Abstract
Bone morphogenetic proteins (BMPs) are crucial regulators of chondrogenesis. BMPs transduce their signals through three type I receptors: BMPR1A, BMPR1B, and ACVR1/ALK2. Fibrodysplasia ossificans progressiva (FOP), a rare disorder characterized by progressive ossification of connective tissue, is caused by an activating mutation in Acvr1 (the gene that encodes ACVR1/ALK2). However, there are few developmental defects associated with FOP. Thus, the role of ACVR1 in chondrogenesis during development is unknown. Here we report the phenotype of mice lacking ACVR1 in cartilage. Acvr1CKO mice are viable but exhibit defects in the development of cranial and axial structures. Mutants exhibit a shortened cranial base, and cervical vertebrae are hypoplastic. Acvr1CKO adult mice develop progressive kyphosis. These morphological defects were associated with decreased levels of Smad1/5 and p38 activation, and with reduced rates of chondrocyte proliferation in vertebral cartilage. We also tested whether ACVR1 exerts coordinated functions with BMPR1A and BMPR1B through analysis of double mutants. Acvr1/Bmpr1a and Acvr1/Bmpr1b mutant mice exhibited generalized perinatal lethal chondrodysplasia that was much more severe than in any of the corresponding mutant strains. These findings demonstrate that ACVR1 is required for chondrocyte proliferation and differentiation, particularly in craniofacial and axial elements, but exerts coordinated functions with both BMPR1A and BMPR1B throughout the developing endochondral skeleton.
Keywords: BMP, ALK2, ACVR1, chondrogenesis, mouse
Introduction
Bone morphogenetic proteins (BMP) are crucial regulators of chondrogenesis. BMPs transduce their signals through three type I receptors: BMP receptor type 1A (BMPR1A, also known as ALK3), BMPR1B (ALK6), and activin receptor type 1A (ACVR1/ActR1/ALK2). BMPR1A and BMPR1B are structurally similar and bind BMPs (1). ACVR1 binds to a more diverse set of ligands, including TGFβs, activins, and multiple BMPs (2-4).
The majority of the vertebrate skeleton forms through endochondral ossification, and BMPs play essential roles at many steps in this process. BMP signaling is required to maintain expression of Sox9, a transcription factor essential for commitment to the chondrogenic fate (5,6), and for these cells to differentiate as chondrocytes and organize into a growth plate. The growth plate consists of zones of proliferating cells, followed by post-mitotic prehypertrophic and hypertrophic chondrocytes (7). BMP signaling is required to promote proliferation and differentiation (8-10). Growth plates form in mice deficient in Bmpr1a or Bmpr1b, but chondrogenesis is arrested at the condensation stage in mice lacking both receptors in cartilage (6,10,11). The function of ACVR1/ALK2 is not as well characterized. The ability of ACVR1 to promote chondrogenesis has been established in vitro (12,13). Its importance in vivo is demonstrated by the fact that fibrodysplasia ossificans progressiva (FOP), a rare disorder characterized by progressive ossification of connective tissue (14), is caused by an activating mutation in Acvr1 (the gene that encodes ALK2) (15,16). Constitutively active ACVR1 can cause ectopic endochondral ossification (17). The activating mutant can also enhance chondrogenic commitment of progenitor cells and predispose mesenchymal cells to an osteoblastic fate (18 {Culbert, 2014 #46}). However, FOP patients have only subtle developmental defects (14). Therefore, whether ACVR1 activity is required for normal chondrogenesis is unknown. Moreover, whether this receptor has unique functions in line with its unique ability to engage diverse TGFβ ligands is unknown. We generated mice lacking ACVR1 in cartilage to address these questions.
Materials and Methods
Generation of Acvr1CKO and Bmpr1aCKO;Acvr1CKO, and Bmpr1b−/−;Acvr1CKO mice
Generation and genotyping of Bmpr1b, Bmpr1a, Acvr1, and Col2-Cre mice was described previously (11,19-21). To generate cartilage-specific Acvr1 null mice, Acvr1fx/+;Col2-Cre mice were intercrossed to generate Acvr1fx/fx;Col2-Cre (Acvr1CKO) mice. Acvr1f+/-;Bmpr1a fx/+;Col2-Cre mice were intercrossed to generate Acvr1CKO;Bmpr1aCKO;mice (referred to as Acvr1/Bmpr1aCKO). An analogous scheme was used to generate Acvr1CKO;Bmpr1b−/− mice. All procedures were approved by the Institutional Animal Care and Use Committee at UCLA (IACUC protocol number 95-018).
Skeletal preparation and histology
Skeletal preparations were performed as described (22). For histology, embryos were fixed in 4% paraformaldehyde, decalcified, and embedded in paraffin. Sections were stained with hematoxylin and eosin or alcian blue and nuclear fast red (23).
Immunohistochemistry
Primary antibodies were ACVR1/ALK2 (Sigma SAB1306388-40TST, 1:100), PCNA (Zymed, 13-3900), phospho-SMAD1/5 (Cell Signaling Technology), or p-p38 (Cell Signaling Technology, 9211 ,1:00). As a control for specificity, a second ACVR1 antibody (Abcam 155981, 1:100) was used for IHC; similar results were obtained (data not shown). Sections were quenched in 3% H2O2 in methanol, blocked with 0.5% blocking reagent (TSA Biotin System, Perkin Elmer, Waltham, MA, USA, NEL700A) in TBS (100 mM Tris pH 7.5, 150 mM NaCl), incubated with primary antibody overnight at 4° C, and then washed and incubated overnight at 4°C with Alexa-Fluor-488 or −555-conjugated rabbit secondary antibody (Invitrogen). Detection of antibody binding was performed using the TSA Biotin System according to the manufacture's instructions. Fluorescence detection was conducted using Streptavidin–AlexaFluor -488 or -555 (Invitrogen) secondary antibodies; sections were counterstained with DAPI (Invitrogen, D1306). Quantitation of pSmad1/5 and PCNA-positive cells was performed as described (24); at least three sections from 5 mice per genotype were examined by individuals blinded to genotype. Total cells (DAPI-positive) and the percentage of total cells (DAPI-positive cells that were also pSmad1/5-positive) were determined and statistical significance assessed using Students t test (24).
