a single injection of morphine (10mg/kg, s.c.; KLH + morphine-challenge group and morphine-TFCS-KLH + morphine-challenge group) or saline (0.9%, s.c.; KLH + saline-challenge group and morphine-TFCS-KLH + saline-challenge group). Five minutes after the challenge injections, the animals were decapitated. The brains were extracted, frozen in N-hexane at −60ºC, and stored at −80ºC until analysis. Using a freezing cryostat (−20ºC; Reichert-Jung 2800 Frigocut E), bilateral tissue of the NAc was taken from 1-mm–thick coronal sections approximately 2.2mm from bregma with 16-gauge punches (Xu et al., 2009; Wang et al., 2010). Each sample was homogenized in 40 μL of 0.1M perchloric acid (10–15 seconds × 3 at 5-second intervals) at 4ºC on ice with an electrical disperser (Wiggenhauser, SdnBhd). After centrifugation at 13300 × g at 4ºC for 15 minutes, the supernatants were stored at −80ºC until analysis.
The concentration of dopamine was quantified by C18 HPLC (150×4.60mm column; Phenomenex, Torrance, CA) coupled to a Coul Array II5600A electrochemical detector as previously described (Mayer et al., 2006). Briefly, the mobile phase (0.76M NaH2PO4•H2O, 0.5mM EDTA, 1.2mM 1-octane sulfonic acid, and 5% acetonitrile) was perfused at a flow rate of 0.6mL/min. The dopamine concentrations were calculated from the peak heights of the chromatographic data according to the standard curve (BAS, West Lafayette, IN).
Locomotor Sensitization
The Animal Locomotor Video Analysis System (JLBehv-LAR-8, Shanghai Jiliang Software Technology Co. Ltd, Shanghai, China) consisted of 8 identical light- and sound-controlled black Plexiglas chambers (40×40×65cm). Each chamber was equipped with a video camera (winfast vc100) connected to a computer to record the rats’ movements (Xu et al., 2009). Locomotor activity was analyzed using DigBehv analysis software (Shanghai Jiliang Software Technology Co. Ltd) and expressed as the total distance traveled (in millimeters).
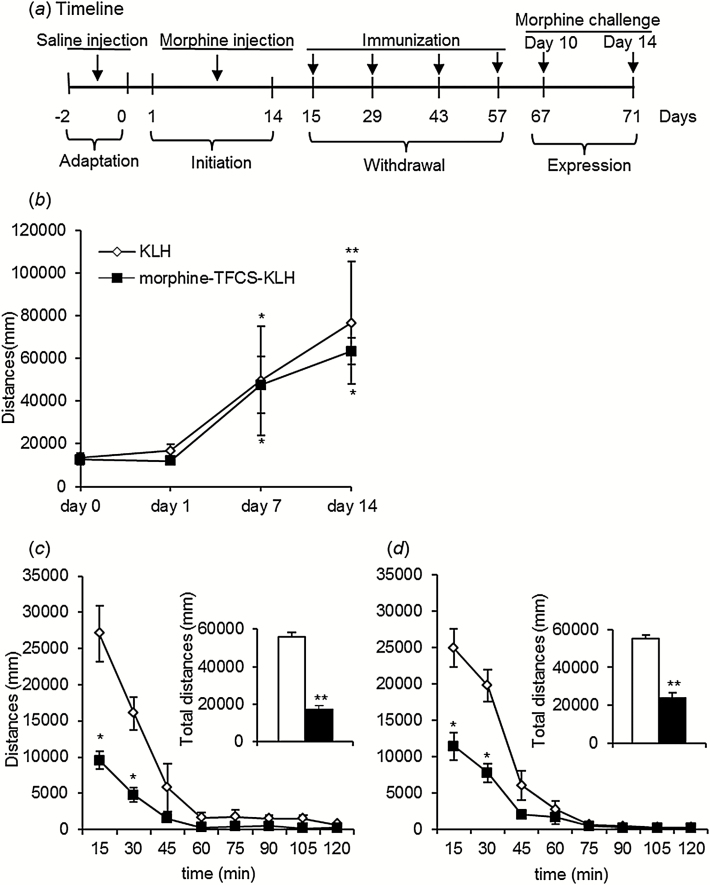
The procedure for locomotor sensitization, which was the same as previously described (Lu et al., 2000, 2005b; Xu et al., 2009), consisted of 4 phases: adaptation, initiation, withdrawal, and expression. In the adaptation phase, locomotor activity was measured after a daily injection of normal saline (0.9%, s.c.) for 3 days, and locomotor activity on the third day was defined as the baseline. For the next 14 consecutive days (ie, initiation phase), the rats were injected daily with morphine (10mg/kg, s.c.) followed by locomotor activity assessment. In the withdrawal phase for the next 43 days, locomotor activity was measured without morphine injections. The rats were actively immunized with the vaccine 4 times on days 15, 29, 43, and 57. During the expression phase, the rats received a morphine challenge on days 10 and 14 after the last immunization (10mg/kg, s.c.), immediately after which locomotor activity was monitored. The duration of each measurement was 2 hours.
Heroin Self-Administration and Reinstatement
Intravenous Cannulation Surgery
Rats (weighing 300–320g at the time of surgery) were anesthetized with sodium pentobarbital (60mg/kg, i.p.). Intravenous cannulation surgery was performed as previously described (Lu et al., 2005a; Xue et al., 2012). Briefly, catheters were connected to modified cannulae and inserted into the right jugular vein, with the tip terminating at the opening of the right atrium. The cannulae were anchored to the skull with stainless-steel screws and dental cement. A stainless-steel stylet blocker was inserted into each cannula to maintain patency and prevent infection. All of the rats were allowed to recover for 5 to 7 days after surgery.
