Abstract
Arsenic trioxide (As2O3) is commonly used to treat acute promyelocytic leukemia and solid tumors. However, the clinical application of the agent is limited by its cyto- and genotoxic effects on normal cells. Thus, relief of As2O3 toxicity in normal cells is essentially necessary for improvement of As2O3-mediated chemotherapy. In this study, we have identified a series of protective effects of resveratrol against As2O3-induced oxidative damage in normal human bronchial epithelial (HBE) cells. We showed that treatment of HBE cells with resveratrol significantly reduced cellular levels of DNA damage, chromosomal breakage and apoptosis induced by As2O3. The effect of resveratrol against DNA damage was associated with a decreased level of reactive oxygen species and lipid peroxidation in cells treated by As2O3, suggesting that resveratrol protects against As2O3 toxicity via a cellular anti-oxidative stress pathway. Further analysis of the roles of resveratrol demonstrated that it modulated biosynthesis, recycling and consumption of glutathione (GSH), thereby promoting GSH homeostasis in HBE cells treated by As2O3. This was further supported by results showing that resveratrol prevented an increase in the activities and levels of caspases, Fas, Fas-L and cytochrome c proteins induced by As2O3. Our study indicates that resveratrol relieves As2O3-induced oxidative damage in normal human lung cells via maintenance of GSH homeostasis and suppression of apoptosis.
Keywords: Arsenic trioxide, Resveratrol, Oxidative damage, Glutathione homeostasis, Apoptosis
1. Introduction
Arsenic, a metalloid, exists ubiquitously in the environment. Chronic exposure to low concentrations of arsenic through drinking water, food and air is associated with an increased risk of skin, bladder, liver and lung cancer (Kitchin and Conolly 2010). Interestingly, one form of inorganic arsenic, arsenic trioxide (As2O3) can also serve as a potent anticancer agent for treatment of acute promyelocytic leukemia (APL) and solid tumors and has been commonly used in clinics for years. As2O3 is currently used as a first-line chemotherapeutic drug for APL because it can effectively induce apoptosis in leukemia cells (Zhang et al. 2001). However, the clinical application of the agent is limited by its side effects that include hepatotoxicity (Hao et al. 2013), cardiotoxicity (Barbey et al. 2003), and nephrotoxicity (Vuky et al. 2002), as well as sudden death (Westervelt et al. 2001). The adverse effects of As2O3 are usually caused by its cyto- and genotoxic effects that are mediated by oxidative stress in normal cells. Thus, relief of As2O3 toxic effects on normal cells is essential for improvement of the chemotherapeutic efficacy of As2O3. It has been suggested that chemo-protective agents that alleviate the toxic effects of As2O3 may be used to improve its efficacy on cancer therapy (Evens et al. 2004). Because As2O3 toxicity mainly results from reactive oxygen species (ROS) that subsequently induce oxidative DNA damage and apoptosis, as well as alter glutathione (GSH) homeostasis (Miller et al. 2002), it is conceivable that a ROS scavenger and/or an antioxidant can reduce the toxic effects of As2O3 on normal cells, thereby improving the selectivity of As2O3 chemotherapy.
Resveratrol (3,4’,5-trihydroxystilbene) is a natural polyphenolic compound that exists in plants such as grapes, mulberries and peanuts (Bishayee 2009). It has been used in conjunction with chemotherapy for the treatment of lung, breast and colon cancer (Aggarwal et al. 2004; Shankar et al. 2007; Bishayee 2009). Recent studies indicate that resveratrol can relieve As2O3-induced cardiotoxicity, hepatotoxicity and nephrotoxicity in animal models (Zhao et al. 2008; Yu et al. 2013; Zhang et al. 2013a; Zhang et al. 2013b). This may be associated with its potent antioxidant function through scavenging of ROS in cells (Aggarwal et al. 2004; Bishayee 2009). Our previous studies showed that resveratrol relieved oxidative damage and cell death induced by sodium arsenite in both human bronchial epithelial (HBE) cells (Chen et al. 2013a) and lung adenocarcinoma epithelial (A549) cells (Chen et al. 2013c). This suggests that resveratrol protects normal cells against the adverse effects of arsenite by decreasing production of ROS and apoptosis. Thus, it is possible that resveratrol can relieve As2O3-induced toxic effects on normal cells, thereby reducing its side effects.
It has been shown that apoptosis plays an important role in mediating the therapeutic effects and toxicity of As2O3 (Miller et al. 2002; Evens et al. 2004; Cui et al. 2008). As2O3 can induce cellular apoptosis in leukemia and solid tumor cells such as liver and lung cancer cells leading to cell death (Zhang et al. 2001; Miller et al. 2002). It has been suggested that ROS and oxidative damage are responsible for As2O3-induced apoptosis (Flora 2011). Thus, a decrease in ROS and oxidative damage by antioxidants may improve As2O3 chemotherapy. Apoptosis is usually mediated by the release of cytochrome c from mitochondria into the cytoplasm that activates caspase 3, 8 and 9, and in turn leads to single-stranded and double-stranded DNA breaks (Brown and Borutaite 2008; Fiandalo and Kyprianou 2012). Moreover, apoptotic progression can be modulated by the cellular redox level (Lushchak 2012) that is regulated by GSH. This further indicates that GSH homeostasis is also critical for mediating cellular apoptosis (Sakurai et al. 2004; Circu and Aw 2008). Thus, it is possible that relief of oxidative damage and prevention of apoptosis may be achieved by altering the cellular GSH level and decreasing the levels of critical enzymatic activities and proteins that are involved in apoptosis. We further hypothesize that resveratrol protects against As2O3-induced oxidative damage by promoting GSH homeostasis and/or suppressing an increase in the activities and levels of critical apoptotic enzymes and proteins. To test this hypothesis, we explored the roles of resveratrol in alleviating As2O3-induced oxidative damage and its underlying mechanisms in normal human HBE cells. We found that resveratrol effectively protected against As2O3-induced oxidative damage in HBE cells by facilitating cellular GSH homeostasis and preventing an increase of the activities and levels of critical enzymes and proteins that mediate apoptosis. Our study provides a new insight into the mechanisms by which resveratrol protects normal cells against As2O3 toxicity and side effects.
2. Materials and methods
2.1 Cell culture
HBE cells were generously provided by Stem Cells and Tissue Engineering Laboratory, State Key Laboratory of Biotherapy, Sichuan University, China. Cells were grown in Dulbecco's Modified Eagle Medium (DMEM) (Invitrogen, Grand Island, NY, USA) supplemented with 10% (v/v) fetal bovine serum (FBS), penicillin (100 unit/ml) and streptomycin (100 μg/ml). Cells were cultured in a 37°C incubator supplied with 5% CO2 and 95% air at constant humidity.
2.2 Cytotoxicity assay
Cytotoxicity of As2O3 (purchased from YiDa Pharmaceutical Co. Ltd, Harbin Medical University, Heilongjiang, China) in the absence or presence of resveratrol (≥98% purity)(purchased from Keddia Reagent Co. Ltd. Chengdu, China) was determined by 3-(4,5-dimethyithiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay as described previously (Chen et al. 2013a). HBE cells were seeded in 96-well plates at a density of 5 × 104 cells (200 μl) per well. After overnight incubation, cells were treated with As2O3 in the absence or presence of resveratrol that was dissolved in dimethylsulfoxide (DMSO) for 24 h. Subsequently, 100 μl of 0.5 mg/ml MTT solution (Amresco Co., Ohio, USA) was added into each well, and cells were incubated at 37°C for additional 4 h. 100 μl DMSO was then employed to dissolve formazan crystals. Absorbance at 570 nm was measured by a Bio-Rad micro-plate reader (Bio-Rad, Hercules, CA, USA). Cell viability was calculated according to the equation: Cell viability (%)=[treatment group OD570/control group OD570]×100.
2.3 Measurement of cellular ROS production
Cellular ROS production was detected with a fluorescent probe, 2’,7’-dichlorofluorescin diacetate (DCFH-DA, Applygen Technologies Inc., Beijing, China). In brief, 1 × 106 cells per well were seeded in 6-well plates with sterilized coverslips (24 mm × 24 mm). Cells were then treated with As2O3 in the absence or presence of 5 μM resveratrol for 24 h. Subsequently, cells were incubated with 10 μM DCFH-DA diluted with DMEM medium at 37°C for 1 h in the dark. Cells were visualized and imaged under a fluorescence microscope (DMLB2, Leica, Wetzlar, Germany) at an excitation/emission wavelength of 485 nm/530 nm. Production of ROS was quantified with Image-Pro@ Plus 6.0 software (Media Cybemetics Inc. USA). Sum integrated optical density (IOD) and sum areas were obtained. Average optical density (AOD) was calculated according to the equation: AOD= Sum IOD/the sum area.
2.4 Detection of superoxide dismutase (SOD) activity
Total SOD activity in HBE cells was determined using a commercially available kit from Nanjing Jiancheng Bioengineering Institute (Nanjing, China) according to the manufacturer's instructions. Briefly, 1 × 106 cells were treated with As2O3 in the absence or presence of resveratrol for 24 h, respectively. Cells were then harvested by 0.2% (w/v) ethylene diamine tetraacetic acid (EDTA) and lysed. Cell lysates were centrifuged at 1,000 X g at 4°C for 5 min. The supernatant was collected for determining cellular SOD activity. Absorbance at 550 nm was measured with a spectrometer (V-1100D, Mapada instruments Co., Ltd. Shanghai, China). Total cellular SOD activity was normalized by protein concentrations measured by bicinchoninic acid method.
2.5 Detection of lipid peroxidation
Lipid peroxidation was determined by measuring the formation of malondialdehyde (MDA) in HBE cells. After exposure to 20 μM As2O3 in the absence or presence of resveratrol for 24 h, cells were harvested and resuspended in lysis buffer. Cell lysates were then subjected to centrifugation at 12,000 X g at 4°C for 10 min. The supernatant was used to measure cellular MDA level according to the manufacturer's instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Cellular MDA level was illustrated as nmol/mg protein.
