Abstract
Objective
The objective of this study was to assess the conditions under which Zambia women with a history of cervical cancer screening by visual inspection with acetic acid might switch to HPV-based testing in the future.
Methods
We conducted a choice-based conjoint survey in a sample of women recently screened by visual inspection in Lusaka, Zambia. The screening attributes considered in hypothetical choice scenarios included: screening modality, sex and age of the examiner, whether screening results would be presented visually, distance from home to the clinic, and wait time for results.
Results
Of 238 women in the sample, 208 (87.4%) provided responses sufficiently reliable for analysis. Laboratory testing on a urine sample was the preferred screening modality, followed by visual screening, laboratory testing on a self-collected vaginal specimen, and laboratory testing on a nurse-collected cervical specimen. Market simulation suggested that only 39.7% (95% confidence interval, 33.8, 45.6) of respondents would prefer urine testing offered by a female nurse in her 30's over visual inspection of the cervix conducted by a male nurse in his 20's if extra wait time were as short as one hour and the option of viewing how their cervix looks like were not available.
Conclusion
Our study suggests that, for some women, level of preference for HPV-based screening strategies may depend highly on the process and conditions of service delivery.
Keywords: Zambia, uterine cervical neoplasms, patient preference, secondary prevention, continuity of patient care
Introduction
The Cervical Cancer Prevention Program in Zambia (CCPPZ) is the largest cervical cancer prevention program linked to HIV care in sub-Saharan Africa.1,2 The screening strategy is currently based on visual inspection of the cervix with acetic acid, supplemented by digital photographic images acquired using a commercial brand digital camera. Women who screen positive are offered immediate cryotherapy, if eligible. Repeated screening is recommended one year after treatment for a cervical abnormality or in three years otherwise.
One option for scaling up cervical cancer prevention services in Zambia is the use of HPV DNA-based screening.1 An important question for the program is therefore to determine the conditions under which women, and in particular high-risk women, might elect to undergo HPV testing in the future.
Choice-based conjoint methodology is a powerful tool to assess patients’ preferences for modifiable aspects of health care, including new services and innovations in service delivery.3,4 The approach is based on the premise that any product can be described with a set of attributes important to the target population and that a person's overall preference for a particular product is driven by their relative preference for the combination of attributes of this product compared to that of the other products available.
In this study we used choice-based conjoint methodology to investigate the preferences of Zambia women recently screened by visual inspection for a range of clinic-based screening alternatives built around key attributes of visual screening and HPV testing. We also aimed to estimate the relative importance of service delivery characteristics in determining these preferences.
Materials and Methods
Setting and Participants
This study was embedded within a larger qualitative and quantitative study of women's experience with cervical cancer screening conducted at Kanyama clinic.5,6 Located in the outskirt of Lusaka, this government-run health center provides a wide range of preventive and curative services to the surrounding community, including cervical cancer preventive care, and HIV, maternal and child care. Cervical cancer screening is currently available every week day, free of charge. Peer educators visit the clinic waiting rooms in the early morning to offer visual cervical screening to all women aged 18-49 years and refer those who accept to the screening nurse. Screening results are immediately communicated to the woman through the use of the magnified digital picture of their cervix. In 2011, an average of 10-15 women were examined each day. Of those 1-to-3 had indications for immediate treatment of cervical abnormality by cryotherapy.
For the main study, we recruited a consecutive sample of 300 women who consented to participate and undergo screening. All women were invited to complete three face-to-face interviews. The first interview took place immediately before screening, the second immediately after screening, and the third 6-to-8 weeks later, when women diagnosed with a cervical abnormality were scheduled for early follow-up. Participants in the choice-based conjoint sub-study (hereafter referred to as stated choice survey) were the subset of 238 women who completed the third interview. Socio-demographic, locator, and medical record data were obtained from the screening team. Participants had no history of cervical cancer screening or treatment for cancer of the cervix.
