Abstract
Purpose
Reovirus is a naturally occurring human virus that is cytopathic to malignant cells possessing an activated Ras signaling pathway. We conducted a phase I trial of Reolysin, a manufactured, proprietary isolate of purified reovirus, in children with relapsed/refractory extracranial solid tumors to define the recommended phase 2 dose (RP2D), toxicities and pharmacokinetic properties when administered as a single agent or in combination with cyclophosphamide.
Experimental Design
Reolysin was administered intravenously for 5 consecutive days, every 28 days. Using a 3 + 3 design, the following dose levels were evaluated: 3 × 108 Tissue Culture Inhibitory Dose 50% (TCID50)/kg; 5 × 108 TCID50/kg (maximum dose was 3 × 1010 TCID50); and 5 × 108 TCID50/kg plus oral cyclophosphamide (50 mg/m2/day × 21 days).
Results
Twenty-nine patients were enrolled; 28 were eligible and 24 were evaluable for toxicity and response. There were no hematologic dose-limiting toxicities. Grade 5 respiratory failure and a Grade 5 thromboembolic event were reported, both in the setting of progressive disease. The median time to clear the reovirus viremia was 6.5 days. Eight of twenty-four patients were viremic beyond the five days of therapy, all were negative by day 17. No patient had detectable viral RNA in saliva or stool. There were no objective responses.
Conclusions
Reolysin at a dose of 5 × 108 TCID50/kg daily for 5 days was well tolerated in children alone and in combination with oral cyclophosphamide. Virus was cleared rapidly from the serum and shedding in stool and salivawas not detectable.
Keywords: Virotherapy, Phase 1, Pediatric Cancer
Introduction
Reovirus is a naturally occurring, ubiquitous, human virus that consists of 10 segments of double-stranded RNA[1]. Community-acquired reovirus infection in humans is generally mild, however, reovirus replicates in and causes a cytopathic effect in cells transformed by an activated Ras signaling pathway[2-6]. Reovirus Serotype 3 – Dearing Strain is being developed as a cancer therapeutic and is now in multiple clinical trials in adult patients. The prevalence of reovirus antibodies in normal adults ranges from > 50% to 100%[7-10]. In healthy infants and children age 1 month to 5 years, 23.5% had detectable anti-reovirus antibodies. Highest prevalence was 50% among 5 year old children, suggesting exposure is common in early childhood[11].
Reoviruses initially infect epithelial cells of the ileum[4, 13] then through lymphatic and hematogenous dissemination spread to extraintestinal organs and the central nervous system[14]. Studies in which volunteers were inoculated with the three serotypes of reovirus resulted in symptoms of illness in approximately one-third of volunteers[3]. There have been isolated reports implicating reovirus with other disease processes such as hepatobiliary[15], neurological[16], and respiratory[17], disease.
The preferential lysis of cells with activated Ras by reovirus may be due in part to inhibition of double-stranded RNA-activated protein kinase (PKR) [5]. In non-Ras activated cells, PKR is autophosphorylated and activated in the presence of viral transcripts resulting in inhibition of viral protein synthesis and replication. Ras activated cells inhibit PKR autophosphorylation, maintaining the inactive state, and allowing viral translation and oncolysis to occur [18]. Some studies suggest that MEK, downstream of Ras, directly inhibits PKR[19]. Specificity of reovirus for Ras-transformed cells, coupled with its relatively nonpathogenic nature in humans, makes it an attractive anti-cancer therapy candidate.
Over 20 completed or ongoing phase 1 or 2 clinical studies of reovirus have been conducted in Canada, the United Kingdom, Europe and the United States with single or multiple doses administered intratumorally or intravenously, either alone or in combination with radiotherapy or chemotherapy. In phase 1 studies, evaluations of intravenous administration of reovirus as monotherapy or combined with chemotherapy have been completed with no defined maximum tolerated dose for reovirus at doses up to 3 × 1010 TCID50/dose for five consecutive days[20-22].
The principal adverse effects (AEs) of reovirus are primarily “flu-like syndrome”. Symptoms include fever, chills, headache, fatigue, rhinorrhea, sweating, rigors, myalgia and cough[20, 23-25]. Reovirus monotherapy has been associated with transient elevations of ALT and/or troponin-I (mainly Grade 1 or 2) that resolve within 1-2 weeks[27]. Other treatment-related laboratory AEs include mild nausea, Grade 3 neutropenia and Grade 2-3 lymphopenia[20, 23, 26]. In combination with either radiotherapy or chemotherapy, reovirus toxicity does not appear to be increased and it also does not appear to increase toxicity of chemotherapies with which it has been combined.
There is no prior experience with reovirus in patients less than 19 years. Preclinical data demonstrate responses to reovirus in pediatric osteosarcoma and Ewing sarcoma. The Children's Oncology Group Phase 1 Consortium aimed to define the safety and recommended phase 2 dose (RP2D) of reovirus alone and in combination with cyclophosphamide in children with relapsed or refractory extra-cranial solid tumors (ClinicalTrials.gov Identifier: NCT01240538).
Patients and Methods
The study was approved by the Institutional Review Board at participating institutions. Informed consent was obtained from patients 18 years or older and permission was obtained from parents or legal guardians of patients less than 18 years. Child assent was obtained in accordance with local institutional policies.
