Abstract
Background
Fat graft volume retention remains highly unpredictable, but addition of adipose-derived stromal cells (ASCs) to fat grafts has been shown to improve retention. The present study aimed to investigate the mechanisms involved in ASC enhancement of fat grafting.
Methods
ASCs isolated from human lipoaspirate were labeled with green fluorescent protein (GFP) and luciferase. Fat grafts enhanced with ASCs were injected into the scalp and bioluminescent imaging was performed to follow retention of ASCs within the fat graft. Fat grafts were also explanted at days 1, 5, and 10 post-grafting for ASC extraction and single-cell gene analysis. Finally, CD31 immunohistochemical staining was performed on fat grafts enriched with ASCs.
Results
Bioluminescent imaging demonstrated significant reduction in luciferase+ ASCs within fat grafts at five days post-grafting. A similar reduction in viable GFP+ ASCs retrieved from explanted grafts was also noted. Single cell analysis revealed expression of multiple genes/markers related to cell survival and angiogenesis including BMPR2, CD90, CD105, FGF2, CD248, TGFβ1, and VEGFA. Genes involved in adipogenesis were not expressed by ASCs. Finally, CD31 staining revealed significantly higher vascular density in fat grafts explanted at day 10 post-grafting.
Conclusions
Although ASC survival in the hypoxic graft environment decreases significantly over time, these cells provide multiple angiogenic growth factors. Therefore, improved fat graft volume retention with ASC enrichment may be due to improved graft vascularization.
Keywords: fat grafting, fat transfer, ASC, cell-assisted lipotransfer
Introduction
Unpredictability in fat graft outcomes and highly variable reports of graft survival(1–3) have stimulated an interest in methods aimed to enhance fat graft retention and reproducibility of results. Cell-assisted lipotransfer (CAL), first described by Yoshimura and colleagues, involves the addition of adipose-derived stromal cells (ASCs) to fat prior to injection (4). In both laboratory(4–6) and clinical studies(7–11), CAL has been shown to improve graft survival, and in clinical studies, to improve aesthetic outcomes.
Still, the mechanism through which ASC supplementation enhances graft survival remains poorly understood. It has been hypothesized that ASCs may differentiate into adipocytes and therefore augment graft volume(9). Alternatively, ASCs may improve graft survival by stimulating angiogenesis, either through direct differentiation into endothelial cells or through paracrine delivery of cytokines and growth factors involved in graft revascularization(4–6).
Therefore, the aim of the present study was to investigate the role of ASCs in CAL and how these cells may enhance fat graft outcomes. Specifically, we investigated retention of ASCs within fat grafts by tracking their post-graft survival. We also aimed to study the transcriptional activity of ASCs following grafting in vivo using single-cell transcriptional analysis and evaluation of expression profiles over time using quantitative real-time polymerase chain reaction (qRT-PCR). Finally, we studied the effect of CAL on graft tissue vascularity through immunohistochemical staining.
Materials and Methods
Animal Model and Study Design
Twenty-five adult Crl:NU-Foxn1nu CD-1 mice were used for the experiments in this study. All experiments were performed under Stanford University APLAC approval (#9999). Four mice were used to track ASC retention in vivo by following bioluminescent imaging of fat grafts enriched with luciferase+ ASCs. This was performed with an In Vivo Imaging System (IVIS) (Caliper Life Sciences, Hopkinton, MA). Nine mice were used for fat grafting with explantation at days one, five, and ten for ASC extraction and single-cell transcriptional analysis. An additional nine mice were similarly used for fat grafting with subsequent explantation for qRT-PCR on isolated ASCs. Finally, three mice were used for histological analysis following fat grafting with CAL.