Western blot analysis
Primary chondrocytes were isolated from costal cartilage as described (25) and seeded at 1X 105 cells/well in 12-well plates. Cells were maintained in chondrogenic medium for 3 days. They were then washed twice with 1x PBS and treated with ACVR1/ALK2 shRNA lentivirus transmission particles (Sigma-Aldrich SHCLNV-NM_007394; TRCN0000361057) at an MOI of 10 in serum free medium for 24 hrs. Cells were then maintained in chondrogenic medium for 72 hrs. Cells were lysed in RIPA buffer supplemented with protease and phosphatase inhibitors as described (25). Whole cell lysates were run on 10% SDS-polyacrylamide gels and transferred onto PVDF membranes. The membranes were blocked with 5% milk in TBS-Tween (30 mM Tris pH 74, 300 mM NaCl, 0.2% Tween 20), incubated with primary antibody (ACVR1/ALK2, Sigma SAB1306388-40TST (1:100); Tubulin, Cell Signaling 2144 (1:2000)) for 1hr at RT and then incubated with secondary antibody diluted in blocking buffer for 1 h at room temperature. Binding was detected using the ECL Plus kit (GE Healthcare).
X-ray and microCT analysis
X-ray analyses of adult mice were performed using a Faxitron as described (11). MicroCT analysis of neonates was performed as described (22).
Results
Expression of ACVR1/ALK2
We examined ACVR1 protein expression in the skeletal system from E10.5 through birth (P0). Western analysis revealed that the antibody employed in these studies detected a single band of the expected size of approximately 57 kD in primary chondrocytes; lentiviral shRNA against ACVR1 significantly reduced the detection of this band (Supplemental Fig. 1). The results are consistent with and extend previous in situ hybridization studies (12,26,27), which indicated diffuse expression of Acvr1 (Alk2) mRNA throughout the growth plate. In craniofacial elements, expression was detected beginning at E10.5 in mesenchyme (Supplemental Fig. 2A), and by E12.5, in condensing cartilage (Supplemental Fig. 2B). By E14.5, expression was seen in proliferating chondrocytes in the sphenoid and at lower levels in adjacent resting chondrocytes in the spheno-occipital synchondrosis (Supplemental Fig 2C). At E16.5, expression persisted in proliferating chondrocytes, and high levels of ACVR1 protein were seen in hypertrophic chondrocytes (Supplemental Fig. 2D,E). This expression persisted at P0 (data not shown).
ACVR1 protein was not strongly expressed in appendicular elements until a growth plate had formed. At E13.5, ACVR1 protein was detected in proliferating chondrocytes, and at higher levels in hypertrophic chondrocytes (Supplemental Fig. 2F). By E16.5, ACVR1 protein expression persisted in proliferating chondrocytes but was expressed at higher levels in hypertrophic chondrocytes (Supplemental Fig. 2G). This pattern persisted through P0 (data not shown).
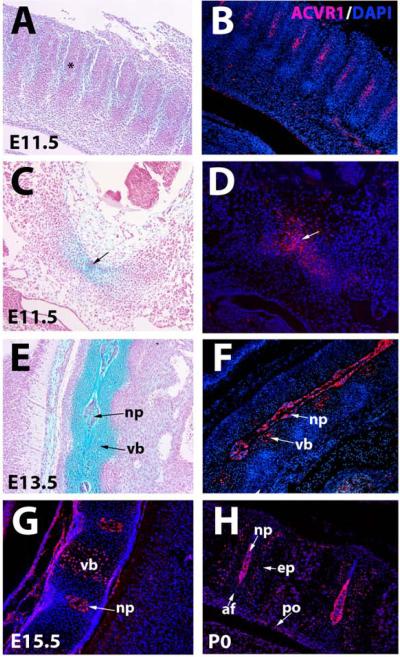
The highest levels and earliest onset of ACVR1 protein expression were detected in axial elements. Expression was first seen at E11.5 in sclerotomal cells (Fig. 1A-D). Consistent with a previous report (28) ACVR1 protein was also expressed in the notochord at this stage (Fig. 1C,D). By E13.5, ACVR1 protein expression was strong in the nucleus pulposus. Expression could also be seen in proliferating chondrocytes in vertebral bodies (Fig. 1E,F). This pattern persisted at E15.5 (Fig. 1G). At P0, expression was strong in the nucleus pulposus, and persisted in the proliferating chondrocytes in cartilage endplates of vertebral bodies (Fig. 1H).
Figure 1. ACVR1/ALK2 protein expression in axial elements.
A, C. and E are stained with alcian blue-nuclear fast red. B, D, F, G, H are immunofluorescent images counterstained with DAPI. A,B, E11.5 cervical region showing ACVR1/ALK2 protein is expressed in sclerotome (asterisk). C,D. E11.5 cervical region showing expression in precartilaginous mesenchyme (detected by alcian blue staining in C), and notochord (arrows). E,F, Sections through E13.5 cervical spine showing expression in nucleus pulposus and chondrocytes in the vertebrae. G, E15.5 cervical spine showing expression in nucleus pulposus and proliferating chondrocytes in vertebral bodies. H, P0 cervical spine showing expression in endplate cartilage and nucleus pulposus. Low levels of expression are present in the periosteum of the vertebrae. Af, annulus fibrosus; ep, endplate; np, nucleus pulposus; po, periosteum; vb, vertebral body.
While our studies of ACVR1 protein localization, indicating expression throughout midgestation stages in proliferating chondrocytes, notochord, and perichondrium are consistent with previous in situ studies (12,26,27) our finding of relatively higher levels of ACVR1 protein in hypertrophic chondrocytes would not be predicted from these studies. It is conceivable that ACVR1 protein produced in less mature chondrocytes persists and accumulates on the surface of maturing chondrocytes. However, we cannot rule out the possibility of cross-reactivity with an unrelated antigen in the hypertrophic zone, as discussed further below.
Phenotype of mice lacking AVCR1/ALK2 in cartilage
We used a conditional allele of Acvr1 (Alk2) (21) to investigate the function of ACVR1 in cartilage. To assess the efficiency of recombination and as a test of specificity of the ACVR1 antibody used to characterize ACVR1 expression, we performed IHC on Acvr1fx/fx;Col2a1-Cre (hereafter referred to as Acvr1CKO) growth plates (Supplemental Figure 3A,B). This analysis revealed that at least 80% of the proliferating chondrocytes had undergone efficient recombination (Supplemental Figure 3C). Immunoreactivity persisted in the nucleus pulposus as expected since this structure is not derived from Col2a1-Cre expressing cells. Within Col2a1-Cre expressing cells, immunoreactivity persisted in hypertrophic chondrocytes in mutants. As discussed above, we cannot rule out the possibility that this represents cross-reactivity of the antibodies we used. However, the strong reduction in ACVR1 protein observed in Acvr1CKO vertebral chondrocytes argues that efficient recombination occurred and that the antibody used to detect ACVR1 protein displays reasonable specificity.