Eligibility
Patients ≥3 and ≤ 21 years with recurrent or refractory solid tumors, excluding tumors originating in or metastatic to the central nervous system or lymphomas, were eligible. Patients were required to have a Karnofsky (age > 15) or Lansky (age < 16) performance score of ≥ 50. Organ function requirements included: adequate bone marrow function (absolute neutrophil count greater ≥ 1,000/mL, and a transfusion-independent platelet count ≥ 100,000/mL); adequate renal (normal serum creatinine for age or a creatinine clearance or radioisotope GFR ≥ 70 ml/min/1.73 m2), and adequate hepatic function (total bilirubin ≤ 1.5 times the upper limit of normal for age, alanine aminotransferase (ALT) ≤ 110 U/L and serum albumin greater ≥ 2 g/dL). Patients must have recovered from the acute toxic effects of prior therapy, including a 3 week interval since the last myelosuppressive therapy, 2 weeks since radiation therapy, 6 weeks since immunotherapy and 1 week since biotherapy. Viral immunizations were not permitted within 7 days of enrollment or during the on-study treatment period. Patients with known germline mutations affecting Ras activation (e.g., neurofibromatosis type 1, cardio-facial-cutaneous syndrome, Noonan syndrome, and Costello syndrome) were excluded from enrollment. Patients requiring ongoing immunosuppression or a history of known viral infections with HIV or hepatitis B or C were also excluded.
Study Drug and Treatment Plan
Reovirus was provided by Oncolytics Biotech Inc. and distributed by the Pharmaceutical Management Branch of the National Cancer Institute. Virus was supplied in 1 ml aliquots at a concentration of 4.5 × 1010 TCID50/ml formulated in phosphate-buffered saline containing 3% mannitol, 2% histidine, 2% sorbitol, 0.01% polysorbate 80 and 2mM MgCl2. The starting dose was two-thirds the recommended phase 2 adult dose adjusted for an average weight of 70 kg, or 3 × 108 TCID50/kg.
Reovirus was handled according to local Institutional Biosafety Committee recommendations. Prior to injection, vials were thawed at room temperature over 10 minutes and the dose prepared using 250 ml 0.9% sodium chloride for patients ≥ 20 kg and 100 ml 0.9% sodium chloride for patients < 20 kg. The reovirus solution was immediately infused over 60 minutes following preparation.
Reovirus was administered for 5 consecutive days, every 28 days. No premedication was required, although non-acetaminophen, non-steroidal anti-pyretics were permitted for patients experiencing a fever. Commercially available cyclophosphamide was administered at a dose of 50 mg/m2/day daily for 21 consecutive days every 28 days to patients enrolled at the third dose level. For patients unable to swallow intact pills, cyclophosphamide was administered reconstituted as a 10 mg/ml suspension in 0.9% sodium chloride and Ora-Plus or simple syrup.
Study Design
The study was designed with a single dose escalation of reovirus (dose levels 1 and 2). A standard 3 + 3 design dose escalation design was used. Briefly, three patients were enrolled at each dose level. If one enrolled patient at risk for a dose-limiting toxicity (DLT) experienced a DLT, 3 additional patients were enrolled at that level. The maximum tolerated dose (MTD) was defined as the maximum dose at which fewer than one-third of patients experience DLT during cycle 1 of therapy. The RP2D was the MTD or highest dose level evaluated that did not exceed the MTD. Once the RP2D was defined a third dose level in which patients received a combination of reovirus at RP2D plus oral cyclophosphamide was evaluated.
Toxicities were graded using the revised NCI Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. DLT was defined as any grade 3 or 4 non-hematological toxicity possibly, probably or definitely attributable to protocol therapy with the exclusion of grade 3 nausea and vomiting, grade 3 liver enzyme elevation, grade 3 or 4 fever of less than 72 hours in duration, grade 3 infection, grade 3 supplement-responsive electrolyte disturbance or grade 3 tumor pain. Additional potential DLTs included non-hematologic toxicities that resulted in a delay of therapy for more than 14 days, allergic reactions that necessitated discontinuation of the study drug, a decreased left ventricular ejection fraction of greater than 10% from baseline, and/or grade 2 heart failure. Hematologic DLTs were defined as grade 4 neutropenia or febrile neutropenia for more than 7 days, a platelet count less than 25,000/mm3 on 2 separate days within a 7 day period, and myelosuppression resulting in more than a 14 day delay between cycles.
Response
Patients who completed at least one cycle of protocol therapy were evaluated for response. Response was assessed according to the Response Evaluation Criteria in Solid Tumors (RECIST)[30]. Response evaluations, performed at the end of cycle 1 and then every 2 cycles, were compared to the baseline evaluation performed at the time of enrollment or the best response. Only patients with stable disease or better received a second or subsequent cycle.
