Abstract
The cell death-inducing DFFA-like effector c (CIDEC; also known in rodents as FSP27 or fat-specific protein 27) is a lipid droplet-associated protein that promotes intracellular triglyceride storage. CIDEC/Fsp27 is highly expressed in adipose tissue but undetectable in normal liver. Its hepatic expression, however, rises during fasting or under genetic or diet-induced hepatosteatosis in both mice and patients. Herein, we demonstrate that CIDEC/Fsp27 is a direct transcriptional target of the nuclear receptor PPARα (peroxisome proliferator activated receptor alpha) in both mouse and human hepatocytes, and that preventing Fsp27 induction accelerates PPARα-stimulated fatty acid oxidation. We show that adenoviral-mediated silencing of hepatic Fsp27 abolishes fasting-induced liver steatosis in the absence of changes in plasma lipids. Finally, we report that anti-Fsp27 shRNA and PPARα agonists synergize to ameliorate hepatosteatosis in mice fed a high fat diet. Together, our data highlight the physiological importance of CIDEC/Fsp27 in triglyceride homeostasis under both physiological and pathological liver steatosis. Our results also suggest that patients taking fibrates likely have elevated levels of hepatic CIDEC, which may limit the efficient mobilization and catabolism of hepatic triglycerides.
Keywords: lipid droplet, liver, steatosis, fatty acid oxidation, fibrates
Non-alcoholic fatty liver disease (NAFLD) is the most common form of chronic liver disease in Western societies and its prevalence is rapidly increasing, paralleling the epidemic of obesity and type 2 diabetes (1, 2). Despite its rampant incidence, the therapeutic toolbox to manage NAFLD patients is notoriously scarce (1, 2). Hepatosteatosis is classically defined as the non-physiological accumulation of triglycerides (TAG) within lipid droplets (LD) in the cytoplasm of hepatocytes. Whether such accumulation is pathological per se, or an adaptive response to ameliorate lipotoxicity is still debated.
Lipid droplets were once thought to function as mere lipid storage depots, but more recent studies have put LD at the forefront of lipid metabolism, transport, and signaling. Several proteins at the surface of LD orchestrate these functions, and determine the metabolic fate of the lipids contained within the organelle. Among them, the CIDE (cell death-inducing DNA fragmentation factor-alpha (DFFA)-like effector) family of LD-associated proteins has been extensively studied. The 3 members of the CIDE family have distinct tissue: CIDEA is abundant in brown adipose tissue; CIDEB in liver; and CIDEC/FSP27 (fat specific protein 27 in rodents) in both white and brown adipose tissue (3). Interestingly, CIDEC/FSP27 is detected in fatty livers, but not in normal livers (3). Overexpression of Fsp27 promoted both the accumulation and an increase in size of LD in multiple cell types (4-6). Conversely, knockdown of Fsp27 decreased LD size and increased LD number in adipocytes (7). Two independent groups reported that Fsp27−/− mice show a lean phenotype paralleled by smaller LD in white fat, enhanced insulin sensitivity and resistance to diet-induced obesity (8, 9). Importantly, a lipodystrophic patient has been identified that carries a homozygous nonsense mutation in CIDEC (10).
Peroxisome proliferator-activated receptors (PPARs) are members of the family of ligand-activated nuclear receptor transcription factors. PPARs function to maintain lipid homeostasis and energy balance, by promoting the transcription of specific targets (11). Both PPARα and PPARδ are expressed at high levels in the hepatocyte, and play a major role in regulating fatty acid oxidation (FAO) (11). In contrast, PPARγ is not expressed in normal hepatocytes, but its abundance is markedly elevated in fatty livers where it promotes a lipogenic phenotype in both rodents and humans (11). Evidence shows that the expression of Fsp27 is induced in the livers of ob/ob, db/db, and ddY-H mice, as well as in normal mice fed a high fat diet (HFD), through a mechanism dependent on PPARγ (4, 12-14). Finally, Vila-Brau et al reported the induction of hepatic Fsp27 expression during early fasting via the canonical PKA/CREB signaling pathway (15). Collectively, these studies suggest a role for CIDEC/Fsp27 during the metabolic adaptations of the liver to dietary insult and fasting.
In this study we tested the functional relevance of hepatic CIDEC/Fsp27 during fasting and in response to a high fat diet (HFD). We also identified CIDEC/Fsp27 as a direct PPARα transcriptional target in the normal liver. Finally, we show that silencing Fsp27 synergized with PPARα agonists to decrease hepatosteatosis in mice, without altering plasma lipid levels. Our data highlight the critical role of CIDEC/Fsp27 during both physiological and pathological hepatic TAG storage, and suggest CIDEC may be a pharmacological target to manage liver TAG contents in patients.