Study protocol was approved by research ethics committees from the University of Alabama at Birmingham and the University of Zambia. The Zambia Ministry of Health authorized the study to be conducted in a government clinic.
Choice-based conjoint survey
In the stated preference surveys the least challenging and most natural for respondents, each participant is presented with a brief set of discrete choice questions; each question compares the attributes of two products; and participants are asked to indicate for each question the product they prefer. In this study, the “products” were two hypothetical screening clinics described in terms of multi-option attributes describing the cervical cancer screening services offered. For each question, the respondent was asked to which clinic they would choose to go.
Attributes retained for the study were: (1) visual screening or laboratory testing modalities, described solely in terms of visual inspection (yes or no), biological specimen (cervical/vaginal sample, urine sample, or neither), and method of specimen collection (nurse-collected or self-collected); (2) examiner, defined by their gender and age; (3) option to view a digital picture of one's cervix; (4) travel distance to the clinic; and (5) wait time to receive screening results (Table 1).
Table 1.
Clinic attributes and attribute options used to compose choice questions
| Attribute | Attribute options |
|---|---|
| Screening modality | • Gynecologic exam and visual inspection: This is the test currently available. You have to undress for a gynecological exam; the wounds of the cervix are detected by visual inspection. |
| • Gynecologic exam and swab of the cervix collected by a nurse: You have to undress for a gynecological exam; the nurse gently rubs your cervix with a cotton swab to collect some fluid, but does not put vinegar on your cervix; the cotton swab is sent to the laboratory for analysis. | |
| • Swab of the virginal privately collected by the woman herself: There is no gynecological exam unless test result is abnormal. You undress, alone in a small room; you introduce a cotton swab in your virginal and rotate it several times to collect fluid; then you remove the swab from the virginal and places it in a small tube; the tube is sent to the laboratory for analysis. | |
| • Urine sample privately collected by the woman herself: There is no gynecological exam unless screening result is abnormal. You undress alone in the bathroom; you pee and collect a little amount of urine in a plastic container; the container is sent to the laboratory for analysis. | |
| Nurse taking care of you | • A female nurse in her twenties |
| • A male nurse in his twenties | |
| • A female nurse in her thirties | |
| • A male nurse in his thirties | |
| To discuss screening results with you... | • Picture: The nurse shows you a picture of how your cervix looks like. |
| • No picture: The nurse does not show you a picture of how your cervix looks like. | |
| Distance of the screening clinic from home | • 10-minute walk: A mobile screening clinic comes in your neighborhood; you need to walk 10 minutes from home to the clinic. |
| • 20-minute walk: You need to walk 20 minutes from home to the nearest screening clinic. | |
| • 15-minute bus trip: It takes you a 15-minute ride by bus to go from home to the nearest screening clinic. | |
| • 30-minute bus trip: It takes you a 30-minute ride by bus to go from home to the nearest screening clinic. | |
| Waiting time for results | • No wait: You do not have to wait at the clinic; the screening result is immediately available. |
| • 1-hour wait: You have to wait 1hour at the clinic until your screening result is back. | |
| • 4-hour wait: You have to wait 4 hours at the clinic until your screening result is back. | |
| • 3-day wait: You have to come back to the clinic 3 days later to learn about your screening result. |
The process of survey development involved a review of the literature, consultations with six Zambian and foreign experts, and eight focus group discussions with women and screening personnel.5,6 We also conducted extensive field testing in local languages to ensure that choice questions were relevant and understood by the respondents. Because the study included low-literacy women, the number of attributes was limited to five and the maximum number of attribute options to four. We did not retain screening fee as an attribute, because cervical cancer screening is offered free of charge in Zambia. Since some scenarios did not involve a speculum examination, participants were told that they would be presented with a “picture of how they cervix looks like”; however, as a shorthand, we will refer to this attribute using the expression ”picture of one's cervix”.