2.6 DNA damage determined by comet assay
DNA damage was determined by alkaline comet assay as described previously (Chen et al. 2013c). In brief, 1 × 105 cells were seeded in 24-well plates. Cells were treated with As2O3 alone or As2O3 along with resveratrol for 24 h. Cell suspension (20 μl) was mixed with 80 μl 0.65% low-melting agarose (Amresco, Solon OH, USA) at 37°C and spread on a slide that was pre-coated with 0.8% normal-melting agarose (Amresco, Solon OH, USA) and subsequently covered by a coverslip. The coverslip was then removed gently, and the slide was immersed in lysis solution (2.5 M NaCl, 100 mM EDTA, 10 mM Tris, pH = 10.0, 1% sodium sarcosinate, 1% Triton X-100, 10% DMSO) at 4°C in the dark for 1 h. After being soaked in electrophoresis buffer (pH>13.0) for 30 min to allow DNA unwinding, the slide was subjected to electrophoresis at 0.75 V/cm. The slide was then washed with distilled water, stained with 20 μg/ml ethidium bromide, and visualized under a fluorescence microscope (DMLB2, Leica, Wetzlar, Germany) at 200 × magnification. For each slide, two-hundred cells were randomly scored for calculation of the percentage of comet cell [Percentage of comet cells (%) = number of comet cells/number of cell counted (200)]. The Olive tail moment (OTM) was obtained using Comet Assay Software Project (CASP, Free Software Foundation. Inc.). Fifty comet cells of each slide were randomly selected for measuring their OTM.
2.7 Chromosomal breakage measured by micronucleus assay
Chromosomal breakage was measured with the micronucleus assay according to the procedure described previously (Chen et al. 2013c). Briefly, cells were seeded in a 6-well plate at a density of 5 × 105 cells/well. Cells were treated with As2O3 in the absence or presence of resveratrol for 24 h. Cells were subsequently collected by centrifugation at 140 X g for 5 min and resuspended in 0.075 M potassium chloride solution. Cells were then fixed in freshly prepared methanol-glacial acetic acid (3:1, v/v) solution three times and subsequently fixed with methanol-glacial acetic acid (99:1, v/v). The cell suspension was then dropped onto −20°C pre-chilled glass slides and stained with 40 μg/ml acridine orange solution (Amresco, Solon OH, USA). Slides were visualized under a fluorescence microscope (DMLB2, Leica, Wetzlar, Germany) at 400 × magnification. For each slide, one thousand cells were randomly selected to identify micronucleated cells. The frequency of micronucleated cells was calculated according to the equation: The frequency of micronucleated cells (‰) = number of micronucleated cells/counted cells (1000).
2.8 Assessment of apoptosis by annexin V-FITC/ PI staining
Cellular apoptosis was examined using an apoptosis detection kit purchased from KeyGen Biotechnology (Nanjing, China). Briefly, cells were cultured in 6-well plates at a density of 1 × 106 cells/well and treated with As2O3 alone or As2O3 in combination with resveratrol for 24 h. Cells were collected by trypsinization and centrifugation at 110 X g for 5 min at 4°C. Cells were then resuspended in binding buffer which contained 5 μl of annexin V-fluorescein isothiocyanate (FITC) and 5 μl of propidium iodide (PI). After incubation at 37°C in the dark for 30 min, cells were subjected to flow cytometry (Beckman Coulter, Indianapolis, IN, USA). 20,000 cells from each sample were analyzed by the software of Windows Multiple Document Interface for Flow Cytometry, version 2.8 (The Scrrips Research Institute, San Diego, CA, USA).
2.9 Detection of cellular level of oxidized (GSSG) and reduced (GSH) glutathione
Cellular levels of total glutathione (T-GSH), reduced GSH and GSSG were determined by commercial assay kits purchased from Beyotime Institute of Biotechnology (Jiangsu, China). In brief, cells were seeded in 6-well plates at a density of 1 × 106 cells/well and treated with 20 μM As2O3 in the absence and presence of resveratrol for 24 h. Subsequently, cells were harvested and lysed. Cell lysates were centrifuged at 16,000 X g at 4°C for 5 min. The supernatant was collected to examine the cellular level of T-GSH and GSSG with a Bio-Rad micro-plate reader (Bio-Rad, Hercules, CA, USA) at a wavelength of 412 nm. Cellular levels of T-GSH and GSSG were obtained from standard curves, and the GSH level was calculated according to the equation: GSH=T-GSH−2×GSSG. The ratio of GSH/GSSG was obtained by dividing the concentration of GSH with that of GSSG. GSH and GSSG levels were normalized by protein concentrations.
2.10 Measurement of activities of GSH reductase (GR), GSH peroxidase (GSH-Px) and γ-glutamylcysteine synthetase (γ-GCS)
The activities of GR and GSH-Px were measured by kits purchased from Beyotime Institute of Biotechnology (Jiangsu, China). Briefly, after exposure to the 20 μM As2O3 in the absence or presence of resveratrol for 24 h, cells were harvested and lysed. Cell lysates were centrifuged at 16,000 X g for 10 min, and the supernatant was collected. The activities of GR and GSH-Px were measured according to the manufacturer's instructions at a wavelength of 340 nm (BioSpec-mini, Shimadzu Corp., Kyoto, Japan) and were normalized by protein concentrations. The activity of γ-GCS was detected with a γ-GCS detection kit purchased from Research Institute of Nanjing Jiancheng Bio-engineering (Nanjing, China). γ-GCS activity was measured by spectrophotometer (V-1100D, Mapada instruments Co., Ltd. Shanghai, China) according to the manufacturer's instructions. The activity of γ-GCS was also normalized by protein concentrations.
2.11 Analysis of protein expression level by Western blot
Western blot was performed as described by Nguygen et al. (Nguyen et al. 2011). Cell lysates (60 μg of protein) were subjected to 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were transferred to a 0.22 μm polyvinylidene fluoride (PVDF) membrane (Millipore, MA, USA). After blocking of non-specific epitopes for 2 h at room temperature with 5% nonfat dry milk, PVDF membranes were incubated with a rabbit polyclonal catalytic subunit of γ-glutamylcysteine synthetase (GCLC) antibody (1:500) (Abgent Inc. San Diego, CA, USA), modifier subunit of γ-glutamylcysteine synthetase (GCLM) antibody (1:500) (Abgent Inc. San Diego, CA, USA), Fas antibody (1:500) (ABclonal Biotech Co., Ltd, USA), Fas-L antibody (1:500) (ABclonal Biotech Co., Ltd, USA), cytochrome c antibody (1:200) (Biosynthesis Biotechnology Co., Ltd. Beijing, China) and a mouse monoclonal β-actin antibody (1:1000) (ZSGB Bio, Beijing, China) overnight at 4°C. A horseradish peroxidase conjugated secondary antibody (1:5000) (ZSGB Bio, Beijing, China) was incubated with PVDF membranes at room temperature for 1 h. Subsequently, PVDF membranes were incubated with an enhanced chemiluminescent substrate (Millipore, Bedford, MA, USA), and chemiluminescent signals were visualized and imaged with a Molecular Imager Gel Doc XR System (Bio-Rad, Hercules, CA, USA). The intensities of chemiluminescence were quantified by the Quantity One software (Bio-Rad, USA).
2.12 Measurement of activities of caspase 3, 8 and 9
The activities of caspase 3, 8 and 9 were detected with kits purchased from Beyotime Institute of Biotechnology (Jiangsu, China). Briefly, after 24 h exposure to As2O3 in the absence or presence of resveratrol, cells were harvested and lysed with lysis buffer for 15 min on ice. Cell lysates were centrifuged at 18,000 X g for 10 min at 4°C, and the supernatant was collected for detecting the activities of caspase 3, 8 and 9 according to the manufacturer's instructions. Enzymatic activities were normalized by protein concentrations.
2.13 Statistical analysis
All experiments were performed at least three times. Data were illustrated as mean ± standard deviation (S.D.). Significant differences among multiple groups were determined by one-way Analysis of Variance (ANOVA) analysis, and a least significant difference (LSD)-t test was used to detect a significant difference. A non-parametric Kruskal-Wallis test was used if original data were not normally distributed. Statistical analysis was performed with the Statistical Package for the Social Sciences (SPSS) software, version 17.0 (SPSS Inc.; Chicago, IL, USA). P < 0.05 indicates a significant difference.
3. Results
3.1 Resveratrol attenuated As2O3-induced cytotoxicity and genotoxicty
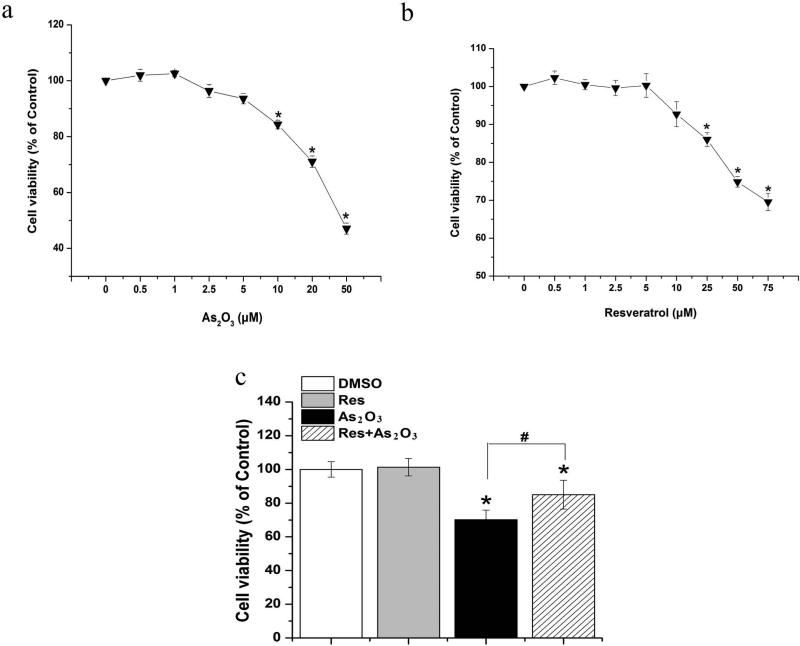
As2O3 causes cytotoxicity and genotoxicity by inducing oxidative damage in cells (Hei and Filipic 2004). This can further lead to death of human normal cells resulting in tissue and organ dysfunction. As an antioxidant, resveratrol may relieve As2O3-induced cytotoxicity and genotoxicity by combating oxidative damage in cells. To test this possibility, we initially examined the effects of resveratrol on As2O3 toxicity in HBE cells. We found that exposure to As2O3 (ranging from 10 μM to 50 μM) significantly decreased HBE cell viability (Fig. 1a). Resveratrol at concentrations lower than 25 μM failed to alter cell viability (Fig. 1b). To determine the effects of resveratrol on As2O3 cytotoxicity, we examined the viability of HBE cells that were treated with 20 μM As2O3 and 5 μM resveratrol simultaneously. The results showed that exposure to 20 μM As2O3 for 24 h significantly reduced the viability of treated cells compared with untreated cells (P<0.05) (Fig. 1c). Simultaneous exposure to both As2O3 (20 μM) and resveratrol (5 μM) significantly increased cell viability (Fig. 1c) (P<0.05), indicating that resveratrol effectively reduced As2O3 cytotoxicity in HBE cells.
