Abstract
Objective
To determine if better cognitive functioning at midlife among more physically fit individuals reflects “neuroprotection,” in which fitness protects against age-related cognitive decline, or “neuroselection,” in which children with higher cognitive functioning select into more active lifestyles.
Methods
Children in the Dunedin Longitudinal Study (N=1,037) completed the Wechsler Intelligence Scales and the Trail-Making, Rey-Delayed-Recall, and Grooved-Pegboard tasks as children and again at midlife (age-38). Adult cardiorespiratory fitness was assessed using a submaximal exercise test to estimate maximum-oxygen-consumption-adjusted-for-body-weight in milliliters/minute/kilogram (VO2max). We tested if more-fit individuals had better cognitive functioning than their less-fit counterparts (which could be consistent with neuroprotection), and if better childhood cognitive functioning predisposed to better adult cardiorespiratory fitness (neuroselection). Finally, we examined possible mechanisms of neuroselection.
Results
Participants with better cardiorespiratory fitness had higher cognitive test scores at midlife. However, fitness-associated advantages in cognitive functioning were present already in childhood. After accounting for childhood-baseline performance on the same cognitive tests, there was no association between cardiorespiratory fitness and midlife cognitive functioning. Socioeconomic and health advantages in childhood, and healthier lifestyles during young adulthood explained most of the association between childhood cognitive functioning and adult cardiorespiratory fitness.
Interpretation
We found no evidence for a neuroprotective effect of cardiorespiratory fitness as of midlife. Instead, children with better cognitive functioning are selecting into healthier lives. Fitness interventions may enhance cognitive functioning. But, observational and experimental studies testing neuroprotective effects of physical fitness should consider confounding by neuroselection.
INTRODUCTION
Higher levels of physical fitness are associated with fewer medical comorbidities, reduced risk for cardiovascular disease, and greater functional capacity.1–4 In addition, there is growing evidence that physical fitness is also associated with better cognitive functioning and lower risk of dementia.5–9 New evidence from a longitudinal studyhas emerged showing a prospective association between physical fitness in young adulthood and later cognitive function in midlife.10 This finding suggested the possibility that young-adult physical activity can have neuroprotective effects that are manifest already by the middle of the life course. The implication is that an active lifestyle during young adulthood could be a prevention strategy to delay or postpone age-related cognitive decline.11
Parallel to research showing that physical fitness predicts better cognitive functioning, longitudinal studies that follow children into adulthood find that better cognitive functioning in childhood predicts better physical and brain health outcomes in older adults.12–16 These findings suggest the possibility that better cognitive functioning in childhood could contribute to higher levels of physical fitness in adulthood. If such “neuroselection” contributes to the correlation between fitness and midlife cognitive function, there is a need to identify mechanisms through which childhood differences in cognitive functioning give rise to fitness disparities later in life.
To advance the science on how physical fitness is related to midlife cognitive functioning, studies are needed that can test neuroprotection and neuroselection hypotheses within a single sample. Data must include measurements of cognitive functioning in midlife, but also in childhood, before adult fitness levels are achieved. Childhood-baseline measurements are necessary to distinguish cognitive benefits of fitness from differences in cognitive functioning that may precede adult fitness attainments.
We examined the relationship between fitness and cognitive functioning in a 4-decade longitudinal study of a single birth cohort. Cohort members completed the same cognitive test battery as children and again when they reached midlife, and also completed cycle ergometry testing to estimate their cardiorespiratory fitness. We tested whether more-fit individuals performed better on a battery of midlife cognitive tests as compared to their less-fit peers (previously interpreted as neuroprotection), and also if children with better cognitive functioning were predisposed to better adult fitness (which we call neuroselection to reflect the non-random patterning of fitness across the cognitive ability distribution). Finally, we examined potential mechanisms that might account for any association between childhood cognition and cardiorespiratory fitness at midlife. We considered two possibilities. The first is that socioeconomic and health advantages might help children develop better cognitive abilities and subsequently, better cardiorespiratory fitness. The second is that children with better cognitive abilities go on to live healthier lives, and that such lifestyle differences mediate the relationship between childhood intelligence and adult fitness.
METHODS
Sample
Participants are members of the Dunedin Multidisciplinary Health and Development Study, a longitudinal investigation of health and behavior in a complete birth cohort. Study members (N=1,037; 91% of eligible births; 52% male) were all individuals born between April 1972 and March 1973 in Dunedin, New Zealand, who were eligible for the longitudinal study based on residence in the province at age 3 and who participated in the first follow-up assessment at age 3. A review of hospital records indicated that the 102 eligible children who did not participate did not differ significantly from the 1,037 participating children in their maternal prenatal complications, their birth weights, their neonatal complications, or the socioeconomic status of their families. The cohort represents the full range of socioeconomic status in the general population of New Zealand’s South Island and is primarily white.17 On adult health, the cohort matches the NZ National Health & Nutrition Survey (e.g., BMI, smoking, GP visits).18 Assessments were carried out at birth and at ages 3, 5, 7, 9, 11, 13, 15, 18, 21, 26, 32, and, most recently, 38 years, when 95% of the 1,007 Study members still alive took part. At each assessment wave, study members are brought to the Dunedin Research Unit for a full day of interviews and examinations. The Otago Ethics Committee approved each phase of the study and informed consent was obtained from all study members.
Measures
Cardiorespiratory Fitness
We estimated maximum oxygen-consumption-adjusted-for-body-weight (in milliliters-per-minute-per-kilogram, “VO2Max”) from measured heart rate in response to a submaximal exercise test on a friction-braked cycle ergometer according standard protocols.19 Details on fitness testing are provided in Table 1.
Table 1.
