Abstract
The use of bone marrow derived mesenchymal stromal cells (BMSC) in the treatment of alloimmune and autoimmune conditions has generated much interest, yet an understanding of the therapeutic mechanism remains elusive. We therefore explored immune modulation by a clinical-grade BMSC product in a model of human-into-mouse xenogeneic GVHD (x-GVHD) mediated by human CD4+ Th1 cells. BMSC reversed established, lethal x-GVHD through marked inhibition of Th1 cell effector function. Gene marking studies indicated BMSC engraftment was limited to the lung; further, there was no increase in regulatory T cells, thereby suggesting a paracrine mechanism of BMSC action. BMSC recipients had increased serum CD73 expressing exosomes that promoted adenosine accumulation ex vivo. Importantly, immune modulation mediated by BMSC was fully abrogated by pharmacologic therapy with an adenosine A2A receptor antagonist. To investigate the potential clinical relevance of these mechanistic findings, patient serum samples collected pre- and post-BMSC treatment were studied for exosome content: CD73 expressing exosomes promoting adenosine accumulation were detected in post-BMSC samples. In conclusion, BMSC effectively modulate experimental GVHD through a paracrine mechanism that promotes adenosine-based immune suppression.
Keywords: MSC, T cells, x-GVHD, Adenosine
Introduction
Bone marrow derived mesenchymal stromal cells (BMSC) have been widely investigated for their therapeutic potential in both autoimmune and alloimmune diseases [1, 2, 3]. Murine BMSC, which clearly have an ability to modulate immune responses during experimental inflammatory conditions, may operate by several proposed mechanisms [4]. By comparison the successful clinical translation of BMSC therapy is still in question [5, 6] and the mechanism of action remains largely unknown [7], representing an obstacle to further clinical applications of BMSC.
Intravenous infusions of human BMSC are immediately sequestered in the lung microvasculature and their therapeutic effect in corneal injury and myocardial infarction has been attributed to paracrine secretory factors such as IL-10 [8] and TSG-6 [9]. These mechanisms, however, do not explain the suppression of adaptive immunity observed when BMSC are used to treat alloimmunity.
Murine data suggests that dampening of the T cell response by BMSC is due to the production of immune-modulatory factors [such as nitric oxide (NO) [10], indoleamine 2,3 dioxygenase (IDO) [11], prostaglandin 2 (PGE2) [12] or through T cell apoptosis via Fas-FasL mechanism [13]. In humans, BMSC produce adenosine on contact with T cells [14], which may cause increased T cell apoptosis. Most of these observations imply BMSC cell-cell contact with alloreactive T cells during GVHD. Such mechanisms are therefore in direct conflict with the observation that adoptively transferred human BMSC can elicit their immunomodulatory action despite being entrapped in the lung vasculature.
To reconcile these issues we evaluated BMSC clinical products recently tested in a pilot clinical trial of BMSC infusion in GVHD and post allogeneic stem cell transplantation tissue injury (NCT01633229) [15]. We used a human-into-mouse xenogeneic graft-versus-host disease (x-GVHD) model caused by CD4+ Th1 cells [16, 17], which are critical for mediation of human alloimmune and autoimmune diseases. Here we describe an adenosine based mechanism by which adoptively transferred BMSC cause systemic immune modulation during ongoing experimental GVHD and find that a similar mechanism may be operational in post transplant recipients receiving adoptive therapy with BMSC in a clinical trial.
Materials and Methods
Mice
Female immune-deficient murine host NODLtSz-scidIL2Rγnull mice (NSG) were obtained from Jackson Laboratory and utilized for humanized GVHD experiments. Experiments were performed according to a protocol approved by the NCI Animal Care and Use Committee. Mice were housed in a sterile facility and received sterile water and pellets. NSG hosts did not undergo conditioning prior to human cell transfer.
Antibodies and Reagents
X-VIVO 20 media was obtained from BioWhitaker and AB serum was from Gem Cell, alphaMEM was from Lonza. CD4 microbeads were from Miltenyi Biotec. Anti-CD3 (clone:OKT3) and anti-CD28 (clone: CD28.6) antibodies were from eBioscience. Recombinant human (rh) IL-2 and IL-12 were from PeproTech. All other antibodies (unless otherwise stated) were purchased from BD Biosciences; anti-human FOXP3 PE was from Biolegend. Formaldehyde and glutaraldehyde for electron microscopy was obtained from Tousimis, uranyl acetate from Electron Microscopy Sciences, oxalic acid adenosine and methylcellulose from Sigma. 13C5-Adenosine is from Cambridge Isotope Laboratories.
Human T cell and BMSC culture
Normal donor peripheral blood cells were collected by apheresis on an IRB-approved protocol (04-C-0055). Total lymphocytes were isolated by elutriation [18] and human CD4+ T cells were isolated with Miltenyi Beads according to the manufacturer's recommendation. Enriched CD4+ T cells were differentiated and expanded for 6 days in Th1 culture conditions prior to being used in both in vitro and in vivo experiments. Briefly, human effector CD4+ cells were differentiated in the presence of plate coated α-CD3 (5μg/ml) and αCD28 (2μg/ml). Soluble rhIL2 (20IU/ml), anti-IL-4 (100ng/ml), rhIL12 (20ng/ml) was added every two days during the 6 day culture protocol. At day 6, cells were harvested, washed once with X-VIVO media and then characterized for Th1 cell chemokine expression, transcription factor expression and cytokine profile by flow cytometry. Differentiated CD4+ Th1 cells expressed >80% Tbet, were CXCR3+ and had significant IFN-γ and TNF-α expression post differentiation.
Human clinical grade BMSC at Passage 3 was obtained from the Department of Transfusion Medicine, NIH under an IRB approved protocol (NCT01071577). BMSC were then expanded in AlphaMEM which was supplemented with 20% FBS for 5 days. Characterization and clinical efficacy of these BMSC has been previously reported[19][15]. Differentiated BMSC were characterized for lineage markers by flow cytometry and were CD45−, CD90+, CD73+, and CD105+.
Xenogeneic GVHD model
Xenogeneic GVHD experiments were set up by adoptive transfer of 5 million human Th1 cells together with 3 million allogeneic human monocytes into immune-deficient NSG mice. Murine recipients were allowed to develop chronic x-GVHD as previously demonstrated [16, 17, 20]. After 20-25 days, when the murine recipients had greater than 10% human Th1 cells in the peripheral blood and showed >50% loss in body hair, either 2 million irradiated monocytes or BMSC were adoptively transferred. Mice were treated 3 times; each treatment was separated by four days. Clinical weight loss, histopathology, and immunology were monitored following treatment. In preliminary experiments, BMSC were administered at a dose of 0.5 million and 1 million per mouse. At this dose, BMSC dosage were found to be ineffective. In certain experiments, cohorts treated with BMSC also received a daily dose of the A2aR antagonist ZM241385 (Tocris; 1.5mg/kg/day) via i.p injections. The number of mice used in each experiment was 5 per cohort unless otherwise specified. The frequency of T cells that were IFN-γ+, TNF-α+ and FoxP3+ were calculated from the human CD45+ population. Absolute numbers were calculated from the spleen and GVHD target organs as follows: Absolute numbers of human CD45+ T cells were calculated using the %hCD45+ T cells from total splenocytes. Absolute numbers of human IFNγ+, TNFα+, FoxP3+ and CD39+ T cells was calculated using the % IFN-γ+, TNF-α+, FoxP3+, and CD39+ T cells from total human CD45+ T cell numbers.
Isolation of T Lymphocytes from GVHD target organs
Lymphocytes were extracted from the skin as previously described [21]. Briefly, a small piece of dorsal skin was removed, minced and incubated with serum free media containing Liberase TL (250 μg/ml; Roche) and 0.05% DNase I (Roche) for 105 min at 37°C. A single cell suspension was prepared by mashing the skin tissue slurry through a 100μm nylon mesh. Cells were sequentially filtered through 70 and 40μm nylon filters [21]. Lung and liver infiltrating lymphocytes were extracted as follows: lung and liver were removed, minced, and digested in serum free media containing Liberase TL (250μg/ml; Roche) and 0.05% DNase I (Roche) for 30 and 15min respectively. Lung and liver infiltrating lymphocytes were separated by centrifugation in 40% Percoll and collected in a cell pellet.
Flow cytometry
Th1 cells were washed with PBS supplemented with 0.1% BSA and 0.01% sodium azide, and stained using CD4 APC (clone: RPA-T4), CD39 PerCP-eFluor 710 or FITC (clone: eBio(A1) from eBioscience), and CD45 PE/FITC/PE-Cy7 (clone: 2D1; H130). For assessment of cell death, Th1 cells were stained for surface markers (CD4 PE and CD39 FITC), re-suspended in Annexin V buffer, and stained with Annexin V APC and 7AAD according to manufacturer's instructions. For in vivo monitoring of human Th1 cells in the spleen, cells were stained with human CD45 PE-Cy7, CD4 Pacific Blue, and CD39 PerCP-eFluor710. For intra-cellular (IC) flow cytometry, fixation and permeabilization buffer was utilized (eBioscience). IC flow cytometry was performed with combinations of CD45 FITC, CD4 Pacific Blue, CD39 PerCP-eFluor 710, and FoxP3 PE (clone 249D). For IC cytokine flow cytometry, human Th1 cells from spleen, bone marrow, lung, liver, and skin were stimulated with PMA (50ng/ml) and ionomycin (0.5mg/ml) for 4hrs together with Golgiplug and Golgistop (BD Biosciences) for the final 2hrs. Cells were subsequently fixed and stained with CD45 PE-Cy7, CD4 Pacific Blue, CD39 PerCP-eFluor 710, IL-2 FITC (clone: MQ1-17H12), IFN-γ PE (clone: 45.B3), TNF-α APC (clone: MAB11; eBioscience). Cells after staining were examined on an LSRII (Becton Dickinson) and data analyzed using FlowJo 9.7.6 (Treestar).