MATERIALS AND METHODS
Experimental Procedures are provided as Supporting Information
RESULTS
Adenovirus-mediated silencing of Fsp27 expression prevents fasting-induced hepatic steatosis
Several laboratories reported that hepatic Fsp27 is highly induced during fasting (15), paralleling the accumulation of TAG (16). To better understand the relevance of Fsp27 on the metabolic adaptation of the liver to fasting, we infused mice with control and anti-Fsp27 adenoviral vectors. Ten days later, animals were fasted 15 h (overnight) or allowed access to food. No changes were noted in body weight between shSCR- and shFsp27-injected mice but, as expected, fasted animals showed ~15% weight loss. Supporting Fig. 1 shows that fasting induced hepatic Fsp27 expression ~70-fold in control mice, consistent with previous reports (15), while mice infused with the shFsp27 vector had significantly reduced Fsp27 levels. We did not detect GFP or shRNA effect in gonadal fat (where Fsp27 is normally expressed; data not shown), suggesting that the adenoviral transduction was restricted to the liver. The levels of hepatic Cideb and other LD-related transcripts were not altered by shFsp27 (Supporting Fig. 1A). As expected, fasted control mice accumulated TAG and FFA in the liver (Fig. 1A–C). In contrast, hepatic steatosis was dramatically reduced in the shFsp27 fasted group (Fig. 1A–C). No changes in hepatic cholesterol contents were noted among groups (Fig. 1C). A detailed lipidomics analysis of the livers showed that silencing Fsp27 expression did not preferentially changed specific TAG or FFA species (Fig. 1D and Supporting Fig. 1B). Since hepatic lipid catabolism during fasting triggers ketogenesis, we measured plasma β-hydroxybutyrate levels: mice transduced with shFsp27 showed a ~50% reduction in this metabolite, compared to control mice (Fig. 1E). In contrast, shFsp27 treatment did not alter other plasma metabolites (Fig. 1E, and Supporting Fig. 1C).
Fig. 1.
Hepatic Fsp27 silencing abolishes fasting-induced hepatosteatosis. Mice (n=5) were infused with scrambled (Ad-shSCR) or anti-Fsp27 (Ad-shFsp27) adenovirus and ten days later fasted or not. (A) Representative macroscopic appearance. (B) Representative oil red O staining of liver frozen sections. (C) Hepatic lipid contents. (D) Lipidomics heat map of hepatic TAG and FFA. (E) Plasma metabolites. *P≤0.05, **P≤0.01, fasted vs. fed; ††P≤0.01, shFsp27 vs. shSCR.
Lastly, we measured the relative abundance of selected transcripts involved in LD metabolism, FAO, and de novo lipogenesis in those same livers. Data from control mice in Supporting Fig. 1A are consistent with previous reports on the effects of nutritional status on hepatic gene expression (17-19). Hence, fasting increased FAO enzymes and specific perilipins, and decreased lipogenic enzymes. Intriguingly, PPARα targets (Plin2, Plin5, Cpt1a, Acox) were slightly decreased in shFsp27 livers, although the fasting-mediated induction was similar compared to shSCR livers (Supporting Fig. 1A). Silencing Fsp27 did not alter the expression of lipogenic genes (Supplementary Fig. 1A). The rapid induction of Fsp27 during early fasting (15) likely mediates the hepatic accumulation of lipids, which will be used as substrates for FAO and ketogenesis in late fasting. We speculate that the inability to store TAG into LD in the shFsp27 hepatocytes from the early stages of fasting results in the gradual, steady oxidation of FFA, depletion of intracellular lipids, and lack of ketogenic burst in late fasting. The remarkable decrease in both liver TAG and plasma ketone bodies noted in fasted mice following Fsp27 silencing is consistent with this proposal.
PPARα regulates CIDEC/Fsp27 expression in human and mouse hepatocytes
Previous studies in obese mice suggested that PPARγ mediates the induction of Fsp27 in steatotic livers (4, 12-14). However, treatment of lean mice with the PPARγ agonist rosiglitazone failed to induce hepatic Fsp27 expression (13), likely because the levels of PPARγ in non-steatotic livers are very low. In contrast, both PPARα and PPARδ are abundant in healthy livers, and function as major regulators of lipid homeostasis during the fasting and post-prandial periods (11). Previous reports using HepG2 cells described a PPRE in the promoter of CIDEC/Fsp27 that confers responsiveness to PPARγ, but not to PPARα or PPARδ (13). Here we used primary hepatocytes and specific agonists to test whether Fsp27 is responsive to the 3 PPARs in normal cells. Data in Fig. 2 show that Fsp27 levels, similar to Cpt1a, were increased following incubation with the PPARα agonist GW7647, but not with the PPARδ or PPARγ agonists. Other transcripts were induced by the other agonists (Fig. 2), thus confirming the biological activity of the different compounds. Contrary to in vitro approaches that require over-expression of the PPAR isotypes, our results in primary hepatocytes suggest that Fsp27 is an exclusive PPARα target in normal mouse hepatocytes, and confirm the remarkable subtleties of targeting of the different isotypes in vivo (20). Importantly, dose-dependent targeting of human CIDEC by the PPARα agonist was confirmed in Huh7 hepatoma cells (Fig. 3A). The inability of GW7647 and Wy14643 to induce Fsp27 expression in either hepatocytes or livers from PPARα−/− mice, compared to wild-type mice (Fig. 3B, 3C), suggest that the effects of these agonists are mediated by PPARα.
