Abstract
Studies show that vitamin D status is associated to obesity but data in Hispanic individuals is scarce. The aim of this study was to assess the association between vitamin D status and obesity in a clinic-based sample in Puerto Rico. We hypothesized that subjects with a higher adiposity would have a lower vitamin D status. We extracted the following data from medical records of a private clinic: age, gender, serum 25(OH)D levels, weight, height, and waist circumference. BMI (kg/m2) and waist-to-height ratio were calculated and categorized according to standard guidelines. Statistical analyses included ANCOVA, Pearson correlations and Chi-square test. From 797 individuals (mean age 53.7±15.4y; 63.5% females), 35.6% were overweight and 43.7% obese. Mean 25(OH)D levels were 24.7±8.7 ng/mL; 5.3% had levels <12 ng/mL, 30.6% had levels 12–20 ng/mL, and 43.5% had levels 21–30 ng/mL. Mean 25(OH)D levels were significantly higher in normal weight and overweight males compared to obese males (p<0.05); and in overweight females compared to obese females (p<0.05). Levels were also higher in those with low-risk compared to high-risk of waist circumference and waist-to-height ratio (p<0.001). BMI, waist circumference, and waist-to-height ratio were inversely correlated to 25(OH)D levels (p<0.001). A greater proportion of obese individuals (41.4%) were vitamin D deficient or insufficient compared to the normal weight (33.9%) and overweight individuals (30.3%) (p<0.05). In conclusion, in this clinic-based sample of Puerto Rican adults, those with higher BMI, WC, and WHtR had a significantly lower vitamin D status.
Keywords: obesity, body mass index, waist circumference, waist-to-height ratio, vitamin D, 25(OH)D, Hispanics, Puerto Rico
1. Introduction
Vitamin D is a liposoluble molecule that can be synthesized in the skin as a result of sun exposure or can be obtained from foods containing this nutrient naturally (e.g., cod liver oil and fatty fishes), vitamin D fortified products, and supplements [1]. Serum vitamin D or 25(OH)D levels are widely accepted as a biomarker to estimate the vitamin D status [2]; it reflects the vitamin D synthesized in the skin and the vitamin D obtained from foods or supplements. Although there are no universally accepted cutoff points for the optimal serum 25(OH)D levels, the Institute of Medicine (IOM) established that levels above 20 ng/mL (>50 nmol/L) are adequate. However, the Endocrinology Society considers that levels 30 ng/mL (≥75 nmol/L) and above are optimal [3].
Vitamin D deficiency is a public health concern around the world [4, 5]. There is a high prevalence of vitamin D deficiency and insufficiency worldwide [6]. In the United States (US), 32% of the total population and 43% of the Hispanics had vitamin D levels below 20 ng/mL [7]. Moreover, in a large sample of Puerto Ricans (n=4,090), 65.8% had vitamin D levels below 30 ng/mL and 24.9% had levels below 20 ng/mL [8].
Several studies have shown a consistent association between adiposity and vitamin D status, in which an increase in adiposity results in lower serum 25(OH)D levels [9–12]. Hence, obese subjects are more likely to have suboptimal vitamin D levels. This association was also found in Hispanics and African Americans in the US [13]. In Puerto Rico (PR), the only study that has addressed this association among 94 overweight and obese adults found a significant inverse correlation of 25(OH)D levels with percent body fat [14]. Further research is needed, particularly in Hispanic populations, to understand the factors that contribute to the high prevalence of vitamin D deficiency, such as obesity [8]. This is important as obesity is now recognized as one of the leading health threats worldwide [15], which is highly prevalent in Hispanics. In the US, 35% of adults (≥20 years) are obese [16]. Similarly, Behavioral Risk Factor Surveillance System (BRFSS) data collected in 2013 showed that 28% of adults were obese and 39% were overweight [17]. However, a study conducted in a representative sample of Puerto Ricans living in the San Juan metropolitan area of PR showed an even higher prevalence of overweight and obesity (40.8% obesity and 36.7% overweight) [18].
To start addressing this gap in knowledge, we sought to assess the association of vitamin D status with obesity in a clinic-based sample from Puerto Rico. Our specific objectives were to (1) assess the correlations of serum 25(OH)D with body composition measures, (2) evaluate the associations of vitamin D status with obesity status, and (3) compare serum 25(OH)D levels across obesity status. Based on prior research, it was hypothesized that subjects with higher adiposity would have a lower vitamin D status. To test our hypothesis, we analyzed a large data set of medical records of individuals attending a private endocrinology clinic with information on vitamin D status and obesity.