ASC Harvest and Labeling
This study was carried out under Stanford University IRB approval (#2188), and all human lipoaspirate was obtained after informed consent. ASCs were isolated from suction-assisted lipoaspirate obtained from the flank, thigh, and abdomen of three healthy female patients (ages 44 to 47). Processing of fat for ASCs was performed as previously described (12). Cells were expanded and passage 1 cells were transduced with copGFP Control Lentivirus Particles (Santa Cruz Biotechnology, Santa Cruz, CA). Additional ASCs underwent viral transduction with a luciferase marker (Vector Biolabs, Philadelphia, PA).
Fat Grafting
For fat grafting, liposuction aspirates were obtained from three healthy female donors (ages 38 to 61). Fat was harvested using the Coleman technique and injectable fat was isolated from the oil layer and blood/debris layer through gravity separation for 30 minutes, as previously described (13–15). Processed fat was then enriched with either 500,000 GFP+ ASCs or luciferase+ ASCs per 200 μl of fat and then transferred to a 1 cc Luer-lock syringe with a 16-gauge needle for injection into mice, all performed by a single surgeon (R.M.G.). A subcutaneous tunnel was first created by passing the needle antegrade beneath the scalp in immunocompromised mice and then fat was injected in retrograde fashion, as previously described (1).
Bioluminescent Imaging
To follow ASC retention within the fat graft, bioluminescent analysis was performed using IVIS at days 0, 5, and 10 and then at weeks 2, 3, 5, and 7 post-grafting. Mice were anesthetized with a 2.5% isoflurane/oxygen mixture at 1–2 L per minute, and D-Luciferin Firefly potassium salt (Biosynth International, Inc, Itasca, IL) was then injected into the peritoneal cavity (200 mg/kg). Animals were imaged immediately for 30 minutes using a 90 second capture and medium binning. Total flux was quantified for a standardized region of the scalp using Living Image Software (Caliper Life Sciences, Hopkinton, MA).
ASC Retrieval, Flow Cytometry, and Microfluidic-Based Single cell Gene Expression Analysis
To extract ASCs for analysis following injection, fat enriched with GFP+ ASCs was harvested from the delivery site at one, five, and ten days post-grafting. Samples were then digested for one hour at 37°C using collagenase I (Roche Applied Science, Indianapolis, IN), followed by centrifugation and resuspension. Viable, GFP+ cells were quantified and sorted as single cells using a FACSAria flow cytometer (Becton Dickinson, Franklin Lakes, NJ) into 6μl of lysis buffer. Cultured ASCs were sorted as a control population. Reverse transcription and low cycle pre-amplification were performed using Cell Direct (Invitrogen, Carlsbad, CA) with Taqman assay primer sets (Applied Biosystems, Grand Island, NY). cDNA was loaded onto 96.96 Dynamic Array (Fluidigm, South San Francisco, CA) for qPCR amplification using Universal PCR Master Mix (Applied Biosystems) with a uniquely compiled Taqman assay primer set, as previously described (16). For confirmation by qRT-PCR analysis, fat enriched with ASCs was similarly explanted at one, five, and ten days post-grafting, and following digestion, sorting of GFP+ ASCs by flow cytometry was performed into TRIzol® reagent (Invitrogen) for phase separation of RNA.
Reverse Transcription and Quantitative Real-Time Polymerase Chain Reaction
RNA was isolated with the RNeasy Mini Kit (Qiagen, San Diego, CA). Reverse transcription was performed and expression of genes involved in cell survival and angiogenesis was examined by qRT-PCR using the Applied Biosystems Prism 7900HT sequence detection system (Applied Biosystems). To generate relative expression levels, target quantities were normalized to endogenous GAPDH expression. Primers for the genes of interest were created from PrimerBank (17, 18).
