Abstract
Background
Supratentorial PNETs (sPNET) are uncommon embryonal malignancies of the central nervous system whose prognosis has historically been poor. We evaluated the outcome and prognostic factors of children with sPNET treated prospectively on a Children’s Oncology Group trial.
Procedure
Following surgery, patients received craniospinal radiotherapy with concurrent carboplatin followed by six months of maintenance chemotherapy with cyclophosphamide and vincristine.
Results
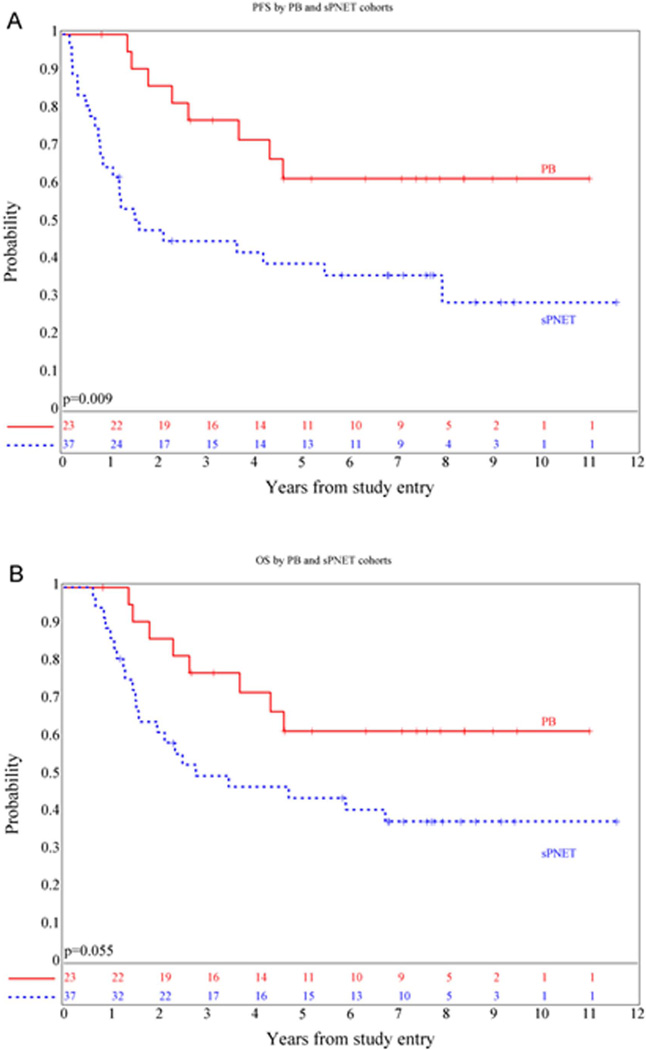
Five-year overall survival (OS) and progression-free survival (PFS) for all patients was 58 ±7% and 48 ±7%. For patients with pineoblastoma (n=23), five-year OS and PFS was 81 ± 9% and 62 ± 11%. Extent of resection but not M-stage was prognostic. Five-year OS and PFS for 37 patients with non-pineal tumors (NPsPNET) was 44 ± 8% and 39 ± 8%, significantly worse than for PB (p=0.055 and 0.009 respectively). Extent of resection and major radiotherapy deviations were prognostic. Five year OS was 59 +/− 11.4% for those undergoing complete resection versus 10.4 +/− 7% for those who did not (p=0.017). Central pathologic review called 14 (38%) “classic” sPNET, 8 (22%) "undifferentiated” and 13 (35%) “malignant gliomas”. There was no significant difference between the subgroups, although survival distributions approached significance when the combined “classic” and “undifferentiated” group was compared to the “malignant gliomas”.
Conclusions
Carboplatin during RT followed by 6 months of non-intensive chemotherapy is a feasible treatment strategy for patients with sPNET. Aggressive surgical resection should be attempted if feasible. The classification of supratentorial small cell malignancies can be difficult.
Keywords: supratentorial PNET, brain tumor, radiosensitizer, prognostic factors, pineoblastoma, pediatrics
INTRODUCTION
Supratentorial primitive neuroectodermal tumors (sPNET) are malignant embryonal tumors of the central nervous system, accounting for only 2–3% of childhood brain tumors [1,2]. PNETs can occur in any location in the central nervous system (CNS) and although they share many morphologic and immunohistochemical features, they differ biologically, even within the same locations [3–6]. These biologic differences almost certainly play a role in the differences in prognosis, with the best outcomes reported in PNET of the posterior fossa i.e. medulloblastoma [7–9]. The outcome for patients with non-pineal sPNET (NPsPNET) has historically been the worst [10,11] with little improvement over time. The rarity of these tumors precludes the ability to undertake a study specific to sPNET, so they are typically included in studies for “high-risk” medulloblastomas and treated with full dose craniospinal radiation therapy (RT) and chemotherapy. An understanding of prognostic factors and the optimal treatment for these tumors is lacking.
Carboplatin not only has significant activity against PNET [12,13] but is also a potent radiosensitizer [14,15]. We previously reported the outcome for children with metastatic medulloblastoma treated on a cooperative group trial using carboplatin concurrently with craniospinal (CS) and boost RT [16] and herein report on the results of this approach in children with pineoblastoma and NPsPNET.
PATIENTS AND METHODS
Patients and Eligibility
Patients between the ages of three and 21 years with newly diagnosed sPNET were eligible. Staging evaluation included a post-operative brain and spine MRI and CSF cytology. Patients were classified as M0 when there was no evidence of metastatic disease and M+ if otherwise. Patients were classified as M1 when they had positive CSF cytology without other evidence of metastatic disease, M2 when they had supratentorial without spinal metastases and M3 when they had spinal metastases with (M3b) or without (M3a) supratentorial disease. A gross total resection (GTR) was defined as no evidence of residual tumor on the post-operative MRI. All patients had to begin therapy within 31 days of definitive surgery. Eligibility criteria based on organ function were previously reported [16]. The study is registered with ClinicalTrials.gov (NCT00003203).