Description of study measures
| Measure | Description |
|---|---|
| Cardiorespiratory Fitness | Cardiorespiratory fitness was assessed by measuring heart rate in response to a submaximal exercise test on a friction-braked cycle ergometer. Dependent on the extent to which heart rate increased during a 2-min 50 W warm-up, the workload was adjusted to elicit a steady heart-rate in the range 130–170 beats per minute. After a further 6-min constant power output stage, the maximum heart rate was recorded and used to calculate predicted maximum oxygen uptake adjusted for body weight in milliliters per minute per kilogram (VO2max) according to standard protocols.19 The mean (standard deviation) VO2Max was 23.72 (4.94) for women and 34.69 (6.23) for men. Sex-specific quartiles were formed based on performance at age 38 years. Quartile 1 is least fit; quartile 4 is most fit. The upper bounds of quartiles 1–3 were, respectively, 20.17, 23.64, and 26.64 for women and 30.46, 34.68, and 38.63 for men. N=107 Study members were either absent from the phase 38 assessment or did not complete fitness testing (34 declined fitness testing or were unable to complete testing due to illness, injury, disability, pregnancy, or other consideration; 27 completed assessments outside of the unit and fitness testing was not conducted). Of this group, 44 had completed cardiorespiratory fitness testing at the age-32 assessment. Based on the high rank order stability of VO2Max across assessments (Pearson’s r=0.84), we imputed age-38 VO2Max for these Study members by predicting it from their age-32 measurements. The resulting analysis sample consisted of n=944 study members. Effect sizes for VO2Max are reported in terms of sex-specific standard deviation units. |
| Cognitive Functioning | We measured IQ from the individually administered Wechsler Intelligence Scale for Children-Revised (WISC-R; averaged across ages 7, 9, 11, and 13)20 and the Wechsler Adult Intelligence Scale-IV (WAIS-IV),21 both with Mean=100 and Standard Deviation=15. In addition to the WISC-R and WAIS-IV, the Rey Auditory Verbal Learning Test,22 the Trail Making Test,23 and the Grooved Pegboard Test were administered at ages 13 and 38 years to assess, respectively, memory, executive, and motor functioning.22 Scores for cognitive tests were scaled to have mean=100 and standard deviation=15 to match the distribution of IQ scores. Scores for the Grooved Pegboard Test and the Trail Making Test were reversed here so that higher values corresponded to better cognitive performance. |
| Childhood Socioeconomic Status | The socioeconomic statuses of cohort members’ families were measured using a 6-point scale that assessed parents’ occupational statuses, defined based on average income and educational levels derived from the New Zealand Census. The highest occupational status of either parent was averaged across the childhood assessments.24 |
| Childhood Health | We measured cohort members’ childhood health from a panel of biomarkers and clinical ratings taken at assessments spanning birth to age 11 years. Motor development was assessed at ages 3, 5, 7, and 9 using the Bailey Motor Scales (age 3),28 McCarthy Motor Scales29 (age 5) and Basic Motor Ability Test30 (ages 7 and 9).31 Children’s overall health at ages 3, 5, 7, 9, and 11 years was rated by two Unit staff members based on review of birth records and assessment dossiers including clinical assessments and reports of infections, diseases, injuries, hospitalizations, and other health problems collected from children’s mothers during standardized interviews. Ratings were made on a five-point scale (inter-rater agreement=0.85). Body mass index was calculated from height and weight measurements taken at ages 5, 7, 9, and 11 years. In addition, tricep and subscapular skinfold thicknesses were measured at ages 7 and 9 years by trained anthropometrists.25 (For calculation of the overall measure, tricep and subscapular skinfold thicknesses were averaged to create a single score.) Systolic and diastolic blood pressure were measured at ages 7, 9, and 11 years using a London School of Hygiene and Tropical Medicine blind mercury sphygmomanometer (Cinetronics Ltd., Mildenhall, United Kingdom).26 Fixed expiratory volume in one second (FEV1) and the ratio of FEV1 to forced vital capacity (FVC) were measured at ages 9 and 11 using a Godart water spirometer.27 To calculate the childhood health measure, assessments were standardized to have mean=0 SD=1 within age and sex specific groups. Cross-age scores for each measure were then computed by averaging standardized scores across measurement ages. Reliabilities for measurements are, for girls/boys Motor Ability 0.79/0.73; Clinician Health Rating 0.66/0.68; BMI 0.92/0.93; Tricep Skinfold Thickness 0.85/0.75; Subscapular Skinfold Thickness 0.90/0.85; Systolic Blood Pressure 0.81/0.84; Diastolic Blood Pressure 0.57/0.69; FEV1 0.92/0.96; FEV1/FVC 0.84/0.85. The final childhood health score was calculated by taking the natural log of the average score across all measures, resulting in a normally distributed childhood health index. |
| Adult Leisure-time Physical Activity | We measured Study members’ adulthood leisure-time physical activity level from data collected during structured interviews at age-32 and -38 assessments. Trained interviewers guided Study members through reporting the different types of physically demanding activities they engaged in during an average week and an average weekend. Study members then indicated number of minutes spent doing each activity at a moderate or more strenuous level of difficulty. Time spent on each activity was converted to metabolic equivalent (MET) units.52 Moderate intensity activity was given a weight of 4; hard activity was given a weight of 6; and very hard activity was given a weight of 10. We summed weekday and weekend METs from moderate or more strenuous leisure activities to calculate physical activity levels at ages 32 and 38 years. METs per week were averaged across the two assessments to calculate adulthood physical activity. Physical activity was distributed as follows: 13% of the cohort (n=123) were sedentary (i.e. they engaged in 0 hours of moderate or more strenuous leisure activity per week at the age 32 and age 38 assessments); 27% (n=266) were non-sedentary, but did not achieve the 500 METs/week minimum recommended dosage of physical activity;53 26% (n=256) achieved 500–1,000 METs/week; and 34% (n=335) exceeded 1,000 METs/week. Because METs/week exhibited a strong right skew, values were log transformed for analyses. |
| Obesity | We measured obesity from anthropometric data at ages 15, 18, 21, 26, 32, and 38 years. Obesity was defined at age 15 as body-mass index exceeding the 90th percentile of the sex-specific US Centers for Disease Control and Prevention reference distribution and thereafter as body-mass index of 30 or greater.25 Between ages 15 and 38 years, 27% of the cohort met criteria for obesity at one or more assessments (n=276). This prevalence is in line with the general New Zealand population (in the most recent Ministry of Health report, obesity prevalence was 28% for New Zealanders 15 years of age or older).54 We calculated the cumulative number of assessments at which a cohort member had been obese during the 25-year interval between IQ assessments, hereafter “life-course cumulative obesity.” |
| Educational Attainment as of Age 38 Years | Education level was measured on a five-point scale relevant to the New Zealand educational system: 1 = no secondary school qualifications (14.7%), 2 = school certificate (14.9%), 3 = high school graduate or equivalent (14.2%), 4 = postsecondary diploma or certificate (27.3%), 5 = bachelor’s degree or higher (28.8%).33 |
| Cigarette Smoking | We measured smoking history as “pack-years,” the number of cigarettes smoked per day divided by 20 and multiplied by the number of years smoked at that rate through 38 years of age.32 |
Cognitive Testing
The Dunedin Study conducted cognitive testing in childhood (when Study members were aged 7, 9, 11, and 13 years, averaged to produce childhood cognitive test performance measures) and at the midlife follow-up at age 38 years. We focused our analysis on the intelligence quotient (IQ), a highly reliable measure of general intellectual functioning that captures overall ability across a variety of cognitive functions.20,21 We also examined performance on IQ subscales and performance on measures testing memory, executive functioning, and motor functioning.22,23 The Study conducted a parallel battery of cognitive testing in childhood and adulthood (see Table 1).