Histological Analysis
For histological analysis, fat grafts enriched with ASCs were explanted at days one, five, and ten post-grafting. The grafts were immediately fixed in 10% formalin and embedded in paraffin for sectioning. To assess fat graft vascularity, 8-micron sections were immunohistochemically stained for CD31 (1° - 1:100 Rb α CD31, Ab28364, Abcam, Cambridge, MA; 2° - 1:200 AF547 Gt α Rb, Life Technologies). Hoechst dye was used for counterstaining of nuclei, and sections were mounted using Fluoromount-G (SouthernBiotech, Birmingham, AL). Three images were obtained from each section at every time point using the 40x objective. CD 31 staining was quantified by recording pixel-positive area per high power field using Image J (NIH, Bethesda, MD). A Leica DM5000 B light microscope (Leica Microsystems, Buffalo Grove, IL) was used for imaging with X-Cite 120 Fluorescence Illumination system (Lumen Dynamics Group Inc., Ontario, Canada) for immunofluorescence.
Statistical Analysis
Data are presented as means with standard deviations. Two-sample t-tests for comparison of means were calculated using StatPlus software (AnalystSoft Inc.), and a *p-value < 0.05 was considered significant. For single cell transcriptional data, a Kolmogorov-Smirnov (K-S) test was used to compare empirical distributions, followed by an adaptive fuzzy c-means clustering algorithm as previously described(16).
Results
Transient ASC Retention Following Cell-Assisted Lipotransfer
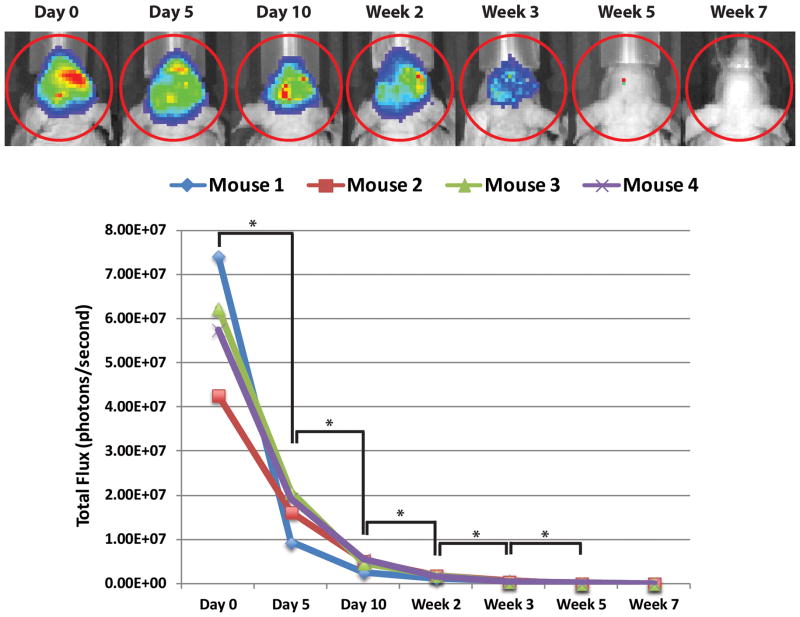
To evaluate retention of viable ASCs, bioluminescent imaging was performed on fat grafts enriched with luciferase+ ASCs over the course of seven weeks (Figure 1A). A significant decrease in total flux was noted between the day of injection and at day 5, with continued decrease observed to week 5 (*p < 0.05). The signal, however, stabilized after week 5, with no significant difference between week 5 and week 7 (p > 0.05) (Figure 1B). The greatest decrease occurred between day 0 (day of injection) and day 5, with an average 71% decrease in total flux between the four mice; subsequently, there was an average 21% decrease in signal between day 5 and day 10, an average 5% decrease between day 10 and week 2, and an average 2% decrease between week 2 and week 3, where the total flux measured was only 1% of the initial signal. At both week 5 and week 7, the total flux measured was less than 1% of the initial signal.
Figure 1.
Bioluminescent imaging of luciferase+ ASCs. (A) IVIS imaging demonstrating progressive decline in bioluminescent signal from ASC enhanced fat grafts following intraperitoneal D-Luciferin injection. (B) Decrease in total flux over time measured by IVIS bioluminescent signal for each mouse. A significant decline was observed between each time point from day 0 to week 5 post-grafting (*p < 0.05). The signal continued to decrease from week 5 to week 7, but the difference in signal between these time points did not reach statistical significance (p > 0.05).