2. Methods and Materials
2.1 Study Population
A retrospective medical record review of all patients who attended the Endocrinology, Diabetes, and Metabolism Clinic located in Utuado, PR was performed between 2005 and 2013. Those medical records that had complete information on body composition measures and laboratory test results for serum 25(OH)D levels were included in the study. The institutional review board of the Medical Sciences Campus of the University of Puerto Rico approved this study.
2.2 Demographic, body composition, and clinical data
A data collection form was used to extract demographic information, body composition measures, and medical history from the medical records. Demographic data included age and sex, and body composition data included weight, height, and waist circumference (WC). Weight (pounds) and height (inches) were measured by the clinic staff using a physician scale (Detecto, Model 338, MO, USA) with height rod and WC (inches) was measured using a measurement tape. These were converted to kg, m and cm, respectively.
Obesity was assessed using the body mass index (BMI), WC, and waist-to-height ratio (WHtR). BMI, calculated as weight in kilograms (kg) divided by height in meters squared (m2), was used as an index of general obesity [19]. Subjects were classified using the World Health Organization (WHO) cutoff points: normal <25.0 kg/m2; overweight 25.0 – 29.9 kg/m2; and obese ≥30 kg/m2 [20]. The WHtR and WC were used as central or abdominal obesity indices [19]. WHtR was calculated by dividing the WC (cm) by height (cm). High WHtR was defined as values greater than 0.5 [21], whereas elevated WC was defined as ≥102 cm (40 in) in men and ≥88 cm (35 in) in women [22]. In addition, co-morbidities including hypertension, diabetes, and hyperlipidemia were collected.
2.3 Circulating 25(OH)D
Blood test results for serum 25(OH)D levels in nanograms per milliliter (ng/mL) were extracted from the records. We also extracted the method used to measure vitamin D levels. Most of the serum vitamin D tests in our sample (96.9%) were performed using immunoassays (92.9% immunochemiluminometric assay [ICMA]; 0.9% chemiluminescence immunoassay [CLIA]; and 3.1% IDS enzymeimmunoassay [IDS-EIA]). The remaining tests (3.1%) were performed with liquid chromatography–mass spectrometry (LC/MS/MS).
There is an ongoing debate on the cutoff values for vitamin D status. The IOM established that a person with levels below 12 ng/mL (<30 nmol/L) is at risk of deficiency, levels between 12 and 20 ng/mL (30 – 50 nmol/L) are inadequate, and levels of 20 ng/mL and above (≥50 nmol/L) are adequate [2]. However, the Endocrine Society classifies the vitamin D status as deficient if serum 25(OH)D levels are 20 ng/mL or below (≤50 nmol/L), insufficient if levels are between 21 and 29 ng/mL (52 – 72 nmol/L), and sufficient if levels are 30 ng/mL and above (≥75 nmol/L) [3]. For the present study, the cutoff values used to describe the vitamin D status were: <12 ng/mL for deficiency, 12 – 20 ng/mL for inadequacy, >20 ng/mL for adequacy (sub-classified as insufficiency if levels were 21 – 29 ng/mL and optimal if levels were ≥30 ng/mL).
2.4 Statistical Analyses
We conducted sex-specific analyses given the sex differences in vitamin D levels and obesity observed in previous studies. Baseline characteristics of patients were summarized by age-adjusted means for the continuous variables and age-adjusted percentages for the categorical variables. Sex-specific comparisons of baseline characteristics were performed using Student’s t-test or chi-square test. Age-adjusted Pearson’s correlation coefficients were used to assess the relationship of serum 25(OH)D with body composition measures (BMI, WC, and WHtR). The chi-square test (linear-by-linear association) was used to evaluate the association of vitamin D status with obesity status. Analysis of covariance (ANCOVA) was used to assess differences in age-adjusted means of serum 25(OH)D levels across BMI status, WC, and WHtR categories. Post hoc pair-wise comparisons were assessed using Bonferroni’s method where indicated. A p value below 0.05 was considered as statistically significant. Statistical analyses were performed using the Statistical Package for Social Sciences (SPSS) Software, version 21.0 (Chicago, IL, USA).
3. Results
A total of 1,379 medical records were reviewed, of which, 914 met the inclusion criteria. Fifty-one records of children and adolescents and 66 individuals (7.2%) who reported the use of vitamin D supplements were excluded. Thus, a total of 797 records (291 males and 506 females) were included in the present analysis. Baseline characteristics of patients are summarized in Table 1. The mean age of patients was 53.7±15.4 years, and women outnumbered men by a ratio of 1.7:1. Mean BMI was 30.0±6.5 kg/m2, which was significantly higher in males than in females (p=0.032). Overall, 35.6% were overweight and 43.7% were obese. Although males had significantly higher WC compared to females (p<0.001), a greater proportion of females were classified in the high-risk WC category compared to males (p<0.001). Males also had significantly higher WHtR than females (p=0.016). With respect to co-morbidities, diabetes, hypertension, and hyperlipidemia were significantly (p<0.05) more common among men than among women.