Data collection
Stated choice data were collected face-to-face using laptop computer-aided technology (SSI Web 6.6.2, Sawtooth Software, Sequin, WA). All participants were interviewed privately in the language of their choice by trained female interviewers of the same cultural background. Interviewers introduced the stated choice survey with the following statement: “The method currently available at Kanyama Clinic to detect sores of the “mouth of the womb” is called visual screening. Within the next few years new methods should become available. We would like to know which methods women like you might prefer in the future and why.” For clarity's sake, the steps of visual cervical screening were briefly reviewed and compared to those of the “new laboratory testing methods”. Then, the interviewer read the automatically-generated choice questions one at a time, making sure that the computer screen was in the respondent's sight.
Four attributes including four response options and one attribute including two response options could be combined into 512 different pairs of clinic profiles and as many choice questions. To reduce the number of questions submitted to each participant to a statistically appropriate subset, we opted for a full-profile, balanced overlap, computer randomized design,4,7 where each woman was presented with a set of 8 randomly-generated questions and 2 fixed, validation questions. Pilot testing indicated that 10 choice questions was the maximum number that women would respond to before getting tired. The balanced overlap design allows investigators to determine if respondents’ preference weights for the options of an attribute depend on the option presented for another attribute (i.e., interaction between attributes).8
Statistical analysis
We used hierarchical Bayes (HB) random-effects logit modeling to estimate an importance score for each attribute, that is an indicator of the relative influence of an attribute on choice decisions for a clinic expressed in percent of the combined influence of all the attributes (Sawtooth CBC/HB software version 4.6.4).9 For each attribute, we calculated the proportion of respondents who selected a particular option as their preferred option, controlling for the other attributes. Model-derived preference scores of each individual woman for each one of the attribute option in the study were expressed as zero-centered difference scores. A property of these scores is that, for each attribute, the range of mean scores for the different options of the attribute reflects the importance of this attribute relative to that of the other attributes.10 Finally we used a market simulator (Sawtooth SMRT version 4.20.2)11 to predict participants’ sensitivity to increasing wait time when comparing preferences for laboratory-based options to visual cervical screening as currently offered.
Results
Choice-based interviews were carried out between January and June 2011. Of 238 women who presented for the third interview (79.3% of baseline sample), 237 (99.6%) successfully completed the stated choice survey. All of them were comfortable with the idea of possibly undergoing cervical screening again in the future. Mean time to complete the survey was 13.4 minutes (SD, 5.6). HB analysis indicated that preferences for an attribute did not meaningfully influence preferences for another attribute (e.g., the characteristics of the examiners did not substantially influence preferences for screening modality). We restricted further analyses to the 208 women (87.4%) who provided sufficiently reliable responses to the choice questions (root likelihood fit score ≥ 800) .12 Among these women, the HB model correctly predicted 396 (95.2%) of the 416 responses to the fixed, validation questions.
Mean age of women retained for analysis was 32 years (SD, 8); 141 (68%) were married; 102 (49%) had primary school education or less; 129 (62%) had a monthly household income ≤ $45 (ZMK, 200,000); and 19 (9%) had formal employment. Eighty (38.5%) were HIV-infected (all but 2 on antiretroviral therapy), 76 (36.5%) were HIV-uninfected, and 52 (25.0%) had an unknown HIV status. Visual screening results were abnormal for 29 women: 17 HIV infected women (prevalence, 21.3%; 95% CI, 13.3, 31.2), 7 HIV uninfected women (prevalence, 9.2%; 95% CI, 4.1, 17.4), and 5 women with unknown HIV status (prevalence, 9.6%; 95% CI, 3.6, 20.0; chi-square test, P=0.05).
In terms of relative influence of attributes on choice of clinics, respondent gave highest importance to wait time (37.4% of total influence; 95% CI, 35.3, 39.5), followed by examiner (25.1%; 95% CI, 21.7, 28.5), picture of one's cervix (18.7%; 95% CI, 15.6, 21.8), screening modality (10.9%; 95% CI, 10.2, 11.7), and distance from home (7.8%; 95% CI, 6.8, 8.9; P<0.001).