Apparatus
The drug self-administration setup (AniLab Software and Instruments, Ningbo, China) consisted of 16 chambers (34cm long × 28cm wide × 34cm high) made of Plexiglas. Each chamber was placed in a light- and sound-controlled box equipped with an exhaust fan to ensure air renewal, a white illumination house light, and a tone stimulator. Each chamber was equipped with a green cue light located 2cm below the top, 2 nosepoke holes located 9cm above the floor, and a pump-driven syringe located on the top. The pump-driven syringe contained heroin·HCl solution and was connected to the modified cannula on the rat’s skull (Wang et al., 2010; Xue et al., 2012).
Acquisition
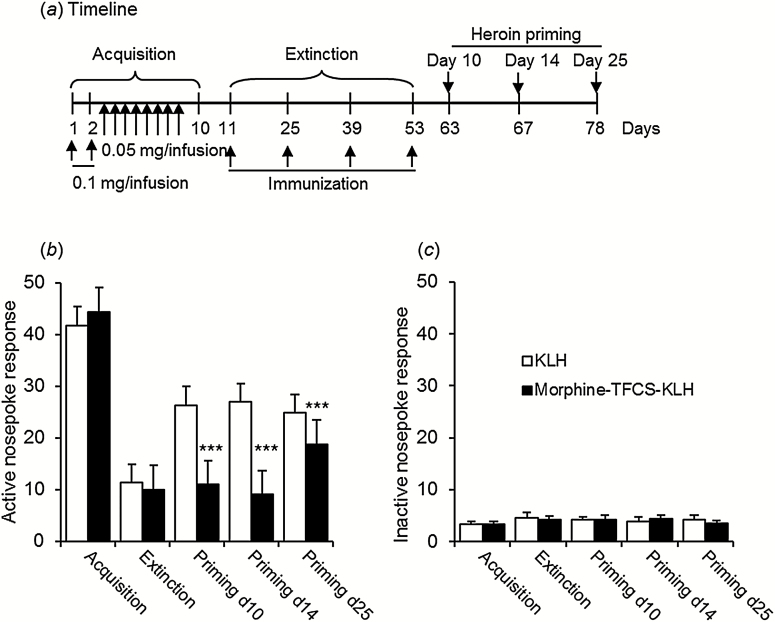
The heroin self-administration procedure was performed according to our previous studies (Wang et al., 2010; Xue et al., 2012). The acquisition of heroin self-administration began during the dark cycle and lasted 10 days (0.1mg/kg/infusion on days 1 and 2 and 0.05mg/kg/infusion on days 3–10). Daily training consisted of 3 sessions separated by 5-minute intervals. Each session lasted 1 hour and began with illumination of the house light, which remained on for the entire session. During the 5-minute intervals, the house light was turned off. A fixed ratio 1 reinforcement schedule was used, in which a nosepoke in the active hole led to an infusion of heroin accompanied by a 5-second tone-light cue. Nosepokes in the inactive hole were also recorded but had no scheduled consequences. A 40-second timeout period occurred after each infusion, during which nosepoke responses were recorded but did not result in infusions. To avoid drug overdose, after a rat earned 20 infusions in 1 hour, active nosepokes led to neither drug infusions nor the accompanying tone-light cue. After acquisition, the rats were divided into 2 groups that were matched for the number of heroin infusions.
Extinction
The extinction conditions were the same as those during acquisition, with the exception that heroin was unavailable. The rats were subjected to extinction training every 2 days during the following 52 days of immunization. The extinction procedure was stopped once active nosepoke responding decreased to <20% of the mean nosepoke responding on the last 3 days of acquisition for at least 2 consecutive days (Goeders and Clampitt, 2002; Goeders et al., 2009; Xue et al., 2012). During the extinction procedure, one group of rats received standard active immunization with morphine-TFCS-KLH, and the other group received immunization with KLH on days 11, 25, 39, and 53.
Reinstatement
The reinstatement test was conducted after extinction training. The test conditions were the same as those during extinction. Briefly, the rats received a single injection of heroin (0.5mg/kg, s.c.) and were then placed in the self-administration chambers 5 minutes later for a 30-minute test. Active nosepokes during the test resulted in contingent presentation of the tone-light cues, which were paired with heroin infusions during acquisition. The reinstatement tests were conducted on days 10, 14, and 25 after the fourth immunization, and the rats were housed in their homecages between tests.
Statistical Analysis
The data were expressed as mean ± standard error of the mean (SEM) and analyzed using analysis of variance (ANOVA) with appropriate between- and within-group factors for different experiments (see Results). Significant main effects and interactions (P < .05, 2-tailed) in the factorial ANOVAs were followed by Tukey’s posthoc test. Values of P < .05 were considered statistically significant.
RESULTS
Antibody Titers Triggered by the Vaccine
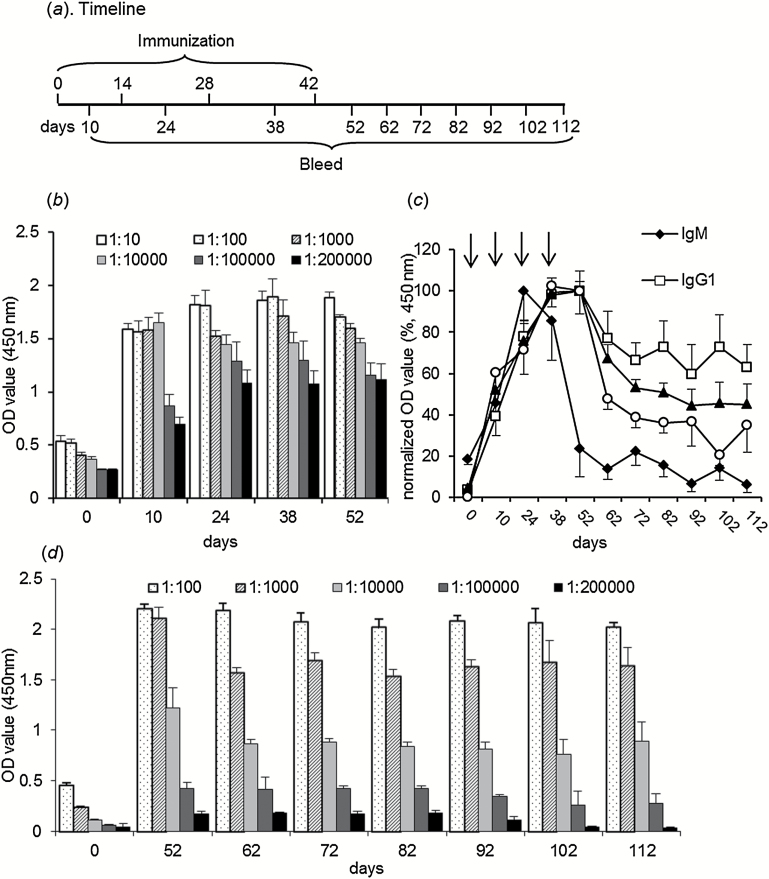
The immunoreactivity of morphine-TFCS-KLH was investigated by ELISA (Figure 2a). After active immunization, anti-morphine antibody titers reached a peak after the third and fourth vaccinations. The maximal antibody titers were 1:200000 after the third injection (Figure 2b). Isotype-specific antibody detection revealed that anti-morphine IgM rapidly reached a peak on day 24 (10 days after the second immunization) and dropped below the level of detection on day 52 (10 days after the fourth immunization). IgG1, IgG2a, and IgG2b increased more slowly, reaching a peak after the third immunization and decreasing after the last booster injection, but the levels remained detectable for at least 70 days (on day 112) (Figure 2c).