Viral Titers and Neutralizing Antibody Evaluations
Blood was collected prior to cycle 1 then twice weekly during cycles 1, 2 and 6 for assessment of viral clearance and neutralizing antibody. Plasma was separated by centrifugation at 2,300 × g for 10 min at 4°C and stored at -70°C. Buccal mucosa and anal swabs were collected at the same time for assessment of viral clearance. Swabs were incubated in 250μL Universal Transport Medium (Gibco, Grand Island, NY) for 10 min at room temperature, centrifuged and used for PCR. RNA was extracted from 140μL of patient serum, stool and saliva samples using the QIAamp Viral RNA Mini Kit (Qiagen, Valencia, CA). Total RNA was reverse-transcribed to cDNA using the High Capacity cDNA Reverse Transcription Kit (Invitrogen, Grand Island, NY). cDNA was amplified by real time-PCR using the S3 gene specific TaqMan probe 5′-/6-FAM/CACTGTCAG/ZEN/CGGAACAGCTTCTGGACGA/IABkFQ/-3′ and S3 primers (F–5′-ATACTGTGGTTCCTGTCGCTCCAA-3′ and R–5′-ATTCGCGTCCACCTCACATCCATA-3′). Standards (105 – 109 viral particles) were prepared by dilutions of Reolysin stock solutions, subjected to RNA isolation and simultaneously reverse transcribed and amplified with patient specimens. The reactions were run in triplicates. Absolute quantification of virus was calculated using the SDS version 2.3 algorithm (Applied Biosystems, Grand Island, NY). Duration of viremia is defined as the period between first infusion and the first negative serum sampling.
An ELISA was developed for anti-reovirus immunoglobin IgG. 96-well Maxisorp plates (Nunc) were coated with 100μl Reolysin lysate (1 × 103 viral particles/well) diluted in 0.05 M sodium carbonate buffer (pH 9.6) and incubated overnight at 4°C. Plates were blocked with 100μl of 5% BSA in PBS containing 0.05% Tween-20 (PBST) for 1 hour at room temperature, then wells were washed three times with PBST. Serum specimens were heat-inactivated at 56°C for 30 mins, diluted 1:5,000 in PBST and added to wells and incubated for 1 hour at 37C. Wells were washed 3 times with PBST. Horseradish peroxidase-conjugated goat anti-human IgG (Promega, Madison, WI), diluted 1:5,000 in 0.1% BSA was added to each well for 1 hour at 37°C. Wells were washed 5 times with PBST and 100μl of 3,3′,5,5′-tetramethylbenzidine (TMB; Promega) substrate was added. The reaction was stopped after 5 mins with 50μl of 2M H2SO4 and the optical density of wells measured at 450nm (VICTOR × Multilabel Plate Reader, Perkin Elmer, Waltham, MA). Serum samples were evaluated in triplicates. Each plate assay included a negative and positive control serum diluted at 1:5,000. A positive cutoff value was determined by the mean absorption at 450nm in negative serum samples plus two standard error (SE) measurements.
Statistics
An unpaired t-test was used to compare peak viral titer, duration of viremia, and age for patients with and without a baseline anti-reovirus antibody absorbance level above 0.2.
Results
Patient Characteristics
Twenty-nine patients were enrolled onto the study from April 2011 to August 2013. Twenty-four were evaluable. Five patients were not evaluable for the following reasons: one patient was found to have central nervous system metastases after enrollment but prior to the initiation of therapy; four did not receive the entire course of prescribed therapy (n=1), or not all the required observations were obtained (n=3). Patient characteristics are summarized in Table I.
Table I. Patient Characteristics for Eligible Patients.
| Characteristic | Number (%) |
|---|---|
| Age (years) | |
| Median | 12.5 years |
| Range | 3.0-20.2 years |
| Sex | |
| Male | 19 (67.9) |
| Female | 9 (32.1) |
| Race | |
| White | 18 (64.3) |
| Asian | 2 (7.1) |
| Native American | 1 (3.6) |
| Pacific Islander | 1(3.6) |
| Black or African American | 4 (14.3) |
| Unknown | 2 (7.1) |
| Ethnicity | |
| Non-Hispanic | 24 (85.7) |
| Hispanic | 4 (14.3) |
| Diagnosis | |
| Alveolar rhabdomyosarcoma | 2 (7.1) |
| Chondroblastic osteosarcoma | 1 (3.6) |
| Clear cell sarcoma | 1 (3.6) |
| Desmoplastic small round cell tumor | 1 (3.6) |
| Embryonal rhabdomyosarcoma | 3 (10.7) |
| Ewing sarcoma | 3 (10.7) |
| Germ cell tumor | 1 (3.6) |
| Hemangiosarcoma/Angiosarcoma | 1 (3.6) |
| Hepatoblastoma | 2 (7.1) |
| Neoplasm, malignant/Tumor, malignant, NOS | 1 (3.6) |
| Wilms,tumor | 3 (10.7) |
| Neuroblastoma | 2 (7.1) |
| Osteosarcoma | 3 (10.7) |
| Retinoblastoma | 1 (3.6) |
| Rhabdomyosarcoma | 1 (3.6) |
| Synovial sarcoma | 2 (7.1) |
| Prior Therapy | |
| Chemotherapy Regimens | |
| Median | 3 |
| Range | 1-8 |
| Number of Patients with Prior Radiation Therapy | 20 (71.4) |
Toxicities
Dose limiting toxicities are listed in Table II. At dose level 1, 3 × 108 TCID50/kg/dose, one patient experienced grade 5 respiratory failure and died on cycle 1, day 9. Death was attributed to progressive disease and attribution to drug was deemed unlikely by the treating physicians. Nonetheless, 5 additional patients were enrolled at dose level 1 given the severity of the event. Respiratory complaints were rare and mild in subsequently enrolled patients. All additional dose-limiting toxicities associated with a grade 5 thromboembolism occurred in a single patient with synovial sarcoma who was enrolled in the expansion cohort at the RP2D, 5 × 108 TCID50/kg/dose. On day 20 of cycle 1 this patient presented with a 1 day history of worsening shortness of breath and was found to have a lower extremity deep venous thrombosis, bilateral pulmonary emboli, and progressive metastatic disease. Attribution was possibly related to Reolysin, probably related to synovial sarcoma, and probably related to progressive metastatic disease. There are 5 reported thromboembolic events reported through the Adverse Event Expedited Reporting System in the United States (three Grade 3, two Grade 2). The Grade 2 events are possibly related, the Grade 3 events are either unlikely related or unrelated to virus. The thromboembolic event in the current study occurred in the setting of progressive disease, 15 days after the last dose of Reolysin. Day 10 was the last day reovirus was detected in the serum of this patient.