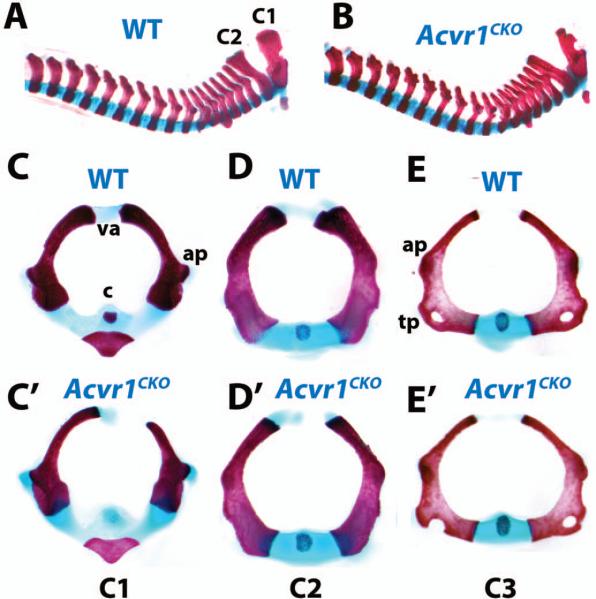
Acvr1CKO mice were recovered in Mendelian ratios. No abnormalities were seen in heterozygotes. However, Acvr1CKO neonates exhibited axial defects with 100% penetrance (n = 23). The cervical vertebral column was compressed, and cervical elements were thinner and broader than in WT littermates (Fig. 2A,B). Transverse views showed that the diameters of cervical vertebrae were enlarged in mutants (Fig. 2C-E’). The vertebral arches of C (cervical) 1 (Fig. 2C,C’) and C2 (Fig. 2D,D’) vertebrae were hypoplastic and failed to fuse. Transverse processes on C3 through C7 were incomplete (Fig. 2E,E’, and data not shown), and the centra exhibited delayed ossification. Histological analyses did not reveal obvious defects in the sizes of condensations, or in chondrocyte differentiation in axial or appendicular elements at E13.5 (Supplemental Fig. 4), suggesting either a subtle defect, and/or later onset.
Figure 2. Axial defects in Acvr1fx/fx;Col2a1-Cre(Acvr1CKO) mutants.
All images are whole-mount skeletal preparations of neonatal (P0) littermates stained with alcian blue/alizarin red. A, B, Cervical and thoracic spines of P0 WT (A) and Acvr1CKO (B) littermates. C-E, transverse views of isolated WT cervical (C1-C3) vertebrae. C’-E’, C1, C2, and C3 vertebrae, respectively, from Acvr1CKO P0 littermate. ap, anterior process; c, centrum; tp, transverse process; va, vertebral arch.
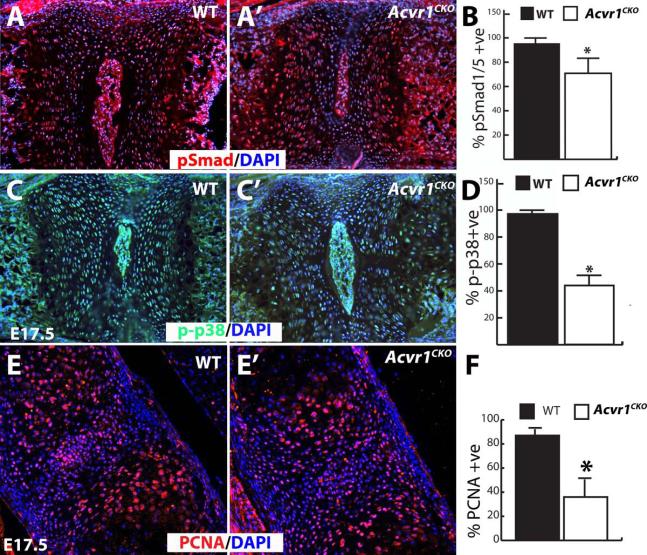
BMPs exert their effects through canonical (Smad) and non-canonical (e.g., p38) pathways. Because a subtle defect was detected in Acvr1CKO axial elements at P0 (Fig. 2), pSmad1/5/8 levels were assessed in vertebral bodies. A small but statistically significant decrease in the percentage of cells positive for pSmad1/5/8 was seen in Acvr1CKO mutants at E17.5 (Fig. 3A,B) but not at E13.5 (data not shown). p-p38 levels were examined to determine whether differences in non-canonical BMP pathway activity could be detected. This analysis revealed an impairment in non-canonical pathway activity in mutants at E17.5 (Fig. 3C,D). Proliferation, assessed by PCNA, was indistinguishable between WT and mutant littermates at E13.5 (data not shown), but was reduced at E17.5 in Alk2CKO vertebrae (Fig. 3E,F). Therefore, loss of ACVR1/ALK2 leads to decreased canonical and non-canonical signaling in axial chondrocytes, and this is correlated with reduced rates of cell proliferation.
Figure 3. Impaired BMP signaling in Acvr1fx/fx;Col2a1-Cre(Acvr1CKO) mutant axial elements.
All images are sagittal sections through the vertebral column of E17.5 mice counterstained with DAPI. A,A’, Immunofluorescence staining for pSmad1/5 in sections through E17.5 cervical vertebrae from WT and Acvr1CKO littermate. B, Quantitation revealed a reduction in Acvr1CKO mice in the percentages of cells positive for pSmad1/5 compared to WT littermates (n = 16, p < 0.0001). C,C’, Immunofluorescence staining for p-p38 in sections through E17.5 cervical vertebrae from WT and Acvr1CKO littermate. D, Quantitation revealed a reduction in Acvr1CKO mice in the percentages of cells positive for p-p38 compared to WT littermates (n = 9, asterisk, p < 0.0001). E,E’, PCNA immunofluorescence on E17.5 cervical vertebrae from WT and Acvr1CKO littermate. F, Quantitation of percentage of PCNA positive cells reveals a significant decrease in mutants. (n = 6, Asterisk, p < 0.005). Size differences in the nucleus pulposus for WT vs. Acvr1CKO mice in panels A and C are not real and are a result of plane of section.