Fig. 1. Effects of resveratrol on As2O3 cytotoxicity.
HBE cells were treated with As2O3 alone, or resveratrol alone or As2O3 in the presence of resveratrol for 24h. Cell viability was determined by MTT assay. Panel (a) illustrates the effects of various concentrations of As2O3 (0.5-50 μM) on cell viability. Panel (b) illustrates the effects of various concentrations of resveratrol (0.5-75 μM) on cell viability. Panel (c) illustrates the protective effects of resveratrol against As2O3 cytotoxcity. Data were obtained from three independent experiments and illustrated as mean ± standard deviation. “*” indicates a significant difference between treated groups and the untreated group. “#” denotes a significant difference between HBE cells treated by As2O3 alone and cells treated by As2O3 in the presence of 5 μM resveratrol. P<0.05 is denoted as significant difference.
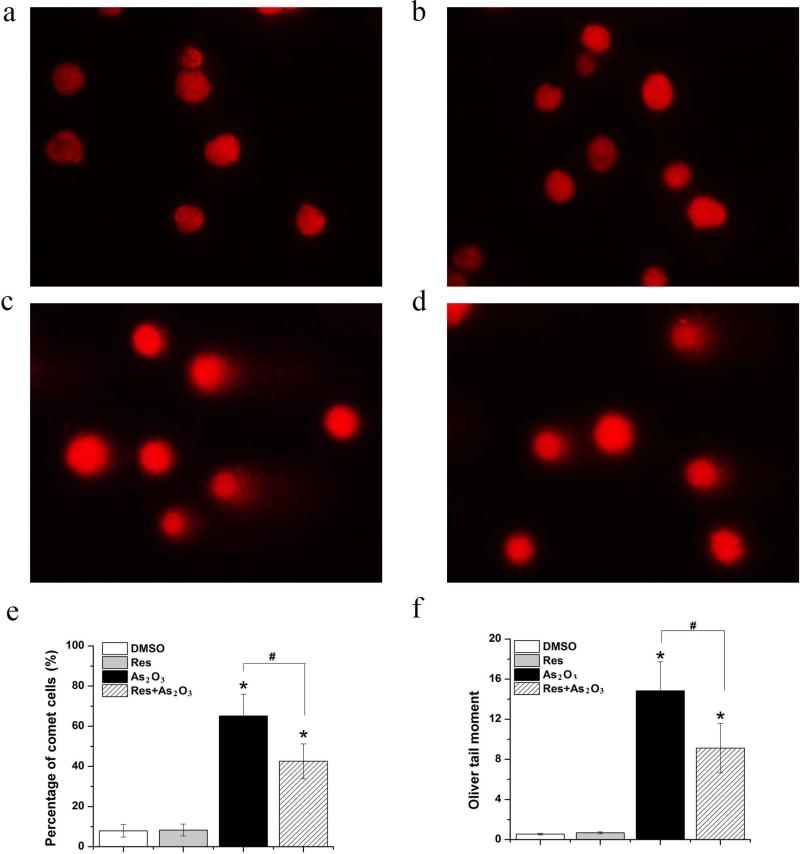
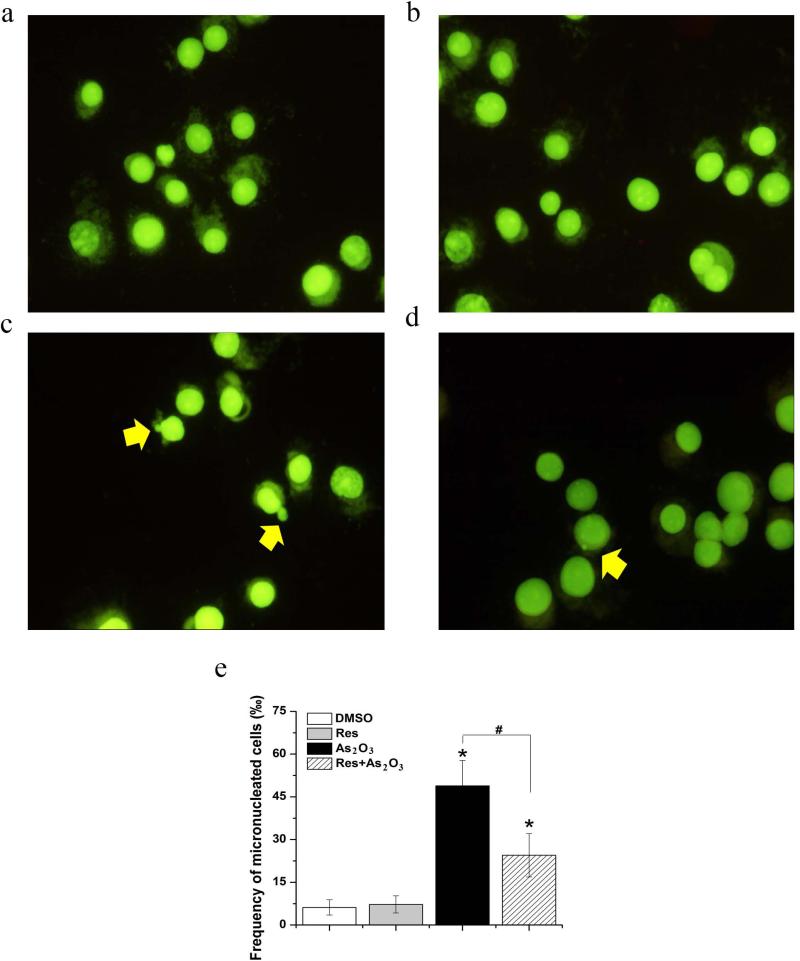
Because As2O3-induced oxidative DNA damage can result in chromosomal breakage (Hughes et al. 2011), we further examined whether resveratrol may relieve As2O3 genotoxicity using the micronucleus test and comet assay. The results showed that HBE cells treated by As2O3 exhibited a significantly higher percentage of comet cells and a larger OTM value than untreated cells (P<0.05) (Fig. 2c and Fig. 2e-2f). 5 μM resveratrol alone failed to increase the percentage of comet cells and the OTM value (P>0.05) (compared Fig. 2b with Fig. 2a and Fig. 2e-2f), indicating that resveratrol at 5 μM did not induce DNA damage. However, exposure to 5 μM resveratrol and 20 μM As2O3 simultaneously significantly decreased the percentage of comet cells and the OTM value (P<0.05)(Fig. 2d and Fig. 2e-2f). The results also showed that exposure to As2O3 for 24 h increased the frequency of micronucleated HBE cells significantly (P<0.05) (compared Fig. 3c with Fig. 3a). Similar to the results from the comet assay, exposure to resveratrol alone (5 μM) did not significantly alter the frequency of micronucleated cells (P>0.05) (compared Fig. 3b with Fig. 3a) (Fig. 3e). Simultaneous exposure to 20 μM As2O3 and 5 μM resveratrol significantly reduced the frequency of micronucleated cells (P<0.05) (compared Fig. 3d with Fig. 3a) (Fig. 3e). Taken together, our results indicate that resveratrol can attenuate As2O3-induced DNA damage and chromosomal breakage and can protect HBE cells against As2O3 genotoxicity.
Fig. 2. Effects of resveratrol on As2O3-induced DNA damage in HBE cells.
Cells were treated with 20 μM As2O3 in the absence or presence of 5 μM resveratrol for 24 h. DNA damage in HBE cells was measured by comet assay. Representative images from the comet assay were shown. Panel (a) represents untreated cells. Panel (b) illustrates cells treated by 5 μM resveratrol alone. Panel (c) represents cells treated by 20 μM As2O3 alone. Panel (d) represents cells treated by 20 μM As2O3 in the presence of 5 μM resveratrol. Panel (e) represents the percentage of comet cells obtained from cells treated with DMSO, resveratrol alone, As2O3 alone and As2O3 along with resveratrol, respectively. The percentage of comet cells was calculated according to the equation: Percentage of comet cells (%) = number of comet cells/200 counted cells. Panel (f) illustrates OTM of cells treated with DMSO, resveratrol alone, As2O3 alone, and As2O3 along with resveratrol. Fifty comet cells from each slide were randomly chosen for analyzing OTM as described in “Materials and Methods”. Data were illustrated as mean ± standard deviation. The data were the average of three independent experiments. “*” indicates a statistically significant difference between treated groups and the untreated control, “#” denotes a significant difference between the group treated by As2O3 alone and the group treated by resveratrol in the presence of As2O3 (P<0.05).
Fig. 3. Effects of resveratrol on As2O3-induced chromosomal breakage in HBE cells.