Additional Measures
Measurements of childhood socioeconomic status, childhood health (a composite including repeated measures of adiposity, blood pressure, lung function, motor function, and diseases and injuries taken when Study members were aged 3–11 years), adult physical activity, obesity, smoking, and educational attainment are described in Table 1.24–33
Analysis
Analyses included n=944 cohort members with cardiorespiratory fitness data. As compared to participants at age 38, Study members who did not participate (including 30 who were deceased) had scored 6.6 IQ points lower on childhood IQ (p<0.001).
To test for a cross-sectional association that could be consistent with a neuroprotective effect of cardiorespiratory fitness, we used linear regression to test whether individuals with good cardiorespiratory fitness performed better on midlife cognitive testing as compared to peers with poor cardiorespiratory fitness.
To test “neuroselection” of children with better cognitive function into healthier lives, we used linear regression to test whether children with better cognitive functioning grew up to have better cardiorespiratory fitness as compared to peers with poorer cognitive functioning.
To test neuroprotection effects after accounting for any neuroselection into better fitness, we regressed the adult cognitive test score on adult cardiorespiratory fitness with adjustment for the childhood score on the same cognitive test. If the effect of fitness on adult cognitive test performance were reduced and no longer statistically significant after adjusting for childhood cognitive test performance, it would suggest that fitness-associated differences in cognitive function were already present from childhood.
To test whether neuroselection effects could be explained by differences in children’s lives leading up to childhood cognitive testing, we re-estimated regression models testing neuroselection, this time adding childhood socioeconomic status and childhood health measures as antecedent covariates. If covariate adjustment attenuated the neuroselection effect, it would suggest that neuroselection effects were attributable to these childhood factors.
To test whether neuroselection effects came about through differences in the lives Study members lived during the 25-year interval between childhood cognitive testing and adult fitness assessment, we conducted mediation analyses. For each potential mediator (physical activity, obesity, smoking, educational attainment), we tested associations between childhood IQ and the mediator; we tested associations between the mediator and adult fitness; and we tested the association between childhood IQ and adult fitness, including the mediator as a covariate. We used the system of equations described by Baron and Kenny34 and the methods described by Preacher et al.35,36 to calculate total, direct, and indirect effects, and to estimate the proportion of the neuroselection effect mediated by each of the mediators.
We analyzed cardiorespiratory fitness in population-defined quartiles to facilitate comparison with the analysis conducted by Zhu and colleagues.10 We also analyzed cardiorespiratory fitness as a continuous Z-score (transformed to have mean=0, SD=1). Fitness quartiles and Z-scores were calculated separately for men and women. We used linear regression models to analyze continuous dependent variables (e.g. cognitive test performance). We used Poisson regression models to estimate relative risks (RRs) for dichotomous dependent variables (e.g. being in the top fitness quartile). We used negative binomial regression models to estimate incident rate ratios (IRRs) for count-dependent variables (lifetime cumulative obesity).
Effect sizes for cardiorespiratory fitness are reported in terms of SD units. Effect sizes for cognitive tests are reported in IQ points (mean=100, SD=15). In analyses where childhood IQ is the predictor, effect sizes are reported for a 15-point (1 SD) change in IQ. For analyses of other study variables, effect-sizes are reported in terms of relative risks or standardized coefficients (equivalent to Pearson’s r). Because the sample is a representative birth cohort, it forms its own norms for the purpose of estimating effect sizes. Regression models were adjusted for sex to account for differences in cardiorespiratory fitness. Analyses were conducted using Stata 13.0.
RESULTS
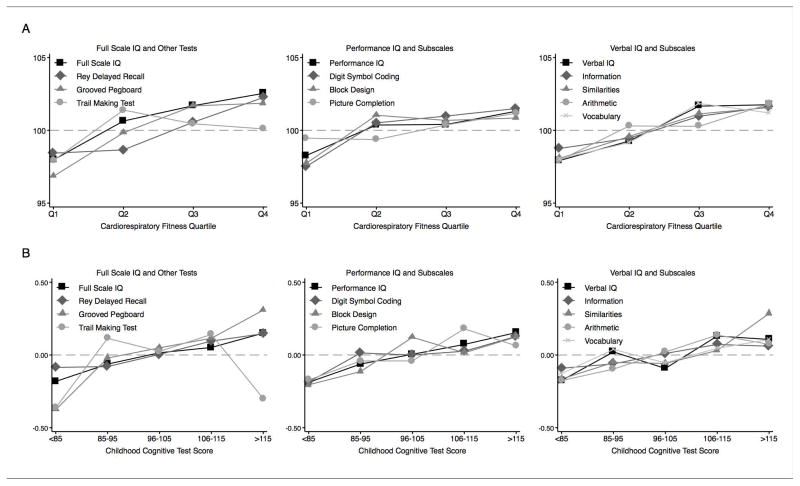
Cross sectional Association of Cardiorespiratory Fitness with Cognitive Functioning in Adulthood. Dunedin Study members with better cardiorespiratory fitness scored higher on cognitive tests at age 38 years. Study members in the highest fitness quartile had Full-Scale IQ scores 4.57 points higher as compared to Study members in the lowest fitness quartile (95% CI [1.88–7.27], p=0.001). Across the cardiorespiratory fitness distribution, each standard deviation increase in fitness predicted a 1.31-point increase in Full-Scale IQ score (SE 0.50, p=0.009). Differences of similar magnitude were observed for performance and verbal intelligence subscales and for the Rey Delayed Recall task and the Grooved Pegboard task (Figure 1 Panel A, Table 2 Panel A).
Figure 1. Association of cardiorespiratory fitness with midlife cognitive functioning (Panel A) and association of childhood cognitive functioning with midlife cardiorespiratory fitness (Panel B).
Data are plotted for cardiorespiratory fitness quartiles and for categories of childhood cognitive functioning for illustrative purposes. Linear associations with continuous variables are reported in Table 2.