X-ray analysis of adult mice revealed no differences between WT and heterozygotes (n = 15). However, 100% of Acvr1CKO (9/9) mice developed thoracic kyphosis (Supplemental Fig. 5A). Mutant cervical and upper thoracic vertebrae exhibited wedging characteristic of butterfly vertebrae (Supplemental Fig. 5B). Consistent with the cervical defects seen at P0 (Fig. 2), cleared skeletal preparations revealed deformations in cervical vertebrae (Supplemental Fig. 5C,D). Lumbar and sacral vertebrae were indistinguishable in adult WT and mutant littermates (data not shown). Acvr1CKO mice also exhibited broader skulls as a result of a shortened cranial base (n = 9/9) (Supplemental Fig. 5E). Measurements of the lengths of appendicular bones revealed no differences at any stage (data not shown).
Overlapping functions with other BMP receptors
Loss of BMPR1A in cartilage leads to generalized chondrodysplasia and perinatal lethality (10). Bmpr1b−/− mice are viable, but exhibit appendicular skeletal defects, including brachypodism and shortened long bones (11). The pattern of ACVR1/ALK2 expression overlaps with those of BMPR1A and BMPR1B (10,26,29). Double mutants were constructed to test whether ACVR1 shares overlapping functions with these receptors.
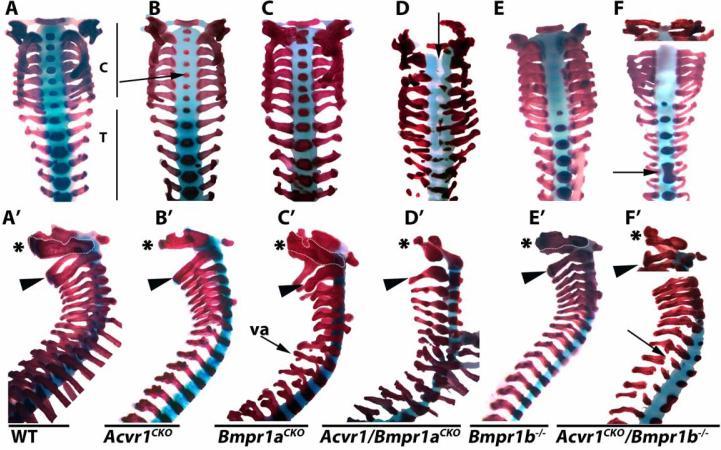
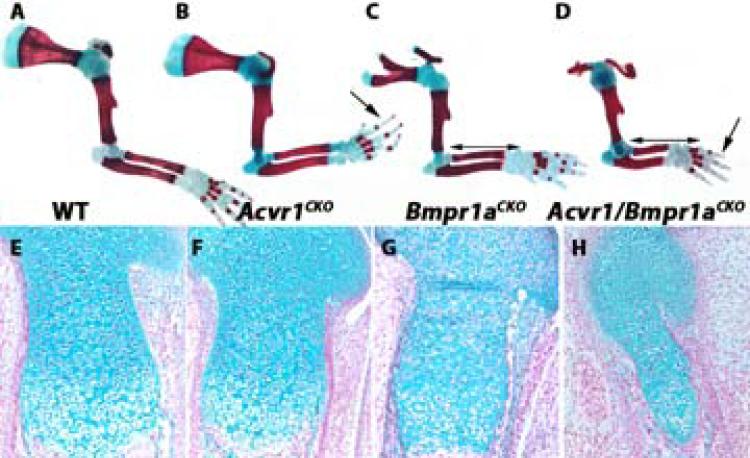
Analysis of craniofacial (data not shown) and axial elements revealed a considerable degree of cooperative function between ACVR1 and BMPR1A. As discussed, C1 and C2 are hypoplastic in neonatal Acvr1CKO mice (Fig. 1A,B; Fig. 4AB’; n = 6). Bmpr1aCKO mutants also exhibited axial defects (n = 5) (Fig. 4C,C’). In contrast to Acvr1CKO mice, where defects are most severe in cervical vertebrae, Bmpr1aCKO mice exhibit more severe defects in thoracic vertebrae (Fig. 4B,C); vertebral arches are severely malformed in Bmpr1aCKO (Fig. 4A’-C’).
Figure 4. ACVR1/ALK2 exhibits overlapping functions with BMPR1A and BMPR1B in axial elements.
All images are cleared skeletal preparations of P0 spines (disarticulated from ribs) stained with alcian blue/alizarin red. A-F, dorsal views. A’-F’, lateral views. Arrow in B highlights reduced ossification of the centra in Acvr1CKO mice. Arrow in D highlights lack of segmentation, evidenced by gaps in staining in regions where ossified centra should be located. Arrow in F highlights vertebral fusion. Arrow in F’ highlights discontinuous vertebral arch. C, cervical; T, thoracic; va, vertebral arch. Asterisks in A’-F’ demarcate the axis (C1). Arrowheads in A’-F’ demarcate the atlas (C2).
In Acvr1/Bmpr1aCKO double mutants, the entire vertebral column is severely malformed. Centra are absent, and the vertebral arches are diminished (Fig. 4D,D’, and data not shown). In the upper cervical region, there is a failure in segmentation (evidenced by the non-alcian blue-stained center of the spinal column (arrow in Fig. 4D). Disorganized and incomplete ossification is seen in the lower cervical and thoracic regions (Fig. 4D). At E12.5, vertebral bodies in Acvr1/Bmpr1aCKO mutants are slightly smaller than in WT littermates, and alcian blue staining appears less intense (Supplemental Fig. 6A,B). Consistent with reduced proliferation in cervical vertebrae in E17.5 Acvr1CKO mutants (Supplemental Fig. 3E,F), vertebral bodies appear smaller in Alk2CKO mice at E17.5 (Supplemental Fig. 6C,D). Acvr1/Bmpr1aCKO axial elements are disorganized (Supplemental Fig. 6C,E). There is a failure in segmentation and formation of nuclei pulposi.
