Summary
Intracerebral hemorrhage (ICH) is a subtype of stroke involving formation of hematoma within brain parenchyma, which accounts for 8–15% of all strokes in Western societies and 20–30% among Asian populations, and has a 1‐year mortality rate >50%. The high mortality and severe morbidity make ICH a major public health problem. Only a few evidence‐based targeted treatments are used for ICH management, and interventions focus primarily on supportive care and comorbidity prevention. Even in patients who survive the ictus, extravasated blood (including plasma components) and subsequent intrahematoma hemolytic products trigger a series of adverse events within the brain parenchyma, leading to secondary brain injury, edema and severe neurological deficits or death. Although the hematoma in humans gradually resolves within months, full restoration of neurological function can be slow and often incomplete, leaving survivors with devastating neurological deficits. During past years, peroxisome proliferator‐activated receptor gamma (PPAR γ) transcription factor and its agonists received recognition as important players in regulating not only glucose and lipid metabolism (which underlies its therapeutic effect in type 2 diabetes mellitus), and more recently, as an instrumental pleiotropic regulator of antiinflammation, antioxidative regulation, and phagocyte‐mediated cleanup processes. PPAR γ agonists have emerged as potential therapeutic target for stroke. The use of PPAR γ as a therapeutic target appears to have particularly strong compatibility toward pathogenic components of ICH. In addition to its direct genomic effect, PPAR γ may interact with transcription factor, NF‐κB, which may underlie many aspects of the antiinflammatory effect of PPAR γ. Furthermore, PPAR γ appears to regulate expression of Nrf2, another transcription factor and master regulator of detoxification and antioxidative regulation. Finally, the synergistic costimulation of PPAR γ and retinoid X receptor, RXR, may play an additional role in the therapeutic modulation of PPAR γ function. In this article, we outline the main components of the role of PPAR γ in ICH pathogenesis.
Keywords: Catalase, CD36, Cerebral Hemorrhage, NF‐kappa B, Nrf2, Oxidative stress, PPAR gamma
Intracerebral Hemorrhage Pathobiology and PPARγ
Intracerebral hemorrhage (ICH) accounts for 8–15% of all strokes in Western societies and 20–30% among Asian populations with a 1‐year mortality rate >50–60% 1, 2, 3, 4. Despite advances in the field of stroke and neurocritical care, the 30‐day mortality has not changed significantly over the past two decades. The therapeutic interventions that are currently available focus primarily on supportive care and comorbidity management and prevention 5, 6, 7. Even in patients who survive the acute ictus (resulting in mass effect and increased intracranial pressure and primary brain injury 8, 9), the extravasated blood and, subsequently, the hemolytic products trigger a series of adverse events within brain parenchyma, causing secondary brain injury, edema, and neurological deficits 4, 10, 11, 12, 13, 14. Only half of ICH‐related deaths occur in the first 2 days after ICH onset 15, strongly pointing at the unique role of secondary brain injury in development of delayed mortality. It is generally accepted that the delayed aspect of ICH injury is multifactorial and, at least in part, is related to hematoma toxicity 16, 17, 18, 19, 20, the presence of noxious cellular debris, and robust inflammation 11, 21, 22. Hemolytic products such as hemoglobin (Hb) and its catabolic by‐products (heme and iron), free‐radical formation (notably through iron involving Fenton‐type mechanism), thrombin, metalloproteinases, complement (and other proteases), formation of oxy‐modified lipid mediators, and excitotoxicity are generally listed as central components of the delayed damage after ICH 10, 23, 24, 25, 26, 27. Although the hematoma in humans gradually resolves within months, restoration of neurological function is slow and most often incomplete, and the neurological deficits can be devastating. Therefore, management of hematoma stability (e.g., preventing rebleeding) during the acute phase followed by the control of timely clearance of hematoma‐deposited blood components (to speed up hematoma resolution) may represent unique targets for the treatment of ICH 28, 29, 30.
The peroxisome proliferator‐activated receptors (PPARs) including α, γ, and δ/β are encoded by separate genes and are members of a type II nuclear hormone receptor superfamily of ligand‐activated nuclear transcription factors 31, 32. Three different PPARγ transcripts (PPARγ 1, 2, and 3), each a derivative of the PPARγ gene through differential promoter usage 33, 34, have been identified. While PPARγ 2 is the isoform primarily expressed in adipose tissue, PPARγ 1 has a broader tissue distribution including presence in the brain 33, 35. The PPARγ regulates target gene expression by binding to conserved DNA sequences termed peroxisome proliferator response elements (PPREs), as heterodimers with the retinoic acid receptor (RXR) 36, 37. PPARγ functions as a therapeutic target for the treatment of metabolic disorders, for example diabetes 32, 38, 39. Phosphorylation of serine 112 at the N‐terminus of PPARγ2 by MAP kinase and SUMOylation was suggested to regulate its transcriptional activities 40, 41. The ligands for PPARγ include oxidized fatty acids, monounsaturated and polyunsaturated fatty acids such as oleic acid or linoleic acid 42, nonsteroidal antiinflammatory drugs 43, 15‐deoxy‐∆12,14‐Prostaglandin J2 (15d‐PGJ2) 44, and a class of compounds, the thiazolidinediones (TZDs) 45. The PPARγ receptor subtype was originally characterized in adipose tissue as an important regulator of the expression of various key enzymes involved in glucose and lipid metabolism to regulate efficient energy storage 32, 38, 39. Through selective activation of PPARγ, the TZDs control insulin sensitivity 44, 46. Two of the TZDs, pioglitazone and rosiglitazone, are approved by the FDA for the treatment of type 2 diabetes mellitus (DM2). It is important to stress that these drugs do not change blood insulin levels; rather, they make cells more sensitive to its effect (Figure 1).
Figure 1.
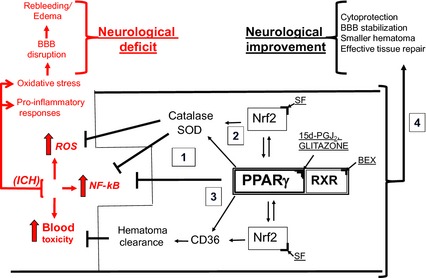
PPAR γ as therapeutic targets for ICH. In response to ICH, the local generation of reactive oxygen species (ROS), accumulation of toxic blood components (e.g., hemolytic products) in brain parenchyma, and activation of pro‐inflammatory transcription factor NF‐κB (causing generation of pro‐inflammatory cytokines and enzymes) lead to brain injury, often referred to as secondary brain damage, which manifest itself with blood brain barrier (BBB) disruption, rebleeding, edema and ultimately neurological deficit or death. Activation of PPAR γ with, for example, 15d‐PGJ2 or thiazolidinediones (known as glitazones) leads to: upregulation of the antioxidative enzymes, catalase and superoxide dismutase (SOD); scavenger receptor (e.g., CD36 on macrophages/microglia MMΦ) for RBC and hematoma clearance. Both PPAR γ and Nrf2 (which can be activated with sulforaphane, SF) regulate transcription of these genes. PPAR γ suppresses NF‐κB to limit the pro‐inflammatory response. Also, activation of RXR, an obligatory heterodimeric partner for PPAR γ activity (e.g., with 9‐cis retinoic acid or bexarotene, BEX), could augment the effect of PPAR γ ligand acting alone. Thus, PPAR γ activation may benefit the acute ICH and post‐ICH recovery by (1) downregulating the production of pro‐inflammatory mediators, (2) upregulating the antioxidative enzymes production, (3) promoting endogenous hematoma clearance thus eliminating the source of inflammation and allowing for more effective repair, and (4) direct and indirect cytoprotection.
In response to stroke, it appears that PPARγ mRNA is robustly upregulated in the affected brain tissue, suggesting that the endogenous system is attempting to activate PPARγ pathway via increasing PPARγ transcript 47, 48. While immunohistochemistry confirms that PPARγ protein is increased in the ischemia‐affected hemisphere, it seems that the PPARγ DNA binding and PPARγ gene target expression in this region is not increased, unless animals are treated with PPARγ activator 48. This may suggest that following brain injury, the endogenous activators of PPARγ are not available or in deficit and that the whole system requires exogenous agonist to activate the PPARγ pathway.
Intracerebral hemorrhage, primarily in the case of large hematomas, could lead to alteration in cerebral perfusion in proximity to the hematoma 49, 50. While, generally, no support exists for direct ischemic penumbra in ICH‐affected tissue 50, 51, it is likely that even modestly reduced perfusion at the hematoma site in combination with local hypermetabolism 52 (an event demonstrated in the brain in response to intracerebral injection of hemolysates) could lead to restricted cellular injury. PPARγ agonists, by controlling expression of the glucose transporter GLUT‐3 53, could improve glucose utilization and local metabolism and, as such, contribute to cytoprotection after ICH. In addition, the arcuate nucleus, an energy homeostasis and glucose metabolism control center in the brain, contains many neurons that show high expression of PPARγ 54, suggesting a potential role of PPARγ agonists in regulating metabolism by also affecting hypothalamic functions.
