Abstract
Many neurological diseases of the CNS are underpinned by malfunctions of the immune system including disorders involving opportunistic infections. Progressive multifocal leukoencephalopathy (PML) is a lethal CNS demyelinating disease caused by the human neurotropic polyomavirus JC (JCV) and is found almost exclusively in individuals with immune disruption including HIV/AIDS, patients receiving therapeutic immunomodulatory monoclonal antibodies to treat conditions such as multiple sclerosis (MS), transplant recipients, etc. Thus, the public health significance of this disease is high because of the number of individuals that constitute the at-risk population. The incidence of PML is very low whereas seroprevalence for virus is high suggesting infection by virus is very common and so it is thought that virus is restrained but it persists in an asymptomatic state that can only occasionally be disrupted to lead to viral reactivation and PML. When JCV actively replicates in oligodendrocytes and astrocytes of the CNS, it produces cytolysis leading to formation of demyelinated lesions with devastating consequences. Defining the molecular nature of persistence and events leading to reactivation of virus to cause PML has proved to be elusive. In this review, we examine the current state of knowledge of the JCV life cycle and mechanisms of pathogenesis. We will discuss the normal course of the JCV life cycle including transmission, primary infection, viremia and establishment of asymptomatic persistence as well as pathogenic events including migration of virus to the brain, reactivation from persistence, viral infection and replication in the glial cells of the CNS and escape from immunosurveillance.
Keywords: Progressive multifocal leukoencephalopathy, Polyomavirus JC, demyelination, viral persistence
INTRODUCTION
Progressive multifocal leukoencephalopathy (PML) is a fatal demyelinating disease caused by the human neurotropic polyomavirus, JC (JCV), which lytically infects the oligodendrocytes of the brain which are responsible for myelin production and maintenance for neuronal axons and, to a lesser extent, astrocytes which are responsible for a wide variety of activities in the CNS. Destruction of oligodendrocytes in the CNS gives multiple expanding regions of demyelination that eventually coalesce to form larger lesions and these together with oligodendrocytes containing inclusion bodies and bizarre astrocytes constitute the triad of histopathological features that are characteristic of PML. The inclusion bodies of oligodendrocytes in PML consist of crystalline arrays of virions that are visible under EM and are immunopositive for the JCV capsid protein VP1, while the bizarre astrocytes also produce virions that can be detected by electron microscopy and express VP1, demonstrating that JCV replicates in both cell types in PML1,2. With the exception of those predisposing conditions associated with PML in which the accompanying immunosuppression can be reversed, e.g., the antiretroviral-naïve person with HIV-associated PML or natalizumab-associated PML, the disease typically follows an inexorable course with death ensuing within a few months. The clinical signs and symptoms of patients depend on the location of the demyelinated lesions3. In almost all patients, some form of immunocompromise is a predisposing factor for PML. PML was first recognized as an obscure disorder predominantly affecting patients with chronic lymphocytic leukemia (CLL) and lymphoma. With the onset of the AIDS pandemic in the early 1980s, the number of PML patients with HIV-1/AIDS overwhelmed other predisposing conditions. As shown in Figure 1, HIV-1 infection remains the most frequent immunodeficiency setting for PML, accounting for ~80% of cases4. A second wave of PML patients occurred in the mid-2000s with the introduction of new therapeutic monoclonal antibodies, which predispose to PML including natalizumab5, used to treat multiple sclerosis (MS) and Crohn's disease (CD), and efalizumab6 previously used in the treatment of chronic psoriasis. The occurrence of PML in the context of these new therapeutic monoclonals, which affect certain aspects of leukocyte function, has provided new insights into how JCV reactivates to cause PML and the role of leukocytes. Conflicting hypotheses have been proposed that remain to be resolved and these will be discussed below. Other therapeutic agents that perturb the immune system, including rituximab7, used to treat lymphomas and rheumatoid arthritis (RA), mycophenolate mofetil8, and other therapeutic immunosuppressants have also been associated with PML, although their association with PML is not as compelling as with natalizumab and efalizumab9. Recently (8/29/2013), the U.S. Food and Drug Administration (FDA) issued an alert that an MS patient in Europe has developed PML after taking the drug fingolimod (http://www.fda.gov/Drugs/DrugSafety/ucm366529.htm) and the death of a patient from PML while taking tecfidera for MS was reported on 10/31/2014 (http://www.healthline.com/health-news/patient-taking-tecfidera-for-ms-dies-of-pml-103014).
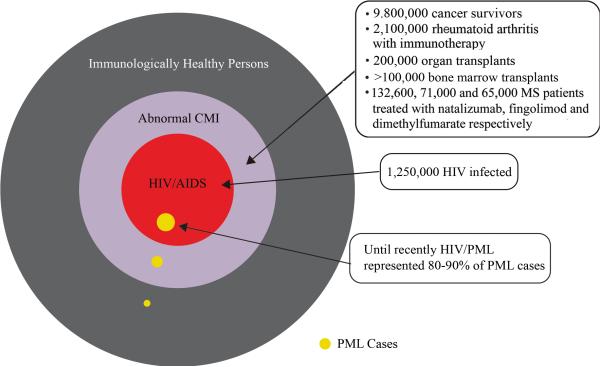
FIGURE 1. Schematic diagram of the occurrence of PML in the US.
A Venn diagram is shown illustrating the occurrence of PML (gold circles) in different populations in the US. The outer circle represents the total population of the US, which was 314 million in 2012. The outer black portion represents the vast majority of the population who are immunologically healthy. Note that the group labeled as immunologically healthy refers to individuals with no apparent cause of immunosuppression and includes the elderly, patients with chronic liver or kidney disease and those with idiopathic or transient lymphocytopenia and, as such, could have an occult immunosuppression that is not recognized. The purple circle indicates the subpopulation of people who have impaired cell-mediated immunity (CMI), e.g., cancer survivors, rheumatoid arthritis patients treated with immunosuppressive agents such as rituximab, bone marrow and solid organ transplant recipients, MS patients receiving natalizumab ((https://medinfo.biogenidec.com/), fingolimod : http://www.fda.gov/Drugs/DrugSafety/ucm366529.htm or dimethyl fumarate98 etc. The inner red circle represents the subpopulation of people with impaired CMI who are infected with HIV-1/AIDS (1.2 million).
As a corollary of these observations, a broad and expanding number of individuals with a variety of immunodeficiency disorders are now at risk for developing PML. It should also be noted that PML can also occur in individuals with no obvious cause of immunosuppression including the elderly, patients with chronic liver or kidney disease, or those with idiopathic or transient lymphocytopenia. Failure to recognize that PML can also occur in this setting may lead to lengthy delays in establishing the diagnosis10-12. The public health significance of this disease lies not only in the number and variety of individuals that constitute the at-risk population but also in the difficulties encountered in the diagnosis of PML, the need for useful biomarkers for PML progression, the absence of any effective therapy to ameliorate or reverse this devastating disease, and our present limited understanding of the immunobiology of JCV and the pathogenesis of PML.
