Summary
As the resident immune cells of the central nervous system, microglia rapidly respond to brain insults, including stroke and traumatic brain injury. Microglial activation plays a major role in neuronal cell damage and death by releasing a variety of inflammatory and neurotoxic mediators. Their activation is an early response that may exacerbate brain injury and many other stressors, especially in the acute stages, but are also essential to brain recovery and repair. The full range of microglial activities is still not completely understood, but there is accumulating knowledge about their role following brain injury. We review recent progress related to the deleterious and beneficial effects of microglia in the setting of acute neurological insults and the current literature surrounding pharmacological interventions for intervention.
Keywords: Brain injury, Inflammation, Microglia, Neurotoxic mediator
Introduction
Inflammation within the central nervous system (CNS) can produce a variety of acute neurological insults including stroke, traumatic brain injury, and others that lead to brain cell damage and death 1. Microglia are the resident macrophages of the brain, and they are a key player in its immune responses 2, 3, 4. Microglia constitute 15% of the total glial population, with predominance in the gray matter 5. They respond to extracellular signals and are involved in clearing debris and toxic substances by phagocytosis, thereby retaining normal cellular homeostasis in the CNS 6. Consequently, under nonpathological states, there is continuous, low‐level microglial activity in the CNS which is primarily involved in activity‐dependent synaptic pruning and repair 2. In the event of acute brain insults such as trauma, ischemia, and neurodegeneration, microglia quickly activate by undergoing morphologic transformation from a “ramified” resting state, characterized by many branching processes, to an active, motile “ameboid” state. In the ameboid state, microglia are difficult to distinguish from circulating macrophages and monocytes, not only morphologically, but also with regard to surface markers and function 7, 8. When activated, microglia have been shown to release a variety of inflammatory and cytotoxic mediators contributing to cell damage and cell death leading to exacerbated brain injury 9, 10. Harmful effects appear to be predominant particularly in the acute stages of brain injury, while beneficial activities emerge in later stages. These studies indicate that different signals lead to two primary activation phenotypes: classically activated (M1) and alternatively activated (M2) 11, 12, 13 (Figure 1). The M1 phenotype, activated by lipopolysaccharide (LPS) and the pro‐inflammatory cytokine interferonγ (IFNγ), promotes transcriptional activation of nuclear factor‐κB (NF‐κB) and generates high levels of pro‐inflammatory cytokines and oxidative metabolites such as interleukin (IL)‐12, tumor necrosis factor (TNF)‐α, IL‐6, IL‐ 1β, and nitric oxide (NO), formerly indicated to cause additional damage. In contrast, the M2 phenotype is triggered by antiinflammatory cytokines such as IL‐4 or IL‐13 14, 15, which are thought to inhibit inflammation and enhance tissue repair and wound healing 16. Microglial activation may initiate with an M1 phenotype that mediated an innate or an adaptive immune response and ultimately exacerbates neuronal damage 17, 18. Microglia may then adopt the M2 phenotype and facilitate repair‐oriented functions by secreting growth factors and by clearing cellular debris via phagocytosis. M2 microglia also tend to limit pro‐inflammatory signal production. This highlights the significance of identifying and understanding the different microglial phenotypes and their unique functions 19, 20. Furthermore, microglia present a potential target for therapeutic intervention with various time windows depending on the functions they contribute. Recent work has also shown that microglia can switch from the M1 phenotype to the M2 phenotype. In HIV‐associated dementia 21 and spinal cord injury 22, microglia have been shown to initiate and maintain a M1 phenotype in the presence of CD40 ligation by CD40L and tumor necrosis factor (TNF), but may switch or “polarize” to the M2 phenotype through upregulation of CD45. In a study of aged rodents subjected to TBI, histological studies indicated that aged brains of injured mice had not only larger lesions and worsened outcome, but microglial polarization toward M1, compared to younger rodents 23. The challenge will be to discover methods for selectively suppressing the detrimental effects of microglial activation without compromising the restorative properties such as repair and remodeling. In related areas such as Alzheimer's and HIV‐related dementia, strategies to turn “off” CD40 or turn “on” CD45 and favoring a M2 phenotype appear to improve neurological outcome 21 and suggest potential therapeutic targets for stroke and brain trauma. However, very little work in this area has been carried out in stroke and brain trauma.
Figure 1.
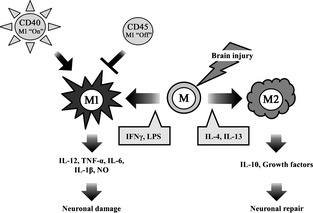
Microglial activation in brain injury. Microglia are activated by brain injury such as ischemic stroke and traumatic brain injury, which affect the polarization status of the cell. In brain injury, resting microglia (M) can be polarized to the M1 phenotype, or alternatively to the antiinflammatory, pro‐phagocytic M2 phenotype by the some of factors shown in the gray boxes. M1 microglia take part in the neuronal damage by elaborating pro‐inflammatory molecules, whereas M2 microglia promote neuronal protection, and through phagocytic functions, set the stage for recovery and repair. While not extensively studied in brain injury, CD40 leads to the M1 phenotype, whereas CD45 turns this “off.”
Microglial Receptors for Activation
Microglia respond to specific receptors in the extracellular space and adjacent cell surfaces that modulate their activation and effector function. Microglial phagocytosis may require different types of receptors to initiate function 24. Microglial receptors are able to bind foreign microbial pathogens as well as cellular debris. Such receptors include the Toll‐like receptors (TLRs), the purinergic receptors, which bind nucleotides, and a relatively newly recognized family, triggering receptor expressed on myeloid cells (TREM).
Toll‐like receptors
The exact initiating stimulus of acute brain damage has not been fully characterized, although some recent research has now identified a number of candidate factors. Several studies have shown that brain damage does induce activation of the TLRs 25, a family of transmembrane proteins involved in the identification of and defense against microbes. TLRs are observed in several cell types in the CNS and are a critical activating mechanism of innate immunity. They have been found on microglia and perivascular macrophages. Microglia are activated by stimulation of TLR4, which in turn leads to the upregulation of several proinflammatory genes. Lehnardt et al. 26 have shown that TLR4 is necessary for microglial activation after hypoxia and TLR4‐knockout mice have better neurological outcome following ischemic stroke 27, 28, 29, 30.
Moreover, reports investigating the real trigger of microglial activation in brain injury have hypothesized that the ischemic brain produces substances which can bind TLRs. Substances released by dying cells which then bind immune receptors have been categorized as damage‐associated molecular pattern molecules (DAMPs). There are also studies implicating the high mobility group box‐1 (HMGB‐1) 31, 32, which is an endogenous TLR agonist and is related to cytokines. HMGB‐1 is released by neurons as well as other immune cell types that contribute to cell death; HMGB‐1 effectively functions as a pro‐inflammatory cytokine 33. Other examples of DAMPs are hyaluronan, surfactant protein, uric acid, and heat shock proteins. These substances can bind to and stimulate microglia and other immune cells, resulting in the upregulation of many immune mediators by activation of several pro‐inflammatory transcription factors, including NF‐κB 34, hypoxia inducible factor 1 (HIF‐1), interferon regulator factor 1 and signal transducer and activator of transcription 3 (STAT3) 35.
Current research suggests that the presence of upstream TLR4 activators may indicate adverse outcomes after brain injury. In experimental stroke, HSP60 has been shown to activate TLR4 and thereby worsen brain injury 36. TLRs are also involved in the phenomenon of tolerance, whereby stimulation of one or more of these receptors with ligands, such as lipopolysaccharide (TLR4) or CpG (TLR9), triggers protection from subsequent lethal insults 37, 38, 39. Mice deficient in these receptors did not achieve tolerance 37.
Purinergic Receptors
Purinergic receptors have emerged as important sensors of brain injury. Of the numerous purinergic receptors identified, P2X7 and P2Y12 have been characterized as such, but P2X4 and adenosine receptors also contribute 40. P2X7 signaling generates the production and release of other cytokines including TNF‐α and IL‐1β via activation of several mitogen‐activated protein (MAP) kinase family members 41, 42, 43, microglial proliferation 44, 45, and superoxide production 46. Furthermore, activated P2X7 can escalate plasminogen release 47 and activation of the transcription factors nuclear factor of activated T cells and NF‐κB, which are key regulators of pro‐inflammatory genes 48, 49. In contrast, microglia also show decreased phagocytosis during P2X7 receptor stimulation 50. A few recent studies have demonstrated that P2X7 antagonists reduce injury and postinjury inflammation when administered acutely after experimental CNS injury model 51, 52. However, other reports have described experiments where stroke appeared to be exacerbated by P2X7 antagonists 53. These investigations are intriguing, but their results are difficult to interpret because these antagonists may also influence other purinergic receptors at concentrations found in vivo, and cell types other than microglia express P2X7, as well as other purinergic receptors.
The earliest reports of P2Y12 receptors described their involvement in microglial membrane ruffling and chemotaxis 54. Activation of P2Y12 contributes to process extension and subsequent microglial migration toward injury and involves the activation of the PI‐3 kinase 55 and Akt 56 signaling pathway. Interestingly, just as P2X4 is beginning to be associated with microglial movement, P2Y12 has recently been implicated in neuropathic pain 57. Clopidogrel, a widely used antiplatelet agent, is also a P2Y12 receptor antagonist. Recent findings from our laboratory demonstrated that clopidogrel was protective in an experimental model of global cerebral ischemia 58. In addition, P2Y12 knockout mice showed neuroprotection and reduced microglial chemotaxis. Nevertheless, it is important to remember that some antagonists such as cangrelor, previously believed to be P2Y12‐specific, can also antagonize P2Y13 58, 59. As both receptors also have common agonists, and both are Gi‐coupled, a role for P2Y13 may not have been eliminated by these pharmacological investigations. Researchers continue to identify novel P2 receptor agonists and antagonists; with an increasing number of available pharmacological agents, there are many more opportunities to study the therapeutic potential of purinergic receptors in regulating microglial activities.
Both P2X7 and P2X12 receptors react to adenosine triphosphate (ATP) as an endogenous agonist. It has been hypothesized that ATP is released extracellularly as a result of tissue injury, but it is unlikely that this occurs simply as a passive result of membrane disruption. For instance, ischemic injury produces energy deficiency and ATP depletion, and ATP released into brain extracellular matrix is rapidly degraded by exonucleases 60. Therefore, an active, ongoing release of ATP is more likely to be the stimulus source acting on microglial purinergic receptors. A feed‐forward, ATP‐induced release of ATP from astrocytes is one feasible mechanism 61.