Measurements of BMSC ATPase Activity
BMSC were co-cultured with human Th1 cells at a ratio of 1:1 in the presence of ATP (100μM; Sigma-Aldrich) for 2 hrs and the supernatant was collected. In certain conditions, CD73 inhibitor adenosine 5’-(α-β-methylene) diphosphate (Sigma-Aldrich) was added at a concentration of 100μM for 15mins prior to the addition of ATP. The co-culture was performed in a 24 well tissue culture plate (Corning). Th1 cells and BMSC were added at a concentration of 1 million cells per ml. For control conditions a single cell type was used (i.e., Th1 cells or BMSC alone), the cell concentration was maintained at 1 million/ml. Similarly, for inhibitor conditions, 1 million Th1 cells and 1 million BMSC were used. Release of phosphate from ATP was measured in the supernatant as previously described [22] using the Malachite Green Phosphate detection assay (R&D systems). In some experiments, supernatants were frozen (−80°C) pending analysis utilizing a modified version of a published GC-MS/MS methodology [23]. Adenosine in the supernatants was measured using GC-MS/MS as follows: solid phase extraction of adenosine was performed on the cell media, followed by automated on-line methylation (derivatization) and quantitation using an Agilent GC-MS/MS system (GC Model 7890A; Triple Quad MS Model 7000A). The extraction provided high recovery of adenosine from the cell media, and the GC-MS/MS method utilized calibration standards and an internal standard (13C5-adenosine).
Isolation of Exosomes
Exosomes were isolated from murine recipients treated or untreated with BMSC. Plasma was collected from consenting patients who underwent treatment at the NIH under an approved clinical protocol (NHLBI protocol #12-H-0010). Total exosomes were isolated from the serum or plasma using the Total Exosome Isolation kit from Invitrogen as per the manufacturer's recommendation. Human exosomes were then enriched using the human CD63 exosome enrichment kit (Invitrogen). Isolated exosomes were characterized by electron microscopy and by flow cytometry. The function of the exosomes was measured by their ability to produce phosphate in the presence of AMP. Exosomes were incubated with AMP for 2 hrs at 37°C in a 96 well round bottom plate (Corning) and the phosphate content in the supernatant was measured using the malachite green phosphate detection assay.
Transwell assay for exosome mediated adenosine production
BMSC from passage 3 were expanded for 5 days to achieve passage 4 and then re-plated in 6 well tissue culture plates in serum free medium at a concentration of 1 million per ml. Exosome secretion inhibitor GW4869 was added at a concentration of 10μM. BMSC were incubated overnight with GW4869 (Santa Cruz Biotechnology), washed and then tested for the inhibition of exosome secretion using the exosome ELISA kit from Systems Biosciences. Briefly, supernatants were collected from BMSC cultures that were either treated with DMSO or GW4869. Exosome enrichment and protein isolation was carried out as per the manufacturer's instructions. The enriched protein was then analyzed for CD81 expression by ELISA. BMSC that were either treated with DMSO or GW4869 were then utilized for transwell assays. Human Th1 cells (0.5 million per ml) were placed at the bottom of the transwell and the upper chambers consisted of BMSC or BMSC pretreated with GW4869 (0.5 million per ml). ATP was added to the culture and the supernatant was collected after 4 hrs. Adenosine production was measured in the supernatant by GC-MS/MS.
Electron Microscopy
Electron microscopy of the exosomes was performed as previously described [24]. Briefly, exosomes from serum were fixed with 2% paraformaldehyde, adsorbed to carbon film grids, fixed with 1% glutaraldehyde, washed with water, and then stained with uranyl-oxalate. The samples were then embedded in a mixture of 9:1 of 2% methylcellulose and 4% uranyl acetate. The embedded samples were then analyzed by electron microscopy.
Transduction of human Th1 and BMSC with LeGO vectors
Transduction of cells was carried out in RetroNectin (Takara) treated 12-wells plates as previously described [25]. Th1 cells and BMSC were respectively transduced with LeGO-T2, encoding tdTomato, and with LeGO-V2 encoding Venus. Cells were seeded at 2×105 cells per well and transduced at a MOI of 20 in a final volume of 1mL of culture media supplemented with protamine sulfate (Sigma-Aldrich). After 6 hrs of incubation at 37°C/5% CO2, 1 mL of media was added to the cells. Transduction efficiency was assessed 4 days later by flow cytometry.
Two Photon/Confocal Microscopy
Microscopy was carried out using a Leica TCS SP5-AOBS 5-channel confocal and multi-photon system (Leica Microsystems) and an HCX-IRAPO-L 25X/0.95 NA water-dipping objective (WD 2.5 mm), as previously described [25]. Second Harmonic Generation (920nm) was used to detect organ structures by 2-photon microscopy. Confocal microscopy was used to detect Venus (514 nm excitation, 523-558 nm detection) and tdTomato (561nm excitation, 579-597 nm detection). Imaris software (Bitplane) was used to reconstruct and visualize three-dimensional images from acquired tiled stacks of the different organs (lungs, spleen, and liver).
Histopathology
Lung, liver, and skin from NSG recipients were fixed in 10% (vol/vol) formyl saline and were embedded on paraffin blocks. Tissue sections were stained with eosin and hematoxylin and GVHD scoring was performed as previously described [16]. A detailed description of the histology score is explained in Supplementary Table 1.
Statistical Analysis
Flow cytometry and cytokine data were analyzed using student's 2-tailed t tests. Comparison values of p ≤ 0.05 were considered statistically significant.
Results
BMSC administration reverses clinical symptoms of established severe x-GVHD
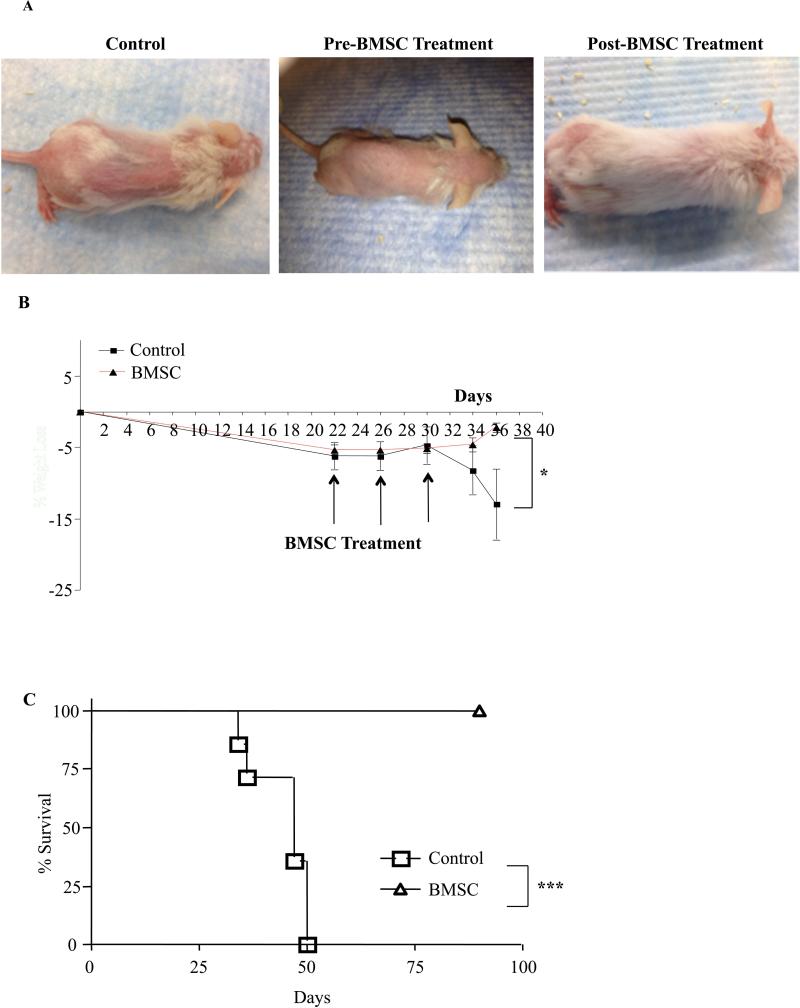
Murine recipients of human Th1 cells developed severe x-GVHD manifesting as loss of hair (Fig. 1A) and loss of weight (Fig. 1B). Multiple adoptive transfers of human BMSC at day 22 post Th1 cell infusion when x-GVHD was fully established, reversed clinical signs of cutaneous GVHD (Fig. 1A) together with a reversal of weight loss (Fig. 1B). Two months post transplant all the animals that received human Th1 cells alone had died (Fig. 1C). In marked contrast, recipients of human Th1 cells and multiple human BMSC infusions, remained healthy for 100 days post-BMSC infusion (Fig. 1C). Murine recipients of human Th1 cells, in addition to exhibiting clinical symptoms of x-GVHD also had profound changes in the histopathology of the GVHD target organs liver and skin. A significant decrease in mononuclear cell infiltration in the lungs was noted in the human BMSC treated cohorts as compared to the control animals (Supplemental Fig. 1A&B). In the liver and skin, BMSC treatment resulted in diminished periductal infiltration of mononuclear cells (Supplemental Fig. 1C&D). The skin of the control mice showed acanthosis and significant thickening of the epidermis. In the BMSC treated mice, skin acanthosis was reduced along with minimal thickening of the epidermis layer. (Supplemental Fig. 1E&F).