JCV belongs to the polyomavirus family of small non-enveloped DNA tumor viruses, with small, circular, double-stranded DNA genomes and was first isolated in 1971 from the brain of a PML patient13. It is the proven causative agent of PML. Polyomavirus BK (BKV) was also isolated in 1971 from a renal transplant recipient with polyomavirus-associated nephropathy (PVAN), which, like PML, is associated with immunosuppression. Recently, at least eight more human polyomaviruses have been discovered, which are distantly related to JCV and BKV14. The JCV genome has two protein-coding regions (early and late) transcribed in opposite directions starting from a common non-coding control region (NCCR), a bidirectional element containing promoter/enhancer elements and the origin of viral DNA replication15. The early region encodes the regulatory proteins: large T-antigen and small t-antigen. During early phase, T-antigen (T-Ag) accumulates and directs initiation of viral DNA replication and the transcriptional switch from early to late gene expression at the onset of late phase16. The late region encodes viral capsid proteins VP1, VP2, and VP3 and a small regulatory protein, agnoprotein, which is an important factor in the regulation of the viral life cycle.
JC virus infection is very common and seroepidemiological studies show that initial infection occurs early in life with the exact proportion of seropositivity varying somewhat between different studies (Table 1). The high seroprevalence of JCV infection and the remarkable rarity of PML suggest that multiple barriers likely exist to development of disease. Altered immune function appears to underlie all the identified conditions that predispose to PML, therefore, the immune system must be critical in keeping the virus in a persistent state and preventing disease expression when the virus finds purchase within the brain. Changes in immune status due to immunodysregulation can lead to viral reactivation and progression of the viral life cycle causing the destruction of glial cells and the development of PML. The molecular events that are involved in reactivation, while not well understood, are important because PML remains an untreatable and incurable disease with substantial mortality between 6 to 15 months following diagnosis. A major obstacle comes from our lack of fundamental understanding of molecular events that control JCV persistence and the cascade of biological events that orchestrates the reactivation of JCV and the various stages of the viral lytic cycle. In this review, we will discuss what is known about the life cycle of JCV and outline our basic understanding of the viral life cycle and disease pathogenesis highlighting areas that are not well understood with an emphasis on newer findings and areas of ongoing controversy within the field.
TABLE 1.
IMMUNOASSAYS FOR JCV: RATES VARY BETWEEN 33% TO 91%
| Country | N | Age (Yrs) | Population | Seropositive | Ref |
|---|---|---|---|---|---|
| Finland | 590 | 0-13 | Normal | 33% (VLPEIA) | 86 |
| 50 | >25 | 72% (VLPEIA) | |||
| UK | 2435 | 1-69 | Normal | 35% (HI) | 87 |
| 356 | 60-69 | 50% (HI) | |||
| USA | 1501 | >21 | Normal | 39% (VP1 EIA) | 88 |
| USA | 415 | 19-78 | Normal | 44% (VLPEIA) | 89 |
| 90 | 40-64 | Cancer | 52% (VLPEIA) | ||
| Belgium | 106 | 20-80 | Normal | 52% (VLPEIA) | 90 |
| 225 | 11-70 | Crohn's/GI | 76% (VLPEIA) | ||
| Switzerland | 400 | 20-59 | Normal | 58% (VLPEIA) | 91 |
| USA | 622 | 20-74 | Normal | 59% (VLPEIA) | 92 |
| 724 | 20-74 | Cancer | 49% (VLPEIA) | ||
| USA | 277 | >20 | Normal | 69% (HI) | 93 |
| Japan | 480 | All ages | Normal | 71% (HI) | 94 |
| Germany | 49 | 4-81 | Normal | 86% (VP1 EIA) | 95 |
| 36 | 17-55 | MS | 76% (VP1 EIA) | ||
| Portugal | 171 | 3-75 | Normal | 91% (VLPEIA) | 96 |
| 63 | 25-75 | HIV | 91% (VLPEIA) | ||
VLPEIA - Virus-Like Particle Enzyme Immunoassay
HI - Haemagglutination Inhibition
VP1 EIA - VP1 Enzyme Immunoassay
VIRAL TRANSMISSION AND PRIMARY INFECTION
Seroepidemiological evidence (Table 1) indicates that most cases of primary infection occur late in childhood. Two different forms of JCV exist based on the structure of the NCCR, the archetype and the neurotropic or prototypical form, often referred to as PML-type or Mad-1-like type, found in PML. The archetype (CY) has a simple highly conserved NCCR structure and may be the “wild-type” that is transmitted between individuals, since it is the most abundant form in the environment. Neurotropic forms have deletions, duplications and rearrangements in the NCCR compared to the archetype and have more variability in sequence (Mad-1, Mad-4, GS/B, Her1, Tokyo-1, etc) and possibly arise within an individual by neuroadaptation although direct evidence for this is lacking. Since prototype has deletions and duplications compared to archetype, it seems reasonable to assume that if any interconversion occurs, it is in the direction of archetype to prototype. However, other models can be conceived for the relationship between archetype and prototype, e.g., rare quasispecies of virus may exist and the abundance of these might vary depending on circumstances and selective pressures placed on the virus. Increased pathogenesis or neurotropism of such rare quasispecies would help explain the rarity of PML, despite nearly ubiquitous exposure of humans to virus. Such a model could be tested by deep sequencing. Recently, Van Loy et al (2014) used deep sequencing to perform a JC virus quasispecies analysis on NCCR DNA amplified from body fluid samples from PML patients17. This revealed a complex mixture composed of multiple viral variants suggestive of a highly dynamic process of the JCV NCCR reorganization in vivo and viral dissemination towards the brain via the hematogenous route17. This approach is important in understanding of the JCV life cycle and will be a fruitful area for future research.
Archetype is the predominant form of JCV in the urine of normal non-immunocompromised individuals18. After primary infection, archetype JCV may establish low-level, asymptomatic, persistent infection in renal tissue where it is released from polarized kidney tubular epithelial cells from the apical face into the tubule and thus shed into urine. Interestingly, analysis of multiple paired urine samples isolated from the same patients show them to have the same sequence indicating persistent infection rather than repeated infections of the same host19.
JCV is highly prevalent and stable in sewage from divergent geographical areas and archetype was reported in nearly all isolates suggesting that archetype virus might be transmitted environmentally20. Genotyping analysis suggests that JCV is transmitted both within and outside the family. Genotyping of JCV is based on DNA sequence polymorphisms in a 610 nucleotide region (VT-IR) containing the C-termini of the VP1 and T-Ag genes and the intergenic region21. Interestingly, some studies suggest subtype polymorphisms in VP1 C-terminus may be associated with PML pathogenesis22,23. Recently, a novel JCV variant was reported with a 10 bp deletion in VP1 C- terminus in JCV granule cell neuronopathy, which results from lytic infection of cerebellar granule cell neurons leading to cerebellar atrophy in patients with HIV-1/AIDS24. JCV infection of cerebellar granule cell neurons has been reported in PML25,26 and also in a patient with cerebellar atrophy and no clinical or radiological evidence of PML where it was named JCV granule cell neuronopathy (JCV GCN)27. Recent studies indicate that JCV infection of cerebral cortical neurons is an important component of PML pathogenesis28. Also recognized recently are JC virus encephalopathy caused by productive infection of cortical pyramidal neurons29 and JCV meningitis with productive JCV infection of leptomeningeal and choroid plexus cells30.
JCV has been found in tonsil tissue leading to the hypothesis that tonsils may serve as an initial site of viral infection31,32, i.e., virus might enter the mouth or nose by close interpersonal contact or via fomites and infect the tonsils. JCV is rarely detected in oropharyngeal fluids32 even in those obtained from immunosuppressed individuals, but is commonly detected in urine suggesting urine contributes substantially to JCV transmission33. Finally, JCV has been reported in epithelial cells of the GI tract34 although it is not known if the GI tract is a portal for infection. The life cycle of JCV and the pathogenic events described below are illustrated in Figure 2.