Table II. Summary of Dose-Limiting Toxicities.
| Dose Level | No. Patients Entered | No. Patients Evaluable | No. Patients with DLT | Type of DLT (n) |
|---|---|---|---|---|
| 3 × 108 TCID50/kg | 7 | 6 | 1 | Respiratory failure (1) |
| 5 × 108 TCID50/kg | 8 | 6 | 0 | |
| 5 × 108 TCID50/kg Expansion cohort | 7 | 7* | 1 | Thromboembolism(1) Hypokalemia (1) Hypocalcemia (1) Hypophosphatemia (1) Hypernatremia (1) Hypoalbuminemia (1) Acidosis (1) |
| 5 × 108 TCID50/kg plus cpm | 6 | 5 | 0 |
One patient was enrolled in the expansion cohort but received DL3.
Cpm - cyclophosphamide
Hematologic toxicities are summarized in Table III, and common non-hematologic toxicities attributable to reovirus are summarized in Table IV. Common toxicities reported include grade 2 and 3 leukopenia, neutropenia and lymphopenia; grade 2 fever, and grade 2 elevation in liver transaminases. These toxicities were reported at all dose levels and are consistent with previous reports in adults. There was no increase in incidence or severity of the toxicities reported in the 15 patients who were antibody negative at baseline or when reovirus was administered in combination with oral cyclophosphamide.
Table III. Non-dose limiting hematologic toxicities observed in evaluable patients irrespective of attribution (n=24).
| Maximum grade of toxicity across cycle 1 (Total, 24 cycles) |
Maximum grade of toxicity across cycles 2 to 3 (Total, 5 cycles) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Toxicity Type | Gr 1 | Gr 2 | Gr 3 | Gr 4 | Gr 1 | Gr 2 | Gr 3 | Gr 4 |
| Anemia | 10 | 5 | 3 | 4 | 1 | 1 | 1 | |
| Leukopenia | 8 | 4 | 8 | 1 | 2 | |||
| Lymphopenia | 1 | 3 | 8 | 3 | 1 | 2 | 1 | |
| Neutropenia | 3 | 10 | 1 | 2 | ||||
| Thrombocytopenia | 11 | 2 | 1 | 1 | ||||
Table IV. Non-dose limiting non-hematologic toxicities related to protocol therapy and observed in more than 10 percent* of evaluable patients (n=24).
| Maximum grade of toxicity across cycle 1 (total, 24 cycles) |
Maximum grade of toxicity across cycles 2 to 3 (Total, 5 cycles) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Toxicity Type | Gr 1 | Gr 2 | Gr 3 | Gr 4 | Gr 1 | Gr 2 | Gr 3 | Gr 4 |
| Abdominal pain | 3 | |||||||
| ALT increased | 7 | 1 | 3 | |||||
| Anorexia | 3 | 2 | ||||||
| AST increased | 4 | 3 | 2 | 1 | ||||
| Chills | 3 | |||||||
| Diarrhea | 3 | |||||||
| Fatigue | 4 | 1 | 2 | |||||
| Fever | 3 | 9 | 3 | 1 | 1 | |||
| Headache | 9 | 3 | 1 | 1 | ||||
| Hypoalbuminemia | 2 | 1 | ||||||
| Myalgia | 1 | 2 | 1 | |||||
| Nausea | 6 | 1 | 1 | |||||
Criteria for a maximum tolerated dose were not met. As a result, the recommended phase 2 dose is 5 × 108 TCID50/kg/ dose for 5 consecutive days to a maximum dose of 3 × 1010 TCID50/dose.
Virology and Antibody Response
The lower threshold absorbance for detection of the anti-reovirus antibody was defined as 0.2 absorbance at 450nm. This represents 2 standard error measurements above the mean of 5 negative samples. On serum samples taken prior to the first dose of reovirus, 9 patients had an absorbance greater than 0.2 (antibody positive) and 15 patients less than 0.2 (antibody negative).
Duration of viremia and percent change in antibody level above baseline for cycle 1 are represented in Figure 1A. Eight patients (30%) were viremic beyond the 5 treatment days. Seven of the eight patients were negative for reovirus in the serum by Day 12, and all by Day 17. All patients developed an increase in anti- reovirus antibody above baseline following cycle 1 treatment. There was no difference in the peak viremia among patients that were antibody negative (mean 1.33 × 106, median 1.38 × 106, range 0.125 to 2.57 × 106) or positive (mean 1.24 × 106, median 0.89 × 106, range 0.15 to 3.76 × 106) at baseline (p= 0.35). Similarly, there was no difference in duration of viremia based on baseline anti-reovirus antibody titer. (p=0.86) The mean age of patients with an antibody absorbance value less than 0.2 is 11.7 years and 12.2 years for patients above 0.2 (p=0.46). Three patients with stable disease at the end of cycle 1 received a second cycle of reovirus. Peak viremia and antibody response are represented in Figure 2A for patients receiving the second cycle. Peak reovirus values for the second cycle were below cycle 1, and baseline antibody value prior to cycle 2 was markedly higher than cycle 1. One patient had no detectable virus in the immediate post infusion specimen (Figure 2A).
