Abstract
The transition to out-of-home childcare brings a number of challenges for children, including complex peer interactions and extended separations from parents. Children often show a midmorning-to-afternoon rise in cortisol on childcare days, compared to the typical diurnal decline seen at home. Changes in cortisol were examined in a wide age range of children (N = 168; 1.2mos–8yrs, M = 3.27yrs) during the 10-week transition to a new childcare setting. Structural equation modeling using latent change scores showed that children experienced an increase in the cortisol rise at childcare across the 10-week transition. Further, child age moderated the difference between home and childcare cortisol patterns. Findings are placed in a developmental context, and potential implications and future directions are discussed.
Full-day childcare poses challenges for young children, including extended separations from caregivers, imposed daily routines, and complex social interactions. One robust and well-replicated finding is that children’s cortisol production on childcare days differs from children’s cortisol production on non-childcare days. Specifically, many children exhibit a rise in cortisol from mid-morning to mid-afternoon on childcare days, as opposed to the typical diurnal decrease on days at home (Dettling, Gunnar, & Donzella, 1999; Dettling, Parker, Lane, Sebanc, & Gunnar, 2000; Geoffroy, Cote, Parent, & Seguin, 2006; Vermeer & van IJzendoorn, 2006; Watamura, Donzella, Alwin, & Gunnar, 2003; Watamura, Donzella, Kertes, & Gunnar, 2004). There are developmental differences in this rise in cortisol across the childcare day, such that toddlers and preschool-aged children tend to show a larger increase across the day, relative to infants and school-aged children who may show no change or a decline in cortisol across the day (Geoffroy et al., 2006; Vermeer & van IJzendoorn, 2006). Because past studies have tended to compare cortisol patterns at home and at childcare at one point in time, less is known about how the rise in cortisol across the childcare day emerges and changes during the transition from home to a new childcare setting. By examining the pattern of cortisol production among children of different ages during the transition to a new childcare setting, we aimed to examine changes in children’s physiological responses to out-of-home care that emerge over time. A better understanding of how cortisol at childcare changes over time is critical for considering developmental and environmental factors that influence children’s adjustment to the childcare context.
Cortisol, an end product of the hypothalamic-pituitary-adrenal (HPA) axis, exhibits a diurnal rhythm, which plays a major role in maintaining circadian patterns of daily activity (Gunnar & Cheatham, 2003). Among human infants, the diurnal rhythm of cortisol secretion emerges after the first several weeks of life and becomes increasingly stable during early childhood (Larson, White, Cochran, Donzella, & Gunnar, 1998; Price, Close, & Fielding, 1983). Cortisol levels peak approximately 30 minutes after wake-up, drop quickly by mid-morning, and then gradually decline across the day to a bedtime nadir. Children under 3 years of age show the mid-morning to afternoon decline less reliably than do older children (Bruce, Davis, & Gunnar, 2002; Watamura et al., 2003). In addition to exhibiting a diurnal rhythm, cortisol is produced in response to stress. Whereas cortisol responses are reliably elicited in adolescents and adults to stressors involving uncontrollability and social threat (Dickerson & Kemeny, 2004), young children appear to be relatively hyporesponsive to most stressors in terms of cortisol production (see Gunnar, Talge, Herrera, 2009 for a review). Given that high levels of glucocorticoids early in life are associated with negative effects on brain development (McEwen, Gould, & Sakai, 1992; Sapolsky & Meaney, 1986), this dampened reactivity may reflect an evolutionary adaptation that protects the young child during especially sensitive developmental periods.
Cortisol Production on Days in Childcare
The experience of full-day childcare is of particular interest because young children often show patterns of cortisol production that are opposite of those observed at home. Whereas young children typically show no change or a drop across the day at home, a number of studies have shown that young children show rises in cortisol levels from morning to afternoon on days in full-day childcare (Dettling et al., 1999, Dettling et al., 2000; Legendre, 2003; Watamura et al., 2003). This rise across the day appears to reflect a normative response to full-day childcare, strongly moderated by age (Geoffroy et al., 2006; Vermeer & van IJzendoorn, 2006). Specifically, preschool-age children (i.e., 2- to 4-year-olds) show the largest increase across the day at childcare compared with infants and school-age children who tend to show no change or a decrease (Dettling et al., 1999; Watamura, Sebanc, & Gunnar, 2002).
Although we only examine age-related effects on childcare cortisol patterns in the current study, other studies have examined other characteristics of children and of settings that influence cortisol patterns at childcare. The magnitude of the rise in cortisol has been linked with worse quality of childcare (Legendre, 2003; Lisonbee, Mize, Payne, & Granger, 2008; Sims, Guilfoyle, & Parry, 2006) and child temperament (Tout et al., 1998; Dettling et al., 1999; Watamura et al., 2003). With regard to child temperament, findings are mixed, with no clear behavioral disposition associated with the increase. For example, whereas Watamura and colleagues (2003) found that children high in social fearfulness showed greater increases across the day relative to children low in social fearfulness, Dettling and colleagues (1999) found that highly aggressive children, who represent a very different group than socially fearful children, exhibited a greater rise across the day relative to non-aggressive children. In a recent meta-analysis, Vermeer and van IJzendoorn (2006) reviewed the complex findings, concluding that childcare attendance was associated with an increase in cortisol across a range of conditions, with age emerging as the most significant moderator of this effect.
Cortisol Production during the Transition to Childcare
In the majority of these studies, cortisol measurements were obtained at one point in time after children had been in care for at least several weeks, when cortisol production patterns were assumed to be stable. An important step in clarifying the significance of the seemingly anomalous pattern of cortisol production in childcare is to examine how these patterns emerge and change over time, particularly during the transition to a new childcare setting. However, few studies to date have examined how cortisol production changes during periods of transition to a new childcare setting, and the majority of these focused on changes in morning cortisol, rather than changes in the mid-morning to afternoon pattern. Furthermore, the studies that have examined changes during the transition to childcare or school have focused primarily on infants (i.e., attending childcare) or school-age children (i.e., attending regular classrooms in primary education settings). Thus, there is less information about how cortisol patterns may change during the transition to childcare among preschool-age children, who are most likely to show the mid-morning to afternoon rise based on meta-analytic findings (Vermeer & van IJzendoorn, 2006).
Ahnert, Gunnar, Lamb, and Barthel (2004), for example, examined a sample of 15-month-old infants enrolled in childcare in Germany. Time-matched morning cortisol samples collected at home were compared with samples obtained during an adaptation period when mothers accompanied their infants to childcare, during the first 10 days at childcare without the parent, and after 5 months of childcare. Three saliva samples were obtained in the morning at childcare: upon arrival, 30 minutes post-arrival, and 60 minutes post-arrival. Cortisol levels at childcare were elevated above levels at home during the transition to childcare and remained elevated 5 months later. Furthermore, cortisol levels increased in the hour following mothers’ departure during the 10 days after the initial adaptation period. By 5 months, although children appeared to show some adaptation (less of an increase after separation), cortisol levels at childcare remained higher than home baseline levels. Findings suggested that childcare continued to be a challenging experience for infants.
Numerous studies have examined school-age children’s cortisol production during the transition to a new school year (e.g., Boyce et al., 1995; Bruce, Davis, & Gunnar, 2002; Davis, Donzella, Krueger, & Gunnar, 1999; Gutteling, de Weerth, & Buitelaar, 2005; Quas, Murowchick, Bedsadoun, & Boyce, 2002; Smider et al., 2002; Turner-Cobb, Rixon, & Jessup, 2008). Bruce and colleagues (2002) found a steeper wake-up to afternoon decline in cortisol levels on the first day of school compared with weekend samples at home, an effect driven by higher morning cortisol levels on the first day of school. At the group level, this difference disappeared by the fifth day of school. Davis et al. (1999), on the other hand, did not find evidence for significant elevations in cortisol during the first week of a new school year. Several studies reported associations between cortisol production during the transition to the school year and temperament characteristics, such as surgency/extraversion (Davis et al., 1999; Quas et al., 2002; Turner-Cobb et al., 2008). For example, Quas et al. (2002) examined wake-up samples from children one week before and one week after entering kindergarten. Increases in wake-up cortisol levels following the transition to kindergarten were largest for children who would be likely to experience kindergarten as a challenge (rated from parent report), either because the experience was novel or because they would engage readily with new and stimulating experiences (Quas et al., 2002). In a sample of children starting elementary school, Groeneveld et al. (2013) examined hair cortisol concentrations at two months following school entry. Compared with the two months prior to starting school, children had higher concentrations of hair cortisol in the first two months of school. Elevations in hair cortisol concentration during the transition to school was particularly pronounced for fearful children.