ASCs Enhance Angiogenic Gene Expression following In Vivo Injection
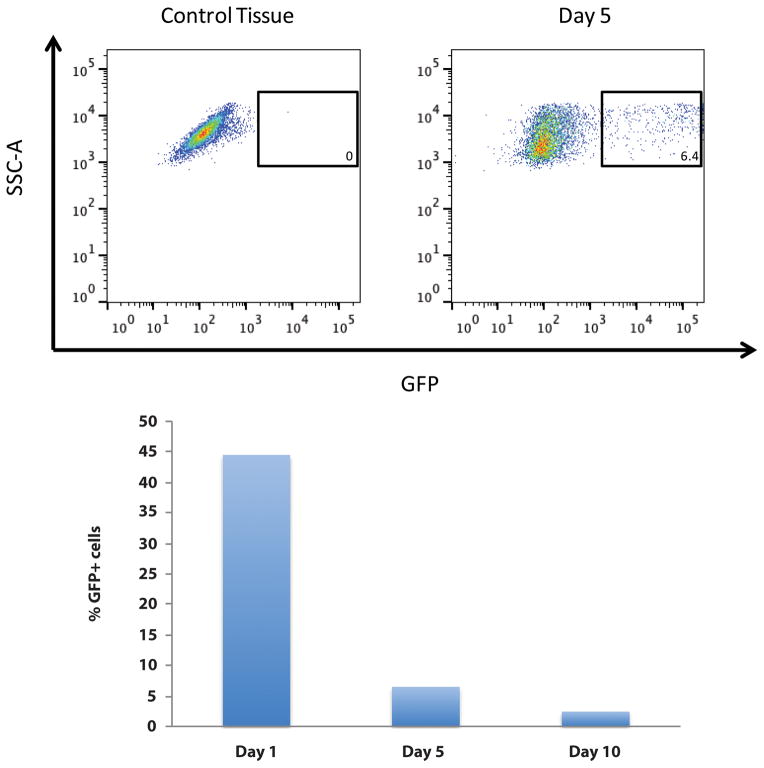
In order to understand the mechanism by which transient retention of viable ASCs may enhance fat grafting, we enriched fat grafts with GFP+ ASCs and then explanted specimens at days 1, 5, and 10 post-grafting for cell extraction. Flow cytometric quantification of digested samples was then performed for GFP+ ASCs, and similar to our bioluminescent imaging data, the percentage of retrievable, viable cells in explanted grafts from the original number of GFP+ ASCs decreased from 44% on day 1, to 6% on day 5, to 2% on day 10 post-injection (Figure 2).
Figure 2.
Flow cytometric quantification of retained, viable ASCs. (A) Representative FACS plot demonstrating gating for sorting of GFP+ ASCs at day 5 post-grafting after retrieval from explanted fat grafts. (B) Progressive decline in percentage of ASC retrieval from digested grafts, measured by quantitative flow cytometry over time. Of the original number of ASCs injected with fat, 44% were retrieved on day 1, 6% on day 5, and 2% on day 10 post-grafting.
