Abstract
The mammalian metanephric kidney is composed of two epithelial components –the collecting duct system and the nephron epithelium– that differentiate from two different tissues –the ureteric bud epithelium and the nephron progenitors, respectively– of intermediate mesoderm origin. The collecting duct system is generated through reiterative ureteric bud branching morphogenesis whereas the nephron epithelium is formed in a process termed nephrogenesis, which is initiated with the mesenchymal-epithelial transition of the nephron progenitors. Ureteric bud branching morphogenesis is regulated by nephron progenitors, and in return the ureteric bud epithelium regulates nephrogenesis. The metanephric kidney is also physiologically divided along the cortico-medullary axis into subcompartments that are enriched with specific segments of these two epithelial structures. Here we provide an overview of the major molecular and cellular processes underlying the morphogenesis and patterning of the ureteric bud epithelium and its roles in the cortical-medullary patterning of the metanephric kidney.
Keywords: branching morphogenesis, collecting duct, medulla
Introduction
The mammalian metanephric kidney performs renal functions in postnatal individuals and adults. It contains two epithelial components –the collecting duct epithelium and the nephron epithelium– that execute vital but distinct renal functions. These two epithelia also employ different cellular and molecular pathways during their ontogeny: the collecting duct system is generated by branching morphogenesis of the ureteric bud epithelium whereas the nephron epithelium is established by a mesenchymal-to-epithelial transition of the mesenchymal nephron progenitors. Yet the formation of these two tissues is also intimately interwoven; in fact, the metanephric kidney is a classic model for studying mesenchymal-epithelial interactions in organ formation (Grobstein, 1953; Grobstein, 1955). Both the collecting duct and the nephron epithelia are patterned such that distinct segments of these epithelia are distributed in the kidney in a spatially organized fashion along the cortico-medullary axis of the kidney.
In this review, we provide a concise overview of the molecular and cellular processes underlying the formation and patterning of the ureteric bud epithelium and of its roles in renal medulla formation. Interested readers are referred to recent, excellent in-depth reviews (Costantini and Kopan, 2010; Little and McMahon, 2012) for more details on specific topics and other developmental processes involved in building the metanephric kidneys.
Morphogenesis of a kidney
Early embryonic events leading to kidney development
The urogenital system, which includes the kidneys and gonads, arises from the intermediate mesoderm that appears along the posterior region of the embryo during gastrulation. The dorsal portion of the intermediate mesoderm gives rise to an epithelial tube, called the nephric or Wolffian duct, whereas the ventral intermediate mesoderm gives rise to the mesenchymal cell population called the nephric cord. The early interactive events between the nephric duct and nephric cord during mouse embryonic day 8.5 to 10.5 (E8.5-E10.5) generate the pronephros and mesonephros, which are transient in nature in higher vertebrates.
Development of the metanephros, the definitive and the permanent adult kidney, begins with the onset of inductive interactions between the caudal end of the nephric duct and a group of mesenchymal cells in the nephric cord called the metanephric mesenchyme (Fig. 1), at E10.5 in mice and at the fifth week of gestation in humans (Costantini and Kopan, 2010; Davidson, 2008; Rosenblum, 2008). The present review focuses mainly on the cellular and molecular mechanisms mediating the metanephric kidney development.
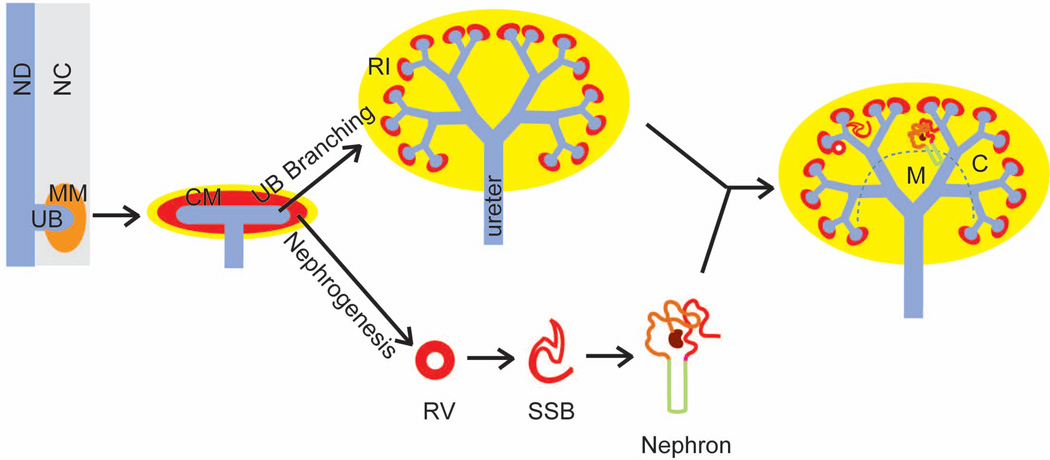
Figure 1. A schematic diagram of mammalian kidney development.
Metanephric kidney development commences when a ureteric bud (UB) outgrowth from the nephric duct (ND) invades the metanephric mesenchyme (MM) of the posterior nephric cord (NC). The ureteric bud undergoes repeated rounds of branching morphogenesis to form the collecting duct system, while a subset of the nephron progenitors in the cap mesenchyme (CM) is induced by the ureteric bud epithelium to undergo nephrogenesis. Nephron progenitors epithelialize to form the renal vesicle (RV), which further develops into the S-shaped body (SSB) before fully develop into a nephron. The kidneys are divided into the cortex (C) and the medulla (M), each enriched with different nephron and collecting duct segments. RI, renal interstitium.
Metanephric kidney development
Metanephric kidney development commences when the caudal end of the nephric duct forms an outgrowth, the ureteric bud, that invades the metanephric mesenchyme (Fig. 1). A subset of metanephric mesenchymal cells, called the cap mesenchyme, surrounds the invading ureteric bud, and induces the ureteric bud epithelium to undergo repeated branching at its tips to form a tree-like structure (Fig. 1). This ureteric tree eventually differentiates into the collect duct system and the ureter of the kidney. Throughout the branching morphogenesis process, the ureteric-bud tips in turn induce its surrounding cap-mesenchyme cells to form nephrons (nephrogenesis) (Fig. 1). Only a subset of nephron progenitor cells, however, are initially induced by the ureteric tip epithelium to form the renal vesicle (Fig. 1); the rest proliferate in preparation for subsequent rounds of nephrogenesis. The renal vesicles differentiate through intermediate stages, such as the comma- and S-shaped bodies, before becoming nephrons (Fig. 1). In this way, ureteric branching morphogenesis, which determines the number of ureteric tips, is a determinant of nephron endowment, which itself is a predisposing factor for hypertension and kidney failure (Luyckx and Brenner, 2005).
While ureteric bud branching morphogenesis and nephron induction occurs at the periphery of the kidney, resulting in the formation of the renal cortex at around E15.5 in mice, the ureteric trunks start to elongate extensively longitudinally, resulting in the emergence of the inner compartment of the kidney, the renal medulla (Fig. 1). The renal cortex and the renal medulla are enriched with different nephron segments (Fig. 2) that perform distinct renal physiology. The medullary collecting ducts open to the calyceal and pelvic spaces, where concentrated urine is collected and passed to the bladder through the ureter.
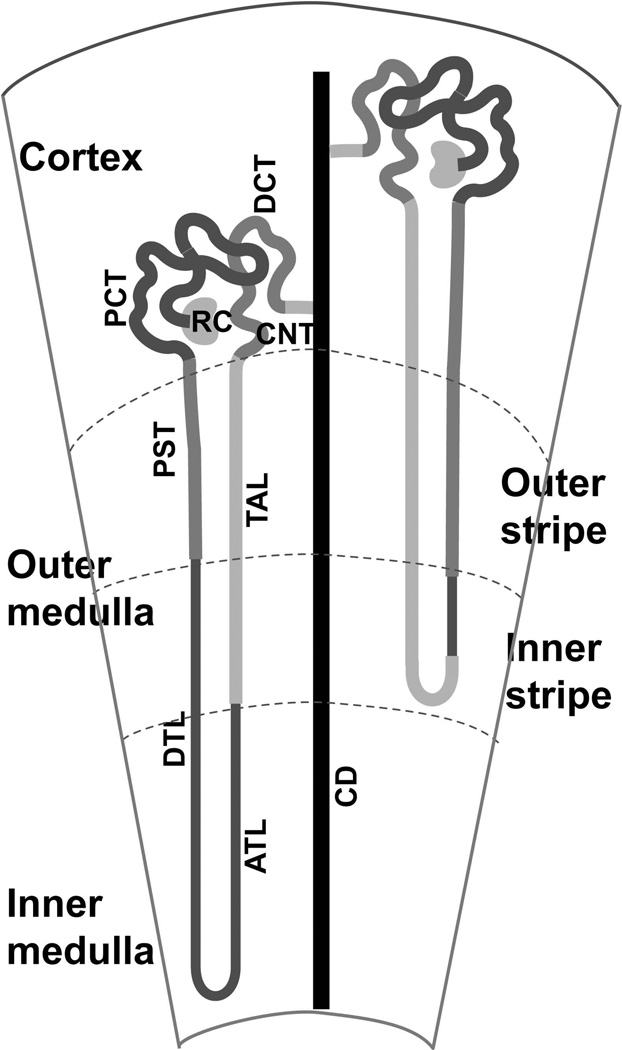
Figure 2. A schematic diagram of the spatial distribution of mature renal epithelial structures along the cortico-medullary axis.
There are two types of nephrons, the cortical nephrons, of which the loops of Henle reach only into the outer medulla, and the juxtamedullary nephrons, of which the loops of Henle reach into the inner medulla. RC, renal corpuscle; PCT, proximal convoluted tubules; PST, proximal straight tubules; DTL, thin descending limb (of the loop of Henle); ATL, thin ascending limb (of the loop of Henle); TAL, thick ascending limb (of the loop of Henle); DCT, distal convoluted tubule; CNT, connecting tubule; CD, collecting duct.
Ureteric bud outgrowth, branching, and collecting duct formation
The entire collecting duct system of the kidney, the final site of urine salt and acid-base regulation, is derived from the ureteric bud epithelium. Following several rounds of branching morphogenesis, the branching tips progress through three recurring morphologies: the ampulla, T-tip, and tri-tip stages (Short et al., 2014). Though the transition of an ampulla to a T-tip is the most common event –occurring during the early kidney development between E12.5-E15.5, when the branching events are very rapid– a T-tip can resolve into a tri-tip that, in turn, can resolve into a T-tip and an ampulla; together, this complexity reflects the asynchronous nature of branching across the kidney during development (Short et al., 2014). In mice, the ureteric bud epithelium undergoes approximately 10 rounds of branching until E15.5; by E16.5, 85% of total branching events is complete, wherein the ureteric bud epithelium has branched to form a network of 2,580 branch and tip segments with an average of 1,300 tips at the periphery. This is further followed by two additional rounds of branching during the remainder of gestation (Cebrian et al., 2004; Short et al., 2014). Branching morphogenesis ceases at about postnatal day 3 in mice (Hartman et al., 2007).
Signaling pathways regulating ureteric bud outgrowth and branching morphogenesis
A wealth of information has accumulated in recent years addressing the cellular and molecular events orchestrating the developmental patterning involved in the establishment of the kidney collecting duct tree. Much of this knowledge have been gained through advancements in molecular and imaging techniques and an expanding repository of transgenic animal models.
Here, we provide a brief description of the major signaling pathways and cellular machineries directing ureteric bud outgrowth and branching morphogenesis that establishes the structure of the collecting duct tree. We direct the reader to several recently published reviews focusing on the kidney patterning and development, including that of the renal collecting ducts, for more extensive details on the morphogenesis (Blake and Rosenblum, 2014; Costantini and Kopan, 2010; Dressler, 2006; Dressler, 2009; Little and McMahon, 2012).