In microglia, P2X4 receptors are primarily related to pain; their expression is markedly increased in spinal cord microglia following peripheral nerve injury which directly contributes to tactile allodynia 46. P2X4 is thought to modulate pain by inducing increased production and release of brain‐derived neurotrophic factor (BDNF) 62, 63, a neurotrophin increased in pain models, which regulates neurotransmitter release and interneuron activity 41. A recent study has also reported a role for P2X4 in microglial chemotaxis 55, which formerly had been mainly associated with P2Y12 54.
PPARγ
Peroxisome proliferator‐activated receptors (PPARs) belong to the superfamily of nuclear hormone receptor, which mediates gene expression related to reproduction, metabolism, development, and immune responses. There are three different PPAR isotypes (α, β/δ, and γ), and each isotype exhibits distinct tissue distributions and ligand specificities 64, 65. In the CNS, PPAR β/δ is generally expressed, while PPARα only appears in astrocytes, and PPARγ is the dominant isoform in microglia 66. In ischemic stroke, inflammation induces the release of cytokines, such as TNF‐α and IL‐1β, and adhesion molecules such as intercellular adhesion molecule 1 (ICAM‐1) and vascular cell adhesion molecule. These mediators drive the accumulation of macrophages and activated microglia in the ischemic tissue regions 67, 68. Infiltrating inflammatory cells express inducible nitric oxide synthases (iNOS) and produce generate large amounts of NO, with the subsequent formation of peroxynitrite 1. The activation of PPARγ can antagonize these harmful effects, suggesting a promising neuroprotective role for PPARγ agonists in ischemic stroke 69, 70.
Triggering receptor expressed on myeloid cells
Triggering receptor expressed on myeloid cells was initially identified on account of its ability to bind pathogens such as bacteria and viruses, as well as for its role in initiating phagocytosis 71. Recent studies show the TREM family consists of two members, TREM1 and TREM2. Both receptors appear on myeloid cells, including microglia. After cross‐linking, TREM recruits and engages its adapter protein, DNAX‐activating protein of 12 (DAP12) and activates signaling, which differs depending on which TREM receptor is activated. In addition to binding anionic patterns on various microbes, TREM was also observed binding an as yet unknown ligand in the CNS 72, 73, 74.
TREM1 signaling is largely pro‐inflammatory. The ligand is constitutively expressed on macrophages, neutrophils, and microglia and is upregulated by various mediators such as the TLR ligands LPS and lipoteichoic acid (LTA) and the pro‐inflammatory cytokine TNF 75, 76. Activation of TREM1 on monocytes and neutrophils boosts secretion of pro‐inflammatory cytokines and chemokines and mediates the upregulation of cell‐surface molecules involved in extravasation, cell activation, and costimulation 77. These phenomena have not been studied extensively. However, TREM1 is gaining recognition as a potentially important molecule in brain injury responses.
Contrary to TREM1, TREM2 signaling is primarily antiinflammatory, while also promoting phagocytosis. Activation of TREM‐2 activates downstream signaling through pathways including phosphatidylinositol 3‐kinase (PI3K), phospholipase Cγ1, and p44‐p42 extracellular signal‐regulated kinase (ERK), but not by classical inflammatory pathways such as NF‐κB and the p38 stress‐activated protein kinase 78, 79. In addition, TREM2 inhibits secretion of pro‐inflammatory factors such as cytokines and ROS 72, 73.
Considerable efforts to identify the endogenous TREM ligand have been unsuccessful in definitively determining what causes TREM activation in the brain. One recent study using a model of autoimmune encephalomyelitis demonstrated that HSP60 is one possible TREM ligand 80. A mitochondrial stress protein, HSP60, can translocate to the cell surface under appropriate conditions 81. Activation of microglia by HSP60 was shown to trigger phagocytosis in TREM2 expressing microglia, but not among microglia that were deficient in TREM2 80. In another study, microglia that appeared to bind TREM2 were activated to phagocytose‐injured cells without stimulating a typical inflammatory response or the release of reactive oxygen species. Conversely, loss of TREM2 blocked phagocytosis and triggered inflammation 82. TREM2 deficiency in cultured microglia was shown to interrupt phagocytosis and expression of microglial proinflammatory responses such as TNFα, while overexpression of TREM2 increased phagocytosis and TNF‐α 82. Restriction of TREM2 using a monoclonal antibody in experimental autoimmune encephalomyelitis resulted in exacerbation of immune responses with increased demyelination and worsened neurological function 83. These findings indicate that TREM2 may play an important role in regulating microglial phagocytosis and inflammatory responses in brain injury.
Activated microglia become highly motile, migrating to the site of injury and phagocytosing remnants from dead or dying neurons. Relevant to brain injury, TREM2 seems to mediate phagocytosis of apoptotic neurons 84. While there is yet no definitive conclusion as to whether this behavior is beneficial or harmful during various stages of brain injury, the common consensus appears to be that microglial and macrophage phagocytoses are important effectors in facilitating recovery and remodeling by clearing necrotic debris as well as infiltrating neutrophils that otherwise exacerbate damage 85, 86. Searches showed two recent articles reporting the effects of TREM2 after ischemic stroke. One study using a model of therapeutic hypothermia in experimental stroke showed that TREM2 positive microglia were increased under conditions of protection, indicating that TREM2 may be correlated with improved outcomes after ischemia 87. However, in a study of TREM2‐deficient mice, the knockouts had similar lesion sizes compared with wild‐type animals, but also fewer activated microglia and overall reduction in inflammatory responses 88—observations which seem to contradict those seen in a neuroinflammation model 83. While the therapeutic potential of TREM2 and microglial phagocytosis yet requires further research, these early studies show that TREM2 could be an important target for treating inflammatory injuries at subacute time points.
Mechanisms of Microglial Cytotoxicity
Microglia, like macrophages, respond to invading pathogens by promoting rapid sequestration and inoculation of microorganisms and by restricting the effects of cell damage and necrosis 89. These acute responses include migration, proliferation, and the release of a variety of effector substances including superoxide, NO, proteases, and cytokines. This is accompanied by phagocytosis of damaged cells. Some of these responses may exacerbate brain injury and thus provide potential therapeutic targets.
Superoxide
Superoxide is a reactive species which interacts with other molecules to form more highly reactive oxygen species, such as peroxynitrite, hypocholorus acid, carbonyl radical, and hydroxyl radical, all of which are directly cytotoxic to neurons and glia. In immune cells such as microglia, superoxide is generated by the partial reduction of molecular oxygen. The result is the production of that are formed by the 1 electron reduction of oxygen using NADPH as the electron donor: 90. Superoxide and other reactive species are also pro‐inflammatory signaling molecules that stimulate microglial activation in a feed‐forward manner 91, 92. The production of superoxide in microglia occurs mainly by NADPH oxidase (NOX), of which a number of isoforms have been identified 93, 94. The isoform predominantly detected in immune cells such as microglia is NOX2, or professional NOX. NOX expression and activation has been strongly linked to ischemic stroke and related brain disorders, and its inactivation or deficiency has been reported to be protective 95. While NOX2 exists in both microglia and circulating immune cells, one study using a bone marrow chimera model indicated that the toxic effects of NOX‐produced superoxide were specifically attributable to NOX expressed in brain cells 96. Work from our own group using a similar experiment showed ischemic brain injury worsened after increases in superoxides that were produced by NOX from both microglia and circulating immune cells 97, 98. The involvement of NOX from circulating immune cells appeared to cause more severe damage than with microglial NOX alone.
The introduction of microglia to endothelial cell and astrocyte cocultures is also known to exacerbate ischemia‐like injury. However, interrupting the production of superoxide protected these elements of the blood–brain barrier (BBB) in in vitro models of ischemia 98. In addition, Walder et al. 96 reported that mice genetically modified to be deficient in the gp91 subunit of NOX2 were protected after experimental ischemia. A few others have investigated the therapeutic potential of treatment with the pharmacological NOX antagonists apocynin 99, 100, 101, 102 and honokiol 103, 104. Their findings characterize NOX as a promising target for treating stroke and brain injury, but it is possible that the efficacy of this therapeutic intervention may be attributed largely or in part to inhibition of NOX in cell types other than microglia 105.
NO and NOS
The free radical gas NO is another substance generated by activated microglia which has been implicated in a wide range of functions following brain injury, including neuronal synaptic activity, host defense, modulating vascular tone, and as an inhibitor of platelet aggregation and leukocyte adhesion 106. NO is produced from l‐arginine through nitric oxide synthases (NOS) 107. At this time, three NOS have been closely examined in brain injury models: endothelial NOS (eNOS, NOS‐3), neuronal NOS (nNOS, NOS‐1), and inducible NOS (iNOS, NOS‐2). Of these isoforms, iNOS is thought to be the most relevant to inflammation. iNOS is expressed mainly by microglia and other macrophages but has also been observed in astrocytes 108, 109, 110, 111. Active microglia increase the inducibility of iNOS and the consequent production of NO. NO generated in microglia may also react with superoxide to produce peroxynitrite, an even more reactive oxygen species that can damage cellular DNA 112, 113. Microglial NO may also be neuroprotective against brain injury 114, 115, but more often, it has proven to be cytotoxic, especially at high levels. In an oligodendrocyte cell culture model of ischemia, one study showed considerable cell death occurred after exposure to microglia‐derived NO 116. NO is also toxic to BBB constituents 117, 118.
Studies investigating potential therapeutic applications have reported that pharmacological suppression of iNOS with aminoguanidine in mice decreases infarct volume after experimental stroke, and iNOS deficient mice also showed better outcomes after injury 110, 119. Moreover, therapeutic hypothermia and neuroprotection by estrogen and progesterone have been linked to reduced iNOS production, suggesting that NO/iNOS play a detrimental role in brain injury 120, 121, 122.