Table 1.
Baseline characteristics of study participants
| Characteristic | n | Males | n | Females | n | Total |
|---|---|---|---|---|---|---|
| Age (years) | 291 | 53.5±14.4 | 506 | 53.8±15.9 | 797 | 53.7±15.4 |
| BMI (kg/m2) | 291 | 30.6±6.1 | 506 | 29.6±6.6* | 797 | 30.0±6.5 |
| Normal weight (<25 kg/m2) | 43 | 14.8% | 122 | 24.1%* | 165 | 20.7% |
| Overweight (25.0 – 29.9 kg/m2) | 108 | 37.1% | 176 | 34.8%* | 284 | 35.6% |
| Obese (≥30 kg/m2) | 140 | 48.1% | 208 | 41.1%* | 348 | 43.7% |
| WC (cm) | 287 | 102±12.8 | 505 | 92.0±15.1** | 792 | 95.8±15.1 |
| Low riska | 162 | 56.4% | 213 | 42.2%* | 375 | 47.3% |
| High riskb | 125 | 43.6% | 292 | 57.8%* | 417 | 52.7% |
| WHtR | 287 | 0.59±0.08 | 505 | 0.58±0.10* | 792 | 0.58±0.09 |
| Low risk (≤0.50) | 28 | 9.8% | 126 | 25.0%* | 154 | 19.4% |
| High risk (>0.50) | 259 | 90.2% | 379 | 75.0%* | 638 | 80.6% |
| 25(OH)D (ng/mL) | 291 | 25.6±8.4 | 506 | 24.1±8.9* | 797 | 24.7±8.7 |
| Vitamin D Status | ||||||
| Deficient (<12 ng/mL) | 11 | 3.8% | 31 | 6.1%* | 42 | 5.3% |
| Inadequate (12 – 20 ng/mL) | 80 | 27.5% | 164 | 32.4%* | 244 | 30.6% |
| Adequate (>20 ng/mL) | 200 | 68.7% | 311 | 61.5%* | 511 | 64.1% |
| Insufficient (21 – 29 ng/mL) | 132 | 45.4% | 215 | 42.5%* | 347 | 43.5% |
| Optimal (≥30 ng/mL) | 68 | 23.4% | 96 | 19.0%* | 164 | 20.6% |
| Co-morbidities | ||||||
| Diabetes | 135 | 46.4% | 180 | 35.6%* | 315 | 39.5% |
| Hypertension | 120 | 41.2% | 132 | 26.1%** | 252 | 31.6% |
| Hyperlipidemia | 156 | 53.6% | 199 | 39.3%** | 355 | 44.5% |
Values are presented as age-adjusted means±SD or percentage (%).
p<0.05 compared to males (Student’s t-test or Chi2).
p<0.001 compared to males (Student’s t-test or Chi2).
Defined as <102 cm in males and <88 cm in females;
Defined as ≥102 cm in males and ≥88 cm in females.
Pearson’s correlations coefficients determined that serum 25(OH)D levels are inversely correlated with BMI, WC, and WHtR (p<0.001), and were stronger for males than for females (Table 2).
Table 2.
Pearson’s correlation coefficients of 25(OH)D levels and obesity indices
| Pearson’s
r
|
|||
|---|---|---|---|
| Obesity indices | Males | Females | Total |
| BMI | − 0.20** | − 0.11* | − 0.14** |
| WC | − 0.21** | − 0.15** | − 0.14** |
| WHtR | − 0.21** | − 0.15** | − 0.17** |
Values are age-adjusted Pearson’s correlation coefficients.
p<0.01;
p<0.001.
Associations between BMI categories and vitamin D status (Table 3) showed that the vitamin D status was significantly associated to BMI status in the overall sample, whereas a greater proportion of individuals in the obese group (41.4%) were categorized as vitamin D deficient or insufficient compared to normal weight (33.9%) and overweight individuals (30.3%) (p=0.02). When analyzed by sex, the association only remained significant in males (p=0.008), whereas a greater proportion of individuals in the overweight (25%) and obese (40%) categories were also categorized as vitamin D deficient or insufficient compared to the normal weight individuals (18.6%). Moreover, 71.5% of overweight and 77.9% of obese subjects had levels below 30 ng/mL (data not shown).
Table 3.