Later work on PPARγ noted that PPARγ plays important roles in regulating antioxidative processes and inflammation 55. It is the antiinflammatory properties of PPARγ ligands that ultimately brought additional attention to the whole class of PPARγ agents 56, 57, 58, 59, 60, 61, 62. As a transcription factor with pleiotropic mechanism of action, in terms of neurological conditions 59, PPARγ was suggested to play important roles in the pathogenesis of Alzheimer's disease 63, 64, Parkinson's disease and neurodegenerative disorders 65, 66, multiple sclerosis 67, 68, ischemic stroke 47, 69, 70, 71, 72, neurotrauma and spinal cord injury 73, 74, 75, 76, and ICH 77, 78. In studies with tissue culture and other injury models, it became clear that PPARγ is protective not only to neurons 47, 72, 79, astrocytes 80, 81, oligodendrocytes 82, endothelia 83, but also to microglia/macrophages (MMΦ) 78, 84, 85, 86. Among many potential mechanisms of action, the beneficial effects of PPARγ agonists are proposed to be due to the following: (i) the suppression of proinflammatory mediators, in part by interference with nuclear factor kappa B (NF‐κB) 87, 88, 89, (ii) the upregulation of antioxidant enzymes including CuZn superoxide dismutase (SOD) and catalase 78, 90, (iii) the inhibition of excitotoxicity 91, 92, and (iv) the promotion of microglia/macrophage‐mediated clearance of toxic cellular debris via mechanism involving upregulation of scavenger receptor CD36 expression 78, 93, 94, 95, or (iv) modification of neutrophil phenotype 61.
In this article, we will focus primarily on the role of PPARγ in ICH. We will discuss the interactions of PPARγ with nuclear factor erythroid 2‐related factor (Nrf2; a master regulator of oxidative responses) and NF‐κB signaling pathways pertaining to regulation of pro‐ and antiinflammatory responses. We will describe a synergistic activation of PPARγ when retinoid X receptor alpha (RXRα) and PPARγ are coactivated to achieve optimal cytoprotection and endogenous cleanup function—the clearance of hematoma‐deposited blood components by brain MMΦ after ICH.
PPARγ and Catalase—Implication in ICH Pathogenesis
The TZDs (e.g., ciglitazone, pioglitazone, and rosiglitazone) and cyclopentanone prostaglandins (e.g., 15d‐PGJ2) are PPARγ agonists which have been proved to act as potent and safe pro‐survival factors for primary neurons subjected to either excitotoxic insult, oxygen–glucose deprivation (OGD), or H2O2‐induced oxidative stress. The exact mechanism behind this protective mechanism is not fully known, but one of several potential candidates is a PPARγ‐mediated induction of potent antioxidative enzymes, such as superoxide dismutase 72, 96 and catalase 72, 74, 97. Catalase is a well‐known gene target for PPARγ 98 and administration of PPARγ agonist, for example 15d‐PGJ2, after ICH was demonstrated to rapidly induce catalase production in the affected brain 77, 78. This boost in production of antioxidative enzymes could be of particular importance for brain cells after ICH, as it was reported that hemoglobin lysis products (a protocol mimicking hematoma environment) reduce tissue levels of free‐radical decomposing enzymes 99, 100, 101. Catalase is a large homotetrameric protein that is highly abundant in the peroxisome (the membrane‐enclosed small organelles that house various oxidation reactions, in which toxic peroxides are generated as side products), where it serves to protect the cells from the toxic effects of H2O2 by catalyzing its decomposition into O2 and H2O (2H2O2 →O2 + 2H2O) without generating free radicals. Interestingly, catalase activity in the brain, as compared with other tissue (e.g., heart, kidney, liver, or lung), is relatively low 102. In response to PPARγ agonist, catalase expression rapidly increased in the ICH‐affected brain, demonstrating two temporal peaks with differential spatial distribution. The first peak reflects, primarily, induction of catalase expression in the ICH‐affected neurons (as early as 1 h after ICH and sustained at higher levels for 6~24 h 77). The second peak is mainly associated with the catalase induction in MMΦ (appeared 3~7 days, unpublished data). The rapid catalase production by neurons may likely reflect an adaptive response aimed at improving the H2O2 buffering capacity of neurons and is linked to direct neuroprotection. On the other hand, the upregulation of catalase in MMΦ could facilitate effective phagocytosis‐mediated cleanup functions by preventing self‐injury to MMΦ. During phagocytosis, MMΦ generate high levels of pro‐oxidative molecules that, unless neutralized by the MMΦ, may adversely affect phagocytes themselves, as well as other perihematomal brain cells 74, 78. Although the benefits of cytoprotective approaches to reduce damage to neurons, oligodendroglia, astroglia, or endothelium have been frequently discussed, the benefits of protecting the phagocytes (MMΦ) from damage at the brain injury site have been seldom addressed. In our ongoing research, subjecting primary microglia to “ICH‐like” (hemolytic products plus mild OGD) injury or high (>50 μM) levels of H2O2 in our hands induced significant morphological and functional damage indicating that these cells can suffer from damage similar to other brain cells. Preincubating the microglia with PPARγ activators improves the expression of antioxidative enzymes and microglia's resistance to H2O2 or “ICH‐like” injury 78 and could increase resistance to ICH‐like damage.
PPARγ and Phagocytosis‐Mediated Hematoma Resolution
The hematoma size after ICH not only predicts the magnitude of mass effect and direct physical injury, but it also reflects the volume of toxic blood breakdown products, which is the cause of “chemical” secondary damage, deposited in the brain. The larger hematomas may require more time for their resolution (blood clearance) and as such may inflict damage to the surrounding brain tissue (or to impair its repair) for a longer duration of time. Thus, it is not surprising that hematoma size is one of the strongest predictors of poor outcome 103, 104. Based on this assumption, several clinical trials targeting surgical hematoma evacuation were initiated 105, 106, 107, 108. While the overall outcome of these studies is generally neutral, some potentially promising results were seen in patients with superficial lobar hematomas without intraventricular hemorrhage 109, 110. Also, in patients subjected to minimally invasive hematoma evacuation surgery plus rt‐PA during hematoma evacuation (MISTIE trial), the procedure was associated with significant reduction in perihematomal edema 107. These suggest that under circumstances when invasiveness of the surgical approaches is low (e.g., manipulations with superficial aspects of the brain or washout of blood with the assistance of thrombolytic rt‐PA vs. application of pressure suction), the clearance of blood from the brain could be beneficial. While surgical approaches to remove blood clots continue to be evaluated, a new concept of nonsurgical approaches to assist blood cleanup through promoting the endogenous microglia/macrophages‐mediated phagocytosis is being tested 18. Normally, depending on the hematoma size, the blood clearance from the brain occurs naturally in 2–4 weeks in rodents 78, 111. Our recent studies indicated that activating PPARγ in microglia/macrophages results in upregulation of expression of CD36, a cell membrane protein, which plays an essential role in phagocytosis‐mediated hematoma cleanup after ICH 12.
CD36, a type II scavenger receptor, has been shown to act as a receptor for phosphatidyl serine, thrombospondin, and oxidized lipids; in addition, it mediates internalization/phagocytosis of brain apoptotic cells 112, 113, 114, sickled/asymmetric/dislocated red blood cells (RBC) 78, 94, 115, and apoptotic neutrophils 116, 117, 118. Interestingly, expression of this phagocytosis‐aiding protein is under transcriptional control of PPARγ 119, so that its expression could effectively be upregulated pharmacologically by PPARγ agonists and inhibited by selective PPARγ antagonists 120, 121, 122. In agreement with this notion, administration of PPARγ activators can efficiently increase expression of CD36 by microglia and improve phagocytosis of RBC, thus promoting hematoma resolution in animal models of ICH 18, 78. This cleanup‐aiding function of CD36 and PPARγ was suggested earlier based on findings that deficiency of CD36 in macrophages due to genetic deletion of PPARγ led to delayed uptake of oxidized low‐density lipoprotein (oxLDL) by macrophages and aggravated atherosclerotic lesions 119, 123, 124. Thus, CD36 upregulation in MMΦ in response to PPARγ activation may ensure a more efficient interaction between MMΦ and their phagocytosis targets for a timely clearance. This line of research prompted us to launch a clinical trial with pioglitazone in ICH patients 108. The underlying hypothesis is that pioglitazone through PPARγ activation will assist the enhancement of the endogenous cleanup process and anti‐oxidative defense, as well as amelioration of pro‐inflammatory responses that altogether will inhibit secondary damage caused by ICH.
PPARγ and Two Faces of Inflammation
After ICH, phagocytosis‐mediated clearance of apoptotic or damaged cells and dislocated blood components by MMΦ is believed to play a beneficial role by minimizing the exposure of the brain tissue to this toxic and pro‐inflammatory milieu 125, 126. Engulfment of apoptotic cells by MMΦ was proposed to actively suppress production of pro‐inflammatory mediators by the phagocyte through promoting release of antiinflammatory mediators, such as transforming growth factors (TGF‐β) and IL‐10 127, 128, 129. Although clearance of hematoma by MMΦ is necessary to achieve elimination of the hematoma, a source of inflammation, the deleterious molecules generated by MMΦ during phagocytosis could injure the neurovascular component of the brain (e.g., neuron, oligodendrocyte, endothelium), and also the phagocytes themselves 11, 130, 131. The main deleterious components of this process include (i) increased release of pro‐inflammatory mediators (e.g., IL‐1β, TNFα), (ii) activation of pro‐inflammatory transcription factor NF‐κB and increased expression of pro‐inflammatory enzymes (e.g., iNOS, COX‐2), (iii) increased synthesis and release of proteinases (e.g., MMP9), (iv) acidification of the environment, and (v) generation of free radicals. These responses are, in part, the reason why in an attempt to control inflammation after ICH, many studies focused on how to reduce microglia/macrophage activation and/or their phagocytosis function. However, as indicated above, phagocytosis is necessary for clearance of the hematoma 18, 108. Thus, it is necessary to find ways to tune up the phagocytosis process, so that effective clearance can be generated under conditions that have minimal adverse effect to the surrounding brain tissue.