Figure 1. Bone Marrow Derived Mesenchymal Stromal Cells Can Treat Human X-GVHD In Mice.
Female NSG mice were reconstituted with human Th1 cells (5 million) and human monocytes (3 million). Between day 20 and day 35 post-transplant, mice developed severe x-GVHD (A; left and middle panel). Within 24 hrs of GVHD onset, mice were treated three times with BMSC (2 million) and each injection was separated by four days. Photographs from a representative mouse from each group are shown (A; right panel). Summary of percent weight loss, from three experiments, in control and treated cohorts (B). Cumulative kaplain-meier survival curve from three experiments (C). For weight loss, P value was determined using a two-way anova test. For survival curve, P values were determined using log-rank test. Each experiment had n=5 animals per cohort, experiments were performed in triplicate with 3 different BMSC clinical products. *=P<0.05, **=P<0.01 and ***=P<0.001.
Reversal of x-GVHD by BMSC was associated with a reduction in Th1 cell numbers
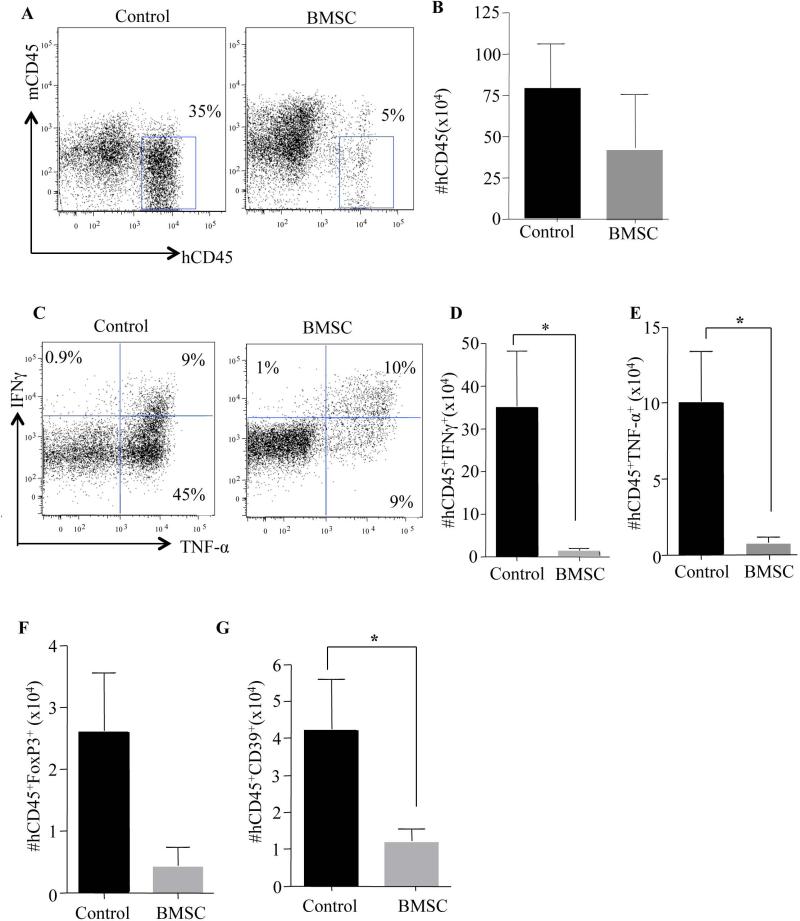
We next investigated the effect of BMSC transfusions on the transferred human Th1 cell population. After the third BMSC transfusion, treated and control animals were compared. BMSC recipients had a significant decrease in the frequency of human Th1 cells that engrafted in the spleen (Fig. 2A), although the absolute number of splenic human Th1 cells was not substantially reduced (Fig. 2B). Human Th1 cells in BMSC recipients were immune suppressed, as indicated by reduced potential for IFN-γ and TNF-α production (frequency analysis, Fig. 2C; absolute number data, Figs. 2D & 2E). In the bone marrow (BM), BMSC recipients had a reduction in both absolute human T cell numbers (Supplementary Fig. 2A) and absolute number of cytokine secreting human Th1 cells (Supplementary Fig. 2B&C). Furthermore, there was a reduction in absolute human T cell number and cytokine-secreting human Th1 cell number in the GVHD target organs of lung, liver, and skin (Supplementary Figure 3). In summary, these data demonstrate that BMSC modulate effector human Th1 cells in vivo through both reduction in Th1 cell number and Th1 cell function, thereby providing a means of characterizing the mechanism of action of clinical BMSC products.
Figure 2. BMSC Treatment Decreased Human Th1 cell Engraftment, Effector Cytokine Production With A Concomitant Reduction In FoxP3+ Tregs.
Female NSG mice were reconstituted with human Th1 cells (5 million) and Monocytes (3 million). Within 24 hrs of GVHD onset (between day 20 to day 35), the BMSC cohort was treated 3 times, each injection separated by four days. Post-treatment, the mice were euthanized and the lymphoid organs such as spleen were evaluated for human Th1 cell engraftment. Representative flow plot showing human CD45+ cells versus mouse CD45+ cells (A). Absolute numbers of human CD45+ Th1 cells were calculated from total splenocyte number (B). Representative flow cytometry plots of IFNγ and TNF-α expression determined in the recovered human Th1 cells by intracellular cytokine staining (C). Cumulative absolute numbers of IFNγ producing human Th1 cells (D) and TNF-α producing human Th1 cells in the spleen (E). Absolute number of FoxP3+ human Tregs determined by intracellular staining (F). Absolute numbers of human CD39+ Th1 cells in the spleen (G). Absolute numbers of cytokine producing cells, T-regs and CD39+ Th1 cells were obtained from the absolute numbers of hCD45+ Th1 cells in the spleen. Each experiment had n=5 animals per cohort, experiments were performed in triplicates with 3 different BMSC clinical products. The data shown are cumulative of all three experiments. *=P<0.05, **=P<0.01 and ***=P<0.001.
Many reports have attributed the beneficial effect of infused BMSC to the induction of regulatory T cells (T-regs) [26, 27]. We next investigated whether BMSC were able to convert human Th1 cells into FoxP3+ T-reg cells. In our model, BMSC recipients showed no increase in absolute numbers of T-regs in the spleen as determined by expression of FoxP3 (Fig. 2F). Since BMSC therapy did not appear to operate by a T-reg cell mechanism, we next evaluated whether BMSC might operate in vivo through an apoptotic mechanism involving adenosine pathway modulation, as has been suggested in previous in vitro studies involving BMSC [14]. Towards this aim, we identified that the absolute number of human Th1 cells expressing the ectonucleoside triphosphate diphosphohydrolase_1 (CD39; responsible for the catabolism of ATP to AMP and previously linked to pathogenic human Th1 cells) in the spleen was significantly reduced in BMSC recipients [28, 29] (Fig. 2G)
BMSC may abrogate established severe x-GVHD through adenosine signaling in Th1 cells
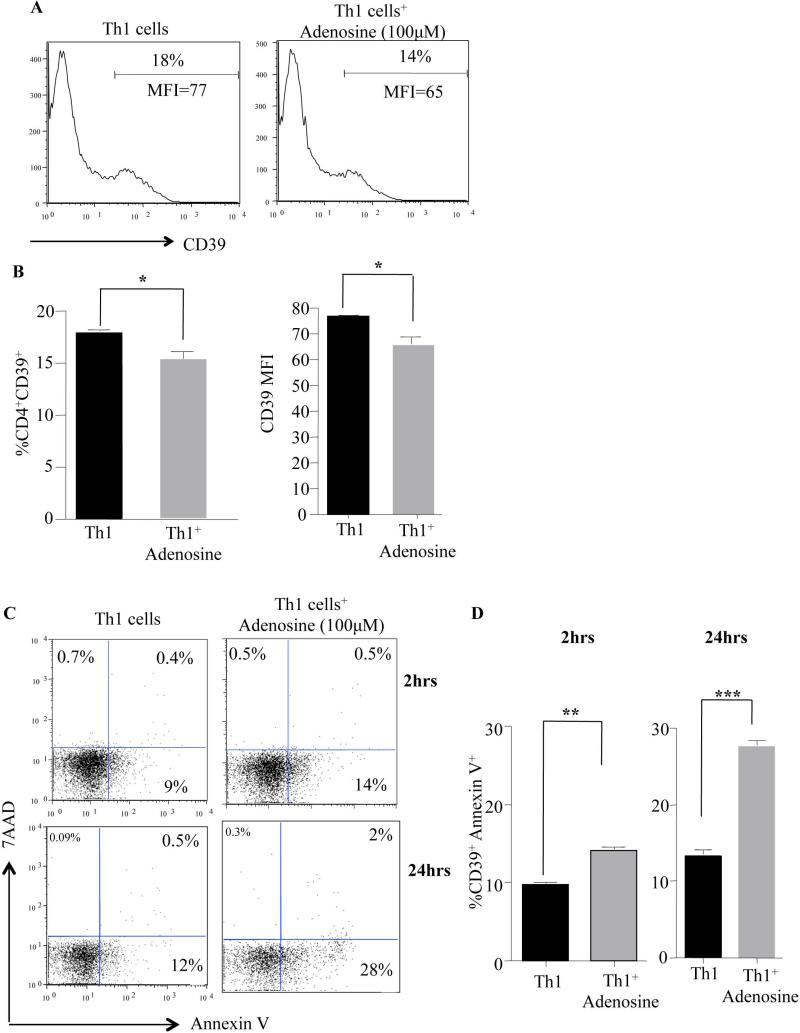
To further investigate this potential interaction between Th1 cell CD39 expression and the adenosine pathway, we next treated in vitro expanded human Th1 cells with adenosine. This nucleoside can be generated from ATP due to the combined effects of CD39 that converts ATP to AMP and the 5’ ectonucleotidase, CD73 that converts AMP to adenosine. Of note, previous reports have studied the roles of CD39 and CD73 in purinergenic signaling and immune suppression of helper T cells [30, 31] (reviewed in [32]). The addition of adenosine to the media of Th1 cells resulted in a significant decrease in CD39 expression after 2 hrs (Fig. 3A&B); along with a significant increase in CD39+ Th1 cell apoptosis (Fig. 3C upper panel & D left panel) and continued to increase further after 24 hrs of exposure. (Fig. 3C lower panel & D right panel). In summary, pathogenic Th1 cells that express CD39 specifically undergo apoptosis in the presence of adenosine.