Relevant to the current study, Ouellet-Morin et al. (2010) conducted a prospective study of toddlers’ cortisol production at childcare. They examined home and childcare cortisol twice per day (morning and afternoon) on two consecutive days when children were 2 years old and then again when they were 3 years old. At 2 years old, children showed a flat pattern of cortisol production from morning to afternoon on childcare days, whereas they showed declining levels on home days. At 3 years old, however, children’s cortisol declined across the day at childcare similar to home patterns. Thus, children appeared to have different patterns of cortisol production on childcare days around age 2, which then returned to normal (i.e., home-like) patterns as they aged. Although this study did not examine repeated measurements of cortisol as children transitioned into childcare, the longitudinal design provided evidence of within-child adaptation across years in care.
The Present Study
The present study was designed to examine changes in cortisol production during the transition to a new childcare setting across a wide age range of children (from infancy to school-age). Midmorning and afternoon cortisol samples were obtained on the first day at the childcare center and at two-week intervals for the first ten weeks of attendance for each child, as well as on two consecutive days at home. We did not posit specific hypotheses about how cortisol patterns would change during the 10-week transition. On one hand, we might expect that patterns would emerge early and remain stable across weeks in care if these patterns of cortisol production reflected normative responses to the ongoing challenges of full-day childcare experiences. On the other hand, we might expect changes as children became adjusted to their environments if these patterns of cortisol production reflected a stress response to entering full-day childcare. We hypothesized that there would be a curvilinear effect of child age on the pattern of cortisol production across the childcare day, such that preschool-age children would show a larger increase across the day relative to infants and older children. Data analysis consisted of Latent Change Score modeling (McArdle, 2008; McArdle & Hamagami, 2001) which allowed us to model reliably latent within-person change in cortisol over the course of the day and how the latent change scores themselves change over the course of the first 10 weeks of transition from home to childcare.
Although we included school-age children in the sample, these children’s exposure to “childcare” was potentially quite different than younger children’s experience. Similar to younger children, school-age children were assessed during their transitions to the same childcare setting. Some of these school-age children attended the childcare setting full-time (i.e., for 10 weeks during the summer), whereas other school-age children attended the childcare setting only for afterschool care after attending a regular school setting during the day. Given that these differences in care arrangements built systematic differences into the study by age group (a key variable of interest), we conducted secondary analyses excluding school-age children to examine the robustness of effects. Further, we discuss considerations for interpreting findings related to school-age children when discussing our results.
Method
Participants
Participants included 168 children enrolled at a university-based childcare center, where participation in research activities was a condition of enrollment. Children were sampled from four infant classrooms, six toddler classrooms, six preschool classrooms, and four school-age classrooms. All classrooms were self-enclosed and had staff-child ratios between 1:3 and 1:6. Children ranged in age from 1.2 months to 8 years old (M = 3.27 years, SD = 2.09). There were 42 infants between 0 and 18 months (25% of sample); 34 toddlers between 18 and 36 months (20% of sample); 58 preschoolers between 36 and 60 months (35% of sample); and 34 school age children over 60 months (20% of sample). Eighty-nine were boys (52%). Eighty-eight were White/non-Hispanic (52%), 51 were African-American (30%), 15 were Asian-American (9%), 13 were Hispanic (7%), and 3 were Biracial (2%). Annual family income ranged from less than $10,000 to greater than $200,000, with the median in the $50,000–$60,000 range. Sixty percent of children had parents who were married, 27% who were single, 11% who were separated or divorced, and 2% who were living together. Most children (75%) had at least one parent who had completed college or earned an advanced degree. Although we did not collect information about children’s previous experiences in other childcare or school settings, all children in the present study were new to the university-based childcare center.
As described above, children ranged in age up to 8 years old when they started at the childcare center. Although all infant-through preschool-age children were enrolled full-time at the center at the outset of their participation, school-age children’s childcare arrangements were more variable than children of the other age groups. There were 34 children over age 5 enrolled in the study. Of these children, 25 were enrolled in full-time care at the center when they started participating in the study (with the majority starting over the summer when regular school was not in session). These children provided varying numbers of morning samples across their 10-week participation, because they switched from full-time care at the center to just after school care at the center at different points during the transition period. Seven were in full-time care at the center for 10 weeks, five for 8 weeks, eight for 6 weeks, and five for 4 weeks or less. The 9 other school-age children (not participating in full-time child care at the center at any point during the study) only provided afternoon samples across the 10 weeks when they were at the center for afterschool care. Due to these varying childcare arrangements, as a group, school-age children’s experiences were notably different than younger children. Given these systematic differences between age groups (both in child care experience and in number of missing morning samples), we conducted analyses with and without school-aged children included. We also discuss issues related to the school-aged sample when describing the handling of missing data and when interpreting results of the study.
Procedure
Morning and afternoon saliva samples at childcare were collected on each child’s first day of attendance, and at weeks 2, 4, 6, 8, and 10. Samples were collected as a classroom activity supervised by the classroom teacher and performed by research staff. Children often approached researchers and actively participated in sampling procedures. Researchers were instructed to conduct mid-morning sampling between 9:30 a.m. and 10:30 a.m. (at least 15 minutes after breakfast and prior to outdoor or gymnasium play), and afternoon sampling between 3:30 p.m. and 4:30 p.m. (at least 30 minutes after napping and either prior to or at least 30 minutes after snack). In infant classrooms, trained research staff performed sampling with each child individually, with morning sample collection scheduled between 8:30 a.m. and 11:30 a.m., and afternoon sample collection scheduled between 3:00 p.m. and 5:00 p.m. The higher range of sampling times among infants was due to nap and feeding schedules; infants often slept for much of the morning and until later in the afternoon. Although average sampling times fell within the specified time intervals, there was variability in sampling times with some falling outside of these intervals (See in Table 1).
Table 1.
Descriptive Statistics for Cortisol Values
| Time of sample
|
Cortisol value (in ug/dl)
|
Log-transformed cortisol value
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | M (SD) | Min | Max | M (SD) | Min | Max | M (SD) | Min | Max | |
| Home (n = 129) | ||||||||||
| AM- Day 1 | 93 | 10:46 (0:46) | 9:27 | 12:45 | .19 (.12) | .032 | .558 | −.79 (.26) | −1.49 | −.25 |
| AM- Day 2 | 90 | 11:00 (0:51) | 8:15 | 12:50 | .21 (.16) | .020 | .872 | −.78 (.31) | −1.70 | −.06 |
| PM- Day 1 | 97 | 3:35 (1:19) | 12:00 | 6:35 | .15 (.11) | .002 | .556 | −.91 (.34) | −2.70 | −.25 |
| PM- Day 2 | 91 | 3:34 (1:17) | 13:00 | 6:47 | .26 (.30) | .005 | 1.24 | −.87 (.39) | −2.30 | .09 |
| Childcare (n = 168) | ||||||||||
| AM- Day 1 | 121 | 10:32 (0:59) | 7:32 | 12:55 | .20 (.14) | .001 | .730 | −.79 (.33) | −3.00 | −.14 |
| AM- Week 2 | 116 | 10:25 (0:53) | 8:45 | 12:30 | .20 (.13) | .008 | .682 | −.79 (.29) | −2.10 | −.17 |
| AM- Week 4 | 91 | 9:55 (0:40) | 8:35 | 11:25 | .21 (.16) | .036 | .848 | −.78 (.28) | −1.44 | −.07 |
| AM- Week 6 | 104 | 10:04 (0:45) | 8:25 | 11:25 | .19 (.13) | .053 | .683 | −.79 (.25) | −1.28 | −.17 |
| AM- Week 8 | 100 | 10:05 (0:54) | 8:10 | 12:00 | .18 (.11) | .021 | .516 | −.80 (.24) | −1.68 | −.29 |
| AM- Week 10 | 85 | 9:57 (0:51) | 8:21 | 11:55 | .17 (.08) | .042 | .407 | −.83 (.21) | −1.38 | −.39 |
| PM- Day 1 | 109 | 3:37 (0:41) | 12:20 | 5:05 | .26 (.18) | .009 | .790 | −.70 (.34) | −2.05 | −.10 |
| PM- Week 2 | 119 | 3:36 (0:39) | 2:10 | 5:15 | .25 (.18) | .025 | .946 | −.69 (.29) | −1.60 | −.02 |
| PM- Week 4 | 89 | 3:36 (0:52) | 1:45 | 5:35 | .21 (.13) | .021 | .516 | −.75 (.29) | −1.68 | −.13 |
| PM- Week 6 | 110 | 3:33 (0:43) | 1:20 | 5:05 | .26 (.17) | .038 | .928 | −.68 (.29) | −1.49 | −.03 |
| PM- Week 8 | 87 | 3:35 (0:35) | 2:00 | 5:15 | .27 (.17) | .032 | .871 | −.66 (.31) | −1.49 | −.06 |
| PM- Week 10 | 88 | 3:35 (0:40) | 12:15 | 5:15 | .23 (.14) | .025 | .622 | −.72 (.26) | −1.60 | −.21 |
For a subset of participants (n = 129), parents also collected saliva samples from children at mid-morning and afternoon for two consecutive days when children were not attending childcare within 2 weeks prior to their children’s first day of childcare. See Table 1 for descriptive statistics of sampling times. On days of sampling, parents also completed questionnaires about children’s health, such as whether children were teething, sick, or had eaten prior to sampling. Parents were asked to delay sampling if children were ill.