Study Design
COG 99701 was a Phase I/II study for patients with “high-risk” PNET that included a dose-escalation phase followed by a comparison of maintanence chemotherapy (MC) with or without cisplatin. The craniospinal axis was treated first and all patients received 36 Gy in 1.8 Gy fractions followed by a boost of 19.8 Gy to the primary tumor site. Focal spinal cord metastases were boosted to 45 Gy if above the termination of the cord and to 50.4 Gy if below. Vincristine 1.5 mg/m2 (maximum 2 mg) IV was administered weekly × six during radiation therapy. Patients received carboplatin over 15–20 minutes, one-four hours before each fraction of radiation. The dose and duration of carboplatin was assigned at study entry using a Phase I dose escalation design, as previously reported. [16] starting with 35 mg/m2/dose × 15 doses during the craniospinal component of therapy. Subsequent dose levels increased the number of doses of carboplatin up to 30 and thereafter the dose of carboplatin. Six to 12 patients were treated at each dose level depending on the number of patients experiencing dose limiting toxicity (DLT) during the 12 week evaluation period. Up to 24 additional patients could be enrolled on the highest safe dose level during evaluation of a higher dose. The maximum tolerated dose (MTD) was defined as the dose level immediately below that at which three or more patients in a cohort of six, or 4 or more in a cohort of 12, experienced a DLT.
Radiation was not withheld for myelosuppression alone but only for a “severe medical condition precluding radiation therapy”, not including fever and neutropenia as long as the patient was clinically stable. If a radiation treatment was not given, carboplatin was also held. Granulocyte-colony stimulating factor (G-CSF) was administered on the weekend if the absolute neutrophil count (ANC) fell ≤1,500/µL on any Friday; if the ANC dropped < 1,000/µL on any Monday or Wednesday, GCSF was administered on that day and the following day. No dose modifications of carboplatin were made for myelosuppression. Vincristine was held for Grade 3 or 4 foot drop, paresis, disabling paresthesias, or ileus and resumed at 1 mg/m2 once symptoms improved.
Six cycles of maintenance chemotherapy (MC) were administered six weeks after radiation was completed or when the ANC > 1,000/µL and platelets >100,000/µL. Patients were initially non-randomly assigned to receive Regimen A MC consisting of cyclophosphamide 1,000 mg/m2 on day zero and one of each four week course and vincristine 1.5 mg/m2 given on day zero and seven. Once the recommended Phase II dose (RP2D) of carboplatin was determined, patients received the same MC with the addition of 75 mg/m2 cisplatin on day zero (Regimen B). The cyclophosphamide dose was reduced by 25% if counts had not recovered by the time the next course was due. Audiograms were obtained prior to each course of cisplatin, with dose reductions dependent on the grade of toxicity.
Follow-up imaging was performed four to six weeks after the completion of RT, at three month intervals during chemotherapy and four to 12-month intervals thereafter. Progressive disease was defined as an increase of >25% in the area of residual disease compared to the best response at that site, or the reappearance of tumor in sites that had responded completely to therapy or the appearance of tumor in previously uninvolved sites.
RT Quality Assurance included central review of simulation fields, tumor coverage, dosimetry data, delivered doses and treatment duration. A dose deviation was considered major if the delivered dose differed from the protocol specified prescription dose by more than 10%. A volume deviation was considered major if the volume transected tumor or tumor-bearing areas.
OS and PFS were estimated using the product limit (Kaplan-Meier) method, with standard error via the Peto-Pike formula[17]. Survival distributions among subgroups were compared using the log-rank test. Association of survival distributions with continuous covariates was investigated using Cox proportional hazards regression models.
Central Review
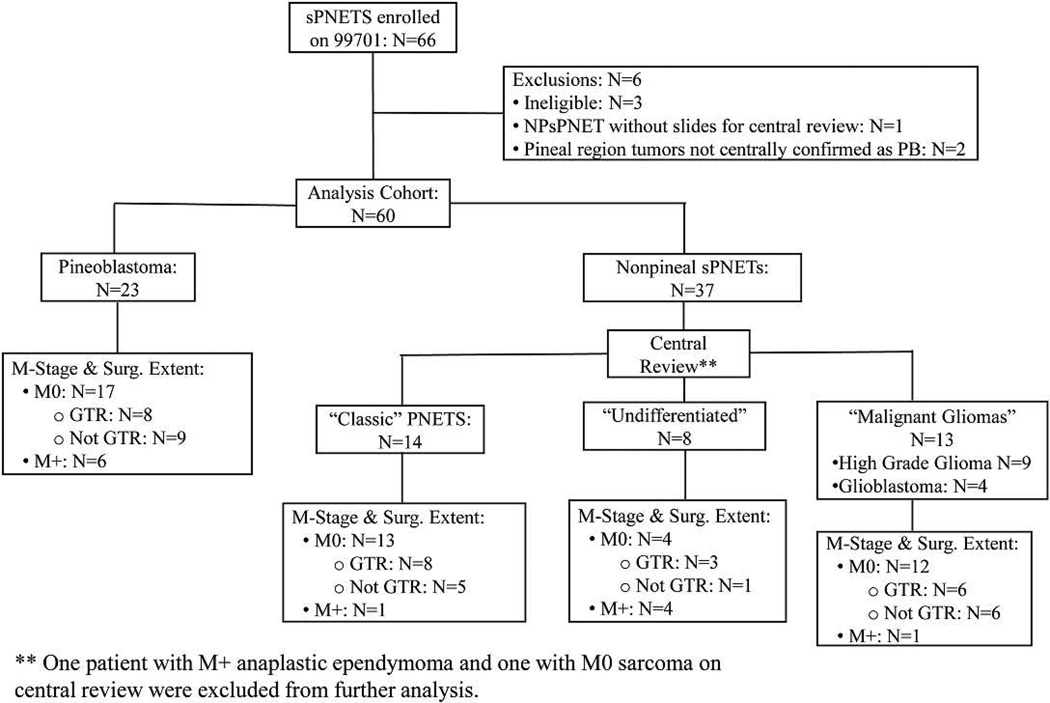
Pathology slides were centrally reviewed by a reviewer blinded to outcome (PCB). Results were not used to determine eligibility. The analysis cohort (Figure 1) consisted of: 1.) all patients considered NPsPNET by the enrolling institution with slides available for central review and 2.) pineoblastomas that were either centrally confirmed following slide review or if no slides were available, considered “centrally confirmed” based on review of their pathology reports, which described the tumors as highly cellular, mitotically active and synaptophysin positive (n=2).