Matrix metalloproteinases
The matrix metalloproteinases (MMPs) are a family of at least 28 zinc‐dependent endopeptidases that, when active, degrade the extracellular matrix. MMPs can cause disruption of the BBB, leading to further infiltration of circulating immune cells, serum proteins, and hemorrhage 123. They are an important part of the inflammatory responses to brain injury. Resting MMPs are typically found in the cytosol in uninjured conditions, but in pathologic states, they transport extracellularly. Once situated outside the cell, they are cleaved to an active form and can degrade substrates of the extracellular matrix 1. MMP‐2, ‐3, and ‐9 have been extensively characterized in stroke and, to a lesser extent, in traumatic brain injury.
vMicroglia are the major main source of MMP release following various forms of brain injury, particularly MMP‐3 and MMP‐9 124, 125. Fibronectin and vitronectin, substances commonly located in the plasma, can stimulate microglial cells to produce pro‐MM‐9 126. Neutrophils can also produce and secrete MMP‐9 127 and studies experiments of bone marrow chimeras have suggested that MMP‐9 derived from circulating immune cells contribute to worsened ischemic injury. The damage contributed by MMPs secreted by leukocytes may even be more significant than those from microglia 128.
Cytokines and Chemokines
Cytokines and chemokines are two classes of proteins that function as signaling molecules for inter‐ and extracellular communication during inflammation. Inactive microglia release many different types of cytokines and chemokines at low levels. The pattern of this production changes drastically in the injured brain 129. During early stages of injury, these factors act primarily as intercellular signaling molecules, and many have feed‐forward effects in driving the inflammatory response. TNF‐α, IFN‐γ, IL‐1, and IL‐6 are some of the best‐studied cytokines. They have been shown to simulate microglia 130 and to have direct cytotoxic effects 131. They are also capable of affecting BBB integrity 132. These molecules have been observed as early as 1 day after experimental stroke ischemic stroke 133, 134.
Although thought to be deleterious in the acute phases of injury, cytokines may have more beneficial effects at a later stage. IL‐10 signaling ultimately inhibits proinflammatory cytokines such as IL‐1 and TNF‐α, as well as other cytokine receptor expression and downstream signals that are upregulated in pathologic states 135. Another study showed that TGF‐β1 overexpression by microglia improved neurobehavioral recovery after experimental ischemia, and these outcomes were correlated with a reduction in the inflammatory response, also attributed to TGF‐β1 overexpression 136, 137. There have also been instances of in vitro work that have observed microglia protecting cultured neurons from ischemia‐like injuries by releasing TGF‐β1 138. In addition, microglia also generate a number of neurotrophic factors, such as TGFβ1, BDNF, and GDNF, and these cytokines are considered important in protecting neuronal integrity after brain injury 139, 140, 141, 142.
Microglia are also activated by and are able to produce chemokines that perform a broad range of functions in the injured brain 143, 144, 145, 146. The expression of monocyte chemoattractant protein‐1 (MCP‐1, CCL2) is triggered by the proinflammatory stimuli, such as IFN‐γ, TNF‐α, and IL‐1β 147. Chemokines can also lead to the disruption of the BBB and are involved in the recruitment of mononuclear leukocytes into the CNS 147, 148, 149.
Mechanisms of Microglial Protection
Microglia play key roles in the tissue repair, remodeling, growth, and homeostasis by releasing antiinflammatory cytokines and growth factors and by clearing cell debris 150. Less has been studied in this area, as it pertains to affecting outcome in stroke and brain trauma. However, recent studies demonstrate that microglia may be beneficial after stroke 151, 152. Transgenic mice in which proliferating microglia were ablated suffered increased lesion size and higher numbers of apoptotic cells after experimental ischemic stroke 153. Moreover, Mac‐2+ resident microglia release neurotrophic molecules such as insulin‐like growth factor (IGF‐1), which leads to neuroprotection 153. In addition, organotypic hippocampal slice cultures exposed to ischemia‐like injury led to decreased neuronal death when cocultured with microglial BV2 cells 150. Other studies suggest that microglia may be beneficial because they engulf neutrophils 152 and release TNF‐α 154 after brain injury. Microglia may also be beneficial through their phagocytic functions. Recent reports demonstrate that dead cells release nucleic acid remnants into the extracellular space where they associate with appropriate receptors on phagocytes. Some of these phagocytosis initiating signals have been referred to as danger‐associated molecular patterns (DAMP) 155. Those identified in stroke and related injury models include purines such as UTP, ADP, and ATP, and signal through purinergic receptor systems to lead to chemotaxis and phagocytosis 156, 157. However, phagocytosis through these signaling systems, while leading to the clearance of injured cells, may also worsen cell death either by causing microglia to phagocytose viable cells or generate more neurotoxic substances 158. Preliminary work in our laboratory suggests that TREM2 may also bind purines, and TREM2 signaling in microglia, which leads to phagocytosis 84, tends to be antiinflammatory 83. Deficiency of TREM2 led to worsened recovery following stroke 159. Finally, no published works are yet available in stroke or brain trauma models regarding the microglial M1/M2 switch, and whether its modulation improves neurological outcome. These beneficial effects of microglia should be explored, as they may provide new therapeutic avenues in brain injury.
Microglia in Ischemia and Traumatic Brain Injury
Activated microglia are key players in neuroinflammation, which is present in many types of neurological disorders. Research in microglia points to many promising therapeutic targets for a broad range of neuroinflammatory diseases, including acute injuries such as ischemic stroke and traumatic brain injury 160. A comparative review of microglial activation in stroke and brain trauma shows considerable overlap. In both injury models, neuroinflammation develops over a time period of hours to days after onset, presenting an ideal time window for therapeutic intervention during which microglia play a central and nuanced role in responding to injury, sustaining inflammatory signaling and gene transcription, and mediating cytotoxic mechanisms 161, 162. Identifying parallels and distinctions between stroke and TBI may also provide useful clues for future research in microglial response to acute injury, as well as help isolate the most robust microglial targets for therapeutic intervention.
Ischemic Stroke
Stroke is the second leading cause of death worldwide and the fourth leading cause of death in the United States. While improvements in acute stroke care have considerably reduced stroke death rates, postischemic morbidity and disability are yet a major and costly public health concern 163. During ischemic stroke, major blood vessels in the brain are occluded, causing oxygen and glucose deprivation in brain tissues. This primary insult initiates subsequent secondary brain damage involving necrotic and apoptotic neuron death as well as the release of cytotoxic and proinflammatory substances such as glutamate, superoxide, NO, chemokines, and cytokines 161. Secondary injury mechanisms are largely responsible for the high rates of poststroke morbidity and disability. Therefore, it is important to identify targets for treatments that can lessen the impact of secondary brain damage. Microglial activation occurs in the early stages of neuroinflammation 98, 164; activated microglia can be detected in lesions as early as 2 h postischemia and can be detected up to 1 week after injury 85. Many studies have shown that the direct application of activated microglia has been shown to effect cell death in neurons 26, 164, 165. The cytotoxic effects begin shortly after insult and can continue to exacerbate injury for a few days afterward. It is thought that the later effects of activated microglia may be important for tissue repair and wound healing 166, 167. Thus, timing is an important element to consider when manipulating microglial activation in stroke. Studies in therapeutic microglial targets will also need to find ways to suppress cytotoxic mechanisms without disrupting beneficial effects.
Traumatic Brain Injury
Brain trauma is another significant cause of morbidity and mortality worldwide, hospitalizing 1.7 million Americans each year 168. As in stroke, microglial activation and neuroinflammation have been implicated as important mediators of secondary damage by releasing proinflammatory cytokines, cytotoxic superoxide, NO, and proteases such as MMP‐9 161, 169. Traumatic brain injury begins with a primary physical insult causing brain deformation 170. Within seconds to minutes, acute injury can trigger a variety of secondary injury mechanisms, including ischemia, hemorrhage, CNS hypoxia, free radical formation, excitotoxicity, BBB disruption, necrotic and apoptotic cell death, and inflammation 162. Microglial activation plays a central role in the inflammatory branch of secondary injury by promoting secretion of proinflammatory cytokines and recruiting circulating immune cells, thereby exacerbating injury 171, 172. One mechanism observed in brain trauma but not in stroke is that activated microglia rapidly migrate to the site of injury, where they fuse to form a barrier between damaged and healthy tissues by about half an hour after injury. This phenomenon appears to be mediated by ATP and P2Y‐receptor‐dependent mechanisms 173. In humans, active microglia appear 72 h after injury, at which time they have been shown to localize at the site of injury 174, 175. These secondary mechanisms can continue long after the time of injury. Rat studies have shown that elevated levels of activated microglia persist long after initial trauma 176, 177 and have been observed in human brains for up to 16 years after injury 174.
Prolonged microglial activation may be among the most neurotoxic consequences of neuroinflammation whether in stroke or TBI 178. The presence of chronically activated microglia is associated with sustained increase in the expression of pro‐inflammatory cytokines such as IL‐1β and TNF‐α 179. Furthermore, a number of clinical studies have shown that brain trauma may increase the risk for dementia and Alzheimer's 180, 181, 182. Based on these findings, experts have hypothesized that microglial activation may link TBI to these neurodegenerative conditions which tend to emerge later in life 169. Identifying microglial targets for treating acute injury may additionally provide greater insight into preventing or mitigating chronic neurodegenerative diseases.
Microglial Targets for Treatment
To date, much work has been performed to identify microglial targets for therapy. The research has produced a considerable body of literature describing a broad repertory of potential pharmacological inducers and suppressors. Most of these approaches protect against the deleterious effects of neuroinflammation, whether by upregulating repressors of microglial activation or by blocking microglia‐mediated proinflammatory signals or substances. Fewer studies focus on upregulating microglial repair and remodeling. While many of these drugs have produced promising preclinical results, there has been little headway in pharmacological treatments for acute injury at the clinical level 183, 184. The difficulty in identifying effective therapy is understandable given the complex, extensive web of immune responses in the injured brain. Successful intervention will most likely require a compound or combination of compounds that can modulate multiple targets. Tailoring such a drug or cocktail demands a detailed understanding of the secondary injury processes 185. A central initiator and mediator of these processes, microglia—its activators, functions, and secretions—can serve as a useful focal point for organizing the current repertoire of known antiinflammatory drugs, highlighting key targets for future investigation.