Figure 2.
Anti-morphine antibody response induced by morphine-N-(ε-trifluoroacetylcaproyloxy) succinimide ester (TFCS)-keyhole limpet hemocyanin (KLH) immunization. (a) Timeline of active immunization. (b) Antibody titers progressively increased along with morphine-TFCS-KLH immunization (n=8). Serum samples were collected 10 days after each immunization (days 10, 24, 38, and 53), and the antibody titers reached detectable levels of 1:200000 after the third and fourth immunizations. (c) Quantification of isotype-specific antibodies. Sera were at a dilution of 1:1000, and optical density (OD) values were normalized to the peak OD values in each isotype, which refers to the value after the second injection in the IgM group and the value after the fourth immunization in the IgG1, IgG2a, and IgG2b groups (n=8). (d) Antibody levels when booster injection was not provided. The antibody titers were examined on days 10, 20, 30, 45, 60, and 70 after the last immunization, with a detectable level of 1:10000 70 days after the last immunization (n=8).
Antibody titers were also examined every 10 days after the fourth immunization for 2 months. Because booster injections were no longer administered, antibody titers declined but remained detectable for at least 2 months. Seventy days after the last vaccination, antibody titers were still detectable (1:10000) (Figure 2d).
Specificity of the Triggered Antibodies
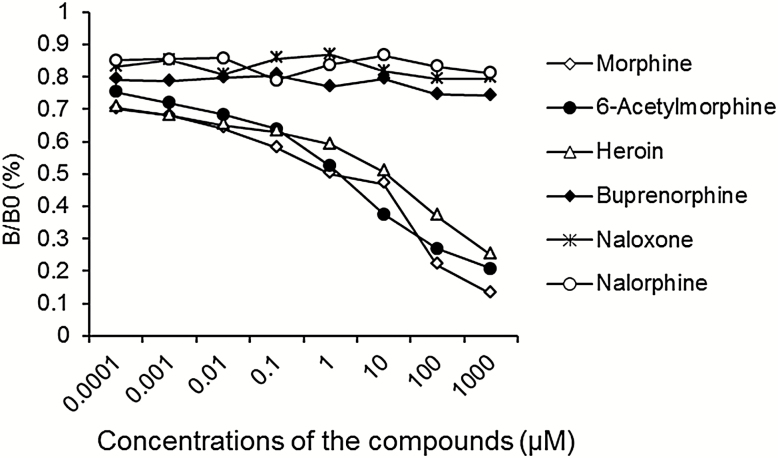
The specificity of morphine-TFCS-KLH–triggered antibodies to opioid-related compounds was determined by competitive ELISA. The IC50 values (ie, the concentration at which the analyte decreases absorbance to 50% of the maximum) for morphine, 6-acetylmorphine, and heroin were 0.227, 0.575, and 1.916 μM, respectively, whereas the IC50 values for buprenorphine, naloxone, and nalorphine were too high to be detected (Table 1; Figure 3). These results demonstrate that the triggered antibodies possessed high and comparable affinity with both morphine and heroin, but not buprenorphine, naloxone, or nalorphine.
Table 1.
IC50 of Related Compounds against Antibodies Triggered by Morphine-TFCS-KLH
| Compound | Structure | IC50 (μM) |
|---|---|---|
| Morphine |
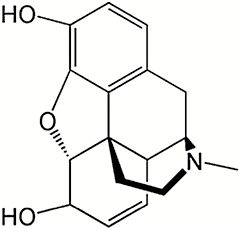
|
0.227 |
| 6-Acetylmorphine |
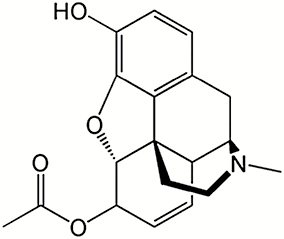
|
0.575 |
| Heroin |
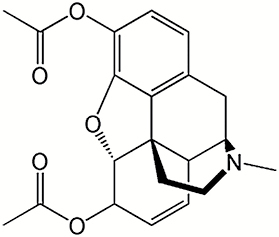
|
1.916 |
| Buprenorphine |
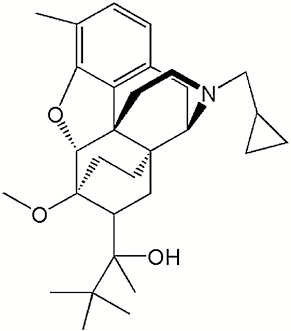
|
ND |
| Naloxone |
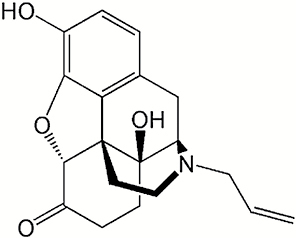
|
ND |
| Nalorphine |
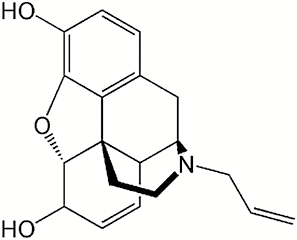
|
ND |
The IC50 is defined as the concentration at which analyte decreases absorbance to 50% of the maximum. ND, not detected (referring to infinite IC50 values that could not be determined by the probit regression analysis); TFCS-KLH, N-(ε-trifluoroacetylcaproyloxy) succinimide ester (TFCS)-keyhole limpet hemocyanin (KLH).