Table 2.
Tests of Association Between Physical Fitness and Cognitive Function.
| Panel A. Association of Physical Fitness with Midlife Cognitive Function
| ||||||
|---|---|---|---|---|---|---|
| Predictor = Physical Fitness | ||||||
| Outcome = Midlife Cognitive Function |
|
|
||||
| Linear Association | Comparison of Top & Bottom Fitness Quartiles | |||||
| b | SE | p-value | b | SE | p-value | |
|
|
|
|||||
| Cognitive Test | ||||||
| Full Scale IQ | 1.31 | (0.50) | 0.009 | 4.57 | (1.37) | 0.001 |
| Performance IQ | 0.86 | (0.51) | 0.092 | 3.05 | (1.42) | 0.032 |
| Digit Symbol Coding | 1.11 | (0.48) | 0.020 | 3.94 | (1.32) | 0.003 |
| Block Design | 0.97 | (0.52) | 0.061 | 3.13 | (1.40) | 0.026 |
| Picture Completion | 0.59 | (0.51) | 0.251 | 1.73 | (1.38) | 0.212 |
| Verbal IQ | 1.12 | (0.48) | 0.019 | 3.85 | (1.33) | 0.004 |
| Information | 0.71 | (0.48) | 0.143 | 2.91 | (1.33) | 0.028 |
| Similarities | 1.07 | (0.51) | 0.034 | 3.51 | (1.41) | 0.013 |
| Arithmetic | 1.12 | (0.49) | 0.023 | 3.90 | (1.35) | 0.004 |
| Vocabulary | 1.02 | (0.47) | 0.031 | 3.16 | (1.31) | 0.016 |
| Rey Delayed Recall | 1.29 | (0.48) | 0.007 | 3.79 | (1.34) | 0.005 |
| Grooved Pegboard | 1.81 | (0.58) | 0.002 | 5.02 | (1.43) | 0.000 |
| Trail Making Test | 0.36 | (0.51) | 0.475 | 2.14 | (1.45) | 0.138 |
| Panel B. Association of Childhood Cognitive Function with Adult Physical Fitness
| ||||||
|---|---|---|---|---|---|---|
| Predictor = Childhood Cognitive Function | ||||||
| Outcome = Physical Fitness |
|
|
||||
| Linear Association | Prob. of Membership in Top Fitness Quartile | |||||
| b | SE | p-value | RR | 95% CI | p-value | |
|
|
|
|||||
| Cognitive Test | ||||||
| Full Scale IQ | 0.12 | (0.04) | 0.002 | 1.20 | [1.07, 1.35] | 0.003 |
| Performance IQ | 0.10 | (0.04) | 0.007 | 1.16 | [1.03, 1.29] | 0.013 |
| Digit Symbol Coding | 0.08 | (0.04) | 0.034 | 1.13 | [1.01, 1.27] | 0.029 |
| Block Design | 0.09 | (0.04) | 0.011 | 1.12 | [1.00, 1.26] | 0.042 |
| Picture Completion | 0.07 | (0.04) | 0.047 | 1.12 | [1.00, 1.26] | 0.043 |
| Verbal IQ | 0.11 | (0.04) | 0.003 | 1.20 | [1.07, 1.34] | 0.002 |
| Information | 0.06 | (0.03) | 0.076 | 1.15 | [1.03, 1.28] | 0.016 |
| Similarities | 0.14 | (0.03) | 0.000 | 1.24 | [1.11, 1.40] | 0.000 |
| Arithmetic | 0.10 | (0.04) | 0.008 | 1.16 | [1.04, 1.30] | 0.009 |
| Vocabulary | 0.06 | (0.03) | 0.065 | 1.13 | [1.02, 1.25] | 0.024 |
| Rey Delayed Recall | 0.11 | (0.04) | 0.004 | 1.19 | [1.04, 1.35] | 0.009 |
| Grooved Pegboard | 0.19 | (0.04) | 0.000 | 1.34 | [1.16, 1.54] | 0.000 |
| Trail Making Test | 0.09 | (0.04) | 0.042 | 1.08 | [0.95, 1.22] | 0.230 |
Prospective Association of Cognitive Functioning in Childhood with Adult Cardiorespiratory Fitness. Consistent with the neuroselection hypothesis that individuals with better cognitive functioning develop and maintain better cardiorespiratory fitness, Dunedin Study members who scored higher on cognitive tests in childhood had better cardiorespiratory fitness 25 years later (Figure 1 Panel B, Table 2 Panel B). Each standard deviation increase in full-scale IQ score measured in childhood predicted a 0.12 standard deviation increase in cardiorespiratory fitness at follow-up (SE 0.04, p=0.002) and a 20% increase in a Study member’s likelihood of being in the top cardiorespiratory fitness quartile (RR=1.20, 95% CI [1.07–1.35], p=0.003). Effect sizes were similar for performance and verbal intelligence subscales and for the Rey Delayed Recall task and Grooved Pegboard task.
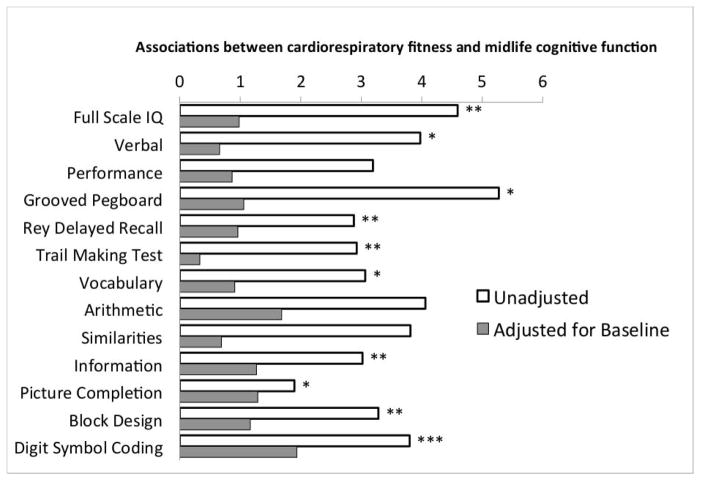
Mechanisms Mediating the Prospective Association of Cognitive Functioning in Childhood with Adult Cardiorespiratory Fitness. When associations between adult cognitive test performance and cardiorespiratory fitness were adjusted for Study members’ performance on the same cognitive tests when they were children, effect-sizes were reduced by as much as 90% and were no longer statistically significant (Figure 2, Table 3). This result indicates that whatever fitness differences developed during the interval between baseline cognitive testing in childhood and follow-up cognitive testing at age 38 years did not contribute to better cognitive functioning at age 38 years. Whatever cognitive advantage was associated with better adult cardiorespiratory fitness was already present since childhood. This evidence supports neuroselection, but not neuroprotection.