Signaling mechanisms regulating ureteric bud budding from the nephric duct
The first event of metanephric kidney development, nascent ureteric bud formation from the nephric duct, is critical for proper kidney formation. Disruption of this budding process can result in renal agenesis, duplex kidneys, and improper positioning of the ureter, which subsequently leads to congenital abnormalities of the urinary tract (CAKUT) symptoms such as urinary-tract obstruction and hydroureter/hydronephrosis.
Several signaling molecules act as critical regulators of initial budding from the nephric duct. For example, ureteric bud evagination from the nephric duct utilizes glial cell line-derived neurotrophic factor (GDNF) signaling through its receptor RET (receptor tyrosine kinase) and co-receptor GFAR1 (Costantini and Kopan, 2010). The significance of GDNF-RET signaling in ureteric bud budding during mammalian kidney development was clearly implicated in mutagenesis analysis of the genes that encode these signaling molecules: RET mutations are found in 5–30% of human CAKUT patients (Davis et al., 2014), while the absence of a ureteric bud and its derivatives was observed in Gdnf- and Ret-mutant mice (Moore et al., 1996; Pichel et al., 1996; Sanchez et al., 1996; Schuchardt et al., 1994). Gdnf is expressed in the metanephric mesenchyme, while Ret and Gfra1 are expressed in the nephric duct epithelium.
Gdnf expression in the metanephric mesenchyme is highly regulated. Many transcription factors/transcriptional regulators expressed in the metanephric mesenchyme –such as the Hox11 cluster (Wellik et al., 2002), Pax2 (Brophy et al., 2001), Eya1 (Sajithlal et al., 2005), Sall1 (Kiefer et al., 2010), Six1 (Kobayashi et al., 2007; Li et al., 2003), Six2 (Brodbeck et al., 2004), and Six4 (Kobayashi et al., 2007)– have been shown to regulate Gdnf transcription. Signaling between the ureteric bud epithelium and extracellular matrix also impacts Gdnf expression in the metanephric mesenchyme, as the mouse mutants for the extracellular matrix genes nephronectin (Npnt) and integrin α8β1 (Itga8 and Itgb1) displayed severe renal defects along with disrupted Gdnf expression at the time of ureteric bud outgrowth (Linton et al., 2007). Furthermore, mice lacking Gdf11, a transforming growth factor β (TGFβ)-family signaling ligand, exhibited kidney agenesis, which was shown to be a result of the loss of Gdnf expression in the metanephric mesenchyme (Esquela and Lee, 2003).
Several negative regulators of Gdnf expression and signaling are also critical during the commencement of kidney development. Such negative regulation is necessary for inhibiting ectopic ureteric bud budding, thereby ensuring the emergence of only a single ureteric bud outgrowth from the nephric duct in response to the branching signals from the metanephric mesenchyme. SLIT2-ROBO2 signaling and FOXC1/C2 transcription factors restrict ureteric bud outgrowth from the nephric duct by limiting the expression domain of Gdnf (Grieshammer et al., 2004; Kume et al., 2000). In contrast, SPRY1, a negative regulator of GDNF-RET signaling, modulates the response of ureteric bud epithelial cells to GDNF levels and thus prevents multiple ureteric bud outgrowths (Basson et al., 2005; Chi et al., 2004). Bone morphogenesis protein 4 (BMP4) and its antagonist gremlin 1 (GREM1) also ensure that only one ureteric bud arises from the nephric duct, although the mechanism used remains elusive (Michos et al., 2007; Miyazaki et al., 2000). Together, these findings demonstrate the finely tuned balance between various signaling pathways during kidney development that ultimately ensures the proper level/domain of Gdnf expression so that one and only one ureteric bud outgrowth forms from the nephric duct. It also highlights the importance of the initial ureteric budding event for proper metanephric kidney development, and the central role of GDNF-RET signaling in its regulation.
Fibroblast growth factor (FGF) signaling pathways also play a role in ureteric bud outgrowth from the nephric duct, as well as contributing to proper positioning of the ureteric bud (Bates, 2011; Michos et al., 2010). Fgf10, an FGF signaling ligand, is expressed in the metanephric mesenchyme. A fraction of Fgf10-mutant mice exhibit renal agenesis and an absence of ureteric buds at E11.5-E12.5 (Michos et al., 2010), thus FGF10 contribtues a minor signaling role from the metanephric mesenchyme during ureteric bud outgrowth. Of note, SPRY1 also serves as a negative regulator of FGF signaling in ureteric bud outgrowth since the loss of Spry1 was required to achieve a complete rescue of the ureteric bud defect by loss of Fgf10 when Gdnf was also absent (Michos et al., 2010). Interestingly, ablation of an FGF receptor from the metanephric mesenchyme resulted in the opposite effect: A majority of mutants lacking Fgfr2 (most likely Fgfr2IIIc) expression in the metanephric mesenchyme displayed multiple ureteric bud outgrowths (Hains et al., 2008). Surprisingly, the expression of Gdnf and known negative regulators of Gdnf signaling was not affected in these mutants (Hains et al., 2008), so the exact mechanism of action is not understood.
Signaling pathways regulating ureteric bud branching and collecting duct arborization
Once the ureteric bud grows out from the nephric duct, at E10.5, it first undergoes bifurcated branching to form a T-shaped bud at E11.5. Each ureteric bud tip or ampulla then undergoes terminal trifurcation, followed by repeated bifurcations. The ureteric tips produced in this manner form a ureteric tree, which further elongates inward to form the collecting ducts, resulting in the establishment of the renal medulla. Several signaling regulators expressed in the ureteric bud epithelium as well as the surrounding metanephric mesenchyme and stromal cells regulate ureteric bud branching morphogenesis in both autocrine and paracrine fashion:
GDNF-RET signaling: GDNF-RET signaling is the major player regulating the iterative ureteric bud branching process (Moore et al., 1996; Pichel et al., 1996; Sanchez et al., 1996; Schuchardt et al., 1994). Studies done with specific inhibitors and transgenic mice revealed the significance of RET-activated intracellular pathways such as phosphoinositol-3-kinase (PI3K), ERK, and phopholipase C gamma (PLCγ) in ureteric bud branching morphogenesis (Jain et al., 2006). Downstream of the PI3K pathway, two ETS transcription factors, ETV4 and ETV5, acts as effectors of GDNF-RET signaling in ureteric bud outgrowth and branching (Lu et al., 2009). GDNF-RET signaling additionally activates the transcription of a group of genes implicated in cell proliferation (Lu et al., 2009), consistent with the role of GDNF-RET signaling in promoting ureteric tip cell proliferation (Michael and Davies, 2004; Pepicelli et al., 1997).
FGF signaling: FGF signaling components FGF7 (Qiao et al., 1999), FGF10 (Michos et al., 2010; Qiao et al., 2001), and FGFR2 (Bates, 2011; Zhao et al., 2004) have been shown to play a critical role in ureteric bud branching. Some downstream genes identified for GDNF-RET signaling, such as Ret and Wnt11, are also regulated by FGFR signaling, indicating that these two receptor-tyrosine-kinase signaling pathways converge, at least in part, to regulate branching events (Bates, 2011) and could partly compensate for each other. Indeed, the ureteric bud branching defects associated with the loss of FGF signaling is more prominent in a reduced-GDNF-signaling background (Michos et al., 2010). On the other hand, unlike GDNF-RET signaling, FGF signaling appears to be sufficient but not necessary for the expression of Etv4 and Etv5 in the ureteric bud epithelium (Lu et al., 2009), illustrating the divergence of actions of these two signaling pathways in the regulation of ureteric bud branching.
WNT11 signaling: WNT11, a WNT-signaling ligand expressed in the ureteric tips, is also important for ureteric bud branching morphogenesis (Kispert et al., 1996). WNT11 forms a feedback loop with GDNF-RET signaling (Lu et al., 2009; Majumdar et al., 2003). It is a downstream transcriptional target of GDNF-RET signaling (Lu et al., 2009); in return, WNT11 regulates Gdnf expression in the metanephric mesenchyme (Majumdar et al., 2003), probably by activating the Frizzled 4/8 (FZD4/8) receptors in the ureteric bud epithelium (Ye et al., 2011). How this signaling activation in the ureteric bud epithelium leads to changes in Gdnf expression in the metanephric mesenchyme remains an open question.
WNT/β-catenin signaling: Genetic models that inactivate β-catenin in the ureteric bud epithelium demonstrate the requirements of the genetic program controlled by the canonical WNT/β-catenin pathway in branching morphogenesis (Bridgewater et al., 2008). WNT/β-catenin signaling is necessary for maintaining ureteric bud epithelial cells in a precursor state that facilitates branching during kidney development (Marose et al., 2008). Conversely, appropriate suppression of the WNT/β-catenin-TCF4-mediated transactivation is also critical for ureteric bud branching morphogenesis, as loss of ICAT, a negative regulator of the canonical Wnt signaling pathway, led to delayed/arrested ureteric bud branching (Hasegawa et al., 2007). Similarly, expression of a constitutively active form of β-catenin in the ureteric bud epithelium also reduced branching (Bridgewater et al., 2011). Interestingly, Tgfb2 is upregulated due to the activation of β-catenin, and may provide some mechanistic insights into the branching defect observed in these mutants (see below). Nevertheless, the phenotypes of the lose- and gain-of-function mutants of WNT/β-catenin signaling highlight the importance of the proper levels of WNT/β-catenin signaling for normal execution of ureteric bud branching morphogenesis.
TGFβ signaling: Inhibition of BMP4 activity by the antagonist gremlin1 (GREM1) is required for the progression of normal ureteric bud branching events (Michos et al., 2007). Studies with mouse heterozygous mutants for Tgfb2 (Sims-Lucas et al., 2008) and the BMP receptor Alk3 (Hartwig et al., 2008; Hu et al., 2003), and ex vivo kidney cultures with manipulation of TGFB1 (Clark et al., 2001) and activin A (Maeshima et al., 2006; Maeshima et al., 2003) revealed a common phenomenon: a decrease in TGFβ signaling leads to an increase in ureteric bud branching. These observations clearly indicate that TGFβ signaling inhibits the ureteric branching process; however, the mechanisms whereby TGFβ signaling regulates branching morphogenesis remains elusive.
Other signaling mechanisms: In addition to GDNF-RET and FGF signaling, receptor-tyrosine kinase pathways mediated by hepatocyte growth factor (HGF)-MET proto-oncogene (Ishibe et al., 2009; Woolf et al., 1995) and epidermal growth factor (EGF)-EGFR (Ishibe et al., 2009) also regulate ureteric bud branching. The cooperative interaction exhibited by vascular endothelial growth factor A (VEGFa)-VEGFR2 signaling (Gao et al., 2005; Marlier et al., 2009) with GDNF-RET signaling (Tufro et al., 2007) and the cross-talk between the renin-angiotensin system and RET signaling (Song et al., 2010) both play an important role in ureteric bud branching events during kidney development. Finally, it was recently reported that the primary cilium kinesin-like KIF3A regulates Ret andWnt11 expression in the ureteric bud epithelium and in ureteric bud branching in a GLI3-dependent fashion (Cain et al., 2009; Chi et al., 2013).
A balance between several signaling mechanisms that promote and inhibit branching morphogenesis is essential for the successful execution of branching morphogenesis and for the formation of the collecting duct system. It is still not clear how this balance coordinates the initiation as well as the progression of ureteric bud branching, and it remains an important research area for further exploration.
Cellular mechanisms mediating ureteric bud outgrowth and branching
Elevated cell proliferation, cell shape changes, and directed cell movements are observed during ureteric bud outgrowth and/or branching events. Studies with mice chimeric for wild-type and Ret- or Spry1-mutant cells expressing distinct fluorescent proteins identified the significance of RET signaling in the regulation of cell migration and re-arrangement during ureteric bud evagination from the nephric duct. Cells with higher RET-signaling activity out-compete cells with lower RET-signaling activity, and migrate towards the budding site where the initial ureteric bud outgrowth subsequently occurs (Chi et al., 2009). The presence of heterogeneous levels of RET signaling observed in the wild-type nephric duct and the preferential migration of a subset of nephric duct cells with higher RET signaling towards the budding site is consistent with the notion that RET-signaling-level-dependent cell sorting is at play during ureteric bud outgrowth (Chi et al., 2009).