Figure 3.
Specificity of antimorphine antibodies induced by morphine- N-(ε-trifluoroacetylcaproyloxy) succinimide ester (TFCS)-keyhole limpet hemocyanin (KLH). The antibodies showed equivalent specificities for morphine, 6-acetylmorphine, and heroin but no cross-reactivity with buprenorphine, naloxone, or nalorphine. The binding of antibodies to morphine-bovine serum albumin (BSA) is expressed as absorbance units at each concentration of the competitive compounds (n=10). B0 refers to absorbance without competitors, and B refers to absorbance in the presence of competitors at each concentration.
Vaccine Effects on Dopamine Levels
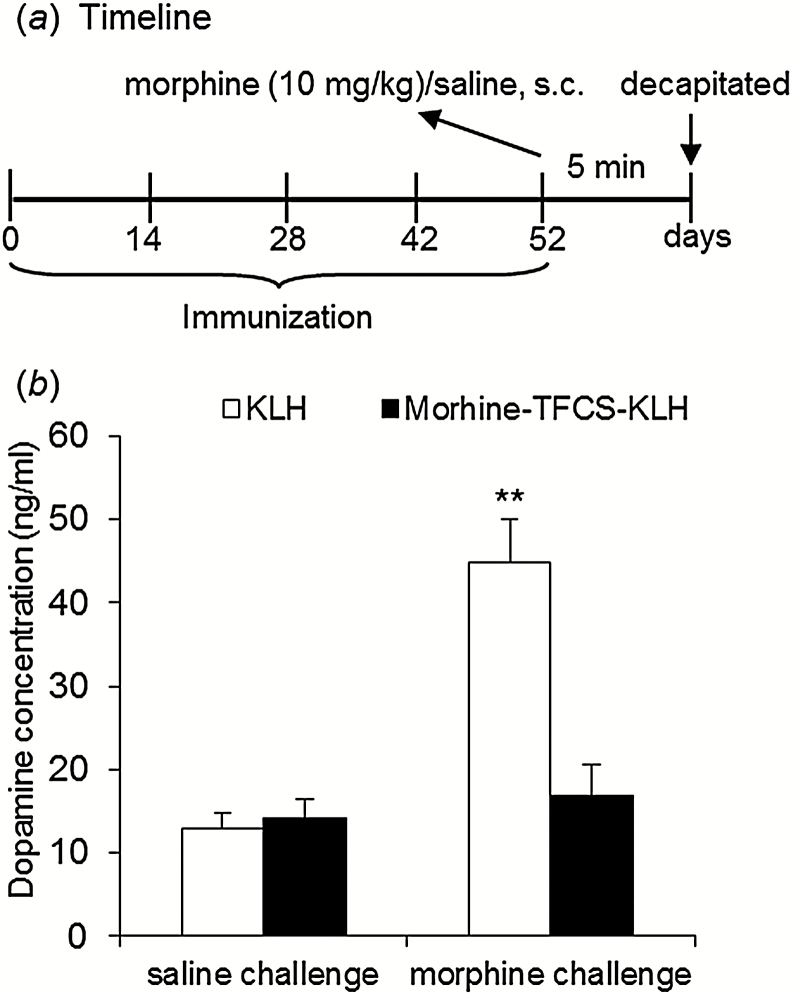
Total dopamine levels in the NAc after an acute injection of morphine (10mg/kg) were measured by HPLC (Figure 4a). Five minutes after morphine challenge, dopamine levels in the NAc were significantly lower in rats that received 4 vaccine immunizations compared with KLH-immunized animals, reflected by a main effect of challenge (F 1,28=21.64, P < .001) and a significant treatment × challenge interaction (F 1,28=15.40, P < .001) in the 2-factor ANOVA (Figure 4b).
Figure 4.
Effects of morphine-N-(ε-trifluoroacetylcaproyloxy) succinimide ester (TFCS)-keyhole limpet hemocyanin (KLH) immunization on morphine-induced dopamine levels. Ten days after active immunization with morphine-TFCS-KLH, the rats received an acute morphine injection (10mg/kg, s.c.) 5 minutes before dopamine detection. The elevation of dopamine levels induced by morphine challenge was significantly suppressed in the morphine-TFCS-KLH group (n=8) compared with the KLH group (n=9). The data are expressed as mean ± SEM. **P < .01, compared with KLH group postmorphine challenge.
Vaccine Effects on the Expression of Morphine-Induced Locomotor Sensitization
The effects of morphine-TFCS-KLH on the psychomotor effect of morphine were determined using a locomotor sensitization model (Figure 5a). During the initiation phase, the analysis revealed a main effect of time (F 3,42 = 8.63, P < .001). Locomotor activity significantly increased on day 7 (P < .001, compared with day 0) and day 14 (P < .001, compared with day 0), with no difference between groups (F 1,14=0.324, P=.578) (Figure 5b). After the morphine challenge (10mg/kg, s.c.) on day 10 after the fourth immunization, locomotor activity in morphine-TFCS-KLH–treated rats was significantly lower than in the KLH group (F 1,14=99.84, P < .05) (Figure 5c, upper), with a significant treatment×time interaction (F 7,98=7.88, P < .001) (Figure 5c, lower). Similarly, after the morphine challenge 14 days after the last immunization, locomotor activity was significantly lower in the morphine-TFCS-KLH group (F 1,14=149.74, P < .05) (Figure 5d, upper), with a significant treatment×time interaction (F 7,98=9.98, P < .001) (Figure 5d, lower). The posthoc analysis revealed a significant difference in locomotor activity between the vaccine and control groups 15 and 30 minutes after both the first and second morphine challenges (all P < .001).