Associations between BMI categories and vitamin D status
| Vitamin D status |
p value* | |||
|---|---|---|---|---|
| <12 ng/mL | 12 – 20 ng/mL | >20 ng/mL | ||
| Males | ||||
| Normal weight | 2.3% | 16.3% | 81.4% | 0.008 |
| Overweight | 4.6% | 20.4% | 75.0% | |
| Obese | 3.6% | 36.4% | 60.0% | |
|
| ||||
| Females | ||||
| Normal weight | 4.9% | 34.4% | 60.7% | 0.177 |
| Overweight | 3.4% | 30.1% | 66.5% | |
| Obese | 9.1% | 33.2% | 57.7% | |
|
| ||||
| Total | ||||
| Normal weight | 4.2% | 29.7% | 66.1% | 0.020 |
| Overweight | 3.9% | 26.4% | 69.7% | |
| Obese | 6.9% | 34.5% | 58.6% | |
Chi-Square test (Linear-by-linear Association).
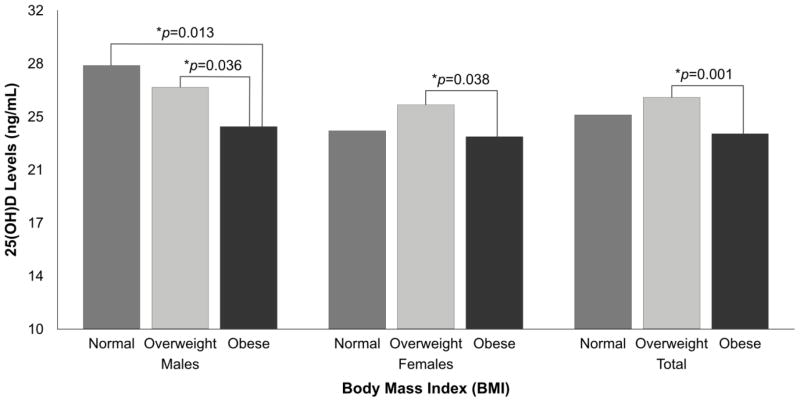
Mean 25(OH)D levels were 24.7±8.7 ng/mL, which was significantly lower in females compared to males (p=0.023). Vitamin D deficiency (<12 ng/mL) was only observed in 5.3% of the participants but vitamin D inadequacy (12 – 20 ng/mL) was observed in 30.6%. According to the IOM cutoff points, most (64.1%) had adequate vitamin D levels (>20 ng/mL); however, according to the Endocrine Society cutoff points, only 20.6% had optimal levels ≥30 ng/mL). Serum 25(OH)D levels by BMI categories according to sex (Figure 1) indicated that mean 25(OH)D levels were significantly higher in normal weight males (28.2±8.8 ng/mL) and overweight males (26.7±8.5 ng/mL) compared to obese males (24.0±7.9 ng/mL) (p<0.05). Overweight females (25.5±9.8 ng/mL) had significantly higher vitamin D levels than obese females (23.3±8.5 ng/mL) (p=0.038), but no significant differences were detected between normal weight women and overweight or obese women. Overall, overweight individuals had higher mean 25(OH)D level compared to obese individuals (p=0.001).
Figure 1.
Differences in 25(OH)D levels by BMI categories according to sex.
Data were analyzed using ANCOVA, adjusting by age.
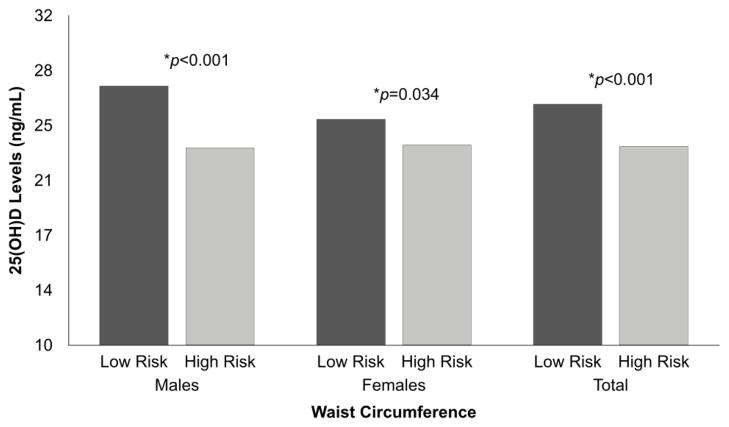
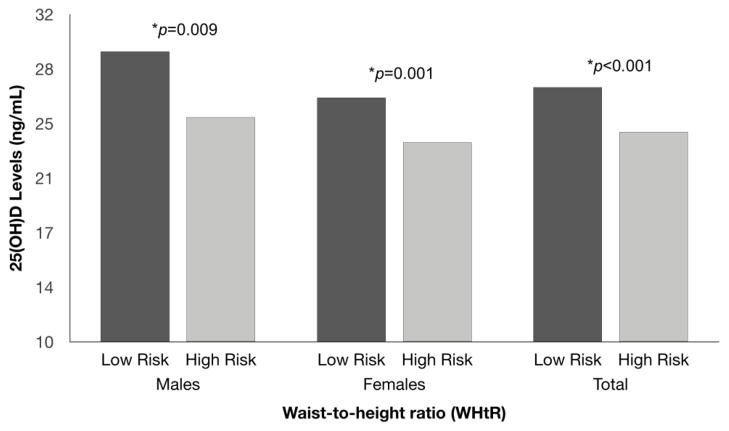
Serum 25(OH)D levels were significantly lower in individuals with a high-risk WC in both males (p<0.001) and females (p=0.034) (Figure 2). Similarly, serum 25(OH)D levels were significantly lower in individuals in the high-risk category of WHtR in both males (p=0.009) and females (p=0.001) (Figure 3).