Acvr1CKO/Bmpr1b−/− P0 double mutants also exhibited craniofacial (data not shown) and vertebral abnormalities not seen in either single mutant strain. Bmpr1b−/− mice exhibited no ossification of the cervical centra at E17.5, but were otherwise normal (Fig. 4E,E’). This was also observed in Acvr1CKO/Bmpr1b double mutants (Fig. 4F). All vertebrae were thinner than in Bmpr1b−/− or Acvr1CKO mutants. Transverse processes were thin or discontinuous along the length of the vertebral column in Acvr1/Bmpr1b double mutants (Fig. 4F’). Vertebral fusions affecting thoracic vertebrae were occasionally observed in double mutants (n = 2/6). These were not seen in Acvr1CKO or Bmpr1b−/− mice (n = 9).
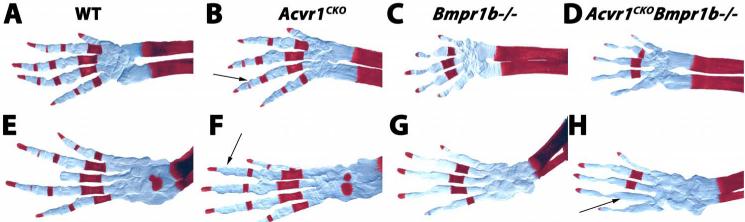
As discussed, appendicular elements in Acvr1CKO mutants were indistinguishable from those in WT littermates, except for a delay in ossification of digits (Fig. 5A,B). Forelimbs of Bmpr1aCKO mice exhibit aplastic scapulae and shortened long bones (10) (Fig. 5C). The radius and ulna are shorter in Acvr1/Bmpr1aCKO mice than in Bmpr1aCKO mice (n = 6) (Fig. 5D). Similar findings were seen in hindlimbs (data not shown). Histological analysis revealed that growth plates from Acvr1CKO mutants are indistinguishable from those of WT littermates (Fig. 5E,F). As previously shown (10), Bmpr1aCKO growth plates exhibit disorganized and short columnar zones (Fig. 5G). In Acvr1/Bmpr1aCKO double mutants, the columnar zone is further reduced and disorganized (Fig. 5H). ACVR1 and BMPR1B also exhibited evidence of coordinated functions in appendicular elements (Fig. 6). As shown previously, Bmpr1b−/− mice exhibit brachypodism accompanied by fusion and reduction of the proximal and middle phalanges, and delayed ossification of metacarpals/metatarsals (Fig. 6A,C,E,F and (11)). The reduction in ossification of the metacarpals/metatarsals is more severe in Acvr1CKO;Bmpr1b−/− mutants than in either single mutant strain (Fig. 6).
Figure 5. Appendicular defects in Acvr1fx/fx;Bmpr1afx/fx;Col2a1-Cre(Acvr1/Bmpr1aCKO) mice.
A-D, skeletal preparations from E17.5 forearms stained with alcian blue/alizarin red. Double-headed arrows in C and D highlight shortening of radius and ulna in Acvr1/Bmpr1aCKO mice compared to Bmpr1aCKO mice. Arrows in B and D highlight delayed ossification of digits in Acvr1CKO mice, which is exacerbated in Acvr1/Bmpr1aCKO mice. E-H, sagittal sections through proximal femurs of E17.5 mice of the indicated genotypes stained with alcian blue/nuclear fast red.
Figure 6. Appendicular defects in Acvr1fx/fx;Bmpr1b−/−;Col2a1-Cre (Acvr1CKO/Bmpr1b−/−) mice.
Cleared skeletal preparations from E17.5 autopods of forelimbs (A-D) and hindlimbs (E-H) stained with alcian blue/nuclear fast red. A blue filter was applied in Photoshop to the images to enhance visualization of alcian blue-stained regions. Arrows in B and F point to reduced or absent ossification in phalangeal elements in Acvr1CKO mice. Arrow in H highlights absence of ossification in the Acvr1CKO/Bmprb−/− hindlimb compared to the Acvr1CKO and Bmpr1b−/− single mutant strains.
Discussion
These studies demonstrate that ACVR1/ALK2 is essential for normal endochondral bone formation. Mice lacking Bmpr1a in cartilage exhibit lethal chondrodysplasia (6). Loss of Bmpr1b in mice and humans is not lethal, and defects are most prominent in appendicular elements (11,30,31). We show here that Acvr1CKO mice are viable but exhibit craniofacial and axial defects. Thus, BMPR1A appears to have global functions, whereas ACVR1/ALK2 and BMPR1B have more restricted roles in committed chondrocytes in axial and appendicular elements, respectively. These differential roles may reflect differences in expression of these receptors or the BMP ligands that activate them (11).
All three type I receptors are expressed to some extent in appendicular, axial, and craniofacial elements, raising the possibility of overlapping or coordinated functions. This was shown for BMPR1A and BMPR1B by the absence of cartilage in Bmpr1aCKO;Bmpr1b−/− mice (6). Here we show that ACVR1 also exerts overlapping functions in committed chondrocytes with BMPR1A and BMPR1B. Combined loss of ACVR1 and either BMPR1A or BMPR1B leads to perinatal lethality and generalized chondrodysplasia.
Fibrodysplasia ossificans progressiva (FOP) patients harbor an activating mutation in Acvr1 (15,16). However, there are few developmental effects of this activating mutation in cartilage, the most penetrant of which is malformation of the great toes. This has also been demonstrated in chimeric mice carrying the FOP mutation (32). Although delayed ossification of digits was observed in Acvr1CKO mice, there were no additional defects in the great toe. It is important to bear in mind, however, that in Acvr1CKO mice, excision occurs only in committed chondrocytes. Therefore limb patterning defects that might affect the great toe would not be expected. Some FOP patients exhibit congenital abnormalities in the cervical spine, the area most severely affected in Acvr1CKO mice. In FOP patients, the cervical spine exhibits large spinous processes and progressive fusion of articular processes (33,34). In Acvr1CKO mice, cervical spinous and articular processes are hypoplastic. Thus, cervical vertebrae are sensitive to both gain and loss of ACVR1 function. The basis for the strong effects of ACVR1 on cervical elements in relation to other vertebrae is unclear, but might be related to the fact that cervical vertebrae are the earliest to be specified in mammals but the last to become ossified, thus requiring a longer period of growth and development (35,36).
We show here that loss of ACVR1 also leads to craniofacial defects. Distinct facial defects have been reported in a subset of FOP patients, including a reduced mandible and underdevelopment of the supra-orbital ridge (37). Defective mandibular development was reported in mice lacking ACVR1 in cranial neural crest cells (21). Distinct craniofacial defects were observed in the present study, which can be attributed to defects in chondrogenesis in the cranial base. It is thus conceivable that some of the facial features seen in FOP patients are a consequence of altered development of the cranial base. Similarly, conductive hearing loss is seen in about 50% of FOP patients (34). It is conceivable that this is due to craniofacial defects affecting the inner ear.
