Abstract
Study Design
Retrospective analysis.
Objective
The objective of this study was to evaluate the efficacy of a surgical site infection (SSI) prevention protocol instituted in the Orthopaedic Spine Department at our institution.
Summary of Background Data
SSI is an undesired complication of orthopaedic spine surgeries. It poses a significant risk to the patient, as well as a financial toll on the healthcare system. A wide range of prophylactic measures have been used to attempt to reduce SSI rates.
Methods
A protocol consisting of a combination of 0.3% Betadine wound irrigation and 1 gram of intra-wound Vancomycin powder application was developed at our institution. Multiple data sources were consolidated for thorough evaluation of changes in SSI rates, patient risk factors, and changes in bacteriology. Identification of risk factors that predispose patients to SSI was performed using mixed effects logistic regression in a univariate fashion. Risk factors with p-values of ≤ 0.05 in univariate analysis were included together in a multivariate mixed effects logistic regression model.
Results
SSI rates were reduced by 50% following the intervention; Chi square analysis comparing the SSI rates between the pre- and post-intervention periods yielded a p-value of 0.042. Rates of methicillin resistant Staphylococcus aureus dropped from 30% to 7% and the rates of multi-bacterial infections dropped from 37% to 27%. The risk factors that were statistically significant in multivariate analysis were the following: age (OR 0.93), anemia (OR 30.73), prior operation (OR 27.45), and vertebral fracture (OR 22.22).
Conclusion
The combination of Betadine wound irrigation and intra-wound vancomycin powder application led to both a clinically and statistically significant decrease in SSI rates by 50%. Bacteriology analysis and risk factor assessment proved to be valuable tools in assessing the efficacy of a new prophylactic measure and in the planning of future protocols.
Keywords: surgical site infection, Spinal Surgery, orthopaedics, neurosurgery, quality of healthcare, Betadine solution, vancomycin powder, risk factors, bacteriology
Introduction
Postoperative surgical site infection (SSI) rates for spinal surgeries have been reported to range from 0.7% to 12.0%.1, 2 This serious complication of spine surgery results in prolonged hospitalization for patients, long term intravenous antibiotics, and reoperation for irrigation and debridement of the wound.3-9 Such additional interventions increase the total cost of care more than four times and are a significant burden to the healthcare system.10 In an attempt to incentivize physicians and hospitals to take the necessary measures to reduce post-operative SSI, the Centers for Medicare and Medicaid Services (CMS) has reduced hospital reimbursements for the management of SSI.9 Due to recent pressures to reduce SSI rates, there have been many studies that have evaluated the efficacy of various prophylactic measures. Such interventions have included pre-surgical application of alcohol foam, use of plastic drapes, wound drains, wound irrigation with Betadine prior to closure, and intra-wound application of vancomycin powder.11-16 Many of these procedures were evaluated individually in previous studies, and were already common practice at our institution. The purpose of this article is to determine the efficacy of a new prophylactic protocol initiated January 1st, 2012, which featured a combination of Betadine wound irrigation & intra-wound use of vancomycin powder. Risk factors that predispose patients to a SSI have already been analyzed at other institutions; however we determined the risk factors specific to the patient demographics at our institution.3, 4, 8, 17-20 In addition to risk factor analysis, a thorough investigation of bacteriology changes before and after our intervention was conducted to further study the efficacy of this new prophylactic protocol and to develop strategies for future measures. With the use of several patient databases and extensive chart review, we were able to accurately determine SSI rates at our institution, review risk factors that predispose to SSI specific to our patient demographics, assess bacteriology changes, and ultimately develop a protocol to reduce SSI rates of orthopaedic spine surgeries at our institution. Our method can be used to help customize prophylactic measures at any institution with a different set of patient demographics to match the goals of preventative medicine.
Materials and Methods
The study period of 2010-2013 was made up of 599, 653, 693, and 480 orthopedic spinal surgeries in 2010, 2011, 2012, and 2013, respectively. These procedures translated to 1252 patients for the 2010-2011 pre-intervention period and 1173 patients for the 2012-2013 post-intervention period, for a total of 2425 patients. The data for these patients was compiled from three databases and one electronic patient record system to determine the effectiveness of a prophylaxis protocol in reducing postoperative SSI in orthopaedic spine surgeries. This information was further used to evaluate both changes in bacteriology before and after the protocol, as well as risk factors and comorbidities that predispose patients to a SSI. Effective January 1st, 2012, all patients undergoing spine surgery under the care of the Orthopaedics Spine Department at our institution received the study intervention: intra-wound irrigation with dilute Betadine solution (0.3% weight/volume) and application of 1 gram of Vancomycin powder throughout the wound prior to wound closure. All spinal surgery cases performed by orthopaedic surgery staff physicians at our institution from January 1st, 2010, through December 31st, 2013, were reviewed with the assistance of Healthcare Infection Management (HIM) and Infection Control findings, which currently archive data via International Classification of Diseases, Ninth Revision (ICD-9) codes, International Classification of Diseases, Tenth Revision (ICD-10) codes, and Current Procedural Terminology (CPT) codes, and data from the National Surgical Quality Improvement Program (NSQIP). Data of interest not available in these databases had to be obtained manually through chart review utilizing the confidential and protected Electronic Medical Record (EMR). To ensure the accuracy of chart review, a second observer verified the data gathered from EMR. The study was approved by the Institutional Review Board (IRB) to review the protected patient information.