Pharmacological Inhibition of Microglial Activation
One of the best‐studied inhibitors of microglial activation, minocycline, is a second‐generation tetracycline known for its ancillary function as an effective suppressor of brain inflammation. The earliest studies in minocycline neuroprotection reported the drug's ability to inhibit microglial activation in stroke and were studied as a promising antiinflammatory drug in many other neurodegenerative diseases, including brain trauma 184, 185, 186, 187, 188, 189, 190, 191, 192. Minocycline inhibits microglia‐mediated neuron death as well as several pro‐inflammatory cytokines secreted by injury‐activated microglia, thereby suppressing both downstream cytotoxic mechanisms in addition to disrupting the inflammatory feed‐forward loop 193, 194. Minocycline is additionally known to block MMP‐9, released by activated microglia, and reduces BBB disruption in stroke and TBI 123, 124, 195, 196. Studies have shown that minocycline treatment can reduce lesion sizes and improves neurobehavioral outcomes after injury 197.
Apocynin has antioxidant and antiinflammatory capabilities, specifically to block the activity of NOX. NOX exists on immune cells (microglia and leukocytes), and apocynin inhibits the release of superoxide through NOX by blocking migration of p47phox to the membrane, thus interfering with assembly of the functional NOX complex 198. Immune cell‐generated NOX also appears important in the maintenance of vascular integrity. The addition of microglia to endothelial cell and astrocyte cocultures worsens ischemia‐like injury, and inhibiting superoxide production with apocynin preserved these BBB constituents in vitro 98. Thus, NOX contributes to BBB disruption downstream events in ischemic stroke. In fact, apocynin attenuated brain edema formation MMP‐9 expression 199, BBB disruption, and hemorrhagic transformation 100 as well as inhibiting immune cell responses 99.
TLR4
Upon exposure to signals released at the site of injury, the transmembrane receptor TLR4 activates microglia and triggers the upregulation of pro‐inflammatory genes 161. Thus, TLR4 may be a critical first step in microglial activation and injury response. Considerable work has been performed to more closely understand TLR4 mechanisms as well as its potential as a therapeutic target. In stroke, several groups have shown that TLR4 may be necessary for microglial activation, and animal studies describe better functional outcomes for TLR4‐deficient mice following experimental stroke 26, 27, 28, 29. Work in trauma continues to compile a range of different neuroprotective TLR4 inhibitors that confer neuroprotection after TBI including resatorvid, curcumin, and ethyl pyruvate 200, 201, 202. Studies have also associated TLR2 and TLR4 with increased inflammation and poorer outcomes among patients admitted for ischemic stroke, and support a clinical need for developing a therapy targeting TLRs 201, 203.
TNF‐α
Tumor necrosis factor (TNF) is an important immune signaling molecule released by activated microglia in acute injury and an especially promising therapeutic target for stroke and TBI 204. Current findings in TNF studies using pharmacological inhibitors such as etanercept suggest that the therapeutic time window for treatments targeting components of microglia‐mediated inflammation may extend long after injury 204. Groups have demonstrated the efficacy of etanercept fused with a decoy BBB‐receptor in mouse models of stroke, and still others have described the antiinflammatory and neuroprotective properties of TNF‐α antagonist such as thalidomides and thalidomide derivatives 205, 206, 207.
PPARγ
Peroxisome proliferator‐activated receptor‐controlled transcription pathways have shown to be antiinflammatory in the brain in both stroke and TBI models. Upon dimerizing with an activating receptor, the PPARγ transcription factor translocates to the nucleus where it activates transcription 208. Although PPARγ expression increases in microglia after stroke and trauma, a reduction in available ligand leads to fewer instances of DNA binding such that PPAR transcription is suppressed 209, 210. PPARγ suppression can be reversed by introducing endogenous ligand or by treatment with agonists such as rosiglitazone and pioglitazone 211, 212. Treatment with PPARγ ligand after stroke decreases microglia and macrophage activation and curbs the expression of inflammatory molecules such as ICAM‐1, MMP‐9, IL‐1β, COX‐2, TNFα, iNOS, and reactive oxygen species 213, 214, 215. PPARγ agonists have also been shown to reduce inflammation and confer neuroprotection in animal models of traumatic injury 211, 216, 217.
Conclusion
The role of inflammation in brain injury has become an increasingly popular area of investigation, given its pleotropic roles in both acute damage and long‐term recovery. Microglia are key players in neuroinflammation and are considered the major immunocompetent cells of the brain. For this reason, much effort has been directed toward an understanding of how microglia become activated and how they mediate neuroinflammation. These efforts have led to many studies which have shown the beneficial and detrimental effects of microglial functions in brain ischemia and trauma. Modulation of microglial behaviors is clearly an important topic of investigation and represents a huge potential for the development of new therapeutic strategies in acute brain injury.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by grants from the National Institutes of Health (NIH) (NS40516 to MY), the Veteran's Merit Awards to MY, and American Hearts Association (13POST14810019) to JYK. Grants to MY and JYK were administered by the Northern California Institute for Research and Education and supported by resources of the Veterans Affairs Medical Center, San Francisco, California.
References
- 1. Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol 2007;184:53–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. El Khoury J, Hickman SE, Thomas CA, Loike JD, Silverstein SC. Microglia, scavenger receptors, and the pathogenesis of Alzheimer's disease. Neurobiol Aging 1998;19:S81–S84. [DOI] [PubMed] [Google Scholar]
- 3. Kreutzberg GW. Microglia: A sensor for pathological events in the CNS. Trends Neurosci 1996;19:312–318. [DOI] [PubMed] [Google Scholar]
- 4. Thomas WE. Brain macrophages. Evaluation of microglia and their functions. Brain Res Brain Res Rev 1992;17:61–74. [DOI] [PubMed] [Google Scholar]
- 5. Mittelbronn M, Dietz K, Schluesener HJ, Meyermann R. Local distribution of microglia in the normal adult human central nervous system differs by up to one order of magnitude. Acta Neuropathol 2001;101:249–255. [DOI] [PubMed] [Google Scholar]
- 6. Hanisch UK, Kettenmann H. Microglia: Active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci 2007;10:1387–1394. [DOI] [PubMed] [Google Scholar]
- 7. Saijo K, Glass CK. Microglial cell origin and phenotypes in health and disease. Nat Rev Immunol 2011;11:775–787. [DOI] [PubMed] [Google Scholar]
- 8. Appel SH, Zhao W, Beers DR, Henkel JS. The microglial‐motoneuron dialogue in ALS. Acta Myol 2011;30:4–8. [PMC free article] [PubMed] [Google Scholar]
- 9. Lai AY, Todd KG. Microglia in cerebral ischemia: Molecular actions and interactions. Can J Physiol Pharmacol 2006;84:49–59. [DOI] [PubMed] [Google Scholar]
- 10. Wood PL. Microglia as a unique cellular target in the treatment of stroke: Potential neurotoxic mediators produced by activated microglia. Neurol Res 1995;17:242–248. [DOI] [PubMed] [Google Scholar]
- 11. Chawla A. Control of macrophage activation and function by PPARs. Circ Res 2010;106:1559–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Geissmann F, Gordon S, Hume DA, Mowat AM, Randolph GJ. Unravelling mononuclear phagocyte heterogeneity. Nat Rev Immunol 2010;10:453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol 2005;5:953–964. [DOI] [PubMed] [Google Scholar]
- 14. Nguyen KD, Qiu Y, Cui X, et al. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature 2011;480:104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nair MG, Guild KJ, Artis D. Novel effector molecules in type 2 inflammation: Lessons drawn from helminth infection and allergy. J Immunol 2006;177:1393–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Colton CA. Heterogeneity of microglial activation in the innate immune response in the brain. J Neuroimmune Pharmacol 2009;4:399–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang G, Zhang J, Hu X, et al. Microglia/macrophage polarization dynamics in white matter after traumatic brain injury. J Cereb Blood Flow Metab 2013;33:1864–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hu X, Li P, Guo Y, et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke 2012;43:3063–3070. [DOI] [PubMed] [Google Scholar]
- 19. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 2008;8:958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 2004;25:677–686. [DOI] [PubMed] [Google Scholar]
- 21. Salemi J, Obregon DF, Cobb A, et al. Flipping the switches: CD40 and CD45 modulation of microglial activation states in HIV associated dementia (HAD). Mol Neurodegener 2011;6:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kroner A, Greenhalgh AD, Zarruk JG, Passos Dos Santos R, Gaestel M, David S. TNF and increased intracellular iron alter macrophage polarization to a detrimental M1 phenotype in the injured spinal cord. Neuron 2014;83:1098–1116. [DOI] [PubMed] [Google Scholar]
- 23. Kumar A, Stoica BA, Sabirzhanov B, Burns MP, Faden AI, Loane DJ. Traumatic brain injury in aged animals increases lesion size and chronically alters microglial/macrophage classical and alternative activation states. Neurobiol Aging 2013;34:1397–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol 1999;17:593–623. [DOI] [PubMed] [Google Scholar]