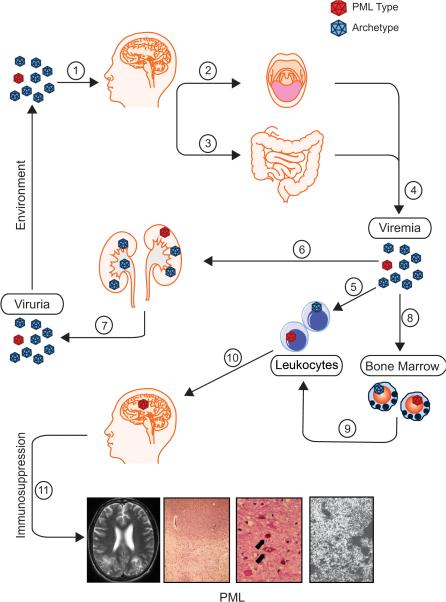
FIGURE 2. Schematic diagram of the JCV life cycle and pathogenesis of PML.
The events thought to occur during the JCV life cycle and pathogenesis of PML are shown with the common non-pathogenic events labeled in green and the rare pathogenic events in red. Virions in the environment (top right hand corner), which are mainly thought to have an archetypal configuration (blue), but sometimes occasionally the neurotropic form (red), are transmitted through the mouth and nose (1) and enter into the bloodstream through either the epithelium of the tonsils and the upper respiratory tract (2) or the GI tract (3) to establish a primary viremia (4). The nature of the primary viremia is not well understood but current hypotheses are that virus may exist as free virions and/or as white blood cell-associated virus (5). Virus is spread hematogenously to the kidney (6) and other organs. In the kidney, JCV can replicate sporadically and at low levels in the epithelium of the kidney tubules from where it can shed from the apical face of the kidney epithelium leading to viruria and transmission of virions into the environment in the urine (7) completing the normal JCV life cycle. Virus may spread to the bone marrow (8), where it has been postulated but is not proven that neurotropic virus (red) may emerge by an unknown mechanism. Subsequently, JCV may undergo hematogenous spread from the bone marrow and possibly other locations in association with leukocytes (9) to other sites including the brain (10) where neurotropic JCV DNA can be detected in healthy, immunocompetent individuals in the absence of expression of detectable levels of viral proteins. Under conditions of immunosuppression, neurotropic JCV can become reactivated and undergoes transcription, DNA replication and spreads to form microlesions, which can coalesce, increase in size and result in PML. The pathological features of PML are shown in the bottom panels and are (from left to right): T2-weighted magnetic resonance image of the brain showing hyperintense signal abnormalities in the white matter of the parieto-occipital lobes due to PML85; hematoxylin and eosin staining of a PML tissue section for lipids showing demyelination; hematoxylin and eosin staining revealing oligodendrocytes bearing nuclear inclusion bodies (upper arrow) and bizarre astrocytes (lower arrow)86; electron microscopy of PML tissue showing crystalline arrays of 45 nm viral particles within a nuclear inclusion body.
EARLY EVENTS IN PATHOGENESIS
Most people are infected with JCV in childhood and for the vast majority, the infection is self-limiting with no clinically evident or pathological consequences. While primary infection is cleared by the immune system to a point where viremia is undetectable, archetype virus may persist in the kidney with occasional shedding when low level replication of persistent virus occurs in tubular epithelial cells. In this sense, JCV can be considered a well-adapted virus that is able to infect a majority of humans relatively asymptomatically depending on age as judged by seropositivity studies (Table 1). However, very rarely, JCV causes PML. For PML to occur, a series of uncommon events needs to take place, which together make the probability of the disease developing very low as discussed below.
Virus must migrate through the blood to establish persistence in the kidney and to the brain for PML to occur
Following primary infection, it is presumed that JCV enters the bloodstream from its portal of entry, which is likely the oropharynx and gut, or possibly the respiratory tract and travels to kidney and perhaps other sites of viral persistence. Accordingly, JCV has been detected in peripheral blood leukocytes from normal immunocompetent individuals35-38. In situ hybridization demonstrated that JCV is localized in the nuclei of blood cells indicating that JCV is able to infect peripheral leukocytes rather than being present in the blood bound to serum proteins or to cell membranes35. Chalkias et al38 sorted PBMCs by FACS and found that JCV DNA can be found in all subpopulations tested. Although archetype has been found in these blood cells35, neurotropic forms are also found35,36 suggesting that blood may be the compartment in which neurotropic virus begins to emerge39. Similarly, JCV is found in leukocytes in bone marrow40 suggesting bone marrow is an important site in the JCV life cycle and may be an intermediate site in PML pathogenesis. Indeed one report failed to detect JCV RNA in blood41. Thus, although various amounts of JCV DNA can be found in different blood cell types, it is important to note that there is no direct evidence of a productive infection, detection of viral RNA, proteins or virions. Thus, the precise role of blood in the life cycle of JCV pathogenesis of PML remains controversial.
How does the neurovirulent form of JCV arise in the pathogenesis of PML?
Different models are possible to account for the relationship between archetypal JCV predominating environmentally and neurotropic JCV present in PML: neuroadaptation of archetype virus to prototype or selection of rare viral quasispecies. In the neuroadaptation model, a conversion of archetype to the neurotropic form of JCV would involve a fairly complex rearrangement of the viral, circular, double-stranded DNA through a series of deletions and duplications. Two possible speculative mechanisms have been proposed: (i) Recombinases of immunoglobulin gene rearrangement in pre-B cells are involved. In this scenario, the somatic recombination events that occur in immunoglobulin genes are hijacked, e.g., RAG1 and RAG2 recombinases. Interestingly, human DNA-binding protein Sμbp-2 (IGHMBP2), which is involved in immunoglobulin μ-chain switch binds to the JCV NCCR41; (ii) Replication-linked DNA homologous recombination events are involved. Johnson and co-workers43 proposed a series of adaptive, DNA replication-driven recombination events in bone marrow cells similar to duplication of the SV40 72 bp enhancer but when virus is grown in tissue culture44. There is no direct evidence in support of either of these mechanisms of neuroadaptation. More research is needed to resolve these issues.
Regulation of JCV in white blood cells
JCV presence in PBMCs and bone marrow and their potential for being a site for neurotropic rearrangement and vehicles for hematogenous spread of virus to CNS has led to many studies of JCV regulation in these cells. Cell cultures studies demonstrated JCV can infect lymphocytes45,46. Early studies reported common gel shifts in B cell nuclear extracts to other permissive cells45. More recent studies suggested a role for specific transcription factors, such as NF-1X46,47 and Spi-B48,49. Again it should be noted that there is no direct evidence of a productive infection of blood cells by JCV in vivo.
VIRAL REACTIVATION IN PML
Before JCV can cause PML, several preconditions need to be met: (i) virus must enter the blood; (ii) neurotropic forms of the virus must be present or emerge; (iii) neurotropic virus must migrate to the brain; (iv) active viral gene expression must occur in the glial cells of the brain; (v) immunosuppression must be present. What is the relationship of these events to viral persistence and reactivation? Clearly, all of these events are necessary for JCV reactivation to cause PML, but no particular step appears to be sufficient. For example, JCV is found in bone marrow of non-immunocompromised individuals35,37 with a neurotropic arrangement of the NCCR50. Therefore, it is possible that events occurring after the bone marrow are involved in transition from persistence to reactivation. Research has focused on three aspects that may be important: (i) migration of virus from bone marrow to brain; (ii) control of JCV gene expression in glial cells; (iii) cell-mediated immunity. Each of these is considered below.