We did not detect obvious changes in appendicular elements in Acvr1CKO mice. However, proximal medial tibial osteochondromas and short broad femoral necks are common but variable features of FOP patients (34). We cannot rule out the possibility that there are subtle defects in appendicular elements in Acvr1CKO mice. The fact that the appendicular skeletons of both Acvr1/Bmpr1aCKO and Acvr1CKO/Bmpr1b−/− mice are more severely affected than those of Bmpr1aCKO or Bmpr1b−/− mice demonstrates that ACVR1 is expressed in and plays a role in the development of appendicular elements.
The precise mechanisms by which ACVR1 exerts its effects during chondrogenesis are unknown. We found that loss of ACVR1 led to reduced canonical (pSmad1/5) and non-canonical (p-p38) activity in axial elements, as has also been seen in mice lacking BMPR1A and/or BMPR1B in cartilage (10,11). The majority of the literature has focused on the ability of ACVR1 to activate canonical Smads 1/5/8. This has been demonstrated most clearly using constitutively active or FOP variants of ACVR1 (16,21). Our observation of a reduction in pSmad1/5 levels in Acvr1CKO mutants is consistent with these findings. ACVR1/ALK2 can activate non-canonical pathways in addition to canonical ones. For example, it was reported that ACVR1 regulates cell proliferation during formation of the vertebrate lens in a Smad-independent manner (38). Thus, the reduced p-p38 levels in Acvr1CKO mutants may contribute to the observed chondrocyte proliferation defects. These are not mutually exclusive possibilities. Regardless of the precise mechanism, the strong synergy in phenotype seen in Acvr1/Bmpr1aCKO and Acvr1CKO/Bmpr1b−/− double mutants strongly argues that ACVR1 exerts its effects through BMP signaling pathways.
The fact that Acvr1/Bmpr1aCKO double mutants exhibit defects along the entire length of the spine indicates that ACVR1 and BMPR1A have coordinated functions in all axial elements. The Acvr1/Bmpr1aCKO phenotype resembles that of Pax1/9 double mutants, in which failure of sclerotomal cells to migrate ventrally leads to the loss of medial structures such as the centra (39). This is consistent with the fact that Col2a1-Cre is expressed in the sclerotome (19). The observation that rates of proliferation are reduced in committed chondrocytes in vertebral elements in Acvr1CKO mice indicates that ALK2 plays a role at multiple stages of chondrogenesis.
The subtlety of the developmental limb phenotype in Acvr1CKO mice can be attributed at least in part to overlapping functions with both BMPR1A and BMPR1B. It is also possible that the Acvr1 limb phenotype is less severe than the axial one because Col2a1-Cre is first expressed in committed chondrocytes in appendicular elements (19). It is conceivable that ACVR1 also has important functions in the limb at an earlier stage; studies utilizing Prx1-Cre would be very informative in this regard, although as discussed above, we (Fig. 1, Supplemental Fig. 2) and others (26) did not detect high levels of ACVR1 expression in prechondrogenic condensations in the limb. Similarly, it is possible that ACVR1 plays a role in maintenance of articular cartilage. Although ACVR1 does not appear to be as highly expressed as BMPR1A or BMPR1B in articular cartilage (40), extra-articular ankylosis of all major joints is a key feature of FOP (41). Post-natal ablation of ACVR1 in cartilage would be required to address this question and to separate direct effects on articular cartilage from indirect effects resulting from abnormal development.
Finally, we show that ACVR1 is strongly expressed in the notochord and nucleus pulposus, raising the possibility that this receptor plays an essential role in the formation/maintenance of these structures. ACVR1 has been shown to regulate the rate of cell proliferation in the node, the transient embryonic structure that gives rise to the notochord (42). The use of a notochord-specific Cre line will be required to address the function, if any, of ACVR1 in the notochord and its derivatives.
In summary, this study shows that all three type I BMP receptors play essential roles in committed chondrocytes. BMPR1A appears to have the most prominent function, as Bmpr1aCKO mutants exhibit generalized chondrodysplasia, while defects in Bmpr1b−/− mice are seen primarily in appendicular elements, whereas those in Acvr1CKO mice are primarily found in axial and craniofacial elements. The extent to which ACVR1 transduces its signals in cartilage through the canonical Smad pathway vs. non-canonical pathways is unknown; we show that loss of ACVR1 impacts levels of both canonical pSmads and noncanonical p-p38. However, we have shown previously that BMPs transduce the majority of their signals in committed chondrocytes through R-Smad pathways, and we have shown here genetically that ACVR1 exerts overlapping functions with BMPR1A and BMPR1B. These findings suggest that ACVR1 also transduces its effects in cartilage to a significant extent through R-Smad-mediated pathways, but future studies are needed to address this issue.
Supplementary Material
Acknowledgements
This work was supported by NIH grant R01 AR044528 (to KML). We thank Austin M. Rike for technical assistance.
Authors roles: Study Design: DR performed the analysis of cleared skeletal preparations, histological analysis, and Western blots. SB performed the ACVR1 immunolocalization and X-ray studies. TA, JKK, and YJL assisted with the histological analyses. DR and KML analyzed and interpreted data. DR and KML designed experiments. DR and KML drafted and revised the manuscript. KML accepts responsibility for integrity of data analysis.