- 25. Trendelenburg G. Acute neurodegeneration and the inflammasome: Central processor for danger signals and the inflammatory response? J Cereb Blood Flow Metab 2008;28:867–881. [DOI] [PubMed] [Google Scholar]
- 26. Lehnardt S, Massillon L, Follett P, et al. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll‐like receptor 4‐dependent pathway. Proc Natl Acad Sci U S A 2003;100:8514–8519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kilic U, Kilic E, Matter CM, Bassetti CL, Hermann DM. TLR‐4 deficiency protects against focal cerebral ischemia and axotomy‐induced neurodegeneration. Neurobiol Dis 2008;31:33–40. [DOI] [PubMed] [Google Scholar]
- 28. Tang SC, Arumugam TV, Xu X, et al. Pivotal role for neuronal Toll‐like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci U S A 2007;104:13798–13803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hua F, Ma J, Ha T, et al. Activation of Toll‐like receptor 4 signaling contributes to hippocampal neuronal death following global cerebral ischemia/reperfusion. J Neuroimmunol 2007;190:101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Caso JR, Pradillo JM, Hurtado O, Lorenzo P, Moro MA, Lizasoain I. Toll‐like receptor 4 is involved in brain damage and inflammation after experimental stroke. Circulation 2007;115:1599–1608. [DOI] [PubMed] [Google Scholar]
- 31. Qiu J, Nishimura M, Wang Y, et al. Early release of HMGB‐1 from neurons after the onset of brain ischemia. J Cereb Blood Flow Metab 2008;28:927–938. [DOI] [PubMed] [Google Scholar]
- 32. Kim JB, Sig Choi J, Yu YM, et al. HMGB1, a novel cytokine‐like mediator linking acute neuronal death and delayed neuroinflammation in the postischemic brain. J Neurosci 2006;26:6413–6421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yang H, Wang H, Czura CJ, Tracey KJ. The cytokine activity of HMGB1. J Leukoc Biol 2005;78:1–8. [DOI] [PubMed] [Google Scholar]
- 34. Ghosh S, May MJ, Kopp EB. NF‐kappa B and Rel proteins: Evolutionarily conserved mediators of immune responses. Annu Rev Immunol 1998;16:225–260. [DOI] [PubMed] [Google Scholar]
- 35. Yilmaz G, Granger DN. Cell adhesion molecules and ischemic stroke. Neurol Res 2008;30:783–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lehnardt S, Schott E, Trimbuch T, et al. A vicious cycle involving release of heat shock protein 60 from injured cells and activation of toll‐like receptor 4 mediates neurodegeneration in the CNS. J Neurosci 2008;28:2320–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pradillo JM, Fernandez‐Lopez D, Garcia‐Yebenes I, et al. Toll‐like receptor 4 is involved in neuroprotection afforded by ischemic preconditioning. J Neurochem 2009;109:287–294. [DOI] [PubMed] [Google Scholar]
- 38. Marsh B, Stevens SL, Packard AE, et al. Systemic lipopolysaccharide protects the brain from ischemic injury by reprogramming the response of the brain to stroke: A critical role for IRF3. J Neurosci 2009;29:9839–9849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stevens SL, Ciesielski TM, Marsh BJ, et al. Toll‐like receptor 9: A new target of ischemic preconditioning in the brain. J Cereb Blood Flow Metab 2008;28:1040–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sperlagh B, Illes P. Purinergic modulation of microglial cell activation. Purinergic Signal 2007;3:117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Suzuki T, Hide I, Ido K, Kohsaka S, Inoue K, Nakata Y. Production and release of neuroprotective tumor necrosis factor by P2X7 receptor‐activated microglia. J Neurosci 2004;24:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Morigiwa K, Quan M, Murakami M, Yamashita M, Fukuda Y. P2 Purinoceptor expression and functional changes of hypoxia‐activated cultured rat retinal microglia. Neurosci Lett 2000;282:153–156. [DOI] [PubMed] [Google Scholar]
- 43. Hide I, Tanaka M, Inoue A, et al. Extracellular ATP triggers tumor necrosis factor‐alpha release from rat microglia. J Neurochem 2000;75:965–972. [DOI] [PubMed] [Google Scholar]
- 44. Monif M, Reid CA, Powell KL, Smart ML, Williams DA. The P2X7 receptor drives microglial activation and proliferation: A trophic role for P2X7R pore. J Neurosci 2009;29:3781–3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bianco F, Ceruti S, Colombo A, et al. A role for P2X7 in microglial proliferation. J Neurochem 2006;99:745–758. [DOI] [PubMed] [Google Scholar]
- 46. Parvathenani LK, Tertyshnikova S, Greco CR, Roberts SB, Robertson B, Posmantur R. P2X7 mediates superoxide production in primary microglia and is up‐regulated in a transgenic mouse model of Alzheimer's disease. J Biol Chem 2003;278:13309–13317. [DOI] [PubMed] [Google Scholar]
- 47. Inoue K, Nakajima K, Morimoto T, et al. ATP stimulation of Ca2+ ‐dependent plasminogen release from cultured microglia. Br J Pharmacol 1998;123:1304–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ferrari D, Stroh C, Schulze‐Osthoff K. P2X7/P2Z purinoreceptor‐mediated activation of transcription factor NFAT in microglial cells. J Biol Chem 1999;274:13205–13210. [DOI] [PubMed] [Google Scholar]
- 49. Ferrari D, Wesselborg S, Bauer MK, Schulze‐Osthoff K. Extracellular ATP activates transcription factor NF‐kappaB through the P2Z purinoreceptor by selectively targeting NF‐kappaB p65. J Cell Biol 1997;139:1635–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fang KM, Yang CS, Sun SH, Tzeng SF. Microglial phagocytosis attenuated by short‐term exposure to exogenous ATP through P2X receptor action. J Neurochem 2009;111:1225–1237. [DOI] [PubMed] [Google Scholar]
- 51. Peng W, Cotrina ML, Han X, et al. Systemic administration of an antagonist of the ATP‐sensitive receptor P2X7 improves recovery after spinal cord injury. Proc Natl Acad Sci U S A 2009;106:12489–12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Melani A, Amadio S, Gianfriddo M, et al. P2X7 receptor modulation on microglial cells and reduction of brain infarct caused by middle cerebral artery occlusion in rat. J Cereb Blood Flow Metab 2006;26:974–982. [DOI] [PubMed] [Google Scholar]
- 53. Yanagisawa D, Kitamura Y, Takata K, Hide I, Nakata Y, Taniguchi T. Possible involvement of P2X7 receptor activation in microglial neuroprotection against focal cerebral ischemia in rats. Biol Pharm Bull 2008;31:1121–1130. [DOI] [PubMed] [Google Scholar]
- 54. Honda S, Sasaki Y, Ohsawa K, et al. Extracellular ATP or ADP induce chemotaxis of cultured microglia through Gi/o‐coupled P2Y receptors. J Neurosci 2001;21:1975–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ohsawa K, Irino Y, Nakamura Y, Akazawa C, Inoue K, Kohsaka S. Involvement of P2X4 and P2Y12 receptors in ATP‐induced microglial chemotaxis. Glia 2007;55:604–616. [DOI] [PubMed] [Google Scholar]
- 56. Irino Y, Nakamura Y, Inoue K, Kohsaka S, Ohsawa K. Akt activation is involved in P2Y12 receptor‐mediated chemotaxis of microglia. J Neurosci Res 2008;86:1511–1519. [DOI] [PubMed] [Google Scholar]
- 57. Tozaki‐Saitoh H, Tsuda M, Miyata H, Ueda K, Kohsaka S, Inoue K. P2Y12 receptors in spinal microglia are required for neuropathic pain after peripheral nerve injury. J Neurosci 2008;28:4949–4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Webster CM, Hokari M, McManus A, et al. Microglial P2Y12 deficiency/inhibition protects against brain ischemia. PLoS ONE 2013;8:e70927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Abbracchio MP, Burnstock G, Boeynaems JM, et al. International Union of Pharmacology LVIII: Update on the P2Y G protein‐coupled nucleotide receptors: From molecular mechanisms and pathophysiology to therapy. Pharmacol Rev 2006;58:281–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev 1998;50:413–492. [PubMed] [Google Scholar]
- 61. Anderson CM, Bergher JP, Swanson RA. ATP‐induced ATP release from astrocytes. J Neurochem 2004;88:246–256. [DOI] [PubMed] [Google Scholar]
- 62. Takenouchi T, Sugama S, Iwamaru Y, Hashimoto M, Kitani H. Modulation of the ATP‐lnduced release and processing of IL‐1beta in microglial cells. Crit Rev Immunol 2009;29:335–345. [DOI] [PubMed] [Google Scholar]
- 63. Brough D, Le Feuvre RA, Iwakura Y, Rothwell NJ. Purinergic (P2X7) receptor activation of microglia induces cell death via an interleukin‐1‐independent mechanism. Mol Cell Neurosci 2002;19:272–280. [DOI] [PubMed] [Google Scholar]
- 64. Kielian T, Drew PD. Effects of peroxisome proliferator‐activated receptor‐gamma agonists on central nervous system inflammation. J Neurosci Res 2003;71:315–325. [DOI] [PubMed] [Google Scholar]
- 65. Desvergne B, Wahli W. Peroxisome proliferator‐activated receptors: Nuclear control of metabolism. Endocr Rev 1999;20:649–688. [DOI] [PubMed] [Google Scholar]
- 66. Basu‐Modak S, Braissant O, Escher P, Desvergne B, Honegger P, Wahli W. Peroxisome proliferator‐activated receptor beta regulates acyl‐CoA synthetase 2 in reaggregated rat brain cell cultures. J Biol Chem 1999;274:35881–35888. [DOI] [PubMed] [Google Scholar]
- 67. Mabuchi T, Kitagawa K, Ohtsuki T, et al. Contribution of microglia/macrophages to expansion of infarction and response of oligodendrocytes after focal cerebral ischemia in rats. Stroke 2000;31:1735–1743. [DOI] [PubMed] [Google Scholar]
- 68. Pantoni L, Sarti C, Inzitari D. Cytokines and cell adhesion molecules in cerebral ischemia: Experimental bases and therapeutic perspectives. Arterioscler Thromb Vasc Biol 1998;18:503–513. [DOI] [PubMed] [Google Scholar]
- 69. Bordet R, Ouk T, Petrault O, et al. PPAR: A new pharmacological target for neuroprotection in stroke and neurodegenerative diseases. Biochem Soc Trans 2006;34:1341–1346. [DOI] [PubMed] [Google Scholar]
- 70. Lin TN, Cheung WM, Wu JS, et al. 15d‐prostaglandin J2 protects brain from ischemia‐reperfusion injury. Arterioscler Thromb Vasc Biol 2006;26:481–487. [DOI] [PubMed] [Google Scholar]