Figure 2. Cardiorespiratory fitness is not associated with midlife cognitive test performance after adjustment for childhood-baseline performance on the same cognitive test.
The Figure shows differences in midlife cognitive test performance between individuals in the top and bottom quartiles of cardiorespiratory fitness. The Figure shows unadjusted effect sizes for associations between cardiorespiratory fitness and midlife cognitive functioning (white bars) and effect sizes after adjusting for childhood baseline performance on the same cognitive test (gray bars). Stars denote statistical significance (*** p<0.001, ** p<0.01, * p<0.05).
Table 3.
Association of cardiorespiratory fitness with midlife cognitive test performance, before and after adjustment for childhood baseline performance on the same cognitive test.
The table presents linear associations between continuously distributed fitness and cognitive function (left side) and also, for illustrative purposes, comparisons of cognitive function in the top and bottom fitness quartiles (right side).
| Linear Associations
|
Comparison of Top and Bottom Fitness Quartiles
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted Effect Size | Effect Size Adjusted for Childhood Baseline | Unadjusted Effect Size | Effect Size Adjusted for Childhood Baseline | |||||||||
| b | SE | p-value | b | SE | p-value | b | SE | p-value | b | SE | p-value | |
|
|
|
|
|
|||||||||
| Cognitive Test | ||||||||||||
| Full Scale IQ | 1.31 | (0.50) | 0.009 | 0.11 | (0.29) | 0.717 | 4.57 | (1.37) | 0.001 | 0.98 | (0.84) | 0.246 |
| Performance IQ | 0.86 | (0.51) | 0.092 | 0.07 | (0.39) | 0.852 | 3.05 | (1.42) | 0.032 | 0.87 | (1.09) | 0.426 |
| Digit Symbol Coding | 1.11 | (0.48) | 0.020 | 0.48 | (0.38) | 0.204 | 3.94 | (1.32) | 0.003 | 1.94 | (1.06) | 0.069 |
| Block Design | 0.97 | (0.52) | 0.061 | 0.24 | (0.38) | 0.521 | 3.13 | (1.40) | 0.026 | 1.17 | (1.04) | 0.263 |
| Picture Completion | 0.59 | (0.51) | 0.251 | 0.35 | (0.49) | 0.471 | 1.73 | (1.38) | 0.212 | 1.29 | (1.32) | 0.330 |
| Verbal IQ | 1.12 | (0.48) | 0.019 | 0.08 | (0.29) | 0.781 | 3.85 | (1.33) | 0.004 | 0.66 | (0.87) | 0.449 |
| Information | 0.71 | (0.48) | 0.143 | 0.16 | (0.34) | 0.639 | 2.91 | (1.33) | 0.028 | 1.27 | (0.96) | 0.187 |
| Similarities | 1.07 | (0.51) | 0.034 | 0.11 | (0.42) | 0.801 | 3.51 | (1.41) | 0.013 | 0.69 | (1.21) | 0.569 |
| Arithmetic | 1.12 | (0.49) | 0.023 | 0.37 | (0.36) | 0.314 | 3.90 | (1.35) | 0.004 | 1.68 | (1.06) | 0.112 |
| Vocabulary | 1.02 | (0.47) | 0.031 | 0.42 | (0.34) | 0.220 | 3.16 | (1.31) | 0.016 | 0.91 | (0.98) | 0.356 |
| Rey Delayed Recall | 1.29 | (0.48) | 0.007 | 0.21 | (0.48) | 0.669 | 3.79 | (1.34) | 0.005 | 0.96 | (1.38) | 0.486 |
| Grooved Pegboard | 1.81 | (0.58) | 0.002 | 0.26 | (0.57) | 0.654 | 5.02 | (1.43) | 0.000 | 1.06 | (1.53) | 0.490 |
| Trail Making Test | 0.36 | (0.51) | 0.475 | −0.51 | (0.51) | 0.316 | 2.14 | (1.45) | 0.138 | 0.33 | (1.54) | 0.830 |
What might account for this neuroselection? One hypothesis is that children’s early socioeconomic status and health influence their cognitive development and, later, their cardiorespiratory fitness. In the Dunedin Cohort, children born into higher socioeconomic status households scored higher on cognitive tests in childhood and had better cardiorespiratory fitness in adulthood as compared to children born into lower socioeconomic status households (for childhood IQ, r=0.38, p<0.001; for adult cardiorespiratory fitness, r=0.10, p=0.001). Similarly, children in better physical health scored higher on cognitive tests in childhood and had better cardiorespiratory fitness twenty-five years later as compared to less healthy children (for childhood IQ, r=0.20, p<0.001; for cardiorespiratory fitness, r=0.21, p<0.001). Adjusting for these characteristics attenuated the association between childhood cognitive ability and adult cardiorespiratory fitness, but the association remained statistically significant (with adjustment for socioeconomic disadvantage, B=0.11 SE=0.04, p=0.011; with adjustment for childhood health, B=0.09 SE=0.04, p=0.014). With adjustment for childhood socioeconomic position and health together, the p-value no longer met the alpha=0.05 threshold for statistical significance (B=0.08 SE=0.04, p=0.054).
A second explanation for why children with higher IQs go on to have better cardiorespiratory fitness as adults is that they select into healthier lives. A proximate cause of cardiorespiratory fitness is physical activity. Children with higher IQ scores were less likely to be sedentary adults (Sedentary RR=0.74 [0.63–0.86], p<0.001). Among those who were non-sedentary, higher childhood IQ predicted increased adult leisure activity (r=0.09, p=0.006). In turn, as expected, a more active lifestyle was associated with better cardiorespiratory fitness (r=0.16, p<0.001). But mediation analysis indicated that adult physical activity accounted for only 16% of the association between childhood IQ and adult cardiorespiratory fitness (p-value for indirect effect=0.005). After accounting for differences in physical activity, the childhood-IQ–adult cardiorespiratory fitness association was only modestly attenuated (B=0.11 SE=0.04, p=0.006).