Figure 2.
Differences in 25(OH)D levels by WC according to sex.
Data were analyzed using ANCOVA, adjusting by age.
Figure 3.
Differences in 25(OH)D levels by WHtR according to sex.
Data were analyzed using ANCOVA, adjusting by age.
4. Discussion
There was a high prevalence of overweight and obesity in our clinic-based sample. Most of the participants (79%) had insufficient vitamin D levels (levels <30 ng/mL) [3]. However, when using the IOM cutoff points, only 35.9% of the study sample had an inadequate vitamin D status (< 20 ng/mL) [2]. We also found that most overweight or obese individuals were vitamin D insufficient or deficient and serum vitamin D levels were inversely correlated to adiposity indices (BMI, WC, and WHtR).
The prevalence of low vitamin D status in our study was higher than the prevalence described in previous studies in PR. A study with a smaller sample (n=219) found that 60.3% had low vitamin D status (<30 ng/mL) [23], while in another study (n=4,090), 68.5% had low vitamin D [8]. However, individuals from the present study were different from other studies conducted in PR, as they were selected among an endocrinology health clinic attenders and thus were unlikely to be representative of all individuals from such area. Thus, confirmation of findings from this clinic-based study in population-based samples is necessary.
The analysis of serum 25(OH)D levels by BMI categories according to sex showed that vitamin D levels were significantly higher in normal weight and overweight males compared to obese males; however, in females, only those overweight had significantly higher vitamin D levels compared to those obese, while vitamin D levels in normal weight females were similar to levels among overweight or obese women. However, when obesity was assessed using WC or WHtR, serum 25(OH)D levels were significantly higher in the low-risk category of WC and WHtR consistently in both males and females. We have previously shown that WC and WHtR are better adiposity measures as related to cardiometabolic risk factors [24], as these take into account fat distribution while BMI does not. Studies have suggested the importance of both visceral and subcutaneous fat in this association [25], but it is in the subcutaneous adipose tissue where vitamin D is more likely to be stored or sequestered [26]. This is important to differentiate as vitamin D is a fat-soluble vitamin that can be stored in the adipose tissue and some researchers have suggested that when more subcutaneous fat is available (e.g. in obese individuals), more vitamin D is sequestered there, resulting in less circulating 25(OH)D levels [26, 27]. Therefore, WC and WHtR may be more appropriate than BMI to assess the association between obesity and vitamin D status.
Other studies have also found that obesity is inversely correlated to serum 25(OH)D levels in different populations, including non-Hispanic White individuals and White adults from New Zealand [28–32, 25], but only a few have included Hispanics [13, 33]. Similarly, others have also reported a higher prevalence of vitamin D inadequacy (<30 ng/mL) among obese subjects (72.0%) compared to non-obese [28]. Moreover, a study conducted in Florida showed that among Hispanics, there was a significantly higher prevalence of vitamin D insufficiency (<30 ng/mL) in obese (63.2%) compared to non-obese individuals (35.8%) [33]. Furthermore, we also reported in another sample of Puerto Ricans that 55.9% of overweight and 61.8% of obese individuals had vitamin D insufficiency (<30 ng/mL) [23].