- 71. N'Diaye EN, Branda CS, Branda SS, et al. TREM‐2 (triggering receptor expressed on myeloid cells 2) is a phagocytic receptor for bacteria. J Cell Biol 2009;184:215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Napoli I, Neumann H. Protective effects of microglia in multiple sclerosis. Exp Neurol 2010;225:24–28. [DOI] [PubMed] [Google Scholar]
- 73. Klesney‐Tait J, Turnbull IR, Colonna M. The TREM receptor family and signal integration. Nat Immunol 2006;7:1266–1273. [DOI] [PubMed] [Google Scholar]
- 74. Daws MR, Sullam PM, Niemi EC, Chen TT, Tchao NK, Seaman WE. Pattern recognition by TREM‐2: Binding of anionic ligands. J Immunol 2003;171:594–599. [DOI] [PubMed] [Google Scholar]
- 75. Schenk M, Bouchon A, Birrer S, Colonna M, Mueller C. Macrophages expressing triggering receptor expressed on myeloid cells‐1 are underrepresented in the human intestine. J Immunol 2005;174:517–524. [DOI] [PubMed] [Google Scholar]
- 76. Bouchon A, Facchetti F, Weigand MA, Colonna M. TREM‐1 amplifies inflammation and is a crucial mediator of septic shock. Nature 2001;410:1103–1107. [DOI] [PubMed] [Google Scholar]
- 77. Bouchon A, Dietrich J, Colonna M. Cutting edge: Inflammatory responses can be triggered by TREM‐1, a novel receptor expressed on neutrophils and monocytes. J Immunol 2000;164:4991–4995. [DOI] [PubMed] [Google Scholar]
- 78. Colonna M. TREMs in the immune system and beyond. Nat Rev Immunol 2003;3:445–453. [DOI] [PubMed] [Google Scholar]
- 79. Bouchon A, Hernandez‐Munain C, Cella M, Colonna M. A DAP12‐mediated pathway regulates expression of CC chemokine receptor 7 and maturation of human dendritic cells. J Exp Med 2001;194:1111–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Stefano L, Racchetti G, Bianco F, et al. The surface‐exposed chaperone, Hsp60, is an agonist of the microglial TREM2 receptor. J Neurochem 2009;110:284–294. [DOI] [PubMed] [Google Scholar]
- 81. Soltys BJ, Gupta RS. Mitochondrial proteins at unexpected cellular locations: Export of proteins from mitochondria from an evolutionary perspective. Int Rev Cytol 2000;194:133–196. [DOI] [PubMed] [Google Scholar]
- 82. Takahashi K, Rochford CD, Neumann H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells‐2. J Exp Med 2005;201:647–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Piccio L, Buonsanti C, Mariani M, et al. Blockade of TREM‐2 exacerbates experimental autoimmune encephalomyelitis. Eur J Immunol 2007;37:1290–1301. [DOI] [PubMed] [Google Scholar]
- 84. Hsieh CL, Koike M, Spusta SC, et al. A role for TREM2 ligands in the phagocytosis of apoptotic neuronal cells by microglia. J Neurochem 2009;109:1144–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kawabori M, Yenari MA. The role of the microglia in acute CNS injury. Metab Brain Dis 2015;30:381–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Fu R, Shen Q, Xu P, Luo JJ, Tang Y. Phagocytosis of microglia in the central nervous system diseases. Mol Neurobiol 2014;49:1422–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kawabori M, Hokari M, Zheng Z, et al. Triggering receptor expressed on myeloid cells‐2 correlates to hypothermic neuroprotection in ischemic stroke. Ther Hypothermia Temp Manag 2013;3:189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sieber MW, Jaenisch N, Brehm M, et al. Attenuated inflammatory response in triggering receptor expressed on myeloid cells 2 (TREM2) knock‐out mice following stroke. PLoS ONE 2013;8:e52982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ransohoff RM, Perry VH. Microglial physiology: Unique stimuli, specialized responses. Annu Rev Immunol 2009;27:119–145. [DOI] [PubMed] [Google Scholar]
- 90. Lassegue B, Clempus RE. Vascular NAD(P)H oxidases: Specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol 2003;285:R277–R297. [DOI] [PubMed] [Google Scholar]
- 91. Kauppinen TM, Higashi Y, Suh SW, Escartin C, Nagasawa K, Swanson RA. Zinc triggers microglial activation. J Neurosci 2008;28:5827–5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Mander PK, Jekabsone A, Brown GC. Microglia proliferation is regulated by hydrogen peroxide from NADPH oxidase. J Immunol 2006;176:1046–1052. [DOI] [PubMed] [Google Scholar]
- 93. Groemping Y, Rittinger K. Activation and assembly of the NADPH oxidase: A structural perspective. Biochem J 2005;386:401–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol 2004;4:181–189. [DOI] [PubMed] [Google Scholar]
- 95. Tang XN, Cairns B, Kim JY, Yenari MA. NADPH oxidase in stroke and cerebrovascular disease. Neurol Res 2012;34:338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Walder CE, Green SP, Darbonne WC, et al. Ischemic stroke injury is reduced in mice lacking a functional NADPH oxidase. Stroke 1997;28:2252–2258. [DOI] [PubMed] [Google Scholar]
- 97. Tang XN, Zheng Z, Giffard RG, Yenari MA. Significance of marrow‐derived nicotinamide adenine dinucleotide phosphate oxidase in experimental ischemic stroke. Ann Neurol 2011;70:606–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Yenari MA, Xu L, Tang XN, Qiao Y, Giffard RG. Microglia potentiate damage to blood–brain barrier constituents: Improvement by minocycline in vivo and in vitro. Stroke 2006;37:1087–1093. [DOI] [PubMed] [Google Scholar]
- 99. Chen H, Song YS, Chan PH. Inhibition of NADPH oxidase is neuroprotective after ischemia‐reperfusion. J Cereb Blood Flow Metab 2009;29:1262–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Tang XN, Cairns B, Cairns N, Yenari MA. Apocynin improves outcome in experimental stroke with a narrow dose range. Neuroscience 2008;154:556–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Tang LL, Ye K, Yang XF, Zheng JS. Apocynin attenuates cerebral infarction after transient focal ischaemia in rats. J Int Med Res 2007;35:517–522. [DOI] [PubMed] [Google Scholar]
- 102. Wang Q, Tompkins KD, Simonyi A, Korthuis RJ, Sun AY, Sun GY. Apocynin protects against global cerebral ischemia‐reperfusion‐induced oxidative stress and injury in the gerbil hippocampus. Brain Res 2006;1090:182–189. [DOI] [PubMed] [Google Scholar]
- 103. Chen CM, Liu SH, Lin‐Shiau SY. Honokiol, a neuroprotectant against mouse cerebral ischaemia, mediated by preserving Na+, K+‐ATPase activity and mitochondrial functions. Basic Clin Pharmacol Toxicol 2007;101:108–116. [DOI] [PubMed] [Google Scholar]
- 104. Liou KT, Shen YC, Chen CF, Tsao CM, Tsai SK. Honokiol protects rat brain from focal cerebral ischemia‐reperfusion injury by inhibiting neutrophil infiltration and reactive oxygen species production. Brain Res 2003;992:159–166. [DOI] [PubMed] [Google Scholar]
- 105. Suh SW, Shin BS, Ma H, et al. Glucose and NADPH oxidase drive neuronal superoxide formation in stroke. Ann Neurol 2008;64:654–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Guix FX, Uribesalgo I, Coma M, Munoz FJ. The physiology and pathophysiology of nitric oxide in the brain. Prog Neurobiol 2005;76:126–152. [DOI] [PubMed] [Google Scholar]
- 107. Droge W. Free radicals in the physiological control of cell function. Physiol Rev 2002;82:47–95. [DOI] [PubMed] [Google Scholar]
- 108. Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: Therapeutic approaches. J Transl Med 2009;7:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Murphy S. Production of nitric oxide by glial cells: Regulation and potential roles in the CNS. Glia 2000;29:1–13. [DOI] [PubMed] [Google Scholar]
- 110. Iadecola C, Zhang F, Xu X. Inhibition of inducible nitric oxide synthase ameliorates cerebral ischemic damage. Am J Physiol 1995;268:R286–R292. [DOI] [PubMed] [Google Scholar]
- 111. Iadecola C, Zhang F, Xu S, Casey R, Ross ME. Inducible nitric oxide synthase gene expression in brain following cerebral ischemia. J Cereb Blood Flow Metab 1995;15:378–384. [DOI] [PubMed] [Google Scholar]
- 112. Cui J, Holmes EH, Liu PK. Oxidative damage to the c‐fos gene and reduction of its transcription after focal cerebral ischemia. J Neurochem 1999;73:1164–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Huang J, Choudhri TF, Winfree CJ, et al. Postischemic cerebrovascular E‐selectin expression mediates tissue injury in murine stroke. Stroke 2000;31:3047–3053. [PubMed] [Google Scholar]
- 114. Cho S, Park EM, Zhou P, Frys K, Ross ME, Iadecola C. Obligatory role of inducible nitric oxide synthase in ischemic preconditioning. J Cereb Blood Flow Metab 2005;25:493–501. [DOI] [PubMed] [Google Scholar]
- 115. Colton CA. Induction of nitric oxide in cultured microglia: Evidence for a cytoprotective role. Adv Neuroimmunol 1995;5:491–503. [DOI] [PubMed] [Google Scholar]
- 116. Merrill JE, Ignarro LJ, Sherman MP, Melinek J, Lane TE. Microglial cell cytotoxicity of oligodendrocytes is mediated through nitric oxide. J Immunol 1993;151:2132–2141. [PubMed] [Google Scholar]
- 117. Kacimi R, Giffard RG, Yenari MA. Endotoxin‐activated microglia injure brain derived endothelial cells via NF‐kappaB, JAK‐STAT and JNK stress kinase pathways. J Inflamm 2011;8:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Thiel VE, Audus KL. Nitric oxide and blood–brain barrier integrity. Antioxid Redox Signal 2001;3:273–278. [DOI] [PubMed] [Google Scholar]
- 119. Zhao X, Haensel C, Araki E, Ross ME, Iadecola C. Gene‐dosing effect and persistence of reduction in ischemic brain injury in mice lacking inducible nitric oxide synthase. Brain Res 2000;872:215–218. [DOI] [PubMed] [Google Scholar]
- 120. Park EM, Cho S, Frys KA, et al. Inducible nitric oxide synthase contributes to gender differences in ischemic brain injury. J Cereb Blood Flow Metab 2006;26:392–401. [DOI] [PubMed] [Google Scholar]
- 121. Coughlan T, Gibson C, Murphy S. Modulatory effects of progesterone on inducible nitric oxide synthase expression in vivo and in vitro. J Neurochem 2005;93:932–942. [DOI] [PubMed] [Google Scholar]