The importance of actin cytoskeletal dynamics during subsequent ureteric bud branching morphogenesis is evident through the analysis of mouse double-knockouts for actin depolymerizing factors cofilin1 (CFL1) and destrin (DSTN), where the lack of Cfl1 and Dstn resulted in disruption of ureteric bud branching morphogenesis and defects in cell shape, cell organization, and cell migration (Kuure et al., 2010). Recently, using live-cell imaging and genetic labeling, ureteric bud cells were shown to exhibit a unique behavior named mitosis-associated cell dispersal during branching morphogenesis. Here, the premitotic ureteric tip cells delaminate from the epithelium, leaving a thin basal process attached to the basement membrane, and divide within the lumen instead of the plane of the ureteric bud epithelium. Of the two daughter cells generated, the one that inherited the basal process reinserted into the site of origin whereas the other daughter cell inserted at a position of one to three cell-diameters away. This unique phenomenon is thought to contribute to the rapid and extensive cellular rearrangements that occur during the ureteric bud branching events (Packard et al., 2013).
An optical-projection tomography-based approach to analyzing kidney development has helped overcome the technological limitations in examining branching morphogenesis in three dimensions, and has allowed for the study of many spatial aspects of kidney development in the mouse, including surface area, surface tip packing, volume, branching angle, and comparative morphology. By this method, it is now possible to analyze even subtle changes during kidney development and to quantify the in vivo contributions of a given gene in ureteric bud epithelial morphogenesis and branching at a high resolution (Short et al., 2014; Short et al., 2010). Despite this technological advance, the functional significance of cellular events in ureteric bud branching morphogenesis and the molecular machinery governing them are largely not well understood. Furthermore, questions remain: Are there other cellular mechanisms –such as cell intercalation– involved in ureteric bud branching morphogenesis, and if so, how they are regulated? Filling these knowledge gaps will not only expand our understanding of ureteric bud branching morphogenesis, but should also facilitate the treatment of collecting duct injuries and the design and development of new therapeutic approaches for kidney repair and regeneration.
Roles of renal interstitial cells in ureteric bud branching
In addition to the cap mesenchyme, the embryonic kidney contains another population of mesenchymal cells, the renal interstitial cells. These cells are divided along the cortico-medullary axis, into the nephrogenic interstitium, the cortical interstitium, and the medullary interstitium (Little et al., 2007). The nephrogenic interstitial cells occupy the outer cortex of the kidney where active nephrogenesis and branching morphogenesis occurs. They express Foxd1, and are the progenitors of the renal interstitium, vascular smooth muscle cells, mesangial cells, pericytes, and adventitial fibroblast (Humphreys et al., 2010; Kobayashi et al., 2014; Sequeira Lopez and Gomez, 2011).
Signaling from interstitial cells also regulate ureteric bud branching morphogenesis. The ureteric tips are surrounded by an inner layer of mesenchymal cells –the cap mesenchyme– and an outer layer of mesenchymal cells –the interstitial cells. During branching morphogenesis, these interstitial cells are passively excluded from the ureteric tips, but increase in number in the cleft region of ureteric buds during bifurcation as new branches are formed, suggesting the possible contribution of interstitial cells in inducing cleft formation (Paroly et al., 2013). A role for interstitial cells in ureteric bud branching morphogenesis was first revealed during examination of mouse mutants lacking the transcription factor Foxd1 (Bf2), which is expressed in the nephrogenic interstitial cells; these mice exhibited impaired ureteric bud branching morphogenesis (Hatini et al., 1996; Levinson et al., 2005). Yet, it remains an open question how FOXD1 regulates ureteric bud branching: Does it regulate branching through the cap mesenchyme residing between the ureteric bud epithelium and the nephrogenic interstitium, as the cap mesenchyme was also abnormal in Foxd1 mutants? Or, as a transcription factor, does it regulate ureteric bud branching by controlling the expression of interstitial signals that acts on branching morphogenesis? Alternatively, does it regulate aspects of interstitial differentiation that generate a population of interstitial cells that regulates ureteric bud branching?
Interstitial cells are the source of another important ureteric bud branching regulator, retinoid acid. These cells express the major retinoic-acid synthesizing enzyme, retinaldehyde dehydrogenase-2 (RALDH2) (Marlier and Gilbert, 2004; Rosselot et al., 2010). RALDH2 facilitates the conversion of an inactive form of retinol (Vitamin A) to an active form of retinoic acid. Raldh2 mutants displayed an impaired ureteric bud branching phenotype (Rosselot et al., 2010). Importantly, based on the kidney phenotype analysis of mouse mutants for Raldh2, Raldh3 (a retinaldehyde dehydrogenase expressed in the ureteric bud lineage), and compound mutants for both genes, it was concluded that RALDH2, and thus the interstitium, generates the major source of retinoic acid during kidney development. Retinoic acid acts as a transcriptional activator on retinoic-acid receptors (RARs), which are expressed in both the ureteric bud epithelium and the interstitial cells. The role of receptors RARA and RARB2 in ureteric bud branching morphogenesis has been inferred from the phenotypes in loss-of-function mutants for both Rara and Rarb2 (Batourina et al., 2002; Batourina et al., 2001; Mendelsohn et al., 1999; Mendelsohn et al., 1994; Rosselot et al., 2010) and by in vitro assays using pan-RAR antagonists in organ culture (Takayama et al., 2014). In all cases, decreased branching is attributed to the failure in the expression of Ret in the ureteric bud epithelium.
The role of retinoic acid signaling in ureteric bud branching morphogenesis has been further established in the in vitro organ-culture model, where embryonic kidneys cultured in the absence of retinoic acid showed significantly reduced ureteric bud branching and decreased Ret expression compared to its counterparts cultured in the presence of retinoic acid (Moreau et al., 1998; Takayama et al., 2014; Vilar et al., 1996). Retinoic acid signaling regulates Ret expression in a cell-autonomous manner in the ureteric tip cells, likely by directly activating its transcription (Moreau et al., 1998; Rosselot et al., 2010; Stine et al., 2011; Takayama et al., 2014).
Recent studies employing microarray analysis of interstitial cells with and without retinoic acid signaling uncovered an additional, novel mechanism of signaling in ureteric bud branching: the direct regulation of the extracellular matrix gene, Ecm1, by retinoic acid signaling in interstitial cells (Paroly et al., 2013; Takayama et al., 2014). Ecm1 is selectively expressed in the cortical interstitial cells, which are in direct contact with the ureteric bud epithelium in the cleft regions and may function in the bifurcation of the ureteric bud by down-regulating Ret expression in the cleft domain. Disruption of Ecm1 results in a broader expression domain for Ret in the ureteric bud epithelium, including the cleft region where it is repressed during normal branching; such ectopic expression of Ret could impair normal cleft formation and bifurcation, resulting in defective ureteric bud branching (Paroly et al., 2013). Thus, retinoic acid signaling in the interstitium appears to restrict the expression of Ret to ureteric tips, via its effects on Ecm1 expression, which is necessary for proper branching morphogenesis. How ECM1 represses Ret expression in the cleft domain is currently unresolved. As a scaffolding protein that brings extracellular matrix structural proteins and growth factors together, however, ECM1 may modulate the levels of growth factors that signals to the ureteric bud epithelium.
The expression of TNFSF13B and IL33 in the interstitium, both of which can activate NF-κB, is also implicated in regulating retinoic acid signaling (Takayama et al., 2014). The significance of NF-κB in ureteric bud branching is implicated by a significant decrease in branching in the embryonic kidney organ cultures treated with NF-κB inhibitors (Takayama et al., 2014), illustrating another mechanism for the regulation of ureteric bud branching morphogenesis by interstitium-derived retinoic acid signaling.
Triple mutants of the Hox10 paralogs (Hoxa10, Hoxc10, and Hoxd10), which are expressed in both the cap mesenchyme and interstitial cells, reveal the importance of the Hox10 gene cluster for interstitial cell development and, in turn, ureteric bud branching morphogenesis. In these mutants, Foxd1-expressing nephrogenic interstitial cells integrated only partly to the kidney periphery and failed to differentiate to form a renal capsule (Yallowitz et al., 2011). In addition, Hox10 triple mutants displayed an aberrant ureteric bud branching phenotype that is most likely due to a dysregulated, broader expression pattern of Ret in the ureteric bud epithelium, which is no longer restricted to the ureteric tips (Yallowitz et al., 2011). The defect in Ret expression raises the question of whether or not the Hox10 paralogs regulate Ecm1 expression.
The gene Pod1 (Tcf21) is also expressed in the cortical interstitium, in addition to the cap mesenchyme and podocytes of the kidney. In Pod1-knockout embryos, Ret expression extended into some of the ureteric bud branches and medullary tips (Quaggin et al., 1999). As ectopic expression of Ret in the ureteric bud epithelium results in the deregulation of ureteric bud branching morphogenesis (Srinivas et al., 1999), the severe reduction of branching in Pod1 mutants is likely a consequence of ectopic Ret expression (Quaggin et al., 1999). Again it will be interesting to examine if Ecm1 expression is reduced in Pod1 mutants, given their overlapping expression domain in the cortical interstitium and their similar loss-of-function effect due to changes in the normal Ret expression pattern in ureteric bud tips.
Collecting duct differentiation
The ureteric bud tips consist of stem cells that give rise to ureteric bud-tip and ureteric bud-trunk cells. Ureteric bud-tip cells are a transient cell type that disappear at the cessation of ureteric bud branching morphogenesis at about postnatal day 3 in mice (Hartman et al., 2007). Ureteric bud-trunk cells differentiate to form the collecting ducts.
The collecting duct cells are composed of principal cells and intercalated cells, and the ratio of principal cells-to-intercalated cells increases from the renal cortex to the renal medulla (Guo et al., 2014). The epithelial cells constituting the terminal portion of the collecting duct, in the renal papilla, are distinct in their cytostructure and function, and are termed inner medullary collecting duct (IMCD) cells (Madsen et al., 1988). Principal cells regulate ion and water transport through the specific expression of transporters, such as the epithelial sodium channel (ENaC) and the water channel aquaporin 2 (AQP2) (Pearce et al., 2014). Intercalated cells regulate acid-base balance, and play a major role in proton and bicarbonate secretion in the collecting ducts. There are two types of intercalated cells, types A and B. Type A cells secrete protons through an apical vacuolar-type H+-ATPase whereas type B cells secrete bicarbonate, which is mediated by an apical Cl−/HCO3− exchanger (Kim et al., 1999). IMCD cells are important for producing concentrated urine –by means of active re-absorption of urea, water, sodium chloride– and for urine acidification –by regulating its final pH. IMCD cells, like principal cells, are also under the influence of anti-diuretic hormone, which stimulates the osmotic water permeability of the collecting duct (Madsen et al., 1988). The IMCD is also the main of source of nitric oxide synthase, which contributes to nitric oxide production in the kidney. Nitric oxide has several physiological functions in the regulation of renal hemodynamics, maintenance of medullary perfusion, promoting natriuresis and diuresis, and renal adaptations to dietary salt intake (Mount and Power, 2006).
Proper collecting duct differentiation is critical for renal physiology. Abberrant collecting duct differentiation from the ureteric bud epithelial cells during kidney development results in renal dysplasia in humans (Woolf et al., 2004). Terminal differentiation of the collecting duct epithelium is evident in mice from E17.5 onwards, and appears to be largely completed by late gestation (Guo et al., 2014). The genetic mechanism that determines the timing of this event, however, is not known. Moreover, genetic programs regulating the ureteric bud-tip versus -trunk cell fate are poorly understood, except that heregulin alpha (HRGA) protein, identified in the conditioned medium derived from metanephric mesenchymal cells, was shown to inhibit ureteric bud tip gene expression and to promote the expression of differentiation markers (Sakurai et al., 2005). WNT/β-catenin signaling in the ureteric bud epithelium was also observed to be sufficient and necessary for maintaining the ureteric bud epithelial cells in a precursor state (Marose et al., 2008).