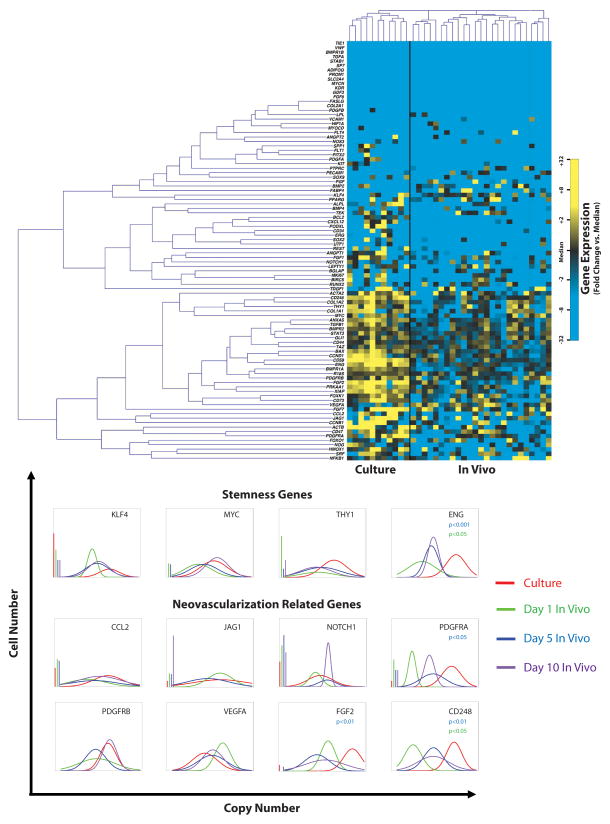
Following retrieval of ASCs from fat grafts, we next performed single-cell transcriptional analysis (16). Individual cell expression patterns for more than 90 gene targets was simultaneously evaluated, specifically looking at genes relating to cell survival, stemness, and neovascularization, as well as genes involved in tissue differentiation processes including adipogenesis, chondrogenesis, osteogenesis, and myogenesis (Supplemental Digital Content 1 - Table 1 shows gene names and assay IDs for microfluidic single-cell gene expression analysis. Genes specifically relating to cell survival, stemness, and neovascularization were chosen, in addition to selected control, cell-cycle, and surface marker related probes (insert link)). Comparison was made to cultured ASCs not injected.
Surface markers associated with ASCs were expressed in both cultured cells and cells isolated from in vivo fat grafts including CD34, CD90, and CD44 (19–21), although significant cellular heterogeneity was observed (Figure 3A, Supplemental Digital Content 2 - Figure 1 shows Whisker plot representing raw qPCR cycle threshold for each gene across conditions. Individual dots represent single gene/cell qPCR reactions, with increased cycle threshold values corresponding to decreased mRNA content. Cycle threshold values of 40 were assigned to all reactions that failed to achieve detectable levels of amplification within 40 qPCR cycles. Cultured and day 1, 5, and 10 ASCs implanted with fat are colored in red, green, blue, and purple, respectively (insert link)). A Kolmogorov-Smirnov analysis was performed on these data to further characterize gene expression across conditions (Figure 3B). Interestingly, while some genes associated with stemness and neovascular processes were comparably expressed between cultured ASCs and ASCs injected with fat, including MYC, GLI1, VEGFA, and JAG1 (22–25), others such as FGF2, PDGFRA, PDGFRB, CD248, and ENG(26–29), were significantly downregulated following in vivo injection. Despite relative downregulation of these genes when compared to cultured ASCs, expression of multiple genes involved in cell survival and angiogenesis was observed at day 5 and 10. Also of note, genes involved in adipogenesis such as PPARγ and LPL and other markers of mesenchymal differentiation (chondrogenesis, osteogenesis, and myogenesis) displayed little or no expression by ASCs when injected with fat.
Figure 3.
Single-cell gene analysis of ASCs. (A) Single-cell gene expression analysis demonstrated both in vitro and in vivo expression of CD34, CD90, and CD44, characteristic of ASCs. Comparable expression of MYC, GLI1, VEGFA, and JAG1, genes involved in stemness and angiogenesis, was observed both in vitro cultured ASCs and among ASCs following grafting and retrieval. Initial downregulation of FGF2, PDGFRA, PDGFRB, CD248, and ENG was noted post-grafting. However, expression of CD248 and multiple other genes involved in angiogenesis, including BMPR2, CD105, FGF2, and TGFβ1, was observed to increase over time. (B) Kolmogorov-Smirnov analysis was performed on these data to further characterize gene expression across conditions.