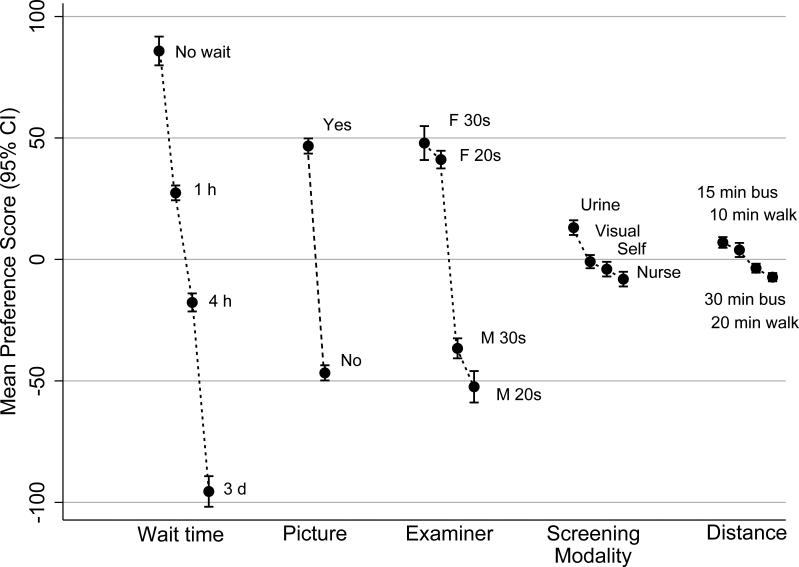
All other attribute options being equal, the vast majority of women (87.5%) preferred to receive screening results immediately (Table 2). Even waiting as little as one hour resulted in a 58.4 point drop in mean preference score (Figure 1). A 3-day wait resulted in 181.3 point drop. Most women preferred a female nurse (88.9%) over a male nurse (11.1%). Age had much less importance in determining the attractiveness of a nurse than gender. All women but two wished to view a picture of their cervix. Urine testing was the screening option most frequently preferred by respondents (51%); the other three options were preferred by equivalent proportions of women (12.5%-to-20.2%). Similarly, in comparison with the estimates obtained for the three previous attributes, differences in mean preference scores for the screening modalities were small (21.2 points or less; Figure 1). Finally, after accounting for the other attributes, equally large proportions of women preferred a 15-minute bus ride (43%), or to walk 10 minutes (42%), to go from home to the screening clinic. Difference in mean preference score between the most attractive option (15-minute bus trip) and the least attractive option (20-minute walk) was only 14.3 points.
Table 2.
Number and percentage of women preferring each attribute option
| Attribute | Preferred option |
|
|---|---|---|
| Number of women (N=208) | Percent (95% CI) | |
| Wait time | ||
| No wait time | 182 | 87.5 (82.5, 91.5) |
| 1 hour | 17 | 8.2 (5.0, 12.5) |
| 4 hours | 4 | 1.9 (0.6, 4.6) |
| 3 days | 5 | 2.4 (0.9, 5.3) |
| Picture | ||
| Yes | 206 | 99.0 (96.9, 99.8) |
| No | 2 | 1.0 (0.2, 3.1) |
| Nurse | ||
| Female in her 30s | 107 | 51.4 (44.7, 58.2) |
| Female in her 20s | 78 | 37.5 (31.1, 44.2) |
| Male in his 30s | 4 | 1.9 (0.6, 4.6) |
| Male in his 20s | 19 | 9.1 (5.8, 13.7) |
| Screening modality | ||
| Urine sample | 106 | 51.0 (44.2, 57.7) |
| Visual inspection | 34 | 16.4 (11.8, 21.8) |
| Self-collected swab | 42 | 20.2 (15.2, 26.1) |
| Nurse-collected swab | 26 | 12.5 (8.5, 17.5) |
| Distance from home | ||
| 15-minute bus trip | 90 | 43.3 (36.7, 50.1) |
| 10-minute walk | 88 | 42.3 (35.7, 49.1) |
| 30-minute bus trip | 21 | 10.1 (6.5, 14.8) |
| 20-minute walk | 9 | 4.3 (2.1, 7.8) |
Attributes (attribute options) are listed by decreasing order of importance (mean preference).