The transcription factor FOXI1 promotes intercalated-cell fate (Blomqvist et al., 2004), while NOTCH signaling promotes principal-cell fate (Guo et al., 2014; Jeong et al., 2009). Ablation of Foxi1 leads to distal renal tubular acidosis in mice due to defects in intercalated-cell differentiation, with an absence of Pendrin, the vacuolar-H+-ATPase AE1, and the anion exchanger AE4 in the intercalated cells tha mediate acid-base homeostasis (Blomqvist et al., 2004). The apical Cl−/HCO3− exchanger AE4, expressed in type B intercalated cells and encoded by the gene Slc4a9, has been demonstrated to be a direct target of FOXI1 transcriptional activation (Kurth et al., 2006). This strongly suggests that FOXI1 positively promotes intercalated-cell fate. On the other hand, in Foxi1 mutants, normal collecting duct epithelium exhibiting defined cell types of principal (Aqp2+CAII− Foxi1−) and intercalated fates (Aqp2−CAII+Foxi1+) has been replaced by a single cell type positive for both the principal and intercalated markers (Aqp2 +CAII+ Foxi1−). Moreover, whole-genome transcription regulator screens performed on kidneys revealed that some of the principal-cell markers are predicted to be direct FOXI1 transcriptional targets (Yu et al., 2012). These data together strongly suggest that FOXI1 suppresses principal-cell fate.
It is observed that constitutive activation of NOTCH signaling results in the differentiation of all renal collecting duct epithelium into Aqp2-expressing principal cells (Jeong et al., 2009), whereas inactivation of NOTCH signaling by ablation of either Mib1 or Adam10 leads to a significant decrease in principal cells and a corresponding increase in the cells expressing Foxi1 (Guo et al., 2014; Jeong et al., 2009). This demonstrates a necessary-and-sufficient role for NOTCH signaling in establishing principal-cell fate, and also implies that the inhibitory effect of NOTCH signaling on FOXI1 function is needed to direct the differentiation of ureteric bud epithelial cells towards principal-cell fate (Guo et al., 2014; Jeong et al., 2009). Taken together, these data illuminate the critical importance of balancing between FOXI1 and NOTCH signaling for the differentiation of ureteric bud cells into principal- or intercalated-cell lineages.
The contribution of the epigenetic program in the choice of principal- versus intercalated-cell fate is evident in mice disrupted for Dot1l, a histone H3K79 methyltransferase, where some principal cells give rise to intercalated cells (Wu et al., 2013). Outside of the ureteric bud epithelium, retinoic acid signaling from the interstitium plays a role in collecting duct differentiation, as evidenced by the dependence of the expression of collecting duct differentiation markers such as Scnn1b (encoding βENAC) and Elf5 on retinoic acid signaling (Takayama et al., 2014). In addition, FGF7 signaling from the interstitium affects the cytoarchitecture of the papilla tip collecting duct cells, as they appeared low cuboidal in shape in Fgf7 mutants instead of the cuboidal/columnar shape seen in controls (Qiao et al., 1999).
A role for cells of the nephron lineage in collecting duct differentiation, however, has not been established. Given the physical separation of the two epithelial components by the interstitium, any possible influence of the nephron epithelium on the ureteric bud epithelium likely has to be mediated by the interstitium.
miRNAs in ureteric bud epithelial morphogenesis
MicroRNAs (miRNAs) are small, non-coding RNAs that exert post-transcriptional regulation on their target gene expression. They play a vital role in a myriad of fundamental biological processes. Dysregulation in miRNA-mediated gene expression has been implicated in several diseases, and miRNA-based therapy in disease amelioration is rapidly evolving. Several studies have identified the significance of miRNA-mediated gene expression in renal development and diseases (Akkina and Becker, 2011; Bhatt et al., 2011; Chandrasekaran et al., 2012; Ho and Kreidberg, 2013; Khella et al., 2013; Ma and Qu, 2013; Pottier et al., 2014).
A miRNA-microarray analysis on the ureteric bud epithelium and the ureteric bud-derived collecting duct epithelial cells revealed the presence of hundreds of miRNAs (Landgraf et al., 2007; Nagalakshmi et al., 2014), suggesting strong gene expression modulation by miRNAs in these cell populations. Though the functional importance of individual miRNAs in ureteric bud epithelial morphogenesis is still evolving, the importance of global miRNA-mediated gene regulation in ureteric bud epithelial development is clearly brought out by studies on conditional Dicer-mutant mice, in which the miRNA biogenesis enzyme DICER was removed from the ureteric bud epithelial compartment using tissue-specific Cre lines (Bartram et al., 2013; Nagalakshmi et al., 2014; Nagalakshmi et al., 2011; Pastorelli et al., 2009; Patel et al., 2012). The ureteric bud epithelium of the conditional Dicer mutants exhibit branching defects as early as E12.5 with ensuing renal hypoplasia (Bartram et al., 2013; Nagalakshmi et al., 2014; Nagalakshmi et al., 2011). This is at least partly due to the compromised ability of the ureteric bud epithelial cells of Dicer mutants to respond to the GDNF-dependent branching signal in the metanephric mesenchyme (Nagalakshmi et al., 2014; Nagalakshmi et al., 2011) since the expression of critical ureteric bud branching regulators, WNT11 and RET, are significantly reduced in the mutant ureteric bud epithelium (Nagalakshmi et al., 2011). Up-regulation and persistent expression of early ureteric bud genes (see below) in the Dicer mutant ureteric bud epithelium may also contribute to the branching defect (Nagalakshmi et al., 2014).
miRNAs are also essential for normal collecting duct elongation as Dicer ablation in the ureteric bud epithelial lineage results in the formation of cysts in the nascent trunks and along the collecting duct epithelium. Increased cell proliferation and cell death and primary cilia defects were observed in Dicer mutant ureteric buds, and are all likely to play a role in the onset and progression of cystic defects in the Dicer-mutant kidneys (Nagalakshmi et al., 2011).
Furthermore, loss of miR-20 and miR-200 in Dicer mutants resulted in the up-regulation of their target, the polycystic kidney disease 1 (Pkd1) gene, which most likely contributes to the onset of the cystic phenotype (Bartram et al., 2013; Patel et al., 2012). On the other hand, up-regulation of miRNAs including the miR-200a and miR-17~92 cluster has been associated with cyst formation (Pandey et al., 2011; Patel et al., 2013) and transgenic expression of the miR-17~92 cluster with Ksp∷Cre led to cyst formation in renal epithelia including the collecting ducts (Patel et al., 2013). It is worth noting that the opposing and apparent contradicting effects of miRNAs illuminate the importance of the expression level of miRNA target genes such as Pkd1 in controlling collecting duct size and the necessity of the role of miRNAs as a rheostat in this process.
Terminal differentiation of the collecting ducts into principal and intercalated cells is significantly compromised in Dicer mutants (Nagalakshmi et al., 2014; Nagalakshmi et al., 2011). The expression of a large group of genes associated with collecting duct differentiation was greatly reduced when Dicer was ablated in the ureteric bud epithelium (Nagalakshmi et al., 2014). Analysis of up-regulated genes in Dicer-mutant ureteric bud cells identified a group of genes whose expression in the wild-type ureteric bud epithelium is present only before E14.5 during kidney development in mice (early ureteric bud genes). Notably, the expression of these early genes increased at the early developmental stages (E12.5 and E13.5) in the Dicer-mutant ureteric bud epithelium, and persisted beyond E13.5 (Nagalakshmi et al., 2014). The persistent expression of early ureteric bud genes and the simultaneous disrupted expression of collecting duct differentiation genes in Dicer-mutant ureteric bud cells strongly suggests that miRNAs suppress ureteric bud progenitor cell fate while promoting collecting duct differentiation. Moreover, early ureteric bud genes with persistent expression in the Dicer-mutant ureteric bud cells are enriched for known and putative let-7-family miRNA targets, pointing to a potential role for the let-7-family miRNAs in inhibiting early ureteric bud gene expression to promote collecting duct differentiation (Nagalakshmi et al., 2014). The timing of the increased expression of let-7-family miRNAs in the wild-type ureteric bud epithelium (Nagalakshmi et al., 2014) also offers a possible mechanism underlying the regulated timing of collecting duct differentiation from the ureteric trunk epithelium. Finally, miRNAs are implicated in collecting duct function, particularly via their action on the renin-angiotensin-aldosterone system in maintaining Na+ transport in renal collecting duct epithelial cells (Edinger et al., 2014).
miRNAs are also important for proper ureter formation and function. Dicer deletion using Ksp∷Cre transgenic mice led to urinary obstruction, probably due to defects in ureteral smooth muscle differentiation (Bartram et al., 2013).
Altogether, these studies indicate that miRNAs tightly regulate ureteric bud epithelial morphogenesis at multiple stages during kidney development, and are essential for collecting duct physiology; together, these reports emphasize a critical and continuous requirement for the appropriate modulation of gene expression by miRNAs during metanephric kidney development and function. The potential use of miRNAs as biomarkers for several kidney anomalies –including acute and chronic kidney injuries and cystic kidney diseases– enhances their importance in the prognosis, diagnosis, and treatment of kidney diseases (Aguado-Fraile et al., 2013; Bellinger et al., 2014; Beltrami et al., 2012; Kanki et al., 2014; Lorenzen and Thum, 2012; Woo and Park, 2013). Indeed, the recent finding that let-7 miRNAs potentially regulate ureteric bud epithelial cell fate from the precursor to the differentiated state (Nagalakshmi et al., 2014) also hints at the possibility of using miRNAs as molecular regulators in reprogramming the ureteric bud epithelial cells in vitro, which could have significant applications in cell-based therapies for human kidney repair and regeneration.
Patterning of the kidney
The metanephric kidney is divided into two compartments, an outer compartment of the renal cortex and an inner compartment of the renal medulla (Fig. 1). The medulla is divided into the outer and inner medulla (the papilla). The outer medulla is further divided into the outer and inner stripe. The renal cortex is where renal corpuscles, proximal and distal convoluted tubules. and cortical collecting ducts reside, whereas medullary collecting ducts and most of the loops of Henle are located in the medullary compartment (Fig. 2) in a parallel fashion. The renal medulla also contains the interstitium and the vasa recta, a network of straight capillaries.
As mentioned earlier, the renal medulla arises late in development compared to the renal cortex. Consistently, renal medulla maturation extends into postnatal development, well after nephrogenesis and ureteric bud branching morphogenesis has ceased. Consequently, the renal medulla gradually acquires functional maturity; full urine-concentration capacity is achieved at 6 weeks of age in the rat (Madsen et al., 2013) and at 18 months of age in humans (Polacek et al., 1965).
Development of the renal medulla has received increased attention in recent years. A variety of molecular pathways has been shown to regulate this process, and the cellular mechanisms governing the process are being elucidated. These studies demonstrate a critical role for the ureteric bud epithelium in renal medulla development: The BMP receptor ALK3, expressed in the ureteric bud epithelium, is implicated in proper renal medulla formation (Hartwig et al., 2008; Qiao et al., 1999). In its absence, renal medulla hypoplasia/dysplasia develops. This is probably due to the critical involvement of ALK3 in UB branching morphogenesis (Hartwig et al., 2008; Qiao et al., 1999) since Alk3 mutant mice present with fewer collecting ducts, which likely results in the observed renal medulla deficiency. The renal medulla defect worsened in postnatal stages, with a complete lack of papilla and dilated and disarrayed medullary collecting ducts.