Saliva samples were obtained using salivettes (Sarstedt catalog # 51.1534). Children older than 12 months were also given a cup containing 0.8 grams of Pathmark™ brand cherry flavored sweetened beverage crystals to stimulate salivation. Recent controlled studies have shown minimal effects of flavored beverage crystals on the enzyme-immunoassay (Gordon, Peloso, Manni, & Dozier, 2005; Talge, Donzela, & Gunnar, 2005). The cotton from the salivette was dampened using the tip of the child’s tongue and then dipped in the beverage crystals. The child then sucked or chewed on the cotton until one end was soaking with saliva. Preschoolers dipped and chewed the cotton on their own with instructions to re-dip the cotton in the drink mix as often as they liked. It is not ideal that we had systematic method differences across age (i.e., youngest children did not have flavored beverage crystals, whereas older children did), given that age is a primary variable of interest; thus, this should be considered a limitation of the study design. The cotton swabs were returned to the salivettes, which were labeled and frozen until the assays were performed (within two weeks of sampling).
Measures
Cortisol
The saliva samples were stored in a freezer at the laboratory at −20 °C prior to being assayed. Samples were assayed using the Salimetrics, Inc. High Sensitivity Salivary Cortisol Enzyme Immunoassay Kit (catalog No. 1-1102/1-1112). All samples from an individual for one day were assayed in duplicate on the same assay plate. Pairs of samples were rerun in a latter assay if coefficients of variation were greater than 10%. Inter- and intra-assay coefficients of variation fell below 7% and 14%, respectively.
Of a possible 2,016 child care samples (2 per day over 6 days), 1219 (60%) were included in final analyses. Child care cortisol data were missing because samples were not collected (e.g., due to absence or child being unavailable at the time of sampling) (36%), there was not sufficient saliva collected (2%), or because cortisol values were over 3 standard deviations above the mean and excluded as outliers (2%), following common procedures (Dettling, Gunnar, & Donzella, 1999). Of a possible 516 home samples (2 per day over 2 days), 371 (72%) were included in final analyses. Home cortisol data were missing either because samples were not collected (12%), there was insufficient saliva collected (12%), or because cortisol values were outliers (3%). As is standard, log 10 transformation was used to normalize the cortisol distributions due to a positive skew.
Results
Preliminary Analyses
Descriptive statistics for cortisol data collection, including raw cortisol values, log-transformed cortisol values, and timing of sampling, are presented in Table 1. For preliminary analyses, log-transformed cortisol values were averaged to compute four values: average mid-morning cortisol level at home, average afternoon cortisol level at home, average mid-morning cortisol level at childcare, and average afternoon cortisol level at childcare. There were no significant differences in averaged cortisol levels predicted by child gender or minority status (p values > .05). Bivariate correlations between child age and the averaged cortisol levels are presented in Table 2. There were significant negative correlations between child age and averaged afternoon log-transformed cortisol levels at home and at childcare. Given that gender and ethnicity were not associated with cortisol levels, these demographic variables were excluded from primary analyses; age was included in primary analyses as it was a variable of interest. As would be expected, average cortisol levels were moderately correlated across time points (mid-morning and afternoon) and settings (home and childcare). Finally, we conducted bivariate correlations to determine whether sample collection time at each individual time point was associated with cortisol values. All of these correlations were non-significant, ranging from r = −.10 to .17 (p > .05). Given these non-significant correlations, we did not include sampling time in the primary model.
Table 2.
Bivariate Correlations Between Child Age and Average Midmorning and Afternoon Log-transformed Cortisol Values at Home and Childcare
| Variable | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 1. Child age | . | . | . | . |
| 2. Average home morning log-transformed cortisol | −.040 | . | . | . |
| 3. Average home afternoon log-transformed cortisol | −.214* | .407** | . | . |
| 4. Average childcare morning log-transformed cortisol | −.154 | .240* | .270** | . |
| 5. Average childcare afternoon log-transformed cortisol | −.277** | .288** | .390** | .275** |
Note.
p< .05,
p< .01
Missing Data
Patterns of missingness for cortisol samples at each time point were examined to determine whether data could be considered missing at random (MAR; Schafer & Graham, 2002). We examined whether missingness (missing vs. not missing) for each cortisol sample was associated with demographic information, using Chi-square tests for categorical variables (i.e., minority status, gender), and t tests for continuous variables (i.e., child age). Minority children were more likely than non-minority children to be missing the home morning sample on day 1 (χ2 = 8.33, p < .01) and day 2 (χ2 = 11.31, p < .01); minority status was unrelated to missingness of home afternoon samples and all 12 childcare samples (p > .05). Gender was not significantly associated with missingness of any of the home or childcare samples (p > .05). In order to account for the association between minority status and missingness, we include minority status as a predictor in the model in secondary analyses.
Age was significantly associated with the missingness of morning samples at childcare at weeks 4 (t = −2.11, p < .05), 6 (t = −2.37, p < .05), 8 (t = −2.39, p < .05), and 10 (t = −2.69, p < .01), with children who were missing morning samples at these time points being older on average than children who were not missing these samples. There were no associations between age and missingness of home samples, child care week 0 or week 2 samples, or any childcare afternoon samples. This association between missingness and age is not surprising given that a proportion of the school-age children were not at the childcare center for the morning period, especially at later weeks of care. In addition to including age as a predictor in primary analyses, we address the issue of systematic missingness by age by analyzing the data without school-age children in secondary analyses.
Data Analytic Strategy
We derived our data analytic strategy from a latent change score (LCS) approach (King et al., 2006; McArdle, 2009; McArdle & Hamagami, 2001) in order to model changes in midmorning to afternoon cortisol patterns during the transition to a new childcare setting. As described by King et al. (2009), LCS models afford important advantages over other statistical approaches when examining change processes over time. First, indices of change within the LCS model are more reliable than traditional approaches that rely on simple difference scores. Simple differences scores can be calculated by subtracting the value at the initial assessment from the value at the subsequent assessment. This simple subtraction method, however, yields a change score with questionable reliability, as it does not take into account measurement error associated with each of the individual assessments (Cronbach & Furby, 1970; King et al., 2006; McArdle, 2009). By adding a set of fixed values to an autoregressive model, latent change scores reflect the change in a variable across two assessment points while also accounting for measurement error (King et al., 2006; McArdle, 2009). Thus, the LCS approach reduces biases in parameter estimates by modeling change in perfectly reliable or “true” scores (i.e., latent variables that separate variance in observed scores from error variance).
A second advantage to the LCS modeling approach is that change can be modeled at multiple levels, allowing for examination of complex trajectories across time. In the present study, for example, we are interested in modeling (1) change in diurnal cortisol from midmorning to afternoon within a day, and (2) change in this diurnal change (i.e., pattern) across days. The LCS approach is similar to traditional growth curve modeling approaches in that the average change in an outcome over time (and individual difference in this within-person change) can be modeled. However, going beyond traditional growth curve modeling, the outcome being modeled is a latent change score. Thus, LCS allows us to capture whether the latent within-person changes in cortisol over the course of the day are themselves changing over the course of weeks of transition from home to childcare.
Third, the LCS approach accounts for the non-independence of a nested data structure by estimating the shared variance of repeated measures within individuals (i.e., multiple cortisol values for each child) separate from between-person differences. Importantly, the LCS approach can also accommodate missing data and/or unbalanced designs because it treats multiple observations as nested within persons (allowing for variability in the spacing and number of data points) and makes use of maximum likelihood estimation (King et al., 2006).