SD stands for standard deviation and 95% CI for 95 percent exact mid-P confidence interval.
Figure 1. Mean preference scores (95% confidence intervals) for the options of each attribute.
“min”=minute, “h”=hour, “d”=day, “F 30s”=female in her 30s, “F 20s”=female in her 20s, “M 30s”=male in his 30s, “M 20s”=male in his 20s”, “visual”=visual inspection with acetic acid, “self”=self-collected vaginal specimen, “nurse”=nurse-collected vaginal specimen.
Note: Attributes are ordered by decreasing importance from the left to the right. Preference scores are expressed as zero-centered difference scores to reduce differences in measurement scale among the attributes. In this interval metric, the sum of the mean preference scores of all the options of an attribute is always equal to zero. Individual scores have no meaningful interpretations; only differences between scores are interpretable.
Market simulations predicted that if participants had to choose between laboratory testing of a self-collected vaginal specimen (without presentation of a cervicogram) and visual cervical screening as currently performed (with presentation of a cervicogram), holding the examiner and travel distance attributes constant, then only 11.5% (95% CI, 8.1, 14.9) would opt for laboratory testing if results were available immediately, 1.5% (95% CI, 0.7, 2.3) if wait time were 1 hour, and 0.8% (95% CI, 0.0, 1.6) if wait time were 3 days. If testing of a urine specimen were proposed instead of testing a self-collected vaginal sample, the corresponding shares barely increased to 14.1% (95% CI, 11.1, 17.1; results immediately available), 3.8% (95% CI, 2.2, 5.4; 1-hour wait), and 2.2% (95% CI, 0.6, 3.9; 3-day wait). Finally, in a scenario were the examiner proposing laboratory testing would be a female in her 30s and the visual examiner a male in his 20s, simulations predicted that 54.7% (95% CI, 48.7, 60.7) of participants would chose laboratory testing of a urine sample if results were available immediately, 39.7% (95% CI, 33.8, 45.6) if wait time were 1 hour, and 22.1% (95% CI, 16.9, 27.3) if wait time were 3 days.
Discussion
In this study, a majority of Zambia women with recent history of screening by visual inspection indicated that for future screening, and all other factors being equal, they would prefer visual screening aided by digital cervicography over clinic-based laboratory testing alternatives not coupled with the option of viewing how their cervix looks. Although urine testing was in itself the preferred screening modality for most women, their hypothetical choice decisions were more heavily influenced by wait time and mode of communication of screening results than by the degree of privacy and invasiveness of the screening method. Since our results support the notion that none of the HPV detection methods currently available, or in development will be automatically considered ideal by all women, they have implications for implementation of new World Health Organization HPV screening guidelines13 in areas where screening programs based on visual inspection methods are already in place.
A distinct advantage of HPV tests from an acceptability perspective is the possibility to avoid speculum examination if testing is performed on a self-collected vaginal sample and results are negative.14 Acceptability of self-sampling is typically high in both developed and developing countries,14,15 especially among affluent and well-educated women.16 In some settings, however, many women declare having more trust in clinician sampling than in self-sampling because they do not have to worry about collecting the vaginal specimen properly.15,16 In our study, and after adjustment for screening process and service delivery variables, women experienced with visual cervical screening gave similar and relatively low preference scores to testing of self-collected samples, testing of nurse-collected samples, and screening by visual inspection.