References
- 1.Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22(4):233–41. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- 2.Clarke TR, Hoshiya Y, Yi SE, Liu X, Lyons KM, Donahoe PK. Mullerian inhibiting substance signaling uses a bone morphogenetic protein (BMP)-like pathway mediated by ALK2 and induces SMAD6 expression. Mol Endocrinol. 2001;15(6):946–59. doi: 10.1210/mend.15.6.0664. [DOI] [PubMed] [Google Scholar]
- 3.Daly AC, Randall RA, Hill CS. Transforming growth factor beta-induced Smad1/5 phosphorylation in epithelial cells is mediated by novel receptor complexes and is essential for anchorage-independent growth. Mol Cell Biol. 2008;28(22):6889–902. doi: 10.1128/MCB.01192-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luo J, Tang M, Huang J, He BC, Gao JL, Chen L, Zuo GW, Zhang W, Luo Q, Shi Q, Zhang BQ, Bi Y, Luo X, Jiang W, Su Y, Shen J, Kim SH, Huang E, Gao Y, Zhou JZ, Yang K, Luu HH, Pan X, Haydon RC, Deng ZL, He TC. TGFbeta/BMP type I receptors ALK1 and ALK2 are essential for BMP9-induced osteogenic signaling in mesenchymal stem cells. J Biol Chem. 2010;285(38):29588–98. doi: 10.1074/jbc.M110.130518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haas AR, Tuan RS. Chondrogenic differentiation of murine C3H10T1/2 multipotential mesenchymal cells: II. Stimulation by bone morphogenetic protein-2 requires modulation of N-cadherin expression and function. Differentiation. 1999;64(2):77–89. doi: 10.1046/j.1432-0436.1999.6420077.x. [DOI] [PubMed] [Google Scholar]
- 6.Yoon BS, Ovchinnikov DA, Yoshii I, Mishina Y, Behringer RR, Lyons KM. Bmpr1a and Bmpr1b have overlapping functions and are essential for chondrogenesis in vivo. Proc Natl Acad Sci U S A. 2005;102(14):5062–7. doi: 10.1073/pnas.0500031102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423(6937):332–6. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- 8.Minina E, Kreschel C, Naski MC, Ornitz DM, Vortkamp A. Interaction of FGF, Ihh/Pthlh, and BMP signaling integrates chondrocyte proliferation and hypertrophic differentiation. Dev Cell. 2002;3(3):439–49. doi: 10.1016/s1534-5807(02)00261-7. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi T, Lyons KM, McMahon AP, Kronenberg HM. BMP signaling stimulates cellular differentiation at multiple steps during cartilage development. Proc Natl Acad Sci U S A. 2005;102(50):18023–7. doi: 10.1073/pnas.0503617102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoon BS, Pogue R, Ovchinnikov DA, Yoshii I, Mishina Y, Behringer RR, Lyons KM. BMPs regulate multiple aspects of growth-plate chondrogenesis through opposing actions on FGF pathways. Development. 2006;133(23):4667–78. doi: 10.1242/dev.02680. [DOI] [PubMed] [Google Scholar]
- 11.Yi SE, Daluiski A, Pederson R, Rosen V, Lyons KM. The type I BMP receptor BMPRIB is required for chondrogenesis in the mouse limb. Development. 2000;127(3):621–30. doi: 10.1242/dev.127.3.621. [DOI] [PubMed] [Google Scholar]
- 12.Zhang D, Schwarz EM, Rosier RN, Zuscik MJ, Puzas JE, O'Keefe RJ. ALK2 functions as a BMP type I receptor and induces Indian hedgehog in chondrocytes during skeletal development. J Bone Miner Res. 2003;18(9):1593–604. doi: 10.1359/jbmr.2003.18.9.1593. [DOI] [PubMed] [Google Scholar]
- 13.Fujii M, Takeda K, Imamura T, Aoki H, Sampath TK, Enomoto S, Kawabata M, Kato M, Ichijo H, Miyazono K. Roles of bone morphogenetic protein type I receptors and Smad proteins in osteoblast and chondroblast differentiation. Mol Biol Cell. 1999;10(11):3801–13. doi: 10.1091/mbc.10.11.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaplan FS, Hahn GV, Zasloff MA. Heterotopic Ossification: Two Rare Forms and What They Can Teach Us. J Am Acad Orthop Surg. 1994;2(5):288–296. doi: 10.5435/00124635-199409000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Shore EM, Xu M, Feldman GJ, Fenstermacher DA, Cho TJ, Choi IH, Connor JM, Delai P, Glaser DL, LeMerrer M, Morhart R, Rogers JG, Smith R, Triffitt JT, Urtizberea JA, Zasloff M, Brown MA, Kaplan FS. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet. 2006;38(5):525–7. doi: 10.1038/ng1783. [DOI] [PubMed] [Google Scholar]
- 16.Shen Q, Little SC, Xu M, Haupt J, Ast C, Katagiri T, Mundlos S, Seemann P, Kaplan FS, Mullins MC, Shore EM. The fibrodysplasia ossificans progressiva R206H ACVR1 mutation activates BMP-independent chondrogenesis and zebrafish embryo ventralization. J Clin Invest. 2009;119(11):3462–72. doi: 10.1172/JCI37412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medici D, Shore EM, Lounev VY, Kaplan FS, Kalluri R, Olsen BR. Conversion of vascular endothelial cells into multipotent stem-like cells. Nat Med. 2010;16(12):1400–6. doi: 10.1038/nm.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Dinther M, Visser N, de Gorter DJ, Doorn J, Goumans MJ, de Boer J, ten Dijke P. ALK2 R206H mutation linked to fibrodysplasia ossificans progressiva confers constitutive activity to the BMP type I receptor and sensitizes mesenchymal cells to BMP-induced osteoblast differentiation and bone formation. J Bone Miner Res. 2010;25(6):1208–15. doi: 10.1359/jbmr.091110. [DOI] [PubMed] [Google Scholar]
- 19.Ovchinnikov DA, Deng JM, Ogunrinu G, Behringer RR. Col2a1-directed expression of Cre recombinase in differentiating chondrocytes in transgenic mice. Genesis. 2000;26(2):145–6. [PubMed] [Google Scholar]
- 20.Mishina Y, Hanks MC, Miura S, Tallquist MD, Behringer RR. Generation of Bmpr/Alk3 conditional knockout mice. Genesis. 2002;32(2):69–72. doi: 10.1002/gene.10038. [DOI] [PubMed] [Google Scholar]
- 21.Dudas M, Sridurongrit S, Nagy A, Okazaki K, Kaartinen V. Craniofacial defects in mice lacking BMP type I receptor Alk2 in neural crest cells. Mech Dev. 2004;121(2):173–82. doi: 10.1016/j.mod.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Retting KN, Song B, Yoon BS, Lyons KM. BMP canonical Smad signaling through Smad1 and Smad5 is required for endochondral bone formation. Development. 2009;136(7):1093–104. doi: 10.1242/dev.029926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luna L. Histopathological Methods and Color Atlas of Special Stains and Tissue Artifacts. American Histologics, Inc.; Gaithersburg, MD.: 1992. [Google Scholar]