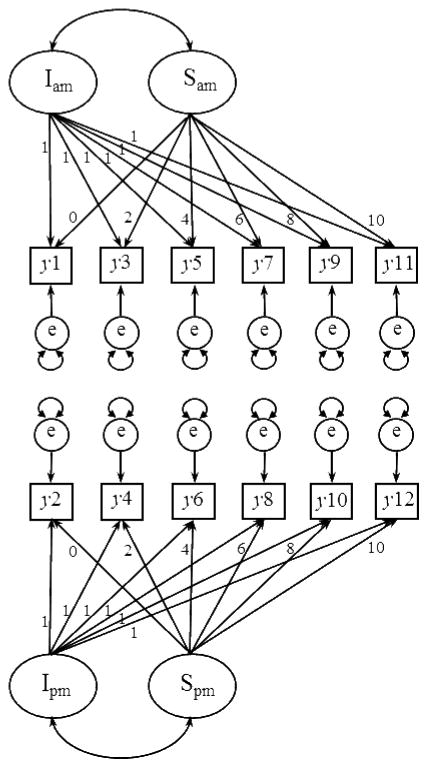
For the present study, an LCS model was constructed using structural equation modeling with Mplus 6.0 (Muthen & Muthen, 1998–2010), basing decisions about model specification on guidelines set forth previously (King et al., 2006; McArdle, 2009). Our approach diverges from other LCS approaches, so we aim to describe our model specifications here in the text as well as in Figure 1. For each child on each day of data collection at childcare, there were potentially two units of data: a midmorning cortisol sample and an afternoon cortisol sample. Figure 1 depicts the full model, with the midmorning cortisol samples represented as y1, y3, y5, y7, y9, and y11 for day 1 and weeks 2, 4, 6, 8, and 10, respectively, and the afternoon cortisol samples represented as y2, y4, y6, y8, y10, and y12 for day 1 and weeks 2, 4, 6, 8, and 10, respectively. A latent factor for each cortisol sample (ly1 to ly12) was estimated from an individual’s observed score and associated error term (e). By fixing pathways as depicted, each latent difference score (dy1 to dy10) reflected change in cortisol across the day (e.g., dy1 = ly2 − ly1). The overall intercept (Idy) was an estimated parameter of children’s average diurnal slope (midmorning to afternoon) at childcare. The overall slope (Sdy) was an estimated parameter of the rate of change in children’s diurnal cortisol pattern across the 10-week transition to childcare.
Figure 1.
Structural model for change in cortisol pattern across weeks in childcare and comparison between childcare and home cortisol patterns. Observed (log-transformed) cortisol values at home in midmorning, hy1, hy3, and in afternoon, hy2, hy4; at childcare in midmorning, y1, y3, y5, y7, y9, y11, and in afternoon, y2, y4, y6, y8, y10. Latent midmorning and afternoon cortisol values (observed + error, e), hly1, hly2, ly1–ly12. Latent change scores at home, hdy1, and at childcare from day 1 through week 10, dy1 – dy10. Childcare latent intercept (latent change score at the start of childcare), Idy. Childcare latent slope (rate of change in latent difference scores across weeks in care), Sdy. Higher order latent change score between home latent difference score (HDLY1) and childcare latent change score intercept (DLY1), DD1. The following model constraints were set for the full latent difference score model: Means set to be 0 for hy1–hy4; y1–y12; dy1, dy2, dy4, dy6, dy8, dy10; hly2; ly2, ly4, ly6, ly8, ly10, ly12. Variances set to be 0 for hly2; ly2, ly4, ly6, ly8, ly10, ly12; dy1. Variances constrained to be equal for ly1, ly3, ly5, ly7, ly9, and ly11; dy2, dy4, dy6, dy8, and dy10. Residual variances constrained to be equal for hy1 and hy2; hy3 and hy4; y1–y12. We constrained the auto-proportional change coefficients whereby the latent change factor is regressed on the starting level to zero. We tested a model with those paths estimated but none of them were statistically different from zero; thus, we constrained these paths to zero for both model simplicity and parsimony.
Given our interest in comparing patterns of cortisol production at home versus patterns of cortisol production at childcare, a latent difference score for home cortisol levels was also estimated, with latent factors for midmorning (hly1) and afternoon (hly2) samples. Notably, cortisol samples at home were collected over two consecutive days; thus, the midmorning and afternoon latent variables each have two indicators (midmorning: hy1 and hy3; afternoon: hy2 and hy4) and associated error terms. The difference between cortisol production at home and cortisol production at childcare was modeled with a higher-order latent difference score. By fixing pathways as depicted, the higher-order latent difference score (DD1) reflected the difference between children’s midmorning to afternoon diurnal cortisol pattern at home and the midmorning to afternoon diurnal cortisol pattern when children begin childcare (e.g., DD1 = DLY1 − HDLY1).
Primary Analyses
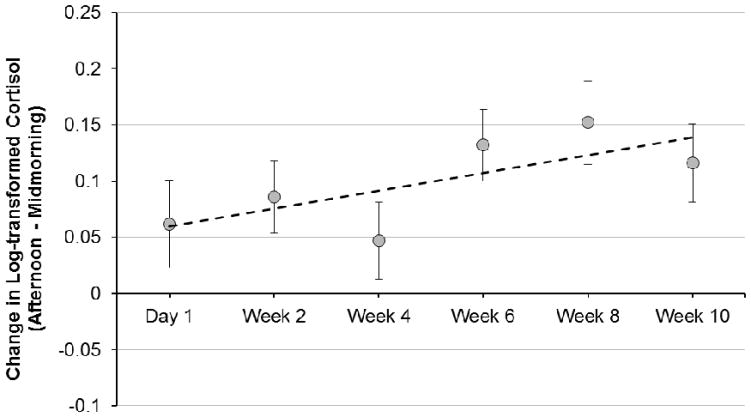
As described above, the LCS model was set up to examine the change in cortisol production across the 10 weeks following entry into a new childcare setting. There was a positive rate of change in the latent difference scores across weeks in childcare (represented by Sdy in Figure 1), Sdy = .009, p = .039 (See Table 3). This significant slope indicated that, on average, the change in cortisol levels across the day became more positive over time (by .009 μg/dL per week). In other words, the rise in cortisol from mid-morning to afternoon became more pronounced across the first 10 weeks in a new childcare setting. There was no effect of child age on the change in cortisol patterns during the transition to childcare (p > .05). Figure 2 displays the midmorning to afternoon change in cortisol for each childcare sampling time point. We note that while the change in difference scores over the 10 weeks can be most easily summarized as a linear increase, Figure 2 suggests some nonlinearity, a point to which we return later in the Results when we address final model fit.
Table 3.
Fit Indices and Parameter Estimate of Full Latent Change Score Model
| Fit indices | Full Latent Change Score Model |
|---|---|
| χ2/df | 163.5/104 (= 1.57) |
| CFI | .800 |
| RMSEA | .058 |
| Parameter Estimates | Estimate (SE) | Z | p-value | 95% CI
|
|
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Intercept, DD1 | .226 (.050) | 4.55 | <.001 | .144 | .307 |
| ChAge (linear) → DD1 | .037 (.017) | 2.17 | .030 | .009 | .065 |
| ChAge (quadratic) → DD1 | −.020 (.007) | −2.68 | .007 | −.032 | −.008 |
| Slope, Sdy | .009 (.004) | 2.06 | .039 | .002 | .016 |
Note. χ2 = Chi square; df = degrees of freedom; CFI = comparative fit index; RMSEA = root mean square error of approximation; DD1 = Higher order latent difference score between average midmorning to afternoon change in cortisol at childcare versus at home; Sdy = Slope of change in latent difference scores across 10-week transition.
Figure 2.
Midmorning to afternoon change in cortisol across weeks in childcare, plotted using LCS model estimates of latent change scores (± 1 SE). Positive values indicate a rise across the day (i.e., that afternoon cortisol was higher than midmorning cortisol). The dotted line depicts the model-estimated slope of latent change scores across the transition (Sdy = .009, p < .05).