The antiinflammatory role of PPARγ in ICH appears to be significant. Many studies using PPARγ activators showed a robust reduction in expression of pro‐inflammatory mediators (TNF‐α, IL‐1β, iNOS, MMP9) in MMΦ with concurrently increased expression of antiinflammatory cytokines (TGF‐β and IL‐10) 59, 78, 89, 132, 133. In rat primary microglia in culture, PPARγ agonists not only increased microglia‐mediated phagocytosis of RBC, but also reduced the production of H2O2 during the process of engulfment 78. Treatment with PPARγ agonist is associated with increased production of antioxidative defense system enzymes such as catalase and superoxide dismutase that may explain reduced pro‐oxidative responses in cells with activated PPARγ 72, 74, 77, 78. It appears that prevention of oxidative stress is obligatory in allowing microglia to show optimal cleanup capacity. We have demonstrated that exogenous application of catalase to primary microglia in culture can enhance internalization of RBC by these cells, suggesting that a self‐protective mechanism (antioxidative defense) from the excessive oxidative stress is critical to ensure MMΦ survival and efficient cleanup function. Interestingly, one of key important gene targets of PPARγ is CD36. As mentioned above, PPARγ‐induced CD36 expression may play an important role in stimulating phagocytotic efficacy of microglia 78. While the process of phagocytosis is overall beneficial from the point of removal of toxic and pro‐inflammatory cellular debris, it is well recognized that microglia‐mediated scavenging activities are associated with generation of massive amount of pro‐oxidants 134 which could adversely affect surrounding brain cells. As such, it is intriguing to note the same transcription factor (PPARγ) not only upregulates genes associated with enhanced phagocytosis (e.g., CD36), but also simultaneously upregulates antioxidative genes (e.g., catalase) that permit more effective neutralization of oxidative stress associated with more robust scavenging activities. Interestingly, this cooperative generation of CD36 and antioxidative enzyme exists not only for PPARγ. In our ongoing research (unpublished results), we have determined that Nrf2, a transcription factor considered a master regulator of cellular antioxidative defense, is also capable of inducing CD36 expression in microglia. These findings strongly suggest that for optimal function of CD36 in hematoma resolution (and likely cleanup after ischemic stroke), the antioxidative defense system needs to be enhanced to eliminate the deleterious consequences (oxidative damage) associated with CD36‐mediated phagocytosis/endocytosis.
Lastly, it should be mentioned that PPARγ is proposed to act as a signaling molecule downstream from the IL‐4 receptor, a pathway that has a key role in an alternative activation of MMΦ 135, 136, 137, which results in formation of a “healing” trophic phenotype of MMΦ. In our ongoing research, we have established that IL‐4 is generated locally in the brain and via IL‐4 receptor activates STAT6 and PPARγ signaling leading to reduction of pro‐inflammatory and induction of antiinflammatory phenotype of microglia after stroke.
Taken together, PPARγ may benefit the inflammation in ICH by directly downregulating the production of pro‐inflammatory mediators and upregulating antiinflammatory mediators. This is in addition to its role in hematoma clearance, the process that leads to removal of the toxic and pro‐inflammatory debris from the intraparenchymal tissue.
PPARγ Activation and Interaction of PPARγ and RXR
PPARγ and RXR, both are ligand‐dependent pleiotropic transcription factors belonging to the nuclear hormone receptor family. Upon dimerization, PPARγ–RXR as “partners” regulate target gene expression by binding to conserved DNA sequences, PPRE 38. There are three RXR isotypes, RXRα (NR2B1), RXRβ (NR2B2), and RXRγ (NR2B3), which have considerable tissue‐specific differences in their expression 138 and are present in various cells of brain tissue 139. The PPARγ–RXR heterodimer complex can be activated either by PPARγ ligands (e.g., TZD or 15d‐PGJ2) and/or by RXR ligands (e.g., 9‐cis retinoic acid) 140; however, the occupancy of both (PPARγ plus RXR) ligand‐binding domains simultaneously could provide maximal receptor activity 32, 141, 142, 143. It is necessary to acknowledge that when comparing PPARγ activation in response to RXR‐ versus PPARγ‐activating ligand, RXR may dimerize with and activate other nuclear receptors (e.g., retinoic acid receptor, RAR; liver X receptor, LXR; pregnane X receptor, PXR; or farnesoid X receptor, FXR). As such, RXR agonists could have broader biological activity than PPARγ. However, it is often proposed that some key effects including antiinflammatory effects of RXR agonists appear to be executed largely through a PPARγ pathway 144. In our laboratory, we have demonstrated that the cotreatment of primary cortical‐cultured neurons with the combination of 15d‐PGJ2 and 9‐cis retinoic acid protected the cells from OGD‐induced damage more robustly than each of the ligands alone. Also, primary rat microglia in response to combined PPARγ activator (e.g., pioglitazone) and RXR activator (e.g., bexarotene) appear to demonstrate a significantly higher phagocytosis efficacy toward erythrocytes, as compared to each of the ligands alone (ongoing studies), further supporting the existence of synergy between PPARγ and RXR activators in various biological processes. These beneficial interactions of PPARγ and RXR ligands are consistent with an earlier report that 15d‐PGJ2 plus 9‐cis retinoic acid was superior in reducing behavioral dysfunction in a mouse model of experimental encephalomyelitis (EAE) 145. Interestingly, it was recently demonstrated that retinoic acid at higher concentration can elicit different, even contrasting effect (to that seen with lower concentration) by activating PPARβδ/RXR heterodimers146.
Interaction of PPARγ and Nrf2 and NF‐κB
NF‐κB is a transcription factor that regulates expression of many pro‐inflammatory enzymes, cytokines, chemokines, proteases, and adhesion molecules, contributing to amplification of the secondary inflammation response and neuronal damage after ICH 11, 147, 148, 149, 150. The functional NF‐κB exists as a dimer, which in neurons is composed primarily of the (NF‐κB1) p50 and (RelA) p65 subunits. Other NF‐κB members of the NF‐κB/Rel family include RelB, c‐Rel, and p52 (NF‐κB2) 151. Numerous studies have confirmed that PPARγ may bind to the NF‐κB subunits, p50 and p65, directly resulting in NF‐κB inactivation 77, 87, 152. PPARγ may also indirectly inhibit NF‐κB by (i) competing for common transcription coactivators such as SRC‐1 153 and p300/CBP (CREB‐binding protein) 154, 155, (ii) upregulating the inhibitor kappa B (IκB), protein that prevents NF‐κB nuclear translocation which is a prerequisite for NF‐κB activation 88, 156, and (iii) indirectly inhibiting NF‐κB by activating transcription factor Nrf2, which reduces generation of pro‐oxidative molecules that are required for NF‐κB activation. Ultimately, inhibition of NF‐κB by PPARγ agonists was reported to reduce generation of pro‐inflammatory mediators/responses 56, 57 and consequently the secondary brain damage associated with these pro‐inflammatory responses 72, 73, 77, 78.
Nrf2 is a ubiquitous pleiotropic transcription factor and a master genomic homeostatic regulator of intracellular stress 157, 158, 159. Combining with Mif family proteins, Nrf2 forms heterodimeric complexes to transactivate the antioxidant response elements (ARE) within the regulatory region of many cytoprotective target genes [e.g., catalase, heme oxygenase‐1 (HO‐1), superoxide dismutase (SOD), glutathione‐S‐transferase (GST), thioredoxin and NAD(P)H dehydrogenase quinone 1 (NQO1)) 160, and also other proteins with important roles in neutralization of oxidative stress and detoxification of hemolytic products (e.g., haptoglobin, hemopexin, ferritin, and hemooxygenase‐1) 30, 161. In most cells, Nrf2 is present at low concentrations due to continuous Nrf2 degradation through the proteasome pathway. Nrf2 contributes to neuroprotection and amelioration of brain damage after cerebral ischemia 162, 163, neurotrauma 164, 165, neurodegenerative diseases 166, 167, 168, and ICH 30, 161, 169 primarily through reducing oxidative stress, inflammation, and generation of detoxifying molecules capable of neutralizing many noxious products generated in response to injury. The interaction between PPARγ and Nrf2 may involve multiple mechanisms. First, PPRE and ARE coexist in the same genes, such as CD36 170 and catalase 171, 172; second, a reciprocal transcriptional regulation exists between Nrf2 and PPARγ genes, Nrf2 gene contains putative PPREs 173, and conversely, PPARγ gene appears to contain the ARE 174, 175; third, an interaction between PPARγ and Nrf2 may be through NF‐κB inhibition. As NF‐κB activation highly depends upon the presence of oxidative stress, then the effect of Nrf2 in ameliorating oxidative stress was proposed to inhibit NF‐κB 176. As different mechanisms are used by Nrf2 and PPARγ in inhibiting NF‐κB, it is likely that the simultaneous activation of both Nrf2 and PPARγ may have a synergistic effect. Due to the interactions among PPARγ, Nrf2, and NF‐κB, it has been suggested that coactivation of PPARγ and Nrf2 may improve outcomes of several neurological disorders 177.
