Abstract
Contrast enhancement magnetic resonance imaging (CE-MRI) of synovial volume is the radiographic gold standard to quantify joint inflammation but cost limits use. Therefore, we examined if power Doppler-ultrasound (PD-US) outcomes of synovitis in tumor necrosis factor transgenic (TNF-Tg) mice correlate with CE-MRI. TNF-Tg mice underwent PD-US of their knees to measure the joint space volume (JSV) and PD volume (PDV), and the results were correlated with synovial volume determined by CE-MRI. Immunohistochemistry for CD31 was performed to corroborate the PD signal. Synovial volume strongly correlated with both JSV and PDV (p<0.01). CD31+ blood vessels were observed in inflamed synovium proximal to the joint surface, which corresponded to areas of intense PD signals. JSV and PDV are valid measures of joint inflammation that correlate with synovial volume determined by CE-MRI and are associated with vascularity. Given the emergence of PD-US as a non-quantitative outcome of joint inflammation, we find JSV and PDV to be feasible and highly cost-effective for longitudinal studies in animal models. Furthermore, given the increasing utilization of PD-US in standard clinical practice, JSV and PDV could be translated to better quantify joint flare and response to therapy in RA patients.
Keywords: Bone ultrasound, Rheumatoid Arthritis (RA), Magnetic Resonance Imaging (MRI)
INTRODUCTION
Based on its high resolution, magnetic resonance imaging (MRI) is the preferred imaging standard to quantify joint inflammation in clinical arthritis studies. Synovial volume assessed via MRI provides clinically useful information about disease activity and treatment response due to the fact that synovial contrast enhancement correlates with vascularity (1), and synovial volume is a marker of disease activity that decreases following successful therapy (2). However, the prohibitively high costs of MRI and limited access have contributed to a marked increase in ultrasound (US) imaging utilization, due to the advantages of real-time imaging, accessibility, cost-efficiency, and absence of ionizing radiation (3). US is particularly useful to assess joint inflammation in rheumatoid arthritis (RA) patients, as power Doppler (PD) US can highlight synovial hyperemia to diagnose active synovitis, predict flares, and provide semi-quantitative analyses (PD Score) in clinical research studies (4–7). The challenges with MRI outlined above also pertain to pre-clinical research. A recent study outlined methods to perform PD-US imaging in murine models of inflammatory-erosive arthritis, including validation studies of B-mode scans with histology (8). However, truly quantitative US outcome measures remain an important unmet need, as most pre-clinical and clinical studies assign semi-quantifiable grades for B mode and/or power Doppler observations (8–11). Thus, the absence of accurate US-based volumetric outcome measures of joint inflammation remains a major limitation for arthritis research. Therefore, we sought to develop methods for quantification of PD-US volume in the joint space of tumor necrosis TNF-Tg mice (12), an established model of RA.
METHODS
Animals
A total of twelve knee joints from six 8–10 month-old TNF-Tg mice (3647 line on a C57BL/6 background) were studied. The research was conducted with approval by the University of Rochester Institutional Animal Care and Use Committee (IACUC).