Fig. 2.

Fsp27 is a PPARα-specific target. Mouse primary hepatocytes were cultured overnight in the presence of 1 μmol/L agonists for PPARα (GW4767), PPARδ (GW0742) or PPARγ (rosiglitazone). The expression of selected transcripts was measured by RT-qPCR. Data from 3 independent experiments (n=4); *P≤0.05, **P≤0.01 compared to vehicle.
Fig. 3.
Transcriptional control of CIDEC/Fsp27 by PPARα. (A) Dose-dependent induction of CIDEC by GW7647 in Huh7 cells. (B) GW7647-mediated induction of Fsp27 is lost in primary hepatocytes from PPARα−/− mice. (C) Wy14643-mediated induction of Fsp27 is lost in the livers of PPARα−/− mice. (D) Relative expression of CIDEC and CPT1A in Huh7 cells cultured 16 h in media supplemented with GW7647, with or without 10 μg/mL cycloheximide (CHX). (E) Relative expression of CIDEC and CPT1A in Huh7 cells cultured 1 h in media supplemented with 5 μg/mL actinomycin D, then with or without GW7647 for an extra 16 h. (F) Relative expression of CIDEC and CPT1A in Huh7 cells cultured 16 h in media supplemented with GW7647, then with or without 5 μg/mL actinomycin D for an extra 6–24 h. (G) Normalized activities of luciferase reporters under control of a 3.3 kb fragment containing the Fsp27 proximal promoter and first intron which includes the putative PPRE, or the same fragment carrying a mutated PPRE (PPRE*), or the proximal promoter of ApoA5 (positive control), in the presence or absence of an expression vector for human PPARα and/or 1 μmol/L GW7647. (H) Relative chromatin enrichment shows the specific recruitment of PPARα to the proximal promoters of Fsp27 and Plin2 (positive control), but not to intronic regions (negative controls). See Experimental Procedures. Data from 3 independent experiments (n=4); *P≤0.05, **P≤0.01, agonist vs. DMSO; †P≤0.05, ††P≤0.01 PPARα−/− (or CHX, or actinomycin D, or PPRE*) vs. wild-type (or DMSO, or PPRE).
To determine whether CIDEC/Fsp27 is a direct transcriptional target of PPARα, we used cycloheximide (a protein translation inhibitor) or actinomycin D (a transcription inhibitor). Data in Fig. 3D show that pre-treatment with cycloheximide before addition of GW7647 did not impair the induction of either CIDEC or CPT1A, suggesting that these are a direct response that occurs in the absence of protein synthesis. In contrast, pre-treatment with actinomycin D abolished the induction of both PPARα targets (Fig. 3E), suggesting a direct transcriptional effect of the agonist on the CIDEC promoter. Finally, to study the potential impact of the agonist on mRNA stability, cells were cultured in the presence or absence of the agonist to induce PPARα targets, and then actinomycin D was introduced to prevent further transcription. Data in Fig. 3F show that the half-life of CIDEC is long and not altered by PPARα activation. Collectively, results from Fig. 3D–F demonstrate that the induction of CIDEC/Fsp27 is mediated by PPARα-dependent transcription.
To map the promoter sequences that mediate the response to PPARα, we amplified a 3.3 kb region of the Fsp27 promoter that contained the previously identified PPRE (13). Activity assays using this fragment driving a luciferase reporter showed that ectopic PPARα and GW7647 increased Fsp27 promoter activity (Fig. 3G). Directed mutagenesis of the PPRE abolished PPARα- and GW7647-mediated regulation (Fig. 3G). A reporter containing the proximal promoter of ApoA5 was used as positive control. Finally, the recruitment of PPARα to the Fsp27 promoter was verified by chromatin immunoprecipitation (ChIP), using extracts from mouse primary hepatocytes transduced with a Flag-PPARα adenovirus and anti-Flag or unrelated IgG antibodies. Data in Fig. 3H show that Flag-PPARα was specifically recruited to the Fsp27 and Plin2 promoters, but not to intronic sequences.
Taken together, data in Figs. 2 and 3 demonstrate that CIDEC/Fsp27 is a direct transcriptional target of PPARα in the hepatocyte.