HBE cells were treated with 20 μM As2O3 alone, or 5 μM resveratrol alone or 20 μM As2O3 in the presence of 5 μM resveratrol. Chromosomal breakages were determined by the micronucleus assay. Representative images from the micronucleus assay were shown. Panel (a) represents untreated cells. Panel (b) represents cells treated by 5 μM resveratrol alone. Panel (c) illustrates cells treated by 20 μM As2O3 alone. Panel (d) illustrates cells treated by 20 μM As2O3 in the presence of 5 μM resveratrol. Panel (e) represents the frequency of micronucleated cells. Data are illustrated as mean ± standard deviation and represent the average of the data from three independent experiments. “*” indicates a statistically significant difference between treated groups and the untreated group, whereas “#” indicates a significant difference (P<0.05) between cells treated by As2O3 and cells treated by As2O3 and resveratrol simultaneously.
3.2 Resveratrol attenuated As2O3-induced oxidative stress and lipid peroxidation
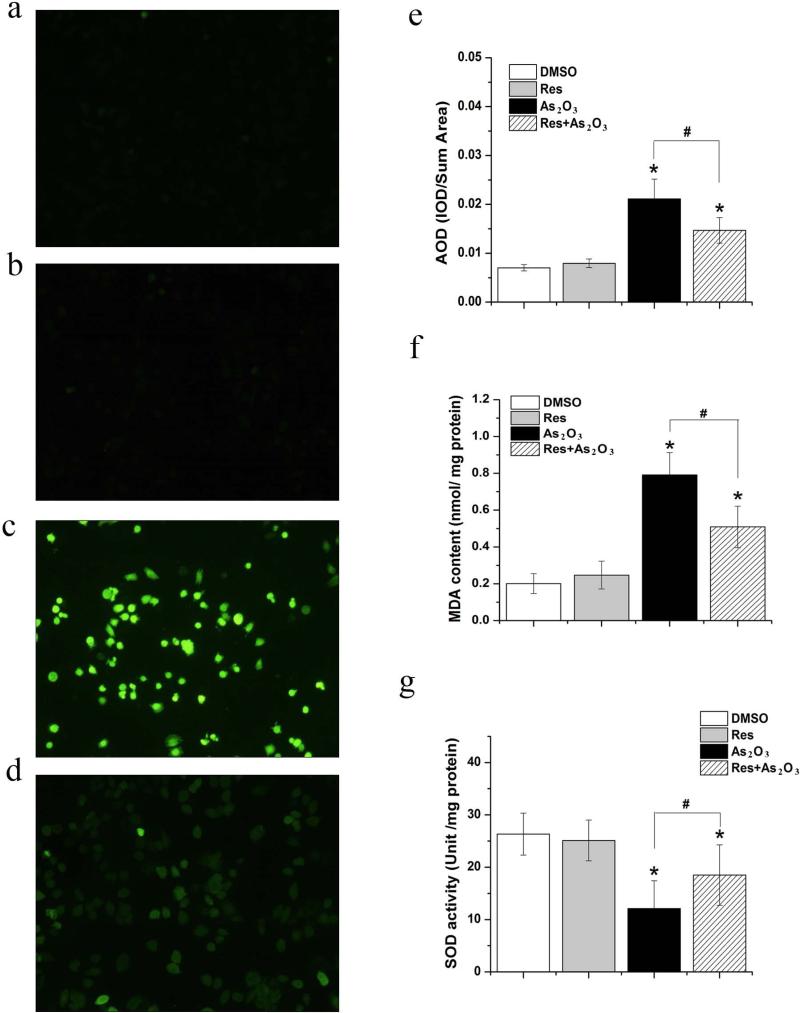
As2O3 can induce oxidative stress and lipid peroxidation in various types of cells by promoting production of ROS (Flora 2011). It is possible that resveratrol may relieve As2O3-induced oxidative stress by inhibiting production of ROS. To test this, we examined the production of ROS and MDA in HBE cells treated by As2O3 in the absence or presence of resveratrol. We found that DCF fluorescence intensity, an indicator of production of ROS, in As2O3-treated HBE cells was significantly increased compared with the untreated control (P<0.05) (compared Fig. 4c with Fig. 4a) (Fig. 4e). Production of ROS in HBE cells that were treated simultaneously by 20 μM As2O3 and 5 μM resveratrol, was significantly decreased (P<0.05) (compared with Fig. 4d with Fig. 4a) (Fig. 4e), indicating that resveratrol reduced the production of ROS in these cells. Consistent with its effect on production of ROS, we found that 20 μM As2O3 significantly increased the production of MDA, indicative of lipid peroxidation in HBE cells (P<0.05) (Fig. 4f). However, the effect of As2O3 was reduced significantly by resveratrol (P<0.05) (Fig. 4f), indicating that the antioxidant can also protect HBE cells from As2O3–induced lipid peroxidation. For both experiments, no significant differences in DCF fluorescence intensity and MDA level between HBE cells treated by 5 μM resveratrol alone and untreated cells were detected (P>0.05) (compared with Fig. 4b with Fig. 4a) (Fig. 4e-4f). This indicated that resveratrol alone did not induce production of ROS and lipid peroxidation. Our results demonstrate that resveratrol can effectively protect HBE cells from As2O3-induced oxidative stress and lipid peroxidation.
Fig. 4. Effects of resveratrol on the level of oxidative stress induced by As2O3 in HBE cells.
Cells were treated by 20 μM As2O3 in the absence or presence of 5 μM resveratrol for 24 h. Cellular level of ROS, MDA, and SOD activity were measured according to the procedures described in the “Materials and Methods”. Panel (a) represents untreated cells. Panel (b) represents cells treated by 5 μM resveratrol alone. Panel (c) represents cells treated by 20 μM As2O3 alone. Panel (d) illustrates cells treated by 20 μM As2O3 in the presence of 5 μM resveratrol. Panel (e) represents cellular production of ROS determined by a fluorescent probe. Panel (f) illustrates the cellular level of MDA. Panel (g) represents SOD activity in HBE cells. “*” indicates a statistically significant difference (P<0.05) between the untreated control and the treated groups. “#” indicates a significant difference between cells treated by As2O3 alone and cells treated with As2O3 in the presence of 5 μM resveratrol.
Because oxidative damage is caused by excessive oxidative stress that exceeds the capacity of cellular antioxidative defense systems (Flora 2011), we have examined whether resveratrol can also relieve As2O3-induced oxidative damage via modulation of HBE antioxidative capability. We determined cellular antioxidative capacity by measuring SOD activity in HBE cells treated with 20 μM As2O3 alone or with 20 μM As2O3 and 5 μM resveratrol simultaneously. The results showed that 20 μM As2O3 decreased SOD activity in HBE cells (P<0.05) (Fig. 4g), whereas resveratrol (5 μM) increased SOD activity in HBE cells treated by As2O3 (P<0.05) (Fig. 4g). No significant difference was detected in SOD activity between HBE cells treated by resveratrol alone and untreated cells (P>0.05) (Fig. 4g), indicating that resveratrol alone failed to affect SOD activity. Collectively, our results indicated that resveratrol alleviated As2O3-induced oxidative stress and lipid peroxidation in HBE cells by reducing the production of ROS and MDA, thereby indirectly increasing HBE cellular SOD availability and activity.
3.3 Resveratrol facilitated GSH homeostasis
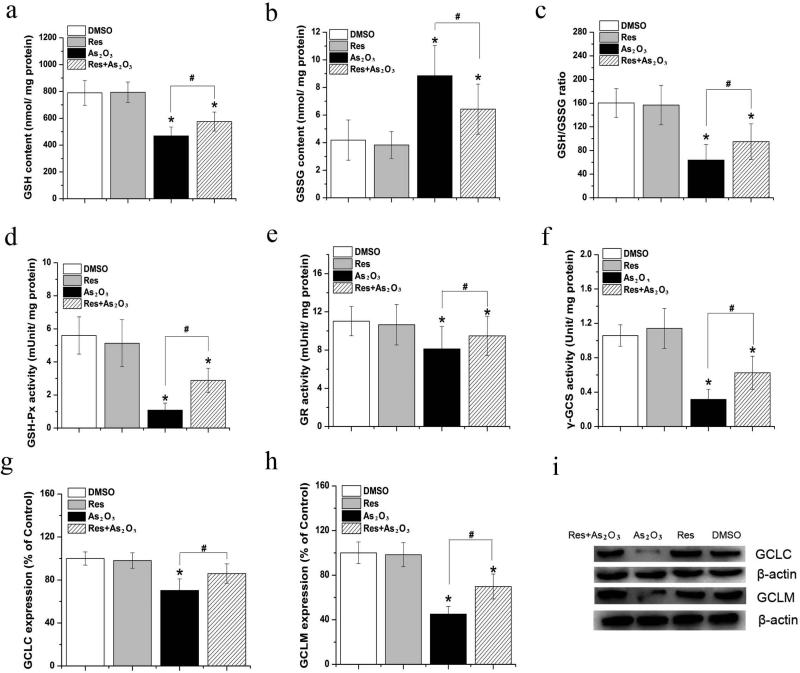
GSH homeostasis also constitutes a significant portion of the cellular capacity of antioxidative damage (Lushchak 2012). Thus, resveratrol may restore GSH homeostasis that is disrupted by As2O3 to suppress oxidative damage in HBE cells. To test this, we examined the levels of GSH and GSSG, the ratio of GSH to GSSG, as well as the activities of the enzymes that are involved in GSH metabolism such as GR, GSH-Px and γ-GCS in HBE cells. The results showed that As2O3 decreased the levels of GSH and the ratio of GSH to GSSG (P<0.05) (Fig. 5a and Fig. 5c) and increased the level of GSSG in HBE cells (P<0.05) (Fig. 5b). However, resveratrol increased GSH level and decreased GSSG level in As2O3-treated HBE cells (p<0.05), thereby increasing the ratio of GSH to GSSG (P<0.05) (Fig. 5a-5c). Resveratrol alone did not alter the levels of GSH and GSSG or the ratio of GSH to GSSG in HBE cells (P>0.05) (Fig. 5a-5c), suggesting that resveratrol alone did not affect GSH homeostasis (P>0.05). To further explore the molecular basis underlying the effects of resveratrol on GSH homeostasis in HBE cells, we have determined whether resveratrol can increase the activities of GSH-Px, GR and γ-GCS that are involved in GSH metabolism in cells. We found that 20 μM As2O3 decreased GSH-Px activity in HBE cells (P<0.05) (Fig. 5d), and resveratrol enhanced GSH-Px activity in the cells treated by As2O3 (P<0.05) (Fig. 5d). Further characterization of the activity of GR that can regenerate GSH (Lushchak 2012) in HBE cells showed a significant decrease in GR activity in the cells treated with As2O3 (P<0.05) (Fig. 5e). However, the effect was relieved by 5 μM resveratrol (P<0.05) (Fig. 5e). Similar to its effects on GSH-Px and GR, resveratrol also increased the activity of γ-GCS, a rate-limiting enzyme for GSH biosynthesis (Lushchak 2012), in the As2O3-treated HBE cells (P<0.05) (Fig. 5f). For all the experiments, resveratrol alone failed to affect the activities of GSH-Px (Fig. 5d), GR (Fig. 5e), and γ-GCS (Fig. 5f) (P>0.05). Thus, our results indicate that resveratrol can promote GSH homeostasis by increasing the activities of these enzymes that produce and restore cellular GSH levels to near normal.