Spine surgery cases with high clinical suspicion of infection that required reoperation and subsequent irrigation and debridement (I&D) were our primary outcome; these were identified using CPT code data from the HIM and Infection Control databases. During I&D procedures, wound cultures were obtained and results were used to assess changes in bacteriology before and after SSI prophylaxis. The SSI rate for a given year was determined by dividing the number of SSI cases by the number of total procedures for the same year. A Χ2 test was performed to compare SSI rates from 2010-2011 (pre-intervention) to those from 2012-2013 (post-intervention).
The NSQIP database featured more comprehensive data for every 8th day of a given year on our patient population than the HIM and Infection Control databases. For this reason, it was the main database used in the risk factor analysis portion of this project in which SSI cases were compared to non-SSI cases. Data for risk factors of interest that was not included in this database was obtained via chart review in EMR. The consistent compilation of the NSQIP data every 8th day was deemed an adequately random and representative subset of an entire year's procedures. To achieve an adequate comparison, the number of non-SSI cases was chosen to be at least four times the number of SSI cases for any given year. Identification of risk factors that predispose a patient to a SSI was determined using mixed effects logistic regression. Analyses were first conducted in a univariate fashion for one risk factor at a time. Risk factors with p-values of ≤ 0.05 in univariate analysis were accepted as statistically significant and then included together in a multivariate mixed effects logistic regression model. The parameter estimates reported from logistic regression analyses were odds ratios (OR) with 95% confidence intervals. The odds of a SSI were defined as the probability of experiencing a SSI divided by the probability of not experiencing a SSI. The OR was defined as the ratio of the odds between a patient with and without a given risk factor, or for continuous risk factors, as the change in odds for a one unit change in a continuous variable (ex. age). For categories that lacked a sufficient sample size, logistic regression procedure failed to converge and no results could be reported. Analyses were conducted using SAS software for Windows, version 9.3 (SAS Institute, Cary, NC).
Results
The rates of SSI in patients at our institution undergoing orthopaedic spine surgery from January 2010 to December 2013 are shown in Figure 1. Prior to the SSI prophylaxis protocol, SSI rates were 2.2% (13 patients), and 2.6% (17 patients) for 2010 and 2011, respectively, with a combined SSI rate of 2.4% for 1252 total procedures. After initiation of the protocol in January 2012, the rate of SSI decreased to 1.7% (12 patients), and 0.625% (3 patients) for 2012 and 2013, respectively, with a combined SSI rate of 1.3% for 1173 total procedures. The Chi-square test p-value comparing rates of SSI in the pre-intervention interval, 2010-2011 (2.4%), to the post-intervention interval, 2012-2013 (1.3%), was 0.042.
Figure 1.
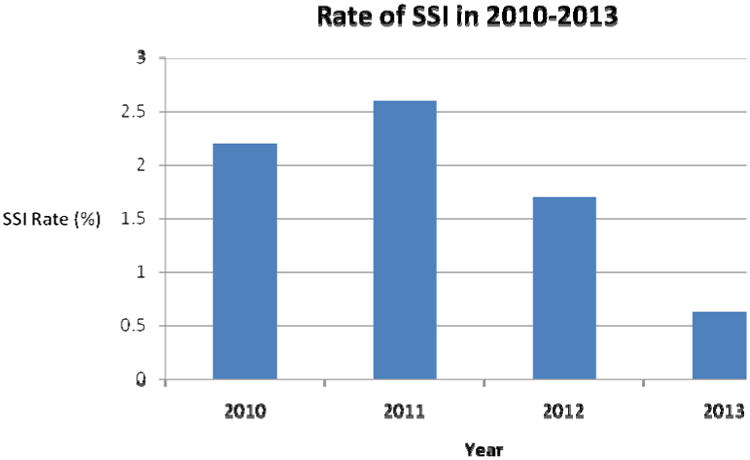
Rate of SSI in Orthopaedic Spine Surgeries at our institution for 2010-2013.