Migration of JCV to the CNS in viral reactivation
It has been suggested that a key event in reactivation is mobilization of neurotropic JCV in leukocytes from bone marrow to CNS where glial cells become infected. The occurrence of PML in patients receiving therapeutic monoclonal antibodies that target cells of the immune system has focused attention on these potential mechanisms51. One such immunomodulatory drug found to be associated with the occurrence of PML is natalizumab5, which inhibits T cell migration into CNS and therefore interferes with cell-mediated immunity and immunoserveillance. Additionally, natalizumab mobilizes mononuclear cells from the bone marrow and it has been proposed that mononuclear cells, such as CD34+ hematopoietic precursor cells, convey JCV to brain52. Frohman and coworkers53 reported finding JCV in CD34+ and CD19+ cells from MS patients but other studies failed to detect virus54,55. Thus, the role of natalizumab-mediated hematopoietic precursor cell mobilization from the bone marrow in the pathogenesis of PML is still a contentious issue. Since there is evidence from many labs that JCV is found in brain of normal individuals without PML (reviewed by White and Khalili56), i.e., JCV has the capacity to traffic into normal brain, other later events occurring in glial cells and immunosuppression are likely more important in viral reactivation.
Role of regulation of JCV gene expression in glial cells in viral persistence and reactivation
Persistent JCV DNA in the brain of normal immunocompetent individuals
While the role of various organs and tissues as reservoirs for viral persistence has been much debated, it is clear that JCV may be present in brain of normal individuals without PML57-59. Nevertheless, it has been suggested that JCV harbored in brain is not functionally important52. Proponents of models of pathogenicity, which envision blood trafficking of virus as the key step in PML initiation, dismiss the role of JCV persistence in the brain52 However, data clearly suggest that, even if there is not a sizable reservoir of virus in normal brains, JCV has full access to all regions of brain in nonimmunocompromized individuals without PML. Thus, once the immune system becomes impaired, transitory and/or resident virus may have the opportunity to initiate its lytic cycle in astrocytes and oligodendrocytes.
Reactivation of JCV in glial cells of the CNS and initiation of PML pathogenesis
Since JCV can access the brain of healthy individuals, the question then becomes as to the mechanism whereby virus becomes reactivated. Since viral protein expression is not found in normal brain, it is possible reactivation of JCV transcription might be an initial event in PML pathogenesis. The mechanism of initiation likely involves molecular processes that regulate viral gene expression at the NCCR, which controls expression of viral early and late proteins including T-antigen, which is essential for viral DNA replication59. The JCV NCCR is regulated by NF-κB and C/EBPβ60, which in turn are activated by proinflammatory cytokines, such as TNF-α. PML occurs in the context of HIV-1/AIDS, which can lead to the production of pro-inflammatory cytokines in CNS, including TNF-α, which stimulates JCV transcriptional reactivation61,62 Atwood et al63 reported that TNF-α does not stimulate JCV transcription but the JCV reporter construct used in the study was incomplete and did not contain the NF-κB binding site. Other studies indicate transcription factors NFAT464,65 and Egr-166 may also be involved in JCV regulation. Histone deacetylase inhibitors such as trichostatin A (TSA) and sodium butyrate strongly increase JCV transcription67,68 but not the DNA methylation inhibitor 5-azacytidine68. This suggests that JCV DNA may persist in the non-PML brain in a silent form perhaps associated with an epigenetic state with chromatin deacetylation. Once PML is initiated, infected, viral antigen-expressing cells must then escape the host immune system, in particular immune surveillance by the cellular immune system.
Role of immunosuppression
PML occurs almost exclusively in individuals with some type of dysfunction or modulation of the immune system69. In HIV-1/AIDS, JCV-specific cellular immune responses in patients with PML correlate with outcome70. This suggests that JCV-specific cellular immunity has a role in containing PML. CTLs are associated with early control of PML71 and PML nonsurvivors have impaired JCV-specific T-cell responses, highlighting the importance of JCV-specific CTL responses in containing viral replication and explaining the role of immunosuppression in PML pathogenesis.
The involvement of cellular immunity in suppressing JCV provides interesting insights into PML association with new immunomodulatory therapies, e.g., natalizumab. Natalizumab inhibits T cell migration and extravasation interfering with cell-mediated immunity and immunoserveillance in CNS71. As of as of December 3, 2014, there have been 517 (514 MS, 3 Crohn's disease [CD]) confirmed PML cases. Through Sep 30, 2014, approximately 132,600 patients received natalizumab in the post-marketing setting worldwide (https://medinfo.biogenidec.com/). Risk mitigation strategies have been developed based on three indicators that have been demonstrated to allow stratification of risk in patients taking natalizumab: JCV seropositivity, duration of natalizumab use and preceding use of any immunsuppressive therapy. Risk varies from 0.1/1000 in JCV seronegative patients to 11.2/1000 in JCV seropostive patients with preceding immunsuppresant use who have taken natalizumab for more than 24 months5. The occurrence of PML in patients receiving natalizumab is due, at least in part, to decreased CNS immunoserveillance. MS patients showed reduced peripheral blood and CSF TCR repertoire during natalizumab therapy, which may contribute to PML development71,72 . Chen and coworkers73 followed 19 MS patients treated with natalizumab and observed increases in JC viruria and viremia although no patients showed clinical or radiologic signs of PML.
Efalizumab inhibits lymphocyte activation and extravasation and is also associated with high incidence of PML. After this link was observed in 200974, efalizumab was withdrawn from the market.
Rituximab is a therapeutic monoclonal antibody targeting CD20+ B cells and is associated with PML75. However it is difficult to define the role of rituximab in PML development because most patients receiving rituximab had lymphoproliferative diseases, which in and of itself is an underlying cause of PML, and are treated with other immunosuppressive drugs76. Rituximab has been suggested to facilitate PML by depleting mature B cells and thereby inducing expansion of pre-B cells, which have been proposed to convey JCV to the CNS52. However, there is no evidence that pre-B cells convey JCV to the CNS and some studies have failed to detect virus in these cells so this hypothesis is unsupported. Alternatively, a depletion in cerebral B cells caused by rituximab may reduce the cellular immune response76. In this scenario, the pathogenesis of rituximab is due to a decrease of B lymphocytes in cerebral perivascular spaces as has been reported by Martin Mdel et al77 resulting in decreased antigen presentation to T lymphocytes and cellular immunity to JCV76. The role of rituximab in PML induction is still an unresolved issue. Other therapeutic agents, such as, mycophenolate mofetil, brentuximab vedotin, etc., may also be associated with PML risk and carry FDA mandated “black box” warnings for the disorder. However, the risk of PML with these agents appears to be significantly less than that with natalizumab and efalizumab. Today, extra precautions are taken when new immunomodulatory therapeutics are introduced to monitor for possible JCV reactivation.