Figure 3. Th1 Cells Exposed To Adenosine Show Significant Reduction In CD39 Expression And Enhanced Apoptosis.
Human CD4+ T cells were expanded in Th1 conditions for 6 days. Th1 cells were washed in media and then dispensed into 24 well tissue culture plates at a concentration of 1 million cells per well in 1 ml of media. Adenosine was added at a concentration of 100μM. Effect of adenosine on Th1 cell CD39 frequency and protein expression (A). Summary of CD39 frequency (B; left panel) and CD39 protein expression (B; right panel) in three different normal donors. Apoptosis of human Th1 cells in the presence of adenosine at 2hrs (C upper panels) and 24 hrs (C lower panels). Summary of Annexin V+ for three different normal donors at 2 hrs (D; left panel) and at 24 hrs (D; right panel). N=5 animals per cohort, experiments were repeated 3 times with 3 different BMSC clinical products. P values were obtained by performing a student t- test where *=P<0.05, **=P<0.01 and ***=P<0.001.
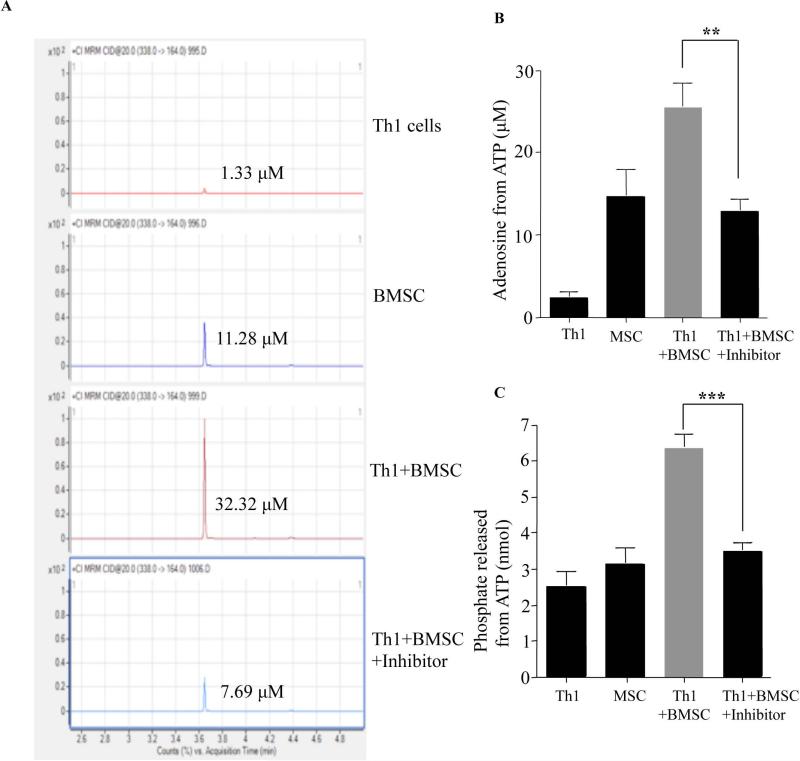
BMSC on co-culture with Th1 cells produce significant amounts of adenosine
Expanded BMSC, which were positive for CD73 (Supplementary Fig. 4A) and negative for CD39 expression by flow cytometry were co-cultured with polarized CD39+ Th1 cells in the presence of ATP and resultant adenosine production was measured by GC-MS/MS (representative example, Fig. 4A). The co-culture of Th1 cells with BMSC enhanced adenosine production from ATP in a CD73 dependent manner (Fig. 4B). BMSC addition to co-culture also increased phosphate levels, further supporting the contention that BMSC promoted the adenosine pathway (Fig. 4C). We next evaluated if co-culture of Th1 cells with BMSC had an impact on CD39 expression. Similar to our findings with adenosine in co-culture, flow cytometric analysis of gated CD4+ Th1 cells post co-culture had decreased CD39 expression (Supplementary Fig. 4B & C) and significantly enhanced apoptosis (Supplementary Fig. 4D & E). Taken together, these results support a model whereby the human BMSC population modulated human Th1 cells through an adenosine signaling pathway, which has been shown to be mediated via Th1 cell expression of the adenosine A2A receptor (Supplementary Fig. 4F).
Figure 4. BMSC Exposure Enhances Purine Metabolism In Human Th1 Cells In Vitro.
Human Th1 cells from 3 different donors were co-cultured with clinical grade BMSC from 3 different donors in the presence of ATP (100μM) for 2 hrs at a 1:1 ratio. Some culture conditions included the inhibitor for CD73 (Adenosine 5’-(α,β-methylene) diphosphate sodium; 100μM). The supernatants were harvested and adenosine measured by GC-MS/MS._Representative GC-MS/MS graphs (A) and cumulative adenosine production in the supernatant from three experiments (B). The co-culture was repeated in a phosphate free reaction buffer and the amount of phosphate released in the supernatant was measured using Malachite Green Phosphate detection assay (C). P values were obtained by performing a student t- test where *=P<0.05, **=P<0.01 and ***=P<0.001.
BMSC modulate adenosine signaling in human Th1 cells through a paracrine mechanism involving exosomes
The requirement for both CD39 and CD73 to convert ATP to adenosine would imply that the transfused BMSC would have to co-localize with their target Th1 cells in order to suppress them. To confirm this, we evaluated whether there was substantial accumulation of BMSC in the GVHD target organs to support such a mechanism. At 24 hrs following adoptive transfer, BMSC transduced with LV (Supplementary Fig. 5A) were detected by flow cytometry nearly exclusively in the lung (Supplementary Fig. 5B); consistent with prior studies [33, 34, 35]. Experiments using two-photon microscopy confirmed the flow cytometry findings (Supplementary Fig. 5C). Importantly, there was also significant_depletion of human Th1 cells within 24 hrs of BMSC infusion (Supplementary Fig. 5C). Yet BMSC engraftment was consistently negative in GVHD target organs such as the spleen, liver, lung, and skin at later points (72 hrs and 96 hrs) as monitored by flow cytometry.