Figures 2 and 3 depict the breakdown of the specific bacterial isolates associated with the SSI. Wound cultures were negative for three SSI cases in 2010, one SSI case in 2011, one SSI case in 2012, and one SSI case in 2013; these cases are also represented in the figures. Figure 2 represents the bacteriology of patients with SSI during the pre-intervention period; methicillin resistant Staphylococcus aureus (MRSA) (30%, 9 patients), and mixed flora (37%, 11 patients) predominated in these patients. Mixed flora infections consisted of a positive culture with at least 2 or more of the following organisms: coagulase negative Staphylococcus, Fusobacterium nucleatum, Prevotella, Bacteroides, Actinomyces, Streptococcus viridans, Escherichia coli, Proteus, methicillin sensitive Staphyloccus aureus (MSSA), Corynebacterium, Streptococcus agalactiae, MRSA, Enterococcus faecalis, Enterobacter, Pseudomonas. The bacteriology of patients with a SSI following the implementation of the study intervention is shown in Figure 3; methicillin sensitive Staphylococcus aureus (MSSA) (20%, 3 patients) and mixed flora (27%, 4 patients) accounted for most of the post-intervention SSI, with a reduction in the rate of MRSA infection to 7% (1 patient). Mixed flora infections consisted of a positive culture with at least 2 or more of the following organisms: Escherichia coli, Peptostreptococcus, Klebsiella, Enterobacter, MRSA, MSSA, coagulase negative Staphylococcus aureus. Eighty percent of the cultures pre-intervention were positive for at least one gram positive organism, while this rate was reduced to 53% after the initiation of the protocol.
Figure 2.
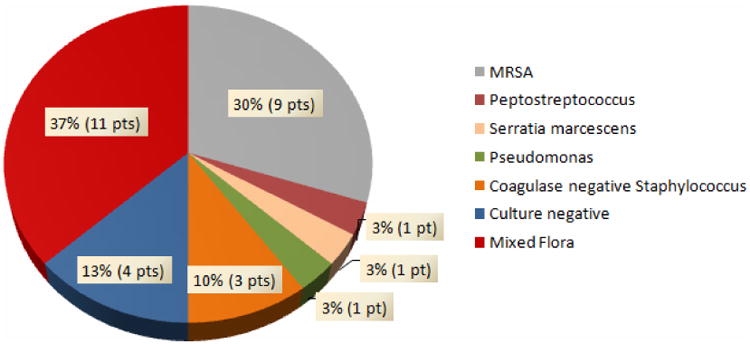
Bacteriology of SSI patients for 2010 and 2011 (Pre-intervention).
Figure 3.
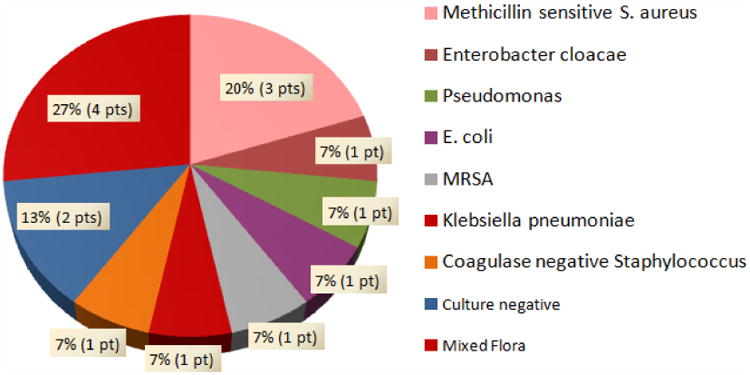
Bacteriology of SSI patients for 2012 and 2013 (Post-intervention).
Demographics of the patient population undergoing orthopaedic spine surgery from 2010-2013 is depicted in Table 1. Increased patient age showed a statistically significant trend toward decreasing rates of SSI (mean age of 57.4 years old for non-SSI cases, and a mean age of 46.0 years old for SSI cases; P = 0.0001). The odds ratio for age is the incremental change in odds for each additional year of age; thus the odds ratio of 0.961 signifies that there is a 4% decrease in odds of SSI for each additional year of age. Gender was not statistically significant for predisposition to a SSI (59% of SSI cases were female while 41% of SSI cases were male; P = 0.255 for female vs. male in univariate mixed-effects logistic regression analysis). As evidenced in the procedural characteristics of Table 2, operations involving the thoracic spine showed an increased likelihood of infection, making up 46% of all SSI cases (P = 0.0012), while contributing to only 21% of non-SSI cases. Global tests for any differences among categories suggested an effect of operative time and surgical approach on likelihood of SSI; post-hoc testing showed significantly higher odds of SSI among patients who had an operation lasting more than 5 hours compared to those who had an operation lasting < 2 hours, as well as those who underwent both an anterior and posterior approach in the same surgery compared to an anterior or posterior approach alone. The type of procedure and transfusions data depicted in Table 3 demonstrates that the orthopaedic spine procedures least likely to result in an operative infection were decompression (P = 0.0150) and discectomy (P = 0.0093). The patient comorbidities with the highest likelihood of SSI predisposition were the following: history of smoking (P = 0.0126), history of alcohol use (P = < 0.0001), anemia (P = < 0.0001), dyspnea (P = 0.0002), renal failure (P = 0.0058), malignant cancer (P = 0.0119), coagulopathy (P = 0.0063), and a prior operation (P = < 0.0001). Patients diagnosed with a disc herniation (P = 0.0292) or stenosis (P = 0.0042) had the lowest risk of SSI, whereas instrumentation failure (P = 0.0037) and vertebral fractures (P = < 0.0001) were diagnoses predisposing to SSI (Table 4). After multivariate analysis, the following risk factors and ORs with confidence intervals were associated with SSI: anemia (30.73 (3.52-268.54); P = 0.0021), prior operation (27.45 (6.75-111.73); P = < 0.0001), and a diagnosis of a vertebral fracture (22.22 (2.25-219.48); P = 0.0081) (Table 5). Dyspnea was a risk factor that was on the verge of statistical significance in multivariate analysis (6.39 (0.97-42.12); P = 0.0538). The SSI prophylaxis proved to be protective against SSI in a multivariate analysis comparing the 2012-2013 period to the 2010-2011 period; OR of 0.23 (CI 0.060-0.86) and P-value of 0.0287.