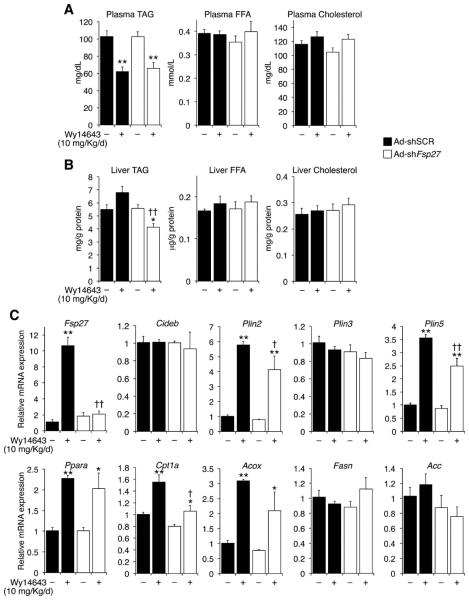
Concomitant PPARα activation and Fsp27 silencing decrease hepatic triglyceride contents in chow-fed mice
To characterize the physiological relevance of PPARα-mediated induction of hepatic Fsp27, chow-fed mice were transduced with shSCR or shFsp27 vectors and then gavaged vehicle or Wy14643 for 7 days. No changes in body weight or circulating transaminases were noted. The significant drop in circulating TAG in mice dosed with Wy14643 (Fig. 4A) validated the efficacy of the agonist, while no changes were observed in plasma FFA or cholesterol (Fig. 4A). PPARα activation did not change the overall hepatic lipid contents in control mice, although a trend towards elevated TAG was noted (Fig. 4B). In contrast, simultaneous PPARα activation and Fsp27 silencing synergized to decrease liver TAG, but not FFA or cholesterol, contents (Fig. 4B). Analysis of selected transcripts in the same livers confirmed that shFsp27 was effective in abrogating PPARα-mediated induction of Fsp27 (Fig. 4C). Similar to Fig. 1, the lower Fsp27 expression in mice receiving both shFsp27 and Wy14643 resulted in a modest decrease in PPARα targets (although agonist-mediated induction was similar), compared to mice treated with shSCR and Wy14643 (Fig. 4C). On the other hand, overexpression of Fsp27 in HuH7 cells did not change basal or agonist-induced expression of PPARα targets (Supplementary Fig. 2). Taken together, data in Fig. 4 suggest that PPARα activation in normal mice can only reduce hepatic TAG contents if the induction of Fsp27 is blunted.
Fig. 4.
Hepatic Fsp27 silencing and PPARα activation reduce triglyceride contents in chow-fed mice. Mice (n=5) were infused with scrambled (Ad-shSCR) or anti-Fsp27 (Ad-shFsp27) adenovirus and 3 days later gavaged with vehicle or 10 mg/kg/d Wy14643 for 7 days, and sacrificed without prior fasting. (A) Hepatic lipid contents. (B) Plasma metabolites. (C) Relative expression of selected transcripts. *P≤0.05, **P≤0.01 Wy14643 vs. vehicle; †P≤0.05, ††P≤0.01, Ad-shFsp27 vs. Ad-shSCR.
Fsp27 silencing and PPARα activation synergize to ameliorate hepatosteatosis in HFD-fed mice
The data above suggest that the combination of PPARα activation with Fsp27 silencing may curb diet-induced hepatosteatosis. Hence, we fed mice a HFD for 8 weeks and then performed adenoviral transduction followed by a 7-day agonist gavage. No changes in body weight or circulating transaminases were noted, although both parameters were significantly elevated compared to chow-fed mice. As expected, HFD raised both plasma TAG and cholesterol, and hepatic TAG contents, compared to chow-fed animals (Fig. 5A, B vs. 4A, B). PPARα activation was again effective in reducing plasma TAG, compared to vehicle (Fig. 5A), but did not impact liver TAG contents (Fig. 5B, C). Importantly, Fsp27 silencing alone was sufficient to reduce hepatic TAG levels, and when combined with Wy14643 led to further reduction of TAG contents (Fig. 5B, C). Remarkably, hepatic TAG in the HFD-fed mice treated with both shFsp27 and Wy14643 were comparable to those from control chow-fed animals (compare Figs. 5B and 4B). These effects were specific, since no changes were noted in liver FFA or cholesterol (Fig. 5B). Analysis of selected transcripts in the same livers did not reveal significant changes in gene expression, except for Fsp27 (Fig. 5D). Together, data in Fig. 5 suggest that PPARα agonists improve diet-induced hepatosteatosis only if the induction of Fsp27 is prevented.
Fig. 5.
Hepatic Fsp27 silencing and PPARα activation synergize to reverse diet-induced hepatosteatosis. Mice (n=5) were fed a HFD for 8 weeks, then infused with scrambled (Ad-shSCR, closed bars) or anti-Fsp27 (Ad-shFsp27, open bars) adenovirus, 3 days later gavaged with vehicle or Wy14643 for 7 days, and sacrificed without prior fasting. (A) Hepatic lipid contents. (B) Plasma metabolites. (C) Representative oil red O of liver frozen sections. (D) Relative expression of selected transcripts. *P≤0.05, **P≤0.01, Wy14643 vs. vehicle; ††P≤0.01, Ad-shFsp27 vs. Ad-shSCR.
Loss of Fsp27 activity potentiates fatty acid oxidation upon PPARα activation in primary hepatocytes
We hypothesized that the induction of CIDEC/Fsp27 counteracts the effect of PPARα on FAO. To test this proposal, we transduced hepatocytes with shSCR or shFsp27 vectors, and cultured them in media supplemented with 0.3 mmol/L oleic acid (to promote TAG accumulation), followed by treatment with vehicle or GW7647. Data in Fig. 6A show that shFsp27 robustly decreased Fsp27 expression without altering the expression of other transcripts. Consistent with the in vivo data above (Figs. 4 and 5), simultaneous Fsp27 silencing and GW7647 treatment significantly reduced cell TAG contents, compared to either treatment alone (Fig. 6B).