HPV testing in urine samples would be easier and, as suggested by our study and others, more acceptable to women than HPV testing in self-collected vaginal samples.17 However, in our market simulations, HPV testing was nearly consistently dominated by visual cervical screening augmented with digital cervicography when HPV test results were communicated without pictorial aid. For instance, this was the case even under the assumptions that the examiner would be a male- rather than a female nurse, and that time spent at the clinic would be increased by one hour only.
An appealing screening option not considered in this study is that of community-based screening by HPV testing of vaginal specimen self-collected at home.14 However, further evaluation of this approach is needed as past studies suggest that women's preference for self-sampling at home or at the clinic might vary across settings.18-20 For instance, in a Uganda study, lack of privacy at home was cited as a barrier to self-sampling.19 In Italy, direct mailing of a self-sampling kit to women who failed to respond to a screening invitation increased screening coverage in urban areas, but not in rural areas.20
A limitation of our study is that women never screened for cervical cancer were excluded. Although these women might have had different preferences, pilot testing suggested that most of them would not have been able to provide reliable responses to the choice questions. Finally, for the sake of limiting participant burden, test accuracy was not included among the attributed to be evaluated. Accounting for the superior accuracy of HPV testing over screening by visual inspection might have influenced the balance of women's preference between test options.
Conclusion
The factors that drive women's preferences for a particular cervical cancer screening option are complex and variable. Although recent advances in HPV testing offer the perspective of widening access to cervical cancer prevention in resource-limited settings, to be successful at a population level, HPV-based screening programs will need to continue paying great attention to obstacles to participation beyond discomfort and embarrassment.
Précis.
In Zambia, women's preference for cervical screening based on HPV testing rather than visual inspection is contingent on wait time and method of result communication.
Acknowledgements
The project described was supported by Award Number R21CA124336 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Conflict of Interest: We have no conflict of interest to report.
Disclaimers: none.
References
- 1.African Center of Excellence for Women's Cancer Control (ACEWCC) [July 23, 2014];Cervical Cancer Prevention Program. http://www.acewcc.org/what-we-do/cervical-cancer-prevention-program/.
- 2.Mwanahamuntu MH, Sahasrabuddhe VV, Blevins M, Kapambwe S, Shepherd BE, Chibwesha C, et al. Utilization of cervical cancer screening services and trends in screening positivity rates in a ‘screen-and-treat’ program integrated with HIV/AIDS care in Zambia. PloS one. 2013;8:e74607. doi: 10.1371/journal.pone.0074607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryan M, Farrar S. Using conjoint analysis to elicit preferences for health care. BMJ. 2000;320:1530–3. doi: 10.1136/bmj.320.7248.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bridges JF, Hauber AB, Marshall D, Lloyd A, Prosser LA, Regier DA, et al. Conjoint analysis applications in health—a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Health. 2011;14:403–13. doi: 10.1016/j.jval.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 5.White HL, Mulambia C, Sinkala M, Mwanahamuntu MH, Parham GP, Moneyham L, et al. ‘Worse than HIV’ or ‘not as serious as other diseases’? Conceptualization of cervical cancer among newly screened women in Zambia. Soc Sci Med. 2012;74:1486–93. doi: 10.1016/j.socscimed.2012.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White HL, Mulambia C, Sinkala M, Mwanahamuntu MH, Parham GP, Kapambwe S, et al. Motivations and experiences of women who accessed “see and treat” cervical cancer prevention services in Zambia. J Psychosom Obstet Gynecol. 2012;33:91–8. doi: 10.3109/0167482X.2012.656161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chrzan K, Orme B. An overview and comparison of design strategies for choice-based conjoint analysis. [July 23, 2014];Sawtooth software research paper series, 2000. http://www.sawtoothsoftware.com/download/techpap/desgncbc.pdf.
- 8.Orme B. Fine-tuning CBC and adaptive CBC questionnaires. [July 23, 2014];Sawtooth software research paper series, 2009. http://www.sawtoothsoftware.com/support/technical-papers/adaptive-cbc-papers/fine-tuning-cbc-and-adaptive-cbc-questionnaires-2009.
