Abstract
Background:
It is generally accepted that chronic treatment with antidepressants increases hippocampal neurogenesis, but the molecular mechanisms underlying their effects are unknown. Recently, glycogen synthase kinase-3 beta (GSK-3β)/β-catenin signaling was shown to be involved in the mechanism of how antidepressants might influence hippocampal neurogenesis.
Methods:
The aim of this study was to determine whether GSK-3β/β-catenin signaling is involved in the alteration of neurogenesis as a result of treatment with fluoxetine, a selective serotonin reuptake inhibitor. The mechanisms involved in fluoxetine’s regulation of GSK-3β/β-catenin signaling pathway were also examined.
Results:
Our results demonstrated that fluoxetine increased the proliferation of embryonic neural precursor cells (NPCs) by up-regulating the phosphorylation of Ser9 on GSK-3β and increasing the level of nuclear β-catenin. The overexpression of a stabilized β-catenin protein (ΔN89 β-catenin) significantly increased NPC proliferation, while inhibition of β-catenin expression in NPCs led to a significant decrease in the proliferation and reduced the proliferative effects induced by fluoxetine. The effects of fluoxetine-induced up-regulation of both phosphorylation of Ser9 on GSK-3β and nuclear β-catenin were significantly prevented by the 5-hydroxytryptamine-1A (5-HT1A) receptor antagonist WAY-100635.
Conclusions:
The results demonstrate that fluoxetine may increase neurogenesis via the GSK-3β/β-catenin signaling pathway that links postsynaptic 5-HT1A receptor activation.
Keywords: β-catenin, cell proliferation, fluoxetine, glycogen synthase kinase-3 beta, neural precursor cells
Introduction
Growing evidence supports the notion that new neurons are generated continuously throughout life from a pool of neural stem/progenitor cells, primarily in the hippocampal dentate gyrus, that ultimately form functional synaptic connections with the existing hippocampal circuitry (van Praag et al., 2002). Recently, studies have shown that hippocampal neurogenesis may play an important role in the effects of clinical antidepressant drugs. First, chronic treatment with a variety of antidepressants, including selective serotonin (5-HT) reuptake inhibitors (SSRIs), selective norepinephrine (NE) reuptake inhibitors (SNRIs), dual 5-HT/NE reuptake inhibitors, and monoamine oxidase inhibitors (MAOIs), increases basal adult hippocampal neurogenesis (Malberg et al., 2000; Kodama et al., 2004; Malberg, 2004; Dranovsky and Hen, 2006). In addition, chronic treatment with SSRIs reverses the inhibition of hippocampal neurogenesis induced by glucocorticoids and improves depression-like behaviors (Sairanen et al., 2007). Furthermore, blockade of neurogenesis by irradiation of the hippocampus abolishes the behavioral and proliferative effects of fluoxetine (Santarelli et al., 2003), which suggests that pathways involved in hippocampal neurogenesis might be an essential target of antidepressants. Therefore, it would be of value to focus on the mechanisms underlying hippocampal neurogenesis that are associated with the use of antidepressant medication.
The glycogen synthase kinase-3 beta (GSK-3β)/β-catenin pathway has been studied extensively in the context of the canonical Wnt pathway, which is an important regulator of mammalian neural development (Logan and Nusse, 2004; Ciani and Salinas, 2005). GSK-3 is a ubiquitous cellular serine/threonine protein kinase. A well-known mechanism that regulates the activity of the two isoforms of GSK-3, GSK-3α, and GSK-3β is the phosphorylation of regulatory serine residues (Ser21 in GSK-3α and Ser9 in GSK-3β), which inhibits GSK-3 activity (Hughes et al., 1993; Wang et al., 1994). Depending on its phosphorylation state, β-catenin can be found in the membrane, in the cytoplasm, or in the nucleus. In the absence of a Wnt signal, β-catenin is phosphorylated by GSK-3β and is degraded by the ubiquitin-proteasome system. In the presence of a Wnt signal, GSK-3β activity is inhibited, and nonphosphorylated β-catenin accumulates in the cytoplasm. It then translocates into the nucleus, where it promotes the transactivation of a variety of genes, including the cell cycle regulator genes myc and cyclinD1, which are important for development.
Both in vitro and in vivo studies demonstrate that the GSK-3β/β-catenin pathway plays an important role in the regulation of hippocampal neurogenesis. Activation of the Wnt/β-catenin pathway is sufficient to increase hippocampal neurogenesis both in vitro and in vivo (Lie et al., 2005; Adachi et al., 2007). Stabilized β-catenin also causes excessive proliferation of neural progenitor cells, which results in a grossly enlarged brain (Chenn and Walsh, 2002). Mao et al. (2009) report that Disrupted in Schizophrenia 1 regulates the proliferation of embryonic and adult neural progenitor cells through the GSK-3β/β-catenin pathway, which indicates a pivotal role of this pathway in the control of hippocampal neurogenesis.
It has been reported that GSK-3β/β-catenin signaling is regulated by different classes of antidepressants. Lithium, which is used for the treatment of bipolar disorder as well as depression, inhibits the activity of GSK-3β (Hedgepeth et al., 1997; Wexler et al., 2008). In addition, fluoxetine and imipramine have both been found to increase the level of phospho-Ser9-GSK-3β in vivo in the mammalian brain (Li et al., 2004). More recently, Okamoto’s study demonstrated that chronic administration of antidepressants can alter hippocampal expression of multiple components of the Wnt/β-catenin signaling cascade, including the Wnt-related proteins Fz, β-catenin, and TCF (Okamoto et al., 2010). Accordingly, these findings have led to the assumption that antidepressants might regulate hippocampal neurogenesis via GSK-3β/β-catenin signaling.
In the present study, we first determined the impact of fluoxetine, a widely prescribed antidepressant, on the proliferation, differentiation, and apoptosis of embryonic neural precursor cells (NPCs). Secondly, we explored the effects of fluoxetine on the expression of different molecules that are involved in the GSK-3β/β-catenin signaling pathway. In addition, we investigated the proliferation of embryonic NPCs under two opposing systems, where β-catenin was overexpressed after transfection with a stabilized β-catenin or suppressed by β-catenin-specific siRNAs; we then evaluated whether β-catenin is required for the proliferative effects of fluoxetine. Finally, we explored the mechanisms involved in fluoxetine’s regulation of the GSK-3β/β-catenin signaling pathway.
Experimental Procedures
NPC Culture
Hippocampal NPCs were prepared as previously described (Xi et al., 2011, 2013). Hippocampal tissues were isolated from embryonic day 12.5 fetal Sprague-Dawley rats and placed in ice-cold phosphate-buffered saline (PBS). After the tissues were mechanically dissected, the dissociated cells were passed through a 70 µm nylon cell strainer (Falcon 2350, BD Bioscience) and centrifuged at 1300rpm for 3min. The pellets were re-suspended in Dulbecco’s Modified Eagle’s Medium (DMEM) with F12 (Sigma) and supplemented with 1% N-2 and 2% B-27 supplements (Invitrogen), 2 mmol/L of glutamine, 20ng/ml of epidermal growth factor (EGF), 20ng/ml of basic fibroblast growth factor (bFGF), 100U/ml of penicillin, and 100 μg/ml of streptomycin. Cells were cultured in Petri dishes at 37°C in 5% CO2, and neurospheres appeared within 2–3 days. After 5–6 days, the spheres were gently dissociated and collected after centrifugation for 3min at 1300rpm. The cells were re-suspended into an appropriate volume of medium containing fluoxetine, CHIR99021 (CHIR), XAV939 (XAV), or WAY-100635 (all from Sigma), as indicated. All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the experiments were approved by the Jiang Su Animal Care and Use Committee.
Immunocytochemistry
After fixation in 4% paraformaldehyde for 20min, NPCs on poly-L-lysine-coated coverslips were permeabilized with 0.5% Triton X-100 in PBS for 20min and blocked with 5% bovine serum albumin for 1h. The cells were then incubated overnight at 4°C with mouse anti-nestin (1:400, Chemicon) or mouse anti-sox2 (1:100; Santa Cruz Biotechnology) to identify NPCs. After washing with PBS, the cells were incubated for 1h with an Alexa Fluor 488-conjugated goat anti-mouse IgG antibody. Cell nuclei were counterstained with 4,6-diamidino-2-phenylindole (DAPI, Sigma). A coverslip that was incubated with the same concentration of normal immunoglobulin G instead of the primary antibody was also included as a negative control.
Cell Proliferation Assay
NPCs were plated in six-well plates at a density of 1×106 cells per well and cultured in the presence of different concentrations of fluoxetine, CHIR99021, XAV939 (XAV), or WAY-100635 as indicated in the figures. After 2 d, 5’-bromo-2-deoxy-uridine (BrdU; 10 μM, Sigma) was added, and after a further incubation for 24h, the cells were dissociated and plated onto poly-L-lysine-coated glass coverslips (Sigma). After attachment, NPCs were fixed for 20min in 4% paraformaldehyde and treated with 2M HCl for 30min at room temperature. Cells were washed with PBS and incubated overnight with rat anti-BrdU (1:200, Abcam) at 4°C. After washing in PBS, the cells were incubated with rhodamine-conjugated rabbit anti-rat lgG (1:100, Jackson ImmunoResearch) for 1h at room temperature. Labeled cells were further incubated with DAPI (Sigma) at 0.1 μg/mL for 30min at room temperature prior to mounting with Gel Mount. The numbers of total cells and BrdU-positive cells were counted using fluorescence microscopy in four non-overlapping fields per coverslip. Cells incubated without the primary antibody served as a negative control.