Fig. 6.
Fsp27 silencing and PPARα activation accelerate FAO. Mouse primary hepatocytes were transduced with scrambled (Ad-shSCR, closed bars) or anti-Fsp27 (Ad-shFsp27, open bars) and cultured in media supplemented with 0.3 mmol/L oleate, and vehicle or 1 μmol/L GW7647 for 24 h. (A) Relative expression of selected transcripts. (B) Intracellular TAG contents. (C) Cells pulsed with [14C]-oleate overnight, then transduced with adenovirus and chased in media containing Triacsin-C. Lipid extracts were resolved on TLC plates, autoradiographed, and the TAG spots (left panel) scraped and counted by scintillation. (D) Fatty acid oxidation capacity calculated from the release of [3H]-water into culture medium. See Experimental Procedures for details on metabolic labeling experiments. Data from 3 independent experiments (n=4); *P≤0.05, **P≤0.01, GW7647 vs. DMSO; †P≤0.05, ††P≤0.01, Ad-shFsp27 vs. Ad-shSCR.
Cells cultured in parallel were used for metabolic labeling experiments. Consistent with previous reports (13), Fsp27 silencing resulted in accelerated TAG turnover (Fig. 6D). Data in Fig. 6E show that, as expected, GW7647 induced the catabolism of FFA in control cells; Fsp27 silencing alone, on the other hand, had no effect. However, combination of both GW7647 and shFsp27 resulted in accelerated FFA β-oxidation, compared to GW7647 alone (Fig. 6E). Additional experiments showed that Fsp27 silencing did not change fatty acid uptake or de novo lipogenesis (Supplementary Fig. 3). Collectively, these results suggest that loss of CIDEC/FSP27 activity potentiates the stimulatory effects of PPARα on FAO.
DISCUSSION
This report highlights the critical role of CIDEC/FSP27 in liver TAG homeostasis under both physiological and pathological conditions, and provides evidence that disruption of Fsp27 activity interferes with TAG storage and reverses hepatosteatosis in mice fed a HFD. Consistent with a recent report from Xu et al. (21), Fsp27β was the only isoform expressed in liver (even in HFD-fed mice) and primary hepatocytes, while Fsp27α was abundant in adipose tissue (data not shown). CIDEC/FSP27 was originally described as an adipocyte-specific LD protein that promoted intracellular lipid accumulation (5-8). Further studies demonstrated that Fsp27 is also expressed in the livers of genetically and diet-induced obese mice in a PPARγ-dependent manner (4, 12-14), as well as early during fasting via the canonical PKA-CREB pathway (15). Further studies showed that in adipocytes FSP27 interacts with PLIN1 at the contact sites of nascent LD to facilitate lipid transfer and the formation of unilocular LDs (22, 23). Other investigators showed that FSP27 also acts as a lipolytic barrier by interacting with ATGL and preventing lipid utilization (24). Consistent with a role in mediating LD growth, Fsp27−/− mice were lean and protected from diet-induced obesity and insulin resistance (8, 9), while Fsp27 overexpression promoted TAG accumulation in both adipocytes and hepatocytes (13). These studies show that Fsp27 is a critical mediator for LD homeostasis, and suggest that reduced Fsp27 expression accelerates lipid utilization. In agreement with that proposal, investigators reported that gastric bypass surgery (25) and caloric restriction (26) reduced both hepatic TAG contents and CIDEC expression in obese subjects. Importantly, a lipodystrophic patient was reported carrying a homozygous nonsense mutation in CIDEC (10). Intriguingly, this patient also showed dyslipidemia, hepatosteatosis, insulin resistance, and diabetes (10). This clinical profile is typical of deleterious lipodystrophies (27), and contrasts with the lean, healthy phenotype of the Fsp27−/− mice. The reasons for the discrepancies between the mouse and human loss-of-function models remain obscure, but the presence of the 185-aminoacid truncated CIDEC (78% of full length) in this patient might affect intracellular lipid homeostasis and confound the liver phenotype. Nevertheless, these studies show that under pathological conditions CIDEC/Fsp27 is highly induced in the liver where its expression correlates with lipid accumulation, and suggest that CIDEC might be an attractive therapeutic target to manage patients with hepatosteatosis.
We present data to support the proposal that CIDEC/Fsp27 is a direct transcriptional target of PPARα. Consequently, we demonstrate that both Wy14643- and GW7647-induced expression of Fsp27 are lost in the livers and hepatocytes, respectively, of PPARα−/− mice. Importantly, this regulation is conserved in human Huh7 hepatocytes. Further evidence of PPARα targeting can be found in prior observations that the Wy14643 compound was able to up regulate CIDEC/Fsp27 in both human (28) and mouse (28, 29) primary hepatocytes, and in transgenic mice expressing a constitutively active PPARα (30). This is in contrast with results by Vila-Brau et al (15), in which incubation of HepG2 cells with Wy14643 failed to induce CIDEC expression. The reasons for this discrepancy are unknown. Nevertheless, herein we established that PPARα binds and activates the CIDEC/Fsp27 promoter via a conserved PPRE that had been previously characterized to drive PPARγ responsiveness (13). Interestingly, although several studies reported that PPARγ was necessary for the elevated expression of Fsp27 in the livers of obese, insulin-resistant mice (4, 12, 13), treatment with rosiglitazone failed to induce hepatic Fsp27 expression in lean mice (13). These latter results are likely explained by the very low expression of PPARγ in non-steatotic livers (11), and question the relevance of PPARγ signaling in normal hepatic physiology. Our data show that only agonists for PPARα, but not for PPARδ or PPARγ, alter the expression of Fsp27 in mouse primary hepatocytes.