Figure 1.
Flow diagram of the analysis cohort and central review subgroups of NPsPNET
A “classic” NPsPNET required a densely cellular, at least in part, tumor with evidence of neuronal differentiation, such as the presence of ganglion cells or smaller “ganglioid” cells, neuroblastic (Homer Wright) rosettes, or unequivocal immunoreactivity for synaptophysin. “Malignant gliomas” were defined as highly cellular GFAP-positive tumors with absent or only focally present synaptophysin staining. “Undifferentiated” tumors were densely cellular tumors that were only focally if at all immunoreactive for synaptophysin and GFAP.
RESULTS
Between December, 1998 and September, 2004, 66 patients with sPNET were enrolled. Three patients were considered ineligible: one patient developed a second tumor prior to RT and was determined to have an atypical teratoid-rhabdoid tumor (ATRT) and two patients who did not undergo a pre-contrast spine MRI. Additionally, one patient with NPsPNET did not have pathology slides available for central review and two patients with pineoblastoma did not meet the criteria for central confirmation. These six patients were excluded from the analysis. Of the remaining 60 patients (median age 11.3 yrs, range 3.1–21.6 yrs, 31 females), 23 had pineoblastomas and 37 had NPsPNET. Median follow-up time for surviving patients is 7.6 years. Five-year OS and PFS for all 60 patients were 58 ±7 and 48 ±7%. No patient with sPNET developed a second malignancy.
Toxicity
There were no treatment-related deaths. Delayed myelosuppression occurring 2–3 weeks after the completion of RT and occasionally causing a delay in the initiation of MC was seen in a subset of patients starting at dose level 7 (35 mg/m2). Specific radiation-related toxicities were not common, occurring in < 10% of patients at each dose level except at the highest dose level tested (50 mg/m2 × 6 weeks) where 2 of 8 patients developed grade 3 skin breakdown and severe esophagitis. The majority of patients required G-CSF and/or transfusion support toward the end of radiation, particularly at the higher dose levels. Although the MTD was not determined as defined by the protocol, a logistic-regression dose-response analysis showed a clear increase in platelet transfusions as the total dose of carboplatin increased. Dose level 7 (35mg/m2 × 6 weeks) was therefore chosen as the RP2D.
Pineal sPNET (pineoblastoma)
Twenty-three patients with centrally confirmed pineoblastomas were enrolled; all but 2 patients were treated on Regimen A. Median age was 10.7 years (range 3.5–17.9 years); 14 (61%) were male. Seventeen patients (74%) had localized tumors (M0) while six patients had metastatic disease to the spine (M3a, n=3) or throughout the brain and spine (M3b,n=3) at the time of diagnosis. OS and PFS are 86 ± 7% and 77% ± 9% at three years and 81 ± 9% and 62 ± 11% at five years. Median time to failure for those who developed progressive disease was 2.5 years (range: 1.4–4.6), and median time to death for those who died was 4.1 years (range: 1.9–5.8 years). Two of the five recurrences among 17 M0 patients had no local component (Table I).
Table I.
Sites of relapse for M0 patients
| Institutional Pathology | Central Pathology | Relapse Type | All Patients |
||
|---|---|---|---|---|---|
| Local | Distant | Local and Distant |
|||
| Pineoblastoma | Pineoblastoma | 2 | 2 | 1 | 17 |
| sPNET | Classic sPNET | 5 | 1 | 0 | 13 |
| Malignant Glioma | 8 | 0 | 2 | 13 | |
| Undifferentiated Malignant Neoplasm/PNET | 2 | 2 | 0 | 4 | |
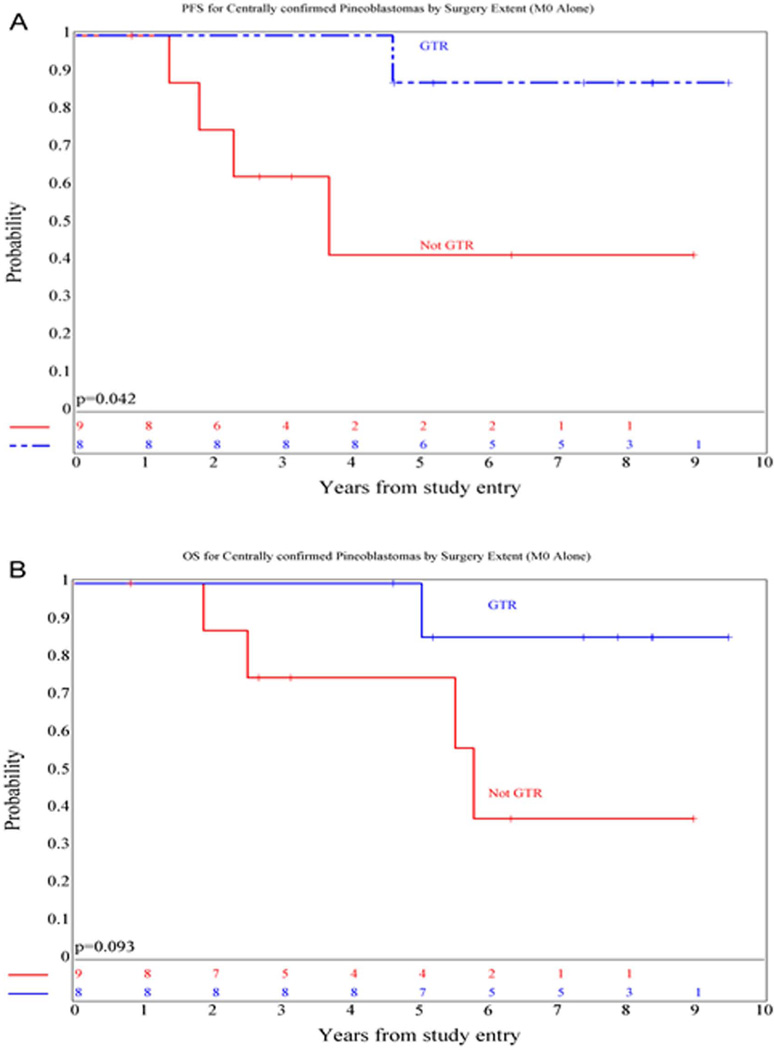
In patients with localized disease, extent of surgical resection was a significant prognostic factor for PFS (p=0.04) (Figure 2) and approached significance for OS (p=0.09). Five year PFS was 87.5 ± 12% for those who underwent a GTR (n=8) versus 41.7 ± 18.4% for those who did not (n=9). There was no difference in outcome distributions based on M stage (M+ versus M0) (p=0.49 and 0.53 for PFS and OS, respectively). Only one of 16 patients with available RT records had a major deviation, precluding further analysis.