Gender differences have been previously described in some studies [34–36], while others have not found these differences [37]. The stronger associations observed in the present study in men could be explained by other conditions and co-morbidities in women. Although we controlled our analyses for age, it has been suggested that age is an independent predictor of vitamin D levels in women but not in men [36]. Previous studies have found differences in vitamin D levels among pre- and post-menopausal women [38–40]. Interestingly, the menopause onset varies within females [41–43] and this may be altering our results, but we did not collect such information. Also, gender differences could be explained by differences in the presence of conditions or disorders that affect vitamin D metabolism, such as reduced or altered absorption, abnormal metabolism due to chronic kidney disease or hepatic dysfunction [44], and use of medications, such as anticonvulsants, steroids and glucocorticoids [45]. However, we were unable to assess the effects of such variables as these were not collected in the study.
The present study provided the opportunity to describe the association between serum 25(OH)D levels and obesity indices using a large sample of Hispanics living in PR. Moreover, serum 25(OH)D levels were measured in most samples using immunoassays, thereby reducing the inter-method variability between laboratory results. Also, the measurement of serum 25(OH)D levels is the most objective measure to describe the vitamin D status, which reflects both vitamin D intake and the vitamin D synthetized in the skin. Nevertheless, the present study has some limitations that merit discussion. First, the use of a clinic-based sample and the cross-sectional design of our study do not allow us to to generalize our findings or infer causality. In addition, variables such as menopausal status and pharmacologic treatment that could affect vitamin D metabolism were not available and thus not accounted in the analysis.
Our results support our hypothesis confirming that obesity is inversely associated to low vitamin D status in this group of Hispanic adults living in Puerto Rico. These findings, if confirmed by additional research, would have important public health implications. There is a need for developing recommendations to increase vitamin D status in overweight and obese individuals by either increasing sun exposure and/or increasing vitamin D intake through foods or supplementation. These findings can also help to develop health promotion programs to encourage healthy behaviors (e.g., weight loss) that may be important to increase vitamin D levels.
Highlights.
We examined the association between vitamin D status and obesity in a large Clinic-based sample in Puerto Rico.
Vitamin D levels were higher in normal weight and overweight males compared to obese males and in overweight females compared to obese females as assessed by BMI.
Subjects in the low-risk categories of WC and WHtR had higher vitamin D levels compared to those in the high-risk categories.
Serum vitamin D levels were inversely correlated to BMI, WC, and WHtR.
A greater proportion of individuals in the obese category were categorized as vitamin D insufficient or deficient compared to normal weight and overweight subjects.
Acknowledgments
Supported in part by Award Number 5G12MD007600 from the National Institute on Minority Health and Health Disparities.
Abbreviations
- 25(OH)D
25-hydroxyvitamin D or vitamin D
- BMI
body mass index
- WC
waist circumference
- WHtR
waist-to-height ratio
- IOM
Institute of Medicine
- WHO
World Health Organization
- BRFSS
Behavioral Risk Factor Surveillance System
- US
United States
- PR
Puerto Rico
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors have no conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hossein-nezhad A, Holick MF. Vitamin D for health: A global perspective. Mayo Clin Proc. 2013;88:720–55. doi: 10.1016/j.mayocp.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Institute of Medicine (IOM) Dietary reference intakes for calcium and vitamin D. Washington, DC: The National Academy Press; 2011. [Google Scholar]
- 3.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 201;96:1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 4.Lips P. Worldwide status of vitamin D nutrition. J Steroid Biochem Mol Biol. 2010;121:297–300. doi: 10.1016/j.jsbmb.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 5.Mezza T, Muscogiuri G, Sorice GP, Prioletta A, Salomone E, Pontecorvi A, et al. Vitamin D deficiency: A new risk factor for type 2 diabetes? Ann Nutr Metab. 2012;61:337–48. doi: 10.1159/000342771. [DOI] [PubMed] [Google Scholar]