Cell Differentiation
NPCs were plated onto poly-L-lysine-coated 24-well culture dishes (150 000 cells per well) and cultured in medium supplemented with 10% fetal bovine serum (FBS). After 7 days in culture, immunocytochemical staining was performed as described in the Methods section. The following primary antibodies were used during an overnight incubation at 4°C: mouse anti-βIII tubulin (1:500; Sigma) and rabbit anti-glial fibrillary acidic protein (1:500; Santa Cruz Biotechnology). An Alexa Fluor 488-conjugated goat anti-mouse IgG antibody and an Alexa Fluor 546–conjugated goat anti-rabbit IgG antibody (Invitrogen) were each used at a dilution of 1:3 000. The number of immunoreactive cells in each well was counted using fluorescent microscopy in four independent fields.
In Situ Detection of Cell Death
To assess apoptosis, the cells were fixed in 4% paraformaldehyde in PBS for 1h at 25°C. After washing in PBS, the cells were permeabilized with 0.1% Triton X-100 in 0.1% sodium citrate for 2min on ice and incubated with 50 μL terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) reaction mixture (Promega) for 1h at 37°C. Cells were then counterstained with DAPI. The number of TUNEL-positive cells was counted using fluorescence microscopy and was normalized to the number of DAPI-positive cells.
Western Blotting
Western blotting was performed according to a standard protocol. Nuclear and cytoplasmic proteins were extracted using a NE-PER Nuclear Protein Extraction Kit (Thermo). In brief, 200 μL of cytoplasmic extraction reagent I was added to each 20 μL of collected cell precipitation and then violently vortexed for 15 s. The mixture was incubated in an ice bath for 10min. Then, 11 μL cytoplasmic extraction reagent II was added to the mixture, which was violently vortexed for 5 s and incubated in an ice bath for another 1min. The solution was then centrifuged at 16 000 g for 10min at 4°C. After removal of supernatant, 100 μL nuclear protein extraction reagent was added to the nuclear precipitate and vortexed on the highest setting for 15 s every 10min for a total of 40min. The mixture was centrifuged at 16 000 g for 15min at 4°C, the supernatant was saved, and protein concentrations were detected by the Bradford method. Equal quantities of protein were loaded onto a 10% polyacrylamide gel containing 0.2% sodium dodecyl sulfate for separation. The separated proteins were transferred onto a polyvinylidene fluoride membrane (Millipore) and incubated overnight at 4°C with the following primary antibodies: GSK-3α (1:1 000, Cell Signaling); phospho-Ser21-GSK-3α (1:1 000, Abcam); GSK-3β (1:1 000, Cell Signaling); phospho-Ser9-GSK-3β (1:1 000, Cell Signaling); phospho-Tyr216-GSK-3β (1:1 000, Abcam); β-catenin (1:2 000, BD Bioscience); α-tubulin (1:2 000, Invitrogen); c-myc (1:500, Santa Cruz Biotechnology); and cyclinD1 (1:500, Santa Cruz Biotechnology). After washing, the membranes were incubated with a secondary antibody solution (goat anti-mouse or goat anti-rabbit IgG-HRP, 1:5 000, Santa Cruz) at room temperature for 2h, followed by detection using the enhanced chemiluminescence method.
TOPflash and FOPflash Reporter Assays
Transient transfection studies using a luciferase expression assay in NPCs were performed essentially as previously described (Kim et al., 2013). The neurospheres were dissociated and seeded into six-well plates at a density of 5×104 cells/ml. Generally, cells in each well were transfected with 4 μg T-cell factor reporter plasmid (TOPflash) or mutant T cell factor binding sites (FOPflash) vector (Millipore) using Lipofectamine 2 000 (Invitrogen) according to the manufacturer’s instructions. Forty-eight hours after transfection, the luciferase expression was measured in a luminometer using the Dual Luciferase Reporter Assay System (Promega), according to the manufacturer’s instructions.
Vector Construction
The pcDNA3 beta-catenin and pcDNA3 deltaN89 beta-catenin were purchased from Addgene. We subcloned beta-catenin and deltaN89 beta-catenin complementary DNAs into the pSin-EF2-Pur retrovirus vector. The plasmids were excised using NotI, blunted with Klenow DNA polymerase (New England BioLabs) and digested with BamHI. The vector backbone was generated from pSin-EF2-Nanog-Pur (Addgene) by digesting with NsiI, blunting with Klenow DNA polymerase (New England BioLabs), and digesting again with BamHI. After ligation by T4 DNA ligase (New England BioLabs), the pSin-EF2-beta-catenin-Pur and pSin-EF2-deltaN89 beta-catenin-Pur were further confirmed after a digest with the BamHI and SalI enzymes.
Lentiviral Production
For lentiviral production, 293-T cells were cultured in DMEM with 10% FBS and plated at a density of 8×106 cells in T-75 flasks. The following day, the cells were transfected using Lipofectamine 2 000 (Invitrogen) with 5 μg psPAX2, 3 μg pHCMV-VSV-G and 8μg of either the pSin-EF2-beta-catenin-Pur or the pSin-EF2-deltaN89 beta-catenin-Pur plasmid. After 48h, the supernatant containing the viruses was collected, centrifuged for 3min at 3 000g, and filtered through a 0.45 μm sterile syringe filter. Clarified supernatant was concentrated by ultracentrifugation at 30 000rpm for 2h at 4°C and resuspended in Optimem (Invitrogen) supplemented with 8 μg/ml polybrene (Sigma). Final viral titres ≥1×108/ml were consistently obtained. NPCs were infected on day 0, and after 3 days of incubation cells were either pulsed with BrdU or lysed for protein analysis. For transduction efficiency, NPCs were cultured in poly-L-lysine and laminin-coated dishes with monolayer and transduced with lentiviral vectors carrying an enhanced green fluorescent protein (pSin-EF2-EGFP-Pur). After 3 days, the cells expressing green fluorescent protein (GFP) were counted.
Small interfering RNA Transfection
The β-catenin Small interfering RNA (siRNA) (a mixture of three different sequences against rat β-catenin) and a fluorescein-conjugated control siRNA were purchased from Santa Cruz Biotechnology. The day before the transfection, cells were plated in six-well dishes at a confluence of 60% to 80%. Transfection of each siRNA (final concentration, 50nM) was performed using Lipofectamine 2 000 (Invitrogen) according to the manufacturer’s instructions. Forty-eight hours after transfection, the cells were collected for the cell proliferation assay.
5-HT Enzyme-Linked Immunosorbent Assay (ELISA)
The NPCs were plated in 24-well plates in growth medium in either the absence or presence of fluoxetine (1 μM) for 48h. The levels of 5-HT in the culture media were then measured using a serotonin enzyme-linked immunosorbent kit (DRG Instruments) according to the manufacturer’s instructions. The optical density was read at 450nm using a microplate reader (Bio-Rad).
Statistical Analysis
All data are expressed as the mean ± standard deviation. Paired student’s t-tests were used to compare two experimental groups; in all other cases, one-way or two-way analyses of variance (ANOVA) were used. Post hoc analyses were performed by the Bonferroni’s test for selected or multiple comparisons, when p < 0.05. Data normality was assessed by the Kolmogorov-Smirnov test. The results showed that all Sig > 0.05.
Results
Fluoxetine Promotes the Proliferation of NPCs
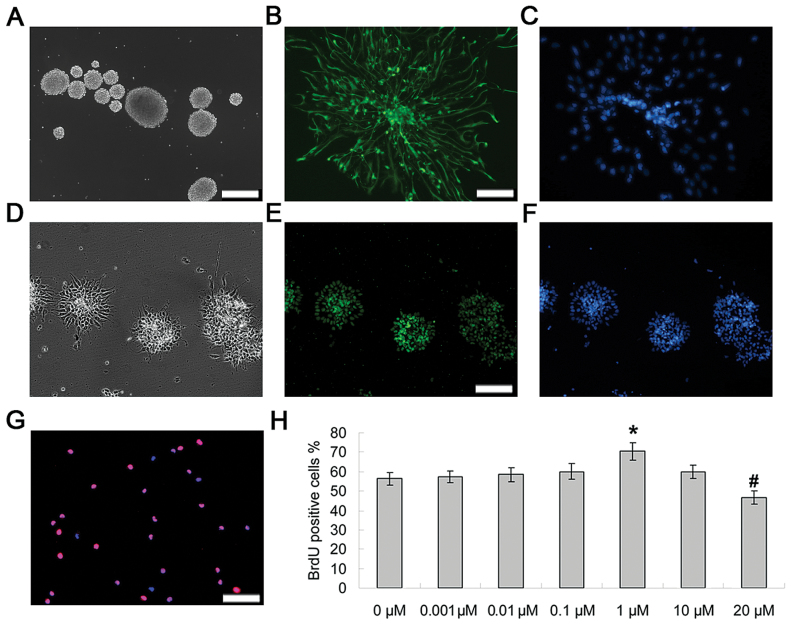
NPC cultures revealed aggregations and formation of typical neurospheres (Figure 1A), which was demonstrated by the immunocytochemical detection of nestin and sox2, two markers of undifferentiated NPCs (Figure 1B and 1E). We assessed the effects of increasing concentrations (0.001–20 μM) of fluoxetine on proliferation by BrdU labelling (Figure 1G). ANOVA revealed a main effect of treatment [F (5, 24) = 9.67, p < 0.0005]. These dose-response experiments, summarized in Figure 1H, show that after exposure to fluoxetine for 48h, cell proliferation was significantly increased at a concentration of 1 μM (p < 0.01, n = 5), whereas the highest concentration used (20 μM) actually decreased cell proliferation (p < 0.005, n = 5). Quantification of the data revealed that the percentage of BrdU-positive cells increased from 56.4±3.21% in untreated control cells to 70.40±4.39% in 1 μM fluoxetine-treated cells, but decreased to 46.80±3.42% in 20 μM fluoxetine-treated cells (Figure 1H).