- 24.Estrada KD, Retting KN, Chin AM, Lyons KM. Smad6 is essential to limit BMP signaling during cartilage development. J Bone Miner Res. 2011;26(10):2498–510. doi: 10.1002/jbmr.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Estrada KD, Wang W, Retting KN, Chien CT, Elkhoury FF, Heuchel R, Lyons KM. Smad7 regulates terminal maturation of chondrocytes in the growth plate. Dev Biol. 2013;382(2):375–84. doi: 10.1016/j.ydbio.2013.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verschueren K, Dewulf N, Goumans MJ, Lonnoy O, Feijen A, Grimsby S, Vandi Spiegle K, ten Dijke P, Moren A, Vanscheeuwijck P, Heldin CH, Miyazono K, Mummery C, Van Den Eijnden-Van Raaij J, Huylebroeck D. Expression of type I and type IB receptors for activin in midgestation mouse embryos suggests distinct functions in organogenesis. Mech Dev. 1995;52(1):109–23. doi: 10.1016/0925-4773(95)00395-h. [DOI] [PubMed] [Google Scholar]
- 27.Minina E, Schneider S, Rosowski M, Lauster R, Vortkamp A. Expression of Fgf and Tgfbeta signaling related genes during embryonic endochondral ossification. Gene Expr Patterns. 2005;6(1):102–9. doi: 10.1016/j.modgep.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 28.Yoshikawa SI, Aota S, Shirayoshi Y, Okazaki K. The ActR-I activin receptor protein is expressed in notochord, lens placode and pituitary primordium cells in the mouse embryo. Mech Dev. 2000;91(1-2):439–44. doi: 10.1016/s0925-4773(99)00320-2. [DOI] [PubMed] [Google Scholar]
- 29.Takae R, Matsunaga S, Origuchi N, Yamamoto T, Morimoto N, Suzuki S, Sakou T. Immunolocalization of bone morphogenetic protein and its receptors in degeneration of intervertebral disc. Spine (Phila Pa 1976) 1999;24(14):1397–401. doi: 10.1097/00007632-199907150-00002. [DOI] [PubMed] [Google Scholar]
- 30.Demirhan O, Turkmen S, Schwabe GC, Soyupak S, Akgul E, Tastemir D, Karahan D, Mundlos S, Lehmann K. A homozygous BMPR1B mutation causes a new subtype of acromesomelic chondrodysplasia with genital anomalies. J Med Genet. 2005;42(4):314–7. doi: 10.1136/jmg.2004.023564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lehmann K, Seemann P, Boergermann J, Morin G, Reif S, Knaus P, Mundlos S. A novel R486Q mutation in BMPR1B resulting in either a brachydactyly type C/symphalangism-like phenotype or brachydactyly type A2. Eur J Hum Genet. 2006;14(12):1248–54. doi: 10.1038/sj.ejhg.5201708. [DOI] [PubMed] [Google Scholar]
- 32.Chakkalakal SA, Zhang D, Culbert AL, Convente MR, Caron RJ, Wright AC, Maidment AD, Kaplan FS, Shore EM. An Acvr1 R206H knock-in mouse has fibrodysplasia ossificans progressiva. J Bone Miner Res. 2012;27(8):1746–56. doi: 10.1002/jbmr.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schaffer AA, Kaplan FS, Tracy MR, O'Brien ML, Dormans JP, Shore EM, Harland RM, Kusumi K. Developmental anomalies of the cervical spine in patients with fibrodysplasia ossificans progressiva are distinctly different from those in patients with Klippel-Feil syndrome: clues from the BMP signaling pathway. Spine (Phila Pa 1976) 2005;30(12):1379–85. doi: 10.1097/01.brs.0000166619.22832.2c. [DOI] [PubMed] [Google Scholar]
- 34.Kaplan FS, Xu M, Seemann P, Connor JM, Glaser DL, Carroll L, Delai P, Fastnacht- Urban E, Forman SJ, Gillessen-Kaesbach G, Hoover-Fong J, Koster B, Pauli RM, Reardon W, Zaidi SA, Zasloff M, Morhart R, Mundlos S, Groppe J, Shore EM. Classic and atypical fibrodysplasia ossificans progressiva (FOP) phenotypes are caused by mutations in the bone morphogenetic protein (BMP) type I receptor ACVR1. Hum Mutat. 2009;30(3):379–90. doi: 10.1002/humu.20868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bagnall KM, Harris PF, Jones PR. A radiographic study of the human fetal spine. 2 The sequence of development of ossification centres in the vertebral column. J Anat. 1977;124(Pt 3):791–802. [PMC free article] [PubMed] [Google Scholar]
- 36.Sofaer JA. Developmental stability in the mouse vertebral column. J Anat. 1985;140(Pt 1):131–41. [PMC free article] [PubMed] [Google Scholar]
- 37.Hammond P, Suttie M, Hennekam RC, Allanson J, Shore EM, Kaplan FS. The face signature of fibrodysplasia ossificans progressiva. Am J Med Genet A. 2012;158A(6):1368–80. doi: 10.1002/ajmg.a.35346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rajagopal R, Huang J, Dattilo LK, Kaartinen V, Mishina Y, Deng CX, Umans L, Zwijsen A, Roberts AB, Beebe DC. The type I BMP receptors, Bmpr1a and Acvr1, activate multiple signaling pathways to regulate lens formation. Dev Biol. 2009;335(2):305–16. doi: 10.1016/j.ydbio.2009.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peters H, Wilm B, Sakai N, Imai K, Maas R, Balling R. Pax1 and Pax9 synergistically regulate vertebral column development. Development. 1999;126(23):5399–408. doi: 10.1242/dev.126.23.5399. [DOI] [PubMed] [Google Scholar]
- 40.Muehleman C, Kuettner KE, Rueger DC, Ten Dijke P, Chubinskaya S. Immunohistochemical localization of osteogenetic protein (OP-1) and its receptors in rabbit articular cartilage. J Histochem Cytochem. 2002;50(10):1341–50. doi: 10.1177/002215540205001007. [DOI] [PubMed] [Google Scholar]
- 41.Kaplan FS, Chakkalakal SA, Shore EM. Fibrodysplasia ossificans progressiva: mechanisms and models of skeletal metamorphosis. Dis Model Mech. 2012;5(6):756–62. doi: 10.1242/dmm.010280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Komatsu Y, Kaartinen V, Mishina Y. Cell cycle arrest in node cells governs ciliogenesis at the node to break left-right symmetry. Development. 2011;138(18):3915–20. doi: 10.1242/dev.068833. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.