- 122. Han HS, Qiao Y, Karabiyikoglu M, Giffard RG, Yenari MA. Influence of mild hypothermia on inducible nitric oxide synthase expression and reactive nitrogen production in experimental stroke and inflammation. J Neurosci 2002;22:3921–3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Candelario‐Jalil E, Yang Y, Rosenberg GA. Diverse roles of matrix metalloproteinases and tissue inhibitors of metalloproteinases in neuroinflammation and cerebral ischemia. Neuroscience 2009;158:983–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. del Zoppo GJ, Milner R, Mabuchi T, et al. Microglial activation and matrix protease generation during focal cerebral ischemia. Stroke 2007;38:646–651. [DOI] [PubMed] [Google Scholar]
- 125. Rosenberg GA, Cunningham LA, Wallace J, et al. Immunohistochemistry of matrix metalloproteinases in reperfusion injury to rat brain: Activation of MMP‐9 linked to stromelysin‐1 and microglia in cell cultures. Brain Res 2001;893:104–112. [DOI] [PubMed] [Google Scholar]
- 126. del Zoppo GJ, Frankowski H, Gu YH, et al. Microglial cell activation is a source of metalloproteinase generation during hemorrhagic transformation. J Cereb Blood Flow Metab 2012;32:919–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Montaner J, Alvarez‐Sabin J, Molina C, et al. Matrix metalloproteinase expression after human cardioembolic stroke: Temporal profile and relation to neurological impairment. Stroke 2001;32:1759–1766. [DOI] [PubMed] [Google Scholar]
- 128. Gidday JM, Gasche YG, Copin JC, et al. Leukocyte‐derived matrix metalloproteinase‐9 mediates blood–brain barrier breakdown and is proinflammatory after transient focal cerebral ischemia. Am J Physiol Heart Circ Physiol 2005;289:H558–H568. [DOI] [PubMed] [Google Scholar]
- 129. Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br J Pharmacol 2006;147(Suppl 1):S232–S240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Schmitz T, Chew LJ. Cytokines and myelination in the central nervous system. ScientificWorldJournal 2008;8:1119–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Shohami E, Ginis I, Hallenbeck JM. Dual role of tumor necrosis factor alpha in brain injury. Cytokine Growth Factor Rev 1999;10:119–130. [DOI] [PubMed] [Google Scholar]
- 132. Vexler ZS, Tang XN, Yenari MA. Inflammation in adult and neonatal stroke. Clin Neurosci Res 2006;6:293–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Gregersen R, Lambertsen K, Finsen B. Microglia and macrophages are the major source of tumor necrosis factor in permanent middle cerebral artery occlusion in mice. J Cereb Blood Flow Metab 2000;20:53–65. [DOI] [PubMed] [Google Scholar]
- 134. Schielke GP, Yang GY, Shivers BD, Betz AL. Reduced ischemic brain injury in interleukin‐1 beta converting enzyme‐deficient mice. J Cereb Blood Flow Metab 1998;18:180–185. [DOI] [PubMed] [Google Scholar]
- 135. Strle K, Zhou JH, Shen WH, et al. Interleukin‐10 in the brain. Crit Rev Immunol 2001;21:427–449. [PubMed] [Google Scholar]
- 136. Pang L, Ye W, Che XM, Roessler BJ, Betz AL, Yang GY. Reduction of inflammatory response in the mouse brain with adenoviral‐mediated transforming growth factor‐ss1 expression. Stroke 2001;32:544–552. [DOI] [PubMed] [Google Scholar]
- 137. Flanders KC, Ren RF, Lippa CF. Transforming growth factor‐betas in neurodegenerative disease. Prog Neurobiol 1998;54:71–85. [DOI] [PubMed] [Google Scholar]
- 138. Lu YZ, Lin CH, Cheng FC, Hsueh CM. Molecular mechanisms responsible for microglia‐derived protection of Sprague–Dawley rat brain cells during in vitro ischemia. Neurosci Lett 2005;373:159–164. [DOI] [PubMed] [Google Scholar]
- 139. Lee TH, Kato H, Chen ST, Kogure K, Itoyama Y. Expression disparity of brain‐derived neurotrophic factor immunoreactivity and mRNA in ischemic hippocampal neurons. NeuroReport 2002;13:2271–2275. [DOI] [PubMed] [Google Scholar]
- 140. Suzuki S, Tanaka K, Nogawa S, et al. Temporal profile and cellular localization of interleukin‐6 protein after focal cerebral ischemia in rats. J Cereb Blood Flow Metab 1999;19:1256–1262. [DOI] [PubMed] [Google Scholar]
- 141. Batchelor PE, Liberatore GT, Wong JY, et al. Activated macrophages and microglia induce dopaminergic sprouting in the injured striatum and express brain‐derived neurotrophic factor and glial cell line‐derived neurotrophic factor. J Neurosci 1999;19:1708–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Lehrmann E, Kiefer R, Christensen T, et al. Microglia and macrophages are major sources of locally produced transforming growth factor‐beta1 after transient middle cerebral artery occlusion in rats. Glia 1998;24:437–448. [DOI] [PubMed] [Google Scholar]
- 143. Terao Y, Ohta H, Oda A, Nakagaito Y, Kiyota Y, Shintani Y. Macrophage inflammatory protein‐3alpha plays a key role in the inflammatory cascade in rat focal cerebral ischemia. Neurosci Res 2009;64:75–82. [DOI] [PubMed] [Google Scholar]
- 144. Terao S, Yilmaz G, Stokes KY, et al. Blood cell‐derived RANTES mediates cerebral microvascular dysfunction, inflammation, and tissue injury after focal ischemia‐reperfusion. Stroke 2008;39:2560–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol 2004;173:3916–3924. [DOI] [PubMed] [Google Scholar]
- 146. Hanisch UK. Microglia as a source and target of cytokines. Glia 2002;40:140–155. [DOI] [PubMed] [Google Scholar]
- 147. Semple BD, Kossmann T, Morganti‐Kossmann MC. Role of chemokines in CNS health and pathology: A focus on the CCL2/CCR2 and CXCL8/CXCR2 networks. J Cereb Blood Flow Metab 2010;30:459–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Mildner A, Mack M, Schmidt H, et al. CCR2+Ly‐6Chi monocytes are crucial for the effector phase of autoimmunity in the central nervous system. Brain 2009;132:2487–2500. [DOI] [PubMed] [Google Scholar]
- 149. King IL, Dickendesher TL, Segal BM. Circulating Ly‐6C+ myeloid precursors migrate to the CNS and play a pathogenic role during autoimmune demyelinating disease. Blood 2009;113:3190–3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol 2011;11:762–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Neumann J, Gunzer M, Gutzeit HO, Ullrich O, Reymann KG, Dinkel K. Miroglia provide neuroprotection after ischemia. FASEB J 2006;20:714–716. [DOI] [PubMed] [Google Scholar]
- 152. vNeumann J, Sauerzweig S, Rönicke R, et al. Microglia cells protect neurons by direct engulfment of invading neutrophil granulocytes: A new mechanism of CNS immune privilege. J Neurosci 2008;28:5965–5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Lalancette‐Hébert M, Gowing G, Simard A, Weng YC, Kriz J. Selective ablation of proliferation microglial cell exacerbates ischemic injury in the brain. J Neurosci 2007;27:2596–25605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Lambertsen KL, Clausen BH, Babcock AA, et al. J Neurosci 2009;29:1319–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. An C, Shi Y, Li P, et al. Molecular dialogs between the ischemic brain and the peripheral immune system: Dualistic roles in injury and repair. Prog Neurobiol 2014;115:6–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Koizumi S, Shigemoto‐Mogami Y, Nasu‐Tada K, et al. UDP acting at P2Y6 receptors is a mediator of microglia phagocytosis. Nature 2007;446:1091–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Haynes SE, Hollopeter G, Yang G, et al. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci 2006;9:1512–1519. [DOI] [PubMed] [Google Scholar]
- 158. Emmrich JV, Hornik TC, Neher JJ, Brown GC. Rotenone induces neuronal death by microglial phagocytosis of neurons. FEBS J 2013;280:5030–5038. [DOI] [PubMed] [Google Scholar]
- 159. Kawabori M, Kacimi R, Kauppinen T, et al. Triggering receptor expressed on myeloid cells 2 (TREM2) deficiency attenuates phagocytic activities of microglia and exacerbates ischemic damage in experimental stroke. Stroke 2014;45:A9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Benarroch EE. Microglia: Multiple roles in surveillance, circuit shaping, and response to injury. Neurology 2013;81:1079–1088. [DOI] [PubMed] [Google Scholar]
- 161. Yenari MA, Kauppinen TM, Swanson RA. Microglial activation in stroke: Therapeutic targets. Neurotherapeutics 2010;7:378–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Loane DJ, Byrnes KR. Role of microglia in neurotrauma. Neurotherapeutics 2010;7:366–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Towfighi A, Saver JL. Stroke declines from third to fourth leading cause of death in the United States: Historical perspective and challenges ahead. Stroke 2011;42:2351–2355. [DOI] [PubMed] [Google Scholar]
- 164. Giulian D, Corpuz M, Chapman S, Mansouri M, Robertson C. Reactive mononuclear phagocytes release neurotoxins after ischemic and traumatic injury to the central nervous system. J Neurosci Res 1993;36:681–693. [DOI] [PubMed] [Google Scholar]
- 165. Huang WC, Qiao Y, Xu L, et al. Direct protection of cultured neurons from ischemia‐like injury by minocycline. Anat Cell Biol 2010;43:325–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Kriz J. Inflammation in ischemic brain injury: Timing is important. Crit Rev Neurobiol 2006;18:145–157. [DOI] [PubMed] [Google Scholar]
- 167. Zhao BQ, Wang S, Kim HY, et al. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med 2006;12:441–445. [DOI] [PubMed] [Google Scholar]
- 168. Coronado VG, Xu L, Basavaraju SV, et al. Surveillance for traumatic brain injury‐related deaths–United States, 1997–2007. MMWR Surveill Summ 2011;60:1–32. [PubMed] [Google Scholar]
- 169. Smith C. Review: The long‐term consequences of microglial activation following acute traumatic brain injury. Neuropathol Appl Neurobiol 2013;39:35–44. [DOI] [PubMed] [Google Scholar]
- 170. Povlishock JT, Katz DI. Update of neuropathology and neurological recovery after traumatic brain injury. J Head Trauma Rehabil 2005;20:76–94. [DOI] [PubMed] [Google Scholar]