Figure 1.
Fluoxetine increased the proliferation of NPCs. (A) Typical neurosphere morphology of rat embryonic neural precursor cells maintained in growth medium. (B–C) Immunostaining of Nestin (green) and DAPI (blue) in NPCs. Scale bars = 20 μm. (D–F) Immunostaining of sox2 (green) and DAPI (blue) in NPCs. (G) For cell proliferation, NPCs were incubated for 2 d in the presence of increasing concentrations (0–20 μM) of fluoxetin. Values represent means ± standard deviation (n = 5). BrdU-positive cells and nuclei (DAPI) were labeled with red and blue. Scale bars = 20 μm. (H) Quantification of data. ANOVA revealed a main effect of treatment [F (5, 24) = 9.67, p < 0.0005]. *p < 0.01 versus control (0 μM); #p < 0.005 versus control. BrdU, 2 d, 5’-bromo-2-deoxy-uridine; DAPI, 4,6-diamidino-2-phenylindole; NPCs, neural precursor cells.
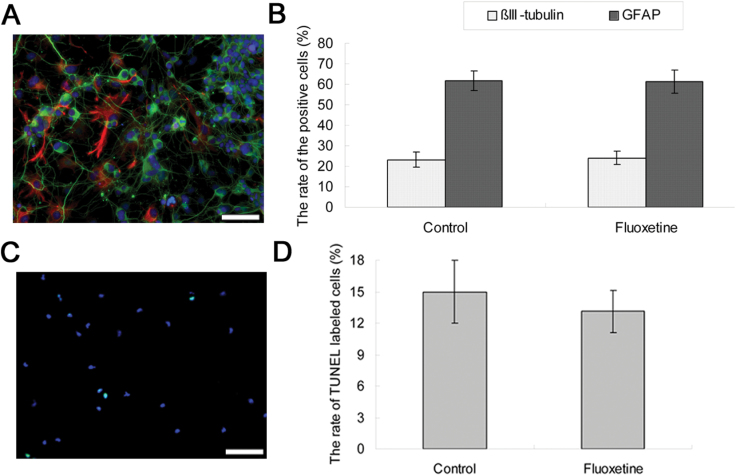
When the NPCs were incubated with medium containing FBS, the cells were able to spontaneously differentiate into neurons and gliocytes, as revealed by immunocytochemical analysis (Figure 2A). After 7 days in pro-differentiation conditions, the percentages of NPCs that differentiated into neurons and gliocytes in the control group were 23.17±3.76% and 61.83±4.83%. Similarly, the percentages of NPCs that differentiated into neurons and glia in fluoxetine treatment group were 24.00±3.22% and 61.33±5.72%. After a t-test was performed, no significant difference (p > 0.05, n = 6) was found between the two groups in terms of the percentage of neuronal and glial cells in each culture (Figure 2B).
Figure 2.
Fluoxetine treatment of 1 μM had no effect on cell differentiation or apoptosis of NPCs. (A) After 7 days cultured in differentiation condition, cells were collected for immunostaining detection. βIII-tubulin- and GFAP-positive cells were shown green and red, respectively. Blue DAPI staining showed the nuclei. Scale bars = 20 μm. (B) Quantitative analyses of βIII-tubulin- and GFAP-positive cells. The percentages of positive cells were shown. Values represent means ± SD (n = 6). (C) After 48h incubation, NPCs were used for TUNEL/DAPI staining. TUNEL-positive cells were green. Scale bars = 20 μm. (D) Quantitative analyses of TUNEL-positive cells. Values represent means ± SD (n = 6). DAPI, 4,6-diamidino-2-phenylindole; GFAP, anti-glial fibrillary acidic protein; NPCs, neural precursor cells; SD, standard deviation; TUNEL, transferase-mediated dUTP nick end labeling.
A TUNEL assay was performed to determine if fluoxetine treatment caused NPCs to undergo apoptosis. The proportion of TUNEL-positive cells was 14.98±3.00% under normal culture conditions in our experiments. There was no significant difference (p > 0.05, n = 6) in the proportion of TUNEL-positive cells (13.12±2.02%) after the treatment of 1 μM fluoxetine for 48h (Figure 2C and D).
Effects of Fluoxetine on the GSK-3β/β-Catenin Signaling Pathway
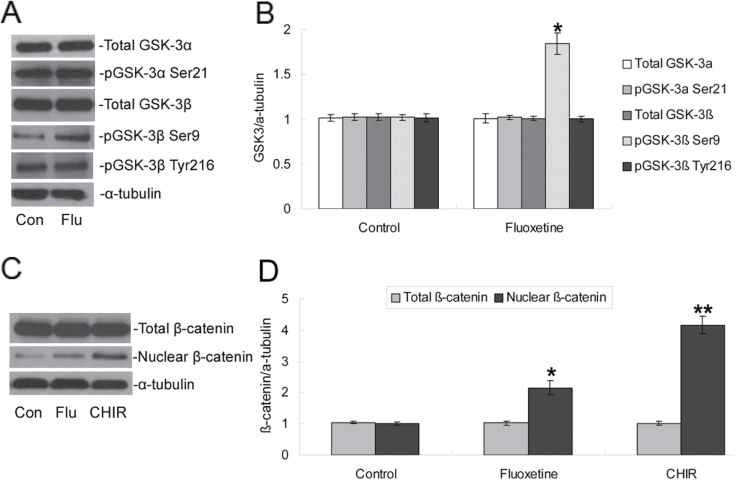
Fluoxetine treatment had no significant effect on the total content of either the GSK-3α or the GSK-3β proteins (both p > 0.05, n = 5, Figure 3A and B). We further examined the phosphorylation state of GSK-3 (Figure 3A) and showed phosphorylation only on the Ser9 residue of GSK-3β had a significantly increased expression after treatment with 1 μM fluoxetine compared to the control group (1.85±0.12-fold vs 1.02±0.03-fold, p < 0.05, n = 5), while phosphorylation on the Tyr216 of GSK-3β or on the Ser21 of GSK-3α did not show significant changes (both p > 0.05, n = 5).
Figure 3.
Effects of fluoxetine on GSK-3β/β-catenin expression in NPCs. NPCs were cultured for 48h in the presence of 1 μM fluoxetine or 3μM CHIR99021. (A) Representative Western blotting of total GSK-3α, phospho-Ser21-GSK-3α, total GSK-3β, phospho-Ser9-GSK-3β, phospho-Tyr216-GSK-3β, and α-tubulin proteins. (B) Quantification of Western blotting signals of GSK3 and α-tubulin proteins. Data were ratios compared with α-tubulin protein. Values represent means ± SD. n = 5 for each group. *p < 0.05 compared with the control group. (C) Representative Western blotting of total β-catenin, nuclear β-catenin, and α-tubulin proteins. (D) Quantification of Western blotting signals of β-catenin and α-tubulin proteins. Data were ratios compared with α-tubulin protein. Values represent means ± SD. n = 5 for each group. *p < 0.01, **p < 0.005 compared with the control group. GSK3, glycogen synthase kinase-3; NPCs, neural precursor cells; SD, standard deviation.
Active GSK-3β protein promotes the degradation of β-catenin, whereas a reduction of GSK-3β activity increases the cytosolic level of β-catenin and allows for its translocation from the cytoplasm to the nucleus. Therefore, the effects of fluoxetine on the total cellular level of β-catenin and the nuclear level of β-catenin were determined. Figure 3C shows that treatment with fluoxetine and CHIR, a selective inhibitor of GSK-3β, significantly increased the levels of nuclear β-catenin up to 2.15±0.22-fold (p < 0.01, n = 5) and 4.16±0.28-fold (p < 0.005, n = 5), respectively.