Figure 2.
Kaplan-Meier curves showing PFS (A) and OS (B) distributions comparing patients with non-metastatic pineoblastoma who did or did not undergo a gross-total resection
Non-Pineal sPNET
Thirty-seven patients with centrally reviewed NPsPNET were enrolled: 33 were treated on Regimen A and 4 on Regimen B. Median age was 12 years, (range 3.1–21.6 years); 15 (40%) were male. Three and 5 year OS and PFS for the group as a whole were 50 ± 8% and 45 ± 8% and 44 ± 8% and 39 ± 8%, significantly worse than for those with pineoblastoma ((p=0.055 and 0.009 respectively) (Figure 3). Median time to failure for those who developed progressive disease was 0.81 years (range: 0.15–7.9 years), and median time to death was 1.5 years (range: 0.6–6.7 years). Seventeen of 20 (85%) recurrences among 30 M0 patients had at least a component of local failure (Table I).
Figure 3.
Kaplan-Meier curves showing PFS (A) and OS (B) distributions for patients with non-pineal sPNET compared with pineal region PNET.
Prognostic factors
Seven of 37 (18.9%) patients with sPNET had M+ disease at the time of diagnosis, including two patients with primary leptomeningeal tumors (both with M3 disease) and three patients with thalamic or intraventricular tumors with positive ventricular fluid cytologies (M1). No patient had M2 disease. There was no significant difference in outcome based on M stage (p=0.21 and 0.36 for PFS and OS respectively).
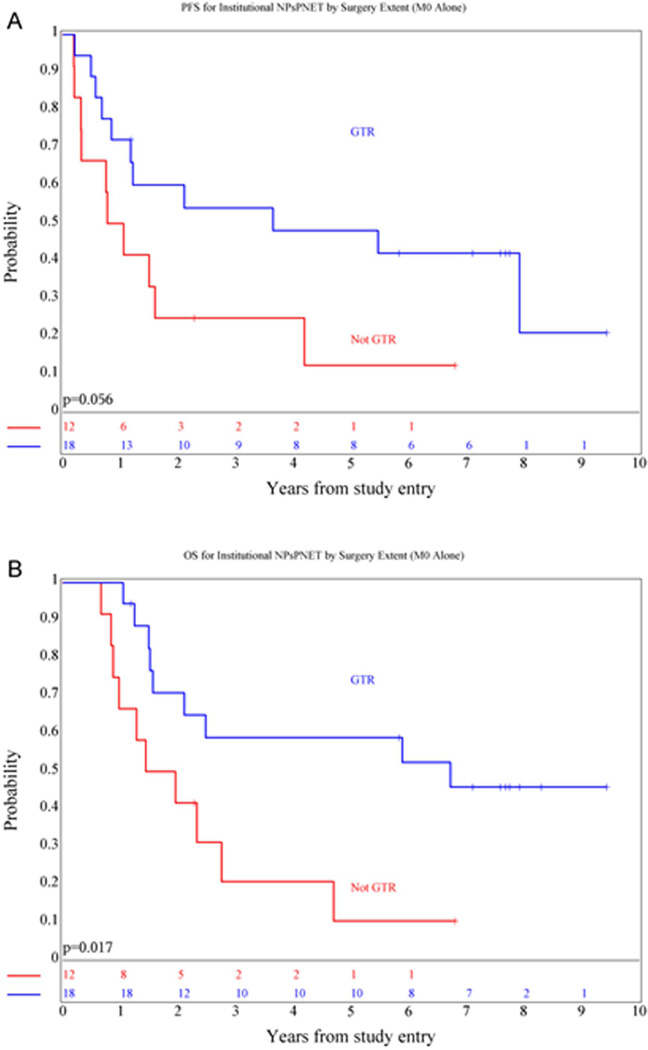
Extent of surgical resection was a significant prognostic factor for OS (p=0.017) in patients with localized disease and approached significance for PFS (p=0.056). Five-year OS was 59 ± 11.4% for those who underwent a GTR versus 10 ± 7% for those who did not (Figure 4). Eight of 29 (28%) patients with radiation therapy records available for review had a major deviation, which negatively impacted survival (p=0.023 and 0.13 for PFS and OS respectively). Five-year PFS was 55 ± 11% for those who received RT as per the protocol guidelines versus 12 ± 8% for those with a major RT deviation. All of the major deviations involved the administration of inadequate brain or boost volumes; no patient received a lower dose to the CS axis or primary tumor site.