In contrast, glypican-3 (GPC3), EGF receptor, and α3 integrin are all required for collecting duct survival and at least partly regulate renal medulla elongation (Cano-Gauci et al., 1999; Grisaru et al., 2001; Kreidberg et al., 1996; Liu et al., 2009). In the case of Gpc3 mutants, the reduction in collecting duct number is possibly a consequence of the over-proliferation of ureteric bud epithelial cells earlier. α3 integrin probably regulates renal papilla elongation by serving as the laminin receptor, as renal papilla extension was similarly retarded in α5 laminin (Lama5)-null kidneys, and by activating/maintaining Wnt7b expression in the collecting duct in conjunction with HGF-MET signaling. Further, α3 integrin promotes renal papilla cell survival by acting in conjunction with HGF and canonical WNT signaling (Liu et al., 2009).
The renin-angiotensin system also acts on the ureteric bud epithelium to regulate renal medulla formation/maintenance, in addition to its effects on other renal tissues. It does so by regulating multiple aspects of ureteric bud development –including ureteric bud branching morphogenesis and papillary collecting duct proliferation and survival– and modulating the expression of genes implicated in renal medulla development –such as Wnt7b, Fgf7, Ctnnb (β-catenin), Ppp3r2 (calcineurin B1), and Itga3 (α3 integrin) (Esther et al., 1996; Miyazaki et al., 1999; Miyazaki et al., 1998; Nagata et al., 1996; Niimura et al., 1995; Nishimura et al., 1999; Oliverio et al., 1998; Song et al., 2012; Song et al., 2011; Song et al., 2009; Takahashi et al., 2005; Tsuchida et al., 1998; Yosypiv et al., 2006).
Ablation of Wnt7b revealed a central role of this ureteric bud-derived signaling ligand in renal medulla formation (Yu et al., 2009). No renal medulla ever forms in the absence of Wnt7b, and cortical structures, such as the renal corpuscles and the proximal and distal convoluted tubules, lie essentially right above the renal pelvis. WNT7B regulates renal medulla formation most likely by activating the WNT/β-catenin pathway in the adjacent renal interstitium. Consistent with the importance of this signaling activation in renal medulla formation, ablation of β-catenin from the renal interstitium also led to renal medulla agenesis (Yu et al., 2009). Downstream of WNT7B and WNT/β-catenin signaling in the renal interstitium, p57kip2 (Cdkn1c) is expressed in the medullary interstitium and contributes to renal medulla formation, as p57kip2-mutant mice displayed renal medulla hypoplasia (Zhang et al., 1997). It is not known how the renal interstitium signals back to the collecting ducts to regulate their elongation. Regardless, oriented cell division in the prospective medullary collecting duct was disrupted in Wnt7b mutants such that the prospective medullary collecting duct cells tended to divide along an axis that preferentially increase the collecting duct diameter in the mutants at the expense of duct elongation. It remains to be determined if other cellular mechanisms, such as cell intercalation, are involved in collecting duct elongation in response to WNT7B and interstitial signals.
Live imaging of kidneys in a recently developed low-volume organ-culture system suggests a phenomenon of “node retraction”, where the branch points (nodes) of the ureteric tree retract toward the pelvis in absolute term, generating parallel arrangements of ureteric trunks similar to the organization of collecting ducts of the renal medulla (Lindstrom et al., 2015). If this ex vivo observation reflects renal medulla formation in vivo, it will be worth examining if WNT7B participates in the regulation of branching during renal medulla formation. Which molecular and/or cellular cues establish the longitudinal axis of the collecting ducts (or the cortico-medullary axis) and how the collecting ducts sense them remain unknown. It was suggested, however, that the process of node retraction generates stress that can orient cell division (Lindstrom et al., 2015), a notion awaiting experimental testing.
The ureteric bud-derived dickkopf (DKK1), an antagonist of canonical WNT signaling and other non-WNT growth factors, inhibits collecting duct proliferation and thus regulates renal medulla elongation (Pietila et al., 2011). In its absence, a longer-than-normal renal medulla forms. Most likely, DKK1 regulates renal medulla elongation at least in part by acting on the WNT/β-catenin pathway in the renal interstitium, as the activity of this pathway increased in the renal interstitium of Dkk1-mutant mice (Pietila et al., 2011). DKK1 likely antagonizes WNT7B function during renal medulla development, based on their strikingly opposing effects on renal medulla elongation, the canonical WNT response in the medullary interstitium, and the fact that DKK1 can indeed subdue WNT7B canonical signaling in vitro (Pietila et al., 2011; Yu et al., 2009). On the other hand, the difference in their effects on collecting duct cell proliferation suggests that either DKK1 additionally acts on other, unknown signaling pathways that also regulates renal medulla formation, or that redundant WNT ligand(s) masks the role of WNT7B in collecting-duct cell proliferation.
No renal papilla was present in estrogen-related receptor gamma (Esrrg/Nr3b3)-mutant embryos (Berry et al., 2011). The expression of Esrrg in ureteric trunks, with this expression gradually becoming restricted to medullary collecting ducts later in embryonic development, suggests a direct role for Esrrg in the UB epithelium during renal papilla elongation, though the underlying molecular and cellular mechanism remains elusive (Berry et al., 2011).
Concluding remarks
With a combination of genetic studies in model organisms and manipulations conduected on in vitro organ and cell cultures, our knowledge into the molecular and cellular mechanisms underpinning kidney formation has expanded greatly over the past decades. Yet, these studies also revealed the gaps in our understanding of and generated more questions on the formation of the metanephric kidney. What cellular mechanisms mediate differential RET activity-regulated nephric duct cell migration? What is the function of mitosis-associated cell dispersal of the ureteric bud cells? How are the fates between the ureteric tip and the ureteric trunk or between the ureteric trunk and the collecting ducts determined? How does the renal interstitium regulate renal medulla elongation? With an increasing population inflicted with chronic renal diseases, effective strategies for kidney repair and regeneration are in high demand. A deep understanding of renal development is also critical to practices employing cell-based therapy and development of repair programs in vivo.
Acknowledgements
Research in the Yu laboratory was supported by funding from American Heart Association Grant-in-Aid 12GRNT12060070 and National Institutions of Health (NIDDK) R01DK085080 to J.Y.
List of abbreviations
- BMP
bone morphogenetic protein
- E#
mouse embryonic day #
- EGF[R]
epidermal growth factor [receptor]
- FGF
fibroblast growth factor
- GDNF
glial cell-derived neurotrophic factor
- HGF
hepatocyte growth factor
- miRNA/miR
microRNA
- RET
receptor tyrosine kinase that binds GDNF
- TGFβ
transforming growth factor beta
References
- Aguado-Fraile E, Ramos E, Conde E, Rodriguez M, Liano F, Garcia-Bermejo ML. MicroRNAs in the kidney: novel biomarkers of acute kidney injury. Nefrologia. 2013;33:826–834. doi: 10.3265/Nefrologia.pre2013.Aug.12198. [DOI] [PubMed] [Google Scholar]
- Akkina S, Becker BN. MicroRNAs in kidney function and disease. Transl Res. 2011;157:236–240. doi: 10.1016/j.trsl.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartram MP, Hohne M, Dafinger C, Volker LA, Albersmeyer M, Heiss J, Gobel H, Bronneke H, Burst V, Liebau MC, et al. Conditional loss of kidney microRNAs results in congenital anomalies of the kidney and urinary tract (CAKUT) J Mol Med (Berl) 2013;91:739–748. doi: 10.1007/s00109-013-1000-x. [DOI] [PubMed] [Google Scholar]
- Basson MA, Akbulut S, Watson-Johnson J, Simon R, Carroll TJ, Shakya R, Gross I, Martin GR, Lufkin T, McMahon AP, et al. Sprouty1 Is a Critical Regulator of GDNF/RET-Mediated Kidney Induction. Developmental Cell. 2005;8:229–239. doi: 10.1016/j.devcel.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Bates CM. Role of fibroblast growth factor receptor signaling in kidney development. Am J Physiol Renal Physiol. 2011;301:F245–F251. doi: 10.1152/ajprenal.00186.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batourina E, Choi C, Paragas N, Bello N, Hensle T, Costantini FD, Schuchardt A, Bacallao RL, Mendelsohn CL. Distal ureter morphogenesis depends on epithelial cell remodeling mediated by vitamin A and Ret. Nat Genet. 2002;32:109–115. doi: 10.1038/ng952. [DOI] [PubMed] [Google Scholar]
- Batourina E, Gim S, Bello N, Shy M, Clagett-Dame M, Srinivas S, Costantini F, Mendelsohn C. Vitamin A controls epithelial/mesenchymal interactions through Ret expression. Nat Genet. 2001;27:74–78. doi: 10.1038/83792. [DOI] [PubMed] [Google Scholar]
- Bellinger MA, Bean JS, Rader MA, Heinz-Taheny KM, Nunes JS, Haas JV, Michael LF, Rekhter MD. Concordant changes of plasma and kidney microRNA in the early stages of acute kidney injury: time course in a mouse model of bilateral renal ischemia-reperfusion. PLoS One. 2014;9:e93297. doi: 10.1371/journal.pone.0093297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltrami C, Clayton A, Phillips AO, Fraser DJ, Bowen T. Analysis of urinary microRNAs in chronic kidney disease. Biochem Soc Trans. 2012;40:875–879. doi: 10.1042/BST20120090. [DOI] [PubMed] [Google Scholar]
- Berry R, Harewood L, Pei L, Fisher M, Brownstein D, Ross A, Alaynick WA, Moss J, Hastie ND, Hohenstein P, et al. Esrrg functions in early branch generation of the ureteric bud and is essential for normal development of the renal papilla. Hum Mol Genet. 2011;20:917–926. doi: 10.1093/hmg/ddq530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt K, Mi QS, Dong Z. microRNAs in kidneys: biogenesis, regulation, and pathophysiological roles. Am J Physiol Renal Physiol. 2011;300:F602–F610. doi: 10.1152/ajprenal.00727.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake J, Rosenblum ND. Renal branching morphogenesis: Morphogenetic and signaling mechanisms. Semin Cell Dev Biol. 2014 doi: 10.1016/j.semcdb.2014.07.011. [DOI] [PubMed] [Google Scholar]