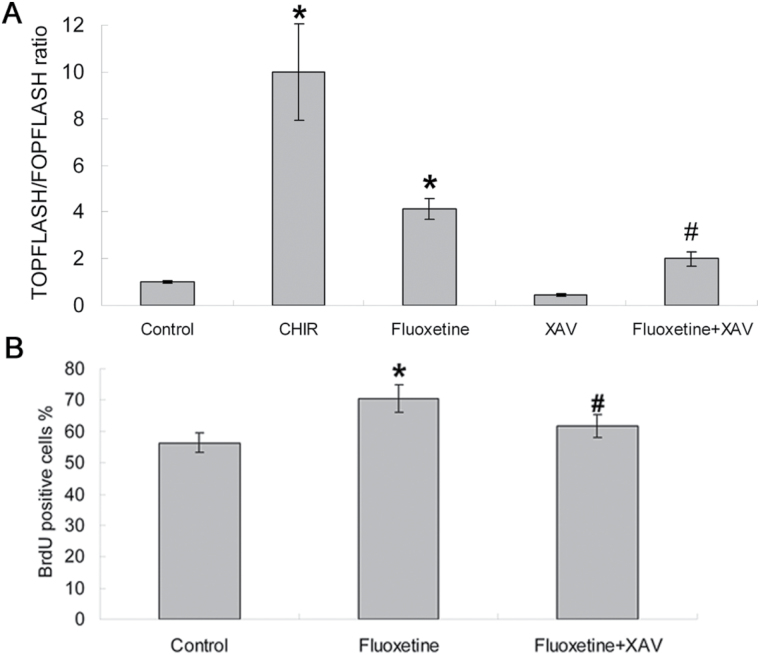
To measure β-catenin activity directly, we used the TOPflash assay. ANOVA revealed a significant effect of treatment [F (4, 25) = 98.12, p < 0.0001]. Post hoc tests showed that the reporter activity was significantly increased by CHIR (10.00±2.08-fold) and fluoxetine (4.11±0.45-fold) compared to the control group (both p < 0.001, n = 6, Figure 4A). Although a strong decrease in reporter activity was measured (0.43±0.06-fold) with the treatment of XAV, which facilitated the degradation of β-catenin protein by stabilizing the APC/Axin/GSK-3β complex and selectively inhibited β-catenin-mediated transcription, this effect was not significant (p > 0.05, n = 6, Figure 4A). However, 5 μM XAV had a substantial effect in reducing the fluoxetine-induced enhancement of reporter activity (1.98±0.30-fold vs 4.11±0.45-fold, p < 0.005, n = 6). In addition, ANOVA revealed main effects for fluoxetine and XAV treatment on NPC proliferation [F (1, 13) = 34.75, p < 0.0001 for fluoxetine; F (1, 13) = 13.11, p = 0.004 for XAV]. Post hoc tests demonstrated that inhibition of GSK-3β activity by fluoxetine (that is, the up-regulation of the nuclear level of β-catenin) significantly enhanced NPC proliferation compared to the control group (70.40±4.39% vs 56.4±3.21%, p < 0.01, n = 5), while treatment with XAV, which facilitated the degradation of β-catenin protein, significantly reversed the fluoxetine-induced proliferation (61.8±3.56% vs 70.40±4.39%, p < 0.05, n = 5, Figure 4B).
Figure 4.
Inhibition of β-catenin signaling reduced the effects of fluoxetine on cell proliferation in NPCs. (A) TOPFlash assay in NPCs treated with the indicated treatments for 48h. ANOVA revealed a significant effect of treatment [F (4, 25) = 98.12, p < 0.0001]. Values represent means ± SD (n = 6). *p < 0.001 versus control group; #p < 0.005 versus fluoxetine group. (B) XAV reversed fluoxetine-mediated proliferation of NPC. NPCs were incubated for 2 d with 1 μM fluoxetine in the absence or presence of 5 μM XAV. ANOVA revealed main effects for fluoxetine and XAV treatment on NPC proliferation [F (1, 13) = 34.75, p < 0.0001 for fluoxetine; F (1, 13) = 13.11, p = 0.004 for XAV]. Values represent means ± SD (n = 5). *p < 0.01 versus control (NPCs); #p < 0.05 versus fluoxetine group. ANOVA, analysis of variance; NPCs, neural precursor cells; SD, standard deviation; XAV, XAV939.
The Proliferative Effects of Fluoxetine are Attributable to β-Catenin Activation
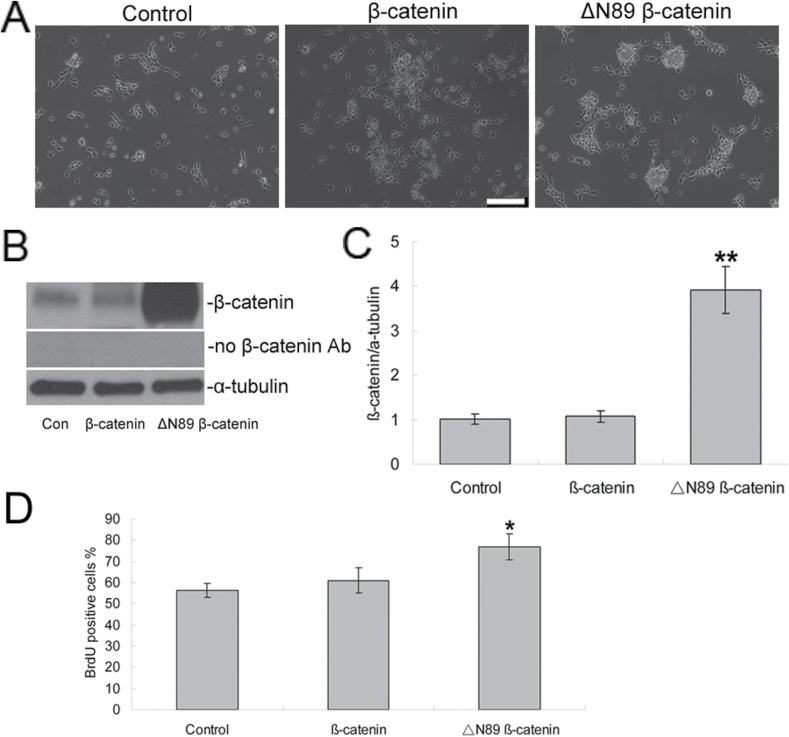
To test the hypothesis that GSK-3β-induced accumulation of β-catenin was responsible for enhancing the proliferation of NPCs, we experimentally elevated the level of cytosolic β-catenin by using a lentivirus to transduce NPCs with β-catenin. The transduction efficiency averaged 68.0±4.2% (n = 40 fields in four plates) by counting the green fluorescent (GFP-positive) cells after 3 days. Western blots (Figure 5B) indicated that there was no significant change in the level of cytosolic β-catenin, compared to vehicle-only treatment (p > 0.05, n = 5, Figure 5C), after transduction with a β-catenin–containing lentivirus. However, there was a robust increase (1.01±0.11-fold vs 3.93±0.53-fold, p < 0.005, n = 5, Figure 5C) in the expression of stabilized active β-catenin after transduction with a form of β-catenin that lacks the NH2-terminal 89 amino acids (ΔN89 β-catenin), retains cadherin/catenin binding, and is insensitive to ubiquitination. NPCs that overexpress ΔN89 β-catenin expanded faster and formed more aggregations compared with cells treated with vehicle (Figure 5A). The effects of a constitutively-expressed β-catenin in NPCs further demonstrated a significant enhancement in cell proliferation, increasing the percentage of BrdU positive cells from 56.40±3.21% in controls to 77.00±6.12% in ΔN89 β-catenin (p < 0.01, n = 5, Figure 5C).
Figure 5.
Transduction of stabilized β-catenin increased the proliferation of NPCs. NPCs were transduced with pSin-EF2-beta-catenin, pSin-EF2-deltaN89 beta-catenin, and pSin-EF2-GFP. (A) Phase contrast images of NPCs after transduction for 48h. Scale bars = 20 μm. (B) The protein expression of β-catenin was further detected by Western blotting. (C) Quantification of Western blotting signals of β-catenin and α-tubulin proteins. Data were ratios compared with α-tubulin protein. n = 5 for each group. **p < 0.005 compared with the control group. (D) 48h after transduction, cell proliferation was measured by BrdU labeling. Values represent means ± standard deviation (n = 5). *p < 0.01 versus the control group. BrdU, 2 d, 5’-bromo-2-deoxy-uridine; NPCs, neural precursor cells.
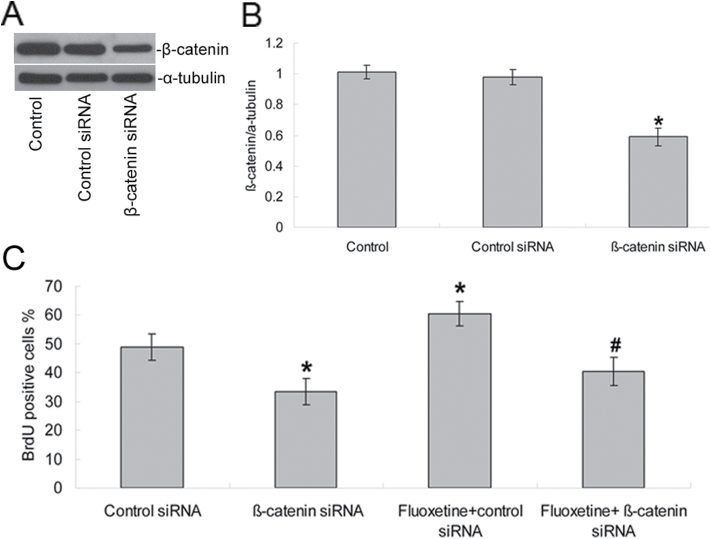
Finally, we performed a knockdown of β-catenin in NPCs using β-catenin siRNA. The efficiency of the transfection averaged 41.00±0.70% (n = 40 fields in four plates) when we counted the green fluorescent (GFP-positive) cells after 4 days. Protein expression was detected by Western blot (Figure 6A), which indicated a decreased expression from 0.98±0.05-fold to 0.59±0.06-fold (p < 0.01, n = 5, Figure 6B). ANOVA revealed main effects for β-catenin siRNA and fluoxetine treatment on NPC proliferation [F (1, 18) = 75.13, p < 0.0001 for β-catenin siRNA; F (1, 18) = 20.74, p < 0.0001 for fluoxetine]. Post hoc tests indicated that down-regulating β-catenin in NPCs significantly decreased (p < 0.01, n = 5) the proportion of BrdU-positive cells to 33.40±4.51% compared with the control siRNA (49.00±4.53%) and reversed the effects of fluoxetine on NPC proliferation (40.60±4.93% vs 60.40±4.28%, p < 0.001, n = 5, Figure 6C).
Figure 6.