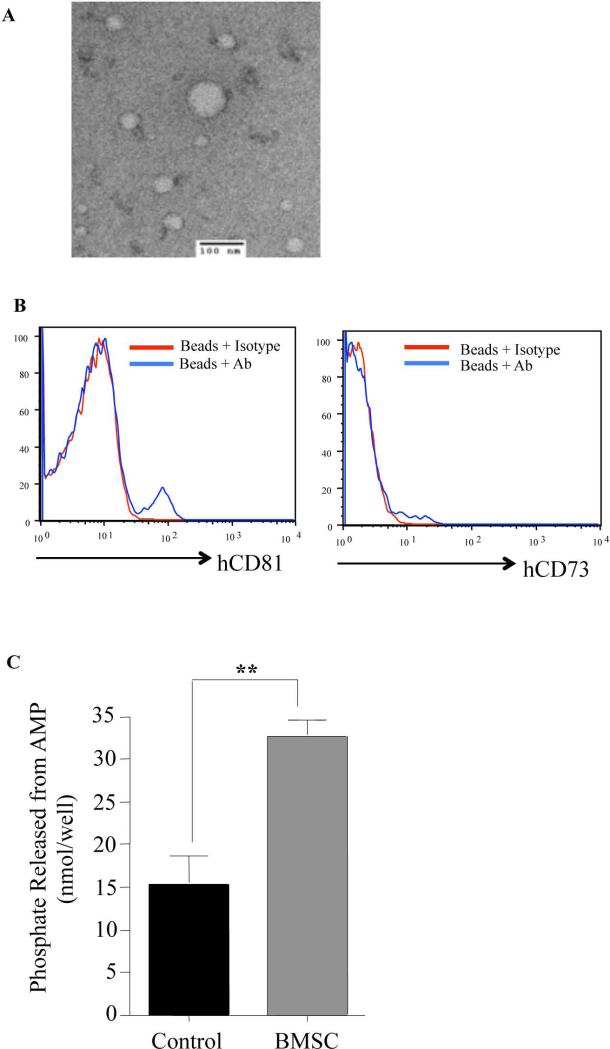
These data suggested that despite the requirement for CD39 and CD73 co-localization, transfused BMSC trapped in the lung were able to suppress their target T cells at a distance. Previous reports have noted that in murine models BMSC mediate tissue repair though the secretion of exosome particles [36, 37]. In order to demonstrate that BMSC derived exosomes generated adenosine from ATP, in vitro experiments were performed with BMSC that were treated with exosome blocker GW4869. Blocking of exosome secretion by BMSC was first confirmed by ELISA (Supplementary Fig.6A). On confirming exosome blocking, BMSC were incubated with human Th1 cells in a transwell plate for 4hrs in the presence of ATP. Adenosine production from ATP by untreated and treated BMSC were measured by GC/MS-MS (Supplementary Fig.6A). Next, we investigated if this phenomenon occurred in vivo on BMSC adoptive transfer. We found that exosomes were readily isolated from the serum of transplant recipients of BMSC (Fig. 5A); such exosomes expressed markers by flow cytometry consistent with a human origin (Fig. 5B), thereby facilitating a human exosome enrichment step. Such enriched exosomes expressed the exosome marker CD81, carried CD73 and were capable of metabolizing AMP (Fig. 5C). These data support a generalized systemic immune suppressive action of lung-restricted BMSC through the export of exosomes capable of adenosine generation.
Figure 5. In Vivo Administration Of Human BMSC Results In Enhanced Human Exosome Presence In The Serum Of Murine Recipients With x-GVHD.
Female NSG mice that had severe x-GVHD were treated with BMSC as previously described. Serum 24 hrs post BMSC infusion, was collected from control and treated mice. Exosomes isolated from the serum were visualized by electron microscopy (A). Flow cytometric analysis of enriched human exosomes (B). Functional phosphate production of exosomes from AMP was detected using the malachite green phosphate assay (C). Experiments were performed in duplicates with BMSC from 3 different donors. P values were obtained by performing a student t- test where *=P<0.05, **=P<0.01 and ***=P<0.001.
Blocking adenosine signaling in human Th1 cells abrogates BMSC effects during severe x-GVHD
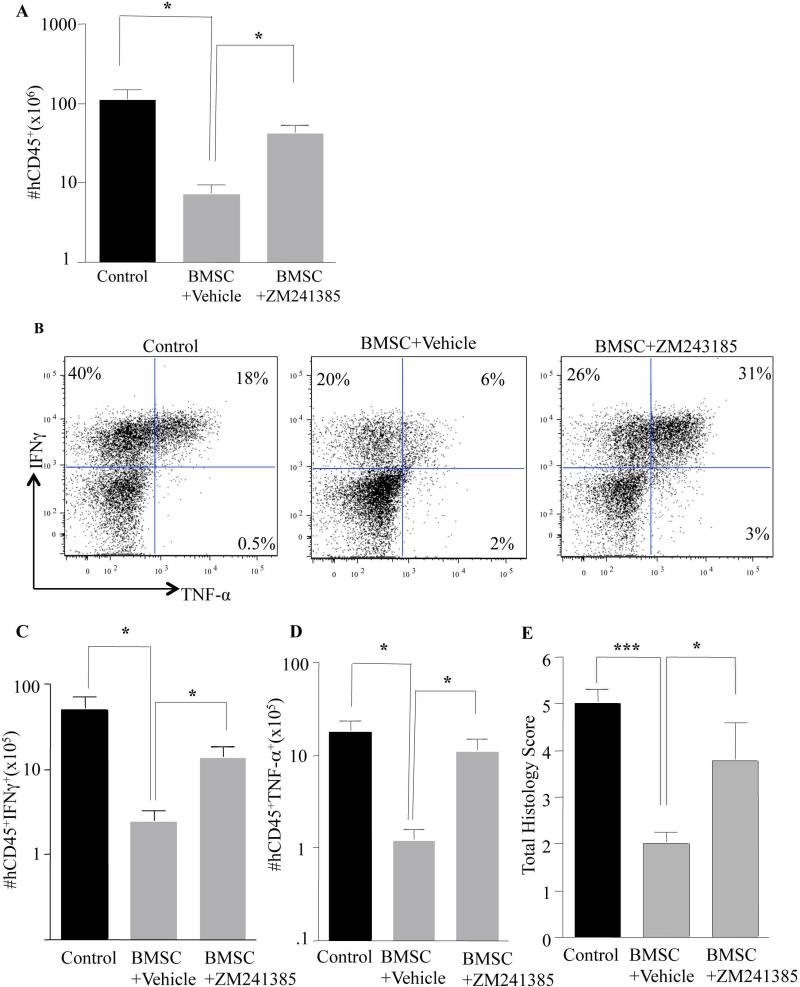
To test our model that the human BMSC modulated human Th1 cells via the adenosine A2A receptor, murine recipients of Th1 cells together with BMSC were treated with either vehicle control (DMSO) or an A2aR antagonist (ZM241385) during BMSC therapy. As hypothesized, pharmacologic therapy abrogated the ability of BMSC to reduce human Th1 cell engraftment (Fig. 6A) and inflammatory cytokine production (Fig. 6B, C & D). Pathological findings further confirmed these observations whereby blocking of adenosine signaling in vivo abrogated BMSC effects in murine recipients (Fig. 6E). These findings indicate that BMSC clinical products modulated pathogenic human Th1 cells through an adenosine pathway.
Figure 6. Inhibiting A2aR In Human Th1 Cells Abrogates The Effect Of BMSC In Vivo.
Female NSG mice were reconstituted with human Th1 cells (5 million) and Monocytes (3 million). Within 24hrs of GVHD onset (between day 20 to day 35), the BMSC cohort was treated 3 times with one injection of BMSC every four days. Some cohorts were treated by I.P. injections with the drug ZM241385 (1.5mg/kg/day) every day for the duration of BMSC treatment. Post-treatment, mice were euthanized and their spleen was evaluated for human Th1 cell engraftment (A). Human Th1 cells from the spleen were stimulated ex-vivo for 4 hrs with PMA/ionomycin along with GolgiStop and GolgiPlug for the last 2 hrs. Cells were then stained for Intracellular cytokine expression. Representative flow cytometry plots for intracellular IFNγ and TNF-α staining (B). Cumulative absolute numbers of IFNγ-producing human Th1 cells (C) and TNF-α-producing human Th1 cells in the spleen (D). Cumulative histological findings in the lung and liver of treated animals (E). Data was pooled from two different experiments using two different BMSC donors. P values were obtained by performing a one way anova where *=P<0.05, **=P<0.01 and ***=P<0.001.
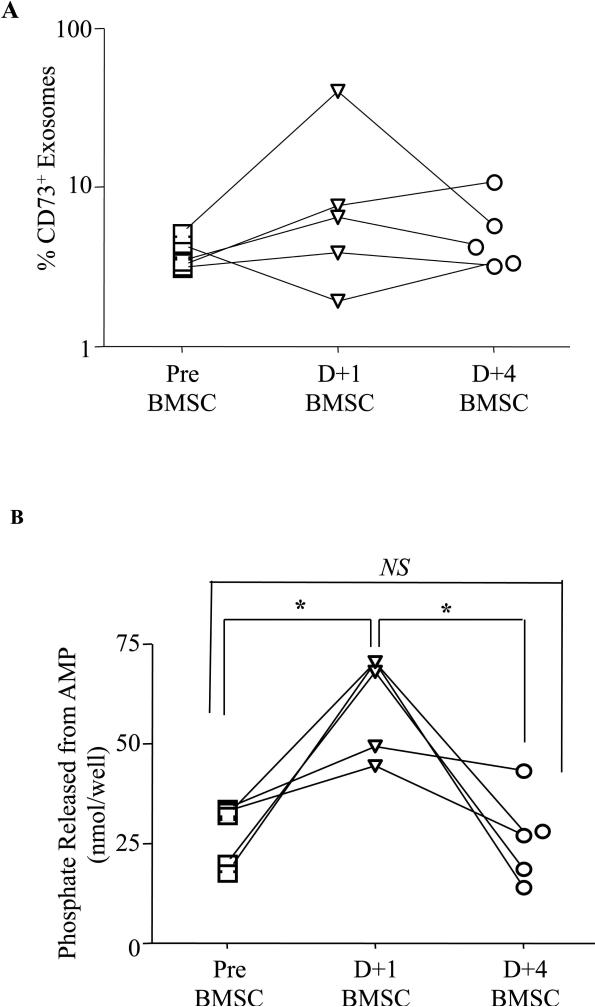
Adenosine producing exosomes are significantly increased in patients post BMSC therapy
We next evaluated if a similar mechanism might occur in humans receiving BMSC in a clinical trial. Patient plasma samples were collected pre-BMSC treatment, and then at day +1 and day +4 post-BMSC infusion. Plasma exosomes were isolated and characterized by flow cytometry (Supplementary Fig. 6C-D) and tested for functional phosphate production. An increase in CD73 expressing exosomes (Fig. 7A) was noted at day +1 post-BMSC treatment. Exosomes from day +1 post-BMSC produced significant amounts of adenosine as compared to samples collected pre-treatment or at day +4 post-BMSC treatment (Fig. 7B). No significant adenosine production was noted when pre-treatment and day +4 exosomes were compared. These results further suggest the need for BMSC reinfusions for sustained immunosuppression
Figure 7. Adenosine Generating Functional Exosomes Are Present In Patient Samples Post BMSC Treatment.