ASC Angiogenic Gene Expression Increases From Day 1 to Day 10 Post-Grafting
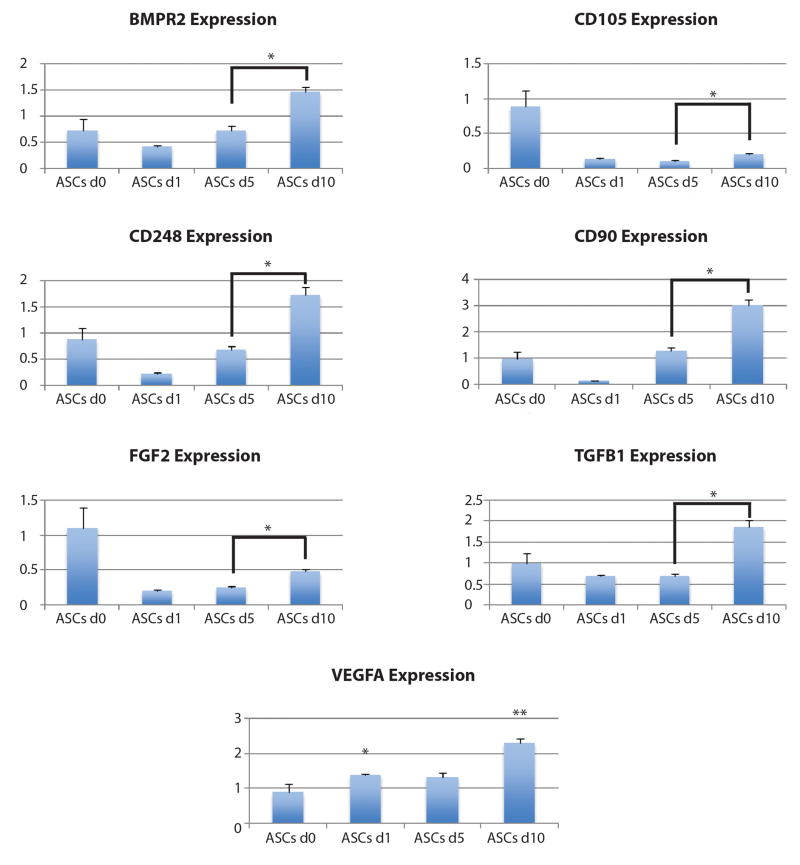
To provide confirmation for our single-cell analysis findings, we quantitatively analyzed ASC gene expression of seven selected genes (BMPR2, CD105, CD248, CD90, FGF2, TGFβ1, VEGFA) whose in vivo expression had been identified. RNA was collected from cultured, non-injected ASCs (day 0) and transcripts for these seven genes were compared to levels in ASCs retrieved from fat grafts at days 1, 5, and 10 post-grafting. Quantitative analysis revealed significantly higher levels of gene expression for all genes tested at day 10 compared to both day 5 and day 1 post-grafting (*p < 0.05) (Figure 4). Interestingly, all genes studied, with the exception of VEGFA, demonstrated an initial downregulation of expression at day 1 when compared to in vitro cells (day 0) and a subsequent trend toward increasing expression up to day 10 (Figure 4). Importantly, significantly increased transcript levels for VEGFA was observed at day 1 following injection, and remaining cells retrieved at day 10 demonstrated the highest level for this angiogenic factor (*p < 0.05, **p < 0.01).
Figure 4.
qRT-PCR analysis of ASC gene expression over time demonstrated an initial downregulation from day 0 (in vitro) to day 1 post-grafting for several genes, with the notable exception of VEGFA. A trend toward increasing expression was observed, however, with significantly higher expression seen at day 10 compared to day 5 for all genes studied (*p < 0.05, **p < 0.01).