Is hepatic PPARα activation the critical driver for the remarkable elevation in Fsp27 during fasting or in response to HFD? Our data comparing wild-type and PPARα−/− mice show that, although the response to HFD was significantly attenuated in PPARα−/− mice, compared to control animals, it was not completely abrogated (Supporting Fig. 4). Additionally, Fsp27 was still induced during fasting in chow-fed PPARα−/− mice (Supporting Fig. 4). We reason that additional factors, besides PPARα, control the hepatic expression of Fsp27 under fasting and in response to HFD. Hence, we provide evidence of a compensatory increase in PPARγ expression in the livers of both wild-type and PPARα−/− mice fed the HFD (Supporting Fig. 4), which likely accounts for the induction of hepatic Fsp27 in those mice. On the other hand, the PKA-CREB pathway is likely sufficient to promote the induction of Fsp27 during early fasting (15). We conclude that, despite being a bona fide PPARα target, CIDEC/Fsp27 can still be induced via alternative mechanisms. However, and crucially, our work suggests that CIDEC expression should be induced in the human liver upon pharmacological PPARα activation.
Synthetic PPARα activators, such as fibrates, are commonly used to treat dyslipidemias and are useful in curbing plasma TAG (11). Although fibrates have been shown to reduce and/or prevent fatty liver in diet-induced murine models of NAFLD (31, 32), others reported an increase in liver TAG content following PPARα activation (19, 33, 34). The efficacy of PPARα agonists on human NAFLD or NASH also remains controversial, as most studies failed to show improved liver histology or decreased hepatic TAG contents in subjects taking fibrates (35). From a mechanistic perspective, however, it is important to consider that hepatic PPARα targets not only FAO genes (i.e., CPT1A, MCAD, ACOX), but also genes involved in LD formation (PLIN2, PLIN5, FSP27) [(17-19) and this report]. It is tempting to speculate that these 2 sets of targets act in concert to modulate intracellular lipid utilization/storage, and prevent lipotoxic effects. We hypothesize that fibrates might be more efficient in promoting hepatic lipid clearance when used in combination with agents that prevent the induction of LD-related genes. Our data provide support for this hypothesis: we show that blocking Fsp27 induction upon PPARα activation potentiates the effect of GW7647 on FAO in hepatocytes, and that Wy14643 and shFsp27 synergize to decrease hepatic TAG contents in both chow- and HFD-fed mice. Importantly, the beneficial effect of Fsp27 silencing on ameliorating hepatosteatosis is not dependent upon further induction of FAO genes, compared to mice treated with fibrates alone, but it is likely the result of increased TAG turnover, which promotes FFA partitioning towards oxidation. In other words, loss of FSP27 activity increases the availability of substrates for FAO, not the amounts or activities of FAO genes. We conclude that hepatic CIDEC/FSP27 acts as a brake for PPARα-stimulated FAO by limiting the release of substrates available for β-oxidation. Our results suggest that specific PPARα modulators (SPPARMs) that were able to selectively induce the expression of target genes involved in FAO, but not in LD biogenesis, might be potent therapeutic tools to decrease TAG in the livers of NAFLD and NASH patients.
Supplementary Material
ACKNOWLEDGMENTS
We thank Erin Touchette for outstanding technical support with in vivo experiments, Dr. David Ford for helping with tissue lipidomics analysis and interpretation, Dr. Maureen Donley for help with statistical analysis, and members of the Baldan laboratory for helpful discussion.
Financial support
This study was supported in part by NIH Grant HL107794 (to Á.B.).