- 6.Palacios C, Gonzalez L. Is vitamin D deficiency a major global public health problem? J Steroid Biochem Mol Biol. 2014;144:138–145. doi: 10.1016/j.jsbmb.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ganji V, Zhang X, Tangpricha V. Serum 25-hydroxyvitamin D concentrations and prevalence estimates of hypovitaminosis D in the U.S. population based on assay-adjusted data. J Nutr. 2012;142:498–507. doi: 10.3945/jn.111.151977. [DOI] [PubMed] [Google Scholar]
- 8.Suarez-Martinez EB, Perez CM, Cruz SK, Khorsandi S, Chardon C, Ferder L. Importance of vitamin D and vitamin D levels status in Puerto Ricans. J Health Care Poor Underserved. 2013;24:38–47. doi: 10.1353/hpu.2014.0000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vimaleswaran KS, Berry DJ, Lu C, Tikkanen E, Pilz S, Hiraki LT, et al. Causal relationship between obesity and vitamin D status: Bi-directional mendelian randomization analysis of multiple cohorts. PLoS Med. 2013;10:e1001383. doi: 10.1371/journal.pmed.1001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forrest KY, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011;31:48–54. doi: 10.1016/j.nutres.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Song Q, Sergeev IN. Calcium and vitamin D in obesity. Nutr Res Rev. 2012;25:130–41. doi: 10.1017/S0954422412000029. [DOI] [PubMed] [Google Scholar]
- 12.Grethen E, McClintock R, Gupta CE, Jones R, Cacucci BM, Diaz D, et al. Vitamin D and hyperparathyroidism in obesity. J Clin Endocrinol Metab. 2011;96:1320–6. doi: 10.1210/jc.2010-2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Young KA, Engelman CD, Langefeld CD, Hairston KG, Haffner SM, Bryer-Ash M, et al. Association of plasma vitamin D levels with adiposity in Hispanic and African Americans. J Clin Endocrinol Metab. 2009;94:3306–13. doi: 10.1210/jc.2009-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palacios C, Gil K, Perez CM, Joshipura K. Determinants of vitamin D status among overweight and obese Puerto Rican adults. Ann Nutr Metab. 2012;60:35–43. doi: 10.1159/000335282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caballero B. The global epidemic of obesity: An overview. Epidemiol Rev. 2007;29:1–5. doi: 10.1093/epirev/mxm012. [DOI] [PubMed] [Google Scholar]
- 16.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–14. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention (CDC) BRFSS prevalence and trends data, overweight and obesity (BMI) in Puerto Rico [Internet] 2012 Available from: http://www.cdc.gov/brfss/index.htm.
- 18.Perez CM, Sanchez H, Ortiz AP. Prevalence of overweight and obesity and their cardiometabolic comorbidities in Hispanic adults living in Puerto Rico. J Community Health. 2013;38:1140–6. doi: 10.1007/s10900-013-9726-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ashwell M, Gunn P, Gibson S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: systematic review and meta- analysis. Obes Rev. 2012;13:275–286. doi: 10.1111/j.1467-789X.2011.00952.x. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization (WHO) Report of a WHO consultation. Geneva: 2000. Obesity: Preventing and managing the global epidemic. (WHO Technical Report Series 894) [PubMed] [Google Scholar]
- 21.Browning LM, Hsieh SD, Ashwell M. A systematic review of waist-to-height ratio as a screening tool for the prediction of cardiovascular disease and diabetes: 0. 5 could be a suitable global boundary value. Nutr Res Rev. 2010;23:247–69. doi: 10.1017/S0954422410000144. [DOI] [PubMed] [Google Scholar]
- 22.National Heart, Lung, and Blood Institute (NHLBI) NIH Publication Number 00-4084. Oct, 2000. The practical guide: Identification, evaluation, and treatment of overweight and obesity in adults. [Google Scholar]
- 23.Caro Y, Negron V, Palacios C. Association between vitamin D levels and blood pressure in a group of Puerto Ricans. PR Health Sci J. 2012;31:123–9. [PMC free article] [PubMed] [Google Scholar]
- 24.Palacios C, Perez CM, Guzman M, Ortiz AP, Ayala A, Suarez E. Association between adiposity indices and cardiometabolic risk factors among adults living in Puerto Rico. Public Health Nutr. 2011;14:1714–23. doi: 10.1017/S1368980011000796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng S, Massaro JM, Fox CS, Larson MG, Keyes MJ, McCabe EL, et al. Adiposity, cardiometabolic risk, and vitamin D status: The Framingham Heart Study. Diabetes. 2010;59:242–8. doi: 10.2337/db09-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–3. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 27.Blum M, Dolnikowski G, Seyoum E, Harris SS, Booth SL, Peterson J, et al. Vitamin D(3) in fat tissue. Endocrine. 2008;33:90–4. doi: 10.1007/s12020-008-9051-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bischof MG, Heinze G, Vierhapper H. Vitamin D status and its relation to age and body mass index. Horm Res. 2006;66:211–5. doi: 10.1159/000094932. [DOI] [PubMed] [Google Scholar]