Contrast-enhanced MRI acquisition and analysis
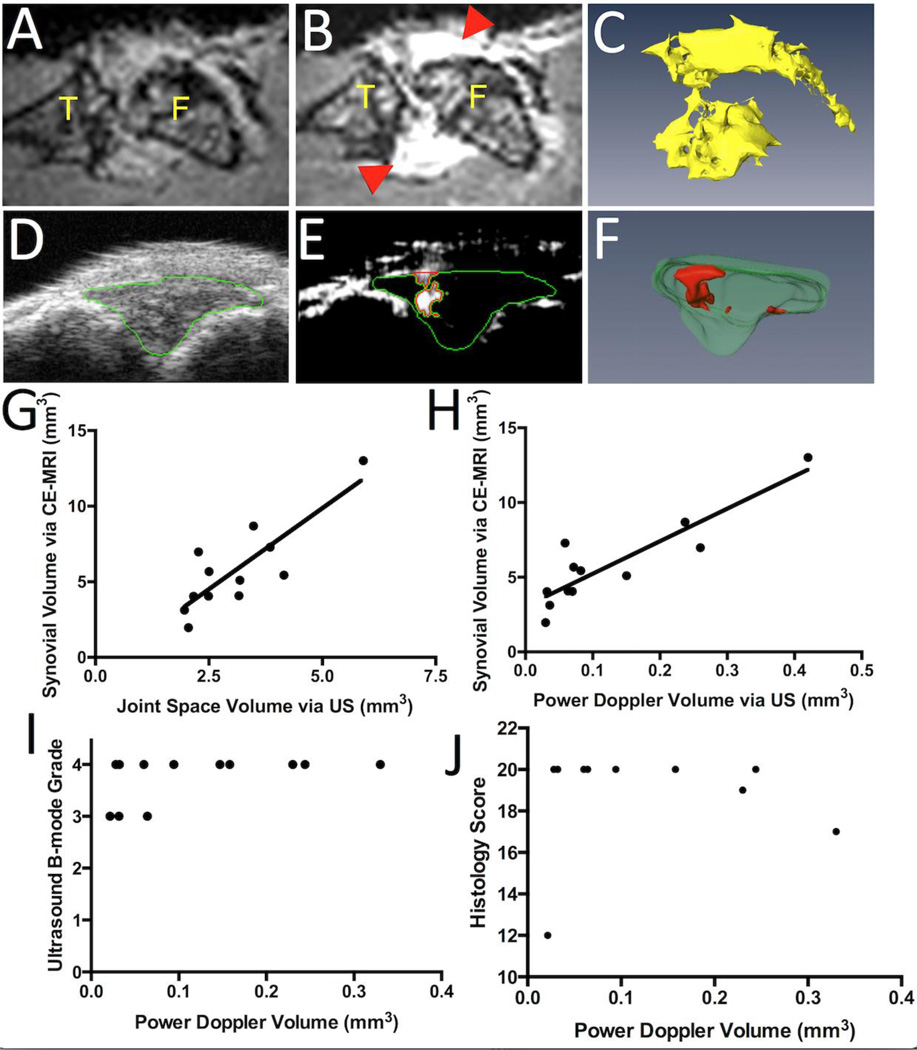
All contrast enhanced MRI (CE-MRI) and PD-US imaging was performed on mice anesthetized with 2% isoflurane as described on IACUC approved protocols. CE-MRI studies were performed with a 3 Tesla Siemens Trio MRI (Siemens Medical Solutions, Erlangen, Germany) and custom surface coils as previously described (13) with the following parameters: Sagittal T1-weighted FLASH, TR=45 ms, TE=9.03 ms, 192×192 pixels, 28 mm×28 mm FOV, 32 slices of 0.16mm slice thickness, flip angle=25°, 1 signal average, time: 9:28 min. First, a pre-contrast scan of the knee joint was obtained (Figure 1A) prior to gadolinium-diethylenetriamine pentaacetic acid (Gd-DTPA) contrast agent (Omniscan, Amer-sham Health, Oslo, Norway) retro-orbital administration (0.5 mL/kg). The post-contrast scan (Figure 1B) was started 5 minutes after Gd-DTPA injection to allow for circulation. The 3D stack of the pre-contrast scan was aligned with the post-contrast scan via automatic registration in Amira (VSG, Burlington, MA, USA). Then, a stack of images was generated by subtracting the pre-contrast scan from the post-contrast scan using the Arithmetic module. Synovial volumes were segmented by manually drawing region of interests (ROIs) on the 3D stack over the entire knee joint and were quantified as voxels above the threshold of 3.5 times the muscle mean signal intensity within the ROI. Enhanced voxels within the bone marrow were subtracted from the synovial volume. A 3D surface was then created using the SurfaceGen and SurfaceView modules (Figure 1C) and quantified using the MaterialStatistics module.
Figure 1. Quantification of Joint Space Volume and Power Doppler Volume determined by US and their correlation with established CE-MRI volumetric outcome measures.