Figure 4.
Kaplan-Meier curves showing PFS (A) and OS (B) distributions comparing patients with non-metastatic non-pineal sPNET who did or did not undergo a gross-total resection.
Impact of central pathology review
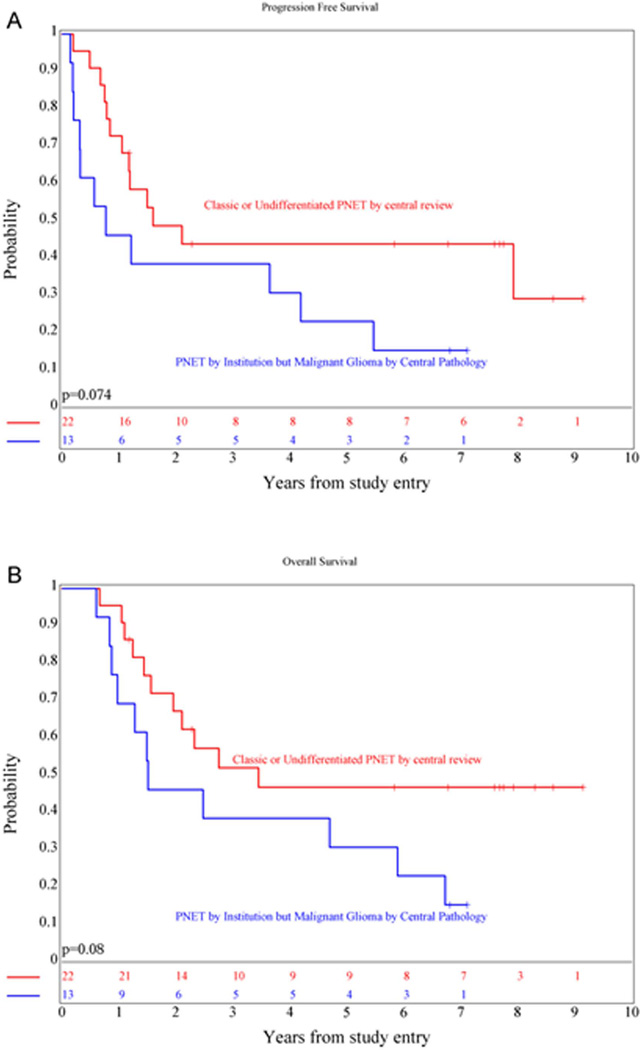
Fourteen of 37 (38%) patients were centrally called “classic” PNETs and eight were considered to be “undifferentiated”. The remaining central pathologic diagnoses included: “malignant gliomas” (high-grade glioma, not otherwise specified, n=9; glioblastoma, n=4), anaplastic ependymoma (n=1), and sarcoma (n=1). Two of the “malignant glioma” cases were verified to be glioblastomas either at the time of second look surgery or at autopsy. There was no statistically significant difference in PFS and OS between the three subgroups of patients (p=0.19 and 0.21, respectively). However, the five-year OS and PFS for the combined “classic” and “undifferentiated” group (Figure 5) was 47 ± 11% and 44 ±11% versus 31 ±11% and 23 ±10 % for those called “malignant glioma” by central review, with survival distribution differences that approached significance, p=0.08 and 0.07 respectively. Subset analysis of prognostic factors was precluded by the small number of patients.
Figure 5.
Kaplan-Meier curves showing PFS (A) and OS (B) distributions comparing NPsPNET patients who were called “classic PNET” or “undifferentiated” by central review versus those who were felt to have malignant gliomas.
DISCUSSION
Because the outcome for sPNET has historically been poor, and data for risk-stratification have been lacking, recent strategies have focused on intensifying treatment, either by using more intensive chemotherapy [18,19] or as in our study, by intensifying the up-front radiation component of treatment through the use of carboplatin, a radiosensitizer that also has efficacy against PNET[12,13]. The Goldie-Coldman model of therapeutic resistance predicts that the use of multiple therapeutic agents during the initial component of treatment reduces the likelihood of the emergence of resistant clones; radiation being modeled as a non-cross-resistant therapeutic agent in this regimen [20]. We previously reported the results using this regimen in medulloblastoma or PNET of the posterior fossa [16]. The regimen is well tolerated and of relatively short duration. Several studies in very young children < 3 years of age treated with chemotherapy alone [10,21], as well as in older children treated with radiation with or without chemotherapy [19,22,23] have illustrated the essential role of radiation therapy in the treatment of sPNET. No study has shown that radiation therapy can be abandoned without sacrificing survival. We showed that major RT violations involving the volume of radiation administered to either the brain or primary tumor site resulted in inferior survival in patients with NPsPNET, further emphasizing the essential role of radiation therapy. The propensity to metastasize, as with all PNETs, makes CSRT the most conservative approach and it is currently considered part of the current standard of care, unless precluded by age. However, with the advent of more sophisticated biologic diagnostic tools to distinguish between sPNETs and high grade gliomas, the decision to use CSRT in the future should certainly take into account the biologic diagnosis, more so than the histologic diagnosis.