Table 1. Patient Demographics.
| Control Cases (N, %) | SSI Cases (N, %) | Odds Ratio (95% CI) | P Value | |
|---|---|---|---|---|
| Age at Operation (Years) | ||||
| Mean (Range) | 57.4 (20-88) | 46.0 (14-81) | 0.96 (0.94-0.98) | 0.0001 |
| Sex (reference group is Male) | ||||
| Female | 131 (49%) | 27 (59%) | 1.45 (0.76-2.77) | 0.255 |
| Male | 134 (51%) | 19 (41%) | - | - |
| Race (failed to converge) | ||||
| Caucasian | 218 (82%) | 28 (61%) | - | - |
| Hispanic | 5 (2%) | 6 (13%) | - | - |
| African American | 13 (5%) | 5 (11%) | - | - |
| American Indian | 0 | 2 (4%) | - | - |
| Asian | 9 (3%) | 1 (2%) | - | - |
| Other | 12 (5%) | 2 (4%) | - | - |
| Unknown | 7 (3%) | 1 (2%) | - | - |
SSI indicates surgical site infection; CI, confidence interval.
Table 2. Procedural Characteristics.
| Control Cases (N, %) | SSI Cases (N, %) | Odds Ratio (95% CI) | P Value | |
|---|---|---|---|---|
| Incision (reference group is posterior) | ||||
| Anterior | 47 (18%) | 3 (7%) | 0.32 (0.09-1.07) | .0158† |
| Anterior/Posterior | 3 (1%) | 3 (7%) | 6.08 (1.14-32.51) | - |
| Posterior | 215 (81%) | 40 (86%) | - | - |
| Region of spine involved | ||||
| Cervical (failed to converge) | 67 (25%) | 4 (9%) | - | - |
| Thoracic | 55 (21%) | 21 (46%) | 3.07 (1.56-6.04) | 0.0012 |
| Lumbar | 191 (72%) | 37 (80%) | 1.66 (0.76-3.66) | 0.203 |
| Sacropelvic | 91 (34%) | 19 (41%) | 1.41 (0.74-2.71) | 0.298 |
| Revision | 31 (12%) | 7 (15%) | 1.55 (0.63-3.86) | 0.341 |
| Estimate blood loss (cc) | ||||
| Mean (Range) (OR is change per 10 cc) | 711.1 (0-7600) | 1110.0 (10-4000) | 1.00 (1.00-1.00) | 0.0618 |
| No. of spinal levels involved (failed to converge) | ||||
| Single | 0 | 3 (6%) | - | - |
| 2-3 | 161 (61%) | 16 (35%) | - | - |
| 4-7 | 68 (26%) | 16 (35%) | - | - |
| 8-12 | 13 (5%) | 5 (11%) | - | - |
| >12 | 22 (8%) | 6 (13%) | - | - |
| Operative time (h) (reference group is <2) | ||||
| <2 | 40 (15%) | 2 (4%) | - | 0.0032† |
| 2-5 | 155 (58%) | 19 (41%) | 2.20 (0.49-9.99) | - |
| >5 | 70 (26%) | 25 (54%) | 6.21 (1.37-28.05) | - |
Indicates P-value for global test of any differences among categories.
SSI indicates surgical site infection; CI, confidence interval; OR, odds ratio.
Table 3. Procedures and Transfusions.