Fig. 5. Effects of resveratrol on cellular GSH homeostasis.
HBE cells were treated by 20 μM As2O3 or 5 μM resveratrol alone or treated by 20 μM As2O3 with 5 μM resveratrol for 24 h. Data were obtained from three independent experiments and illustrated as mean ± standard deviation. Panels (a-c) illustrate the effects of resveratrol on the level of GSH, GSSG and GSH/GSSG ratio. Panels (d-f) show the effects of resveratrol on GSH-Px, GR, and γ-GCS activity. Panels (g-i) illustrate the effects of resveratrol on the level of GCLC and GCLM. “*” denotes a significant difference (P<0.05) between treated groups and the untreated control. “#” indicates a significant difference (P<0.05) between HBE cells treated by As2O3 alone and cells treated by As2O3 in the presence of resveratrol.
γ-GCS is a heterodimer that consists of two subunits, a heavy catalytic (GCLC) subunit and a light modifier (GCLM) subunit (Lushchak 2012). We reason that resveratrol may increase the level of γ-GCS by modulating the γ-GCS protein subunit levels in HBE cells treated by As2O3. The results showed that 20 μM As2O3 depleted both GCLC and GCLM in HBE cells (P<0.05) (Fig. 5g-5i). However, resveratrol restored the level of GCLC and GCLM in the As2O3-treated cells to approximately that of untreated cells (Fig. 5g-5i). Resveratrol alone did not alter GCLC and GCLM protein levels (Fig. 5g-5i) (P>0.05) in HBE cells, suggesting that the antioxidant promotes homeostasis of GSH by increasing the activities of enzymes that maintain GSH homeostasis.
3.4 Resveratrol suppressed As2O3-induced apoptosis
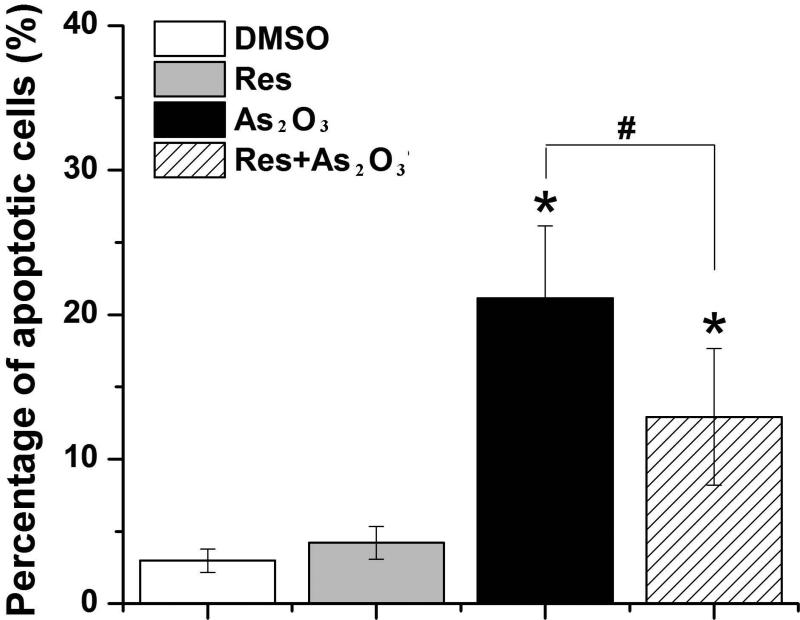
One of the side effects from As2O3-mediated cancer therapy is that the anticancer agent can cause cell death by inducing apoptosis in normal cells. It is possible that resveratrol may protect normal cells from cell death by suppressing As2O3-induced apoptosis. To determine whether resveratrol can inhibit As2O3-triggered apoptosis, we examined apoptosis in HBE cells treated with As2O3 (20 μM) alone or with both As2O3 (20 μM) and resveratrol (5 μM) using the annexin V-FITC/PI assay (Fig. 6). The results showed that As2O3 increased the percentage of apoptotic cells in HBE cells (P<0.05) (Fig. 6). Resveratrol (5 μM) decreased the percentage of apoptotic cells by approximately 2-fold in As2O3–treated HBE cells (P<0.05) (Fig. 6). However, resveratrol alone failed to cause apoptosis (Fig. 6), indicating that resveratrol specifically inhibited As2O3-induced apoptosis in HBE cells.
Fig. 6. Effects of resveratrol on As2O3-induced apoptosis.
Cell apoptosis was determined by the annexin V-FITC/PI staining assay as described in “Materials and Methods”. The effects of resveratrol on the percentage of apoptotic cells were shown. Data were obtained from three independent experiments and illustrated as mean ± standard deviation (SD). “*” indicates a significant difference (P<0.05) between treated cells and untreated cells. “#” indicates a significant difference (P<0.05) between cells treated by As2O3 alone and cells treated by As2O3 in the presence of resveratrol.
3.5 Resveratrol suppressed an increase in the activities of apoptotic enzymes and proteins induced by As2O3
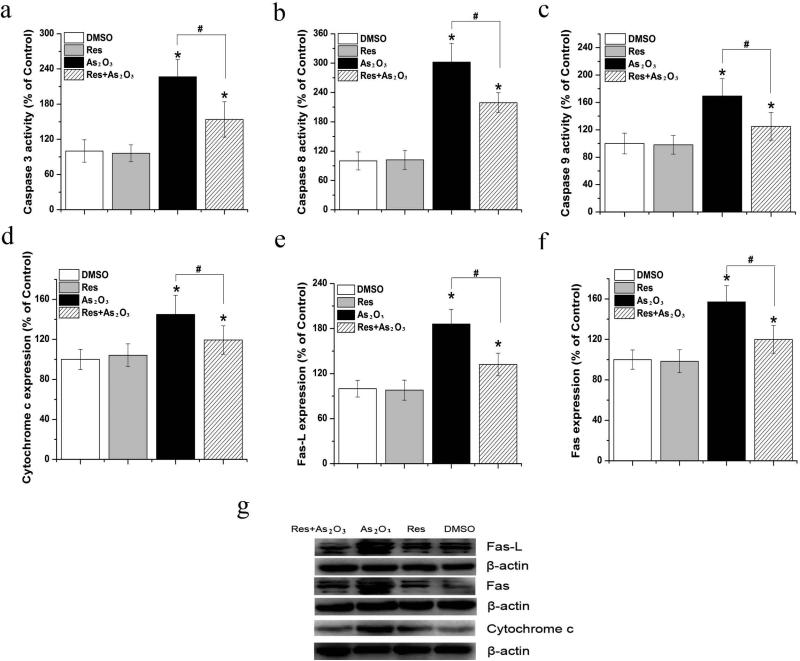
To further explore the mechanisms underlying the effects of resveratrol against As2O3-induced apoptosis in HBE cells, we then asked if resveratrol can prevent an increase in the activities and levels of enzymes and proteins that initiate and mediate apoptosis induced by As2O3. These include cytochrome c, caspases, Fas receptor (Fas) and cell death factors that can bind to Fas such as Fas ligand (Fas-L) (Fiandalo and Kyprianou 2012). Cytochrome c serves as a component of the electron transfer chain located in mitochondria for energy production. However, cytochrome c can also bind to caspases when it is released into the cytoplasm to trigger the initiation of apoptosis (Goodsell 2004). Caspase 8 and 9 serve as proteases and apoptosis initiators, whereas caspase 3 acts as an executor of apoptosis (McIlwain et al. 2013). We found that As2O3 (20 μM) increased the activities of all three caspases (Fig. 7a-7c) and the protein levels of cytochrome c, Fas, and Fas-L in HBE cells (P<0.05) (Fig. 7d and Fig. 7g). However, the adverse effects of As2O3 were effectively suppressed by resveratrol (Fig. 7a-7g). This indicates that resveratrol can protect HBE cells from As2O3-induced apoptosis. The effects of resveratrol appeared to result from its function in scavenging ROS induced by As2O3, because resveratrol alone did not exhibit any effects on caspase activities and protein levels of cytochrome c, Fas and Fas-L (Fig. 7a-7g).
Fig. 7. Effects of resveratrol on the activities and levels of apoptotic enzymes and proteins in HBE cells treated by As2O3.