Neurotoxicity Following PPARγ Activation
Unlike synthetic thiazolidinediones (TZDs; e.g., pioglitazone and rosiglitazone) that have considerable levels of specificity toward PPARγ, prostaglandin D2 derivatives (primarily with cyclopentanone structure), including 15d‐PGJ2, which is proposed to act as endogenous PPARγ ligands, demonstrate rather limited selectivity toward PPARγ with some of its biological activities including activation of Nrf2 168 or inhibition of NF‐κB 87. There is existing evidence on the dose‐dependent neurotoxicity of the 15d‐PGJ2 in cerebellar granule cells, primary cortical neurons, and spinal cord motor neurons 178, 179 which originally were believed to be linked to PPARγ. The mechanism that underlies the neurotoxic effect of 15d‐PGJ2 is chiefly linked to higher doses of the compound. In addition, it is primarily associated with induction of apoptosis and not likely associated with the activation of PPARγ 180, 181. On the other hand, the clinically relevant, more selective PPARγ agonist such as rosiglitazone was linked to peripheral edema, increase in body weight, and cardiomyopathies and heart failure 182. Again the relationship between these side effects and PPARγ is somewhat controversial, as another TZD PPARγ agonist, pioglitazone, may show beneficial effects. The PROACTIVE (PROspective pioglitAzone Clinical Trial In macroVascular Events; NCT00174993) randomized, double‐blinded placebo‐controlled study looked at the impact of pioglitazone on total mortality and macrovascular morbidity in 5238 patients with DM2 and macrovascular disease. This secondary prevention study showed safety and a macrovascular benefit with pioglitazone in terms of major adverse cardiovascular events including all‐cause mortality, MI, and stroke 183, 184. Finally, it should be mentioned that the above side effects of rosiglitazone are described in patients taking TZDs long term for DM2. It is likely that PPARγ agonist treatment for ICH will be short term, potentially avoiding these side effects, although this needs careful testing.
Clinical Trials of PPARγ Agonists in ICH
Preclinical work has shown that PPARγ agonists are capable of promoting endogenous hematoma clearance, decreasing neuronal damage, and improving functional recovery in rodent model of ICH 77, 78. In addition, PPARγ agonists in vitro reduced the production of pro‐inflammatory mediators and free radicals produced during phagocytosis 78. Based on these studies, a clinical trial to evaluate the Safety of Pioglitazone for Hematoma Resolution in ICH has been launched (SHRINC) 108. This is a prospective, randomized, blinded, placebo‐controlled dose‐escalation safety trial in which patients with spontaneous intracerebral hemorrhage are randomly allocated to placebo or treatment. Pioglitazone, one of the PPARγ agonists that are approved by the Food and Drug Administration (FDA) for glycemic control in type II diabetes mellitus, was provided to the patients with escalating dosages. There was an evaluation period of 3~6 months, and the continual reassessment method for dose finding is used to determine the maximum tolerated dose of pioglitazone. Hematoma and edema resolution is evaluated with serial magnetic resonance imaging (MRI) at specified time points. The Phase 2 study with a planned sample size of 84 patients has preliminarily demonstrated safety regarding mortality 108 and is now in the next planning stages (ClinicalTrials.gov Identifier: NCT00827892).
As hematoma absorption is an extremely important objective after ICH, the SHRINC study should provide important information regarding the safety and clinical outcome regarding PPARγ agonists in the endogenous hematoma absorption. Besides primary ICH, secondary brain hemorrhage following brain trauma and brain surgery, subarachnoid hemorrhage (SAH), and hemorrhagic transformation of the ischemic stroke treated with rtPA may also be managed through this endogenous blood reabsorption (clearance) mechanism. Therefore, we are expecting that PPARγ, as a promising therapeutic target, could open a new field for managing hematoma clearance through a nonsurgical mechanism.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
Some studies indicated in this report were supported by NIH R01NS060768, 1R01NS064109 and R01NS084292 grants.
References
- 1. Feigin VL, Lawes CM, Bennett DA, Barker‐Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population‐based studies: A systematic review. Lancet Neurol 2009;8:355–369. [DOI] [PubMed] [Google Scholar]
- 2. van Asch CJ, Luitse MJ, Rinkel GJ, et al. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: A systematic review and meta‐analysis. Lancet Neurol 2010;9:167–176. [DOI] [PubMed] [Google Scholar]
- 3. Sangha N, Gonzales NR. Treatment targets in intracerebral hemorrhage. Neurotherapeutics 2011;8:374–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Keep RF, Hua Y, Xi G. Intracerebral haemorrhage: Mechanisms of injury and therapeutic targets. Lancet Neurol 2012;11:720–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Qureshi AI, Tuhrim S, Broderick JP, et al. Spontaneous intracerebral hemorrhage. N Engl J Med 2001;344:1450–1460. [DOI] [PubMed] [Google Scholar]
- 6. Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet 2009;373:1632–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brouwers HB, Goldstein JN. Therapeutic strategies in acute intracerebral hemorrhage. Neurotherapeutics 2012;9:87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Keep RF, Xi G, Hua Y, Hoff JT. The deleterious or beneficial effects of different agents in intracerebral hemorrhage: Think big, think small, or is hematoma size important? Stroke 2005;36:1594–1596. [DOI] [PubMed] [Google Scholar]
- 9. Keep RF, Xi G, Hua Y, Xiang J. Clot formation, vascular repair and hematoma resolution after ICH, a coordinating role for thrombin? Acta Neurochir Suppl 2011;111:71–75. [DOI] [PubMed] [Google Scholar]
- 10. Wagner KR, Sharp FR, Ardizzone TD, Lu A, Clark JF. Heme and iron metabolism: Role in cerebral hemorrhage. J Cereb Blood Flow Metab 2003;23:629–652. [DOI] [PubMed] [Google Scholar]
- 11. Aronowski J, Hall CE. New horizons for primary intracerebral hemorrhage treatment: Experience from preclinical studies. Neurol Res 2005;27:268–279. [DOI] [PubMed] [Google Scholar]
- 12. Aronowski J, Zhao X. Molecular pathophysiology of cerebral hemorrhage: Secondary brain injury. Stroke 2011;42:1781–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hwang BY, Appelboom G, Ayer A, et al. Advances in neuroprotective strategies: Potential therapies for intracerebral hemorrhage. Cerebrovasc Dis 2011;31:211–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Belur PK, Chang JJ, He S, Emanuel BA, Mack WJ. Emerging experimental therapies for intracerebral hemorrhage: Targeting mechanisms of secondary brain injury. Neurosurg Focus 2013;34:E9. [DOI] [PubMed] [Google Scholar]
- 15. Broderick J, Connolly S, Feldmann E, et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: A guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Stroke 2007;38:2001–2023. [DOI] [PubMed] [Google Scholar]
- 16. Koeppen AH. The history of iron in the brain. J Neurol Sci 1995;134(Suppl):1–9. [DOI] [PubMed] [Google Scholar]
- 17. Xi G, Fewel ME, Hua Y, et al. Intracerebral hemorrhage: Pathophysiology and therapy. Neurocrit Care 2004;1:5–18. [DOI] [PubMed] [Google Scholar]
- 18. Zhao X, Grotta J, Gonzales N, Aronowski J. Hematoma resolution as a therapeutic target: The role of microglia/macrophages. Stroke 2009;40:S92–S94. [DOI] [PubMed] [Google Scholar]
- 19. Lok J, Leung W, Murphy S, et al. Intracranial hemorrhage: Mechanisms of secondary brain injury. Acta Neurochir Suppl 2011;111:63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen‐Roetling J, Sinanan J, Regan RF. Effect of iron chelators on methemoglobin and thrombin preconditioning. Transl Stroke Res 2012;3:452–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang J, Dore S. Inflammation after intracerebral hemorrhage. J Cereb Blood Flow Metab 2007;27:894–908. [DOI] [PubMed] [Google Scholar]
- 22. Wang J. Preclinical and clinical research on inflammation after intracerebral hemorrhage. Prog Neurobiol 2010;92:463–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Regan RF, Panter SS. Hemoglobin potentiates excitotoxic injury in cortical cell culture. J Neurotrauma 1996;13:223–231. [DOI] [PubMed] [Google Scholar]
- 24. Xi G, Keep RF, Hoff JT. Erythrocytes and delayed brain edema formation following intracerebral hemorrhage in rats. J Neurosurg 1998;89:991–996. [DOI] [PubMed] [Google Scholar]
- 25. Huang FP, Xi G, Keep RF, et al. Brain edema after experimental intracerebral hemorrhage: Role of hemoglobin degradation products. J Neurosurg 2002;96:287–293. [DOI] [PubMed] [Google Scholar]