Plasma from patients who underwent BMSC treatment was collected at different time points. Exosomes were isolated and characterized by flow cytometry for the expression of CD73 (A). Functional phosphate production of exosomes from AMP was detected using the malachite green phosphate assay (B). P values were obtained by performing a paired student t- test *=P<0.05, **=P<0.01, ***=P<0.001 and ns=not significant
Discussion
This study is the first to demonstrate a distal mechanism of action of clinical grade BMSC in GVHD patients. Hitherto, despite numerous clinical trials using BMSC, data on the mechanism of action of adoptively transferred BMSC in man has been incomplete. In many BMSC clinical trials, patients do not receive regular infusions of BMSC, which could explain reported disparities in the clinical response. We found that adenosine producing exosomes increased within 24 hours after BMSC infusion but diminished by D+4 post BMSC therapy, indicating a short lived action of BMSC. This latter observation has direct clinical implication, as it indicates that subsequent clinical trials should evaluate more frequent BMSC infusions.
While adenosine metabolism has been proposed as an immunomodulatory mechanism of BMSC [14], this does not explain how BMSC encounter their cell targets. Our study is the first to address this mechanism in human Th1 cells in an in vivo model of humanized GVHD. We found a significant increase in CD39+ Th1 cells during severe x-GVHD. The CD39+ Th1 subset is believed to be a major T cell memory subset at sites of tissue inflammation [28]. It has been proposed that during inflammation, CD39 prevents the deleterious effects of ATP signaling through P2X7receptors [38,39], but a significant lack of CD73 in the microenvironment prevents the resolution of inflammation [28]. We found that adoptively transferred BMSC deliver CD73 to the target tissues via exosomes capable of producing adenosine. Blocking the A2aR receptor on xenoreactive Th1 cells with ZM241385, (one of the most widely used adenosine blocker for immunesuppression and tissue injury) [40, 41], reversed the effects of BMSC on the immunological phenotype of Th1 cells and the pathological manifestations of GVHD. These data thus demonstrate that BMSC use adenosine signaling as the central mechanism of GVHD protection in experimental x-GVHD. Our findings suggest that BMSC modulate both inflammation and tissue injury through adenosine signaling. A recent report on B cells has suggested a role for adenosine in mediating T cell immune suppression [42]. Future studies evaluating the synergistic immune suppression of T cells by B cells and BMSC are warranted.
Interestingly, in our in vivo model, we did not observe a skewing of T cell phenotype into T regulatory cells [26, 27]. Since Th1 cells are major contributors during GVHD, the data presented here suggests that BMSC may not be potent inducers of T-regs during active GVHD. Similar to our in vivo findings, no difference in T-reg cell frequency was noted in patients post BMSC therapy (data not shown). In sum, these observations suggest that BMSC action in both the humanized mouse model and in GVHD patients receiving BMSC may involve an adenosine-based mechanism rather than a T-reg-based mechanism.
Of note, BMSC derived exosomes have been implicated as the main mechanism of action of BMSC in tissue injury models [43], GVHD [44] and in tumors [45]. Currently, clinical trials for treating GVHD with BMSC derived exosomes are underway [46]. These studies suggest that the future of BMSC clinical trials should focus on microvesicles produced by BMSC rather than the cells themselves. The major drawback of such clinical trials is the lack of information on longevity and function of exosomes post-adoptive transfer. While many reports claim the function of exosomes to be mainly in the production of anti-inflammatory cytokines, there is no in vivo evidence to prove such mechanisms. The short lifespan of exosomes as demonstrated by our study suggests that patients should receive BMSC (or BMSC exosomes) adoptive transfer approximately every other day for optimum effect. Clinical trials that incorporate finite infusions of exosomes fail to provide long term remission in patients [46]. In light of these studies, our report is the first and most definitive to demonstrate a functional consequence of BMSC exosomes in patients post adoptive therapy and as such will be of importance in developing next generation clinical trials.
In conclusion, this is the first demonstration that human BMSC modulate systemic inflammation caused by human Th1 cells through an exosome-mediated adenosine pathway. Th1 cell expression of CD39 during x-GVHD related inflammation provides a molecular pathway that allows pathogenic Th1 cells the capacity for auto-regulation as long as CD73 was also present either through intact BMSC or, perhaps more likely, through systemic exposure due to exosomes produced by BMSC. These data have clear and immediate translational significance, as they indicate that CD39 may represent a critical biomarker for determining T cell susceptibility to modulation by clinical BMSC products.
Supplementary Material
Supplemental Figure 1: BMSC Treatment Alleviates Histological Manifestations in Murine Recipients With Severe x-GVHD
Female NSG mice that exhibited severe x-GVHD were treated three times with BMSC. At the end of the treatment, when murine recipients showed clinical signs of improvement from x-GVHD, histology was performed. Mononuclear cell infiltration in the lungs of control and treated cohorts (A&B), GVHD manifestations in the liver of control and treated cohorts (C&D) and the severity of acanthosis in the skin of control and treated cohorts (E&F). Histological score from two different experiments is summarized in B, D and F. P values were obtained by using student t-test *=P<0.05, **=P<0.01 and ***=P<0.001.
Supplemental Figure 2: Human Th1 Cell Engraftment And Cytokine Production Was Diminished In The Bone Marrow Post-BMSC Treatment
Female NSG mice were reconstituted with human Th1 cells (5 million) and Monocytes (3 million). When GVHD was apparent post-transplant, within 24hrs of disease onset (between day 20 to day 35), the BMSC cohort was treated 3 times with one injection of BMSC every four days. Post-treatment, mice were euthanized and bone marrow was evaluated for human Th1 cell engraftment (A). Human Th1 cells from BM were stimulated ex-vivo for 4 hrs with PMA/ionomycin along with GolgiStop and GolgiPlug for the last 2 hrs. Cells were then stained for intracellular cytokine expression. Cumulative absolute numbers of IFNγ producing human Th1 cells (B) and TNF-α producing human Th1 cells (C). P values were obtained by using student t-test *=P<0.05, **=P<0.01 and ***=P<0.001.
Supplemental Figure 3: BMSC Treatment Results In Decreased Human Th1 cell Engraftment And Effector Cytokine Production In GVHD Target Organs
NSG mice were reconstituted with human Th1 cells (5 million) and Monocytes (3 million). When GVHD was apparent post-transplant (between day 20 to day 35), the BMSC cohort was treated 3 times every four days with BMSC. Post-treatment, mice were euthanized and the GVHD target organs such as lung (A), liver (E), and skin (I) were evaluated for human Th1 cell engraftment. Cumulative absolute numbers of human Th1 cells in the lung (B), liver (F) and skin (J). Human Th1 cells from the different organs were stimulated ex-vivo for 4 hrs with PMA/ionomycin along with GolgiStop and GolgiPlug for the last 2 hrs. Cells were then stained for intracellular cytokine expression. Representative flow plots for IFNγ of infiltrating Th1 cells in the lung (C), liver (G) and skin (K). Absolute number of IFNγ producing human Th1 cells in the lung (D), liver (H) and skin (L). N=5 animals per cohort, experiments were repeated 3 times with 3 different BMSC clinical products. P values were obtained by using student t-test *=P<0.05, **=P<0.01 and ***=P<0.001.
Supplemental Figure 4: Effect Of BMSC Adenosine Production On CD39 Expressing Th1 Cells
CD73 expression by BMSC prior to adoptive transfer was analyzed by flow cytometry (A). CD39 expression by human Th1 cells pre and post BMSC treatment in the presence of ATP for 2hrs in vitro (B). Summary of three different normal donors and two different BMSC products is shown (C). Apoptosis analysis of Th1 cells in the presence of BMSC and ATP (D). Summary of Annexin V+ cells 2 hrs post co-culture is shown (E). The proposed mechanism of action of BMSC in vivo is shown. During inflammation and tissue injury the released ATP can be converted to AMP by CD39 expressed on activated human Th1 cells. AMP can be further metabolized to adenosine directly by BMSC or through their exosomes expressing CD73. The resultant adenosine can suppress human Th1 cells through the activation of adenosine receptor A2AR (F). P values were obtained by using student t-test *=P<0.05, **=P<0.01 and ***=P<0.001.