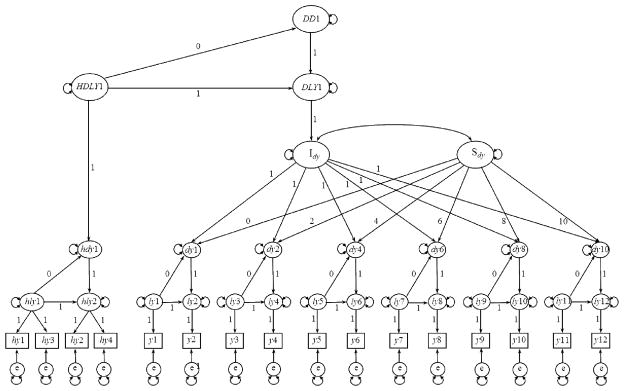
Further probing of the change in cortisol across the transition to the new childcare setting was necessary because the positive slope in latent difference scores could have been driven by increasing afternoon cortisol levels over time, decreasing midmorning cortisol levels over time, or a combination of these two patterns. In order to probe what was driving the effect, we estimated linear growth models to examine the slopes of midmorning cortisol levels and afternoon cortisol levels across weeks in care (See Figure 4 for structural model, Table 4). The slope of midmorning childcare cortisol levels across the 10-week transition was Sam = −.006, p = .057, indicating that midmorning cortisol levels declined at a rate of .006 μg/dL per week. The linear growth model of afternoon cortisol levels revealed a non-significant change in afternoon cortisol across weeks in care, Spm = −.001, p > .05. These data suggest that the positive slope of latent difference scores across the 10-week transition was driven by a decline in midmorning cortisol levels concurrent with stable afternoon levels. We plotted the model-estimated slopes for change in morning cortisol and change in afternoon cortisol across weeks in childcare, superimposed on the raw data of within-day change on childcare days (See Figure 3).
Figure 4.
Structural linear growth models for change in midmorning cortisol levels across weeks in childcare and afternoon cortisol levels across weeks in care. Observed (log-transformed) cortisol values at childcare in midmorning, y1, y3, y5, y7, y9, y11. Intercept (estimate of morning cortisol on day 1), Iam. Slope (rate of change in morning cortisol across weeks in care), Sam. Observed (log-transformed) cortisol values at childcare in afternoon, y2, y4, y6, y8, y10, y12. Intercept (estimate of afternoon cortisol on day 1), Ipm. Slope (rate of change in afternoon cortisol across weeks in care), Spm.
Table 4.
Parameter Estimates from Linear Growth Models of Midmorning Cortisol and Afternoon Cortisol
| Fit indices | Midmorning and Afternoon Linear Growth Models |
|---|---|
| χ2/df | 90.59/64 (= 1.42) |
| CFI | .800 |
| RMSEA | .050 |
| Parameter Estimates | Estimate (SE) | Z | p-value | 95% CI
|
|
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Midmorning Growth Model
|
|||||
| Intercept, Iam | −.756 (.024) | −28.18 | <.001 | −.795 | −.716 |
| Slope, Sam | −.006 (.003) | −1.90 | .057 | −.012 | −.001 |
| Afternoon Growth Model
|
|||||
| Intercept, Ipm | −.685 (.024) | −28.18 | <.001 | −.725 | −.645 |
| Slope, Spm | −.001 (.003) | −.25 | .800 | −.006 | .005 |
| Variances
|
|||||
| Intercept, Iam | .034 (.011) | 3.11 | .002 | .016 | .052 |
| Slope, Sam | .000 (.000) | 1.97 | .049 | .000 | .001 |
| Intercept, Ipm | .035 (.012) | 2.90 | .004 | .015 | .055 |
| Slope, Spm | .000 (.000) | .98 | .325 | .000 | .001 |
Note. Iam = Intercept of linear growth model of midmorning cortisol at childcare (i.e., average across time points); Sam = Slope of linear growth model of midmorning cortisol at childcare; Ipm = Intercept of linear growth model of afternoon cortisol at childcare (i.e., average across time points); Spm = Slope of linear growth model of afternoon cortisol at childcare.
Figure 3.
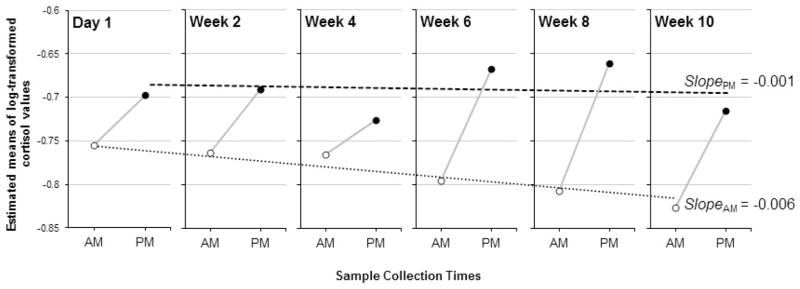
Change in midmorning to afternoon pattern across weeks in care, plotted using means of log-transformed raw cortisol values. Dotted and dashed lines across weeks depict model-estimated slopes of midmorning longitudinal growth across childcare weeks (SlopeAM = −.006, p = .057) and afternoon longitudinal growth across childcare weeks (SlopePM = −.001, ns), respectively.
Using the full LCS model, we also compared patterns of cortisol production on days at childcare to patterns of cortisol production on days at home (represented by DD1 in Figure 1). There was a significant difference between the mid-morning to afternoon change in cortisol at home versus at childcare, DD1 = 0.226, p < .001. Specifically, the change in cortisol across the day at childcare was more positive than the change in cortisol across the day at home. Child age (centered at the average age) was entered in the model as a predictor of the higher-order latent difference score (DD1). In addition to the linear term for child age, we included a quadratic term for child age (calculated by squaring child age [centered]) given that we expected preschool age children to show a greater difference between home and childcare cortisol patterns (driven by the largest increase in cortisol across the childcare day) relative to infants and older children. The quadratic term for child age significantly predicted DD1 (i.e., the difference between the cortisol slope at home and the cortisol slope at childcare), β = −.020, p = .007. Thus, as predicted, there was a quadratic effect of child age on the patterns of cortisol production on childcare days relative to non-childcare days.
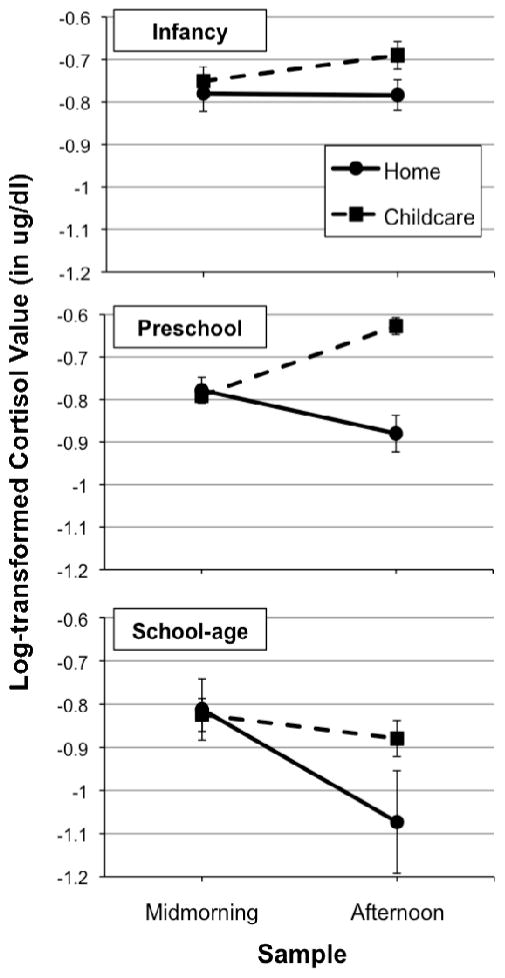
As is further evident when data were plotted, this finding suggested that preschool-aged children showed a greater difference between their childcare and home cortisol than younger infants and older children. Figure 5 depicts the pattern of cortisol production on home versus childcare days (plotted using averaged log-transformed data across cortisol assessments for 2 home days, and 6 childcare days) for children separated into three age groups: infancy (i.e., under 18 months), preschool (i.e., 18 months through 60 months), and school-age (i.e., 60 months and above). These home vs. childcare patterns were further explored by conducting repeated measures ANOVAs separately for each group, with two within-subjects factors (Time: midmorning vs. afternoon; Context: home vs. childcare). For each age group, we were interested in whether there was a significant Time X Context interaction, given that this would indicate that the pattern of cortisol across the day was different depending on the context. For infants, the context X time interaction was non-significant, F = .10, p = .75; for preschoolers, the context X time interaction was significant, F = 32.53, p < .001; for school-aged children, the context X time interaction approached significance, F = 3.95, p = .07. We probed the interactions using pairwise comparisons. For preschoolers, there was a significant difference between midmorning and afternoon cortisol levels at home, indicating a decline across the day, t = −2.88, p < .01, as well as a significant difference between midmorning and afternoon cortisol levels at childcare, indicating an increase across the day, t = 7.32, p < .001. For school-aged children, there was a significant difference between midmorning and afternoon cortisol levels at home, indicating a decline across the day, t = −2.71, p < .05, but the difference between midmorning and afternoon cortisol levels at childcare was non-significant, t = 1.45, p = .16.