HBE cells were treated by As2O3 alone or resveratrol alone or by As2O3 in the presence of resveratrol for 24 h. Panels (a-c) illustrate the effects of resveratrol on the activities of caspase 3, 8, and 9 in HBE cells. Panels (d)-(g) represent the effects of resveratrol and As2O3 on the levels of cytochrome c, Fas-L, and Fas proteins. The levels of cytochrome c, Fas-L and Fas proteins were detected by Western blot. Data were obtained from three independent experiments and illustrated as mean ± standard deviation. “*” indicates a significant difference (P<0.05) between treated and untreated cells. “#” indicates a significant difference (P<0.05) between cells treated by As2O3 alone and those treated by As2O3 in the presence of resveratrol.
4. Discussion
In this study, we provided evidence that resveratrol can relieve As2O3-induced oxidative damage that leads to cytotoxicity, genotoxicity and apoptotic cell death in HBE cells (Figs. 1-3). The protective effects of resveratrol were achieved by its function in combating the cellular production of ROS and lipid peroxidation, promoting GSH homeostasis and suppressing apoptotic cell death (Figs. 4-7). We found that resveratrol effectively suppressed an increase of ROS and MDA and a decrease of SOD activities induced by As2O3 in HBE cells (Fig. 4a-4f). We further demonstrated that resveratrol promoted GSH homeostasis in HBE cells treated with As2O3 by increasing the cellular level of GSH, the activities and levels of GSH metabolic enzymes (GSH-Px, GR and γ-GCS), and cellular levels of GCLC and GCLM proteins (Fig. 5). We showed that resveratrol protected HBE cells against As2O3-induced apoptosis by inhibiting the activities of caspase 3, 8 and 9 (Fig. 6 and Fig.7a-7c) and decreasing the levels of cytochrome c, Fas, and Fas-L (Fig. 7d-7g). The results allow us to propose several pathways that illustrate how resveratrol may protect HBE cells from As2O3 toxicity. First, resveratrol can prevent the generation of excessive amount of ROS induced by As2O3 in HBE cells. This subsequently leads to prevention of As2O3-induced cellular redox imbalance via lipid peroxidation, depletion of GSH, and decreased activities of SOD and GSH metabolic enzymes, thereby alleviating As2O3 genotoxicity. Second, resveratrol can disrupt the critical steps of intrinsic and extrinsic apoptotic pathways (Figs. 6-7) that can be initiated by As2O3 (Emadi and Gore 2010). The effects of resveratrol may be achieved by decreasing the activities of caspases and the levels of cytochrome c, Fas, and Fas-L. The anti-apoptotic effects of resveratrol may also be partially mediated by its function in facilitating GSH homeostasis that inhibits apoptosis (Lushchak 2012). Our results suggest that resveratrol protects normal HBE cells against As2O3-induced lung injury by combating the production of ROS and lipid peroxidation, promoting GSH homeostasis and suppressing apoptosis.
ROS play a crucial role in mediating both the toxic and therapeutic effects of As2O3 (Flora 2011). A moderate level of ROS facilitates cell proliferation, whereas a high level of ROS leads to cell death (Simon et al. 2000; Flora 2011). We found that 20 μM As2O3 resulted in an increased level of ROS that led to apoptosis of HBE cells (Fig. 4c and Fig. 6), suggesting a high level of ROS induced by As2O3 in these cells. Because As2O3 at this dosage also increased the activities and levels of caspases and cytochrome c, Fas and Fas-L proteins (Fig. 7), this further indicates that a high level of ROS induced by As2O3 in HBE cells can trigger apoptotic processes by modulating cellular levels and activities of apoptotic proteins and enzymes (Alarifi et al. 2013; Jiang et al. 2013). Our results have demonstrated that 5 μM resveratrol can combat As2O3-induced apoptosis in HBE cells efficiently (Fig. 6). This suggests that a low dose of resveratrol is sufficient for preventing ROS from reaching to a high level, thereby preventing cellular apoptotic processes.
Apoptosis can be triggered by various factors, such as cell injury and death receptor-mediated signals. Each stimulus activates the apoptotic process with a unique pathway (Hampton et al. 1998). These pathways can be classified into an intrinsic mitochondrial pathway or extrinsic death receptor pathway (Takeda et al. 2011). For both pathways, activation of caspases is an essential step for initiating apoptosis (Fan et al. 2005). The intrinsic apoptotic pathway is activated by cellular stressors, including DNA damage and oxidative stress such as a high level of ROS, that can trigger the release of cytochrome c from mitochondria into the cytoplasm. Cytochrome c then binds to apoptotic protease-activating factor-1 (APAF1), an adapter protein, and caspase 9 to form a large protein complex known as the apoptosome that subsequently activates an apoptotic executor, caspase 3, thereby initiating apoptosis (Fiandalo and Kyprianou 2012). The extrinsic apoptotic pathway is triggered by extracellular stimuli that serve as ligands to bind to death receptors, such as Fas and Fas-L. This further leads to dimerization of caspase 8 that activates the enzyme. Activated caspase 8 then cleaves caspase 3, an apoptotic executor, initiating apoptotic cell death (McIlwain et al. 2013). Because environmental toxicants and anticancer agents usually initiate the extrinsic apoptotic pathway, it is conceivable that As2O3 induces apoptosis via activation of caspases 8 and 3 to mediate arsenic carcinogenesis and its anticancer therapeutic effects (Miller et al. 2002; Cui et al. 2008). Our results suggest that As2O3 can activate both the intrinsic caspase 9-dependent and the extrinsic caspase 8-dependent apoptotic pathways in HBE cells by increasing the cellular production of ROS (Fig. 4c and Fig. 4e) and lipid peroxidation (Fig. 4f), as well as by increasing cellular levels of cytochrome c, Fas, and Fas-L proteins (Fig. 7). This is consistent with the results reported by a previous study (Hayashi et al. 2002). However, we found that resveratrol suppressed As2O3-induced apoptosis by blocking several critical steps that mediate intrinsic and extrinsic apoptotic cell death pathways. This indicates that resveratrol is an effective agent that protects normal HBE cells from As2O3-induced apoptosis (Zhang et al. 2001).
Our results indicate that resveratrol can also effectively protect against As2O3 genotoxicity in HBE cells by relieving oxidative damage. We showed that 20 μM As2O3 significantly increased single-stranded DNA breaks and chromosome breakage in HBE cells via promoting the production of ROS (Figs. 2-3). This is supported by previous findings showing that As2O3-induced ROS can result in DNA strand breaks and chromosome breakage (Miller et al. 2002; Hei and Filipic 2004; Selvaraj et al. 2012; Chen et al. 2013b). We demonstrated that As2O3 genotoxicity was effectively suppressed by 5 μM resveratrol (Figs. 2-3). This appears to be accomplished by resveratrol's function in increasing the activity of SOD and combating the production of ROS and MDA in HBE cells (Fig. 4e-4g).
In this study, we also demonstrated that resveratrol effectively restored cellular antioxidant defense mechanisms in HBE cells by promoting GSH homeostasis. Given that it plays a key regulatory role in redox signaling in normal cells (Sakurai et al. 2004), disruption of GSH homeostasis might be an early event in the apoptotic cascades that activate death receptor-mediated caspase 8 and mitochondrial caspase 9 (Circu and Aw 2008). It was shown that depletion of cellular GSH significantly increased susceptibility of lung tissue to As2O3-mediated oxidative stress (Yang et al. 1999; Davison et al. 2003). Thus, it is conceivable that sustainment of GSH homeostasis by resveratrol protects HBE cells against As2O3-induced toxic effects. This is supported by our results shown in Figs. 1-3 and Fig. 5. Thus, our results further suggest that resveratrol can enhance the efficacy of As2O3 anticancer activity or reduce As2O3 toxicity by maintaining cellular balance of GSH (Hughes 2002; Miller et al. 2002; Cui et al. 2008).
As an antioxidant, resveratrol exerts its effects against oxidative damage in a dose-dependent manner (Aggarwal et al. 2004). At a concentration lower than 15 μM, resveratrol can protect cells through several functions. These include its anti-apoptosis, anti-inflammation, and anti-senescence functions (Alarcón de la Lastra and Villegas 2005; Shankar et al. 2007). However, at a concentration higher than 15 μM, resveratrol can act as a pro-apoptotic agent through inhibition of DNA and protein synthesis, induction of chromosomal breakage and disruption of cellular redox balance (Nakagawa et al. 2001; Kuwajerwala et al. 2002). This suggests that the intake of a moderate level of resveratrol may promote human health. We found that resveratrol concentrations lower than 10 μM (Fig. 1b) did not affect cell viability, and that 5 μM resveratrol is sufficient to protect against As2O3-induced toxic effects. This suggests that a low dose of 5 μM resveratrol may be used for relieving the side effects resulting from As2O3-mediated cancer treatment on normal HBE cells.
In this study, we examined the effects of resveratrol on a variety of toxic effects induced by 20 μM As2O3. This dosage of As2O3 has been commonly used in many studies to determine the toxic effects and mechanisms of As2O3 anticancer functions. Pharmacological studies indicate that 20 μM As2O3 can cause cytotoxicity and apoptosis of many cancer cells (Shim et al. 2002; Walker et al. 2010; Sun et al. 2011; Wang et al. 2012), indicating that As2O3 at this dosage can efficiently induce apoptosis. In addition, previous studies have shown that at a relatively high dose of 25 μM, As2O3 toxicity can be alleviated by lipoic acid, nitric oxide and zinc (Milton et al. 2004; Cheng et al. 2007; Jin et al. 2010). This indicates that it is likely resveratrol can also protect against the toxic effects of As2O3 at a dose of 20 μM. Thus, in our study, 20 μM As2O3 was employed to specifically examine the inhibitory effects of resveratrol on As2O3-induced apoptosis in HBE cells. Although this dosage is higher than the clinically used dosage that ranges from 1 to 10 μM (Emadi and Gore 2010; Lam et al. 2014), we demonstrate that this dosage of As2O3 significantly increased the percentage of apoptotic cells (Fig. 6), which was effectively reduced by 5 μM resveratrol. Thus, it appears that 20 μM As2O3 is an appropriate concentration for determining the inhibitory effects of resveratrol on As2O3-induced apoptosis in HBE cells. Our study indicates that resveratrol can effectively relieve cyto- and genotoxic effects induced by As2O3 at 20 μM in normal human cells. However, it is conceivable that resveratrol may exhibit little effect on the cyto- and genotoxicity resulting from a chemotherapeutic dosage of As2O3, which is lower than 20 μM. This would potentially help to sustain the chemotherapeutic efficacy of As2O3. Thus, it is of interest to study the effects of resveratrol on the toxic effects induced by a low dose of As2O3.