Figure 5.
Effects of morphine-N-(ε-trifluoroacetylcaproyloxy) succinimide ester (TFCS)-keyhole limpet hemocyanin (KLH) immunization on morphine-induced locomotor sensitization. (a) Experimental timeline. (b) Initiation of morphine-induced locomotor sensitization. During 14 daily injections of morphine (10mg/kg, s.c.), locomotor activity significantly increased on days 7 and 14 of initiation, with no difference between groups. Locomotor activity on day 0 served as baseline. (c) Locomotor activity in morphine-TFCS-KLH-treated rats was significantly lower compared with KLH-treated rats after the morphine challenge on day 10. (d) Locomotor activity in morphine-TFCS-KLH–treated rats was significantly lower compared with KLH-treated rats after the morphine challenge on day 14. The data are expressed as mean ± SEM. *P < .05, compared with KLH group; **P < .01, compared with KLH group (n=10 per group).
Vaccine Effects on Heroin-Induced Reinstatement of Heroin Seeking
A heroin self-administration model was used to determine whether the morphine-TFCS-KLH vaccine prevents the reinstatement of drug-seeking behavior induced by heroin priming (Figure 6a). The baselines (defined as the average number of heroin infusions over the last 3 days of acquisition) were equivalent between groups (KLH group: 28.72±0.98; morphine-TFCS-KLH group: 27.58±1.44). After acquisition, the animals were subjected to 52 days of discontinuous extinction until all of the rats presented extinction-like levels of responding, during which they received 4 active immunizations. On days 10, 14, and 25 after the last immunization, the rats were subjected to reinstatement tests 5 minutes after heroin priming (0.5mg/kg). The 2-factor repeated-measures ANOVA (treatment×time) revealed a main effect of treatment (F 1,21=21.60, P < .001) and a significant treatment×priming test interaction (F 4,84=10.06, P < .001). The posthoc analysis revealed that nosepoke responding in the morphine-TFCS-KLH group was not reinstated by a single priming injection 10 days (P < .001) or 14 days (P < .001) after the last immunization. The significant inhibition of heroin-primed nosepoke responding lasted at least 25 days after the final immunization (P < .001) (Figure 6b). Inactive nosepokes were not significantly different between groups during any of the reinstatement tests (Figure 6c).
Figure 6.
Effects of morphine-N-(ε-trifluoroacetylcaproyloxy) succinimide ester (TFCS)-keyhole limpet hemocyanin (KLH) immunization on heroin-induced reinstatement. (a) Experimental timeline. (b) Active nosepokes during acquisition, extinction, and reinstatement of heroin self-administration. The numbers of active nosepokes during acquisition and extinction were not significantly different between groups. Heroin-primed (0.5mg/kg) reinstatement was significantly inhibited in the morphine-TFCS-KLH group (n=11) compared with the KLH group (n=12) on days 10, 14, and 25 after the last immunization. (c) Inactive nosepokes during acquisition, extinction, and reinstatement were not significantly different between groups. The data are expressed as mean ± SEM. ***P < .001, compared with KLH group.
Discussion
In the present study, we synthesized a novel vaccine, morphine-TFCS-KLH, with M-6-G conjugated to KLH via a lengthened linker of 6 carbon atoms. As expected, the vaccine triggered a robust and sustained immunological response, reflected by high and long-lasting serum antibody titers. The antibodies showed equivalent specificities to morphine, 6-acetylmorphine, and heroin, reflecting its efficacy for heroin and its active metabolites. The vaccine possibly reduced the central entry of morphine, reflected by a significant decline in dopamine levels induced by morphine challenge. Vaccination also decreased the expression of behavioral sensitization and heroin-primed drug-seeking behavior. These results indicate that the present vaccine might be a potential approach to prevent the relapse of opioid use, including both heroin and morphine.
The hapten M-6-G, which was first designed and reported in our previous work (Li et al., 2011), has 2 major properties. First, M-6-G is possibly more stable than the commonly used 6-hemiglutarate hapten, because aflatoxin B2a-hemisuccinate hydrolyzes more rapidly than aflatoxin B2a-hemiglutarate in aqueous solution (Lau et al., 1980). Slower hydrolysis is speculated to result in a higher sustained immunoconjugate concentration and thus a higher antibody titer (Tew et al., 1980; Gray and Skarvall, 1988). Second, the 6-glutarate hapten contained one more carbon atom than 6-hemiglutarate, which makes the morphine structure less likely to be fettered by the carrier protein, possibly leading to better antibody affinity to morphine. In addition to hapten design, we also introduced a longer linker arm between the hapten and carrier protein as reported for a morphine-tetanus toxoid vaccine (Anton and Leff, 2006), which probably allows further exposure of the morphine structure to the vaccine and contributes to higher recognition affinity to exogenous morphine by the antibodies.
A small-molecule drug per se does not possess immunoreactivity, but when conjugated to a large carrier, the exposed drug structure becomes an epitope that is able to trigger an immune reaction (Kosten and Owens, 2005). According to studies of cocaine and methamphetamine vaccines, the space between the hapten, carrier, and heteroatoms determines the titers of triggered antibodies (Carrera et al., 2001; Carroll et al., 2011; Moreno et al., 2011). Enhanced stability and sufficient epitope exposure are highly predictive of a higher antibody titer and affinity (Carrera et al., 2001; Anton et al., 2009). Compared with our previous vaccine (Li et al., 2011), the present vaccine triggered an antibody response with a 2-fold higher peak titer and longer persistence. Moreover, the antibody titers reached a peak more rapidly (after the third immunization) compared with our previous vaccine (after the fourth immunization) and decayed more slowly. Although both vaccines induced antibodies with comparable affinity to morphine, the present vaccine robustly increased the antibody affinity to heroin (1.916 μM vs 162.054 μM), suggesting better application in heroin addiction. Although the active immunization procedures and adjuvants were not the same among studies, our present vaccine showed an advantage of maximal antibody titers that were comparable with other morphine or heroin vaccines (Anton et al., 2009; Kinsey et al., 2009; Kosten et al., 2013; Schlosburg et al., 2013).