TNF-Tg mice (8–10 months, n=6) underwent bilateral CE-MRI and US imaging of their knees (n=12) with analysis performed in Amira. Joints were then harvested for histology (n=10). 2D images of representative pre-contrast MRI (A), and CE-MRI highlighting the inflamed synovium (red arrowheads in B) are shown to illustrate the primary images of the knee (F, femur; T, tibia) used to generate the synovial volume via subtractive 3D rendering (C), as previously described (7). Similarly, representative 2D images of B mode scans with highlighted joint space (green outline in D), and thresholded PD signal within the joint space (red in E), are presented to illustrate the primary data used to generate the 3D surfaces of the joint space volume (JSV, green in F) and power Doppler volume (PDV) in the JSV (red in F). To validate PD-US measurements, correlation analyses were performed between synovial volume and joint space volume (JSV) (G; r=0.8301, r2=0.6891, p<0.01); and between synovial volume and power Doppler volume (PDV) within the joint space (H; r=0.8916, r2=0.7950, p<0.001). PDV was then compared to B-mode grades (I; r=0.4780, r2=0.2285, p=0.1160) and histology scores (J; r=0.0489, r2=0.0023, p=0.8932).
Ultrasound acquisition and analysis
Subsequently, the joints of these animals were imaged using a high-resolution small-animal US system (VisualSonics 770 with 704 scanhead, FujiFilm VisualSonics Inc., Toronto, ON, Canada) as previously described (14). Mice were placed in the supine position with their knees flexed over a customized mold and both B-mode and PD scans of the joint space were collected with a wall filter of 3 mm/s, scan speed of 2 mm/s, dynamic range of 13.13– 23.24 dB and the number of pulses to radio-frequency (RF) cycles as two. Amira was used for data analysis. First, the joint space was manually segmented as the anterior portion between the joint surfaces of the femur and tibia (green, Figure 1D), and a threshold was applied to the PD signal (>64 arbitrary units; a.u.) to encompass the PD signal. Then, a mask was created using the Arithmetic module with the expression A*(B>0), where A refers to the PD signal and B refers to the mask (joint space). This mask (green, Figure 1E) segments the PD data so the positive Doppler signal volume in the joint space (red, Figure 1E) can be delineated. These volumes were then quantified via the MaterialStatistics module, and the SurfaceGen and SurfaceView modules were used to generate 3D surfaces of both the joint space volume (JSV) and PD volume (PDV) in the joint space (green and red, respectively, Figure 1F).
Statistical Analysis
Linear regressions of PD-US and CE-MRI data, B-mode grades and histology scores were performed and tested for significance using Prism (GraphPad Software, San Diego, CA, USA). To assess the inter-user variability of analyzing JSV and PDV, these outcomes were quantified independently by two observers (E.M.B. and P.D.B.). The results demonstrated reasonable inter-user variability as determined by interclass coefficient correlation (ICC) analysis (JSV = 0.8582; PDV = 0.8438). Histology scoring according to Mould et al (15), was performed by two independent observers (E.M.B. and H.R.) and an ICC of 0.9022 was found.
Histology
For immunohistochemistry, knees were harvested and fixed in 10% neutral buffered formalin. The joints were then decalcified in 14% ethylenediaminetetraacetic acid at room temperature for 21 days. The joint was then embedded in paraffin and cut into 5 µm sections. Sections were then deparaffinized and underwent either an alcian blue hematoxylin/orange G stain for histology scoring, or were stained for the blood vessel marker CD31 (rabbit anti-mouse CD31, Abcam, Cambridge, MA, USA).