Although sPNETs can be at least temporarily responsive to chemotherapy [18,21] the role of adjuvant chemotherapy is less clear. A meta-analysis of the outcome of patients with pineoblastoma showed marginal, if any benefit with the addition of chemotherapy to surgery and radiation therapy [24]. Pizer [19] showed that the administration of pre-RT chemotherapy provided no benefit compared with RT alone in patients with sPNET. Our results in both the non-pineal and pineal groups compare favorably to previously published cooperative group series using CSRT with or without standard chemotherapy. The International Society for Paediatric Oncology (SIOP) PNET 3 study [19] reported a five year OS and event-free survival (EFS) of 42.5% (95% CI: 29.3–55.7) and 40.7% (95% CI: 27.6–53.8) for NPsPNET and 71.4% (95% CI: 47.8–95.1) and 71.4% (95% CI: 47.8–95.1) for PB. The Children’s Oncology Group 921 study reported a five year OS of 34 ± 20% for NPsPNET [25] and a three year OS and PFS of 73 ± 12% and 61 ± 13% for pineoblastoma [10]. The German HIT 88/89 and 91 trial reported a three year PFS of 33.9% (95% CI: 20–47) for NPsPNET and 63.6 % (95% CI: 35.2–92.1) for PB [22]. Two smaller studies in patients with sPNET evaluated the role of higher dose chemotherapy with stem cell support and risk adapted radiotherapy. One found a 24 ± 10 % five-year EFS[26] while the other found a 68 ± 14% five year EFS [27]. The small size of these studies, particularly when subdivided by location, limits the ability to draw definitive conclusions.
We also found that patients with both pineal and NPsPNETs who underwent a gross total resection had a better outcome than those who did not, confirming results from a systematic literature review of pineoblastoma [24] as well as trends reported in smaller studies [8,25]. Therefore, even though the locations of these tumors may make surgical resection risky, neurosurgeons should at least consider an attempt at gross total resection.
The rate of discordance between institutional and central review diagnoses (14/37) was similar to a previous Children’s Oncology Group study for high-risk PNET where 11/38 patients with sPNET were not centrally confirmed [25]. This reflects clinical reality, as pediatric small cell supratentorial malignancies can be notoriously difficult to categorize, particularly in cases without specific histological or immunohistochemical features [28]. Whereas pineal sPNETs are typically compact lesions and strongly synaptophysin positive, many NPsPNET are infiltrative, with little synaptophysin immunoreactivity as seen in the 8 patients classified as “undifferentiated”. Thirteen of the 15 remaining cases were felt to be malignant gliomas by central review and two of these were confirmed to be glioblastomas at the time of second-look surgery or autopsy. An increasing number of studies are confirming the biologic heterogeneity of tumors that morphologically appear to be sPNET [6,29,30]. In a molecular analysis of 142 institutionally categorized sPNETs, transcriptional and copy number profiles identified three molecular subgroups, categorized as being of primitive neural, oligoneural (likely glial) and mesenchymal lineage [6]. Moreover, further stratification of these tumors on the basis of their molecular features may support genomically-based categorization and potentially, treatment in future studies [30].
In conclusion, the use of carboplatin during RT followed by six months of non-intensive chemotherapy for patients with sPNET is well tolerated with at least comparable results to those seen with more intensive and longer-duration regimens. Gross total resection should be attempted if possible. Finally, classification of pediatric supratentorial non-pineal malignant neoplasms by histology alone is fraught with difficulties and future studies should prospectively incorporate the results of molecular studies.
Acknowledgments
Funding Source: This study was supported by the National Institute of Health grant U10 CA 098543 to the Children’s Oncology Group
Conflict of Interest Statement: Regina Jakacki is employed by Astra Zeneca; Joel Goldwein is employed by Elekta AB, Stockholm Sweden; Minesh Mehta is a consultant for Abbot, Bristol-Myers Squibb, Elekta, Phillips and Genentech and has stock options with Pharmacydics and Accuray
Glossary
- CSRT
Craniospinal radiation therapy
- GFAP
Glial fibrillary acidic protein
- GTR
Gross total resection
- M-stage
Metastatic stage
- MC
Maintenance chemotherapy
- MTD
Maximum tolerated dose
- NPsPNET
Non-pineal supratentorial PNET
- OS
Overall Survival
- PFS
Progression free survival
- PNET
Primitive neuroectodermal tumor
- RP2D
Recommended Phase II dose
- RT
Radiation Therapy
- sPNET
Supratentorial primitive neuroectodermal tumor
Contributor Information
Regina I. Jakacki, AstraZeneca, One Medimmune Way, Gaithersburg, MD 20878.
Peter C. Burger, Department of Pathology, Sheikh Zayed Tower, Rm M2101, 1800 Orleans Street, Baltimore, MD 21231.
Mehmet Kocak, Department of Preventive Medicine, University of Tennessee, 603 Doctors Office Building, Memphis, TN 38163.
James M. Boyett, Department of Biostatistics, St. Jude Children's Research Hospital, 262 Danny Thomas Place, Memphis, TN 38105-3678.
Joel Goldwein, Department of Radiation Oncology, Perelman Center for Advanced Medicine, 3400 Civic Center Blvd., Philadelphia, PA 19104.
Minesh Mehta, Radiation Oncology, University of Maryland School of Medicine, 655 W. Baltimore Street, Baltimore MD 21201.
Roger J. Packer, Neurology & Pediatrics, Children's National Medical Center, 111 Michigan Avenue, NW, Washington, DC 20010-2970.
Nancy J. Tarbell, Radiation Oncology, Harvard Medical School, 25 Shattuck Street, Boston, MA 0211.
Ian F. Pollack, Pediatric Neurosurgery, Children’s Hospital of Pittsburgh, Department of Neurological Surgery, Children’s Hospital of Pittsburgh of UPMC, 4401 Penn Avenue, Pittsburgh, PA 15224, Phone 412-692-5881, Fax: 412-692-5921, Ian.pollack@chp.edu.