The dopamine system, particularly the mesolimbic system, underlies drug reward processing (Di Chiara and Imperato, 1988; Le Moal and Simon, 1991). Extracellular dopamine levels in the NAc core are increased by acute heroin administration (Gerasimov et al., 1999; Sorge and Stewart, 2006). Typically, as large proteins, antibodies bind to drugs and retain them in peripheral regions, thus blocking their permeation of the blood-brain barrier and rewarding effects (Orson et al., 2008). One limitation of the present study is that we measured total dopamine concentrations rather than dopamine release by microdialysis. We found that the vaccine blocked the increase in total dopamine levels in the NAc after a single morphine injection. Previous studies found that NAc lesion with 6-hydroxydopamine attenuated the increase in total dopamine induced by heroin (Spyraki et al., 1983), and the level of 3,4-dihydroxy-phenylacetic acid, a major metabolite of dopamine, was increased in the striatum 1 hour after an acute morphine injection (Airavaara et al., 2006). Total dopamine levels in the NAc homogenate reflect dopamine in synaptic vesicles, synaptic cleft, and cell bodies, and we speculate that NAc undergoes a general increase in dopamine transmission after an acute morphine injection (Lammel et al., 2014). The locomotor-stimulating effect of opiates is presumed to be modulated by dopamine transmission (Le Moal and Simon, 1991). Our novel vaccine inhibited the expression of morphine-induced locomotor sensitization, suggesting that it is able to attenuate the psychoactive effects of morphine and heroin.
Reexposure to an abused drug provokes relapse in addicts, which is the most difficult problem encountered in drug addiction treatment (Shaham et al., 2003). Notably, the results of the heroin reinstatement tests indicated that heroin-primed heroin-seeking behavior was inhibited at least 25 days after the last immunization, reflecting a longer-lasting effect than the vaccine in our previous work (Li et al., 2011). This is consistent with our antibody detection results. The higher affinity of the present vaccine to heroin may also indicate better relapse prevention in a heroin self-administration task. Because the vaccine does not target opioid receptors and the antibodies persisted sufficiently long in the body, such an approach could minimize the side effects related to opioid receptor activation and provide long-lasting protection against the psychoactive effects of specific opioid drugs. Consequently, less effort would be needed to maintain patient compliance.
A recently published study showed that a heroin vaccine with the metabolically reactive heroin region exposed for immunization recognition induced antibodies that specifically recognized heroin and its metabolite 6-acetylmorphine but not morphine (Schlosburg et al., 2013). Our vaccine triggered high and sustained antibody levels, and the design of the lengthened linker, together with the lengthened hapten, might be applied in the synthesis of other more specific vaccines with different epitope exposure.
A potential limitation of anti-addiction vaccines, including morphine and heroin vaccines, is that patients may take drug doses that are several-fold higher than their usual doses to overcome the effect of the vaccine, similar to the results in rats subjected to pharmacological opioid receptor blockade (Koob et al., 1984). However, several clinical trials of cocaine and nicotine vaccines did not show any evidence of compensatory smoking or cocaine use and found that a higher antibody titer indeed resulted in significantly higher abstinence rates (Hatsukami et al., 2005, 2011; Cornuz et al., 2008; Martell et al., 2009). Another limitation of drug vaccines is that antibodies gradually decrease after the last vaccine injection, and reexposure to nonconjugated drug is not sufficient to trigger immunological memory. However, long-term immunological memory that is generated during the humoral antibody response facilitates a more rapid response to the future booster injections with the vaccine (Kosten and Owens, 2005). Therefore, repeated booster injections of the vaccine are required to maintain a sustained antibody level.
In summary, we provided a novel KLH-conjugated vaccine that attenuated the behavioral and psychoactive effects of heroin and morphine. The vaccine produced high and sustained antibody titers against both morphine and heroin. After being conjugated with clinically approved carriers, our hapten may be therapeutically applied to morphine and heroin addiction.
Interest Statement
None.
Acknowledgments
This work was supported by the National Science Technology Major Project (no. 2013ZX09103003) and Beijing Technological Innovation Base Project (no. Z131102002813096). We thank Dr. Yan-Ping Bao for helpful comments on the manuscript.