Figure 5.
Descriptive presentation of home versus childcare cortisol patterns by age group using raw (rather than model-estimated) data. Plotted values are averaged log-transformed midmorning and afternoon cortisol values. For home data, midmorning values across the two days were averaged to calculate a mean midmorning level, and afternoon values were averaged to create a mean afternoon cortisol level. For childcare data, cortisol values for midmorning and for afternoon were averaged across all 10 weeks in care. Averaged cortisol levels are plotted separately for infants (under 18 months; n = 42), preschool-aged children (18 months to 5 years; n = 92), and school-aged children (over 5 years old; n = 34).
For further description of the findings, we calculated the percentage of children from each of these age groups that showed a rise in cortisol across the day, using raw cortisol values. In order to use conservative estimates, we defined a rise as a percentage increase that was above the assay coefficient of variation (following procedures used by Watamura et al., 2003). On average across weeks, 45% of infants, 62% of preschoolers, and 35% of school-age children showed a rise across the childcare day. Percentages of children showing a rise at each time point are reported in Table 5.
Table 5.
Percentage of Children Showing Rise in Cortisol on Childcare Days
| Child care time point | Infant (0–18 months) | Preschool-age (18–60 months) | School-age (60+ months) |
|---|---|---|---|
| Day 1 | 39% | 50% | 30% |
| Week 2 | 30% | 61% | 33% |
| Week 4 | 38% | 52% | 40% |
| Week 6 | 54% | 69% | 54% |
| Week 8 | 60% | 77% | 11% |
| Week 10 | 47% | 60% | 43% |
Absolute model fit for the full LCS model (See Table 3) was assessed with the relative chi-square (χ2/df), the root mean square error of approximation (RMSEA), and the comparative fit index (CFI). Overall, these indices indicated adequate to good fit to the data. A relative chi-square (i.e., ratio of chi-square to degrees of freedom) in the range of 2 to 1 is considered indicative of an acceptable fit between the estimated model and observed data (Byrne, 1989; Carmines & McIver, 1981; Marsh & Hocevar, 1985; Wheaton, 1987). Based on these criteria, the relative chi-square was in the acceptable range (χ2/df = 1.57). The root mean square error of approximation (RMSEA) is a type of absolute fit index, which measures the discrepancy between the model-implied covariance matrix and observed covariance matrix, taking into account the degrees of freedom (Chen, 2007; Steiger, 1998). For the tested model, the RMSEA met criteria (≤ .08) for adequate fit to the sample data (RMSEA = .058) (Browne & Cudeck, 1993). Finally, the comparative fit index (CFI) measures whether the examined model is better than an alternative model at replicating the observed covariance matrix (Chen, 2007). For the hypothesized model, the CFI = .80, falling below what is recommended as the criteria for goodness of fit (≥ .90) (Chen, Sousa, & West, 2005). Notably, some recommend that the CFI be avoided given its sensitivity to correlations within the model (Kenny & McCoach, 2003). There is considerable debate about the reliance on fit indices when interpreting results of a model due to different reporting standards (i.e., which indices to report, appropriate criteria/cutoffs) across studies and sensitivity of fit indices to variations in observed data (i.e., sample size, correlations) (Barrett, 2007; Hayduk, Cummings, Boadu, Pazderka-Robinson, & Bouulianne, 2007). Lastly, as suggested by Figure 2, change in the latent change scores increased overall, but in a nonlinear fashion. Model fit could be improved somewhat by using a latent basis approach (Meredith & Tisak, 1990) by estimating the loadings that define the latent change growth trajectory, but we felt this approach would make the pattern of change more difficult to interpret than a more parsimonious linear latent change model.
Secondary Analyses
We conducted secondary analyses to address issues related to missingness and systematic differences in sampling. First, we re-ran primary analyses including minority status as an additional predictor of the higher order latent difference score, due to the issue that minority status was associated with missingness of morning cortisol samples at home. The effect of minority status was non-significant, and the effect of age and quadratic age remained unchanged. Second, we re-ran primary analyses after excluding school-age children (children over age 5), due to conceptual issues (e.g., childcare may mean something different for these children compared to younger children), methodological issues (e.g., school-age children were not all enrolled full-time at the childcare center across the full 10 weeks) and statistical issues (e.g., systematic differences in missingness of morning samples). In this follow-up analysis, we were interested in whether (1) the slope of change in morning-to-afternoon cortisol across weeks in care (i.e., Sdy) remained significant, and (2) the quadratic effect of age on the higher order latent difference score (i.e., DD1) remained significant. Indeed, when removing school-age children from analyses (with a reduced sample of n = 134), there was still a significantly positive rate of change in the latent difference scores across weeks in childcare (Sdy = .009, p = .036) and still a significant quadratic effect of age on the higher order latent difference score (β = −.034, p = .024).
Discussion
We examined the change in midmorning to afternoon cortisol patterns across the first 10 weeks during children’s transition to a new childcare setting. Findings from the present study add to our understanding of the rise in cortisol that is often observed on childcare days and how this pattern changes during the transition to a new childcare setting. Using a latent difference score approach in a structural equation modeling framework, we found that children showed a significant increase in the midmorning to afternoon change in cortisol during the first 10 weeks following entry into the childcare setting. Whereas children’s afternoon cortisol levels remained relatively stable, their midmorning cortisol levels showed a decline across the 10-week transition, suggesting that the change in midmorning-to-afternoon patterns across weeks was driven by decreasing midmorning levels. On average, the midmorning to afternoon change in cortisol was more positive on childcare days than it was on home days (when children showed the typical decline across the day). However, we found a curvilinear effect of age on this difference between cortisol patterns at home versus at childcare. Specifically, preschool-aged children showed a larger rise in cortisol at childcare relative to home compared to younger infants and older school-aged children. Thus, there was an effect of age on the difference between home patterns and childcare patterns across the day; however, there was no effect of age on the change in cortisol pattern across weeks. Although a similar pattern of child age was described in the Vermeer & van IJzendoorn (2006) meta-analysis, this is the first study to our knowledge to include a wide age range (i.e., infancy through age 8) in the same study.
One might have suspected that the rise in cortisol at childcare would diminish as children became adjusted to a new setting, new caregivers/teachers, childcare routines, and peers. However, our findings show that, rather than resuming a typical diurnal pattern (i.e., a midmorning to afternoon decline in cortisol), children continued to show an increase in cortisol on childcare days across the first 10 weeks in a new childcare setting. As is evident by afternoon cortisol levels that remained elevated, childcare continued to serve as a context that elicited a cortisol rise, relative to the home cortisol decline, even months into the transition.
Taken together, our findings that (1) children continued to show a midmorning to afternoon cortisol rise across 10 weeks in childcare, and (2) preschool-aged children showed larger rises in cortisol than infants or school-aged children, help us speculate about mechanisms that elicit the rise in cortisol at childcare. One of the key developmental tasks of children as they enter school is interacting with peers. Preschool-aged children, in particular, are likely to spend a significant amount of time engaging with peers during the childcare day. Given that young children are still developing skills in emotion regulation, behavioral control, and social communications, they are likely to face a number of challenges when interacting with peers, such as negotiating about conflicts that arise during play. Even positive peer interactions may involve certain complexities for young children as they learn to navigate appropriate rules for social behavior. For adults, social-evaluative threat (e.g., public speaking) is associated with the largest increase in cortisol (Dickerson & Kemeny, 2004). Although the types of situations perceived as threatening differ across ages, it is possible that young children experience interactions with new or familiar peers as unpredictable and challenging.
There are other aspects of entering a new childcare setting that may elicit elevations in cortisol, such as being separated from parents. We found that children’s midmorning cortisol levels showed a decrease across the first 10 weeks of care. This finding may indicate that morning levels were elevated above typical levels observed at childcare during the early weeks of entering a new childcare setting, when children were adjusting to separations from parents, new caregivers at childcare, and a novel setting. Elevated morning cortisol levels (collected after arrival) have been previously reported for infants during the transition to childcare (Ahnert et al., 2004). Similarly, elevated wake-up cortisol levels have been previously reported for kindergarten and school-age children starting a new school year (e.g., Bruce et al., 2002; Quas et al., 2002). It is also possible that midmorning levels declined across the week for other reasons. Other studies have found that morning levels of cortisol on childcare days are lower than morning levels on home days (e.g., Gunnar, Tout, de Haan, Pierce, & Stansbury, 1997; Ouellet-Morin et al., 2010), and it has been suggested that this effect my reflect anticipatory drops (Jessop & Turner-Cobb, 2007; Ouellet-Morin et al., 2010). It is possible that children increasingly anticipate next-day childcare attendance and have higher HPA axis activity overnight due to this anticipation. If children’s HPA axes are more active overnight, resulting in higher nocturnal outputs of cortisol, they may have lower levels of cortisol (i.e., hypoactivation) the following morning. Additionally, as children become used to the childcare routine, their sleep schedules may change. Children may begin to wake-up earlier than they initially did during the first couple weeks of care. This change in waking time would result in more time between when children wake up and when midmorning samples were collected during later weeks in care, which could contribute to increasingly lower midmorning levels. Unfortunately, we did not collect information about wake-up time that would allow us to examine this issue.