Figure 1.
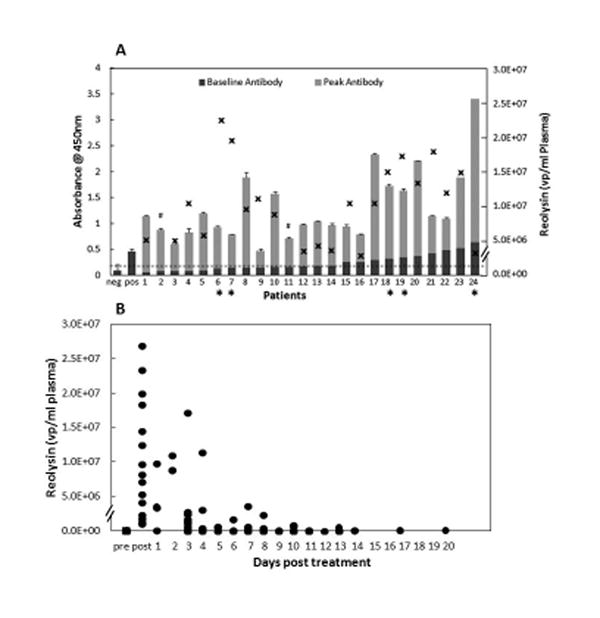
A) Baseline anti-reovirus antibody levels in plasma samples from 24 evaluable patients (dark gray bars), taken prior to the first dose of reovirus expressed as absorbance at 450 nm (left-sided y-axis). The light gray bars represent the peak antibody titer post infusion of reovirus. An absorbance of 0.2 is defined as the threshold for detection of anti-reovirus antibody. This value represents the mean of known negative samples plus 2 standard errors. A negative control (neg) and a positive control (pos) are provided for reference. Error bars are provided and represent the standard deviation of anti-reovirus antibody samples tested in triplicate. Patients who received reovirus in combination with cyclophosphamide are identified by an apteryx (*). Additionally, the peak viral-particles per ml of plasma (x) for each patient is presented using the right-sided y-axis. Samples are numbered continuously based on the baseline anti-reovirus titer. A hash mark (#) indicates no sample was available for testing. B) Reovirus is quantified in plasma by real-time PCR and expressed as viral particles (●) based on comparison to a standard curve generated using reovirus standards. Twice weekly measurements were evaluated for virus until samples were negative.
Figure 2.
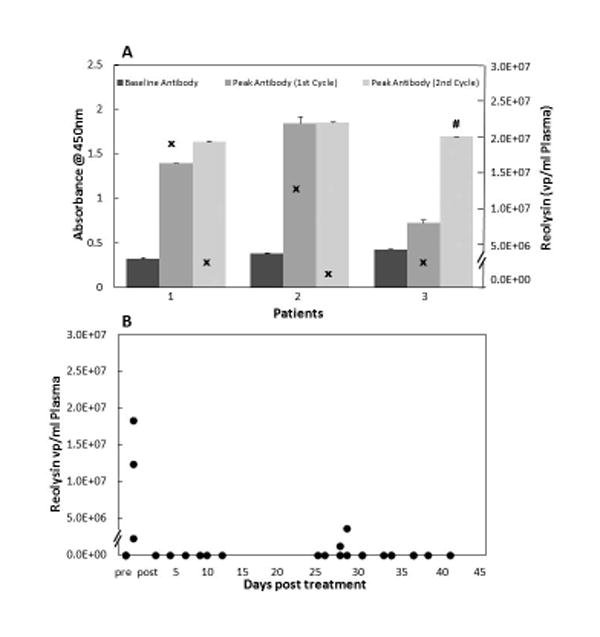
A) Three patients received a second cycle of reovirus. Antibody level at baseline, as well as the peak level after the first and second cycles are presented (left-sided y-axis). The peak viral-particles per ml of plasma (x) for each patient is presented using the right-sided y-axis. A hash mark (#) indicates no sample was available for testing. B) Reovirus is quantified in plasma by real-time PCR and expressed as viral particles (●) based on comparison to a standard curve generated using reovirus standards. Twice weekly measurements for each cycle were evaluated for virus until samples were negative.
In Figure 1A, viremia peak and antibody response are presented in the five evaluable patients who received reovirus in combination with cyclophosphamide. Cyclophosphamide had no impact on the duration of viremia or on emergence of anti-reovirus antibody.
Tumor Response
Three patients with stable disease received a second cycle and 2 patients a third cycle of therapy prior to progressive disease. There were no complete or partial responses reported and all other patients progressed within 28 days.
Discussion
Reovirus is a double-stranded RNA virus that replicates preferentially in cells with increased Ras pathway signaling [5, 31]. Clinical trials in adults demonstrated that reovirus is well tolerated following intratumoral or systemic administration, either as monotherapy or in combination with chemotherapy and/or radiation therapy[20-22, 24, 33-35]. In the current trial, reovirus was found to be safe in children when administered as a single agent and in combination with oral cyclophosphamide. A maximum tolerated dose was not identified at the dose levels studied. The recommended phase 2 dose is 5 × 108 TCID50/kg (not exceeding a total dose of 3 × 1010 TCID50/dose) daily for 5 consecutive days every 28 days via intravenous infusion over 60 minutes, which is equivalent RP2D for adults adjusted for weight. No viral shedding was detectable in stool or saliva.