RESULTS
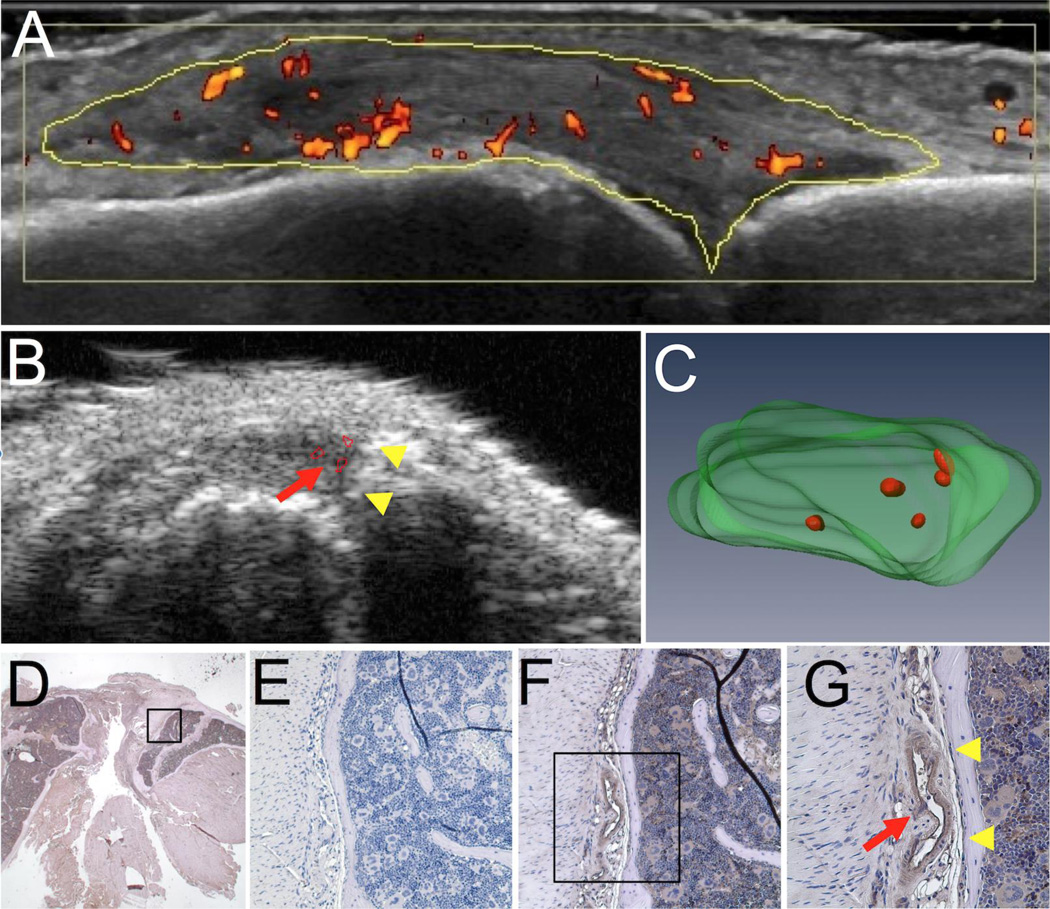
To validate PD-US as a volumetric outcome measure of joint inflammation, correlation analysis was performed using the CE-MRI measurement of synovial volume (Figure 1G and H). Both JSV and PDV demonstrated a significant correlation with synovial volume (Pearson’s r = 0.8301 and 0.8916, respectively). To compare our modality with previously published B-mode and histology scores (8, 15), we sought to identify correlations between PDV and these scoring systems (Figure 1I and J, respectively). Due to the limited range of these scoring systems, no significant correlation was found between the PDV and B-mode grade, or the PDV and histology score (p=0.1160 and 0.8932, respectively). This finding demonstrates that directly measuring joint inflammation independent of a discrete scoring system is beneficial, as it allows for continuous data such that differences are more easily detected. A clinical image of a RA metacarpophalangeal (MCP) joint space with hyperemia is shown to illustrate the similarities between patient and murine US images (Figure 2A and B, respectively). Note the high PD signal (heat map) present in the RA MCP joint space (yellow outline) (Figure 2A). To demonstrate that the PD signal localizes with blood vessels in the JS, knees were harvested after PD-US and CD31 staining was performed. The PD-US 2D and 3D images (Figure 2B and C, respectively) and histology (Figure 2D–G) from the same animal are shown to illustrate the presence of large blood vessels in the tissues that produced strong PD signals. Higher magnification clearly shows the CD31+ blood vessel (red arrow, Figure 2G) adjacent to the joint surface (yellow arrowheads, Figure 2G), with similar anatomy as the PD signal (red arrow, Figure 2B) to the joint surface (yellow arrowheads, Figure 2B) in the PD-US B-mode scan.
Figure 2. Corroboration of the PD-US signal with immunohistochemistry for CD31+ blood vessels in the joint space.
A PD-US image is shown to illustrate the resolution and the sensitivity of the PD signal (red heat map) in the metacarpophalangeal joint space (yellow outline) from a rheumatoid arthritis patient experiencing hyperemia of the synovium (A). TNF-Tg mice (8–10 months) underwent PD-US as described in Figure 1, and the knee joints were harvested for immunohistochemistry. A representative 2D image of the B-mode with PD signal (red arrow in B), with the corresponding 3D rendering of the joint space and PD signal (green and red, respectively in C) from PD-US are shown to highlight the vascular regions in the tissue that were subsequently evaluated by immunohistochemistry. Histology sections of the joint corresponding to the vascular regions in the PD-US images were processed for immunohistochemistry with antibodies specific for CD31. Micrographs of the joint space are shown at low magnification (1.25×; D) where the box highlighted is shown at 20× for the negative control (E) and CD31+ blood vessels (brown in F). The box highlighted in F is shown in G at 40×. Note the proximity of the blood vessel (red arrow in G) to the joint surface (yellow arrowheads in G), corresponding to the close proximity of the PD signal (red arrow in B) to joint surface (yellow arrowheads in B) shown in PD-US.