Inhibition of β-catenin expression decreased NPC proliferation and reduced the proliferative effects induced by fluoxetine. NPCs were transfected with β-catenin siRNA or a fluorescein-conjugated control siRNA. (A) β-catenin protein expression was detected by Western blotting and indicated a decrease expression (B). Values represent means ± SD (n = 5). *p < 0.01 versus control siRNA group. (C) 48h after transfection, some of the cells (as noted) were treated with 1 μM fluoxetine for an additional 48h before their cell proliferation was measured by BrdU labeling. Analyses of variance revealed main effects for β-catenin siRNA and fluoxetine treatment on NPC proliferation [F (1, 18) = 75.13, p < 0.0001 for β-catenin siRNA; F (1, 18) = 20.74, p < 0.0001 for fluoxetine]. Values represent means ± SD (n = 5). *p < 0.01 versus control siRNA group; #p < 0.001 versus fluoxetine + control siRNA group. BrdU, 2 d, 5’-bromo-2-deoxy-uridine; NPCs, neural precursor cells; SD, standard deviation; siRNA,.
Effects of Fluoxetine on the GSK-3β/β-Catenin Signaling Pathway Depends on 5-HT1A Receptors
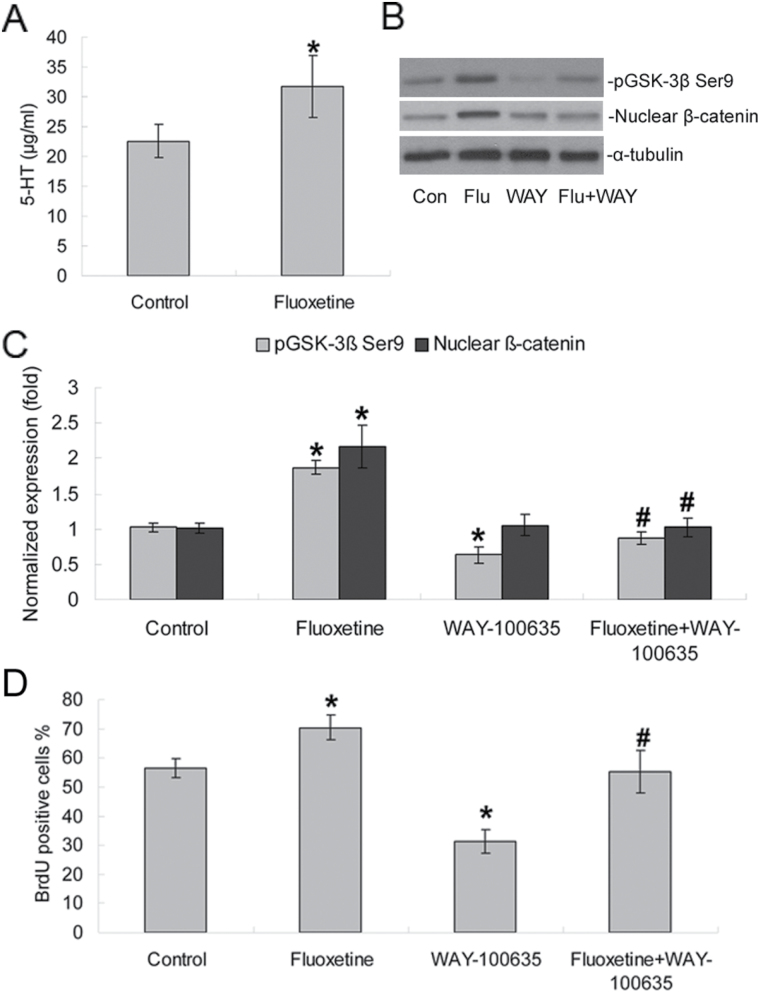
Since the 5-hydroxytryptamine-1A (5-HT1A) receptor is essential for the fluoxetine-induced increase in hippocampal neurogenesis (Santarelli et al., 2003; Huang and Herbert, 2005; Zusso et al., 2008; Benninghoff et al., 2010), we next examined whether 5-HT1A receptors are involved in the phosphorylation of Ser9 on GSK-3β induced by fluoxetine. Because we have previously demonstrated that the expression of 5-HT, 5-HT1A receptors, the serotonin transporter, and tryptophan hydroxylase in NPCs by reverse transcription-polymerase chain reaction and immunostaining (Wang et al., 2014), we further examined the levels of serotonin in the culture medium. Compare to the control group, 1 μM fluoxetine caused a significant increase (22.57±2.73 μg/ml vs 31.72±5.24 μg/ml, p < 0.01, n = 5) in 5-HT concentration in the culture medium during the proliferation phases of NPCs (Figure 7A).
Figure 7.
Fluoxetine-induced enhancement of phosphorylation of Ser9 on GSK-3β and nuclear β-catenin depends on 5-HT1A receptors. (A) The ELISA showed that 1 μM fluoxetine increased 5-HT concentration in the culture media in NPC proliferation phases (n = 5 each). *p < 0.01 versus the control group. (B) Fluoxetine enhanced the phosphorylation of Ser9 on GSK-3β and nuclear β-catenin protein expression via 5-HT1A receptors. (C) Quantification of Western blotting signals of phospho-Ser9-GSK-3β, nuclear β-catenin, and α-tubulin proteins. Data were ratios compared with α-tubulin protein. Values represent means ± SD. n = 5 for each group. *p < 0.05 compared with the control group, #p < 0.05 versus the fluoxetine group. (D) WAY-100635 reversed fluoxetine-mediated proliferation of NPC. Analyses of variance revealed significant effects of fluoxetine and WAY-100635 on NPC proliferation [F (1, 18) = 72.20, p < 0.0001 for fluoxetine; F (1, 18) = 81.61, p < 0.0001 for WAY-100635]. Values represent means ± SD (n = 5). *p < 0.01 versus control; #p < 0.05 versus fluoxetine group. 5-HT1A, 5-hydroxytryptamine-1A; GSK-3β, glycogen synthase kinase-3 beta; NPCs, neural precursor cells; SD, standard deviation.
WAY-100635, a selective antagonist of 5HT1A receptors, significantly decreased the phosphorylation of Ser9 on GSK-3β compared to the control group (0.63±0.12-fold vs 1.02±0.06-fold, p < 0.05, n = 5), but had no significantly effect on the levels of nuclear β-catenin (p > 0.05, n = 5, Figure 7 B and C). However, the stimulatory effects of fluoxetine on phosphorylation of both Ser9 on GSK-3β and nuclear β-catenin were significantly blocked by WAY-100635 (1.87±0.10-fold vs 0.87±0.09-fold and 2.17±0.30-fold vs 1.02±0.13-fold, both p < 0.05, n = 5, Figure 7 B and C). In addition, we investigated whether 5-HT1A receptors are involved in the fluoxetine-induced alteration of hippocampal NPC neurogenesis. Cultured NPCs were treated with fluoxetine and WAY-100635 for 48h. ANOVA revealed significant effects of fluoxetine and WAY-100635 on NPC proliferation [F (1, 18) = 72.20, p < 0.0001 for fluoxetine; F (1, 18) = 81.61, p < 0.0001 for WAY-100635]. Post hoc analyses revealed that WAY-100635 substantially decreased the proportion of BrdU-positive cells compared with the control group (31.20±4.15% vs 56.40±3.21%, p < 0.05, n = 5) and significantly reversed the effects of fluoxetine on NPC proliferation (55.20±7.29% vs 70.40±4.39%, p < 0.05, n = 5, Figure 7D).
Discussion
The results of the present study demonstrated that fluoxetine increased the proliferation of embryonic NPCs by up-regulating the phosphorylation of Ser9 of GSK-3β as well as the level of β-catenin in the nucleus. Overexpression of the stabilized form of β-catenin (ΔN89 β-catenin) significantly increased proliferation of NPCs, while inhibition of β-catenin expression in NPCs led to a significant decrease in proliferation and abolished the proliferative effects induced by fluoxetine. Furthermore, fluoxetine-induced up-regulation of phosphorylation of both Ser9 on GSK-3β and nuclear β-catenin were significantly prevented by WAY-100635. Our studies implied that the GSK-3β/β-catenin signaling pathway might underlie fluoxetine’s neurogenic effects.
Numerous studies have reported that fluoxetine treatment can increase the proliferation of hippocampal neural stem/progenitor cells both in vivo and in vitro (Malberg et al., 2000; Kodama et al., 2004; Xi et al., 2011). Consistent with previous findings, we showed that 1 μM fluoxetine significantly enhanced the proliferation of NPCs. However, at the highest concentration tested (20 μM) in the present study, fluoxetine significantly decreased cell proliferation. This is likely a consequence of the cytotoxicity of fluoxetine at such a high concentration, which may relate to its interaction with the CYP1A function (Thibaut and Porte, 2008). In addition, the effects of fluoxetine on cell proliferation at different concentrations are in agreement with previous findings in primary rat cerebellar granule cells and hippocampal neural stem cells (Chiou et al., 2006; Zusso et al., 2008). Moreover, the 1 μM fluoxetine used in our present study is relevant to the therapeutic plasma concentration of this drug (Karson et al., 1993; Komoroski et al., 1994; Strauss et al., 2002).