List of Abbreviations
- ALT
alanine amino transferase
- AST
aspartate amino transferase
- CIDE
cell death-inducing DFFA-like effector
- CHX
cycloheximide
- FAO
fatty acid oxidation
- FFA
free fatty acid
- FSP27
fat-specific protein 27
- LD
lipid droplet
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- PPAR
peroxisome proliferator activated receptor
- PPRE
PPAR responsive element
- SCR
scrambled
- sh
small hairpin
- TAG
triacylglycerides
REFERENCES
- 1.Caldwell S, Argo C. The natural history of non-alcoholic fatty liver disease. Dig Dis. 2010;28:162–168. doi: 10.1159/000282081. [DOI] [PubMed] [Google Scholar]
- 2.Lomonaco R, Sunny NE, Bril F, Cusi K. Nonalcoholic fatty liver disease: current issues and novel treatment approaches. Drugs. 2013;73:1–14. doi: 10.1007/s40265-012-0004-0. [DOI] [PubMed] [Google Scholar]
- 3.Xu L, Zhou L, Li P. CIDE proteins and lipid metabolism. Arterioscler Thromb Vasc Biol. 2012;32:1094–1098. doi: 10.1161/ATVBAHA.111.241489. [DOI] [PubMed] [Google Scholar]
- 4.Yu S, Matsusue K, Kashireddy P, Cao WQ, Yeldandi V, Yeldandi AV, Rao MS, et al. Adipocyte-specific gene expression and adipogenic steatosis in the mouse liver due to peroxisome proliferator-activated receptor gamma1 (PPARgamma1) overexpression. J Biol Chem. 2003;278:498–505. doi: 10.1074/jbc.M210062200. [DOI] [PubMed] [Google Scholar]
- 5.Danesch U, Hoeck W, Ringold GM. Cloning and transcriptional regulation of a novel adipocyte-specific gene, FSP27. CAAT-enhancer-binding protein (C/EBP) and C/EBP-like proteins interact with sequences required for differentiation-dependent expression. J Biol Chem. 1992;267:7185–7193. [PubMed] [Google Scholar]
- 6.Keller P, Petrie JT, De Rose P, Gerin I, Wright WS, Chiang SH, Nielsen AR, et al. Fat-specific protein 27 regulates storage of triacylglycerol. J Biol Chem. 2008;283:14355–14365. doi: 10.1074/jbc.M708323200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Puri V, Konda S, Ranjit S, Aouadi M, Chawla A, Chouinard M, Chakladar A, et al. Fat-specific protein 27, a novel lipid droplet protein that enhances triglyceride storage. J Biol Chem. 2007;282:34213–34218. doi: 10.1074/jbc.M707404200. [DOI] [PubMed] [Google Scholar]
- 8.Nishino N, Tamori Y, Tateya S, Kawaguchi T, Shibakusa T, Mizunoya W, Inoue K, et al. FSP27 contributes to efficient energy storage in murine white adipocytes by promoting the formation of unilocular lipid droplets. J Clin Invest. 2008;118:2808–2821. doi: 10.1172/JCI34090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toh SY, Gong J, Du G, Li JZ, Yang S, Ye J, Yao H, et al. Up-regulation of mitochondrial activity and acquirement of brown adipose tissue-like property in the white adipose tissue of fsp27 deficient mice. PLoS One. 2008;3:e2890. doi: 10.1371/journal.pone.0002890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubio-Cabezas O, Puri V, Murano I, Saudek V, Semple RK, Dash S, Hyden CS, et al. Partial lipodystrophy and insulin resistant diabetes in a patient with a homozygous nonsense mutation in CIDEC. EMBO Mol Med. 2009;1:280–287. doi: 10.1002/emmm.200900037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desvergne B, Michalik L, Wahli W. Transcriptional regulation of metabolism. Physiol Rev. 2006;86:465–514. doi: 10.1152/physrev.00025.2005. [DOI] [PubMed] [Google Scholar]
- 12.Aibara D, Matsusue K, Matsuo K, Takiguchi S, Gonzalez FJ, Yamano S. Expression of hepatic fat-specific protein 27 depends on the specific etiology of fatty liver. Biol Pharm Bull. 2013;36:1766–1772. doi: 10.1248/bpb.b13-00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsusue K, Kusakabe T, Noguchi T, Takiguchi S, Suzuki T, Yamano S, Gonzalez FJ. Hepatic steatosis in leptin-deficient mice is promoted by the PPARgamma target gene Fsp27. Cell Metab. 2008;7:302–311. doi: 10.1016/j.cmet.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Satoh H, Ide N, Kagawa Y, Maeda T. Hepatic steatosis with relation to increased expression of peroxisome proliferator-activated receptor-gamma in insulin resistant mice. Biol Pharm Bull. 2013;36:616–623. doi: 10.1248/bpb.b12-01000. [DOI] [PubMed] [Google Scholar]
- 15.Vila-Brau A, De Sousa-Coelho AL, Goncalves JF, Haro D, Marrero PF. Fsp27/CIDEC is a CREB target gene induced during early fasting in liver and regulated by FA oxidation rate. J Lipid Res. 2013;54:592–601. doi: 10.1194/jlr.M028472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guan HP, Goldstein JL, Brown MS, Liang G. Accelerated fatty acid oxidation in muscle averts fasting-induced hepatic steatosis in SJL/J mice. J Biol Chem. 2009;284:24644–24652. doi: 10.1074/jbc.M109.034397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dalen KT, Dahl T, Holter E, Arntsen B, Londos C, Sztalryd C, Nebb HI. LSDP5 is a PAT protein specifically expressed in fatty acid oxidizing tissues. Biochim Biophys Acta. 2007;1771:210–227. doi: 10.1016/j.bbalip.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 18.Dalen KT, Ulven SM, Arntsen BM, Solaas K, Nebb HI. PPARalpha activators and fasting induce the expression of adipose differentiation-related protein in liver. J Lipid Res. 2006;47:931–943. doi: 10.1194/jlr.M500459-JLR200. [DOI] [PubMed] [Google Scholar]