In our experiments, HBE cells were used to study the roles of resveratrol in protecting against As2O3-induced side effects in normal cells of bronchi and lung tissue. These cells were used because human bronchi and lung tissue are sensitive to As2O3 toxicity. In addition, HBE cells are derived from the epithelium of normal human bronchi that have been widely used to study the mechanisms of carcinogenesis and the toxic effects of arsenic (Xu et al. 2012; Sherwood et al. 2013; Li et al. 2014; Qi et al. 2014). Recent studies have shown that As2O3 exhibits a high efficacy for lung cancer therapy by triggering apoptotic death of lung cancer cells (Mandegary et al. 2013; Gatti et al. 2014; Lam et al. 2014). This indicates that As2O3 chemotherapy may cause a series of side effects on bronchi epithelium cells. Thus, employment of HBE cells to study the roles of resveratrol in relieving As2O3-induced side effects would help to improve As2O3 chemotherapeutic efficacy in lung cancer treatment. In fact, our results demonstrated that HBE cells indeed served as an appropriate system for us to elucidate the mechanisms underlying As2O3 toxicity in the lung and to explore a potential role for resveratrol in combating As2O3-induced lung toxicity.
In summary, in this study, we have shown that resveratrol can protect against As2O3-induced toxic effects in HBE cells by processes involving suppression of apoptosis and GSH homeostasis maintenance. We suggest that resveratrol can be developed as a potential chemotherapeutic supplementary agent to relieve oxidative damage resulting from As2O3 anti-cancer treatment.
Acknowledgments
The authors thank Ping Zhang, State key laboratory of dental diseases, West China Hospital of Stomatology, Sichuan University, for his helpful assistance on the assays by flow cytometry and data analysis of the apoptotic assay. The authors thank Jill Beaver for critical readings and insightful comments. This work was supported by the Grant No. 81172632 and 81372945 from the National Natural Science Foundation of China to Zunzhen Zhang and Grant No. ES023569 from National Institutes of Health of USA to Yuan Liu.
Abbreviations
- As2O3
Arsenic trioxide
- γ-GCS
γ-Glutamylcysteine synthetase
- GCLC
Catalytic subunit of γ-glutamylcysteine synthetase
- GCLM
Modifier subunit of γ-glutamylcysteine synthetase
- GSH
Glutathione
- GSH-Px
Glutathione peroxidase
- GR
Glutathione reductase
- GSSG
Glutathione disulfide
- MDA
Malondialdehyde
- ROS
Reactive oxygen species
- SOD
Superoxide dismutase
Footnotes
Statement of Author Contributions
Chengzhi Chen designed and performed the experiments. Xuejun Jiang performed the experiments and prepared the figures. Chengzhi Chen and Yanhao Lai analyzed the data and drafted the manuscript. Zunzhen Zhang and Yuan Liu participated in study design, reconstructed and revised the manuscript.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Reference
- Aggarwal BB, Bhardwaj A, Aggarwal RS, Seeram NP, Shishodia S, Takada Y. Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Res. 2004;24(5A):2783–2840. [PubMed] [Google Scholar]
- Alarcón de la Lastra C, Villegas I. Resveratrol as an anti-inflammatory and anti-aging agent: Mechanisms and clinical implications. Mol Nutr Food Res. 2005;49(5):405–430. doi: 10.1002/mnfr.200500022. [DOI] [PubMed] [Google Scholar]
- Alarifi S, Ali D, Alkahtani S, Siddiqui MA, Ali BA. Arsenic trioxide-mediated oxidative stress and genotoxicity in human hepatocellular carcinoma cells. Onco Targets Ther. 2013;6:75–84. doi: 10.2147/OTT.S38227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbey JT, Pezzullo JC, Soignet SL. Effect of arsenic trioxide on QT interval in patients with advanced malignancies. J Clin Oncol. 2003;21(19):3609–3615. doi: 10.1200/JCO.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Bishayee A. Cancer prevention and treatment with resveratrol: from rodent studies to clinical trials. Cancer Prev Res (Phila) 2009;2(5):409–418. doi: 10.1158/1940-6207.CAPR-08-0160. [DOI] [PubMed] [Google Scholar]
- Brown GC, Borutaite V. Regulation of apoptosis by the redox state of cytochrome c. Biochim Biophys Acta. 2008;1777(7-8):877–881. doi: 10.1016/j.bbabio.2008.03.024. [DOI] [PubMed] [Google Scholar]
- Chen C, Jiang X, Hu Y, Zhang Z. The protective role of resveratrol in the sodium arsenite-induced oxidative damage via modulation of intracellular GSH homeostasis. Biol Trace Elem Res. 2013a;2013155(1):119–131. doi: 10.1007/s12011-013-9757-x. [DOI] [PubMed] [Google Scholar]
- Chen C, Jiang X, Ren Y, Zhang Z. Arsenic trioxide co-exposure potentiates benzo (a) pyrene genotoxicity by enhancing the oxidative stress in human lung adenocarcinoma cell. Biol Trace Elem Res. 2013b;156(1-3):338–349. doi: 10.1007/s12011-013-9819-0. [DOI] [PubMed] [Google Scholar]
- Chen C, Jiang X, Zhao W, Zhang Z. Dual role of resveratrol in modulation of genotoxicity induced by sodium arsenite via oxidative stress and apoptosis. Food Chem Toxicol. 2013c;59C:8–17. doi: 10.1016/j.fct.2013.05.030. [DOI] [PubMed] [Google Scholar]
- Cheng TJ, Wang YJ, Kao WW, Chen RJ, Ho YS. Protection against arsenic trioxide-induced autophagic cell death in U118 human glioma cells by use of lipoic acid. Food Chem Toxicol. 2007;45(6):1027–1038. doi: 10.1016/j.fct.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Circu ML, Aw TY. Glutathione and apoptosis. Free Radic Res. 2008;42(8):689–706. doi: 10.1080/10715760802317663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X, Kobayashi Y, Akashi M, Okayasu R. Metabolism and the paradoxical effects of arsenic: carcinogenesis and anticancer. Curr Med Chem. 2008;15(22):2293–2304. doi: 10.2174/092986708785747526. [DOI] [PubMed] [Google Scholar]
- Davison K, Cote S, Mader S, Miller WH. Glutathione depletion overcomes resistance to arsenic trioxide in arsenic-resistant cell lines. Leukemia. 2003;17(5):931–940. doi: 10.1038/sj.leu.2402876. [DOI] [PubMed] [Google Scholar]
- Emadi A, Gore SD. Arsenic trioxide - An old drug rediscovered. Blood Rev. 2010;24(4-5):191–199. doi: 10.1016/j.blre.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evens AM, Tallman MS, Gartenhaus RB. The potential of arsenic trioxide in the treatment of malignant disease: past, present, and future. Leuk Res. 2004;28(9):891–900. doi: 10.1016/j.leukres.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Fan TJ, Han LH, Cong RS, Liang J. Caspase family proteases and apoptosis. Acta Biochim Biophys Sin (Shanghai) 2005;37(11):719–727. doi: 10.1111/j.1745-7270.2005.00108.x. [DOI] [PubMed] [Google Scholar]
- Fiandalo MV, Kyprianou N. Caspase control: protagonists of cancer cell apoptosis. Exp Oncol. 2012;34(3):165–175. [PMC free article] [PubMed] [Google Scholar]
- Flora SJS. Arsenic-induced oxidative stress and its reversibility. Free Radic Biol Med. 2011;51(2):257–281. doi: 10.1016/j.freeradbiomed.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Gatti L, Cossa G, Tinelli S, Carenini N, Arrighetti N, Pennati M, Cominetti D, De Cesare M, Zunino F, Zaffaroni N, Perego P. Improved apoptotic cell death in drug-resistant non-small-cell lung cancer cells by tumor necrosis factor-related apoptosis-inducing ligand-based treatment. J Pharmacol Exp Ther. 2014;348(3):360–371. doi: 10.1124/jpet.113.210054. [DOI] [PubMed] [Google Scholar]
- Goodsell DS. The molecular perspective: cytochrome c and apoptosis. Oncologist. 2004;9(2):226–227. doi: 10.1634/theoncologist.9-2-226. [DOI] [PubMed] [Google Scholar]
- Hampton MB, Fadeel B, Orrenius S. Redox regulation of the caspases during apoptosis. Ann N Y Acad Sci. 1998;854:328–335. doi: 10.1111/j.1749-6632.1998.tb09913.x. [DOI] [PubMed] [Google Scholar]
- Hao L, Zhao J, Wang X, Wang H, Wang H, Xu G. Hepatotoxicity from arsenic trioxide for pediatric acute promyelocytic leukemia. J Pediatr Hematol Oncol. 2013;35(2):e67–70. doi: 10.1097/MPH.0b013e31827e91bc. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Hideshima T, Akiyama M, Richardson P, Schlossman RL, Chauhan D, Munshi NC, Waxman S, Anderson KC. Arsenic trioxide inhibits growth of human multiple myeloma cells in the bone marrow microenvironment. Mol Cancer Ther. 2002;1(10):851–860. [PubMed] [Google Scholar]
- Hei TK, Filipic M. Role of oxidative damage in the genotoxicity of arsenic. Free Radic Biol Med. 2004;37(5):574–581. doi: 10.1016/j.freeradbiomed.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Hughes MF. Arsenic toxicity and potential mechanisms of action. Toxicol Lett. 2002;133(1):1–16. doi: 10.1016/s0378-4274(02)00084-x. [DOI] [PubMed] [Google Scholar]