Apart from cell proliferation, the present study demonstrated that there was no significant change in the relative proportion of neurons and glia generated from NPCs, indicating no influence of fluoxetine on differentiation of NPCs. This is consistent with the finding that chronic antidepressant treatments in vivo showed similar ratios of labeled neurons and glia in BrdU-positive cells compared to the control group (Malberg et al., 2000; Santarelli et al., 2003). Although a published paper showed that fluoxetine treatment for up to 5 days induced a significant increase in neuronal phenotypes of cerebellar neural progenitors (Zusso et al., 2008), differences in the cell types used and differentiation conditions may account for the discrepancies. Interestingly, the proportion of new cells that differentiate into neurons in vitro is starkly different those that differentiate in vivo. The mechanism is unknown and might be attributed to the extracellular microenvironment. The in vivo clonal analysis reveals that, after neurogenic cell division, the adult hippocampal radial glia-like precursors returned to quiescence, whereas the intermediate progenitor cells entered cell cycles, proliferated, and differentiated into neurons (Bonaguidi et al., 2011). In contrast, after gliogenic cell division, both the radial glia-like and astroglia cells became quiescent. So, the difference of cell proliferative property between neurogenic and gliogenic cell division may contribute to the high proportion of neural differentiation and low proportion of glial differentiation during in vivo neurogenesis. However, hippocampal NPCs cultured in vitro seem to regain some glial characteristics and predominately differentiate into glia (Reynolds and Weiss, 1992; Dromard et al., 2007). In addition, our results found no significant change of TUNEL-positive cells after fluoxetine treatment, while an in vivo study showed fluoxetine simultaneously increased both survival and apoptosis of hippocampal neural stem cells (Sairanen et al., 2005). These findings raise the possibility that the self-renewal and fate specification of in vivo hippocampal neurogenesis might be regulated by the specific niche architecture in the subgranular zone (SGZ), including their cellular niche components and extracellular niche signals, whereas the extracellular microenvironment is not available for NPCs cultured in vitro.
In the present study, we found that exposure to fluoxetine for 48h increased NPC proliferation in vitro, while prolonged treatment enhanced neurogenesis in vivo (Malberg et al., 2000; Santarelli et al., 2003). The apparent differences of fluoxetine treatment on neurogenesis in vivo and in vitro could be attributed to the alterations in the extracellular microenvironment. Adult NPCs within the lateral ventricle divide slowly and have an average cell cycle time of 15 days (Morshead et al., 1998). We cultured NPCs in serum-free medium containing bFGF and EGF, which have been demonstrated to significantly enhance the division of NPCs in vit ro (Gensburger et al., 1987; Cattaneo and McKay, 1990; Murphy et al., 1990). Thus, the differences in the baseline levels of cell proliferation in vivo and in vitro may be responsible for the slower and faster effects of fluoxetine on neurogenesis that were observed in vivo and in vitro, respectively. In addition, other drugs that increase neurogenesis in vivo after a prolonged treatment can also enhance neurogenesis at a much faster rate in vitro, which is consistent with our hypothesis (Peng et al., 2008; Xi et al., 2011; Ohira et al., 2013).
Several recent publications have implicated the GSK-3β/β-catenin pathway as the mechanism of action of some antidepressants (Chen et al., 2012; Garza et al., 2012; Polter et al., 2012; Basar et al., 2013; Duman and Aghajanian, 2014). For example, the antidepressants fluoxetine and imipramine have been found to inhibit GSK-3β activity by increasing phosphorylation of its N-terminus in the prefrontal cortex of mouse brains (Li et al., 2004). Okamoto et al. (2010) reported that GSK-3β/β-catenin signaling in the hippocampus is regulated by different classes of antidepressant therapies, including SSRIs, SNRIs, dual 5-HT/NE reuptake inhibitors, and chronic electroconvulsive shock. Furthermore, GSK-3β inhibitors, which allow for the stabilization of β-catenin, have been reported to exert antidepressant-like behavioral effects in animal models (Gould et al., 2004, 2007; Kaidanovich-Beilin et al., 2004; Beaulieu et al., 2008). We found that stimulation of cultured NPCs with fluoxetine promoted the phosphorylation of Ser9 of GSK-3β and up-regulated the level of β-catenin in the nucleus, which is in agreement with previous studies.
On the other hand, abnormal GSK-3β/β-catenin signaling has been implicated in the pathophysiology of mood disorders. Increases in GSK-3β activity have been found in the prefrontal cortices of post-mortem depressed suicide victims (Karege et al., 2012). Furthermore, the GSK-3β gene may play a role in determining the regional gray matter volume differences of the right hippocampus and bilateral superior temporal gyri in patients with recurrent major depressive disorder (Inkster et al., 2009). In addition, decreased levels of phosphorylated GSK-3β and β-catenin in hippocampi have been demonstrated in rats subjected to forced swim stress for 14 consecutive days and exhibiting depression-like behaviors (Liu et al., 2012). These findings suggested that dysregulated GSK-3β/β-catenin pathways contribute to the pathophysiology of mood disorders, and the pathway might be a common therapeutic target of antidepressants.
Growing evidence indicates that the enhancement of hippocampal neurogenesis is crucial in the mechanisms of antidepressant efficacy (Malberg et al., 2000; Duman et al., 2001). However, the molecular pathways underlying such effects have not been fully understood. It has been suggested that up-regulation of the brain-derived neurotrophic factor could be involved (Taliaz et al., 2010). Previous study showed that sertraline increased human hippocampal neurogenesis by activating the glucocorticoid receptor (Anacker et al., 2011). In addition, Warner-Schmidt and Duman (2007) reported that vascular endothelial growth factor signaling is required for fluoxetine-induced cell proliferation in the SGZ. Recently, numerous studies have reported that the GSK-3β/β-catenin signaling pathway plays a potential role in mood disorders and regulates various neurobiological functions, including axon and dendrite remodelling and development, synaptogenesis, neuroplasticity, and neurogenesis (Hirabayashi et al., 2004; Lie et al., 2005; Clevers, 2006; Tang et al., 2010; Maguschak and Ressler, 2011, 2012). Inhibition of GSK-3 with a small molecule, NP03112, induced neurogenesis in the dentate gyri of the hippocampi of adult rats (Morales-Garcia et al., 2012). GSK-3 promotes apoptotic signaling in cultured neural precursor cells derived from embryonic mouse brains subjected to apoptotic conditions, while pharmacological inhibition of GSK-3 activity significantly reduced cell apoptosis (Eom et al., 2007). Importantly, in vivo expression of GSK-3 cannot be inhibited by serine-phosphorylation–impaired neurogenesis in mice and blocked the enhancement of neurogenesis induced by co-administration of lithium and fluoxetine (Eom and Jope, 2009). Moreover, in adult transgenic mice that express the stabilized form of β-catenin, which lacks the GSK-3β phosphorylation site, the SVZ is enlarged (Chenn and Walsh, 2002). Retroviral-mediated expression of a stabilized β-catenin protein or administration of a specific inhibitor of GSK-3β promoted the proliferation of progenitor cells in the adult mouse brain (Adachi et al., 2007). In the present study, our results showed that overexpression of a stabilized β-catenin protein significantly increased NPC proliferation, while inhibition of β-catenin expression decreased cell proliferation and reduced the proliferative effects induced by fluoxetine. This is consistent with the essential role of GSK-3β/β-catenin in neurogenesis and indicated that β-catenin might be an important target for the therapeutic effects of fluoxetine.
The role of β-catenin in changing gene expression is likely mediated by its interaction with lymphoid enhancer factor/T-cell factor DNA-binding proteins in the nucleus (Novak and Dedhar, 1999). The target genes would most likely include cell cycle regulator genes, such as myc and cyclinD1. In agreement with this notion, fluoxetine was shown to up-regulate the level of nuclear β-catenin, which leads to increased NPC proliferation. Unfortunately, we found no significant changes in the protein or mRNA levels of myc and cyclinD1 after fluoxetine treatment in this study (data not shown). Interestingly, the Wnt/β-catenin signaling has been shown to regulate the synthesis of brain-derived neurotrophic factor and vascular endothelial growth factor (Zhang et al., 2001; Seitz et al., 2010), which are two important regulators of adult hippocampal neurogenesis and behavioral effects of antidepressants. Further work is required to determine the mechanisms by which β-catenin increases NPC proliferation after treatment with antidepressants.
The mechanism of the phosphorylation of GSK-3β induced by fluoxetine is unknown. The primary action of a chronic SSRI is based on the inhibition of serotonin reuptake to elevate synaptic 5-HT concentrations, thereby activating postsynaptic 5-HT receptors and triggering downstream intracellular signaling cascades. Our previous study demonstrated the existence of a complete circuit for antidepressants that regulates the neurobiological effect of 5-HT in an in vitro NPC system (Wang et al., 2014). Moreover, our results further show that fluoxetine significantly increases 5-HT concentrations in culture solution, indicating that fluoxetine is able to inhibit serotonin reuptake and consequently increase 5-HT receptor stimulation in hippocampal NPCs. Because activation of the 5HT1A receptor is required for the neurogenic effects of fluoxetine (Santarelli et al., 2003; Huang and Herbert, 2005; Zusso et al., 2008; Benninghoff et al., 2010), we speculate the mechanisms underlying the increased phosphorylation of GSK-3β induced by fluoxetine may involve 5-HT1A and the phosphoinositide 3-kinase (PI3K)/Akt pathway. The present study has found that the effects of fluoxetine-induced up-regulation of phosphorylation of Ser9 on GSK-3β can be blocked by the 5-HT1A receptor antagonist WAY-100635. In addition, administration of the 5-HT1A agonist 8-hydroxy-N,N-dipropyl-2-aminotetralin enhanced the active phosphorylation of Akt, leading to increased phosphorylation of Ser9 on GSK-3β, which was blocked by LY294002, an inhibitor of PI3Ks (Mercado-Gomez et al., 2008). There are reports that Akt phosphorylates GSK-3β on Ser9, and thereby inhibits GSK-3β activity (Fukumoto et al., 2001; Mercado-Gomez et al., 2008). We suggest that phosphorylation of Ser9 on GSK-3β is induced by fluoxetine through the activation of the 5-HT1A receptor and the PI3-kinase/Akt pathway, which is consistent with these previous findings. While the present study provides evidence of the ability of fluoxetine to regulate phosphorylation of GSK-3β via 5-HT1A receptors, further studies are needed to fully define the mechanisms involved.