CONCLUSIONS
In the more than forty years since the isolation of JCV, important advances have been made in the understanding of the biology of the virus and the pathogenesis of PML. However, there are still important areas where our knowledge is incomplete and certain aspects of the viral life cycle remain unresolved. For example, there is a view that has received acceptance by some researchers that white blood cells in the bone marrow are the site of initiation of PML pathogenesis by converting the archetype form of the virus to the neurotropic form and mobilizing it to travel to the brain. However, careful examination of the available evidence reveals that neurotropic virus may exist, on occasion, in the bone marrow and brain of normal individuals who do not develop PML. Despite the development of elaborate proposed models, the notion that archetype undergoes conversion to a neurotropic form in the bone marrow still lacks direct support. This could be a fruitful area for future research. A comprehensive analysis by deep sequencing of the viral populations present in different tissues at different times during the development of PML may shed throw light on this. Further insight may also be gained by the development of in vitro model systems to study potential neuroadaptation of JCV. Lack of a suitable animal model for PML due to the virus’ inability to replicate in non-human hosts has also hampered JCV research. Several early studies on JCV T-antigen transgenic mice, with the native viral promoter driving T-antigen expression, observed dysmyelination in the absence of late gene expression, suggesting an important role for T-antigen in this process78,79. More recently, a mouse model was generated by engrafting bipotential human glial progenitor cells into neonatal immunodeficient and myelin-deficient mice, whose forebrain glial populations became substantially humanized with age80. When injected intracerebrally with JCV, productive infection occurred and subsequent demyelination. In these mice, JCV was principally spread by infection of glial precursor cells and astrocytes while oligodendrocytes expressed T-antigen and showed cell death by apoptosis, which was a secondary occurrence and led to demyelination. Indeed, previous studies have shown that viral infection or expression of viral proteins can inhibit differentiation of oligodendrocyte progenitor cells and glial cells81,82. In these respects, the new mouse model has important differences to clinical PML. In clinical infection, oligodendrocytes express VP183 and their nuclei contain large numbers of JC virions in the nucleus (inclusion bodies) indicating robust productive infection83. Astrocytes are also infected and also express VP183 and contain virions84 but to a lesser extent. The explanation for this is not clear but may be related to the differing nature of immunosuppression in the mouse and the delivery of virus by intracranial injection into the animals. It will be interesting to see what we can learn from new animal models which may be more relevant to PML.
Answering the fundamental questions is important in advancing our knowledge of the JCV life cycle and the pathogenesis of PML, which remains a significant public health problem. PML is still an important complication of HIV-1/AIDS despite widespread treatment with combination antiretroviral therapy (cART). The use of important efficacious immunomodulatory therapies for diseases such as MS, RA and Crohn's disease has been hampered by the occurrence of PML as a debilitating and often deadly side effect. There is neither effective prophylactic therapy to be provided to individuals at risk of PML nor therapy for established PML and so there is a great need to advance our understanding of the biology of JCV and the pathogenesis of PML so as to address these issues.
ACKNOWLEDGEMENTS
We thank past and present members of the Center for Neurovirology for their insightful ideas and discussion. We also wish to thank C. Papaleo for editorial assistance. This work was supported by grants awarded by the NIH to MKW, JG and KK.
Footnotes
AUTHOR CONTRIBUTIONS
JRB, JG, KK, HSW and MKW conceived of the concept and organization of the review
HSW, KK and MKW
KK, JG, HSW and MKW wrote and edited the manuscript
JRB contributed Table 1 and Figure 1 and edited the manuscript
KK directed and organized the writing of the manuscript, contributed Figure 2 and edited the manuscript
POTENTIAL CONFLICTS OF INTEREST
None.
REFERENCES
- 1.Watanabe I, Preskorn SH. Virus-cell interaction in oligodendroglia, astroglia and phagocyte in progressive multifocal leukoencephalopathy. An electron microscopic study. Acta Neuropathol (Berl) 1976;36:101–115. doi: 10.1007/BF00685273. [DOI] [PubMed] [Google Scholar]
- 2.Del Valle L, Piña-Oviedo S. HIV disorders of the brain: pathology and pathogenesis. Front Biosci. 2006;11:718–732. doi: 10.2741/1830. [DOI] [PubMed] [Google Scholar]
- 3.Berger JR. The clinical features of PML. Cleve Clin J Med. 2011;78(Suppl 2):S8–12. doi: 10.3949/ccjm.78.s2.03. [DOI] [PubMed] [Google Scholar]
- 4.Tavazzi E, White MK, Khalili K. Progressive multifocal leukoencephalopathy: clinical and molecular aspects. Rev Med Virol. 2012;22:18–32. doi: 10.1002/rmv.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chalkley JJ, Berger JR. Progressive multifocal leukoencephalopathy in multiple sclerosis. Curr Neurol Neurosci Rep. 2013;13:408. doi: 10.1007/s11910-013-0408-6. [DOI] [PubMed] [Google Scholar]
- 6.Schwab N, Ulzheimer JC, Fox RJ, Schneider-Hohendorf T, et al. Fatal PML associated with efalizumab therapy: insights into integrin αLβ2 in JC virus control. Neurology. 2012;78:458–467. doi: 10.1212/WNL.0b013e3182478d4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clifford DB, Ances B, Costello C, Rosen-Schmidt S, et al. Rituximab-associated progressive multifocal leukoencephalopathy in rheumatoid arthritis. Arch Neurol. 2011;68:1156–1164. doi: 10.1001/archneurol.2011.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagayama S, Gondo Y, Araya S, Minato N, Fujita-Nakata M, Kaito M, et al. Progressive multifocal leukoencephalopathy developed 26 years after renal transplantation. Clin Neurol Neurosurg. 2013;115:1482–1484. doi: 10.1016/j.clineuro.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 9.Zaheer F, Berger JR. Treatment-related progressive multifocal leukoencephalopathy: current understanding and future steps. Ther Adv Drug Saf. 2012;3:227–239. doi: 10.1177/2042098612453849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gheuens S, Pierone G, Peeters P, Koralnik IJ. Progressive multifocal leukoencephalopathy in individuals with minimal or occult immunosuppression. J Neurol Neurosurg Psychiatry. 2010;81:247–254. doi: 10.1136/jnnp.2009.187666. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan IL, Koralnik IJ, Rumbaugh JA, Burger PC, et al. Progressive multifocal leukoencephalopathy in a patient without immunodeficiency. Neurology. 2011;77:297–299. doi: 10.1212/WNL.0b013e318225ab3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delgado-Alvarado M, Sedano MJ, González-Quintanilla V, de Lucas EM, et al. Progressive multifocal leukoencephalopathy and idiopathic CD4 lymphocytopenia. J Neurol Sci. 2013;327:75–79. doi: 10.1016/j.jns.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Padgett BL, Zu Rhein GM, Walker DL, Echroade R, et al. Cultivation of papova-like virus from human brain with progressive multifocal leukoencephalopathy. Lancet. 1971;1(7712):1257–1260. doi: 10.1016/s0140-6736(71)91777-6. [DOI] [PubMed] [Google Scholar]