| Control Cases (N, %) | SSI Cases (N, %) | Odds Ratio (95% CI) | P Value | |
|---|---|---|---|---|
| Procedure | ||||
| Arthrodesis | 187 (71%) | 39 (85%) | 2.16 (0.92-5.09) | 0.0772 |
| Osteotomy | 24 (9%) | 6 (13%) | 1.46 (0.55-3.90) | 0.451 |
| Decompression | 181 (68%) | 23 (50%) | 0.45 (0.24-0.86) | 0.0150 |
| Discectomy | 118 (45%) | 11 (24%) | 0.38 (0.18-0.79) | 0.0093 |
| Corpectomy | 16 (6%) | 5 (11%) | 1.76 (0.60-5.16) | 0.300 |
| Laminoplasty | 10 (4%) | 0 | - | - |
| Kyphoplasty/vertebroplasty | 6 (2%) | 0 | - | - |
| Artificial disk | 0 | 0 | - | - |
| Transfusions | ||||
| BMP Units (reference group is 0 units) | ||||
| 0 | 167 (63%) | 28 (61%) | - | 0.896† |
| 1 | 78 (29%) | 12 (26%) | 0.95 (0.45-1.99) | - |
| 2 | 16 (6%) | 5 (11%) | 1.48 (0.48-4.60) | - |
| >2 | 4 (2%) | 1 (2%) | 1.38 (0.14-13.3) | - |
| CellSaver Units (reference group is 0 units) | ||||
| 0 | 200 (75%) | 26 (57%) | - | 0.107† |
| 1 | 31 (12%) | 7 (15%) | 1.61 (0.64-4.08) | - |
| 2 | 17 (6%) | 6 (13%) | 2.56 (0.91-7.20) | - |
| >2 | 17 (6%) | 7 (15%) | 2.86 (1.07-7.65) | - |
| Fresh Frozen Plasma Units (reference group is 0 units) | ||||
| 0 | 233 (88%) | 34 (74%) | - | 0.139† |
| 1 | 5 (2%) | 3 (7%) | 3.80 (0.84-17.33) | - |
| 2 | 11 (4%) | 4 (9%) | 2.55 (0.75-8.62) | - |
| >2 | 16 (6%) | 5 (11%) | 1.98 (0.67-5.88) | - |
| Packed Red Blood Cell Units (reference group is 0 units) | ||||
| 0 | 201 (76%) | 26 (57%) | - | 0.0630† |
| 1 | 10 (4%) | 5 (11%) | 3.79 (1.18-12.14) | - |
| 2 | 16 (6%) | 3 (7%) | 1.41 (0.38-5.29) | - |
| >2 | 38 (14%) | 12 (26%) | 2.28 (1.04-4.98) | - |
| Platelets Units (failed to converge) | ||||
| 0 | 251 (95%) | 39 (85%) | - | - |
| 1 | 10 (4%) | 3 (7%) | - | - |
| 2 | 3 (1%) | 0 | - | - |
| >2 | 1 (0%) | 4 (9%) | - | - |
Indicates P-value for global test of any differences among categories.
SSI indicates surgical site infection; CI, confidence interval; BMP, bone morphogenic protein.
Table 4. Comorbidities and Diagnoses.
| Control Cases (N, %) | SSI Cases (N, %) | Odds Ratio (95% CI) | P Value | |
|---|---|---|---|---|
| Comorbidities | ||||
| Diabetes | 41 (15%) | 8 (17%) | 1.26 (0.54-2.93) | 0.596 |
| Tobacco history | 37 (14%) | 13 (28%) | 2.59 (1.23-5.45) | 0.0126 |
| Alcohol history | 17 (6%) | 14 (30%) | 6.10 (2.72-13.7) | <.0001 |
| Arthropathy | 118 (45%) | 17 (37%) | 0.81 (0.42-1.56) | 0.529 |
| Anemia | 4 (2%) | 8 (17%) | 17.59 (4.83-64.08) | <.0001 |
| Dyspnea | 8 (3%) | 8 (17%) | 7.64 (2.63-22.21) | 0.0002 |
| COPD | 7 (3%) | 2 (4%) | 1.92 (0.38-9.81) | 0.431 |
| Coronary artery disease | 13 (6%) | 6 (14.3%) | 2.39 (0.93-6.14) | 0.0699 |
| CHF | 6 (2%) | 3 (7%) | 3.78 (0.88-16.16) | 0.0729 |
| Previous cardiac surgery | 8 (4%) | 1 (2%) | 0.48 (0.06-3.82) | 0.484 |
| Angina | 8 (3%) | 4 (9%) | 3.38 (0.95-12.05) | 0.0600 |
| Hypertension | 131 (49%) | 17 (37%) | 0.64 (0.33-1.23) | 0.177 |
| PVD | 1 (0%) | 2 (5%) | 3.68 (0.78-17.41) | 0.101 |
| Malignant Cancer | 2 (1%) | 3 (7%) | 11.07 (1.71-71.88) | 0.0119 |
| Open Wound | 5 (2%) | 1 (2%) | 1.36 (0.15-12.19) | 0.784 |
| Overweight/Obese | 195 (74%) | 34 (74%) | 1.06 (0.52-2.19) | 0.869 |
| Coagulopathy | 3 (1%) | 4 (9%) | 8.95 (1.87-42.94) | 0.0063 |
| Sepsis | 4 (2%) | 1 (2%) | 1.25 (0.13-11.76) | 0.843 |
| Prior Operation | 43 (16%) | 31 (67%) | 10.74 (5.29-21.80) | <.0001 |
| Diagnoses | ||||
| Degenerative spondylosis | 31 (12%) | 4 (9%) | 0.67 (0.22-2.06) | 0.486 |
| Deformity | 35 (13%) | 9 (20%) | 1.53 (0.66-3.52) | 0.319 |
| Disc Herniation | 57 (22%) | 3 (7%) | 0.26 (0.077-0.87) | 0.0292 |
| Stenosis | 139 (52%) | 13 (28%) | 0.36 (0.18-0.72) | 0.0042 |
| Myelopathy | 29 (11%) | 3 (7%) | 0.51 (0.15-1.80) | 0.297 |
| Spondylolisthesis | 45 (17%) | 5 (11%) | 0.63 (0.23-1.70) | 0.363 |
| Instrumentation Failure | 11 (4%) | 7 (15%) | 4.65 (1.65-13.07) | 0.0037 |
| Vertebral Fracture | 3 (1%) | 10 (22%) | 23.08 (5.97-89.15) | <.0001 |
| Bone/CT Neoplasm | 3 (1%) | 2 (4%) | 5.21 (0.79-34.43) | 0.0863 |
SSI indicates surgical site infection; CI, confidence interval; COPD, chronic obstructive pulmonary disease; CHF, congestive heart failure ; PVD, peripheral vascular disease; CT, connective tissue.