- Blomqvist SR, Vidarsson H, Fitzgerald S, Johansson BR, Ollerstam A, Brown R, Persson AE, Bergstrom GG, Enerback S. Distal renal tubular acidosis in mice that lack the forkhead transcription factor Foxi1. J Clin Invest. 2004;113:1560–1570. doi: 10.1172/JCI20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgewater D, Cox B, Cain J, Lau A, Athaide V, Gill PS, Kuure S, Sainio K, Rosenblum ND. Canonical WNT/beta-catenin signaling is required for ureteric branching. Dev Biol. 2008;317:83–94. doi: 10.1016/j.ydbio.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Bridgewater D, Di Giovanni V, Cain JE, Cox B, Jakobson M, Sainio K, Rosenblum ND. beta-catenin causes renal dysplasia via upregulation of Tgfbeta2 and Dkk1. J Am Soc Nephrol. 2011;22:718–731. doi: 10.1681/ASN.2010050562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodbeck S, Besenbeck B, Englert C. The transcription factor Six2 activates expression of the Gdnf gene as well as its own promoter. Mech Dev. 2004;121:1211–1222. doi: 10.1016/j.mod.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Brophy PD, Ostrom L, Lang KM, Dressler GR. Regulation of ureteric bud outgrowth by Pax2-dependent activation of the glial derived neurotrophic factor gene. Development. 2001;128:4747–4756. doi: 10.1242/dev.128.23.4747. [DOI] [PubMed] [Google Scholar]
- Cain JE, Islam E, Haxho F, Chen L, Bridgewater D, Nieuwenhuis E, Hui CC, Rosenblum ND. GLI3 repressor controls nephron number via regulation of Wnt11 and Ret in ureteric tip cells. PLoS One. 2009;4:e7313. doi: 10.1371/journal.pone.0007313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano-Gauci DF, Song HH, Yang H, McKerlie C, Choo B, Shi W, Pullano R, Piscione TD, Grisaru S, Soon S, et al. Glypican-3-deficient mice exhibit developmental overgrowth and some of the abnormalities typical of Simpson-Golabi-Behmel syndrome. J Cell Biol. 1999;146:255–264. doi: 10.1083/jcb.146.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebrian C, Borodo K, Charles N, Herzlinger DA. Morphometric index of the developing murine kidney. Dev Dyn. 2004;231:601–608. doi: 10.1002/dvdy.20143. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran K, Karolina DS, Sepramaniam S, Armugam A, Wintour EM, Bertram JF, Jeyaseelan K. Role of microRNAs in kidney homeostasis and disease. Kidney Int. 2012;81:617–627. doi: 10.1038/ki.2011.448. [DOI] [PubMed] [Google Scholar]
- Chi L, Galtseva A, Chen L, Mo R, Hui CC, Rosenblum ND. Kif3a controls murine nephron number via GLI3 repressor, cell survival, and gene expression in a lineage-specific manner. PLoS One. 2013;8:e65448. doi: 10.1371/journal.pone.0065448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi L, Zhang S, Lin Y, Prunskaite-Hyyrylainen R, Vuolteenaho R, Itaranta P, Vainio S. Sprouty proteins regulate ureteric branching by coordinating reciprocal epithelial Wnt11, mesenchymal Gdnf and stromal Fgf7 signalling during kidney development. Development. 2004;131:3345–3356. doi: 10.1242/dev.01200. [DOI] [PubMed] [Google Scholar]
- Chi X, Michos O, Shakya R, Riccio P, Enomoto H, Licht JD, Asai N, Takahashi M, Ohgami N, Kato M, et al. Ret-dependent cell rearrangements in the Wolffian duct epithelium initiate ureteric bud morphogenesis. Dev Cell. 2009;17:199–209. doi: 10.1016/j.devcel.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AT, Young RJ, Bertram JF. In vitro studies on the roles of transforming growth factor-beta 1 in rat metanephric development. Kidney Int. 2001;59:1641–1653. doi: 10.1046/j.1523-1755.2001.0590051641.x. [DOI] [PubMed] [Google Scholar]
- Costantini F, Kopan R. Patterning a Complex Organ: Branching Morphogenesis and Nephron Segmentation in Kidney Development. Developmental Cell. 2010;18:698–712. doi: 10.1016/j.devcel.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AJ. Mouse kidney development. 2008 [PubMed] [Google Scholar]
- Davis TK, Hoshi M, Jain S. To bud or not to bud: the RET perspective in CAKUT. Pediatr Nephrol. 2014;29:597–608. doi: 10.1007/s00467-013-2606-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressler GR. The cellular basis of kidney development. Annu Rev Cell Dev Biol. 2006;22:509–529. doi: 10.1146/annurev.cellbio.22.010305.104340. [DOI] [PubMed] [Google Scholar]
- Dressler GR. Advances in early kidney specification, development and patterning. Development. 2009;136:3863–3874. doi: 10.1242/dev.034876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edinger RS, Coronnello C, Bodnar AJ, Laframboise WA, Benos PV, Ho J, Johnson JP, Butterworth MB. Aldosterone Regulates MicroRNAs in the Cortical Collecting Duct to Alter Sodium Transport. J Am Soc Nephrol. 2014 doi: 10.1681/ASN.2013090931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esquela AF, Lee Se-J. Regulation of metanephric kidney development by growth/differentiation factor 11. Developmental Biology. 2003;257:356–370. doi: 10.1016/s0012-1606(03)00100-3. [DOI] [PubMed] [Google Scholar]
- Esther CR, Jr., Howard TE, Marino EM, Goddard JM, Capecchi MR, Bernstein KE. Mice lacking angiotensin-converting enzyme have low blood pressure, renal pathology, and reduced male fertility. Lab Invest. 1996;74:953–965. [PubMed] [Google Scholar]
- Gao X, Chen X, Taglienti M, Rumballe B, Little MH, Kreidberg JA. Angioblast-mesenchyme induction of early kidney development is mediated by Wt1 and Vegfa. Development. 2005;132:5437–5449. doi: 10.1242/dev.02095. [DOI] [PubMed] [Google Scholar]
- Grieshammer U, Le M, Plump AS, Wang F, Tessier-Lavigne M, Martin GR. SLIT2-Mediated ROBO2 Signaling Restricts Kidney Induction to a Single Site. Developmental Cell. 2004;6:709–717. doi: 10.1016/s1534-5807(04)00108-x. [DOI] [PubMed] [Google Scholar]
- Grisaru S, Cano-Gauci D, Tee J, Filmus J, Rosenblum ND. Glypican-3 modulates BMP- and FGF-mediated effects during renal branching morphogenesis. Dev Biol. 2001;231:31–46. doi: 10.1006/dbio.2000.0127. [DOI] [PubMed] [Google Scholar]
- Grobstein C. Inductive epithelio-mesenchymal interaction in cultured organ rudiments of the mouse. Science. 1953;118:52–55. doi: 10.1126/science.118.3054.52. [DOI] [PubMed] [Google Scholar]
- Grobstein C. Inductive interaction in the development of the mouse metanephros. Journal of experimental zoology. 1955;130:319–339. [Google Scholar]
- Guo Q, Wang Y, Tripathi P, Manda KR, Mukherjee M, Chaklader M, Austin PF, Surendran K, Chen F. Adam10 Mediates the Choice between Principal Cells and Intercalated Cells in the Kidney. J Am Soc Nephrol. 2014 doi: 10.1681/ASN.2013070764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains D, Sims-Lucas S, Kish K, Saha M, McHugh K, Bates CM. Role of fibroblast growth factor receptor 2 in kidney mesenchyme. Pediatr Res. 2008;64:592–598. doi: 10.1203/PDR.0b013e318187cc12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman HA, Lai HL, Patterson LT. Cessation of renal morphogenesis in mice. Dev Biol. 2007;310:379–387. doi: 10.1016/j.ydbio.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig S, Bridgewater D, Di Giovanni V, Cain J, Mishina Y, Rosenblum ND. BMP receptor ALK3 controls collecting system development. J Am Soc Nephrol. 2008;19:117–124. doi: 10.1681/ASN.2007010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa Y, Satoh K, Iizuka-Kogo A, Shimomura A, Nomura R, Akiyama T, Senda T. Loss of ICAT gene function leads to arrest of ureteric bud branching and renal agenesis. Biochem Biophys Res Commun. 2007;362:988–994. doi: 10.1016/j.bbrc.2007.08.085. [DOI] [PubMed] [Google Scholar]
- Hatini V, Huh SO, Herzlinger D, Soares VC, Lai E. Essential role of stromal mesenchyme in kidney morphogenesis revealed by targeted disruption of Winged Helix transcription factor BF-2. Genes Dev. 1996;10:1467–1478. doi: 10.1101/gad.10.12.1467. [DOI] [PubMed] [Google Scholar]
- Ho J, Kreidberg JA. MicroRNAs in renal development. Pediatr Nephrol. 2013;28:219–225. doi: 10.1007/s00467-012-2204-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu MC, Piscione TD, Rosenblum ND. Elevated SMAD1/beta-catenin molecular complexes and renal medullary cystic dysplasia in ALK3 transgenic mice. Development. 2003;130:2753–2766. doi: 10.1242/dev.00478. [DOI] [PubMed] [Google Scholar]
- Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 2010;176:85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibe S, Karihaloo A, Ma H, Zhang J, Marlier A, Mitobe M, Togawa A, Schmitt R, Czyczk J, Kashgarian M, et al. Met and the epidermal growth factor receptor act cooperatively to regulate final nephron number and maintain collecting duct morphology. Development. 2009;136:337–345. doi: 10.1242/dev.024463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S, Encinas M, Johnson EM, Jr., Milbrandt J. Critical and distinct roles for key RET tyrosine docking sites in renal development. Genes Dev. 2006;20:321–333. doi: 10.1101/gad.1387206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong HW, Jeon US, Koo BK, Kim WY, Im SK, Shin J, Cho Y, Kim J, Kong YY. Inactivation of Notch signaling in the renal collecting duct causes nephrogenic diabetes insipidus in mice. J Clin Invest. 2009;119:3290–3300. doi: 10.1172/JCI38416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanki M, Moriguchi A, Sasaki D, Mitori H, Yamada A, Unami A, Miyamae Y. Identification of urinary miRNA biomarkers for detecting cisplatin-induced proximal tubular injury in rats. Toxicology. 2014 doi: 10.1016/j.tox.2014.05.004. [DOI] [PubMed] [Google Scholar]
- Khella HW, Bakhet M, Lichner Z, Romaschin AD, Jewett MA, Yousef GM. MicroRNAs in kidney disease: an emerging understanding. Am J Kidney Dis. 2013;61:798–808. doi: 10.1053/j.ajkd.2012.09.018. [DOI] [PubMed] [Google Scholar]
- Kiefer SM, Robbins L, Stumpff KM, Lin C, Ma L, Rauchman M. Sall1-dependent signals affect Wnt signaling and ureter tip fate to initiate kidney development. Development. 2010;137:3099–3106. doi: 10.1242/dev.037812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kim YH, Cha JH, Tisher CC, Madsen KM. Intercalated cell subtypes in connecting tubule and cortical collecting duct of rat and mouse. J Am Soc Nephrol. 1999;10:1–12. doi: 10.1681/ASN.V1011. [DOI] [PubMed] [Google Scholar]
- Kispert A, Vainio S, Shen L, Rowitch DH, McMahon AP. Proteoglycans are required for maintenance of Wnt-11 expression in the ureter tips. Development. 1996;122:3627–3637. doi: 10.1242/dev.122.11.3627. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Mugford JW, Krautzberger AM, Naiman N, Liao J, McMahon AP. Identification of a multipotent self-renewing stromal progenitor population during mammalian kidney organogenesis. Stem Cell Reports. 2014;3:650–662. doi: 10.1016/j.stemcr.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H, Kawakami K, Asashima M, Nishinakamura R. Six1 and Six4 are essential for Gdnf expression in the metanephric mesenchyme and ureteric bud formation, while Six1 deficiency alone causes mesonephric-tubule defects. Mech Dev. 2007;124:290–303. doi: 10.1016/j.mod.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Kreidberg JA, Donovan MJ, Goldstein SL, Rennke H, Shepherd K, Jones RC, Jaenisch R. Alpha 3 beta 1 integrin has a crucial role in kidney and lung organogenesis. Development. 1996;122:3537–3547. doi: 10.1242/dev.122.11.3537. [DOI] [PubMed] [Google Scholar]
- Kume T, Deng K, Hogan BL. Murine forkhead/winged helix genes Foxc1 (Mf1) and Foxc2 (Mfh1) are required for the early organogenesis of the kidney and urinary tract. Development. 2000;127:1387–1395. doi: 10.1242/dev.127.7.1387. [DOI] [PubMed] [Google Scholar]