We acknowledge that the current studies on rat embryonic hippocampal NPCs do not necessarily extrapolate either to hippocampal NPCs in adult animals or humans, and of the antidepressant drugs only fluoxetine has been studied here. Further studies in adult animals will be needed to confirm the relevance of our findings to understanding mechanisms underlying the treatment of depression.
In summary, our results suggest that fluoxetine increases neurogenesis via the GSK-3β/β-catenin signaling pathway that links postsynaptic 5-HT1A receptor activation. These results have important implications for enhancing our understanding of the molecular mechanisms of neurogenesis induced by SSRI antidepressants.
Supplementary Material
For supplementary material accompanying this paper, visit http://www.ijnp.oxfordjournals.org/
Statement of Interest
The authors declare no conflicts of interest.
Acknowledgments
This research was supported by National Natural Science Foundation of China (No.81201051, Dr Xi; No.81401619, Dr Hui; No.81061120529, Dr Z Zhang), Natural Science Foundation of Jiangsu Province (No.BK2012097, Dr Xi), and Jiangsu Health International Exchange Program scholarship (2012). The authors would like to express thanks to the members of the Ying lab for technical assistance and Professor G Reynolds for critical reading of the manuscript.
References
- Adachi K, Mirzadeh Z, Sakaguchi M, Yamashita T, Nikolcheva T, Gotoh Y, Peltz G, Gong L, Kawase T, Alvarez-Buylla A, Okano H, Sawamoto K. (2007). Beta-catenin signaling promotes proliferation of progenitor cells in the adult mouse subventricular zone. Stem Cells 25:2827–2836. [DOI] [PubMed] [Google Scholar]
- Anacker C, Zunszain PA, Cattaneo A, Carvalho LA, Garabedian MJ, Thuret S, Price J, Pariante CM. (2011). Antidepressants increase human hippocampal neurogenesis by activating the glucocorticoid receptor. Mol Psychiatry 16:738–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basar K, Eren-Kocak E, Ozdemir H, Ertugrul A. (2013). Effects of acute and chronic electroconvulsive shocks on glycogen synthase kinase 3beta level and phosphorylation in mice. J ECT 29:265–270. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Zhang X, Rodriguiz RM, Sotnikova TD, Cools MJ, Wetsel WC, Gainetdinov RR, Caron MG. (2008). Role of GSK3 beta in behavioral abnormalities induced by serotonin deficiency. Proc Natl Acad Sci USA 105:1333–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benninghoff J, Gritti A, Rizzi M, Lamorte G, Schloesser RJ, Schmitt A, Robel S, Genius J, Moessner R, Riederer P, Manji HK, Grunze H, Rujescu D, Moeller HJ, Lesch KP, Vescovi AL. (2010). Serotonin depletion hampers survival and proliferation in neurospheres derived from adult neural stem cells. Neuropsychopharmacology 35:893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaguidi MA, Wheeler MA, Shapiro JS, Stadel RP, Sun GJ, Ming GL, Song H. (2011). In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell 145:1142–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo E, McKay R. (1990). Proliferation and differentiation of neuronal stem cells regulated by nerve growth factor. Nature 347:762–765. [DOI] [PubMed] [Google Scholar]
- Chen YC, Tan QR, Dang W, Wang HN, Zhang RB, Li ZY, Lin H, Liu R. (2012). The effect of citalopram on chronic stress-induced depressive-like behavior in rats through GSK3beta/beta-catenin activation in the medial prefrontal cortex. Brain Res Bull 88:338–344. [DOI] [PubMed] [Google Scholar]
- Chenn A, Walsh CA. (2002). Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science 297:365–369. [DOI] [PubMed] [Google Scholar]
- Chiou SH, Chen SJ, Peng CH, Chang YL, Ku HH, Hsu WM, Ho LL, Lee CH. (2006). Fluoxetine up-regulates expression of cellular FLICE-inhibitory protein and inhibits LPS-induced apoptosis in hippocampus-derived neural stem cell. Biochem Biophys Res Commun 343:391–400. [DOI] [PubMed] [Google Scholar]
- Ciani L, Salinas PC. (2005). WNTs in the vertebrate nervous system: from patterning to neuronal connectivity. Nat Rev Neurosci 6:351–362. [DOI] [PubMed] [Google Scholar]
- Clevers H. (2006). Wnt/beta-catenin signaling in development and disease. Cell 127:469–480. [DOI] [PubMed] [Google Scholar]
- Dranovsky A, Hen R. (2006). Hippocampal neurogenesis: regulation by stress and antidepressants. Biol Psychiatry 59:1136–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dromard C, Bartolami S, Deleyrolle L, Takebayashi H, Ripoll C, Simonneau L, Prome S, Puech S, Tran VB, Duperray C, Valmier J, Privat A, Hugnot JP. (2007). NG2 and Olig2 expression provides evidence for phenotypic deregulation of cultured central nervous system and peripheral nervous system neural precursor cells. Stem Cells 25:340–353. [DOI] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK. (2014). Neurobiology of rapid acting antidepressants: role of BDNF and GSK-3beta. Neuropsychopharmacology 39:233–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Nakagawa S, Malberg J. (2001). Regulation of adult neurogenesis by antidepressant treatment. Neuropsychopharmacology 25:836–844. [DOI] [PubMed] [Google Scholar]
- Eom TY, Jope RS. (2009). Blocked inhibitory serine-phosphorylation of glycogen synthase kinase-3alpha/beta impairs in vivo neural precursor cell proliferation. Biol Psychiatry 66:494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eom TY, Roth KA, Jope RS. (2007). Neural precursor cells are protected from apoptosis induced by trophic factor withdrawal or genotoxic stress by inhibitors of glycogen synthase kinase 3. J Biol Chem 282:22856–22864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto S, Hsieh CM, Maemura K, Layne MD, Yet SF, Lee KH, Matsui T, Rosenzweig A, Taylor WG, Rubin JS, Perrella MA, Lee ME. (2001). Akt participation in the Wnt signaling pathway through Dishevelled. J Biol Chem 276:17479–17483. [DOI] [PubMed] [Google Scholar]
- Garza JC, Guo M, Zhang W, Lu XY. (2012). Leptin restores adult hippocampal neurogenesis in a chronic unpredictable stress model of depression and reverses glucocorticoid-induced inhibition of GSK-3beta/beta-catenin signaling. Mol Psychiatry 17:790–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensburger C, Labourdette G, Sensenbrenner M. (1987). Brain basic fibroblast growth factor stimulates the proliferation of rat neuronal precursor cells in vitro . FEBS Lett 217:1–5. [DOI] [PubMed] [Google Scholar]
- Gould TD, Einat H, Bhat R, Manji HK. (2004). AR-A014418, a selective GSK-3 inhibitor, produces antidepressant-like effects in the forced swim test. Int J Neuropsychop 7:387–390. [DOI] [PubMed] [Google Scholar]
- Gould TD, Einat H, O DK, Picchini AM, Schloesser RJ, Manji HK. (2007). Beta-catenin overexpression in the mouse brain phenocopies lithium-sensitive behaviors. Neuropsychopharmacology 32:2173–2183. [DOI] [PubMed] [Google Scholar]
- Hedgepeth CM, Conrad LJ, Zhang J, Huang HC, Lee VM, Klein PS. (1997). Activation of the Wnt signaling pathway: a molecular mechanism for lithium action. Dev Biol 185:82–91. [DOI] [PubMed] [Google Scholar]
- Hirabayashi Y, Itoh Y, Tabata H, Nakajima K, Akiyama T, Masuyama N, Gotoh Y. (2004). The Wnt/beta-catenin pathway directs neuronal differentiation of cortical neural precursor cells. Development 131:2791–2801. [DOI] [PubMed] [Google Scholar]
- Huang GJ, Herbert J. (2005). The role of 5-HT1A receptors in the proliferation and survival of progenitor cells in the dentate gyrus of the adult hippocampus and their regulation by corticoids. Neuroscience 135:803–813. [DOI] [PubMed] [Google Scholar]
- Hughes K, Nikolakaki E, Plyte SE, Totty NF, Woodgett JR. (1993). Modulation of the glycogen synthase kinase-3 family by tyrosine phosphorylation. EMBO J 12:803–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inkster B, Nichols TE, Saemann PG, Auer DP, Holsboer F, Muglia P, Matthews PM. (2009). Association of GSK3beta polymorphisms with brain structural changes in major depressive disorder. Arch Gen Psychiatry 66:721–728. [DOI] [PubMed] [Google Scholar]
- Kaidanovich-Beilin O, Milman A, Weizman A, Pick CG, Eldar-Finkelman H. (2004). Rapid antidepressive-like activity of specific glycogen synthase kinase-3 inhibitor and its effect on beta-catenin in mouse hippocampus. Biol Psychiatry 55:781–784. [DOI] [PubMed] [Google Scholar]
- Karege F, Perroud N, Burkhardt S, Fernandez R, Ballmann E, La Harpe R, Malafosse A. (2012). Protein levels of beta-catenin and activation state of glycogen synthase kinase-3beta in major depression. A study with postmortem prefrontal cortex. J Affect Disord 136:185–188. [DOI] [PubMed] [Google Scholar]
- Karson CN, Newton JE, Livingston R, Jolly JB, Cooper TB, Sprigg J, Komoroski RA. (1993). Human brain fluoxetine concentrations. J Neuropsychiatry Clin Neurosci 5:322–329. [DOI] [PubMed] [Google Scholar]