- 19.Edvardsson U, Ljungberg A, Linden D, William-Olsson L, Peilot-Sjogren H, Ahnmark A, Oscarsson J. PPARalpha activation increases triglyceride mass and adipose differentiation-related protein in hepatocytes. J Lipid Res. 2006;47:329–340. doi: 10.1194/jlr.M500203-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Nielsen R, Grontved L, Stunnenberg HG, Mandrup S. Peroxisome proliferator-activated receptor subtype- and cell-type-specific activation of genomic target genes upon adenoviral transgene delivery. Mol Cell Biol. 2006;26:5698–5714. doi: 10.1128/MCB.02266-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu X, Park JG, So JS, Lee AH. Transcriptional activation of Fsp27 by the liver-enriched transcription factor CREBH promotes lipid droplet growth and hepatic steatosis. Hepatology. 2014 doi: 10.1002/hep.27371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun Z, Gong J, Wu H, Xu W, Wu L, Xu D, Gao J, et al. Perilipin1 promotes unilocular lipid droplet formation through the activation of Fsp27 in adipocytes. Nat Commun. 2013;4:1594. doi: 10.1038/ncomms2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gong J, Sun Z, Wu L, Xu W, Schieber N, Xu D, Shui G, et al. Fsp27 promotes lipid droplet growth by lipid exchange and transfer at lipid droplet contact sites. J Cell Biol. 2011;195:953–963. doi: 10.1083/jcb.201104142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grahn TH, Kaur R, Yin J, Schweiger M, Sharma VM, Lee MJ, Ido Y, et al. Fat-specific protein 27 (FSP27) interacts with adipose triglyceride lipase (ATGL) to regulate lipolysis and insulin sensitivity in human adipocytes. J Biol Chem. 2014;289:12029–12039. doi: 10.1074/jbc.M113.539890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall AM, Brunt EM, Klein S, Finck BN. Hepatic expression of cell death-inducing DFFA-like effector C in obese subjects is reduced by marked weight loss. Obesity (Silver Spring) 2010;18:417–419. doi: 10.1038/oby.2009.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magnusson B, Gummesson A, Glad CA, Goedecke JH, Jernas M, Lystig TC, Carlsson B, et al. Cell death-inducing DFF45-like effector C is reduced by caloric restriction and regulates adipocyte lipid metabolism. Metabolism. 2008;57:1307–1313. doi: 10.1016/j.metabol.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 27.Garg A. Acquired and inherited lipodystrophies. N Engl J Med. 2004;350:1220–1234. doi: 10.1056/NEJMra025261. [DOI] [PubMed] [Google Scholar]
- 28.Rakhshandehroo M, Hooiveld G, Muller M, Kersten S. Comparative analysis of gene regulation by the transcription factor PPARalpha between mouse and human. PLoS One. 2009;4:e6796. doi: 10.1371/journal.pone.0006796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou L, Xu L, Ye J, Li D, Wang W, Li X, Wu L, et al. Cidea promotes hepatic steatosis by sensing dietary fatty acids. Hepatology. 2012;56:95–107. doi: 10.1002/hep.25611. [DOI] [PubMed] [Google Scholar]
- 30.Qu A, Shah YM, Matsubara T, Yang Q, Gonzalez FJ. PPARalpha-dependent activation of cell cycle control and DNA repair genes in hepatic nonparenchymal cells. Toxicol Sci. 2010;118:404–410. doi: 10.1093/toxsci/kfq259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagai Y, Nishio Y, Nakamura T, Maegawa H, Kikkawa R, Kashiwagi A. Amelioration of high fructose-induced metabolic derangements by activation of PPARalpha. Am J Physiol Endocrinol Metab. 2002;282:E1180–1190. doi: 10.1152/ajpendo.00471.2001. [DOI] [PubMed] [Google Scholar]
- 32.Shiri-Sverdlov R, Wouters K, van Gorp PJ, Gijbels MJ, Noel B, Buffat L, Staels B, et al. Early diet-induced non-alcoholic steatohepatitis in APOE2 knock-in mice and its prevention by fibrates. J Hepatol. 2006;44:732–741. doi: 10.1016/j.jhep.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 33.Hertz R, Arnon J, Bar-Tana J. The effect of bezafibrate and long-chain fatty acids on peroxisomal activities in cultured rat hepatocytes. Biochim Biophys Acta. 1985;836:192–200. doi: 10.1016/0005-2760(85)90066-9. [DOI] [PubMed] [Google Scholar]
- 34.Waterman IJ, Zammit VA. Differential effects of fenofibrate or simvastatin treatment of rats on hepatic microsomal overt and latent diacylglycerol acyltransferase activities. Diabetes. 2002;51:1708–1713. doi: 10.2337/diabetes.51.6.1708. [DOI] [PubMed] [Google Scholar]
- 35.Nseir W, Mograbi J, Ghali M. Lipid-lowering agents in nonalcoholic fatty liver disease and steatohepatitis: human studies. Dig Dis Sci. 2012;57:1773–1781. doi: 10.1007/s10620-012-2118-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.