To date, systemic administration of reovirus has yielded responses in sarcomas, breast cancer, lung cancer, ovarian cancer, colorectal cancer and head and neck cancers. In adults, neutralizing anti-reovirus antibodies are detectable pre-treatment. Titers subsequently increase 1 to 2 weeks after initial treatment and plateau with additional courses. Viral shedding is rare in urine, saliva or stool[22, 34]. In this study, more than half of the pediatric patients were antibody negative pre-treatment. All patients had increased anti-reovirus titers following the first course of therapy. There was with no apparent difference in the peak or duration of viremia, or in viral shedding among patients with low or negative baseline anti-reovirus antibody titers.
Viral titers were undetectable for most patients beyond the treatment period and beyond 13 days post infusion. Little is reported on the correlation of viral circulation and delivery in the setting of a humoral immune response. In a completed phase 2 trial of reovirus in patients with metastatic melanoma, 2 of 13 patients demonstrated productive viral replication despite an increase in neutralizing antibody titer[20]. One could hypothesize that the dose of reovirus, 3 × 1010 TCID50/dose, was sufficient to overcome circulating antibodies delivering virus to tumor cells. Further, Adair et al., reported viral evasion of the humoral immune response through uptake and transport in mononuclear cells[36]. In two phase 1 trials of single agent systemic reovirus administered daily via intravenous infusion, in adults with advanced solid tumors (n=51) [22, 33], one partial response was reported in a patient with taxane-resistant breast cancer. Seventeen patients (33%) with various histologies receiving up to 6 cycles had stable disease [22, 33]. In a phase 2 study of 3 × 1010 TCID50/dose intravenous reovirus for 5 days in patients with metastatic melanoma (n=21), no responses were reported. In this study, 3 of 24 evaluable patients with a heterogeneous mix of pediatric tumors had stable disease at the end of 1 cycle. All progressed by cycle 3. Given the absence of objective responses, and the published response in wild-type Ras tumors, we did not pursue further analysis of Ras pathway mutation status in the setting of this phase 1 trial.
Objective responses to single agent reovirus administered via intravenous infusion are rare[22]. However, Morris et al., reported 1 complete response, 2 partial responses and 4 patients with stable disease among 19 patients receiving intralesional reovirus for advanced solid tumors[37]. Intralesional administration has the advantage of delivering higher viral loads to the tumor, expediting delivery and perhaps avoiding rapid immune clearance. Two completed phase 1 trials of intralesional reovirus for malignant glioma have not identified dose-limiting toxicities to a dose of 1 × 1010 TCID50/dose[24, 38], and underscore the feasibility of intralesional reovirus. Further, the combination of intralesional reovirus with radiation therapy or chemotherapy may enhance response[21, 25, 39]. Efficacy of platinum and radiation based combinations have been reported in pediatric osteosarcoma xenografts[40]. Given the primary safety concern of viral replication in children following high titer bolus administration, safety data of reovirus in immunosuppressed children is needed before multi-agent combination chemotherapy trials are attempted. In this study, we tested the hypothesis that reovirus could be administered safely with immunosuppressive cyclophosphamide therapy. Cyclophosphamide can inhibit T-regulatory cell and NK cell function [41] and increase intratumoral virus levels and tumor response[28, 42]. This study was not designed to determine if cyclophosphamide increased efficacy of reovirus, only to assess the safety of the combination. Cyclophosphamide did not impact peak anti-reovirus antibody levels, or viral clearance in the 5 patients evaluated (Figure 1A) and there were no unanticipated or dose limiting toxicities associated with the combination.
In summary, reovirus can be administered safely to heavily pre-treated children with relapsed and refractory solid tumors and no maximum tolerated dose was reached. The recommended Phase 2 dose is 5 × 108 TCID50/kg (not exceeding a total dose of 3 × 1010 TCID50/dose) daily for 5 consecutive days every 28 days via intravenous infusion over 60 minutes. Reovirus was cleared from the serum in most patients within 48 hours of completion of the 5-day course and from all patients within 2 weeks of the last dose. Viral shedding in saliva and stool was not seen. Although this study was not designed to test antitumor efficacy, the low incidence of tumor responses we observed suggests the utility of reovirus will likely require combination therapies as is currently being explored in adults.
Acknowledgments
This research reported in this publication was supported in part by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number (P20GM103464 and P20GM103446) as well as the NIH Pediatric Phase 1/Pilot Consortium 5UM1 CA097452-12 grant. Further support was provided by Cookies for Kids. We would also like to thank Biljana Georgievska, Thalia Beeles, and Catalina Martinez of the COG Phase 1/Pilot Consortium Coordinating Center for outstanding administrative and data management support throughout the development and conduct of this trial.
Footnotes
Conflict of interest statement: There are no actual or perceived conflicts of interest.