- 26. Wang X, Mori T, Sumii T, Lo EH. Hemoglobin‐induced cytotoxicity in rat cerebral cortical neurons: Caspase activation and oxidative stress. Stroke 2002;33:1882–1888. [DOI] [PubMed] [Google Scholar]
- 27. Kuramatsu JB, Huttner HB, Schwab S. Advances in the management of intracerebral hemorrhage. J Neural Transm 2013;120(Suppl 1):S35–S41. [DOI] [PubMed] [Google Scholar]
- 28. Babu R, Bagley JH, Di C, Friedman AH, Adamson C. Thrombin and hemin as central factors in the mechanisms of intracerebral hemorrhage‐induced secondary brain injury and as potential targets for intervention. Neurosurg Focus 2012;32:E8. [DOI] [PubMed] [Google Scholar]
- 29. Brouwers HB, Greenberg SM. Hematoma expansion following acute intracerebral hemorrhage. Cerebrovasc Dis 2013;35:195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhao X, Aronowski J. Nrf2 to pre‐condition the brain against injury caused by products of hemolysis after ICH. Transl Stroke Res 2013;4:71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kliewer SA, Forman BM, Blumberg B, et al. Differential expression and activation of a family of murine peroxisome proliferator‐activated receptors. Proc Natl Acad Sci U S A 1994;91:7355–7359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med 2002;53:409–435. [DOI] [PubMed] [Google Scholar]
- 33. Fajas L, Auboeuf D, Raspe E, et al. The organization, promoter analysis, and expression of the human PPARgamma gene. J Biol Chem 1997;272:18779–18789. [DOI] [PubMed] [Google Scholar]
- 34. Willson TM, Lambert MH, Kliewer SA. Peroxisome proliferator‐activated receptor gamma and metabolic disease. Annu Rev Biochem 2001;70:341–367. [DOI] [PubMed] [Google Scholar]
- 35. Cristiano L, Bernardo A, Ceru MP. Peroxisome proliferator‐activated receptors (PPARs) and peroxisomes in rat cortical and cerebellar astrocytes. J Neurocytol 2001;30:671–683. [DOI] [PubMed] [Google Scholar]
- 36. Greene ME, Blumberg B, McBride OW, et al. Isolation of the human peroxisome proliferator activated receptor gamma cDNA: Expression in hematopoietic cells and chromosomal mapping. Gene Expr 1995;4:281–299. [PMC free article] [PubMed] [Google Scholar]
- 37. Elbrecht A, Chen Y, Cullinan CA, et al. Molecular cloning, expression and characterization of human peroxisome proliferator activated receptors gamma 1 and gamma 2. Biochem Biophys Res Commun 1996;224:431–437. [DOI] [PubMed] [Google Scholar]
- 38. Mangelsdorf DJ, Thummel C, Beato M, et al. The nuclear receptor superfamily: The second decade. Cell 1995;83:835–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lemberger T, Desvergne B, Wahli W. Peroxisome proliferator‐activated receptors: A nuclear receptor signaling pathway in lipid physiology. Annu Rev Cell Dev Biol 1996;12:335–363. [DOI] [PubMed] [Google Scholar]
- 40. Adams M, Reginato MJ, Shao D, Lazar MA, Chatterjee VK. Transcriptional activation by peroxisome proliferator‐activated receptor gamma is inhibited by phosphorylation at a consensus mitogen‐activated protein kinase site. J Biol Chem 1997;272:5128–5132. [DOI] [PubMed] [Google Scholar]
- 41. Pascual G, Fong AL, Ogawa S, et al. A SUMOylation‐dependent pathway mediates transrepression of inflammatory response genes by PPAR‐gamma. Nature 2005;437:759–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Michaud SE, Renier G. Direct regulatory effect of fatty acids on macrophage lipoprotein lipase: Potential role of PPARs. Diabetes 2001;50:660–666. [DOI] [PubMed] [Google Scholar]
- 43. Lehmann JM, Lenhard JM, Oliver BB, Ringold GM, Kliewer SA. Peroxisome proliferator‐activated receptors alpha and gamma are activated by indomethacin and other non‐steroidal anti‐inflammatory drugs. J Biol Chem 1997;272:3406–3410. [DOI] [PubMed] [Google Scholar]
- 44. Forman BM, Tontonoz P, Chen J, et al. 15‐Deoxy‐delta 12, 14‐prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell 1995;83:803–812. [DOI] [PubMed] [Google Scholar]
- 45. Lehmann JM, Moore LB, Smith‐Oliver TA, et al. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator‐activated receptor gamma (PPAR gamma). J Biol Chem 1995;270:12953–12956. [DOI] [PubMed] [Google Scholar]
- 46. Stumvoll M, Haring HU. Glitazones: Clinical effects and molecular mechanisms. Ann Med 2002;34:217–224. [PubMed] [Google Scholar]
- 47. Ou Z, Zhao X, Labiche LA, et al. Neuronal expression of peroxisome proliferator‐activated receptor‐gamma (PPARgamma) and 15d‐prostaglandin J2–mediated protection of brain after experimental cerebral ischemia in rat. Brain Res 2006;1096:196–203. [DOI] [PubMed] [Google Scholar]
- 48. Victor NA, Wanderi EW, Gamboa J, et al. Altered PPARgamma expression and activation after transient focal ischemia in rats. Eur J Neurosci 2006;24:1653–1663. [DOI] [PubMed] [Google Scholar]
- 49. Etminan N, Beseoglu K, Turowski B, Steiger HJ, Hanggi D. Perfusion CT in patients with spontaneous lobar intracerebral hemorrhage: Effect of surgery on perihemorrhagic perfusion. Stroke 2012;43:759–763. [DOI] [PubMed] [Google Scholar]
- 50. Zazulia AR, Diringer MN, Videen TO, et al. Hypoperfusion without ischemia surrounding acute intracerebral hemorrhage. J Cereb Blood Flow Metab 2001;21:804–810. [DOI] [PubMed] [Google Scholar]
- 51. Qureshi AI, Wilson DA, Hanley DF, Traystman RJ. No evidence for an ischemic penumbra in massive experimental intracerebral hemorrhage. Neurology 1999;52:266–272. [DOI] [PubMed] [Google Scholar]
- 52. Ardizzone TD, Lu A, Wagner KR, et al. Glutamate receptor blockade attenuates glucose hypermetabolism in perihematomal brain after experimental intracerebral hemorrhage in rat. Stroke 2004;35:2587–2591. [DOI] [PubMed] [Google Scholar]
- 53. Garcia‐Bueno B, Caso JR, Perez‐Nievas BG, Lorenzo P, Leza JC. Effects of peroxisome proliferator‐activated receptor gamma agonists on brain glucose and glutamate transporters after stress in rats. Neuropsychopharmacology 2007;32:1251–1260. [DOI] [PubMed] [Google Scholar]
- 54. Sarruf DA, Yu F, Nguyen HT, et al. Expression of peroxisome proliferator‐activated receptor‐gamma in key neuronal subsets regulating glucose metabolism and energy homeostasis. Endocrinology 2009;150:707–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Neve BP, Fruchart JC, Staels B. Role of the peroxisome proliferator‐activated receptors (PPAR) in atherosclerosis. Biochem Pharmacol 2000;60:1245–1250. [DOI] [PubMed] [Google Scholar]
- 56. Jiang C, Ting AT, Seed B. PPAR‐gamma agonists inhibit production of monocyte inflammatory cytokines. Nature 1998;391:82–86. [DOI] [PubMed] [Google Scholar]
- 57. Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator‐activated receptor‐gamma is a negative regulator of macrophage activation. Nature 1998;391:79–82. [DOI] [PubMed] [Google Scholar]
- 58. Heneka MT, Landreth GE. PPARs in the brain. Biochim Biophys Acta 2007;1771:1031–1045. [DOI] [PubMed] [Google Scholar]
- 59. Kapadia R, Yi JH, Vemuganti R. Mechanisms of anti‐inflammatory and neuroprotective actions of PPAR‐gamma agonists. Front Biosci 2008;13:1813–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ballesteros I, Cuartero MI, Pradillo JM, et al. Rosiglitazone‐induced CD36 up‐regulation resolves inflammation by PPARgamma and 5‐LO‐dependent pathways. J Leukoc Biol 2014;95:587–598. [DOI] [PubMed] [Google Scholar]
- 61. Cuartero MI, Ballesteros I, Moraga A, et al. N2 neutrophils, novel players in brain inflammation after stroke: Modulation by the PPARgamma agonist rosiglitazone. Stroke 2013;44:3498–3508. [DOI] [PubMed] [Google Scholar]
- 62. Zhao X, Aronowski J. The role of PPARg in stroke. Immunological mechanisms and therapies in brain injuries and stroke. Springer Ser Transl Stroke Res 2014;301–318, Chapter 17. [Google Scholar]
- 63. Landreth GE, Heneka MT. Anti‐inflammatory actions of peroxisome proliferator‐activated receptor gamma agonists in Alzheimer's disease. Neurobiol Aging 2001;22:937–944. [DOI] [PubMed] [Google Scholar]
- 64. Feinstein DL. Therapeutic potential of peroxisome proliferator‐activated receptor agonists for neurological disease. Diabetes Technol Ther 2003;5:67–73. [DOI] [PubMed] [Google Scholar]
- 65. Randy LH, Guoying B. Agonism of peroxisome proliferator receptor‐gamma may have therapeutic potential for neuroinflammation and Parkinson's disease. Curr Neuropharmacol 2007;5:35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Clark J, Simon DK. Transcribe to survive: Transcriptional control of antioxidant defense programs for neuroprotection in Parkinson's disease. Antioxid Redox Signal 2009;11:509–528. [DOI] [PubMed] [Google Scholar]
- 67. Feinstein DL, Galea E, Gavrilyuk V, et al. Peroxisome proliferator‐activated receptor‐gamma agonists prevent experimental autoimmune encephalomyelitis. Ann Neurol 2002;51:694–702. [DOI] [PubMed] [Google Scholar]