Supplementary Figure 5: In Vivo Kinetics Of LV Transduced BMSC
Human BMSC were transduced with LV expressing Venus (A) and then adoptively transferred into NSG murine recipients. The kinetics of BMSC homing in the lymphoid organs and GVHD target organs was measured at 24 hrs by flow cytometry (B). Mice were allowed to develop GVHD after injection of human Th1 cells labeled with tdTomato expressing LV. Mice were then treated with BMSC labeled with Venus expressing LV. Confocal and two-photon microscopy was performed to image fluorescently labeled BMSC (yellow) and Th1 cells (magenta) in lung, spleen, and liver. The structure of the organs was revealed by second harmonic generation from the collagen fibers (white). Imaging was performed at 24 and 48 hrs post BMSC adoptive transfer (C).
Supplemental Figure 6: In vitro assays demonstrate that functional exosomes are secreted by BMSC
BMSC were treated with either Vehicle (BMSCveh;DMSO) or Inhibitor (BMSCINH;GW48619) overnight in serum free media and then supernatant was collected. Number of exosome particles present in the supernatant in vehicle treated and Inhibitor treated BMSC cultures were measured by ELISA (A). In vitro transwell experiments with T cells and BMSC, that were either untreated or treated with exosome blocker GW48619, were performed. ATP was added at a concentration of 100μM to the bottom wells that had Th1 cells. Each condition had 0.5 million BMSC in the top well and 0.5 million Th1 cells in the bottom well in 1 ml of media. Adenosine production in the supernatant was measured by GC-MS/MS (B). Representative example of a BMSC donor is shown; ND is not detected. Total exosomes were isolated from plasma of patient samples pre-BMSC treatment, day+1 and day +4 post BMSC treatment. Isolated exosomes were enriched for CD63 and flow cytometry characterization of exosomes were performed. Human exosomes CD63 expression (A) and CD81 expression (B) was monitored. P values were obtained by using a paired student t-test *=P<0.05, **=P<0.01 and ***=P<0.001.
Acknowledgements
The Intramural Research Program, Center supported this work for Cancer Research, National Cancer Institute; DIR, National Institute of Dental and Craniofacial Research; NIH Clinical Center; the NIH Bone Marrow Stromal Cell Transplantation Center; and the National Heart, Lung and Blood Institute, National Institutes of Health. We would like to thank Dr. Ulrich Baxa, (Leidos Biomedical Research, Frederick National Laboratory for cancer research, NCI, NIH) for his technical expertise on electron microscopy. We would also like to thank Dr. Cynthia E. Dunbar for critical reading of the manuscript and for support of the two-photon microscopy studies.
Footnotes
Author Contributions S.A designed experiments, analyzed data and wrote the manuscript, D.H.F, A.J.B, R.E.G, designed experiments and wrote the manuscript. D.E.F performed and analyzed GC/MS-MS experiments, M.A.E performed histological analysis, J.Y.M performed and analyzed Two Photon Microscopic experiments, A.L, J.R., F.A.H, J.E.F, T.C.F, A.V.C performed experiments, P.G.R, D.E.S, M.S provided the BMSC cell products. M.B and S.I provided patient care and performed BMSC clinical trial.
References
- 1.Ciccocioppo R, Bernardo ME, Sgarella A, et al. Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn's disease. Gut. 2011;60:788–798. doi: 10.1136/gut.2010.214841. [DOI] [PubMed] [Google Scholar]
- 2.Ringden O, Uzunel M, Rasmusson I, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81:1390–1397. doi: 10.1097/01.tp.0000214462.63943.14. [DOI] [PubMed] [Google Scholar]
- 3.Ball LM, Bernardo ME, Roelofs H, et al. Multiple infusions of mesenchymal stromal cells induce sustained remission in children with steroid-refractory, grade III-IV acute graft-versus-host disease. Br J Haematol. 2013;163:501–509. doi: 10.1111/bjh.12545. [DOI] [PubMed] [Google Scholar]
- 4.English K. Mechanisms of mesenchymal stromal cell immunomodulation. Immunol Cell Biol. 2013;91:19–26. doi: 10.1038/icb.2012.56. [DOI] [PubMed] [Google Scholar]
- 5.Kebriaei P, Robinson S. Mesenchymal stem cell therapy in the treatment of acute and chronic graft versus host disease. Front Oncol. 2011;1:16. doi: 10.3389/fonc.2011.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baron F, Storb R. Mesenchymal stromal cells: a new tool against graft-versus-host disease? Biol Blood Marrow Transplant. 2012;18:822–840. doi: 10.1016/j.bbmt.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amarnath S, Barrett AJ. Mesenchymal stromal cells: heroes or non-combatants? Cytotherapy. 2013;15:253–254. doi: 10.1016/j.jcyt.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Batten P, Sarathchandra P, Antoniw JW, et al. Human mesenchymal stem cells induce T cell anergy and downregulate T cell allo-responses via the TH2 pathway: relevance to tissue engineering human heart valves. Tissue Eng. 2006;12:2263–2273. doi: 10.1089/ten.2006.12.2263. [DOI] [PubMed] [Google Scholar]
- 9.Lee MJ, Kim J, Kim MY, et al. Proteomic analysis of tumor necrosis factor-alpha-induced secretome of human adipose tissue-derived mesenchymal stem cells. J Proteome Res. 9:1754–1762. doi: 10.1021/pr900898n. [DOI] [PubMed] [Google Scholar]
- 10.Ren G, Zhang L, Zhao X, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 11.Meisel R, Zibert A, Laryea M, et al. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103:4619–4621. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- 12.Bouffi C, Bony C, Courties G, et al. IL-6-dependent PGE2 secretion by mesenchymal stem cells inhibits local inflammation in experimental arthritis. PLoS One. 5:e14247. doi: 10.1371/journal.pone.0014247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akiyama K, Chen C, Wang D, et al. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell. 2012;10:544–555. doi: 10.1016/j.stem.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saldanha-Araujo F, Ferreira FI, Palma PV, et al. Mesenchymal stromal cells up-regulate CD39 and increase adenosine production to suppress activated T-lymphocytes. Stem Cell Res. 2011;7:66–74. doi: 10.1016/j.scr.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Yin F, Battiwalla M, Ito S, et al. Bone marrow mesenchymal stromal cells to treat tissue damage in allogeneic stem cell transplant recipients: Correlation of biological markers with clinical responses. Stem Cells. 2014 doi: 10.1002/stem.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amarnath S, Flomerfelt FA, Costanzo CM, et al. Rapamycin generates anti-apoptotic human Th1/Tc1 cells via autophagy for induction of xenogeneic GVHD. Autophagy. 2010;6:523–541. doi: 10.4161/auto.6.4.11811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amarnath S, Costanzo CM, Mariotti J, et al. Regulatory T cells and human myeloid dendritic cells promote tolerance via programmed death ligand-1. PLoS Biol. 2010;8:e1000302. doi: 10.1371/journal.pbio.1000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abrahamsen TG, Carter CS, Read EJ, et al. Stimulatory effect of counterflow centrifugal elutriation in large-scale separation of peripheral blood monocytes can be reversed by storing the cells at 37 degrees C. J Clin Apher. 1991;6:48–53. doi: 10.1002/jca.2920060110. [DOI] [PubMed] [Google Scholar]
- 19.Ren J, Jin P, Sabatino M, et al. Global transcriptome analysis of human bone marrow stromal cells (BMSC) reveals proliferative, mobile and interactive cells that produce abundant extracellular matrix proteins, some of which may affect BMSC potency. Cytotherapy. 2011;13:661–674. doi: 10.3109/14653249.2010.548379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Rijn RS, Simonetti ER, Hagenbeek A, et al. Quantitative assessment of human T lymphocytes in RAG2(−/−)gammac(−/−) mice: the impact of ex vivo manipulation on in vivo functionality. Exp Hematol. 2007;35:117–127. doi: 10.1016/j.exphem.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 21.Hwang J, Kita R, Kwon HS, et al. Epidermal ablation of Dlx3 is linked to IL-17-associated skin inflammation. Proc Natl Acad Sci U S A. 2011;108:11566–11571. doi: 10.1073/pnas.1019658108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jenabian MA, Seddiki N, Yatim A, et al. Regulatory T cells negatively affect IL-2 production of effector T cells through CD39/adenosine pathway in HIV infection. PLoS Pathog. 2013;9:e1003319. doi: 10.1371/journal.ppat.1003319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farthing DE, Buxbaum NP, Bare CV, et al. Sensitive GC-MS/MS method to measure deuterium labeled deoxyadenosine in DNA from limited mouse cell populations. Anal Chem. 2013;85:4613–4620. doi: 10.1021/ac400309d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thery C, Amigorena S, Raposo G, et al. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006 doi: 10.1002/0471143030.cb0322s30. Chapter 3:Unit 3 22. [DOI] [PubMed] [Google Scholar]
- 25.Malide D, Metais JY, Dunbar CE. Dynamic clonal analysis of murine hematopoietic stem and progenitor cells marked by 5 fluorescent proteins using confocal and multiphoton microscopy. Blood. 2012;120:e105–116. doi: 10.1182/blood-2012-06-440636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maccario R, Podesta M, Moretta A, et al. Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica. 2005;90:516–525. [PubMed] [Google Scholar]
- 27.Melief SM, Schrama E, Brugman MH, et al. Multipotent stromal cells induce human regulatory T cells through a novel pathway involving skewing of monocytes toward anti-inflammatory macrophages. Stem Cells. 2013;31:1980–1991. doi: 10.1002/stem.1432. [DOI] [PubMed] [Google Scholar]