- 29.Parikh SJ, Edelman M, Uwaifo GI, Freedman RJ, Semega-Janneh M, Reynolds J, et al. The relationship between obesity and serum 1,25-dihydroxy vitamin D concentrations in healthy adults. J Clin Endocrinol Metab. 2004;89:1196–9. doi: 10.1210/jc.2003-031398. [DOI] [PubMed] [Google Scholar]
- 30.McGill AT, Stewart JM, Lithander FE, Strik CM, Poppitt SD. Relationships of low serum vitamin D3 with anthropometry and markers of the metabolic syndrome and diabetes in overweight and obesity. Nutr J. 2008;7:4. doi: 10.1186/1475-2891-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muscogiuri G, Sorice GP, Prioletta A, Policola C, Della Casa S, Pontecorvi A, et al. 25-hydroxyvitamin D concentration correlates with insulin-sensitivity and BMI in obesity. Obesity (Silver Spring) 2010;18:1906–10. doi: 10.1038/oby.2010.11. [DOI] [PubMed] [Google Scholar]
- 32.Vashi PG, Lammersfeld CA, Braun DP, Gupta D. Serum 25-hydroxyvitamin D is inversely associated with body mass index in cancer. Nutr J. 2011;10:51. doi: 10.1186/1475-2891-10-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Florez H, Martinez R, Chacra W, Strickman-Stein N, Levis S. Outdoor exercise reduces the risk of hypovitaminosis D in the obese. J Steroid Biochem Mol Biol. 2007;103:679–81. doi: 10.1016/j.jsbmb.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 34.Jacques PF, Felson DT, Tucker KL, Mahnken B, Wilson PW, Rosenberg IH, et al. Plasma 25-hydroxyvitamin D and its determinants in an elderly population sample. Am J Clin Nutr. 1997;66:929–936. doi: 10.1093/ajcn/66.4.929. [DOI] [PubMed] [Google Scholar]
- 35.Barnes MS, Bonham MP, Robson PJ, Strain JJ, Lowe-Strong AS, Eaton-Evans J, et al. Assessment of 25-hydroxyvitamin D and 1, 25-dihydroxyvitamin D3 concentrations in male and female multiple sclerosis patients and control volunteers. Mult Scler. 2007;13:670–2. doi: 10.1177/1352458506072666. [DOI] [PubMed] [Google Scholar]
- 36.Bolland MJ, Grey AB, Ames RW, Mason BH, Horne AM, Gamble GD, et al. Determinants of vitamin D status in older men living in a subtropical climate. Osteoporos Int. 2006;17:1742–8. doi: 10.1007/s00198-006-0190-2. [DOI] [PubMed] [Google Scholar]
- 37.Jenab M, Bueno-de-Mesquita HB, Ferrari P, van Duijnhoven FJ, Norat T, Pischon T, et al. Association between pre-diagnostic circulating vitamin D concentration and risk of colorectal cancer in European populations: a nested case-control study. BMJ. 2010;340:b5500. doi: 10.1136/bmj.b5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shirazi L, Almquist M, Malm J, Wirfält E, Manjer J. Determinants of serum levels of vitamin D: a study of life-style, menopausal status, dietary intake, serum calcium, and PTH. BMC Womens Health. 2013;13:33. doi: 10.1186/1472-6874-13-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lerchbaum E. Vitamin D and menopause-a narrative review. Maturitas. 2014;79:3–7. doi: 10.1016/j.maturitas.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 40.Touvier M, Deschasaux M, Montourcy M, Sutton A, Charnaux N, Kesse-Guyot E, et al. Determinants of vitamin D status in caucasian adults: influence of sun exposure, dietary intake, sociodemographic, lifestyle, anthropometric, and genetic factors. J Invest Dermatol. 2015;135:378–88. doi: 10.1038/jid.2014.400. [DOI] [PubMed] [Google Scholar]
- 41.Gold EB, Bromberger J, Crawford S, Samuels S, Greendale GA, Harlow SD, et al. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol. 2001;153:865–74. doi: 10.1093/aje/153.9.865. [DOI] [PubMed] [Google Scholar]
- 42.Henderson KD, Bernstein L, Henderson B, Kolonel L, Pike MC. Predictors of the timing of natural menopause in the Multiethnic Cohort Study. Am J Epidemiol. 2008;167:1287–94. doi: 10.1093/aje/kwn046. [DOI] [PubMed] [Google Scholar]
- 43.Thomas F, Renaud F, Benefice E, de Meeüs T, Guegan JF. International variability of ages at menarche and menopause: patterns and main determinants. Hum Biol. 2001;73:271–90. doi: 10.1353/hub.2001.0029. [DOI] [PubMed] [Google Scholar]
- 44.Nair S. Vitamin D Deficiency and Liver Disease. Gastroenterol Hepatol. 2010;6:491–493. [PMC free article] [PubMed] [Google Scholar]
- 45.Gröber U, Kisters K. Influence of drugs on vitamin D and calcium metabolism. Dermatoendocrinol. 2012;4:158–66. doi: 10.4161/derm.20731. [DOI] [PMC free article] [PubMed] [Google Scholar]