- 68. Racke MK, Gocke AR, Muir M, et al. Nuclear receptors and autoimmune disease: The potential of PPAR agonists to treat multiple sclerosis. J Nutr 2006;136:700–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sundararajan S, Gamboa JL, Victor NA, et al. Peroxisome proliferator‐activated receptor‐gamma ligands reduce inflammation and infarction size in transient focal ischemia. Neuroscience 2005;130:685–696. [DOI] [PubMed] [Google Scholar]
- 70. Zhao Y, Patzer A, Gohlke P, Herdegen T, Culman J. The intracerebral application of the PPARgamma‐ligand pioglitazone confers neuroprotection against focal ischaemia in the rat brain. Eur J Neurosci 2005;22:278–282. [DOI] [PubMed] [Google Scholar]
- 71. Vemuganti R. Therapeutic potential of PPARgamma activation in stroke. PPAR Res 2008;2008:461981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zhao X, Strong R, Zhang J, et al. Neuronal PPARgamma deficiency increases susceptibility to brain damage after cerebral ischemia. J Neurosci 2009;29:6186–6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Park SW, Yi JH, Miranpuri G, et al. Thiazolidinedione class of peroxisome proliferator‐activated receptor gamma agonists prevents neuronal damage, motor dysfunction, myelin loss, neuropathic pain, and inflammation after spinal cord injury in adult rats. J Pharmacol Exp Ther 2007;320:1002–1012. [DOI] [PubMed] [Google Scholar]
- 74. Yi JH, Park SW, Brooks N, Lang BT, Vemuganti R. PPARgamma agonist rosiglitazone is neuroprotective after traumatic brain injury via anti‐inflammatory and anti‐oxidative mechanisms. Brain Res 2008;1244:164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sauerbeck A, Gao J, Readnower R, et al. Pioglitazone attenuates mitochondrial dysfunction, cognitive impairment, cortical tissue loss, and inflammation following traumatic brain injury. Exp Neurol 2011;227:128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Thal SC, Heinemann M, Luh C, et al. Pioglitazone reduces secondary brain damage after experimental brain trauma by PPAR‐gamma‐independent mechanisms. J Neurotrauma 2011;28:983–993. [DOI] [PubMed] [Google Scholar]
- 77. Zhao X, Zhang Y, Strong R, Grotta JC, Aronowski J. 15d‐Prostaglandin J2 activates peroxisome proliferator‐activated receptor‐gamma, promotes expression of catalase, and reduces inflammation, behavioral dysfunction, and neuronal loss after intracerebral hemorrhage in rats. J Cereb Blood Flow Metab 2006;26:811–820. [DOI] [PubMed] [Google Scholar]
- 78. Zhao X, Sun G, Zhang J, et al. Hematoma resolution as a target for intracerebral hemorrhage treatment: Role for peroxisome proliferator‐activated receptor gamma in microglia/macrophages. Ann Neurol 2007;61:352–362. [DOI] [PubMed] [Google Scholar]
- 79. Tureyen K, Kapadia R, Bowen KK, et al. Peroxisome proliferator‐activated receptor‐gamma agonists induce neuroprotection following transient focal ischemia in normotensive, normoglycemic as well as hypertensive and type‐2 diabetic rodents. J Neurochem 2007;101:41–56. [DOI] [PubMed] [Google Scholar]
- 80. Aleshin S, Grabeklis S, Hanck T, Sergeeva M, Reiser G. Peroxisome proliferator‐activated receptor (PPAR)‐gamma positively controls and PPARalpha negatively controls cyclooxygenase‐2 expression in rat brain astrocytes through a convergence on PPARbeta/delta via mutual control of PPAR expression levels. Mol Pharmacol 2009;76:414–424. [DOI] [PubMed] [Google Scholar]
- 81. Wang HM, Zhao YX, Zhang S, et al. PPARgamma agonist curcumin reduces the amyloid‐beta‐stimulated inflammatory responses in primary astrocytes. J Alzheimers Dis 2010;20:1189–1199. [DOI] [PubMed] [Google Scholar]
- 82. Hucke S, Flossdorf J, Grutzke B, et al. Licensing of myeloid cells promotes central nervous system autoimmunity and is controlled by peroxisome proliferator‐activated receptor gamma. Brain 2012;135:1586–1605. [DOI] [PubMed] [Google Scholar]
- 83. Ramirez SH, Heilman D, Morsey B, et al. Activation of peroxisome proliferator‐activated receptor gamma (PPARgamma) suppresses Rho GTPases in human brain microvascular endothelial cells and inhibits adhesion and transendothelial migration of HIV‐1 infected monocytes. J Immunol 2008;180:1854–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Petrova TV, Akama KT, Van Eldik LJ. Cyclopentenone prostaglandins suppress activation of microglia: Down‐regulation of inducible nitric‐oxide synthase by 15‐deoxy‐Delta12,14‐prostaglandin J2. Proc Natl Acad Sci U S A 1999;96:4668–4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Heneka MT, Sastre M, Dumitrescu‐Ozimek L, et al. Acute treatment with the PPARgamma agonist pioglitazone and ibuprofen reduces glial inflammation and Abeta1‐42 levels in APPV717I transgenic mice. Brain 2005;128:1442–1453. [DOI] [PubMed] [Google Scholar]
- 86. Mandrekar‐Colucci S, Karlo JC, Landreth GE. Mechanisms underlying the rapid peroxisome proliferator‐activated receptor‐gamma‐mediated amyloid clearance and reversal of cognitive deficits in a murine model of Alzheimer's disease. J Neurosci 2012;32:10117–10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Rossi A, Kapahi P, Natoli G, et al. Anti‐inflammatory cyclopentenone prostaglandins are direct inhibitors of IkappaB kinase. Nature 2000;403:103–108. [DOI] [PubMed] [Google Scholar]
- 88. Cernuda‐Morollon E, Rodriguez‐Pascual F, Klatt P, Lamas S, Perez‐Sala D. PPAR agonists amplify iNOS expression while inhibiting NF‐kappaB: Implications for mesangial cell activation by cytokines. J Am Soc Nephrol 2002;13:2223–2231. [DOI] [PubMed] [Google Scholar]
- 89. Delerive P, Fruchart JC, Staels B. Peroxisome proliferator‐activated receptors in inflammation control. J Endocrinol 2001;169:453–459. [DOI] [PubMed] [Google Scholar]
- 90. Shimazu T, Inoue I, Araki N, et al. A peroxisome proliferator‐activated receptor‐gamma agonist reduces infarct size in transient but not in permanent ischemia. Stroke 2005;36:353–359. [DOI] [PubMed] [Google Scholar]
- 91. Zhao X, Ou Z, Grotta JC, Waxham N, Aronowski J. Peroxisome‐proliferator‐activated receptor‐gamma (PPARgamma) activation protects neurons from NMDA excitotoxicity. Brain Res 2006;1073–1074:460–469. [DOI] [PubMed] [Google Scholar]
- 92. Romera C, Hurtado O, Mallolas J, et al. Ischemic preconditioning reveals that GLT1/EAAT2 glutamate transporter is a novel PPARgamma target gene involved in neuroprotection. J Cereb Blood Flow Metab 2007;27:1327–1338. [DOI] [PubMed] [Google Scholar]
- 93. Moore KJ, Rosen ED, Fitzgerald ML, et al. The role of PPAR‐gamma in macrophage differentiation and cholesterol uptake. Nat Med 2001;7:41–47. [DOI] [PubMed] [Google Scholar]
- 94. Patel SN, Serghides L, Smith TG, et al. CD36 mediates the phagocytosis of Plasmodium falciparum‐infected erythrocytes by rodent macrophages. J Infect Dis 2004;189:204–213. [DOI] [PubMed] [Google Scholar]
- 95. Majai G, Sarang Z, Csomos K, Zahuczky G, Fesus L. PPARgamma‐dependent regulation of human macrophages in phagocytosis of apoptotic cells. Eur J Immunol 2007;37:1343–1354. [DOI] [PubMed] [Google Scholar]
- 96. Shimazu T, Greenberg JH. A PPAR gamma agonist reduces infarct size in transient but not in permanent ischemia. Stroke 2005;36:353–359. [DOI] [PubMed] [Google Scholar]
- 97. Straus DS, Glass CK. Cyclopentenone prostaglandins: New insights on biological activities and cellular targets. Med Res Rev 2001;21:185–210. [DOI] [PubMed] [Google Scholar]
- 98. Moreno S, Mugnaini E, Ceru MP. Immunocytochemical localization of catalase in the central nervous system of the rat. J Histochem Cytochem 1995;43:1253–1267. [DOI] [PubMed] [Google Scholar]
- 99. Koeppen AH, Dickson AC, McEvoy JA. The cellular reactions to experimental intracerebral hemorrhage. J Neurol Sci 1995;134(Suppl):102–112. [DOI] [PubMed] [Google Scholar]
- 100. Hall NC, Packard BA, Hall CL, de Courten‐Myers G, Wagner KR. Protein oxidation and enzyme susceptibility in white and gray matter with in vitro oxidative stress: Relevance to brain injury from intracerebral hemorrhage. Cell Mol Biol (Noisy‐le‐grand) 2000;46:673–683. [PubMed] [Google Scholar]
- 101. Nakamura T, Keep RF, Hua Y, Hoff JT, Xi G. Oxidative DNA injury after experimental intracerebral hemorrhage. Brain Res 2005;1039:30–36. [DOI] [PubMed] [Google Scholar]
- 102. Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev 1979;59:527–605. [DOI] [PubMed] [Google Scholar]
- 103. Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage. A powerful and easy‐to‐use predictor of 30‐day mortality. Stroke 1993;24:987–993. [DOI] [PubMed] [Google Scholar]
- 104. Jordan LC, Kleinman JT, Hillis AE. Intracerebral hemorrhage volume predicts poor neurologic outcome in children. Stroke 2009;40:1666–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Rincon F, Mayer SA. Novel therapies for intracerebral hemorrhage. Curr Opin Crit Care 2004;10:94–100. [DOI] [PubMed] [Google Scholar]