DISCUSSION
In conclusion, PD-US is able to accurately quantify synovial inflammation by measuring the PD signal volume that resides within the joint space. We have also confirmed that the PD signal corresponds to highly vascular tissue in the synovium. As this approach provides a translational cost-efficient alternative to CE-MRI, PDV has great potential as a primary outcome measure in arthritis intervention trials. Previous studies have shown that CE of the synovial volume correlates with vascularity (1), supporting PDV as measure of synovitis in the clinic. Further, despite the widespread use PD-US in rheumatology, the lack of quantifiable parameters from PD-US remains a major limitation. Therefore, our study offers methodology to quantify JSV and PDV, two parameters that provide a valid measure of the extent of active synovitis and tissue response following therapy in RA patients.
ACKNOWLEGEMENTS
This work was supported by research grants from the National Institutes of Health PHS awards (T32 AR053459; R01 AR056702; P01 AI078907; and P30 AR061307).
Footnotes
Authors’ Roles: Study design: EMB, RWW, and EMS. Data collection: EMB and RGT. Data analysis: EMB, PDB and HR. Drafting manuscript: EMB. Revising manuscript content: All authors. Approving final version of manuscript: All authors. EMB takes responsibility of the integrity of the data analysis.
Disclosures
All authors state that they have no conflicts of interest.
REFERENCES
- 1.Gaffney K, Cookson J, Blades S, Coumbe A, Blake D. Quantitative assessment of the rheumatoid synovial microvascular bed by gadolinium-DTPA enhanced magnetic resonance imaging. Ann Rheum Dis. 1998 Mar;57(3):152–157. doi: 10.1136/ard.57.3.152. PubMed PMID: 9640130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ostergaard M, Hansen M, Stoltenberg M, Gideon P, Klarlund M, Jensen KE, et al. Magnetic resonance imaging-determined synovial membrane volume as a marker of disease activity and a predictor of progressive joint destruction in the wrists of patients with rheumatoid arthritis. Arthritis Rheum. 1999 May;42(5):918–929. doi: 10.1002/1529-0131(199905)42:5<918::AID-ANR10>3.0.CO;2-2. PubMed PMID: 10323447. [DOI] [PubMed] [Google Scholar]
- 3.Tan YK, Ostergaard M, Conaghan PG. Imaging tools in rheumatoid arthritis: ultrasound vs magnetic resonance imaging. Rheumatology (Oxford) 2012 Dec;51(Suppl 7):vii36–vii42. doi: 10.1093/rheumatology/kes329. PubMed PMID: 23230093. Epub 2012/12/19. eng. [DOI] [PubMed] [Google Scholar]
- 4.van de Stadt LA, Bos WH, Meursinge Reynders M, Wieringa H, Turkstra F, van der Laken CJ, et al. The value of ultrasonography in predicting arthritis in auto-antibody positive arthralgia patients: a prospective cohort study. Arthritis Res Ther. 2010;12(3):R98. doi: 10.1186/ar3028. PubMed PMID: 20487531. Pubmed Central PMCID: PMC2911885. Epub 2010/05/22. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foltz V, Gandjbakhch F, Etchepare F, Rosenberg C, Tanguy ML, Rozenberg S, et al. Power Doppler ultrasound, but not low-field magnetic resonance imaging, predicts relapse and radiographic disease progression in rheumatoid arthritis patients with low levels of disease activity. Arthritis Rheum. 2012 Jan;64(1):67–76. doi: 10.1002/art.33312. PubMed PMID: 21904998. Epub 2011/09/10. eng. [DOI] [PubMed] [Google Scholar]