Recent research has examined whether full-day childcare confers risk for children’s development (e.g., Belsky et al., 2007; Roisman et al., 2009; Watamura, Coe, Laudenslager, & Robertson, 2010). Given that chronic elevations in cortisol can pose risks for typical brain development, behavioral outcomes, and physical health (Kaufman & Charney, 2001; Kiecolt-Glaser et al., 2003; Sapolsky, 1996), examining the long-term effects of the anomalous cortisol increase across the childcare day is important. Watamura et al. (2010) examined associations between rising afternoon cortisol levels at childcare, a marker of immune function (i.e., antibody secretion) at home and at childcare, and parent-reported illness in young children who were attending full-day child care. They found that higher levels of afternoon cortisol at childcare were associated with lower antibody secretion rates on weekend mornings at home (presumably reflecting a delayed effect of elevated cortisol levels during the week). The Watamura et al. (2010) study highlights potential links between elevated cortisol and aspects of physical health measured concurrently in 3- to 5-year-old children. More longitudinal research is needed to examine whether elevated afternoon cortisol associated with childcare leads to lasting effects for children into later childhood or adolescence.
In addition to the question of potential consequences of frequent (although moderate) increases in cortisol associated with childcare, future research should continue to explore moderators of children’s cortisol patterns. By examining child characteristics, particularly those related to child temperament and social behavior (e.g., behavioral inhibition, extraversion, aggression), we may be able to more clearly delineate what may lead to afternoon elevations. Additionally, future research might take into account specific experiences during the childcare day (e.g., frequency of peer conflicts, time spent in solitary play) that may be associated with different patterns of cortisol activity.
An important consideration for interpreting findings of our study relates to the inclusion of a wide age range of children. In particular, the school-age children included in this study had varying degrees of involvement at the childcare setting. Some of these children were attending regular school settings and then coming to the center for after school care. This complicates our interpretation of findings, given that that the exposure to childcare is systematically different across ages. However, it is notable that our effects hold when excluding school-age children from analyses. This suggests that the findings are robust and age-related effects are observable within a more limited age range of children that all participated in full-time care at the center.
The current study had a number of strengths, including the longitudinal design with repeated cortisol measurements at home and at childcare, large age range of children all starting at the same childcare center, relatively large sample, and advanced data analytic strategy that was appropriate for the questions of interest. There were also a number of limitations that are important to consider. We only assessed cortisol at home at one point in time (prior to the start of childcare), and therefore were unable to examine how home cortisol changed across the 10-week period. It is possible that there were concurrent changes occurring in home cortisol patterns across time that we were unable to capture, given this design decision. There was considerable attrition in the sample over time, particularly with respect to the school-age children. We did not have information about children’s previous experiences in childcare. For some children, this was likely their first time out-of-home care, whereas other children (especially those in the school-age group) had likely been in out-of-home care before. Given that this information was not available, we were not able to examine whether previous childcare experience influenced patterns of cortisol activity in the current study. Related to this issue, we did not have information about factors that might have influenced parents’ decisions to enroll their children in childcare at younger versus older ages. Factors related to these selection effects (e.g., parent work schedules) could pose threats to validity, but we were unable to account for these possible confounds given limited data about these variables. Additionally, we did not have data about differences in child care schedules (e.g., drop-off and pick-up times), routines, or activities (e.g., napping) that could have varied systematically by age group.
Out-of-home childcare is a context that elicits a unique pattern of cortisol production, characterized by an increase in cortisol from midmorning to afternoon. This increase is especially evident for children in the preschool-age range, and continues even months into the transition to a childcare setting. Future research can help identify what aspects of childcare (e.g., peer interactions) elicit the cortisol rise, and whether there are long-term implications of these elevations in cortisol at childcare.
Acknowledgments
The project described was supported by funding to M. Dozier from the National Institute of Mental Health (Award numbers: R01MH052135, R01MH074374, and R01MH084135).
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health.
References
- Ahnert L, Gunnar MR, Lamb ME, Barthel M. Transition to child care: Associations with infant-mother attachment, infant negative emotion, and cortisol elevations. Child Development. 2004;75:639–650. doi: 10.1111/j.1467-8624.2004.00698.x. [DOI] [PubMed] [Google Scholar]
- Barrett P. Structural equation modelling: adjudging model fit. Personality and Individual Differences. 2007;42:815–824. [Google Scholar]
- Belsky J, Burchinal M, McCartney K, Vandell DL, Clarke-Stewart KA, Owen MT, et al. Are there long-term effects of early child care? Child Development. 2007;78:681–701. doi: 10.1111/j.1467-8624.2007.01021.x. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Chapoux M, Suomi SJ, Gunnar MR. Salivary cortisol in nursery reared rhesus monkeys: Reactivity to peer interactions and altered circadian activity. Developmental Psychobiology. 1995;28:257–267. doi: 10.1002/dev.420280502. [DOI] [PubMed] [Google Scholar]
- Bruce J, Davis EP, Gunnar MR. Individual differences in children’s cortisol response to the beginning of a new school year. Psychoneuroendocrinology. 2002;27:635–650. doi: 10.1016/S0306-4530(01)00031-2. [DOI] [PubMed] [Google Scholar]
- Byrne BM. A primer of LISREL: Basic applications and programming for confirmatory factor analytic models. New York: Springer-Verlag; 1989. [Google Scholar]
- Carmines EG, McIver JP. Analyzing models with unobserved variables. In: Bohrnstedt GW, Borgatta EF, editors. Social measurement: Current issues. Beverly Hills: Sage Publications; 1981. [Google Scholar]
- Cronbach LJ, Furby L. How we should measure “change”: Or should we? Psychological Bulletin. 1970;74:68–80. doi: 10.1037/h0029382. [DOI] [Google Scholar]
- Davis EP, Donzella B, Krueger WK, Gunnar MR. The start of a new school year: Individual differences in salivary cortisol response in relation to child temperament. Developmental Psychobiology. 1999;35:188–196. doi: 10.1002/(SICI)1098-2302(199911)35:3<188::AID-DEV3>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Dettling AC, Gunnar MR, Donzella B. Cortisol levels of young children in full-day childcare centers: Relations with age and temperament. Psychoneuroendocrinology. 1999;24:519–536. doi: 10.1016/S0306-4530(99)00009-8. [DOI] [PubMed] [Google Scholar]
- Dettling AC, Parker SW, Lane SK, Sebanc AM, Gunnar MR. Quality of care and temperament determine whether cortisol levels rise over the day for children in full-day childcare. Psychoneuroendocrinology. 2000;25:819–836. doi: 10.1016/S0306-4530(00)00028-7. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Geoffroy MC, Cote SM, Parent S, Seguin JR. Daycare attendance, stress, and mental health. The Canadian Journal of Psychiatry. 2006;51:607–615. doi: 10.1177/070674370605100909. [DOI] [PubMed] [Google Scholar]
- Gordon MK, Peloso E, Auker A, Dozier M. Effect of flavored beverage crystals on salivary cortisol enzyme immunoreactive assay measurements. Developmental Psychobiology. 2005;4:189–195. doi: 10.1002/dev.20081. [DOI] [PubMed] [Google Scholar]