- 14.White MK, Gordon J, Khalili K. The rapidly expanding family of human polyomaviruses: recent developments in understanding their life cycle and role in human pathology. PLoS Pathog. 2013;9:e1003206. doi: 10.1371/journal.ppat.1003206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frisque RJ, Bream GL, Cannella MT. Human polyomavirus JC virus genome. J Virol. 1984;51:458–469. doi: 10.1128/jvi.51.2.458-469.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khalili K, Gordon J, White MK. The polyomavirus, JCV and its involvement in human disease. Adv Exp Med Biol. 2006;577:274–287. doi: 10.1007/0-387-32957-9_20. [DOI] [PubMed] [Google Scholar]
- 17.Van Loy T, Thys K, Ryschkewitsch C, Lagatie O, et al. JC virus quasispecies analysis reveals complex viral population underlying PML and supports viral dissemination via the hematogenous route. J Virol. 2014 doi: 10.1128/JVI.02565-14. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yogo Y, Kitamura T, Sugimoto C, Ueki T, et al. Isolation of a possible archetypal JC virus DNA sequence from nonimmunocompromised individuals. J Virol. 1990;64:3139–3143. doi: 10.1128/jvi.64.6.3139-3143.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitamura T, Sugimoto C, Kato A, Ebihara H, et al. Persistent JC virus (JCV) infection is demonstrated by continuous shedding of the same JCV strains. J Clin Microbiol. 1997;35:1255–1257. doi: 10.1128/jcm.35.5.1255-1257.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bofill-Mas S, Clemente-Casares P, Major EO, Curfman B, et al. Analysis of the excreted JC virus strains and their potential oral transmission. J. Neurovirol. 2003;9:498–507. doi: 10.1080/13550280390218887. [DOI] [PubMed] [Google Scholar]
- 21.Ault GS, Stoner GL. Two major types of JC virus defined in progressive multifocal leukoencephalopathy brain by early and late coding region DNA sequences. J Gen Virol. 1992;73:2669–2678. doi: 10.1099/0022-1317-73-10-2669. [DOI] [PubMed] [Google Scholar]
- 22.Agostini HT, Ryschkewitsch CF, Mory R, Singer EJ, et al. JC virus (JCV) genotypes in brain tissue from patients with progressive multifocal leukoencephalopathy (PML) and in urine from controls without PML: increased frequency of JCV type 2 in PML. J Infect Dis. 1997;176:1–8. doi: 10.1086/514010. [DOI] [PubMed] [Google Scholar]
- 23.Zheng HY1, Takasaka T, Noda K, Kanazawa A, et al. New sequence polymorphisms in the outer loops of the JC polyomavirus major capsid protein (VP1) possibly associated with progressive multifocal leukoencephalopathy. J Gen Virol. 2005;86:2035–2045. doi: 10.1099/vir.0.80863-0. [DOI] [PubMed] [Google Scholar]
- 24.Dang X, Vidal JE, Oliveira AC, Simpson DM, et al. JC virus granule cell neuronopathy is associated with VP1 C terminus mutants. J Gen Virol. 2012;93:175–183. doi: 10.1099/vir.0.037440-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richardson EP. Progressive multifocal leukoencephalopathy. N Engl J Med. 1961;265:815–823. doi: 10.1056/NEJM196110262651701. 1961. [DOI] [PubMed] [Google Scholar]
- 26.Du Pasquier RA, Corey S, Margolin DH, Williams K, et al. Productive infection of cerebellar granule cell neurons by JC virus in an HIV+ individual. Neurology. 2003;61:775–782. doi: 10.1212/01.wnl.0000081306.86961.33. [DOI] [PubMed] [Google Scholar]
- 27.Koralnik IJ, Wüthrich C, Dang X, Rottnek M, et al. JC virus granule cell neuronopathy: a novel clinical syndrome distinct from progressive multifocal leukoencephalopathy. Ann Neurol. 2005;57:576–580. doi: 10.1002/ana.20431. [DOI] [PubMed] [Google Scholar]
- 28.Wüthrich C, Koralnik IJ. Frequent infection of cortical neurons by JC virus in patients with progressive multifocal leukoencephalopathy. J Neuropathol Exp Neurol. 2012;71:54–65. doi: 10.1097/NEN.0b013e31823ede59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dang X, Wüthrich C, Gordon J, Sawa H, Koralnik IJ. JC virus encephalopathy is associated with a novel agnoprotein-deletion JCV variant. PLoS One. 2012;7:e35793. doi: 10.1371/journal.pone.0035793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agnihotri SP, Wuthrich C, Dang X, Nauen D, et al. A fatal case of JC virus meningitis presenting with hydrocephalus in a human immunodeficiency virus-seronegative patient. Ann Neurol. 2014;76:140–147. doi: 10.1002/ana.24192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monaco MC, Jensen PN, Hou J, Durham LC, et al. Detection of JC virus DNA in human tonsil tissue: evidence for site of initial viral infection. J Virol. 1998;72:9918–9923. doi: 10.1128/jvi.72.12.9918-9923.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kato A, Kitamura T, Takasaka T, Tominaga T, et al. Detection of the archetypal regulatory region of JC virus from the tonsil tissue of patients with tonsillitis and tonsilar hypertrophy. J Neurovirol. 2004;10:244–249. doi: 10.1080/13550280490468663. [DOI] [PubMed] [Google Scholar]
- 33.Berger JR, Miller CS, Mootoor Y, Avdiushko SA, et al. JC virus detection in bodily fluids: clues to transmission. Clin Infect Dis. 2006;43:e9–12. doi: 10.1086/504947. [DOI] [PubMed] [Google Scholar]
- 34.Del Valle L, White MK, Enam S, Piña Oviedo S, et al. Detection of JC virus DNA sequences and expression of viral T antigen and agnoprotein in esophageal carcinoma. Cancer. 2005;103:516–527. doi: 10.1002/cncr.20806. [DOI] [PubMed] [Google Scholar]
- 35.Dörries K, Vogel E, Günther S, Czub S. Infection of human polyomaviruses JC and BK in peripheral blood leukocytes from immunocompetent individuals. Virology. 1994;198:59–70. doi: 10.1006/viro.1994.1008. [DOI] [PubMed] [Google Scholar]
- 36.Gallia GL, Houff SA, Major EO, Khalili K. Review: JC virus infection of lymphocytes--revisited. J Infect Dis. 1997;176:1603–1609. doi: 10.1086/514161. [DOI] [PubMed] [Google Scholar]
- 37.Ciappi S, Azzi A, De Santis R, Leoncini F, et al. Archetypal and rearranged sequences of human polyomavirus JC transcription control region in peripheral blood leukocytes and in cerebrospinal fluid. J Gen Virol. 1999;80:1017–1023. doi: 10.1099/0022-1317-80-4-1017. [DOI] [PubMed] [Google Scholar]
- 38.Chalkias S, Dang X, Bord E, Stein MC, et al. JC virus reactivation during prolonged natalizumab monotherapy for multiple sclerosis. Ann Neurol. 2014;75:925–934. doi: 10.1002/ana.24148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jensen PN, Major EO. Viral variant nucleotide sequences help expose leukocytic positioning in the JC virus pathway to the CNS. J Leukoc Biol. 1999;65:428–438. doi: 10.1002/jlb.65.4.428. [DOI] [PubMed] [Google Scholar]