- 6.Peluso G, Michelutti A, Bosello S, Gremese E, Tolusso B, Ferraccioli G. Clinical and ultrasonographic remission determines different chances of relapse in early and long standing rheumatoid arthritis. Ann Rheum Dis. 2011 Jan;70(1):172–175. doi: 10.1136/ard.2010.129924. PubMed PMID: 21097799. Epub 2010/11/26. eng. [DOI] [PubMed] [Google Scholar]
- 7.Manzo A, Caporali R, Vitolo B, Alessi S, Benaglio F, Todoerti M, et al. Subclinical remodelling of draining lymph node structure in early and established rheumatoid arthritis assessed by power Doppler ultrasonography. Rheumatology (Oxford) 2011 Aug;50(8):1395–1400. doi: 10.1093/rheumatology/ker076. PubMed PMID: 21378108. Epub 2011/03/08. eng. [DOI] [PubMed] [Google Scholar]
- 8.Clavel G, Marchiol-Fournigault C, Renault G, Boissier MC, Fradelizi D, Bessis N. Ultrasound and Doppler micro-imaging in a model of rheumatoid arthritis in mice. Ann Rheum Dis. 2008 Dec;67(12):1765–1772. doi: 10.1136/ard.2007.083915. PubMed PMID: 18218664. Epub 2008/01/26. eng. [DOI] [PubMed] [Google Scholar]
- 9.Hammer HB, Sveinsson M, Kongtorp AK, Kvien TK. A 78-joints ultrasonographic assessment is associated with clinical assessments and is highly responsive to improvement in a longitudinal study of patients with rheumatoid arthritis starting adalimumab treatment. Ann Rheum Dis. 2010 Jul;69(7):1349–1351. doi: 10.1136/ard.2009.126995. PubMed PMID: 20472599. Epub 2010/05/18. eng. [DOI] [PubMed] [Google Scholar]
- 10.Hartung W, Kellner H, Strunk J, Sattler H, Schmidt WA, Ehrenstein B, et al. Development and evaluation of a novel ultrasound score for large joints in rheumatoid arthritis: one year of experience in daily clinical practice. Arthritis Care Res (Hoboken) 2011 May;64(5):675–682. doi: 10.1002/acr.21574. PubMed PMID: 22183834. Epub 2011/12/21. eng. [DOI] [PubMed] [Google Scholar]
- 11.Szkudlarek M, Court-Payen M, Jacobsen S, Klarlund M, Thomsen HS, Ostergaard M. Interobserver agreement in ultrasonography of the finger and toe joints in rheumatoid arthritis. Arthritis Rheum. 2003 Apr;48(4):955–962. doi: 10.1002/art.10877. PubMed PMID: 12687537. Epub 2003/04/11. eng. [DOI] [PubMed] [Google Scholar]
- 12.Keffer J, Probert L, Cazlaris H, Georgopoulos S, Kaslaris E, Kioussis D, et al. Transgenic mice expressing human tumour necrosis factor: a predictive genetic model of arthritis. EMBO J. 1991 Dec;10(13):4025–4031. doi: 10.1002/j.1460-2075.1991.tb04978.x. PubMed PMID: 1721867. Pubmed Central PMCID: 453150. Epub 1991/12/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Proulx ST, Kwok E, You Z, Papuga MO, Beck CA, Shealy DJ, et al. Longitudinal assessment of synovial, lymph node, and bone volumes in inflammatory arthritis in mice by in vivo magnetic resonance imaging and microfocal computed tomography. Arthritis Rheum. 2007 Nov 29;56(12):4024–4037. doi: 10.1002/art.23128. PubMed PMID: 18050199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bouta EM, Ju Y, Rahimi H, de Mesy-Bentley KL, Wood RW, Xing L, et al. Power Doppler Ultrasound Phenotyping of Expanding versus Collapsed Popliteal Lymph Nodes in Murine Inflammatory Arthritis. PLoS One. 2013;8(9):e73766. doi: 10.1371/journal.pone.0073766. PubMed PMID: 24040061. Pubmed Central PMCID: PMC3767819. Epub 2013/09/17. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mould AW, Tonks ID, Cahill MM, Pettit AR, Thomas R, Hayward NK, et al. Vegfb gene knockout mice display reduced pathology and synovial angiogenesis in both antigen-induced and collagen-induced models of arthritis. Arthritis Rheum. 2003 Sep;48(9):2660–2669. doi: 10.1002/art.11232. PubMed PMID: 13130487. Epub 2003/09/18. eng. [DOI] [PubMed] [Google Scholar]