- 171. Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci 2009;29:13435–13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Dusart I, Schwab ME. Secondary cell death and the inflammatory reaction after dorsal hemisection of the rat spinal cord. Eur J Neurosci 1994;6:712–724. [DOI] [PubMed] [Google Scholar]
- 173. Davalos D, Grutzendler J, Yang G, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 2005;8:752–758. [DOI] [PubMed] [Google Scholar]
- 174. Gentleman SM, Leclercq PD, Moyes L, et al. Long‐term intracerebral inflammatory response after traumatic brain injury. Forensic Sci Int 2004;146:97–104. [DOI] [PubMed] [Google Scholar]
- 175. Engel S, Schluesener H, Mittelbronn M, et al. Dynamics of microglial activation after human traumatic brain injury are revealed by delayed expression of macrophage‐related proteins MRP8 and MRP14. Acta Neuropathol 2000;100:313–322. [DOI] [PubMed] [Google Scholar]
- 176. Bramlett HM, Dietrich WD. Quantitative structural changes in white and gray matter 1 year following traumatic brain injury in rats. Acta Neuropathol 2002;103:607–614. [DOI] [PubMed] [Google Scholar]
- 177. Smith DH, Chen XH, Pierce JE, et al. Progressive atrophy and neuron death for one year following brain trauma in the rat. J Neurotrauma 1997;14:715–727. [DOI] [PubMed] [Google Scholar]
- 178. Block ML, Zecca L, Hong JS. Microglia‐mediated neurotoxicity: Uncovering the molecular mechanisms. Nat Rev Neurosci 2007;8:57–69. [DOI] [PubMed] [Google Scholar]
- 179. Holmin S, Mathiesen T. Long‐term intracerebral inflammatory response after experimental focal brain injury in rat. NeuroReport 1999;10:1889–1891. [DOI] [PubMed] [Google Scholar]
- 180. Guo Z, Cupples LA, Kurz A, et al. Head injury and the risk of AD in the MIRAGE study. Neurology 2000;54:1316–1323. [DOI] [PubMed] [Google Scholar]
- 181. Plassman BL, Havlik RJ, Steffens DC, et al. Documented head injury in early adulthood and risk of Alzheimer's disease and other dementias. Neurology 2000;55:1158–1166. [DOI] [PubMed] [Google Scholar]
- 182. Mayeux R, Ottman R, Tang MX, et al. Genetic susceptibility and head injury as risk factors for Alzheimer's disease among community‐dwelling elderly persons and their first‐degree relatives. Ann Neurol 1993;33:494–501. [DOI] [PubMed] [Google Scholar]
- 183. Tymianski M. Novel approaches to neuroprotection trials in acute ischemic stroke. Stroke 2013;44:2942–2950. [DOI] [PubMed] [Google Scholar]
- 184. Maas AI, Roozenbeek B, Manley GT. Clinical trials in traumatic brain injury: Past experience and current developments. Neurotherapeutics, 2010;7:115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Kumar A, Loane DJ. Neuroinflammation after traumatic brain injury: Opportunities for therapeutic intervention. Brain Behav Immun 2010;26:1191–1201. [DOI] [PubMed] [Google Scholar]
- 186. Choi Y, Kim HS, Shin KY, et al. Minocycline attenuates neuronal cell death and improves cognitive impairment in Alzheimer's disease models. Neuropsychopharmacology 2007;32:2393–2404. [DOI] [PubMed] [Google Scholar]
- 187. Wang X, Zhu S, Drozda M, et al. Minocycline inhibits caspase‐independent and ‐dependent mitochondrial cell death pathways in models of Huntington's disease. Proc Natl Acad Sci U S A 2003;100:10483–10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Zhu S, Stavrovskaya IG, Drozda M, et al. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature 2002;417:74–78. [DOI] [PubMed] [Google Scholar]
- 189. Wu DC, Jackson‐Lewis V, Vila M, et al. Blockade of microglial activation is neuroprotective in the 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine mouse model of Parkinson disease. J Neurosci 2002;22:1763–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Popovic N, Schubart A, Goetz BD, Zhang SC, Linington C, Duncan ID. Inhibition of autoimmune encephalomyelitis by a tetracycline. Ann Neurol 2002;51:215–223. [DOI] [PubMed] [Google Scholar]
- 191. Chen M, Ona VO, Li M, et al. Minocycline inhibits caspase‐1 and caspase‐3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat Med 2000;6:797–801. [DOI] [PubMed] [Google Scholar]
- 192. Yrjanheikki J, Tikka T, Keinanen R, Goldsteins G, Chan PH, Koistinaho J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci U S A 1999;96:13496–13500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Seabrook TJ, Jiang L, Maier M, Lemere CA. Minocycline affects microglia activation, Abeta deposition, and behavior in APP‐tg mice. Glia 2006;53:776–782. [DOI] [PubMed] [Google Scholar]
- 194. Tikka T, Fiebich BL, Goldsteins G, Keinanen R, Koistinaho J. Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J Neurosci 2001;21:2580–2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Koistinaho M, Malm TM, Kettunen MI, et al. Minocycline protects against permanent cerebral ischemia in wild type but not in matrix metalloprotease‐9‐deficient mice. J Cereb Blood Flow Metab 2005;25:460–467. [DOI] [PubMed] [Google Scholar]
- 196. Pfefferkorn T, Rosenberg GA. Closure of the blood–brain barrier by matrix metalloproteinase inhibition reduces rtPA‐mediated mortality in cerebral ischemia with delayed reperfusion. Stroke 2003;34:2025–2030. [DOI] [PubMed] [Google Scholar]
- 197. Sanchez Mejia RO, Ona VO, Li M, Friedlander RM. Minocycline reduces traumatic brain injury‐mediated caspase‐1 activation, tissue damage, and neurological dysfunction. Neurosurgery 2001;48:1393–1401. [DOI] [PubMed] [Google Scholar]
- 198. Touyz RM. Apocynin, NADPH oxidase, and vascular cells: A complex matter. Hypertension 2008;51:172–174. [DOI] [PubMed] [Google Scholar]
- 199. Liu W, Sood R, Chen Q, et al. Normobaric hyperoxia inhibits NADPH oxidase‐mediated matrix metalloproteinase‐9 induction in cerebral microvessels in experimental stroke. J Neurochem 2008;107:1196–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Zhu HT, Bian C, Yuan JC, et al. Curcumin attenuates acute inflammatory injury by inhibiting the TLR4/MyD88/NF‐kappaB signaling pathway in experimental traumatic brain injury. J Neuroinflammation 2014;11:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Zhang D, Li H, Li T, et al. TLR4 inhibitor resatorvid provides neuroprotection in experimental traumatic brain injury: Implication in the treatment of human brain injury. Neurochem Int 2014;75:11–18. [DOI] [PubMed] [Google Scholar]
- 202. Su X, Wang H, Zhao J, Pan H, Mao L. Beneficial effects of ethyl pyruvate through inhibiting high‐mobility group box 1 expression and TLR4/NF‐kappaB pathway after traumatic brain injury in the rat. Mediators Inflamm 2011;2011:807142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Brea D, Blanco M, Ramos‐Cabrer P, et al. Toll‐like receptors 2 and 4 in ischemic stroke: Outcome and therapeutic values. J Cereb Blood Flow Metab 2011;31:1424–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Clark IA. How TNF was recognized as a key mechanism of disease. Cytokine Growth Factor Rev 2007;18:335–343. [DOI] [PubMed] [Google Scholar]
- 205. Yoon JS, Lee JH, Tweedie D, et al. 3,6′‐dithiothalidomide improves experimental stroke outcome by suppressing neuroinflammation. J Neurosci Res 2013;91:671–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Sumbria RK, Boado RJ, Pardridge WM. Brain protection from stroke with intravenous TNFalpha decoy receptor‐Trojan horse fusion protein. J Cereb Blood Flow Metab 2012;32:1933–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Belarbi K, Jopson T, Tweedie D, et al. TNF‐alpha protein synthesis inhibitor restores neuronal function and reverses cognitive deficits induced by chronic neuroinflammation. J Neuroinflammation 2012;9:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med 2002;53:409–435. [DOI] [PubMed] [Google Scholar]
- 209. Victor NA, Wanderi EW, Gamboa J, et al. Altered PPARgamma expression and activation after transient focal ischemia in rats. Eur J Neurosci 2006;24:1653–1663. [DOI] [PubMed] [Google Scholar]
- 210. Chawla A, Barak Y, Nagy L, Liao D, Tontonoz P, Evans RM. PPAR‐gamma dependent and independent effects on macrophage‐gene expression in lipid metabolism and inflammation. Nat Med 2001;7:48–52. [DOI] [PubMed] [Google Scholar]
- 211. Park SW, Yi JH, Miranpuri G, et al. Thiazolidinedione class of peroxisome proliferator‐activated receptor gamma agonists prevents neuronal damage, motor dysfunction, myelin loss, neuropathic pain, and inflammation after spinal cord injury in adult rats. J Pharmacol Exp Ther 2007;320:1002–1012. [DOI] [PubMed] [Google Scholar]
- 212. Luo Y, Yin W, Signore AP, et al. Neuroprotection against focal ischemic brain injury by the peroxisome proliferator‐activated receptor‐gamma agonist rosiglitazone. J Neurochem 2006;97:435–448. [DOI] [PubMed] [Google Scholar]
- 213. Sundararajan S, Gamboa JL, Victor NA, Wanderi EW, Lust WD, Landreth GE. Peroxisome proliferator‐activated receptor‐gamma ligands reduce inflammation and infarction size in transient focal ischemia. Neuroscience 2005;130:685–696. [DOI] [PubMed] [Google Scholar]
- 214. Zhao Y, Patzer A, Gohlke P, Herdegen T, Culman J. The intracerebral application of the PPARgamma‐ligand pioglitazone confers neuroprotection against focal ischaemia in the rat brain. Eur J Neurosci 2005;22:278–282. [DOI] [PubMed] [Google Scholar]
- 215. Pereira MP, Hurtado O, Cardenas A, et al. The nonthiazolidinedione PPARgamma agonist L‐796,449 is neuroprotective in experimental stroke. J Neuropathol Exp Neurol 2005;64:797–805. [DOI] [PubMed] [Google Scholar]
- 216. Yonutas HM, Sullivan PG. Targeting PPAR isoforms following CNS injury. Curr Drug Targets 2013;14:733–742. [DOI] [PubMed] [Google Scholar]
- 217. Bernardo A, Minghetti L. PPAR‐gamma agonists as regulators of microglial activation and brain inflammation. Curr Pharm Des 2006;12:93–109. [DOI] [PubMed] [Google Scholar]