Physical activity during a single decade of adult life may provide an insufficient summary of healthy lifestyle processes that support the development and maintenance of cardiorespiratory fitness. We therefore considered lifetime obesity as a surrogate marker for healthy lifestyle. We analyzed a cumulative obesity measure that captured the onset and persistence of obesity across the twenty-five year interval between the completion of childhood IQ testing at age 13 years and the assessment of cardiorespiratory fitness at age 38 years. Children with higher IQ scores were less likely to become obese during follow-up (RR=0.83 [0.75–0.91], p<0.001) and they were obese at fewer assessments (IRR=0.76 [0.66–0.86], p<0.001). In turn, as expected, study members with less lifetime obesity had better cardiorespiratory fitness (each additional assessment at which a study member remained lean was associated with a 0.43 SD (SE=0.02) increase in their cardiorespiratory fitness, p<0.001. Mediation analysis indicated that 47% of the association between childhood IQ and adult cardiorespiratory fitness was accounted for by lifetime obesity (p-value for indirect effect=0.005). After accounting for differences in obesity histories, the childhood-IQ–adult fitness association was reduced by nearly half and was no longer statistically significant (B=0.05 SE=0.03, p=0.142). Together, physical activity and obesity accounted for a total of 61% of the childhood IQ-adult fitness association.
A history of cigarette smoking and truncated education may also influence physical health. When we added measures of smoking history and educational attainment to the mediation model, the full set of mediators accounted for 74% of the childhood IQ-adult fitness association (Supplemental Table 1).
DISCUSSION
We analyzed data from a 4-decade longitudinal study of a birth cohort to test neuroprotective effects of fitness. More physically fit adults exhibited better cognitive performance at midlife. However, this advantage was almost completely explained by differences in cognitive function already present in childhood. We then examined how children with better cognitive function came to have better fitness twenty-five years later. Dunedin Study children with better cognitive function grew up in higher socioeconomic status households and enjoyed better health before childhood-baseline cognitive testing. These early-life differences accounted for a portion of the “neuroselection” effect. It is possible that other unmeasured characteristics of the children would further account for the better fitness we observed among children with higher IQ scores. In addition, we found that neuroselection into better adult fitness appeared to reflect differences in the lives children led between baseline IQ testing and follow-up twenty-five years later, particularly their lifetime histories of obesity.
We acknowledge limitations. First, the Dunedin cohort comes from a single nation and is predominantly white. The cohort represents the full spectrum of socioeconomic status and health, and has a track record of replication in American, British, and international cohorts. Nonetheless, replication in diverse cohorts is needed. Second, cardiorespiratory fitness was not assessed in childhood. As a result, we could not account for the possibility that poor cardiorespiratory fitness in childhood was a cause of low childhood IQ. However, to address this limitation, our analysis did take into account differences in children’s blood pressure, lung function, adiposity, and their experience of childhood illnesses. Third, cognitive testing data were right censored; the most-recent follow-up was completed at age 38 years. It remains possible that cardiorespiratory fitness will have neuroprotective properties later in the life course. Fourth, we assessed cardiorespiratory fitness as heart rate response to a submaximal exercise test rather than with direct measurement of VO2max.37 We note that epidemiologic studies, including the original investigation reporting possible neuroprotective effects of young adult fitness, use indirect methods to evaluate cardiorespiratory fitness.10,38,39 We do not anticipate that use of directly measured VO2max would substantively alter results because correlations between indirect and direct measures of VO2max are approximately r=0.9 in studies comparing the two.19,40,41
The findings have implications for research, policy, and intervention. There is now evidence from randomized trials that increasing physical activity in late life may improve cognitive function.4,6,42 This evidence has spurred observational research and intervention studies to examine the possibility that active lifestyle promotion for young adults could serve as an early prevention strategy to promote healthy cognitive aging population-wide.43–45 Physical fitness at any age has important physical health benefits. But our data suggest that, at least where young-adult fitness is concerned, protecting cognitive function through midlife is not one of them. This does not rule out the possibility that fitness interventions could improve cognitive functioning. But our data indicate that future studies examining cognitive benefits of fitness at any stage in life must take account of the neuroselection phenomenon, in which children with the best cognitive function select into lives that promote the development and maintenance of cardiorespiratory fitness. Neuroselection is an important consideration even in randomized trials, where cognitive ability may influence who enrolls, who adheres to the physical activity program, and who drops out.
At the level of policy, neuroselection findings lend additional support to efforts to promote early-childhood cognitive health as a means of improving outcomes in adulthood.46 Reducing toxic exposures and increasing nourishing ones (both chemical and psychological) are proven means of enhancing cognitive development.47–51 As our data show, the dividends of healthy cognitive development may extend to adult fitness through the fourth decade of life—a strong indicator of reduced age-related morbidity to follow.
Neuroselection findings also have implications for interventions to promote physical fitness. Obesity prevention is already a public health priority. And some progress is being made. What our study adds is insight into how current population-level prevention strategies promoting active lifestyle and healthy diet may benefit some population segments more than others. We found that children with higher IQ scores grew up to be adults who were less sedentary and less obese, and in turn, had better cardiorespiratory fitness. It is possible that individuals with strong cognitive resources are responding to public-health messages, whereas their peers with fewer cognitive resources may be struggling to take up behavior-change recommendations. Recommendations to eat healthy and exercise are easy to understand, but require cognitive effort to implement. Moreover, eating healthy food and getting more leisure activity are not always cheap or easy. Given that unhealthy calories and sedentary lifestyle are cheap and easy, public health messaging and incentive programs to promote healthy diet and exercise should be paired with resources and services to help at-risk individuals accomplish these objectives.
Supplementary Material
Acknowledgments
We thank the Dunedin Study members, their families, unit research staff, and the Dunedin Study founder Phil Silva. The Dunedin Multidisciplinary Health and Development Research Unit is supported by the New Zealand Health Research Council. This research received support from US National Institute on Aging (NIA) grant AG032282 and UK Medical Research Council grant MR/K00381X. Additional support was provided by the Jacobs Foundation. DWB received support from the NIA through a postdoctoral fellowship T32 AG000029 and P30 AG028716-08. SI was supported by a Rothschild Fellowship from the Yad Hanadiv Rothschild Foundation.
Footnotes
The authors report no conflict of interest.