REFERENCES
- 1.Gaffney CC, Sloane JP, Bradley NJ, Bloom HJ. Primitive neuroectodermal tumours of the cerebrum. Pathology and treatment. J Neurooncol. 1985;3(1):23–33. doi: 10.1007/BF00165168. [DOI] [PubMed] [Google Scholar]
- 2.Gurney J, Bunin GR. In: CNS and miscellaneous intracranial and intraspinal neoplasms. Monograph SP, editor. Bethesda: Surveillance Research Program; 1999. pp. 51–63. [Google Scholar]
- 3.McCabe MG, Ichimura K, Liu L, Plant K, Backlund LM, Pearson DM, Collins VP. High-resolution array-based comparative genomic hybridization of medulloblastomas and supratentorial primitive neuroectodermal tumors. J Neuropathol Exp Neurol. 2006;65(6):549–561. doi: 10.1097/00005072-200606000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pfister S, Remke M, Toedt G, Werft W, Benner A, Mendrzyk F, Wittmann A, Devens F, von Hoff K, Rutkowski S, Kulozik A, Radlwimmer B, Scheurlen W, Lichter P, Korshunov A. Supratentorial primitive neuroectodermal tumors of the central nervous system frequently harbor deletions of the CDKN2A locus and other genomic aberrations distinct from medulloblastomas. Genes Chromosomes Cancer. 2007;46(9):839–851. doi: 10.1002/gcc.20471. [DOI] [PubMed] [Google Scholar]
- 5.Northcott PA, Korshunov A, Witt H, Hielscher T, Eberhart CG, Mack S, Bouffet E, Clifford SC, Hawkins CE, French P, Rutka JT, Pfister S, Taylor MD. Medulloblastoma comprises four distinct molecular variants. J Clin Oncol. 2011;29(11):1408–1414. doi: 10.1200/JCO.2009.27.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Picard D, Miller S, Hawkins CE, Bouffet E, Rogers HA, Chan TS, Kim SK, Ra YS, Fangusaro J, Korshunov A, Toledano H, Nakamura H, Hayden JT, Chan J, Lafay-Cousin L, Hu P, Fan X, Muraszko KM, Pomeroy SL, Lau CC, Ng HK, Jones C, Van Meter T, Clifford SC, Eberhart C, Gajjar A, Pfister SM, Grundy RG, Huang A. Markers of survival and metastatic potential in childhood CNS primitive neuro-ectodermal brain tumours: an integrative genomic analysis. Lancet Oncol. 2012;13(8):838–848. doi: 10.1016/S1470-2045(12)70257-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biswas S, Burke A, Cherian S, Williams D, Nicholson J, Horan G, Jefferies S, Williams M, Earl HM, Burnet NG, Hatcher H. Non-pineal supratentorial primitive neuro-ectodermal tumors (sPNET) in teenagers and young adults: Time to reconsider cisplatin based chemotherapy after cranio-spinal irradiation? Pediatr Blood Cancer. 2009;52(7):796–803. doi: 10.1002/pbc.21899. [DOI] [PubMed] [Google Scholar]
- 8.Reddy AT, Janss AJ, Phillips PC, Weiss HL, Packer RJ. Outcome for children with supratentorial primitive neuroectodermal tumors treated with surgery, radiation, and chemotherapy. Cancer. 2000;88(9):2189–2193. doi: 10.1002/(sici)1097-0142(20000501)88:9<2189::aid-cncr27>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 9.Packer RJ, Gajjar A, Vezina G, Rorke-Adams L, Burger PC, Robertson PL, Bayer L, LaFond D, Donahue BR, Marymont MH, Muraszko K, Langston J, Sposto R. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24(25):4202–4208. doi: 10.1200/JCO.2006.06.4980. [DOI] [PubMed] [Google Scholar]
- 10.Jakacki RI, Zeltzer PM, Boyett JM, Albright AL, Allen JC, Geyer JR, Rorke LB, Stanley P, Stevens KR, Wisoff J, et al. Survival and prognostic factors following radiation and/or chemotherapy for primitive neuroectodermal tumors of the pineal region in infants and children: a report of the Childrens Cancer Group. J Clin Oncol. 1995;13(6):1377–1383. doi: 10.1200/JCO.1995.13.6.1377. [DOI] [PubMed] [Google Scholar]
- 11.Cohen BH, Zeltzer PM, Boyett JM, Geyer JR, Allen JC, Finlay JL, McGuire-Cullen P, Milstein JM, Rorke LB, Stanley P, et al. Prognostic factors and treatment results for supratentorial primitive neuroectodermal tumors in children using radiation and chemotherapy: a Childrens Cancer Group randomized trial. J Clin Oncol. 1995;13(7):1687–1696. doi: 10.1200/JCO.1995.13.7.1687. [DOI] [PubMed] [Google Scholar]
- 12.Mastrangelo R, Lasorella A, Riccardi R, Colosimo C, Iavarone A, Tornesello A, Mastrangelo S, Ausili-Cefaro G, Di Rocco C. Carboplatin in childhood medulloblastoma/PNET: feasibility of an in vivo sensitivity test in an "up-front" study. Med Pediatr Oncol. 1995;24(3):188–196. doi: 10.1002/mpo.2950240309. [DOI] [PubMed] [Google Scholar]
- 13.Gaynon PS, Ettinger LJ, Baum ES, Siegel SE, Krailo MD, Hammond GD. Carboplatin in childhood brain tumors. A Children's Cancer Study Group Phase II trial. Cancer. 1990;66(12):2465–2469. doi: 10.1002/1097-0142(19901215)66:12<2465::aid-cncr2820661204>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 14.Yang L, Douple EB, O'Hara JA, Crabtree RA, Eastman A. Enhanced radiation-induced cell killing by carboplatin in cells of repair-proficient and repair-deficient cell lines. Radiation research. 1995;144(2):230–236. [PubMed] [Google Scholar]
- 15.Yang LX, Douple EB, O'Hara JA, Wang HJ. Carboplatin enhances the production and persistence of radiation-induced DNA single-strand breaks. Radiation research. 1995;143(3):302–308. [PubMed] [Google Scholar]