Table 5. Multivariate Analysis.
| Risk Factor | Odds Ratio | 95% CI | P Value |
|---|---|---|---|
| Operation Year: 2012-2013 vs. 2010-2011 | 0.23 | 0.06-0.86 | 0.0287 |
| Age at Time of Operation | 0.93 | 0.90-0.97 | 0.0004 |
| Anterior approach | 0.55 | 0.06-4.81 | 0.338† |
| Anterior/Posterior approach | 6.14 | 0.38-98.41 | - |
| Thoracic spine | 0.29 | 0.061-1.37 | 0.118 |
| Tobacco History | 1.51 | 0.34-6.59 | 0.584 |
| Alcohol History | 3.23 | 0.81-12.88 | 0.097 |
| Anemia | 30.73 | 3.52-268.54 | 0.0021 |
| Dyspnea | 6.39 | 0.97-42.12 | 0.0538 |
| Renal failure | 2.50 | 0.09-66.86 | 0.583 |
| Coagulopathy | 1.44 | 0.06-36.61 | 0.826 |
| Prior Operation | 27.45 | 6.75-111.73 | <.0001 |
| Decompression | 0.64 | 0.20-2.12 | 0.467 |
| Discectomy | 0.34 | 0.071-1.62 | 0.175 |
| Disc Herniation | 0.98 | 0.14-6.81 | 0.981 |
| Stenosis | 1.31 | 0.35-4.86 | 0.683 |
| Instrumentation Failure | 3.09 | 0.24-40.00 | 0.387 |
| Vertebral Fracture | 22.22 | 2.25-219.48 | 0.0081 |
| Operative Time (h): 2-5 vs. <2 | 1.78 | 0.18-17.45 | 0.218† |
| Operation Time (h): >5 vs. <2 | 5.11 | 0.42-61.71 | - |
Indicates P-value for global test of any differences among categories.
CI, confidence interval
Discussion
There are many studies that have measured the individual effectiveness of the application of intraoperative vancomycin powder, or the use of Betadine solution irrigation, in the prevention of SSI.11, 12, 14, 15, 17 However, our study combined these two procedures and we analyzed the efficacy of this prophylactic measure in reducing the rates of SSI in orthopaedic spine surgery patients at our institution. Both a clinically and statistically significant reduction in an SSI rate to just 1.3% in 2012-2013 (post-intervention), down from 2.4% the pre-intervention period of 2010-2011, was achieved. The determined SSI rates for our institution for every year of the study were found to be within the 0.7% to 12.0% spectrum referenced in previous literature.1
Many previously published risk factors for SSI were supported by our study. With evidence from multivariate analysis, these risk factors ranged from a preoperative diagnosis of a vertebral fracture, to medical history including anemia, and prior operations.1 A few notable procedural characteristics that were linked to SSI in univariate analysis include operation of the thoracic spine, and an operative time greater than 5 hours. Previous publications reported that operations of the lumbar and sacral spine were risk factors for SSI; however we could not achieve statistical significance for these attributes.4, 21-23 An increase in the number of spinal levels involved in a procedure can be linked to a longer operative time, however only the latter was found to be a significant risk factor. Despite a lack of statistical significance, SSI cases were found to have a mean estimated blood loss (EBL) of 400 cc more than non-SSI cases. A risk factor for SSI that seems to be incongruent with literature is the age of our patients. We found the mean age for patients who developed a SSI to be 46.0, while the mean age for the non-SSI group was 57.4. This difference was supported with p-values of 0.0001 in univariate analysis and 0.0287 in multivariate analysis. This result may be attributed to a selection bias, in which surgeons were more conservative in their treatment of older patients based on the age and additional health factors associated with older patients. A pre-surgical diagnosis of a vertebral fracture was also found to be a significant risk factor for SSI with a p-value of 0.0081 in multivariate analysis. This can be explained if many of the patients diagnosed with a vertebral fracture were linked to a significant mechanism of injury, which itself can increase a patient's risk for infection. However, we do not have the data to determine the mechanism of injury for vertebral fractures, thus cannot support this assumption. A risk factor that was not quite statistically significant but that may be important for clinicians to assess is dyspnea (P = 0.0538 in multivariate analysis). Dyspnea is often the result of risk factors that have been linked in other studies to predispose to SSI, including: COPD, tobacco use, and obesity.18 For this reason, it may be an important symptom to evaluate for in the pre-operation risk assessment for likelihood of developing surgical complications such as SSI. Some risk factors that we could not link to the development of SSI were obesity, diabetes, and hypertension. Previous studies have identified these co-morbidities as risk factors for SSI, but we believe that there were more significant factors that overshadowed these categories in our dataset.19 These differences can also be explained by a difference in patient demographics as compared to other studies. A more user-friendly database for assessing risk factors can further allow physicians to assess risk based on patient information in their region.