- 28.Moncrieffe H, Nistala K, Kamhieh Y, et al. High expression of the ectonucleotidase CD39 on T cells from the inflamed site identifies two distinct populations, one regulatory and one memory T cell population. J Immunol. 2010;185:134–143. doi: 10.4049/jimmunol.0803474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou Q, Yan J, Putheti P, et al. Isolated CD39 expression on CD4+ T cells denotes both regulatory and memory populations. Am J Transplant. 2009;9:2303–2311. doi: 10.1111/j.1600-6143.2009.02777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deaglio S, Dwyer KM, Gao W, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mandapathil M, Hilldorfer B, Szczepanski MJ, et al. Generation and accumulation of immunosuppressive adenosine by human CD4+CD25highFOXP3+ regulatory T cells. J Biol Chem. 2010;285:7176–7186. doi: 10.1074/jbc.M109.047423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Regateiro FS. 2013;171:1–7. doi: 10.1111/j.1365-2249.2012.04623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee RH, Pulin AA, Seo MJ, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schrepfer S, Deuse T, Reichenspurner H, et al. Stem cell transplantation: the lung barrier. Transplant Proc. 2007;39:573–576. doi: 10.1016/j.transproceed.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 35.Bianco P, Cao X, Frenette PS, et al. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med. 2013;19:35–42. doi: 10.1038/nm.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reis LA, Borges FT, Simoes MJ, et al. Bone marrow-derived mesenchymal stem cells repaired but did not prevent gentamicin-induced acute kidney injury through paracrine effects in rats. PLoS One. 2012;7:e44092. doi: 10.1371/journal.pone.0044092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lai RC, Arslan F, Lee MM, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 38.Ferrari D, Pizzirani C, Adinolfi E, et al. The P2X7 receptor: a key player in IL-1 processing and release. J Immunol. 2006;176:3877–3883. doi: 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]
- 39.Sluyter R, Dalitz JG, Wiley JS. P2X7 receptor polymorphism impairs extracellular adenosine 5′-triphosphate-induced interleukin-18 release from human monocytes. Genes Immun. 2004;5:588–591. doi: 10.1038/sj.gene.6364127. [DOI] [PubMed] [Google Scholar]
- 40.Sevigny CP, Li L, Awad AS, et al. Activation of adenosine 2A receptors attenuates allograft rejection and alloantigen recognition. J Immunol. 2007;178:4240–4249. doi: 10.4049/jimmunol.178.7.4240. [DOI] [PubMed] [Google Scholar]
- 41.Okusa MD, Linden J, Macdonald T, et al. Selective A2A adenosine receptor activation reduces ischemia-reperfusion injury in rat kidney. Am J Physiol. 1999;277:F404–412. doi: 10.1152/ajprenal.1999.277.3.F404. [DOI] [PubMed] [Google Scholar]
- 42.Saze Z, Schuler PJ, Hong CS, et al. Adenosine production by human B cells and B cell-mediated suppression of activated T cells. Blood. 2013;122:9–18. doi: 10.1182/blood-2013-02-482406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lai RC, Arslan F, Lee MM, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 4:214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 44.Alegre E, Rebmann V, Lemaoult J, et al. In vivo identification of an HLA-G complex as ubiquitinated protein circulating in exosomes. Eur J Immunol. 43:1933–1939. doi: 10.1002/eji.201343318. [DOI] [PubMed] [Google Scholar]
- 45.Roccaro AM, Sacco A, Maiso P, et al. BM mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. J Clin Invest. 123:1542–1555. doi: 10.1172/JCI66517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kordelas L, Rebmann V, Ludwig AK, et al. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia. 2014;28:970–973. doi: 10.1038/leu.2014.41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: BMSC Treatment Alleviates Histological Manifestations in Murine Recipients With Severe x-GVHD
Female NSG mice that exhibited severe x-GVHD were treated three times with BMSC. At the end of the treatment, when murine recipients showed clinical signs of improvement from x-GVHD, histology was performed. Mononuclear cell infiltration in the lungs of control and treated cohorts (A&B), GVHD manifestations in the liver of control and treated cohorts (C&D) and the severity of acanthosis in the skin of control and treated cohorts (E&F). Histological score from two different experiments is summarized in B, D and F. P values were obtained by using student t-test *=P<0.05, **=P<0.01 and ***=P<0.001.
Supplemental Figure 2: Human Th1 Cell Engraftment And Cytokine Production Was Diminished In The Bone Marrow Post-BMSC Treatment
Female NSG mice were reconstituted with human Th1 cells (5 million) and Monocytes (3 million). When GVHD was apparent post-transplant, within 24hrs of disease onset (between day 20 to day 35), the BMSC cohort was treated 3 times with one injection of BMSC every four days. Post-treatment, mice were euthanized and bone marrow was evaluated for human Th1 cell engraftment (A). Human Th1 cells from BM were stimulated ex-vivo for 4 hrs with PMA/ionomycin along with GolgiStop and GolgiPlug for the last 2 hrs. Cells were then stained for intracellular cytokine expression. Cumulative absolute numbers of IFNγ producing human Th1 cells (B) and TNF-α producing human Th1 cells (C). P values were obtained by using student t-test *=P<0.05, **=P<0.01 and ***=P<0.001.
Supplemental Figure 3: BMSC Treatment Results In Decreased Human Th1 cell Engraftment And Effector Cytokine Production In GVHD Target Organs
NSG mice were reconstituted with human Th1 cells (5 million) and Monocytes (3 million). When GVHD was apparent post-transplant (between day 20 to day 35), the BMSC cohort was treated 3 times every four days with BMSC. Post-treatment, mice were euthanized and the GVHD target organs such as lung (A), liver (E), and skin (I) were evaluated for human Th1 cell engraftment. Cumulative absolute numbers of human Th1 cells in the lung (B), liver (F) and skin (J). Human Th1 cells from the different organs were stimulated ex-vivo for 4 hrs with PMA/ionomycin along with GolgiStop and GolgiPlug for the last 2 hrs. Cells were then stained for intracellular cytokine expression. Representative flow plots for IFNγ of infiltrating Th1 cells in the lung (C), liver (G) and skin (K). Absolute number of IFNγ producing human Th1 cells in the lung (D), liver (H) and skin (L). N=5 animals per cohort, experiments were repeated 3 times with 3 different BMSC clinical products. P values were obtained by using student t-test *=P<0.05, **=P<0.01 and ***=P<0.001.
Supplemental Figure 4: Effect Of BMSC Adenosine Production On CD39 Expressing Th1 Cells
CD73 expression by BMSC prior to adoptive transfer was analyzed by flow cytometry (A). CD39 expression by human Th1 cells pre and post BMSC treatment in the presence of ATP for 2hrs in vitro (B). Summary of three different normal donors and two different BMSC products is shown (C). Apoptosis analysis of Th1 cells in the presence of BMSC and ATP (D). Summary of Annexin V+ cells 2 hrs post co-culture is shown (E). The proposed mechanism of action of BMSC in vivo is shown. During inflammation and tissue injury the released ATP can be converted to AMP by CD39 expressed on activated human Th1 cells. AMP can be further metabolized to adenosine directly by BMSC or through their exosomes expressing CD73. The resultant adenosine can suppress human Th1 cells through the activation of adenosine receptor A2AR (F). P values were obtained by using student t-test *=P<0.05, **=P<0.01 and ***=P<0.001.
Supplementary Figure 5: In Vivo Kinetics Of LV Transduced BMSC
Human BMSC were transduced with LV expressing Venus (A) and then adoptively transferred into NSG murine recipients. The kinetics of BMSC homing in the lymphoid organs and GVHD target organs was measured at 24 hrs by flow cytometry (B). Mice were allowed to develop GVHD after injection of human Th1 cells labeled with tdTomato expressing LV. Mice were then treated with BMSC labeled with Venus expressing LV. Confocal and two-photon microscopy was performed to image fluorescently labeled BMSC (yellow) and Th1 cells (magenta) in lung, spleen, and liver. The structure of the organs was revealed by second harmonic generation from the collagen fibers (white). Imaging was performed at 24 and 48 hrs post BMSC adoptive transfer (C).
Supplemental Figure 6: In vitro assays demonstrate that functional exosomes are secreted by BMSC
BMSC were treated with either Vehicle (BMSCveh;DMSO) or Inhibitor (BMSCINH;GW48619) overnight in serum free media and then supernatant was collected. Number of exosome particles present in the supernatant in vehicle treated and Inhibitor treated BMSC cultures were measured by ELISA (A). In vitro transwell experiments with T cells and BMSC, that were either untreated or treated with exosome blocker GW48619, were performed. ATP was added at a concentration of 100μM to the bottom wells that had Th1 cells. Each condition had 0.5 million BMSC in the top well and 0.5 million Th1 cells in the bottom well in 1 ml of media. Adenosine production in the supernatant was measured by GC-MS/MS (B). Representative example of a BMSC donor is shown; ND is not detected. Total exosomes were isolated from plasma of patient samples pre-BMSC treatment, day+1 and day +4 post BMSC treatment. Isolated exosomes were enriched for CD63 and flow cytometry characterization of exosomes were performed. Human exosomes CD63 expression (A) and CD81 expression (B) was monitored. P values were obtained by using a paired student t-test *=P<0.05, **=P<0.01 and ***=P<0.001.