- 40.Houff SA, Major EO, Katz DA, Kufta CV, et al. Involvement of JC virus-infected mononuclear cells from the bone marrow and spleen in the pathogenesis of progressive multifocal leukoencephalopathy. N Engl J Med. 1988;318:301–305. doi: 10.1056/NEJM198802043180507. [DOI] [PubMed] [Google Scholar]
- 41.Dubois V, Dutronc H, Lafon ME, Poinsot V, et al. Latency and reactivation of JC virus in peripheral blood of human immunodeficiency virus type 1-infected patients. J Clin Microbiol. 1997;35:2288–2292. doi: 10.1128/jcm.35.9.2288-2292.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen NN, Kerr D, Chang CF, Honjo T, et al. Evidence for regulation of transcription and replication of the human neurotropic virus JCV genome by the human S(mu)bp-2 protein in glial cells. Gene. 1997;185:55–62. doi: 10.1016/s0378-1119(96)00630-0. [DOI] [PubMed] [Google Scholar]
- 43.Johnson EM, Wortman MJ, Dagdanova AV, Lundberg PS, et al. Polyomavirus JC in the context of immunosuppression: a series of adaptive, DNA replication-driven recombination events in the development of progressive multifocal leukoencephalopathy. Clin Dev Immunol. 2013;2013:197807. doi: 10.1155/2013/197807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lednicky JA, Butel JS. Tissue culture adaptation of natural isolates of simian virus 40: changes occur in viral regulatory region but not in carboxy-terminal domain of large T-antigen. J Gen Virol. 1997;78:1697–1705. doi: 10.1099/0022-1317-78-7-1697. [DOI] [PubMed] [Google Scholar]
- 45.Atwood WJ, Amemiya K, Traub R, Harms J, et al. Interaction of the human polyomavirus, JCV, with human B-lymphocytes. Virology. 1992;190:716–723. doi: 10.1016/0042-6822(92)90909-9. [DOI] [PubMed] [Google Scholar]
- 46.Monaco MC, Atwood WJ, Gravell M, Major EO. JCV infection of hematopoietic progenitor cells, primary B lymphocytes and tonsillar stromal cells: implications for viral latency. J Virol. 1996;70:7004–7012. doi: 10.1128/jvi.70.10.7004-7012.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Monaco MC, Sabath BF, Durham LC, Major EO. JC virus multiplication in human hematopoietic progenitor cells requires the NF-1 class D transcription factor. J Virol. 2001;75:9687–9695. doi: 10.1128/JVI.75.20.9687-9695.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marshall LJ, Dunham L, Major EO. Transcription factor Spi-B binds unique sequences present in the tandem repeat promoter/enhancer of JC virus and supports viral activity. J Gen Virol. 2010;91:3042–3052. doi: 10.1099/vir.0.023184-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marshall LJ, Moore LD, Mirsky MM, Major EO. JC virus promoter/enhancers contain TATA box-associated Spi-B-binding sites that support early viral gene expression in primary astrocytes. J Gen Virol. 2012;93:651–661. doi: 10.1099/vir.0.035832-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tan CS, Dezube BJ, Bhargava P, Autissier P, et al. Detection of JC virus DNA and proteins in the bone marrow of HIV-positive and HIV-negative patients: implications for viral latency and neurotropic transformation. J Infect Dis. 2009;199:881–8. doi: 10.1086/597117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berger JR, Houff SA, Major EO. Monoclonal antibodies and progressive multifocal leukoencephalopathy. MAbs. 2009;1:583–589. doi: 10.4161/mabs.1.6.9884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Major EO. Progressive multifocal leukoencephalopathy in patients on immunomodulatory therapies. Annu Rev Med. 2010;61:35–47. doi: 10.1146/annurev.med.080708.082655. [DOI] [PubMed] [Google Scholar]
- 53.Frohman EM, Monaco MC, Remington G, Ryschkewitsch C, et al. JC Virus in CD34+ and CD19+ Cells in Patients With Multiple Sclerosis Treated With Natalizumab. JAMA Neurol. 2014;71:596–602. doi: 10.1001/jamaneurol.2014.63. [DOI] [PubMed] [Google Scholar]
- 54.Saure C, Warnke C, Zohren F, Schroeder T, et al. Natalizumab and impedance of the homing of CD34+ hematopoietic progenitors. Arch Neurol. 2011;68:1428–1431. doi: 10.1001/archneurol.2011.238. [DOI] [PubMed] [Google Scholar]
- 55.Warnke C, Smolianov V, Dehmel T, Andrée M, et al. CD34+ progenitor cells mobilized by natalizumab are not a relevant reservoir for JC virus. Mult Scler. 2011;17:151–156. doi: 10.1177/1352458510385834. [DOI] [PubMed] [Google Scholar]
- 56.White MK, Khalili K. Pathogenesis of progressive multifocal leukoencephalopathy--revisited. J Infect Dis. 2011;203:578–586. doi: 10.1093/infdis/jiq097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pérez-Liz G, Del Valle L, Gentilella A, Croul S, et al. Detection of JC virus DNA fragments but not proteins in normal brain tissue. Ann Neurol. 2008;64:379–387. doi: 10.1002/ana.21443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tan CS, Ellis LC, Wüthrich C, Ngo L, et al. JC virus latency in the brain and extraneural organs of patients with and without progressive multifocal leukoencephalopathy. J Virol. 2010;84:9200–9209. doi: 10.1128/JVI.00609-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.White MK, Safak M, Khalili K. Regulation of gene expression in primate polyomaviruses. J Virol. 2009;83:10846–10856. doi: 10.1128/JVI.00542-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Romagnoli L, Wollebo HS, Deshmane SL, Mukerjee R, et al. Modulation of JC virus transcription by C/EBPbeta. Virus Res. 2009;146:97–106. doi: 10.1016/j.virusres.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wollebo HS, Safak M, Del Valle L, Khalili K, et al. Role for tumor necrosis factor-α in JC virus reactivation and progressive multifocal leukoencephalopathy. J Neuroimmunol. 2011;233:46–53. doi: 10.1016/j.jneuroim.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nukuzuma S, Nakamichi K, Kameoka M, Sugiura S, et al. TNF-α stimulates efficient JC virus replication in neuroblastoma cells. J Med Virol. 2014;86:2026–2032. doi: 10.1002/jmv.23886. [DOI] [PubMed] [Google Scholar]
- 63.Atwood WJ, Wang L, Durham LC, Amemiya K, et al. Evaluation of the role of cytokine activation in the multiplication of JC virus (JCV) in human fetal glial cells. J Neurovirol. 1995;1:40–49. doi: 10.3109/13550289509111009. [DOI] [PubMed] [Google Scholar]
- 64.Manley K, O'Hara BA, Gee GV, Simkevich CP, et al. NFAT4 is required for JC virus infection of glial cells. J Virol. 2006;80:12079–12085. doi: 10.1128/JVI.01456-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wollebo HS, Melis S, Khalili K, Safak M, et al. Cooperative roles of NF-κB and NFAT4 in polyomavirus JC regulation at the KB control element. Virology. 2012;432:146–154. doi: 10.1016/j.virol.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Romagnoli L, Sariyer IK, Tung J, Feliciano M, et al. Early growth response-1 protein is induced by JC virus infection and binds and regulates the JC virus promoter. Virology. 2008;375:331–41. doi: 10.1016/j.virol.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim SY, Woo MS, Kim WK, Choi EC, et al. Glial cell-specific regulation of the JC virus early promoter by histone deacetylase inhibitors. J Virol. 2003;77:3394–3401. doi: 10.1128/JVI.77.6.3394-3401.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wollebo HS, Woldemichaele B, Khalili K, Safak M, et al. Epigenetic regulation of polyomavirus JC. Virol J. 2013;10:264. doi: 10.1186/1743-422X-10-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Beltrami S, Gordon J. Immune surveillance and response to JC virus infection and PML. J Neurovirol. 2014;20:137–149. doi: 10.1007/s13365-013-0222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koralnik IJ, Du Pasquier RA, Letvin NL. JC virus-specific cytotoxic T lymphocytes in individuals with progressive multifocal leukoencephalopathy. J Virol. 2001;75:3483–3487. doi: 10.1128/JVI.75.7.3483-3487.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Du Pasquier RA, Kuroda MJ, Zheng Y, Jean-Jacques J, et al. A prospective study demonstrates an association between JC virus-specific cytotoxic T lymphocytes and the early control of progressive multifocal leukoencephalopathy. Brain. 2004;127:1970–1978. doi: 10.1093/brain/awh215. [DOI] [PubMed] [Google Scholar]