Identifying the changes in the bacteriology of SSI prior to and after our intervention was crucial in determining a plan for further improving the protocol. During the pre-intervention interval, we found that 80% of the cultures from SSI cases contained at least one gram positive organism. There was a steep drop in the proportion of gram positive cultures to 53% following institution of the prophylactic procedure. The most significant trends included a drop in MRSA infections from 30% (9 patients) to 7% (1 patient), and a decrease in the number of multi-bacterial infections from 37% (11 patients) pre-intervention to 27% (4 patients) post-intervention. Further, the multi-bacterial infections from 2010 and 2011 featured a constitution of 15 different organisms, while the list was reduced by more than half to just 7 organisms after the 2012 intervention. The sharp decline in post-surgical MRSA infections allows for more manageable and less aggressive treatment of patients with SSI. It is evident that the vancomycin powder was an effective means of reducing the number of gram positive organisms in SSI cases. It is possible to consider adding an additional antibiotic targeted for gram positive bacteria to further reduce their proliferation. However, there is also a need for an additional prophylactic agent to reduce the number of gram negative organisms that are still found in the surgical sites of patients. Analysis of bacteriology proves to be a useful tool in identifying the effectiveness of such an intervention, as well as determining the strategy for future prophylaxis.
The use of intra-wound Betadine irrigation and vancomycin powder is a safe, fast, inexpensive, and technically straightforward approach that led to a clinically and statistically significant reduction in SSI rates of orthopaedic spine surgeries of a major academic medical center. This study shows that it is possible to reduce already low SSI rates with such simple procedures; however it also identified challenges to be improved upon in the future. Pooling data from several different databases, each with their own limitations in thoroughness and accuracy, proved to be inefficient and exhaustive. We hope that the pressure to reduce SSI rates will lead to a consolidation of databases for easier and more accurate analysis of risk factors and postoperative patient outcomes. A single database of all patient criteria would allow for larger scale studies and a more efficient method of determining effective prophylactic measures. In addition, this would allow for such analyses as the interactions amongst risk factors and the ultimate likelihood of developing a SSI. This is important because many risk factors that may not be overtly statistically significant in the development of SSI may predispose to other risk factors that are. Such interactions were not analyzed in this study, but would be meaningful to assess in future studies. The methods used in this study can be used to monitor infection rates over time, to identify risk factors, and to quantify efficacy of interventions. Doing so will allow for a more accurate preoperative risk assessment for SSIs and to effectively focus measures designed to reduce SSIs.
Acknowledgments
- Monica Evans, R.N., BSN
- Kimberly Brink-Capps, R.N., BSN
Footnotes
The manuscript submitted does not contain information about medical device(s)/drug(s). The National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR000002 funds were received in support of this work. No relevant financial activities outside the submitted work.