- Hughes MF, Beck BD, Chen Y, Lewis AS, Thomas DJ. Arsenic exposure and toxicology: a historical perspective. Toxicol Sci. 2011;123(2):305–332. doi: 10.1093/toxsci/kfr184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Chen C, Zhao W, Zhang Z. Sodium arsenite and arsenic trioxide differently affect the oxidative stress, genotoxicity and apoptosis in A549 cells: An implication for the paradoxical mechanism. Environ Toxicol Pharmacol. 2013;201336(3):891–902. doi: 10.1016/j.etap.2013.08.002. [DOI] [PubMed] [Google Scholar]
- Jin J-W, Xu Y-F, Huang Y-F. Protective effect of nitric oxide against arsenic-induced oxidative damage in tall fescue leaves. Afr J Biotechnol. 2010;9(11):1619–1627. [Google Scholar]
- Kitchin KT, Conolly R. Arsenic-induced carcinogenesis--oxidative stress as a possible mode of action and future research needs for more biologically based risk assessment. Chem Res Toxicol. 2010;23(2):327–335. doi: 10.1021/tx900343d. [DOI] [PubMed] [Google Scholar]
- Kuwajerwala N, Cifuentes E, Gautam S, Menon M, Barrack ER, Reddy GP. Resveratrol induces prostate cancer cell entry into s phase and inhibits DNA synthesis. Cancer Res. 2002;62(9):2488–2492. [PubMed] [Google Scholar]
- Lam SK, Li YY, Zheng CY, Leung LL, Ho JC. E2F1 downregulation by arsenic trioxide in lung adenocarcinoma. Int J Oncol. 2014 doi: 10.3892/ijo.2014.2609. doi: 10.3892/ijo.2014.2609. [DOI] [PubMed] [Google Scholar]
- Li L, Qiu P, Chen B, Lu Y, Wu K, Thakur C, Chang Q, Sun J, Chen F. Reactive oxygen species contribute to arsenic-induced EZH2 phosphorylation in human bronchial epithelial cells and lung cancer cells. Toxicol Appl Pharmacol. 2014;276(3):165–170. doi: 10.1016/j.taap.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lushchak VI. Glutathione homeostasis and functions: potential targets for medical interventions. J Amino Acids. 2012;2012:736837. doi: 10.1155/2012/736837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandegary A, Torshabi M, Seyedabadi M, Amirheidari B, Sharif E, Ghahremani MH. Indomethacin-enhanced anticancer effect of arsenic trioxide in A549 cell line: involvement of apoptosis and phospho-ERK and p38 MAPK pathways. Biomed Res Int. 2013;2013:237543. doi: 10.1155/2013/237543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlwain DR, Berger T, Mak TW. Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol. 2013;5(4):a008656. doi: 10.1101/cshperspect.a008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WH, Schipper HM, Lee JS, Singer J, Waxman S. Mechanisms of action of arsenic trioxide. Cancer Res. 2002;62(14):3893–3903. [PubMed] [Google Scholar]
- Milton AG, Zalewski PD, Ratnaike RN. Zinc protects against arsenic-induced apoptosis in a neuronal cell line, measured by DEVD-caspase activity. Biometals. 2004;17(6):707–713. doi: 10.1007/s10534-004-1210-3. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Kiyozuka Y, Uemura Y, Senzaki H, Shikata N, Hioki K, Tsubura A. Resveratrol inhibits human breast cancer cell growth and may mitigate the effect of linoleic acid, a potent breast cancer cell stimulator. J Cancer Res Clin Oncol. 2001;127(4):258–264. doi: 10.1007/s004320000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen U, Squaglia N, Boge A, Fung PA. The Simple Western[trade]: a gel-free, blot-free, hands-free Western blotting reinvention. Nat Meth. 2011;8:11. [Google Scholar]
- Qi Y, Zhang M, Li H, Frank JA, Dai L, Liu H, Zhang Z, Wang C, Chen G. Autophagy inhibition by sustained overproduction of IL6 contributes to arsenic carcinogenesis. Cancer Res. 2014;74(14):3740–3752. doi: 10.1158/0008-5472.CAN-13-3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, Ochiai M, Kojima C, Ohta T, Sakurai MH, Takada NO, Qu W, Waalkes MP, Fujiwara K. Role of glutathione in dimethylarsinic acid-induced apoptosis. Toxicol Appl Pharmacol. 2004;198(3):354–365. doi: 10.1016/j.taap.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Selvaraj V, Yeager-Armstead M, Murray E. Protective and antioxidant role of selenium on arsenic trioxide–induced oxidative stress and genotoxicity in the fish hepatoma cell line PLHC-1. Environ Toxicol Chem. 2012;31(12):2861–2869. doi: 10.1002/etc.2022. [DOI] [PubMed] [Google Scholar]
- Shankar S, Singh G, Srivastava RK. Chemoprevention by resveratrol: molecular mechanisms and therapeutic potential. Front Biosci. 2007;12:4839–4854. doi: 10.2741/2432. [DOI] [PubMed] [Google Scholar]
- Sherwood CL, Liguori AE, Olsen CE, Lantz RC, Burgess JL, Boitano S. Arsenic compromises conducting airway epithelial barrier properties in primary mouse and immortalized human cell cultures. PLoS One. 2013;8(12):e82970. doi: 10.1371/journal.pone.0082970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim MJ, Kim HJ, Yang SJ, Lee IS, Choi HI, Kim T. Arsenic trioxide induces apoptosis in chronic myelogenous leukemia K562 cells: possible involvement of p38 MAP kinase. J Biochem Mol Biol. 2002;35(4):377–383. doi: 10.5483/bmbrep.2002.35.4.377. [DOI] [PubMed] [Google Scholar]
- Simon HU, Haj-Yehia A, Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000;5(5):415–418. doi: 10.1023/a:1009616228304. [DOI] [PubMed] [Google Scholar]
- Sun RC, Board PG, Blackburn AC. Targeting metabolism with arsenic trioxide and dichloroacetate in breast cancer cells. Mol cancer. 2011;10:142. doi: 10.1186/1476-4598-10-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Naguro I, Nishitoh H, Matsuzawa A, Ichijo H. Apoptosis signaling kinases: from stress response to health outcomes. Antioxid Redox Signal. 2011;15(3):719–761. doi: 10.1089/ars.2010.3392. [DOI] [PubMed] [Google Scholar]
- Vuky J, Yu R, Schwartz L, Motzer RJ. Phase II trial of arsenic trioxide in patients with metastatic renal cell carcinoma. Invest New Drugs. 2002;20(3):327–330. doi: 10.1023/a:1016270206374. [DOI] [PubMed] [Google Scholar]
- Walker AM, Stevens JJ, Ndebele K, Tchounwou PB. Arsenic trioxide modulates DNA synthesis and apoptosis in lung carcinoma cells. Int J Environ Res Public Health. 2010;7(5):1996–2007. doi: 10.3390/ijerph7051996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wei Y, Zhang H, Shi Y, Li Y, Li R. Arsenic trioxide induces apoptosis of p53 null osteosarcoma MG63 cells through the inhibition of catalase. Med Oncol. 2012;29(2):1328–1334. doi: 10.1007/s12032-011-9848-5. [DOI] [PubMed] [Google Scholar]
- Westervelt P, Brown RA, Adkins DR, Khoury H, Curtin P, Hurd D, Luger SM, Ma MK, Ley TJ, DiPersio JF. Sudden death among patients with acute promyelocytic leukemia treated with arsenic trioxide. Blood. 2001;98(2):266–271. doi: 10.1182/blood.v98.2.266. [DOI] [PubMed] [Google Scholar]
- Xu Y, Li Y, Pang Y, Ling M, Shen L, Jiang R, Zhao Y, Zhou J, Wang X, Liu Q. Blockade of p53 by HIF-2alpha, but not HIF-1alpha, is involved in arsenite-induced malignant transformation of human bronchial epithelial cells. Arch Toxicol. 2012;86(6):947–959. doi: 10.1007/s00204-012-0810-x. [DOI] [PubMed] [Google Scholar]
- Yang CH, Kuo ML, Chen JC, Chen YC. Arsenic trioxide sensitivity is associated with low level of glutathione in cancer cells. Br J Cancer. 1999;81(5):796–799. doi: 10.1038/sj.bjc.6690766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Xue J, Li Y, Zhang W, Ma D, Liu L, Zhang Z. Resveratrol protects against arsenic trioxide-induced nephrotoxicity by facilitating arsenic metabolism and decreasing oxidative stress. Arch Toxicol. 2013;87(6):1025–1035. doi: 10.1007/s00204-013-1026-4. [DOI] [PubMed] [Google Scholar]
- Zhang TD, Chen GQ, Wang ZG, Wang ZY, Chen SJ, Chen Z. Arsenic trioxide, a therapeutic agent for APL. Oncogene. 2001;20(49):7146–7153. doi: 10.1038/sj.onc.1204762. [DOI] [PubMed] [Google Scholar]
- Zhang W, Xue J, Ge M, Yu M, Liu L, Zhang Z. Resveratrol attenuates hepatotoxicity of rats exposed to arsenic trioxide. Food Chem Toxicol. 2013a;51:87–92. doi: 10.1016/j.fct.2012.09.023. [DOI] [PubMed] [Google Scholar]
- Zhang W, Yao C, Ge M, Xue J, Ma D, Liu Y, Liu J, Zhang Z. Attenuation of arsenic retention by resveratrol in lung of arsenic trioxide-exposed rats. Environ Toxicol Pharmacol. 2013b;36(1):35–39. doi: 10.1016/j.etap.2013.02.017. [DOI] [PubMed] [Google Scholar]
- Zhao XY, Li GY, Liu Y, Chai LM, Chen JX, Zhang Y, Du ZM, Lu YJ, Yang BF. Resveratrol protects against arsenic trioxide-induced cardiotoxicity in vitro and in vivo. Br J Pharmacol. 2008;154(1):105–113. doi: 10.1038/bjp.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]