References
- Airavaara M, Mijatovic J, Vihavainen T, Piepponen TP, Saarma M, Ahtee L. (2006). In heterozygous GDNF knockout mice the response of striatal dopaminergic system to acute morphine is altered. Synapse 59:321–329. [DOI] [PubMed] [Google Scholar]
- Anton B, Leff P. (2006). A novel bivalent morphine/heroin vaccine that prevents relapse to heroin addiction in rodents. Vaccine 24:3232–3240. [DOI] [PubMed] [Google Scholar]
- Anton B, Salazar A, Flores A, Matus M, Marin R, Hernandez JA, Leff P. (2009). Vaccines against morphine/heroin and its use as effective medication for preventing relapse to opiate addictive behaviors. Hum Vaccin 5:214–229. [DOI] [PubMed] [Google Scholar]
- Bell JR, Butler B, Lawrance A, Batey R, Salmelainen P. (2009). Comparing overdose mortality associated with methadone and buprenorphine treatment. Drug Alcohol Depend 104:73–77. [DOI] [PubMed] [Google Scholar]
- Bonese KF, Wainer BH, Fitch FW, Rothberg RM, Schuster CR. (1974). Changes in heroin self-administration by a rhesus monkey after morphine immunisation. Nature 252:708–710. [DOI] [PubMed] [Google Scholar]
- Carrera MR, Ashley JA, Wirsching P, Koob GF, Janda KD. (2001). A second-generation vaccine protects against the psychoactive effects of cocaine. Proc Natl Acad Sci U S A 98:1988–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll FI, Blough BE, Pidaparthi RR, Abraham P, Gong PK, Deng L, Huang X, Gunnell M, Lay JO, Jr, Peterson EC, Owens SM. (2011). Synthesis of mercapto-(+)-methamphetamine haptens and their use for obtaining improved epitope density on (+)-methamphetamine conjugate vaccines. J Med Chem 54:5221–5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornuz J, Zwahlen S, Jungi WF, Osterwalder J, Klingler K, van Melle G, Bangala Y, Guessous I, Muller P, Willers J, Maurer P, Bachmann MF, Cerny T. (2008). A vaccine against nicotine for smoking cessation: a randomized controlled trial. PLoS One 3:e2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. (1988). Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A 85:5274–5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duryee MJ, Bevins RA, Reichel CM, Murray JE, Dong Y, Thiele GM, Sanderson SD. (2009). Immune responses to methamphetamine by active immunization with peptide-based, molecular adjuvant-containing vaccines. Vaccine 27:2981–2988. [DOI] [PubMed] [Google Scholar]
- Fareed A, Vayalapalli S, Stout S, Casarella J, Drexler K, Bailey SP. (2011). Effect of methadone maintenance treatment on heroin craving, a literature review. J Addict Dis 30:27–38. [DOI] [PubMed] [Google Scholar]
- Gerasimov MR, Ashby CR, Jr, Gardner EL, Mills MJ, Brodie JD, Dewey SL. (1999). Gamma-vinyl GABA inhibits methamphetamine, heroin, or ethanol-induced increases in nucleus accumbens dopamine. Synapse 34:11–19. [DOI] [PubMed] [Google Scholar]
- Goeders JE, Murnane KS, Banks ML, Fantegrossi WE. (2009). Escalation of food-maintained responding and sensitivity to the locomotor stimulant effects of cocaine in mice. Pharmacol Biochem Behav 93:67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeders NE, Clampitt DM. (2002). Potential role for the hypothalamo-pituitary-adrenal axis in the conditioned reinforcer-induced reinstatement of extinguished cocaine seeking in rats. Psychopharmacology (Berl) 161:222–232. [DOI] [PubMed] [Google Scholar]
- Gray D, Skarvall H. (1988). B-cell memory is short-lived in the absence of antigen. Nature 336:70–73. [DOI] [PubMed] [Google Scholar]
- Haney M, Gunderson EW, Jiang H, Collins ED, Foltin RW. (2010). Cocaine-specific antibodies blunt the subjective effects of smoked cocaine in humans. Biol Psychiatry 67:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Rennard S, Jorenby D, Fiore M, Koopmeiners J, de Vos A, Horwith G, Pentel PR. (2005). Safety and immunogenicity of a nicotine conjugate vaccine in current smokers. Clin Pharmacol Ther 78:456–467. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Jorenby DE, Gonzales D, Rigotti NA, Glover ED, Oncken CA, Tashkin DP, Reus VI, Akhavain RC, Fahim RE, Kessler PD, Niknian M, Kalnik MW, Rennard SI. (2011). Immunogenicity and smoking-cessation outcomes for a novel nicotine immunotherapeutic. Clin Pharmacol Ther 89:392–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks MJ, De BP, Rosenberg JB, Davidson JT, Moreno AY, Janda KD, Wee S, Koob GF, Hackett NR, Kaminsky SM, Worgall S, Toth M, Mezey JG, Crystal RG. (2011). Cocaine analog coupled to disrupted adenovirus: a vaccine strategy to evoke high-titer immunity against addictive drugs. Mol Ther 19:612–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyler DE, Goon DJ, Shelver WL, Ross CA, Nagasawa HT, St Peter JV, Pentel PR. (1994). Redistribution and enhanced urinary excretion of 2,2’,4,4’,5,5’-hexachlorobiphenyl (HCB) in rats using HCB-specific IgG and Fab fragments. Biochem Pharmacol 48:767–773. [DOI] [PubMed] [Google Scholar]
- Kinsey BM, Jackson DC, Orson FM. (2009). Anti-drug vaccines to treat substance abuse. Immunol Cell Biol 87:309–314. [DOI] [PubMed] [Google Scholar]
- Koob GF, Pettit HO, Ettenberg A, Bloom FE. (1984). Effects of opiate antagonists and their quaternary derivatives on heroin self-administration in the rat. J Pharmacol Exp Ther 229:481–486. [PubMed] [Google Scholar]
- Kosten T, Owens SM. (2005). Immunotherapy for the treatment of drug abuse. Pharmacol Ther 108:76–85. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Shen XY, O’Malley PW, Kinsey BM, Lykissa ED, Orson FM, Kosten TR. (2013). A morphine conjugate vaccine attenuates the behavioral effects of morphine in rats. Prog Neuropsychopharmacol Biol Psychiatry 45C:223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR, Kreek MJ, Ragunath J, Kleber HD. (1986). A preliminary study of beta endorphin during chronic naltrexone maintenance treatment in ex-opiate addicts. Life Sci 39:55–59. [DOI] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Malenka RC. (2014). Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology 76 Pt B:351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau HP, Gaur PK, Chu FS. (1980). Preparation and characterization of aflatoxin B2a-hemiglutarate and its use for the production of antibody against aflatoxin B1. J Food Saf 3:1–13. [Google Scholar]