References
- 1.Gerometta A, Rodriguez Olaverri JC, Bitan F. Infections in spinal instrumentation. Int Orthop. 2012;36(2):457–64. doi: 10.1007/s00264-011-1426-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pull ter Gunne AF, et al. The presentation, incidence, etiology, and treatment of surgical site infections after spinal surgery. Spine (Phila Pa 1976) 2010;35(13):1323–8. doi: 10.1097/BRS.0b013e3181bcde61. [DOI] [PubMed] [Google Scholar]
- 3.Blam OG, et al. Risk factors for surgical site infection in the patient with spinal injury. Spine (Phila Pa 1976) 2003;28(13):1475–80. doi: 10.1097/01.BRS.0000067109.23914.0A. [DOI] [PubMed] [Google Scholar]
- 4.Abdul-Jabbar A, et al. Surgical site infection in spinal surgery: description of surgical and patient-based risk factors for postoperative infection using administrative claims data. Spine (Phila Pa 1976) 2012;37(15):1340–5. doi: 10.1097/BRS.0b013e318246a53a. [DOI] [PubMed] [Google Scholar]
- 5.Ahn DK, et al. The degree of bacterial contamination while performing spine surgery. Asian Spine J. 2013;7(1):8–13. doi: 10.4184/asj.2013.7.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hegde V, et al. Management of postoperative spinal infections. World J Orthop. 2012;3(11):182–9. doi: 10.5312/wjo.v3.i11.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lazennec JY, et al. Infections in the operated spine: update on risk management and therapeutic strategies. Orthop Traumatol Surg Res. 2011;97(6 Suppl):S107–16. doi: 10.1016/j.otsr.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Pull ter Gunne AF, Cohen DB. Incidence, prevalence, and analysis of risk factors for surgical site infection following adult spinal surgery. Spine (Phila Pa 1976) 2009;34(13):1422–8. doi: 10.1097/BRS.0b013e3181a03013. [DOI] [PubMed] [Google Scholar]
- 9.Salkind AR, Rao KC. Antiobiotic prophylaxis to prevent surgical site infections. Am Fam Physician. 2011;83(5):585–90. [PubMed] [Google Scholar]
- 10.Calderone RR, et al. Cost of medical care for postoperative spinal infections. Orthop Clin North Am. 1996;27(1):171–82. [PubMed] [Google Scholar]
- 11.Caroom C, et al. Intrawound vancomycin powder reduces surgical site infections in posterior cervical fusion. Spine (Phila Pa 1976) 2013;38(14):1183–7. doi: 10.1097/BRS.0b013e31828fcfb5. [DOI] [PubMed] [Google Scholar]
- 12.Pahys JM, et al. Methods to decrease postoperative infections following posterior cervical spine surgery. J Bone Joint Surg Am. 2013;95(6):549–54. doi: 10.2106/JBJS.K.00756. [DOI] [PubMed] [Google Scholar]
- 13.O'Neill KR, et al. Reduced surgical site infections in patients undergoing posterior spinal stabilization of traumatic injuries using vancomycin powder. Spine J. 2011;11(7):641–6. doi: 10.1016/j.spinee.2011.04.025. [DOI] [PubMed] [Google Scholar]
- 14.Cheng MT, et al. Efficacy of dilute betadine solution irrigation in the prevention of postoperative infection of spinal surgery. Spine (Phila Pa 1976) 2005;30(15):1689–93. doi: 10.1097/01.brs.0000171907.60775.85. [DOI] [PubMed] [Google Scholar]
- 15.Molinari RW, Khera OA, Molinari WJ., 3rd Prophylactic intraoperative powdered vancomycin and postoperative deep spinal wound infection: 1,512 consecutive surgical cases over a 6-year period. Eur Spine J. 2012;21 Suppl 4:S476–82. doi: 10.1007/s00586-011-2104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watters WC., 3rd Antibiotic prophylaxis in spine surgery: an evidence-based clinical guideline for the use of prophylactic antibiotics in spine surgery. Spine J. 2009;9(2):142–6. doi: 10.1016/j.spinee.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Boston KM, et al. Risk factors for spinal surgical site infection, Houston, Texas. Infect Control Hosp Epidemiol. 2009;30(9):884–9. doi: 10.1086/605323. [DOI] [PubMed] [Google Scholar]
- 18.Koutsoumbelis S, et al. Risk factors for postoperative infection following posterior lumbar instrumented arthrodesis. J Bone Joint Surg Am. 2011;93(17):1627–33. doi: 10.2106/JBJS.J.00039. [DOI] [PubMed] [Google Scholar]
- 19.Olsen MA, et al. Risk factors for surgical site infection following orthopaedic spinal operations. J Bone Joint Surg Am. 2008;90(1):62–9. doi: 10.2106/JBJS.F.01515. [DOI] [PubMed] [Google Scholar]
- 20.Rao SB, et al. Risk factors for surgical site infections following spinal fusion procedures: a case-control study. Clin Infect Dis. 2011;53(7):686–92. doi: 10.1093/cid/cir506. [DOI] [PubMed] [Google Scholar]
- 21.Lonjon G, et al. Early surgical site infections in adult spinal trauma: a prospective, multicentre study of infection rates and risk factors. Orthop Traumatol Surg Res. 2012;98(7):788–94. doi: 10.1016/j.otsr.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe M, et al. Risk factors for surgical site infection following spine surgery: efficacy of intraoperative saline irrigation. J Neurosurg Spine. 2010;12(5):540–6. doi: 10.3171/2009.11.SPINE09308. [DOI] [PubMed] [Google Scholar]
- 23.Schuster JM, et al. The influence of perioperative risk factors and therapeutic interventions on infection rates after spine surgery: a systematic review. Spine (Phila Pa 1976) 2010;35(9 Suppl):S125–37. doi: 10.1097/BRS.0b013e3181d8342c. [DOI] [PubMed] [Google Scholar]
- 24.Schwarzkopf R, et al. Effects of perioperative blood product use on surgical site infection following thoracic and lumbar spinal surgery. Spine (Phila Pa 1976) 2010;35(3):340–6. doi: 10.1097/BRS.0b013e3181b86eda. [DOI] [PubMed] [Google Scholar]