References
- 1.Tyler KL, Fields BN. Virology (ed 2) New York: Raven Press; [Google Scholar]
- 2.Bodkin DK, Nibert ML, Fields BN. Proteolytic digestion of reovirus in the intestinal lumens of neonatal mice. J Virol. 1989;63:4676–4681. doi: 10.1128/jvi.63.11.4676-4681.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosen L, Evans HE, Spickard A. Reovirus infections in human volunteers. Am J Hyg. 1963;77:29–37. doi: 10.1093/oxfordjournals.aje.a120293. [DOI] [PubMed] [Google Scholar]
- 4.Rubin DH, Kornstein MJ, Anderson AO. Reovirus serotype 1 intestinal infection: a novel replicative cycle with ileal disease. J Virol. 1985;53:391–398. doi: 10.1128/jvi.53.2.391-398.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strong JE, Coffey MC, Tang D, et al. The molecular basis of viral oncolysis: usurpation of the Ras signaling pathway by reovirus. EMBO J. 1998;17:3351–3362. doi: 10.1093/emboj/17.12.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolf JL, Kauffman RS, Finberg R, et al. Determinants of reovirus interaction with the intestinal M cells and absorptive cells of murine intestine. Gastroenterology. 1983;85:291–300. [PubMed] [Google Scholar]
- 7.Minuk GY, Rascanin N, Paul RW, et al. Reovirus type 3 infection in patients with primary biliary cirrhosis and primary sclerosing cholangitis. J Hepatol. 1987;5:8–13. doi: 10.1016/s0168-8278(87)80054-5. [DOI] [PubMed] [Google Scholar]
- 8.Minuk GY, Paul RW, Lee PW. The prevalence of antibodies to reovirus type 3 in adults with idiopathic cholestatic liver disease. J Med Virol. 1985;16:55–60. doi: 10.1002/jmv.1890160108. [DOI] [PubMed] [Google Scholar]
- 9.Jackson GG, Muldoon RL. Viruses causing common respiratory infections in man. J Infect Dis. 1973;127:328–355. doi: 10.1093/infdis/127.3.328. [DOI] [PubMed] [Google Scholar]
- 10.Stanley NF. Comparative Diagnosis of Viral Diseases. New York: Academic Press; 1977. [Google Scholar]
- 11.Tai JH, Williams JV, Edwards KM, et al. Prevalence of reovirus-specific antibodies in young children in Nashville, Tennessee. J Infect Dis. 2005;191:1221–1224. doi: 10.1086/428911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sabin AB. Reoviruses. A new group of respiratory and enteric viruses formerly classified as ECHO type 10 is described. Science. 1959;130:1387–1389. doi: 10.1126/science.130.3386.1387. [DOI] [PubMed] [Google Scholar]
- 13.Wolf JL, Dambrauskas R, Sharpe AH, et al. Adherence to and penetration of the intestinal epithelium by reovirus type 1 in neonatal mice. Gastroenterology. 1987;92:82–91. doi: 10.1016/0016-5085(87)90842-0. [DOI] [PubMed] [Google Scholar]
- 14.Kauffman RS, Wolf JL, Finberg R, et al. The sigma 1 protein determines the extent of spread of reovirus from the gastrointestinal tract of mice. Virology. 1983;124:403–410. doi: 10.1016/0042-6822(83)90356-2. [DOI] [PubMed] [Google Scholar]
- 15.Brown WR, Sokol RJ, Levin MJ, et al. Lack of correlation between infection with reovirus 3 and extrahepatic biliary atresia or neonatal hepatitis. J Pediatr. 1988;113:670–676. doi: 10.1016/s0022-3476(88)80376-7. [DOI] [PubMed] [Google Scholar]
- 16.Ouattara LA, Barin F, Barthez MA, et al. Novel human reovirus isolated from children with acute necrotizing encephalopathy. Emerg Infect Dis. 2011;17:1436–1444. doi: 10.3201/eid1708.101528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tillotson JR, Lerner AM. Reovirus type 3 associated with fatal pneumonia. N Engl J Med. 1967;276:1060–1063. doi: 10.1056/NEJM196705112761903. [DOI] [PubMed] [Google Scholar]
- 18.Kelly K, Nawrocki S, Mita A, et al. Reovirus-based therapy for cancer. Expert Opin Biol Ther. 2009;9:817–830. doi: 10.1517/14712590903002039. [DOI] [PubMed] [Google Scholar]
- 19.Veerapong J, Bickenbach KA, Shao MY, et al. Systemic delivery of (gamma1)34.5-deleted herpes simplex virus-1 selectively targets and treats distant human xenograft tumors that express high MEK activity. Cancer Res. 2007;67:8301–8306. doi: 10.1158/0008-5472.CAN-07-1499. [DOI] [PubMed] [Google Scholar]
- 20.Galanis E, Markovic SN, Suman VJ, et al. Phase II trial of intravenous administration of Reolysin(®) (Reovirus Serotype-3-dearing Strain) in patients with metastatic melanoma. Mol Ther. 2012;20:1998–2003. doi: 10.1038/mt.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Comins C, Spicer J, Protheroe A, et al. REO-10: a phase I study of intravenous reovirus and docetaxel in patients with advanced cancer. Clin Cancer Res. 2010;16:5564–5572. doi: 10.1158/1078-0432.CCR-10-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gollamudi R, Ghalib MH, Desai KK, et al. Intravenous administration of Reolysin, a live replication competent RNA virus is safe in patients with advanced solid tumors. Invest New Drugs. 2010;28:641–649. doi: 10.1007/s10637-009-9279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vidal L, Pandha HS, Yap TA, et al. A phase I study of intravenous oncolytic reovirus type 3 Dearing in patients with advanced cancer. Clin Cancer Res. 2008;14:7127–7137. doi: 10.1158/1078-0432.CCR-08-0524. [DOI] [PubMed] [Google Scholar]