- Laurenzana EM, Hendrickson HP, Carpenter D, Peterson EC, Gentry WB, West M, Che Y, Carroll FI, Owens SM. (2009). Functional and biological determinants affecting the duration of action and efficacy of anti-(+)-methamphetamine monoclonal antibodies in rats. Vaccine 27:7011–7020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Moal M, Simon H. (1991). Mesocorticolimbic dopaminergic network: functional and regulatory roles. Physiol Rev 71:155–234. [DOI] [PubMed] [Google Scholar]
- Li QQ, Luo YX, Sun CY, Xue YX, Zhu WL, Shi HS, Zhai HF, Shi J, Lu L. (2011). A morphine/heroin vaccine with new hapten design attenuates behavioral effects in rats. J Neurochem 119:1271–1281. [DOI] [PubMed] [Google Scholar]
- Lu L, Huang M, Liu Z, Ma L. (2000). Cholecystokinin-B receptor antagonists attenuate morphine dependence and withdrawal in rats. Neuroreport 11:829–832. [DOI] [PubMed] [Google Scholar]
- Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, Shaham Y. (2005a) Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat Neurosci 8:212–219. [DOI] [PubMed] [Google Scholar]
- Lu L, Chen H, Su W, Ge X, Yue W, Su F, Ma L. (2005b) Role of withdrawal in reinstatement of morphine-conditioned place preference. Psychopharmacology (Berl) 181:90–100. [DOI] [PubMed] [Google Scholar]
- Martell BA, Orson FM, Poling J, Mitchell E, Rossen RD, Gardner T, Kosten TR. (2009). Cocaine vaccine for the treatment of cocaine dependence in methadone-maintained patients: a randomized, double-blind, placebo-controlled efficacy trial. Arch Gen Psychiatry 66:1116–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer MA, Hocht C, Opezzo JA, Peredo HA, Navacchia D, Taira CA, Fernandez BE, Puyo AM. (2006). Role of hypothalamic alpha-adrenoceptor activity in fructose-induced hypertension. Clin Exp Pharmacol Physiol 33:904–909. [DOI] [PubMed] [Google Scholar]
- Megarbane B, Buisine A, Jacobs F, Resiere D, Chevillard L, Vicaut E, Baud FJ. (2010). Prospective comparative assessment of buprenorphine overdose with heroin and methadone: clinical characteristics and response to antidotal treatment. J Subst Abuse Treat 38:403–407. [DOI] [PubMed] [Google Scholar]
- Moreno AY, Mayorov AV, Janda KD. (2011). Impact of distinct chemical structures for the development of a methamphetamine vaccine. J Am Chem Soc 133:6587–6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nik Jaafar NR, Mislan N, Abdul Aziz S, Baharudin A, Ibrahim N, Midin M, Das S, Sidi H. (2013). Risk factors of erectile dysfunction in patients receiving methadone maintenance therapy. J Sex Med 10:2069–2076. [DOI] [PubMed] [Google Scholar]
- Orson FM, Kinsey BM, Singh RA, Wu Y, Gardner T, Kosten TR. (2008). Substance abuse vaccines. Ann N Y Acad Sci 1141:257–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raleigh MD, Pravetoni M, Harris AC, Birnbaum AK, Pentel PR. (2013). Selective effects of a morphine conjugate vaccine on heroin and metabolite distribution and heroin-induced behaviors in rats. J Pharmacol Exp Ther 344:397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter AJ. (2002). Naltrexone in the treatment of heroin dependence: relationship with depression and risk of overdose. Aust N Z J Psychiatry 36:224–228. [DOI] [PubMed] [Google Scholar]
- Sauro MD, Greenberg RP. (2005). Endogenous opiates and the placebo effect: a meta-analytic review. J Psychosom Res 58:115–120. [DOI] [PubMed] [Google Scholar]
- Schlosburg JE, Vendruscolo LF, Bremer PT, Lockner JW, Wade CL, Nunes AA, Stowe GN, Edwards S, Janda KD, Koob GF. (2013). Dynamic vaccine blocks relapse to compulsive intake of heroin. Proc Natl Acad Sci U S A 110:9036–9041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. (2003). The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 168:3–20. [DOI] [PubMed] [Google Scholar]
- Sorge RE, Stewart J. (2006). The effects of long-term chronic buprenorphine treatment on the locomotor and nucleus accumbens dopamine response to acute heroin and cocaine in rats. Pharmacol Biochem Behav 84:300–305. [DOI] [PubMed] [Google Scholar]
- Spyraki C, Fibiger HC, Phillips AG. (1983). Attenuation of heroin reward in rats by disruption of the mesolimbic dopamine system. Psychopharmacology (Berl) 79:278–283. [DOI] [PubMed] [Google Scholar]
- Stowe GN, Vendruscolo LF, Edwards S, Schlosburg JE, Misra KK, Schulteis G, Mayorov AV, Zakhari JS, Koob GF, Janda KD. (2011). A vaccine strategy that induces protective immunity against heroin. J Med Chem 54:5195–5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tew JG, Phipps RP, Mandel TE. (1980). The maintenance and regulation of the humoral immune response: persisting antigen and the role of follicular antigen-binding dendritic cells as accessory cells. Immunol Rev 53:175–201. [DOI] [PubMed] [Google Scholar]
- UNODC (2014). World drug report. In: New York: United Nations. [Google Scholar]
- Wainer BH, Fitch FW, Rothberg RM, Fried J. (1972). Morphine-3-succinyl--bovine serum albumin: an immunogenic hapten-protein conjugate. Science 176:1143–1145. [DOI] [PubMed] [Google Scholar]
- Wang X, Luo YX, He YY, Li FQ, Shi HS, Xue LF, Xue YX, Lu L. (2010). Nucleus accumbens core mammalian target of rapamycin signaling pathway is critical for cue-induced reinstatement of cocaine seeking in rats. J Neurosci 30:12632–12641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu CM, Wang J, Wu P, Zhu WL, Li QQ, Xue YX, Zhai HF, Shi J, Lu L. (2009). Glycogen synthase kinase 3beta in the nucleus accumbens core mediates cocaine-induced behavioral sensitization. J Neurochem 111:1357–1368. [DOI] [PubMed] [Google Scholar]
- Xue YX, Luo YX, Wu P, Shi HS, Xue LF, Chen C, Zhu WL, Ding ZB, Bao YP, Shi J, Epstein DH, Shaham Y, Lu L. (2012). A memory retrieval-extinction procedure to prevent drug craving and relapse. Science 336:241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]