References
- 1.Morey MC, Pieper CF, Crowley GM, Rnc-Bsn, Sullivan RJ, Puglisi CM. Exercise Adherence and 10-Year Mortality in Chronically Ill Older Adults. J Am Geriatr Soc. 2002 Dec 1;50(12):1929–1933. doi: 10.1046/j.1532-5415.2002.50602.x. [DOI] [PubMed] [Google Scholar]
- 2.Demark-Wahnefried W, Morey MC, Sloane R, Snyder DC, Cohen HJ. Promoting healthy lifestyles in older cancer survivors to improve health and preserve function. J Am Geriatr Soc. 2009 Nov;57(Suppl 2):S262–S264. doi: 10.1111/j.1532-5415.2009.02507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peterson MJ, Giuliani C, Morey MC, et al. Physical activity as a preventative factor for frailty: the health, aging, and body composition study. J Gerontol A Biol Sci Med Sci. 2009 Jan;64(1):61–68. doi: 10.1093/gerona/gln001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: The life study randomized clinical trial. JAMA [Internet] 2014 May 27; doi: 10.1001/jama.2014.5616. [cited 2014]; Available from: http://dx.doi.org/10.1001/jama.2014.5616. [DOI] [PMC free article] [PubMed]
- 5.Kelly ME, Loughrey D, Lawlor BA, Robertson IH, Walsh C, Brennan S. The impact of exercise on the cognitive functioning of healthy older adults: A systematic review and meta-analysis. Ageing Res Rev. 2014 Jul;16:12–31. doi: 10.1016/j.arr.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Smith PJ, Blumenthal JA, Hoffman BM, et al. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med. 2010 Apr;72(3):239–252. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angevaren M, Aufdemkampe G, Verhaar HJJ, Aleman A, Vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev. 2008;(3):CD005381. doi: 10.1002/14651858.CD005381.pub3. [DOI] [PubMed] [Google Scholar]
- 8.Bherer L, Erickson KI, Liu-Ambrose T. A review of the effects of physical activity and exercise on cognitive and brain functions in older adults. J Aging Res. 2013;2013:657508. doi: 10.1155/2013/657508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Defina LF, Willis BL, Radford NB, et al. The association between midlife cardiorespiratory fitness levels and later-life dementia: a cohort study. Ann Intern Med. 2013 Feb 5;158(3):162–168. doi: 10.7326/0003-4819-158-3-201302050-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu N, Jacobs DR, Jr, Schreiner PJ, et al. Cardiorespiratory fitness and cognitive function in middle age: The CARDIA Study. Neurology. 2014 Apr 15;82(15):1339–1346. doi: 10.1212/WNL.0000000000000310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reynolds G. Early fitness can improve the middle-age brain [Internet] N Y Times Well. 2014 [cited 2014]. Available from: http://well.blogs.nytimes.com/2014/05/07/a-fit-body-at-25-a-fit-brain-at-50/
- 12.Gottfredson LS, Deary IJ. Intelligence predicts health and longevity, but why? Curr Dir Psychol Sci. 2004 Feb;13:1–4. [Google Scholar]
- 13.Deary IJ, Weiss A, Batty GD. Intelligence and Personality as Predictors of Illness and Death: How Researchers in Differential Psychology and Chronic Disease Epidemiology Are Collaborating to Understand and Address Health Inequalities. Psychol Sci Public Interest. 2010;11(2):53–79. doi: 10.1177/1529100610387081. [DOI] [PubMed] [Google Scholar]
- 14.Gow AJ, Johnson W, Pattie A, Whiteman MC, Starr J, Deary IJ. Mental Ability in Childhood and Cognitive Aging. Gerontology. 2008;54(3):177–186. doi: 10.1159/000118098. [DOI] [PubMed] [Google Scholar]
- 15.Deary IJ, Bastin ME, Pattie A, et al. White matter integrity and cognition in childhood and old age. Neurology. 2006 Feb 28;66(4):505–512. doi: 10.1212/01.wnl.0000199954.81900.e2. [DOI] [PubMed] [Google Scholar]
- 16.Kanazawa S. Childhood intelligence and adult obesity. Obes Silver Spring Md. 2013 Mar;21(3):434–440. doi: 10.1002/oby.20018. [DOI] [PubMed] [Google Scholar]
- 17.Moffitt TE, Caspi A, Rutter M, Silva PA. Sex differences in antisocial behavior: Conduct disorder, delinquency, and violence in the Dunedin longitudinal study. Cambridge: Cambridge University Press; 2001. [Google Scholar]
- 18.Poulton R, Hancox R, Milne B, Baxter J, Scott K, Wilson N. The Dunedin Multidisciplinary Health and Development Study: are its findings consistent with the overall New Zealand population? N Z Med J. 2006;119:U2002. [PubMed] [Google Scholar]
- 19.Cullinane EM, Siconolfi S, Carleton RA, Thompson PD. Modification of the Astrand-Rhyming sub-maximal bicycle test for estimating VO2max of inactive men and women. Med Sci Sports Exerc. 1988 Jun;20(3):317–318. [PubMed] [Google Scholar]
- 20.Wechsler D. Wechsler Intelligence Scale for Children. San Antonio, TX: Harcourt Assessment; 2003. 4th (UK Version) [Google Scholar]
- 21.Wechsler D. Wechsler Adult Intelligence Scale. 4. San Antonio, TX: Pearson Assessment; 2008. [Google Scholar]
- 22.Lezak DM, Howieson DB, Loring DW, Hannay HJ, Fischer JS. Neuropsychological Assessment. 4. Oxford University Press; 2004. [Google Scholar]
- 23.Army Individual Battery: Manual and Directions for Scoring. Washington, DC: War Department, Adjutant General’s Office; 1944. [Google Scholar]
- 24.Poulton R, Caspi A, Milne BJ, et al. Association between children’s experience of socioeconomic disadvantage and adult health: a life-course study. Lancet. 2002 Nov;360(9346):1640–1645. doi: 10.1016/S0140-6736(02)11602-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Belsky DW, Moffitt TE, Houts R, et al. Polygenic Risk, Rapid Childhood Growth, and the Development of Obesity: Evidence from a 4-Decade Longitudinal Study. Arch Pediatr Adolesc Med. 2012;166(6):515–521. doi: 10.1001/archpediatrics.2012.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams S, Poulton R. Birth Size, Growth, and Blood Pressure between the Ages of 7 and 26 Years: Failure to Support the Fetal Origins Hypothesis. Am J Epidemiol. 2002 May 1;155(9):849–852. doi: 10.1093/aje/155.9.849. [DOI] [PubMed] [Google Scholar]
- 27.Sears MR, Greene JM, Willan AR, et al. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003 Oct;349(15):1414–1422. doi: 10.1056/NEJMoa022363. [DOI] [PubMed] [Google Scholar]