- Kim H, Wu J, Ye S, Tai CI, Zhou X, Yan H, Li P, Pera M, Ying QL. (2013). Modulation of beta-catenin function maintains mouse epiblast stem cell and human embryonic stem cell self-renewal. Nat Commun 4:2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama M, Fujioka T, Duman RS. (2004). Chronic olanzapine or fluoxetine administration increases cell proliferation in hippocampus and prefrontal cortex of adult rat. Biol Psychiatry 56:570–580. [DOI] [PubMed] [Google Scholar]
- Komoroski RA, Newton JE, Cardwell D, Sprigg J, Pearce J, Karson CN. (1994). In vivo 19F spin relaxation and localized spectroscopy of fluoxetine in human brain. Magn Reson Med 31:204–211. [DOI] [PubMed] [Google Scholar]
- Li X, Zhu W, Roh MS, Friedman AB, Rosborough K, Jope RS. (2004). In vivo regulation of glycogen synthase kinase-3beta (GSK3beta) by serotonergic activity in mouse brain. Neuropsychopharmacology 29:1426–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie DC, Colamarino SA, Song HJ, Desire L, Mira H, Consiglio A, Lein ES, Jessberger S, Lansford H, Dearie AR, Gage FH. (2005). Wnt signaling regulates adult hippocampal neurogenesis. Nature 437:1370–1375. [DOI] [PubMed] [Google Scholar]
- Liu R, Dang W, Jianting M, Su C, Wang H, Chen Y, Tan Q. (2012). Citalopram alleviates chronic stress induced depression-like behaviors in rats by activating GSK3beta signaling in dorsal hippocampus. Brain Res 1467:10–17. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. (2004). The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 20:781–810. [DOI] [PubMed] [Google Scholar]
- Maguschak KA, Ressler KJ. (2011). Wnt signaling in amygdala-dependent learning and memory. J Neurosci 31:13057–13067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguschak KA, Ressler KJ. (2012). The dynamic role of beta-catenin in synaptic plasticity. Neuropharmacology 62:78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg JE. (2004). Implications of adult hippocampal neurogenesis in antidepressant action. J Psychiatry Neurosci 29:196–205. [PMC free article] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. (2000). Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci 20:9104–9110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y, Ge X, Frank CL, Madison JM, Koehler AN, Doud MK, Tassa C, Berry EM, Soda T, Singh KK, Biechele T, Petryshen TL, Moon RT, Haggarty SJ, Tsai LH. (2009). Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3beta/beta-catenin signaling. Cell 136:1017–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado-Gomez O, Hernandez-Fonseca K, Villavicencio-Queijeiro A, Massieu L, Chimal-Monroy J, Arias C. (2008). Inhibition of Wnt and PI3K signaling modulates GSK-3beta activity and induces morphological changes in cortical neurons: role of tau phosphorylation. Neurochem Res 33:1599–1609. [DOI] [PubMed] [Google Scholar]
- Morales-Garcia JA, Luna-Medina R, Alonso-Gil S, Sanz-Sancristobal M, Palomo V, Gil C, Santos A, Martinez A, Perez-Castillo A. (2012). Glycogen synthase kinase 3 inhibition promotes adult hippocampal neurogenesis in vitro and in vivo . ACS Chem Neurosci 3:963–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morshead CM, Craig CG, van KD. (1998). In vivo clonal analyses reveal the properties of endogenous neural stem cell proliferation in the adult mammalian forebrain. Development 125:2251–2261. [DOI] [PubMed] [Google Scholar]
- Murphy M, Drago J, Bartlett PF. (1990). Fibroblast growth factor stimulates the proliferation and differentiation of neural precursor cells in vitro . J Neurosci Res 25:463–475. [DOI] [PubMed] [Google Scholar]
- Novak A, Dedhar S. (1999). Signaling through beta-catenin and Lef/Tcf. Cell Mol Life Sci 56:523–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohira K, Takeuchi R, Shoji H, Miyakawa T. (2013). Fluoxetine-induced cortical adult neurogenesis. Neuropsychopharmacology 38:909–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H, Voleti B, Banasr M, Sarhan M, Duric V, Girgenti MJ, Dileone RJ, Newton SS, Duman RS. (2010). Wnt2 expression and signaling is increased by different classes of antidepressant treatments. Biol Psychiatry 68:521–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng CH, Chiou SH, Chen SJ, Chou YC, Ku HH, Cheng CK, Yen CJ, Tsai TH, Chang YL, Kao CL. (2008). Neuroprotection by Imipramine against lipopolysaccharide-induced apoptosis in hippocampus-derived neural stem cells mediated by activation of BDNF and the MAPK pathway. Eur Neuropsychopharmacol 18:128–140. [DOI] [PubMed] [Google Scholar]
- Polter AM, Yang S, Jope RS, Li X. (2012). Functional significance of glycogen synthase kinase-3 regulation by serotonin. Cell Signal 24:265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. (1992). Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 255:1707–1710. [DOI] [PubMed] [Google Scholar]
- Sairanen M, Lucas G, Ernfors P, Castren M, Castren E. (2005). Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J Neurosci 25:1089–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sairanen M, O LO, Knuuttila JE, Castren E. (2007). Chronic antidepressant treatment selectively increases expression of plasticity-related proteins in the hippocampus and medial prefrontal cortex of the rat. Neuroscience 144:368–374. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. (2003). Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301:805–809. [DOI] [PubMed] [Google Scholar]
- Seitz R, Hackl S, Seibuchner T, Tamm ER, Ohlmann A. (2010). Norrin mediates neuroprotective effects on retinal ganglion cells via activation of the Wnt/beta-catenin signaling pathway and the induction of neuroprotective growth factors in Muller cells. J Neurosci 30:5998–6010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss WL, Unis AS, Cowan C, Dawson G, Dager SR. (2002). Fluorine magnetic resonance spectroscopy measurement of brain fluvoxamine and fluoxetine in pediatric patients treated for pervasive developmental disorders. Am J Psych 159:755–760. [DOI] [PubMed] [Google Scholar]
- Taliaz D, Stall N, Dar DE, Zangen A. (2010). Knockdown of brain-derived neurotrophic factor in specific brain sites precipitates behaviors associated with depression and reduces neurogenesis. Mol Psychiatry 15:80–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M, Villaescusa JC, Luo SX, Guitarte C, Lei S, Miyamoto Y, Taketo MM, Arenas E, Huang EJ. (2010). Interactions of Wnt/beta-catenin signaling and sonic hedgehog regulate the neurogenesis of ventral midbrain dopamine neurons. J Neurosci 30:9280–9291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibaut R, Porte C. (2008). Effects of fibrates, anti-inflammatory drugs and antidepressants in the fish hepatoma cell line PLHC-1: cytotoxicity and interactions with cytochrome P450 1A. Toxicol In Vitro 22:1128–1135. [DOI] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. (2002). Functional neurogenesis in the adult hippocampus. Nature 415:1030–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QM, Fiol CJ, DePaoli-Roach AA, Roach PJ. (1994). Glycogen synthase kinase-3 beta is a dual specificity kinase differentially regulated by tyrosine and serine/threonine phosphorylation. J Biol Chem 269:14566–14574. [PubMed] [Google Scholar]
- Wang YX, Zhang XR, Zhang ZJ, Li L, Xi GJ, Wu D, Wang YJ. (2014). Protein kinase Mzeta is involved in the modulatory effect of fluoxetine on hippocampal neurogenesis in vitro . Int J Neuropsychop 17:1429–1441. [DOI] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Duman RS. (2007). VEGF is an essential mediator of the neurogenic and behavioral actions of antidepressants. Proc Natl Acad Sci USA 104:4647–4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler EM, Geschwind DH, Palmer TD. (2008). Lithium regulates adult hippocampal progenitor development through canonical Wnt pathway activation. Mol Psychiatry 13:285–292. [DOI] [PubMed] [Google Scholar]
- Xi G, Zhang X, Zhang L, Sui Y, Hui J, Liu S, Wang Y, Li L, Zhang Z. (2011). Fluoxetine attenuates the inhibitory effect of glucocorticoid hormones on neurogenesis in vitro via a two-pore domain potassium channel, TREK-1. Psychopharmacology (Berl) 214:747–759. [DOI] [PubMed] [Google Scholar]
- Xi G, Hu P, Qu C, Qiu S, Tong C, Ying QL. (2013). Induced neural stem cells generated from rat fibroblasts. Genomics Proteomics Bioinformatics 11:312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Gaspard JP, Chung DC. (2001). Regulation of vascular endothelial growth factor by the Wnt and K-ras pathways in colonic neoplasia. Cancer Res 61:6050–6054. [PubMed] [Google Scholar]
- Zusso M, Debetto P, Guidolin D, Barbierato M, Manev H, Giusti P. (2008). Fluoxetine-induced proliferation and differentiation of neural progenitor cells isolated from rat postnatal cerebellum. Biochem Pharmacol 76:391–403. [DOI] [PubMed] [Google Scholar]