- 16.Jakacki RI, Burger PC, Zhou T, Holmes EJ, Kocak M, Onar A, Goldwein J, Mehta M, Packer RJ, Tarbell N, Fitz C, Vezina G, Hilden J, Pollack IF. Outcome of children with metastatic medulloblastoma treated with carboplatin during craniospinal radiotherapy: a Children's Oncology Group Phase I/II study. J Clin Oncol. 2012;30(21):2648–2653. doi: 10.1200/JCO.2011.40.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, Mantel N, McPherson K, Peto J, Smith PG. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. British journal of cancer. 1977;35(1):1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Massimino M, Gandola L, Spreafico F, Luksch R, Collini P, Giangaspero F, Simonetti F, Casanova M, Cefalo G, Pignoli E, Ferrari A, Terenziani M, Podda M, Meazza C, Polastri D, Poggi G, Ravagnani F, Fossati-Bellani F. Supratentorial primitive neuroectodermal tumors (S-PNET) in children: A prospective experience with adjuvant intensive chemotherapy and hyperfractionated accelerated radiotherapy. Int J Radiat Oncol Biol Phys. 2006;64(4):1031–1037. doi: 10.1016/j.ijrobp.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 19.Pizer BL, Weston CL, Robinson KJ, Ellison DW, Ironside J, Saran F, Lashford LS, Tait D, Lucraft H, Walker DA, Bailey CC, Taylor RE. Analysis of patients with supratentorial primitive neuro-ectodermal tumours entered into the SIOP/UKCCSG PNET 3 study. Eur J Cancer. 2006;42(8):1120–1128. doi: 10.1016/j.ejca.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 20.Goldie JH, Coldman AJ. A mathematic model for relating the drug sensitivity of tumors to their spontaneous mutation rate. Cancer Treat Rep. 1979;63(11–12):1727–1733. [PubMed] [Google Scholar]
- 21.Geyer JR, Sposto R, Jennings M, Boyett JM, Axtell RA, Breiger D, Broxson E, Donahue B, Finlay JL, Goldwein JW, Heier LA, Johnson D, Mazewski C, Miller DC, Packer R, Puccetti D, Radcliffe J, Tao ML, Shiminski-Maher T. Multiagent chemotherapy and deferred radiotherapy in infants with malignant brain tumors: a report from the Children's Cancer Group. J Clin Oncol. 2005;23(30):7621–7631. doi: 10.1200/JCO.2005.09.095. [DOI] [PubMed] [Google Scholar]
- 22.Timmermann B, Kortmann RD, Kuhl J, Meisner C, Dieckmann K, Pietsch T, Bamberg M. Role of radiotherapy in the treatment of supratentorial primitive neuroectodermal tumors in childhood: results of the prospective German brain tumor trials HIT 88/89 and 91. J Clin Oncol. 2002;20(3):842–849. doi: 10.1200/JCO.2002.20.3.842. [DOI] [PubMed] [Google Scholar]
- 23.Johnston DL, Keene DL, Lafay-Cousin L, Steinbok P, Sung L, Carret AS, Crooks B, Strother D, Wilson B, Odame I, Eisenstat DD, Mpofu C, Zelcer S, Huang A, Bouffet E. Supratentorial primitive neuroectodermal tumors: a Canadian pediatric brain tumor consortium report. J Neurooncol. 2008;86(1):101–108. doi: 10.1007/s11060-007-9440-1. [DOI] [PubMed] [Google Scholar]
- 24.Tate M, Sughrue ME, Rutkowski MJ, Kane AJ, Aranda D, McClinton L, Barani IJ, Parsa AT. The long-term postsurgical prognosis of patients with pineoblastoma. Cancer. 2012;118(1):173–179. doi: 10.1002/cncr.26300. [DOI] [PubMed] [Google Scholar]
- 25.Albright AL, Wisoff JH, Zeltzer P, Boyett J, Rorke LB, Stanley P, Geyer JR, Milstein JM. Prognostic factors in children with supratentorial (nonpineal) primitive neuroectodermal tumors. A neurosurgical perspective from the Children's Cancer Group. Pediatr Neurosurg. 1995;22(1):1–7. doi: 10.1159/000121292. [DOI] [PubMed] [Google Scholar]
- 26.Friedrich C, von Bueren AO, von Hoff K, Gerber NU, Ottensmeier H, Deinlein F, Benesch M, Kwiecien R, Pietsch T, Warmuth-Metz M, Faldum A, Kuehl J, Kortmann RD, Rutkowski S. Treatment of young children with CNS-primitive neuroectodermal tumors/pineoblastomas in the prospective multicenter trial HIT 2000 using different chemotherapy regimens and radiotherapy. Neuro Oncol. 2013;15(2):224–234. doi: 10.1093/neuonc/nos292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chintagumpala M, Hassall T, Palmer S, Ashley D, Wallace D, Kasow K, Merchant TE, Krasin MJ, Dauser R, Boop F, Krance R, Woo S, Cheuk R, Lau C, Gilbertson R, Gajjar A. A pilot study of risk-adapted radiotherapy and chemotherapy in patients with supratentorial PNET. Neuro Oncol. 2009;11(1):33–40. doi: 10.1215/15228517-2008-079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burger PC. Supratentorial primitive neuroectodermal tumor (sPNET) Brain Pathol. 2006;16(1):86. doi: 10.1111/j.1750-3639.2006.tb00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwalbe EC, Hayden JT, Rogers HA, Miller S, Lindsey JC, Hill RM, Nicholson SL, Kilday JP, Adamowicz-Brice M, Storer L, Jacques TS, Robson K, Lowe J, Williamson D, Grundy RG, Bailey S, Clifford SC. Histologically defined central nervous system primitive neuro-ectodermal tumours (CNS-PNETs) display heterogeneous DNA methylation profiles and show relationships to other paediatric brain tumour types. Acta neuropathologica. 2013;126(6):943–946. doi: 10.1007/s00401-013-1206-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gessi M, von Bueren AO, Treszl A, Muhlen AZ, Hartmann W, Warmuth-Metz M, Rutkowski S, Pietsch T. MYCN amplification predicts poor outcome for patients with supratentorial primitive neuroectodermal tumors of the central nervous system. Neuro Oncol. 2014 doi: 10.1093/neuonc/not302. [DOI] [PMC free article] [PubMed] [Google Scholar]