- 106. Mendelow AD, Gregson BA, Rowan EN, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): A randomised trial. Lancet 2013;382:397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Mould WA, Carhuapoma JR, Muschelli J, et al. Minimally invasive surgery plus recombinant tissue‐type plasminogen activator for intracerebral hemorrhage evacuation decreases perihematomal edema. Stroke 2013;44:627–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Gonzales NR, Shah J, Sangha N, et al. Design of a prospective, dose‐escalation study evaluating the safety of pioglitazone for hematoma resolution in intracerebral hemorrhage (SHRINC). Int J Stroke 2013;8:388–396. [DOI] [PubMed] [Google Scholar]
- 109. Bhattathiri PS, Gregson B, Prasad KS, Mendelow AD, Investigators S. Intraventricular hemorrhage and hydrocephalus after spontaneous intracerebral hemorrhage: Results from the STICH trial. Acta Neurochir Suppl 2006;96:65–68. [DOI] [PubMed] [Google Scholar]
- 110. Abdu E, Hanley DF, Newell DW. Minimally invasive treatment for intracerebral hemorrhage. Neurosurg Focus 2012;32:E3. [DOI] [PubMed] [Google Scholar]
- 111. Hua Y, Schallert T, Keep RF, et al. Behavioral tests after intracerebral hemorrhage in the rat. Stroke 2002;33:2478–2484. [DOI] [PubMed] [Google Scholar]
- 112. Fadok VA, Warner ML, Bratton DL, Henson PM. CD36 is required for phagocytosis of apoptotic cells by human macrophages that use either a phosphatidylserine receptor or the vitronectin receptor (alpha v beta 3). J Immunol 1998;161:6250–6257. [PubMed] [Google Scholar]
- 113. Stolzing A, Grune T. Neuronal apoptotic bodies: Phagocytosis and degradation by primary microglial cells. FASEB J 2004;18:743–745. [DOI] [PubMed] [Google Scholar]
- 114. Ren Y, Silverstein RL, Allen J, Savill J. CD36 gene transfer confers capacity for phagocytosis of cells undergoing apoptosis. J Exp Med 1995;181:1857–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Smith TG, Serghides L, Patel SN, et al. CD36‐mediated nonopsonic phagocytosis of erythrocytes infected with stage I and IIA gametocytes of Plasmodium falciparum. Infect Immun 2003;71:393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Ren Y, Savill J. Proinflammatory cytokines potentiate thrombospondin‐mediated phagocytosis of neutrophils undergoing apoptosis. J Immunol 1995;154e:2366–2374. [PubMed] [Google Scholar]
- 117. Navazo MD, Daviet L, Savill J, et al. Identification of a domain (155‐183) on CD36 implicated in the phagocytosis of apoptotic neutrophils. J Biol Chem 1996;271:15381–15385. [DOI] [PubMed] [Google Scholar]
- 118. Erwig LP, Gordon S, Walsh GM, Rees AJ. Previous uptake of apoptotic neutrophils or ligation of integrin receptors downmodulates the ability of macrophages to ingest apoptotic neutrophils. Blood 1999;93:1406–1412. [PubMed] [Google Scholar]
- 119. Nicholson AC. Expression of CD36 in macrophages and atherosclerosis: The role of lipid regulation of PPARgamma signaling. Trends Cardiovasc Med 2004;14:8–12. [DOI] [PubMed] [Google Scholar]
- 120. Miyahara T, Schrum L, Rippe R, et al. Peroxisome proliferator‐activated receptors and hepatic stellate cell activation. J Biol Chem 2000;275:35715–35722. [DOI] [PubMed] [Google Scholar]
- 121. Han S, Sidell N. Peroxisome‐proliferator‐activated‐receptor gamma (PPARgamma) independent induction of CD36 in THP‐1 monocytes by retinoic acid. Immunology 2002;106:53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Babaev V, Yancey P, Ryzhov S, et al. Conditional knockout of macrophage PPARg increases atherosclerosis in C57BL/6 and low‐density lipoprotein receptor‐deficient mice. Arterioscler Thromb Vasc Biol 2005;8:1648–1653. [DOI] [PubMed] [Google Scholar]
- 123. Ottnad E, Parthasarathy S, Sambrano GR, et al. A macrophage receptor for oxidized low density lipoprotein distinct from the receptor for acetyl low density lipoprotein: Partial purification and role in recognition of oxidatively damaged cells. Proc Natl Acad Sci U S A 1995;92:1391–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Nagy L, Tontonoz P, Alvarez JG, Chen H, Evans RM. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell 1998;93:229–240. [DOI] [PubMed] [Google Scholar]
- 125. Shehab AM, MacFadyen RJ, McLaren M, et al. Sudden unexpected death in heart failure may be preceded by short term, intraindividual increases in inflammation and in autonomic dysfunction: A pilot study. Heart 2004;90:1263–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Aibiki M, Ohtsubo S, Nishiyama T, et al. Elevated serum beta‐D‐glucan level and depressed neutrophil phagocytosis in a heatstroke patient. Resuscitation 2005;65:115–117. [DOI] [PubMed] [Google Scholar]
- 127. Fadok VA. Clearance: The last and often forgotten stage of apoptosis. J Mammary Gland Biol Neoplasia 1999;4:203–211. [DOI] [PubMed] [Google Scholar]
- 128. Ekdahl CT, Kokaia Z, Lindvall O. Brain inflammation and adult neurogenesis: The dual role of microglia. Neuroscience 2009;158:1021–1029. [DOI] [PubMed] [Google Scholar]
- 129. MacLellan CL, Colbourne F. Mild to moderate hyperthermia does not worsen outcome after severe intracerebral hemorrhage in rats. J Cereb Blood Flow Metab 2005;25:1020–1029. [DOI] [PubMed] [Google Scholar]
- 130. Gong C, Hoff JT, Keep RF. Acute inflammatory reaction following experimental intracerebral hemorrhage in rat. Brain Res 2000;871:57–65. [DOI] [PubMed] [Google Scholar]
- 131. Cheret C, Gervais A, Lelli A, et al. Neurotoxic activation of microglia is promoted by a nox1‐dependent NADPH oxidase. J Neurosci 2008;28:12039–12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Sundararajan S, Jiang Q, Heneka M, Landreth G. PPARgamma as a therapeutic target in central nervous system diseases. Neurochem Int 2006;49:136–144. [DOI] [PubMed] [Google Scholar]
- 133. Pisanu A, Lecca D, Mulas G, et al. Dynamic changes in pro‐ and anti‐inflammatory cytokines in microglia after PPAR‐gamma agonist neuroprotective treatment in the MPTPp mouse model of progressive Parkinson's disease. Neurobiol Dis 2014;71:280–291. [DOI] [PubMed] [Google Scholar]
- 134. Splettstoesser WD, Schuff‐Werner P. Oxidative stress in phagocytes–”the enemy within”. Microsc Res Tech 2002;57:441–455. [DOI] [PubMed] [Google Scholar]
- 135. Szanto A, Balint BL, Nagy ZS, et al. STAT6 transcription factor is a facilitator of the nuclear receptor PPARgamma‐regulated gene expression in macrophages and dendritic cells. Immunity 2010;33:699–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: A marker of alternative immunologic macrophage activation. J Exp Med 1992;176:287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Derecki NC, Quinnies KM, Kipnis J. Alternatively activated myeloid (M2) cells enhance cognitive function in immune compromised mice. Brain Behav Immun 2011;25:379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Pelidou SH, Kostulas N, Matusevicius D, et al. High levels of IL‐10 secreting cells are present in blood in cerebrovascular diseases. Eur J Neurol 1999;6:437–442. [DOI] [PubMed] [Google Scholar]
- 139. Cullingford TE, Bhakoo K, Peuchen S, et al. Distribution of mRNAs encoding the peroxisome proliferator‐activated receptor alpha, beta, and gamma and the retinoid X receptor alpha, beta, and gamma in rat central nervous system. J Neurochem 1998;70:1366–1375. [DOI] [PubMed] [Google Scholar]
- 140. Leblanc BP, Stunnenberg HG. 9‐cis retinoic acid signaling: Changing partners causes some excitement. Genes Dev 1995;9:1811–1816. [DOI] [PubMed] [Google Scholar]
- 141. Mukherjee R, Davies PJ, Crombie DL, et al. Sensitization of diabetic and obese mice to insulin by retinoid X receptor agonists. Nature 1997;386:407–410. [DOI] [PubMed] [Google Scholar]
- 142. Mukherjee R, Jow L, Croston GE, Paterniti JR Jr. Identification, characterization, and tissue distribution of human peroxisome proliferator‐activated receptor (PPAR) isoforms PPARgamma2 versus PPARgamma1 and activation with retinoid X receptor agonists and antagonists. J Biol Chem 1997;272:8071–8076. [DOI] [PubMed] [Google Scholar]
- 143. Tontonoz P, Singer S, Forman BM, et al. Terminal differentiation of human liposarcoma cells induced by ligands for peroxisome proliferator‐activated receptor gamma and the retinoid X receptor. Proc Natl Acad Sci U S A 1997;94:237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Sanz MJ, Albertos F, Otero E, et al. Retinoid X receptor agonists impair arterial mononuclear cell recruitment through peroxisome proliferator‐activated receptor‐gamma activation. J Immunol 2012;189:411–424. [DOI] [PubMed] [Google Scholar]
- 145. Diab A, Hussain RZ, Lovett‐Racke AE, et al. Ligands for the peroxisome proliferator‐activated receptor‐gamma and the retinoid X receptor exert additive anti‐inflammatory effects on experimental autoimmune encephalomyelitis. J Neuroimmunol 2004;148:116–126. [DOI] [PubMed] [Google Scholar]