- Groeneveld MG, Vermeer HJ, Linting M, Noppe G, van Rossum EFC, van IJzendoorn MH. Children’s hair cortisol as a biomarker of stress at school entry. Stress: The International Journal on the Biology of Stress. 2013;16:711–715. doi: 10.3109/10253890.2013.817553. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Cheatham CL. Brain and behavior interfaces: Stress and the developing brain. Infant Mental Health Journal. 2003;24:195–211. doi: 10.1002/imhj.10052. [DOI] [Google Scholar]
- Gunnar MR, Talge NM, Herrera A. Stressor paradigms in developmental studies: What does and does not work to produce mean increases in salivary cortisol. Psychoneuroendocrinology. 2009;34:953–967. doi: 10.1016/j.psyneuen.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Tout K, de Haan M, Pierce S, Stansbury K. Temperament, social competence, and adrenocortical activity in preschoolers. Developmental Psychobiology. 1997;31:65–85. doi: 10.1002/(SICI)1098-2302(199707)31:1<65::AID-DEV6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Gutteling BM, de Weerth C, Buitelaar JK. Prenatal stress and children’s cortisol reaction to the first day of school. Psychoneuroendocrinology. 2005;30:541–549. doi: 10.1016/j.psyneuen.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Hayduk L, Cummings GG, Boadu K, Pazderka-Robinson H, Boulianne S. Testing! Testing! One, two three – Testing the theory in structural equation models! Personality and Individual Differences. 2007;42:841–50. [Google Scholar]
- Jessop DS, Turner-Cobb JM. Measurement and meaning of salivary cortisol: A focus on health and disease in children. Stress. 2007;11:1–14. doi: 10.1080/10253890701365527. [DOI] [PubMed] [Google Scholar]
- Larson MC, White BP, Cochran A, Donzella B, Gunnar M. Dampening of the cortisol response to handling at 3 months in humans and its relation to sleep, circadian cortisol activity, and behavioral distress. Developmental Psychobiology. 1998;33:327–337. doi: 10.1002/(SICI)1098-2302(199812)33:4<327::AID-DEV4>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Legendre A. Environmental features influencing toddlers’ bioemotional reactions in day care centers. Environment and Behavior. 2003;35:523–549. doi: 10.1177/0013916503035004005. [DOI] [Google Scholar]
- Lisonbee JA, Mize J, Payne AL, Granger DA. Children’s cortisol and the quality of teacher-child relationships in child care. Child Development. 2008;79:1818–1832. doi: 10.1111/j.1467-8624.2008.01228.x. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Charney D. Effects of early stress on brain structure and function: Implications for understanding the relationship between child maltreatment and depression. Development and Psychopathology. 2001;13:451–471. doi: 10.1017/S0954579401003030. [DOI] [PubMed] [Google Scholar]
- Kenny DA, McCoach DB. Effect of the number of variables on measures of fit in structural equation modeling. Structural Equation Modeling. 2003;10:333–351. doi: 10.1207/S15328007SEM1003_1. [DOI] [Google Scholar]
- Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson K, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. PNAS. 2003;100:9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King DW, King LA, McArdle JJ, Grimm K, Jones RT, Ollendick TH. Characterizing time in longitudinal trauma research. Journal of Traumatic Stress. 2006;19:205–215. doi: 10.1002/jts.20112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh HW, Hocevar D. Application of confirmatory factor analysis to the study of self-concept: First- and higher-order factor models and their invariance across groups. Psychological Bulletin. 1985;97:562–582. doi: 10.1037/0033-2909.97.3.562. [DOI] [Google Scholar]
- McArdle JJ. Latent variable modeling of longitudinal data. Annual Review of Psychology. 2008;60:577–605. doi: 10.1146/annurev.psych.60.110707.163612. [DOI] [PubMed] [Google Scholar]
- McArdle JJ, Hamagami F. Latent difference score stuructural models for linear dynamic analyses with incomplete longitudinal data. In: Collins L, Sayer A, editors. New methods for the analysis of change. Decade of behavior. Washington, DC: APA Press; 2001. pp. 139–175. [Google Scholar]
- McEwen NS, Gould EA, Sakai RR. The vulnerability of the hippocampus to protective and destructive effects of glucocortiocodes in relation to stress. The British Journal of Psychiatry. 1992;160:18–23. [PubMed] [Google Scholar]
- Meredith W, Tisak J. Latent curve analysis. Psychometrika. 1990;55:107–122. doi: 10.1007/BF02294746. [DOI] [Google Scholar]
- Ouellet-Morin I, Tremblay RE, Boivin M, Meaney M, Kramer M, Cote SM. Diurnal cortisol secretion at home and in child care: A prospective study of 2-year-old toddlers. Journal of Child Psychology and Psychiatry. 2010;51:295–303. doi: 10.1111/j.1469-7610.2009.02167.x. doi:10.111j.1469-7610.2009.02167.x. [DOI] [PubMed] [Google Scholar]
- Price DA, Close GC, Fielding BA. Age of appearance of circadian rhythm in salivary cortisol values in infancy. Archives of Disease in Childhood. 1983;58:454–456. doi: 10.1136/adc.58.6.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quas JA, Murowchick E, Bedsadoun J, Boyce WT. Predictors of children’s cortisol activation during the transition to kindergarten. Developmental and Behavioral Pediatrcs. 2002;23:304–313. doi: 10.1097/00004703-200210000-00002. [DOI] [PubMed] [Google Scholar]
- Roisman GI, Susman E, Barnett-Walker K, Booth-LaForce C, Owen MT, Steinberg L. Early family and child-care antecedents of awakening cortisol levels in adolescence. Child Development. 2009;80:907–920. doi: 10.1111/j.1467-8624.2009.01305.x. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Why stress is bad for your brain. Science. 1996;273:749–750. doi: 10.1126/science.273.5276.749. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: Neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Research Reviews. 1986;11:65–76. doi: 10.1016/0165-0173(86)90010-X. [DOI] [PubMed] [Google Scholar]
- Schafer JL, Graham JW. Missing data: Our view of the state of the art. Psychological Methods. 2002;7:147–177. doi: 10.1037//1082-989X.7.2.147. [DOI] [PubMed] [Google Scholar]
- Sims M, Guilfoyle A, Parry TS. Children’s cortisol levels and quality of child care provision. Child: Care, Health, and Development. 2006;32:453–466. doi: 10.1111/j.1365-2214.2006.00632.x. [DOI] [PubMed] [Google Scholar]
- Smider NA, Essex MJ, Kalin NH, Buss KA, Klein MH, Goldsmith HH. Salivary cortisol as a predictor of socioemotional adjustment during kindergarten: A prospective study. Child Development. 2002;73:75–92. doi: 10.1111/1467-8624.00393. [DOI] [PubMed] [Google Scholar]
- Talge NM, Donzella B, Kryzer EM, Gierens A, Gunnar MR. It’s not that bad: Error introduced by oral stimulants in salivary cortisol research. Developmental Psychobiology. 2005;47(4):369–376. doi: 10.1002/dev.20097. [DOI] [PubMed] [Google Scholar]
- Tout K, de Haan M, Campbell EK, Gunnar MR. Social behavior correlates of cortisol activity in childcare: Gender differences and time-of-day effects. Child Development. 1998;69:1247–1262. [PubMed] [Google Scholar]
- Turner-Cobb JM, Rixon L, Jessup DS. A prospective study of diurnal cortisol responses to the social experience of school transition in four-year-old children: Anticipation, exposure, and adaptation. Developmental Psychobiology. 2008;50:377–389. doi: 10.1002/dev.20298. [DOI] [PubMed] [Google Scholar]
- Vermeer HJ, van IJzendoorn MH. Children’s elevated cortisol levels at daycare: A review and meta-analysis. Early Childhood Research Quarterly. 2006;21:390–401. doi: 10.1016/j.ecresq.2006.07.004. [DOI] [Google Scholar]
- Watamura SE, Coe CL, Laudenslager ML, Robertson SS. Child care setting affects salivary cortisol and antibody secretion in young children. Psychoneuroendocrinology. 2010;35:1156–1166. doi: 10.1016/j.psyneuen.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Watamura SE, Donzella B, Alwin J, Gunnar MR. Morning-to-afternoon increases in cortisol concentrations for infants and toddlers at childcare: Age differences and behavioral correlates. Child Development. 2003;74:1006–1020. doi: 10.1111/1467-8624.00583. [DOI] [PubMed] [Google Scholar]
- Watamura SE, Donzella B, Kertes DA, Gunnar MR. Developmental changes in baseline cortisol activity in early childhood: Relations with napping and effortful control. Developmental Psychobiology. 2004;45:125–133. doi: 10.1002/dev.20026. [DOI] [PubMed] [Google Scholar]
- Watamura SE, Sebanc AM, Gunnar MR. Rising cortisol at childcare: Relations with nap, rest, and temperament. Developmental Psychobiology. 2002;40:33–42. doi: 10.1002/dev.10011. [DOI] [PubMed] [Google Scholar]
- Wheaton B. Assessment of fit in overidentified models with latent variables. Sociological Methods and Research. 1987;16:118–154. [Google Scholar]