- 72.Warnke C, Mausberg AK, Stettner M, Dehmel T, et al. Natalizumab affects the T-cell receptor repertoire in patients with multiple sclerosis. Neurology. 2013;81:1400–1408. doi: 10.1212/WNL.0b013e3182a84101. [DOI] [PubMed] [Google Scholar]
- 73.Chen Y, Bord E, Tompkins T, Miller J, et al. Asymptomatic reactivation of JC virus in patients treated with natalizumab. N Engl J Med. 2009;361:1067–74. doi: 10.1056/NEJMoa0904267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Korman BD, Tyler KL, Korman NJ. Progressive multifocal leukoencephalopathy, efalizumab, and immunosuppression: a cautionary tale for dermatologists. Arch Dermatol. 2009;145:937–942. doi: 10.1001/archdermatol.2009.175. [DOI] [PubMed] [Google Scholar]
- 75.Carson KR, Evens AM, Richey EA, Habermann TM, et al. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood. 2009;113:4834–4840. doi: 10.1182/blood-2008-10-186999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tan CS, Koralnik IJ. Progressive multifocal leukoencephalopathy and other disorders caused by JC virus: clinical features and pathogenesis. Lancet Neurol. 2010;9:425–437. doi: 10.1016/S1474-4422(10)70040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Martin Mdel P, Cravens PD, Winger R, Kieseier BC, et al. Depletion of B lymphocytes from cerebral perivascular spaces by rituximab. Arch Neurol. 2009;66:1016–1020. doi: 10.1001/archneurol.2009.157. [DOI] [PubMed] [Google Scholar]
- 78.Small JA, Scangos GA, Cork L, Jay G, et al. The early region of human papovavirus JC induces dysmyelination in transgenic mice. Cell. 1986;46:13–18. doi: 10.1016/0092-8674(86)90855-x. [DOI] [PubMed] [Google Scholar]
- 79.Haas S, Haque NS, Beggs AH, Khalili K, et al. Expression of the myelin basic protein gene in transgenic mice expressing human neurotropic virus, JCV, early protein. Virology. 1994;202:89–96. doi: 10.1006/viro.1994.1325. [DOI] [PubMed] [Google Scholar]
- 80.Kondo Y, Windrem MS, Zou L, Chandler-Militello D, et al. Human glial chimeric mice reveal astrocytic dependence of JC virus infection. J Clin Invest. 2014 doi: 10.1172/JCI76629. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tretiakova A, Krynska B, Gordon J, Khalili K. Human neurotropic JC virus early protein deregulates glial cell cycle pathway and impairs cell differentiation. J Neurosci Res. 1999;55:588–599. doi: 10.1002/(SICI)1097-4547(19990301)55:5<588::AID-JNR6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 82.Darbinyan A, Kaminski R, White MK, Darbinian-Sarkissian N, et al. Polyomavirus JC infection inhibits differentiation of oligodendrocyte progenitor cells. J Neurosci Res. 2013;91:116–127. doi: 10.1002/jnr.23135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Del Valle L, Pina-Oviedo S. HIV disorders of the brain: pathology and pathogenesis. Front Biosci. 2006;11:718–732. doi: 10.2741/1830. [DOI] [PubMed] [Google Scholar]
- 84.Mazlo M, Ressetar HG, Stoner GL. The neuropathology and pathogenesis of progressive multifocal leukencephalopy. In: Khalili K, Stoner GL, editors. Human polyomaviruses: molecular and clinical perspectives. John Wiley & Sons, Inc.; New York: 2001. [Google Scholar]
- 85.Berger JR. Progressive multifocal leukoencephalopathy. Curr Neurol Neurosci Rep. 2007;7:461–469. doi: 10.1007/s11910-007-0072-9. [DOI] [PubMed] [Google Scholar]
- 86.Gordon J, Gallia GL, Del Valle L, Amini S, Khalili K. Human polyomavirus JCV and expression of myelin genes. J Neurovirol. 2000;6(Suppl 2):S92–S97. [PubMed] [Google Scholar]
- 87.Stolt A, Sasnauskas K, Koskela P, Lehtinen M, et al. Seroepidemiology of the human polyomaviruses. J Gen Virol. 2003;84:1499–1504. doi: 10.1099/vir.0.18842-0. [DOI] [PubMed] [Google Scholar]
- 88.Knowles WA, Pipkin P, Andrews N, Vyse A, et al. Population-based study of antibody to the human polyomaviruses BKV and JCV and the simian polyomavirus SV40. J Med Virol. 2003;71:115–123. doi: 10.1002/jmv.10450. [DOI] [PubMed] [Google Scholar]
- 89.Kean JM, Rao S, Wang M, Garcea RL. Seroepidemiology of human polyomaviruses. PLoS Pathog. 2009;5(3):e1000363. doi: 10.1371/journal.ppat.1000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Carter JJ, Madeleine MM, Wipf GC, Garcea RL, et al. Lack of serologic evidence for prevalent simian virus 40 infection in humans. J Natl Cancer Inst. 2003;95:1522–1530. doi: 10.1093/jnci/djg074. [DOI] [PubMed] [Google Scholar]
- 91.Verbeeck J, Van Assche G, Ryding J, Wollants E, et al. JC viral loads in patients with Crohn's disease treated with immunosuppression: can we screen for elevated risk of progressive multifocal leukoencephalopathy? Gut. 2008;57:1393–1397. doi: 10.1136/gut.2007.145698. [DOI] [PubMed] [Google Scholar]
- 92.Egli A, Infanti L, Dumoulin A, Buser A, et al. Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. J Infect Dis. 2009;199:837–846. doi: 10.1086/597126. [DOI] [PubMed] [Google Scholar]
- 93.Engels EA, Rollison DE, Hartge P, Baris D, et al. Antibodies to JC and BK viruses among persons with non-Hodgkin lymphoma. Int J Cancer. 2005;117:1013–1019. doi: 10.1002/ijc.21277. [DOI] [PubMed] [Google Scholar]
- 94.Padgett BL, Walker DL. Prevalence of antibodies in human sera against JC virus, an isolate from a case of progressive multifocal leukoencephalopathy. J Infect Dis. 1973;127:467–470. doi: 10.1093/infdis/127.4.467. [DOI] [PubMed] [Google Scholar]
- 95.Taguchi F, Kajioka J, Miyamura T. Prevalence rate and age of acquisition of antibodies against JC virus and BK virus in human sera. Microbiol Immunol. 1982;26:1057–1064. doi: 10.1111/j.1348-0421.1982.tb00254.x. [DOI] [PubMed] [Google Scholar]
- 96.Weber T, Trebst C, Frye S, Cinque P, et al. Analysis of the systemic and intrathecal humoral immune response in progressive multifocal leukoencephalopathy. J Infect Dis. 1997;176:250–254. doi: 10.1086/514032. [DOI] [PubMed] [Google Scholar]
- 97.Matos A, Duque V, Beato S, da Silva JP, et al. Characterization of JC human polyomavirus infection in a Portuguese population. J Med Virol. 2010;82:494–504. doi: 10.1002/jmv.21710. [DOI] [PubMed] [Google Scholar]
- 98.Sheremata W, Brown AD, Rammohan KW. Dimethyl fumarate for treating relapsing multiple sclerosis. Expert Opin Drug Saf. 2015;14:161–170. doi: 10.1517/14740338.2015.977251. [DOI] [PubMed] [Google Scholar]