- 28.Bayley N. The Bayley Scale of Infant Development. New York, NY: Psychological Corp; 1969. [Google Scholar]
- 29.McCarthy D. Scales of Children’s Abilities. New York, NY: Psychological Corp; 1972. [Google Scholar]
- 30.Arnheim D, Sinclair SW. The Clumsy Child. St Louis Mo: VC Mosby Co; 1974. [Google Scholar]
- 31.Cannon M, Caspi A, Moffitt TE, et al. Evidence for early-childhood, pan-developmental impairment specific to schizophreniform disorder - Results from a longitudinal birth cohort. Arch Gen Psychiatry. 2002 May;59:449–456. doi: 10.1001/archpsyc.59.5.449. [DOI] [PubMed] [Google Scholar]
- 32.Belsky DW, Moffitt TE, Baker TB, et al. Polygenic risk and the developmental progression to heavy, persistent smoking and nicotine dependence: Evidence from a 4-decade longitudinal study. JAMA Psychiatry. 2013 Mar;70(5):534–542. doi: 10.1001/jamapsychiatry.2013.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Israel S, Caspi A, Belsky DW, et al. Credit scores, cardiovascular disease risk, and human capital. Proc Natl Acad Sci. 2014 Nov;17:201409794. doi: 10.1073/pnas.1409794111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baron RM, Kenny DA. The Moderator Mediator Variable Distinction in Social Psychological-Research - Conceptual, Strategic, and Statistical Considerations. J Pers Soc Psychol. 1986 Dec;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 35.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008 Aug;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- 36.Preacher KJ, Kelley K. Effect size measures for mediation models: Quantitative strategies for communicating indirect effects. Psychol Methods. 2011 Jun;16:93–115. doi: 10.1037/a0022658. [DOI] [PubMed] [Google Scholar]
- 37.Barnes DE, Yaffe K, Satariano WA, Tager IB. A longitudinal study of cardiorespiratory fitness and cognitive function in healthy older adults. J Am Geriatr Soc. 2003 Apr;51(4):459–465. doi: 10.1046/j.1532-5415.2003.51153.x. [DOI] [PubMed] [Google Scholar]
- 38.Blair SN, Kampert JB, Kohl HW, et al. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA J Am Med Assoc. 1996 Jul 17;276(3):205–210. [PubMed] [Google Scholar]
- 39.Lee D, Sui X, Artero EG, et al. Long-term effects of changes in cardiorespiratory fitness and body mass index on all-cause and cardiovascular disease mortality in men: the Aerobics Center Longitudinal Study. Circulation. 2011 Dec 6;124(23):2483–2490. doi: 10.1161/CIRCULATIONAHA.111.038422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uth N, Sørensen H, Overgaard K, Pedersen PK. Estimation of VO2max from the ratio between HRmax and HRrest--the Heart Rate Ratio Method. Eur J Appl Physiol. 2004 Jan;91(1):111–115. doi: 10.1007/s00421-003-0988-y. [DOI] [PubMed] [Google Scholar]
- 41.Siconolfi SF, Garber CE, Lasater TM, Carleton RA. A simple, valid step test for estimating maximal oxygen uptake in epidemiologic studies. Am J Epidemiol. 1985 Mar;121(3):382–390. doi: 10.1093/oxfordjournals.aje.a114010. [DOI] [PubMed] [Google Scholar]
- 42.Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci. 2011 Feb 15;108(7):3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci. 2008 Jan;9(1):58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- 44.Åberg MAI, Pedersen NL, Torén K, et al. Cardiovascular fitness is associated with cognition in young adulthood. Proc Natl Acad Sci. 2009 Dec 8;106(49):20906–20911. doi: 10.1073/pnas.0905307106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Middleton LE, Barnes DE, Lui L-Y, Yaffe K. Physical Activity Over the Life Course and Its Association with Cognitive Performance and Impairment in Old Age. J Am Geriatr Soc. 2010 Jul 1;58(7):1322–1326. doi: 10.1111/j.1532-5415.2010.02903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Belsky DW, Caspi A, Goldman-Mellor S, et al. Is obesity associated with a decline in intelligence quotient during the first half of the life course? Am J Epidemiol. 2013 Nov 1;178(9):1461–1468. doi: 10.1093/aje/kwt135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van IJzendoorn MH, Juffer F, Klein W. Adoption and Cognitive Development: A Meta-Analytic Comparison of Adopted and Nonadopted Children’s IQ and School Performance. Psychol Bull. 2005;131(2):301–316. doi: 10.1037/0033-2909.131.2.301. [DOI] [PubMed] [Google Scholar]
- 48.Beckett C, Maughan B, Rutter M, et al. Do the Effects of Early Severe Deprivation on Cognition Persist Into Early Adolescence? Findings From the English and Romanian Adoptees Study. Child Dev. 2006 May 1;77(3):696–711. doi: 10.1111/j.1467-8624.2006.00898.x. [DOI] [PubMed] [Google Scholar]
- 49.Gorman KS. Malnutrition and cognitive development: evidence from experimental/quasi-experimental studies among the mild-to-moderately malnourished. J Nutr. 1995 Aug;125(8 Suppl):2239S–2244S. doi: 10.1093/jn/125.suppl_8.2239S. [DOI] [PubMed] [Google Scholar]
- 50.Bellinger D, Leviton A, Waternaux C, Needleman H, Rabinowitz M. Longitudinal analyses of prenatal and postnatal lead exposure and early cognitive development. N Engl J Med. 1987 Apr 23;316(17):1037–1043. doi: 10.1056/NEJM198704233161701. [DOI] [PubMed] [Google Scholar]
- 51.Schettler T. Toxic threats to neurologic development of children. Environ Health Perspect. 2001 Dec;109(Suppl 6):813–816. doi: 10.1289/ehp.01109s6813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000 Sep;32(9 Suppl):S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 53.Haskell WL, Lee I-M, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007 Aug;39(8):1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 54.University of Otago and Ministry of Health. A Focus on Nutrition: Key findings of the 2008/09 New Zealand Adult Nutrition Survey [Internet] Wellington: Ministry of Health; 2011. Available from: http://www.health.govt.nz/publication/focus-nutrition-key-findings-2008-09-nz-adult-nutrition-survey. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.