- Kurth I, Hentschke M, Hentschke S, Borgmeyer U, Gal A, Hubner CA. The forkhead transcription factor Foxi1 directly activates the AE4 promoter. Biochem J. 2006;393:277–283. doi: 10.1042/BJ20051094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuure S, Cebrian C, Machingo Q, Lu BC, Chi X, Hyink D, D'Agati V, Gurniak C, Witke W, Costantini F. Actin depolymerizing factors cofilin1 and destrin are required for ureteric bud branching morphogenesis. PLoS Genet. 2010;6:e1001176. doi: 10.1371/journal.pgen.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf P, Rusu M, Sheridan R, Sewer A, Iovino N, Aravin A, Pfeffer S, Rice A, Kamphorst AO, Landthaler M, et al. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell. 2007;129:1401–1414. doi: 10.1016/j.cell.2007.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson RS, Batourina E, Choi C, Vorontchikhina M, Kitajewski J, Mendelsohn CL. Foxd1-dependent signals control cellularity in the renal capsule, a structure required for normal renal development. Development. 2005;132:529–539. doi: 10.1242/dev.01604. [DOI] [PubMed] [Google Scholar]
- Li X, Oghi KA, Zhang J, Krones A, Bush KT, Glass CK, Nigam SK, Aggarwal AK, Maas R, Rose DW, et al. Eya protein phosphatase activity regulates Six1-Dach-Eya transcriptional effects in mammalian organogenesis. Nature. 2003;426:247–254. doi: 10.1038/nature02083. [DOI] [PubMed] [Google Scholar]
- Lindstrom NO, Chang CH, Valerius MT, Hohenstein P, Davies JA. Node retraction during patterning of the urinary collecting duct system. J Anat. 2015;226:13–21. doi: 10.1111/joa.12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton JM, Martin GR, Reichardt LF. The ECM protein nephronectin promotes kidney development via integrin alpha8beta1-mediated stimulation of Gdnf expression. Development. 2007;134:2501–2509. doi: 10.1242/dev.005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little MH, Brennan J, Georgas K, Davies JA, Davidson DR, Baldock RA, Beverdam A, Bertram JF, Capel B, Chiu HS, et al. A high-resolution anatomical ontology of the developing murine genitourinary tract. Gene Expr Patterns. 2007;7:680–699. doi: 10.1016/j.modgep.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little MH, McMahon AP. Mammalian kidney development: principles, progress, and projections. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a008300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Chattopadhyay N, Qin S, Szekeres C, Vasylyeva T, Mahoney ZX, Taglienti M, Bates CM, Chapman HA, Miner JH, et al. Coordinate integrin and c-Met signaling regulate Wnt gene expression during epithelial morphogenesis. Development. 2009;136:843–853. doi: 10.1242/dev.027805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzen JM, Thum T. Circulating and urinary microRNAs in kidney disease. Clin J Am Soc Nephrol. 2012;7:1528–1533. doi: 10.2215/CJN.01170212. [DOI] [PubMed] [Google Scholar]
- Lu BC, Cebrian C, Chi X, Kuure S, Kuo R, Bates CM, Arber S, Hassell J, MacNeil L, Hoshi M, et al. Etv4 and Etv5 are required downstream of GDNF and Ret for kidney branching morphogenesis. Nat Genet. 2009;41:1295–1302. doi: 10.1038/ng.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyckx VA, Brenner BM. Low birth weight, nephron number, and kidney disease. Kidney Int Suppl. 2005:S68–S77. doi: 10.1111/j.1523-1755.2005.09712.x. [DOI] [PubMed] [Google Scholar]
- Ma L, Qu L. The function of microRNAs in renal development and pathophysiology. J Genet Genomics. 2013;40:143–152. doi: 10.1016/j.jgg.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Madsen K, Tinning AR, Marcussen N, Jensen BL. Postnatal development of the renal medulla; role of the renin-angiotensin system. Acta Physiol (Oxf) 2013;208:41–49. doi: 10.1111/apha.12088. [DOI] [PubMed] [Google Scholar]
- Madsen KM, Clapp WL, Verlander JW. Structure and function of the inner medullary collecting duct. Kidney Int. 1988;34:441–454. doi: 10.1038/ki.1988.201. [DOI] [PubMed] [Google Scholar]
- Maeshima A, Vaughn DA, Choi Y, Nigam SK. Activin A is an endogenous inhibitor of ureteric bud outgrowth from the Wolffian duct. Dev Biol. 2006;295:473–485. doi: 10.1016/j.ydbio.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Maeshima A, Yamashita S, Maeshima K, Kojima I, Nojima Y. Activin a produced by ureteric bud is a differentiation factor for metanephric mesenchyme. J Am Soc Nephrol. 2003;14:1523–1534. doi: 10.1097/01.asn.0000067419.86611.21. [DOI] [PubMed] [Google Scholar]
- Majumdar A, Vainio S, Kispert A, McMahon J, McMahon AP. Wnt11 and Ret/Gdnf pathways cooperate in regulating ureteric branching during metanephric kidney development. Development. 2003;130:3175–3185. doi: 10.1242/dev.00520. [DOI] [PubMed] [Google Scholar]
- Marlier A, Gilbert T. Expression of retinoic acid-synthesizing and -metabolizing enzymes during nephrogenesis in the rat. Gene Expr Patterns. 2004;5:179–185. doi: 10.1016/j.modgep.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Marlier A, Schmidt-Ott KM, Gallagher AR, Barasch J, Karihaloo A. Vegf as an epithelial cell morphogen modulates branching morphogenesis of embryonic kidney by directly acting on the ureteric bud. Mech Dev. 2009;126:91–98. doi: 10.1016/j.mod.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Marose TD, Merkel CE, McMahon AP, Carroll TJ. Beta-catenin is necessary to keep cells of ureteric bud/Wolffian duct epithelium in a precursor state. Dev Biol. 2008;314:112–126. doi: 10.1016/j.ydbio.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn C, Batourina E, Fung S, Gilbert T, Dodd J. Stromal cells mediate retinoid-dependent functions essential for renal development. Development. 1999;126:1139–1148. doi: 10.1242/dev.126.6.1139. [DOI] [PubMed] [Google Scholar]
- Mendelsohn C, Lohnes D, Decimo D, Lufkin T, LeMeur M, Chambon P, Mark M. Function of the retinoic acid receptors (RARs) during development (II). Multiple abnormalities at various stages of organogenesis in RAR double mutants. Development. 1994;120:2749–2771. doi: 10.1242/dev.120.10.2749. [DOI] [PubMed] [Google Scholar]
- Michael L, Davies JA. Pattern and regulation of cell proliferation during murine ureteric bud development. J Anat. 2004;204:241–255. doi: 10.1111/j.0021-8782.2004.00285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michos O, Cebrian C, Hyink D, Grieshammer U, Williams L, D'Agati V, Licht JD, Martin GR, Costantini F. Kidney development in the absence of Gdnf and Spry1 requires Fgf10. PLoS Genet. 2010;6:e1000809. doi: 10.1371/journal.pgen.1000809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michos O, Goncalves A, Lopez-Rios J, Tiecke E, Naillat F, Beier K, Galli A, Vainio S, Zeller R. Reduction of BMP4 activity by gremlin 1 enables ureteric bud outgrowth and GDNF/WNT11 feedback signalling during kidney branching morphogenesis. Development. 2007;134:2397–2405. doi: 10.1242/dev.02861. [DOI] [PubMed] [Google Scholar]
- Miyazaki Y, Oshima K, Fogo A, Hogan BL, Ichikawa I. Bone morphogenetic protein 4 regulates the budding site and elongation of the mouse ureter. J Clin Invest. 2000;105:863–873. doi: 10.1172/JCI8256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki Y, Tsuchida S, Fogo A, Ichikawa I. The renal lesions that develop in neonatal mice during angiotensin inhibition mimic obstructive nephropathy. Kidney Int. 1999;55:1683–1695. doi: 10.1046/j.1523-1755.1999.00420.x. [DOI] [PubMed] [Google Scholar]
- Miyazaki Y, Tsuchida S, Nishimura H, Pope JCt, Harris RC, McKanna JM, Inagami T, Hogan BL, Fogo A, Ichikawa I. Angiotensin induces the urinary peristaltic machinery during the perinatal period. J Clin Invest. 1998;102:1489–1497. doi: 10.1172/JCI4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MW, Klein RD, Farinas I, Sauer H, Armanini M, Phillips H, Reichardt LF, Ryan AM, Carver-Moore K, Rosenthal A. Renal and neuronal abnormalities in mice lacking GDNF. Nature. 1996;382:76–79. doi: 10.1038/382076a0. [DOI] [PubMed] [Google Scholar]
- Moreau E, Vilar J, Lelievre-Pegorier M, Merlet-Benichou C, Gilbert T. Regulation of c-ret expression by retinoic acid in rat metanephros: implication in nephron mass control. Am J Physiol. 1998;275:F938–F945. doi: 10.1152/ajprenal.1998.275.6.F938. [DOI] [PubMed] [Google Scholar]
- Mount PF, Power DA. Nitric oxide in the kidney: functions and regulation of synthesis. Acta Physiol (Oxf) 2006;187:433–446. doi: 10.1111/j.1748-1716.2006.01582.x. [DOI] [PubMed] [Google Scholar]
- Nagalakshmi VK, Lindner V, Wessels A, Yu J. microRNA-dependent temporal gene expression in the ureteric bud epithelium during mammalian kidney development. Dev Dyn. 2014 doi: 10.1002/dvdy.24221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagalakshmi VK, Ren Q, Pugh MM, Valerius MT, McMahon AP, Yu J. Dicer regulates the development of nephrogenic and ureteric compartments in the mammalian kidney. Kidney Int. 2011;79:317–330. doi: 10.1038/ki.2010.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata M, Tanimoto K, Fukamizu A, Kon Y, Sugiyama F, Yagami K, Murakami K, Watanabe T. Nephrogenesis and renovascular development in angiotensinogen-deficient mice. Lab Invest. 1996;75:745–753. [PubMed] [Google Scholar]
- Niimura F, Labosky PA, Kakuchi J, Okubo S, Yoshida H, Oikawa T, Ichiki T, Naftilan AJ, Fogo A, Inagami T, et al. Gene targeting in mice reveals a requirement for angiotensin in the development and maintenance of kidney morphology and growth factor regulation. J Clin Invest. 1995;96:2947–2954. doi: 10.1172/JCI118366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura H, Yerkes E, Hohenfellner K, Miyazaki Y, Ma J, Hunley TE, Yoshida H, Ichiki T, Threadgill D, Phillips JA, 3rd, et al. Role of the angiotensin type 2 receptor gene in congenital anomalies of the kidney and urinary tract, CAKUT, of mice and men. Mol Cell. 1999;3:1–10. doi: 10.1016/s1097-2765(00)80169-0. [DOI] [PubMed] [Google Scholar]
- Oliverio MI, Kim HS, Ito M, Le T, Audoly L, Best CF, Hiller S, Kluckman K, Maeda N, Smithies O, et al. Reduced growth, abnormal kidney structure, and type 2 (AT2) angiotensin receptor-mediated blood pressure regulation in mice lacking both AT1A and AT1B receptors for angiotensin II. Proc Natl Acad Sci U S A. 1998;95:15496–15501. doi: 10.1073/pnas.95.26.15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard A, Georgas K, Michos O, Riccio P, Cebrian C, Combes AN, Ju A, Ferrer-Vaquer A, Hadjantonakis AK, Zong H, et al. Luminal mitosis drives epithelial cell dispersal within the branching ureteric bud. Dev Cell. 2013;27:319–330. doi: 10.1016/j.devcel.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey P, Qin S, Ho J, Zhou J, Kreidberg JA. Systems biology approach to identify transcriptome reprogramming and candidate microRNA targets during the progression of polycystic kidney disease. BMC Syst Biol. 2011;5:56. doi: 10.1186/1752-0509-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paroly SS, Wang F, Spraggon L, Merregaert J, Batourina E, Tycko B, Schmidt-Ott KM, Grimmond S, Little M, Mendelsohn C. Stromal protein Ecm1 regulates ureteric bud patterning and branching. PLoS One. 2013;8:e84155. doi: 10.1371/journal.pone.0084155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastorelli LM, Wells S, Fray M, Smith A, Hough T, Harfe BD, McManus MT, Smith L, Woolf AS, Cheeseman M, et al. Genetic analyses reveal a requirement for Dicer1 in the mouse urogenital tract. Mamm Genome. 2009;20:140–151. doi: 10.1007/s00335-008-9169-y. [DOI] [PubMed] [Google Scholar]