- 9.Lenk PJ, DeSarbo WS, Green PE, Young MR. Hierarchical Bayes conjoint analysis: recovery of partworth heterogeneity from reduced experimental designs. Market Sci. 1996;15:173–91. [Google Scholar]
- 10.Sawtooth Software Scaling Conjoint Part Worths: Points vs. Zero-Centered Diffs. [July 23, 2014];Sawtooth Solutions. 1999 :10. http://www.sawtoothsoftware.com/about-us/news-and-events/sawtooth-solutions/ss10-cb/1201-scaling-conjoint-part-worths-points-vs-zero-centered-diffs.
- 11.Huber J, Orme B, Miller R. Dealing with product simulation in conjoint simulations. In: Gustafsson A, Herrmann A, Huber F, editors. Conjoint measurement [electronic resource]: methods and applications. Springer; New York: 2007. pp. 347–62. [Google Scholar]
- 12.Orme BK. Software for hierarchical Bayes estimation for CBC data, CBC/HB v5. Sawtooth Software Inc.; Sequim, WA: 2009. [July 23, 2014]. http://www.sawtoothsoftware.com/download/ssiweb/CBCHB_Manual.pdf. [Google Scholar]
- 13.World Health Organization . WHO guidelines for screening and treatment of precancerous lesions for cervical cancer prevention. WHO; Geneva, Switzerland: 2013. 2013. [July 23, 2014]. http://apps.who.int/iris/bitstream/10665/94830/1/9789241548694_eng.pdf. [PubMed] [Google Scholar]
- 14.Gravitt PE, Belinson JL, Salmeron J, Shah KV. Looking ahead: A case for human papillomavirus testing of self-sampled vaginal specimens as a cervical cancer screening strategy. Int J Cancer. 2011;129:517–27. doi: 10.1002/ijc.25974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huynh J, Howard M, Lytwyn A. Self-collection for vaginal human papillomavirus testing: systematic review of studies asking women their perceptions. J Lower Genit Tract Dis. 2010;4:356–62. doi: 10.1097/LGT.0b013e3181dc115b. [DOI] [PubMed] [Google Scholar]
- 16.Stewart DE, Gagliardi A, Johnston M, Howlett R, Barata P, Lewis N, et al. Self-collected samples for testing of oncogenic human papillomavirus: a systematic review. J Obstet Gynaecol Can. 2007;29:817–28. doi: 10.1016/s1701-2163(16)32636-6. [DOI] [PubMed] [Google Scholar]
- 17.Vorsters A, Micalessi I, Bilcke J, Ieven M, Bogers J, Van Damme P. Detection of human papillomavirus DNA in urine. A review of the literature. Eur J Clin Microbiol Infect Dis. 2012;31:627–40. doi: 10.1007/s10096-011-1358-z. [DOI] [PubMed] [Google Scholar]
- 18.Tisci S, Shen YH, Fife D, Huang J, Goycoolea J, Ma CP, et al. Patient acceptance of self-sampling for human papillomavirus in rural China. J Lower Genit Tract Dis. 2003;7:107–16. doi: 10.1097/00128360-200304000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell S, Ogilvie G, Steinberg M, Sekikubo M, Biryabarema C, Money D. Assessing women's willingness to collect their own cervical samples for HPV testing as part of the ASPIRE cervical cancer screening project in Uganda. Int J Gynecol Obstet. 2011;114:111–5. doi: 10.1016/j.ijgo.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 20.Giorgi Rossi P, Marsili LM, Camilloni L, Iossa A, Lattanzi A, Sani C, et al. The effect of self-sampled HPV testing on participation to cervical cancer screening in Italy: a randomised controlled trial (ISRCTN96071600). Br J Cancer. 2010;104:248–54. doi: 10.1038/sj.bjc.6606040. [DOI] [PMC free article] [PubMed] [Google Scholar]