- 24.Forsyth P, Roldán G, George D, et al. A phase I trial of intratumoral administration of reovirus in patients with histologically confirmed recurrent malignant gliomas. Mol Ther. 2008;16:627–632. doi: 10.1038/sj.mt.6300403. [DOI] [PubMed] [Google Scholar]
- 25.Harrington KJ, Karapanagiotou EM, Roulstone V, et al. Two-stage phase I dose-escalation study of intratumoral reovirus type 3 dearing and palliative radiotherapy in patients with advanced cancers. Clin Cancer Res. 2010;16:3067–3077. doi: 10.1158/1078-0432.CCR-10-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forsyth P, Roldán G, George D, et al. A phase I trial of intratumoral administration of reovirus in patients with histologically confirmed recurrent malignant gliomas. Mol Ther. 2008;16:627–632. doi: 10.1038/sj.mt.6300403. [DOI] [PubMed] [Google Scholar]
- 27.Lolkema MP, Arkenau HT, Harrington K, et al. A phase I study of the combination of intravenous reovirus type 3 Dearing and gemcitabine in patients with advanced cancer. Clin Cancer Res. 2011;17:581–588. doi: 10.1158/1078-0432.CCR-10-2159. [DOI] [PubMed] [Google Scholar]
- 28.Qiao J, Wang H, Kottke T, et al. Cyclophosphamide facilitates antitumor efficacy against subcutaneous tumors following intravenous delivery of reovirus. Clin Cancer Res. 2008;14:259–269. doi: 10.1158/1078-0432.CCR-07-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Black AJ, Morris DG. Clinical trials involving the oncolytic virus, reovirus: ready for prime time? Expert Rev Clin Pharmacol. 2012;5:517–520. doi: 10.1586/ecp.12.53. [DOI] [PubMed] [Google Scholar]
- 30.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 31.Coffey MC, Strong JE, Forsyth PA, et al. Reovirus therapy of tumors with activated Ras pathway. Science. 1998;282:1332–1334. doi: 10.1126/science.282.5392.1332. [DOI] [PubMed] [Google Scholar]
- 32.Kelly KR, Espitia CM, Mahalingam D, et al. Reovirus therapy stimulates endoplasmic reticular stress, NOXA induction, and augments bortezomib-mediated apoptosis in multiple myeloma. Oncogene. 2012;31:3023–3038. doi: 10.1038/onc.2011.478. [DOI] [PubMed] [Google Scholar]
- 33.Vidal L, Pandha HS, Yap TA, et al. A phase I study of intravenous oncolytic reovirus type 3 Dearing in patients with advanced cancer. Clin Cancer Res. 2008;14:7127–7137. doi: 10.1158/1078-0432.CCR-08-0524. [DOI] [PubMed] [Google Scholar]
- 34.Karapanagiotou EM, Roulstone V, Twigger K, et al. Phase I/II trial of carboplatin and paclitaxel chemotherapy in combination with intravenous oncolytic reovirus in patients with advanced malignancies. Clin Cancer Res. 2012;18:2080–2089. doi: 10.1158/1078-0432.CCR-11-2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harrington KJ, Karapanagiotou EM, Roulstone V, et al. Two-stage phase I dose-escalation study of intratumoral reovirus type 3 dearing and palliative radiotherapy in patients with advanced cancers. Clin Cancer Res. 2010;16:3067–3077. doi: 10.1158/1078-0432.CCR-10-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adair RA, Scott KJ, Fraser S, et al. Cytotoxic and immune-mediated killing of human colorectal cancer by reovirus-loaded blood and liver mononuclear cells. Int J Cancer. 2013;132:2327–2338. doi: 10.1002/ijc.27918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morris DG, Feng X, DiFrancesco LM, et al. REO-001: A phase I trial of percutaneous intralesional administration of reovirus type 3 dearing (Reolysin(R)) in patients with advanced solid tumors. Invest New Drugs. 2013;31:696–706. doi: 10.1007/s10637-012-9865-z. [DOI] [PubMed] [Google Scholar]
- 38.Kicielinski KP, Chiocca EA, Yu JS, et al. Phase 1 clinical trial of intratumoral reovirus infusion for the treatment of recurrent malignant gliomas in adults. Mol Ther. 2014;22:1056–1062. doi: 10.1038/mt.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kyula JN, Roulstone V, Karapanagiotou EM, et al. Oncolytic reovirus type 3 (Dearing) as a novel therapy in head and neck cancer. Expert Opin Biol Ther. 2012;12:1669–1678. doi: 10.1517/14712598.2012.745507. [DOI] [PubMed] [Google Scholar]
- 40.Hingorani P, Zhang W, Lin J, et al. Systemic administration of reovirus (Reolysin) inhibits growth of human sarcoma xenografts. Cancer. 2011;117:1764–1774. doi: 10.1002/cncr.25741. [DOI] [PubMed] [Google Scholar]
- 41.Ghiringhelli F, Menard C, Puig PE, et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56:641–648. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smakman N, van der Bilt JDW, van den Wollenberg DJM, et al. Immunosuppression promotes reovirus therapy of colorectal liver metastases. Cancer Gene Ther. 2006;13:815–818. doi: 10.1038/sj.cgt.7700949. [DOI] [PubMed] [Google Scholar]