- 146. Liao SL, Chen WY, Raung SL, Chen CJ. Ethanol attenuates ischemic and hypoxic injury in rat brain and cultured neurons. NeuroReport 2003;14:2089–2094. [DOI] [PubMed] [Google Scholar]
- 147. Ghosh S, May MJ, Kopp EB. NF‐kappa B and Rel proteins: Evolutionarily conserved mediators of immune responses. Annu Rev Immunol 1998;16:225–260. [DOI] [PubMed] [Google Scholar]
- 148. Hickenbottom SL, Grotta JC, Strong R, Denner LA, Aronowski J. Nuclear factor‐kappaB and cell death after experimental intracerebral hemorrhage in rats. Stroke 1999;30:2472–2477; discussion 77‐8. [DOI] [PubMed] [Google Scholar]
- 149. Zhao X, Zhang Y, Strong R, et al. Distinct patterns of intracerebral hemorrhage‐induced alterations in NF‐kappaB subunit, iNOS, and COX‐2 expression. J Neurochem 2007;101:652–663. [DOI] [PubMed] [Google Scholar]
- 150. Wagner KR, Dean C, Beiler B, et al. Plasma infusion into porcine cerebral white matter induce early edema, oxidative stress, pro‐inflammatory cytokine gene expression and DNA fragmentation: implications for white matter injury with increased blood‐brain‐barrier permeability Curr Neurovasc Res 2005;63:149–155. [DOI] [PubMed] [Google Scholar]
- 151. Verma IM. Nuclear factor (NF)‐kappaB proteins: Therapeutic targets. Ann Rheum Dis 2004;63 (Suppl 2):ii57–ii61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Straus DS, Pascual G, Li M, et al. 15‐deoxy‐delta 12,14‐prostaglandin J2 inhibits multiple steps in the NF‐ kappa B signaling pathway. Proc Natl Acad Sci U S A 2000;97:4844–4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Chung SW, Kang BY, Kim SH, et al. Oxidized low density lipoprotein inhibits interleukin‐12 production in lipopolysaccharide‐activated mouse macrophages via direct interactions between peroxisome proliferator‐activated receptor‐gamma and nuclear factor‐kappa B. J Biol Chem 2000;275:32681–32687. [DOI] [PubMed] [Google Scholar]
- 154. Dowell P, Ishmael JE, Avram D, et al. p300 functions as a coactivator for the peroxisome proliferator‐activated receptor alpha. J Biol Chem 1997;272:33435–33443. [DOI] [PubMed] [Google Scholar]
- 155. Nolte RT, Wisely GB, Westin S, et al. Ligand binding and co‐activator assembly of the peroxisome proliferator‐activated receptor‐gamma. Nature 1998;395:137–143. [DOI] [PubMed] [Google Scholar]
- 156. Delerive P, Gervois P, Fruchart JC, Staels B. Induction of IkappaBalpha expression as a mechanism contributing to the anti‐inflammatory activities of peroxisome proliferator‐activated receptor‐alpha activators. J Biol Chem 2000;275:36703–36707. [DOI] [PubMed] [Google Scholar]
- 157. Moi P, Chan K, Asunis I, Cao A, Kan YW. Isolation of NF‐E2‐related factor 2 (Nrf2), a NF‐E2‐like basic leucine zipper transcriptional activator that binds to the tandem NF‐E2/AP1 repeat of the beta‐globin locus control region. Proc Natl Acad Sci U S A 1994;91:9926–9930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Kobayashi A, Ohta T, Yamamoto M. Unique function of the Nrf2‐Keap1 pathway in the inducible expression of antioxidant and detoxifying enzymes. Methods Enzymol 2004;378:273–286. [DOI] [PubMed] [Google Scholar]
- 159. van Muiswinkel FL, Kuiperij HB. The Nrf2‐ARE Signalling pathway: Promising drug target to combat oxidative stress in neurodegenerative disorders. Curr Drug Targets CNS Neurol Disord 2005;4:267–281. [DOI] [PubMed] [Google Scholar]
- 160. Ishii T, Itoh K, Yamamoto M. Roles of Nrf2 in activation of antioxidant enzyme genes via antioxidant responsive elements. Methods Enzymol 2002;348:182–190. [DOI] [PubMed] [Google Scholar]
- 161. Zhao X, Sun G, Zhang J, et al. Transcription factor Nrf2 protects the brain from damage produced by intracerebral hemorrhage. Stroke 2007;38:3280–3286. [DOI] [PubMed] [Google Scholar]
- 162. Itoh K, Wakabayashi N, Katoh Y, et al. Keap1 regulates both cytoplasmic‐nuclear shuttling and degradation of Nrf2 in response to electrophiles. Genes Cells 2003;8:379–391. [DOI] [PubMed] [Google Scholar]
- 163. Giudice A, Montella M. Activation of the Nrf2‐ARE signaling pathway: A promising strategy in cancer prevention. BioEssays 2006;28:169–181. [DOI] [PubMed] [Google Scholar]
- 164. Dash PK, Zhao J, Orsi SA, Zhang M, Moore AN. Sulforaphane improves cognitive function administered following traumatic brain injury. Neurosci Lett 2009;460:103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Xu HL, Feng YP. Inhibitory effects of chiral 3‐n‐butylphthalide on inflammation following focal ischemic brain injury in rats. Acta Pharmacol Sin 2000;21:433–438. [PubMed] [Google Scholar]
- 166. Shih AY, Li P, Murphy TH. A small‐molecule‐inducible Nrf2‐mediated antioxidant response provides effective prophylaxis against cerebral ischemia in vivo. J Neurosci 2005;25:10321–10335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Leonard MO, Kieran NE, Howell K, et al. Reoxygenation‐specific activation of the antioxidant transcription factor Nrf2 mediates cytoprotective gene expression in ischemia‐reperfusion injury. FASEB J 2006;20:2624–2626. [DOI] [PubMed] [Google Scholar]
- 168. Satoh T, Okamoto SI, Cui J, et al. Activation of the Keap1/Nrf2 pathway for neuroprotection by electrophilic [correction of electrophilic] phase II inducers. Proc Natl Acad Sci U S A 2006;103:768–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Wang J, Fields J, Zhao C, et al. Role of Nrf2 in protection against intracerebral hemorrhage injury in mice. Free Radic Biol Med 2007;43:408–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Ishii T, Itoh K, Ruiz E, et al. Role of Nrf2 in the regulation of CD36 and stress protein expression in murine macrophages: Activation by oxidatively modified LDL and 4‐hydroxynonenal. Circ Res 2004;94:609–616. [DOI] [PubMed] [Google Scholar]
- 171. Kwak MK, Itoh K, Yamamoto M, Sutter TR, Kensler TW. Role of transcription factor Nrf2 in the induction of hepatic phase 2 and antioxidative enzymes in vivo by the cancer chemoprotective agent, 3H‐1, 2‐dimethiole‐3‐thione. Mol Med 2001;7:135–145. [PMC free article] [PubMed] [Google Scholar]
- 172. Girnun GD, Domann FE, Moore SA, Robbins ME. Identification of a functional peroxisome proliferator‐activated receptor response element in the rat catalase promoter. Mol Endocrinol 2002;16:2793–2801. [DOI] [PubMed] [Google Scholar]
- 173. Shih AY, Imbeault S, Barakauskas V, et al. Induction of the Nrf2‐driven antioxidant response confers neuroprotection during mitochondrial stress in vivo. J Biol Chem 2005;280:22925–22936. [DOI] [PubMed] [Google Scholar]
- 174. Cho HY, Gladwell W, Wang X, et al. Nrf2‐regulated PPAR{gamma} expression is critical to protection against acute lung injury in mice. Am J Respir Crit Care Med 2010;182:170–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Park EY, Cho IJ, Kim SG. Transactivation of the PPAR‐responsive enhancer module in chemopreventive glutathione S‐transferase gene by the peroxisome proliferator‐activated receptor‐gamma and retinoid X receptor heterodimer. Cancer Res 2004;64:3701–3713. [DOI] [PubMed] [Google Scholar]
- 176. Bowie A, O'Neill LA. Oxidative stress and nuclear factor‐kappaB activation: A reassessment of the evidence in the light of recent discoveries. Biochem Pharmacol 2000;59:13–23. [DOI] [PubMed] [Google Scholar]
- 177. Whalen MJ, Carlos TM, Dixon CE, et al. Effect of traumatic brain injury in mice deficient in intercellular adhesion molecule‐1: Assessment of histopathologic and functional outcome. J Neurotrauma 1999;16:299–309. [DOI] [PubMed] [Google Scholar]
- 178. Smith SA, Monteith GR, Holman NA, et al. Effects of peroxisome proliferator‐activated receptor gamma ligands ciglitazone and 15‐deoxy‐delta 12,14‐prostaglandin J2 on rat cultured cerebellar granule neuronal viability. J Neurosci Res 2003;72:747–755. [DOI] [PubMed] [Google Scholar]
- 179. Rohn TT, Wong SM, Cotman CW, Cribbs DH. 15‐deoxy‐delta12,14‐prostaglandin J2, a specific ligand for peroxisome proliferator‐activated receptor‐gamma, induces neuronal apoptosis. NeuroReport 2001;12:839–843. [DOI] [PubMed] [Google Scholar]
- 180. Kondo M, Shibata T, Kumagai T, et al. 15‐Deoxy‐Delta(12,14)‐prostaglandin J(2): The endogenous electrophile that induces neuronal apoptosis. Proc Natl Acad Sci U S A 2002;99:7367–7372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Yagami T, Ueda K, Asakura K, et al. Novel binding sites of 15‐deoxy‐Delta(12,14)‐prostaglandin J(2) in plasma membranes from primary rat cortical neurons. Exp Cell Res 2003;291:212–227. [DOI] [PubMed] [Google Scholar]
- 182. Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007;356:2457–2471. [DOI] [PubMed] [Google Scholar]
- 183. Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): A randomised controlled trial. Lancet 2005;366:1279–1289. [DOI] [PubMed] [Google Scholar]
- 184. Betteridge DJ, DeFronzo RA, Chilton RJ. PROactive: Time for a critical appraisal. Eur Heart J 2008;29:969–983. [DOI] [PubMed] [Google Scholar]