- Patel V, Hajarnis S, Williams D, Hunter R, Huynh D, Igarashi P. MicroRNAs regulate renal tubule maturation through modulation of Pkd1. J Am Soc Nephrol. 2012;23:1941–1948. doi: 10.1681/ASN.2012030321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V, Williams D, Hajarnis S, Hunter R, Pontoglio M, Somlo S, Igarashi P. miR-17~92 miRNA cluster promotes kidney cyst growth in polycystic kidney disease. Proc Natl Acad Sci U S A. 2013;110:10765–10770. doi: 10.1073/pnas.1301693110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce D, Soundararajan R, Trimpert C, Kashlan OB, Deen PM, Kohan DE. Collecting Duct Principal Cell Transport Processes and Their Regulation. Clin J Am Soc Nephrol. 2014 doi: 10.2215/CJN.05760513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepicelli CV, Kispert A, Rowitch DH, McMahon AP. GDNF induces branching and increased cell proliferation in the ureter of the mouse. Dev Biol. 1997;192:193–198. doi: 10.1006/dbio.1997.8745. [DOI] [PubMed] [Google Scholar]
- Pichel JG, Shen L, Sheng HZ, Granholm AC, Drago J, Grinberg A, Lee EJ, Huang SP, Saarma M, Hoffer BJ, et al. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature. 1996;382:73–76. doi: 10.1038/382073a0. [DOI] [PubMed] [Google Scholar]
- Pietila I, Ellwanger K, Railo A, Jokela T, Del Barco Barrantes I, Shan J, Niehrs C, Vainio SJ. Secreted Wnt antagonist Dickkopf-1 controls kidney papilla development coordinated by Wnt-7b signalling. Dev Biol. 2011 doi: 10.1016/j.ydbio.2011.02.019. [DOI] [PubMed] [Google Scholar]
- Polacek E, Vocel J, Neugebauerova L, Sebkova M, Vechetova E. The Osmotic Concentrating Ability in Healthy Infants and Children. Arch Dis Child. 1965;40:291–295. doi: 10.1136/adc.40.211.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottier N, Cauffiez C, Perrais M, Barbry P, Mari B. FibromiRs: translating molecular discoveries into new anti-fibrotic drugs. Trends Pharmacol Sci. 2014;35:119–126. doi: 10.1016/j.tips.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Qiao J, Bush KT, Steer DL, Stuart RO, Sakurai H, Wachsman W, Nigam SK. Multiple fibroblast growth factors support growth of the ureteric bud but have different effects on branching morphogenesis. Mech Dev. 2001;109:123–135. doi: 10.1016/s0925-4773(01)00592-5. [DOI] [PubMed] [Google Scholar]
- Qiao J, Uzzo R, Obara-Ishihara T, Degenstein L, Fuchs E, Herzlinger D. FGF-7 modulates ureteric bud growth and nephron number in the developing kidney. Development. 1999;126:547–554. doi: 10.1242/dev.126.3.547. [DOI] [PubMed] [Google Scholar]
- Quaggin SE, Schwartz L, Cui S, Igarashi P, Deimling J, Post M, Rossant J. The basic-helix-loop-helix protein pod1 is critically important for kidney and lung organogenesis. Development. 1999;126:5771–5783. doi: 10.1242/dev.126.24.5771. [DOI] [PubMed] [Google Scholar]
- Rosenblum ND. Developmental biology of the human kidney. Semin Fetal Neonatal Med. 2008;13:125–132. doi: 10.1016/j.siny.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Rosselot C, Spraggon L, Chia I, Batourina E, Riccio P, Lu B, Niederreither K, Dolle P, Duester G, Chambon P, et al. Non-cell-autonomous retinoid signaling is crucial for renal development. Development. 2010;137:283–292. doi: 10.1242/dev.040287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajithlal G, Zou D, Silvius D, Xu PX. Eya 1 acts as a critical regulator for specifying the metanephric mesenchyme. Dev Biol. 2005;284:323–336. doi: 10.1016/j.ydbio.2005.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai H, Bush KT, Nigam SK. Heregulin induces glial cell line-derived neurotrophic growth factor-independent, non-branching growth and differentiation of ureteric bud epithelia. J Biol Chem. 2005;280:42181–42187. doi: 10.1074/jbc.M507962200. [DOI] [PubMed] [Google Scholar]
- Sanchez MP, Silos-Santiago I, Frisen J, He B, Lira SA, Barbacid M. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature. 1996;382:70–73. doi: 10.1038/382070a0. [DOI] [PubMed] [Google Scholar]
- Schuchardt A, D'Agati V, Larsson-Blomberg L, Costantini F, Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature. 1994;367:380–383. doi: 10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- Sequeira Lopez ML, Gomez RA. Development of the renal arterioles. J Am Soc Nephrol. 2011;22:2156–2165. doi: 10.1681/ASN.2011080818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short KM, Combes AN, Lefevre J, Ju AL, Georgas KM, Lamberton T, Cairncross O, Rumballe BA, McMahon AP, Hamilton NA, et al. Global quantification of tissue dynamics in the developing mouse kidney. Dev Cell. 2014;29:188–202. doi: 10.1016/j.devcel.2014.02.017. [DOI] [PubMed] [Google Scholar]
- Short KM, Hodson MJ, Smyth IM. Tomographic quantification of branching morphogenesis and renal development. Kidney Int. 2010;77:1132–1139. doi: 10.1038/ki.2010.42. [DOI] [PubMed] [Google Scholar]
- Sims-Lucas S, Caruana G, Dowling J, Kett MM, Bertram JF. Augmented and accelerated nephrogenesis in TGF-beta2 heterozygous mutant mice. Pediatr Res. 2008;63:607–612. doi: 10.1203/PDR.0b013e31816d9130. [DOI] [PubMed] [Google Scholar]
- Song R, Preston G, Khalili A, El-Dahr SS, Yosypiv IV. Angiotensin II regulates growth of the developing papillas ex vivo. Am J Physiol Renal Physiol. 2012;302:F1112–F1120. doi: 10.1152/ajprenal.00435.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song R, Preston G, Yosypiv IV. Angiotensin II stimulates in vitro branching morphogenesis of the isolated ureteric bud. Mech Dev. 2011;128:359–367. doi: 10.1016/j.mod.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song R, Spera M, Garrett C, El-Dahr SS, Yosypiv IV. Angiotensin II AT2 receptor regulates ureteric bud morphogenesis. Am J Physiol Renal Physiol. 2009;298:F807–F817. doi: 10.1152/ajprenal.00147.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song R, Spera M, Garrett C, Yosypiv IV. Angiotensin II-induced activation of c-Ret signaling is critical in ureteric bud branching morphogenesis. Mech Dev. 2010;127:21–27. doi: 10.1016/j.mod.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas S, Wu Z, Chen CM, D'Agati V, Costantini F. Dominant effects of RET receptor misexpression and ligand-independent RET signaling on ureteric bud development. Development. 1999;126:1375–1386. doi: 10.1242/dev.126.7.1375. [DOI] [PubMed] [Google Scholar]
- Stine ZE, McGaughey DM, Bessling SL, Li S, McCallion AS. Steroid hormone modulation of RET through two estrogen responsive enhancers in breast cancer. Hum Mol Genet. 2011;20:3746–3756. doi: 10.1093/hmg/ddr291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Lopez ML, Cowhig JE, Jr., Taylor MA, Hatada T, Riggs E, Lee G, Gomez RA, Kim HS, Smithies O. Ren1c homozygous null mice are hypotensive and polyuric, but heterozygotes are indistinguishable from wild-type. J Am Soc Nephrol. 2005;16:125–132. doi: 10.1681/ASN.2004060490. [DOI] [PubMed] [Google Scholar]
- Takayama M, Miyatake K, Nishida E. Identification and characterization of retinoic acid-responsive genes in mouse kidney development. Genes Cells. 2014;19:637–649. doi: 10.1111/gtc.12163. [DOI] [PubMed] [Google Scholar]
- Tsuchida S, Matsusaka T, Chen X, Okubo S, Niimura F, Nishimura H, Fogo A, Utsunomiya H, Inagami T, Ichikawa I. Murine double nullizygotes of the angiotensin type 1A and 1B receptor genes duplicate severe abnormal phenotypes of angiotensinogen nullizygotes. J Clin Invest. 1998;101:755–760. doi: 10.1172/JCI1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufro A, Teichman J, Banu N, Villegas G. Crosstalk between VEGF-A/VEGFR2 and GDNF/RET signaling pathways. Biochem Biophys Res Commun. 2007;358:410–416. doi: 10.1016/j.bbrc.2007.04.146. [DOI] [PubMed] [Google Scholar]
- Vilar J, Gilbert T, Moreau E, Merlet-Benichou C. Metanephros organogenesis is highly stimulated by vitamin A derivatives in organ culture. Kidney Int. 1996;49:1478–1487. doi: 10.1038/ki.1996.208. [DOI] [PubMed] [Google Scholar]
- Wellik DM, Hawkes PJ, Capecchi MR. Hox11 paralogous genes are essential for metanephric kidney induction. Genes Dev. 2002;16:1423–1432. doi: 10.1101/gad.993302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo YM, Park JH. microRNA biomarkers in cystic diseases. BMB Rep. 2013;46:338–345. doi: 10.5483/BMBRep.2013.46.7.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf AS, Kolatsi-Joannou M, Hardman P, Andermarcher E, Moorby C, Fine LG, Jat PS, Noble MD, Gherardi E. Roles of hepatocyte growth factor/scatter factor and the met receptor in the early development of the metanephros. J Cell Biol. 1995;128:171–184. doi: 10.1083/jcb.128.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf AS, Price KL, Scambler PJ, Winyard PJ. Evolving concepts in human renal dysplasia. J Am Soc Nephrol. 2004;15:998–1007. doi: 10.1097/01.asn.0000113778.06598.6f. [DOI] [PubMed] [Google Scholar]
- Wu H, Chen L, Zhou Q, Zhang X, Berger S, Bi J, Lewis DE, Xia Y, Zhang W. Aqp2-expressing cells give rise to renal intercalated cells. J Am Soc Nephrol. 2013;24:243–252. doi: 10.1681/ASN.2012080866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yallowitz AR, Hrycaj SM, Short KM, Smyth IM, Wellik DM. Hox10 genes function in kidney development in the differentiation and integration of the cortical stroma. PLoS One. 2011;6:e23410. doi: 10.1371/journal.pone.0023410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Wang Y, Rattner A, Nathans J. Genetic mosaic analysis reveals a major role for frizzled 4 and frizzled 8 in controlling ureteric growth in the developing kidney. Development. 2011;138:1161–1172. doi: 10.1242/dev.057620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yosypiv IV, Schroeder M, El-Dahr SS. Angiotensin II type 1 receptor-EGF receptor cross-talk regulates ureteric bud branching morphogenesis. J Am Soc Nephrol. 2006;17:1005–1014. doi: 10.1681/ASN.2005080803. [DOI] [PubMed] [Google Scholar]
- Yu J, Carroll TJ, Rajagopal J, Kobayashi A, Ren Q, McMahon AP. A Wnt7b-dependent pathway regulates the orientation of epithelial cell division and establishes the cortico-medullary axis of the mammalian kidney. Development. 2009;136:161–171. doi: 10.1242/dev.022087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Valerius MT, Duah M, Staser K, Hansard JK, Guo JJ, McMahon J, Vaughan J, Faria D, Georgas K, et al. Identification of molecular compartments and genetic circuitry in the developing mammalian kidney. Development. 2012;139:1863–1873. doi: 10.1242/dev.074005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Liegeois NJ, Wong C, Finegold M, Hou H, Thompson JC, Silverman A, Harper JW, DePinho RA, Elledge SJ. Altered cell differentiation and proliferation in mice lacking p57KIP2 indicates a role in Beckwith-Wiedemann syndrome. Nature. 1997;387:151–158. doi: 10.1038/387151a0. [DOI] [PubMed] [Google Scholar]
- Zhao H, Kegg H, Grady S, Truong HT, Robinson ML, Baum M, Bates CM. Role of fibroblast growth factor receptors 1 and 2 in the ureteric bud. Dev Biol. 2004;276:403–415. doi: 10.1016/j.ydbio.2004.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]