Vascularity of ASC-Enhanced Fat Grafts Increases From Day 1 to Day 10 Post-Grafting
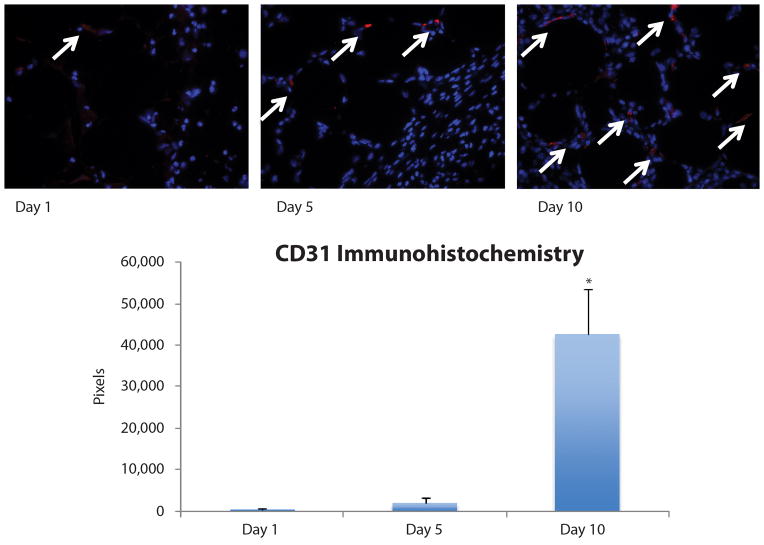
As significant expression of VEGFA was noted in ASCs extracted from fat grafts, staining for vascular markers was subsequently performed. CD31 immunohistochemical staining demonstrated a progressive increase in expression of this endothelial marker over time following fat grafting (day 1: 3,249 ± 5,628 red pixels, day 5: 18,104 ± 26,679 red pixels, and day 10: 426,148 ± 189,071 red pixels). Quantification of staining revealed vascularity at day 5 was higher than day 1, but the difference did not reach statistical significance (p > 0.05). However, graft vascularity at day 10 post-grafting was significantly higher than that observed in grafts at both day 1 and day 5 (*p < 0.05) (Figure 5).
Figure 5.
CD31 Immunohistochemical staining. Increased graft vascularization was detected by CD31 immunohistochemical staining (red pixels highlighted by white arrows) at day 10 (426,148 ± 189,071 pixels) compared to day 1 (3,249 ± 5,628 pixels, *p < 0.05) and day 5 (18,104 ± 26,679 pixels, *p < 0.05) post-grafting. Vascularity at day 5 was higher than day 1, but the difference did not reach statistical significance (p > 0.05).
Discussion
Cell-assisted lipotransfer has been promoted as a means of increasing fat graft viability and making the process of fat grafting more predictable and reproducible(30). In 2006, Matsumoto et al. first described the process of CAL and, using a mouse model injected with human aspirated fat both with and without supplemental ASCs, showed increased survival in the CAL group, with an average of 35% greater survival measured by explanted graft weight(4). Similarly, Zhu et al. showed ASC supplementation led to a 2-fold increase in explanted graft weight, and Lu et al. showed a graft retention rate of 60.1% with CAL compared to 27.1% in non-CAL(5, 6). Histologically, CAL grafts demonstrate less necrosis with more viable adipocytes, less fibrosis, and fewer cysts/vacuoles, along with increased microvasculature and capillary density when compared to non-CAL grafts(4–6). Even in grafts without the addition of supplemental ASCs, Philips et al. observed greater survival of grafts that naturally contained a higher proportion of ASCs(31).
With evidence supporting the effectiveness of CAL over non-CAL fat grafting, the mechanism of enhancement has become of great interest. Importantly, studies with human fat grafting using immunocompromised mice have been well described in recent literature, making this model well-suited to investigate ASC contribution to fat graft retention (1, 13–15). Whether this occurs through secretion of paracrine factors by ASCs or a direct contribution to fat through adipogenic differentiation remains undetermined. Interestingly, in our study we noted only transient retention of viable ASCs within the fat graft. Through bioluminescent imaging, only 29% of total initial flux from transplanted ASCs remained detectable five days after grafting, and this dropped to 8% by day 10. Similar findings were noted by a more quantitative approach using flow cytometry on extracted cells. These data suggest either progressive loss of ASCs through cell death or diffusion away from the graft site. In either case, a significant contribution to the adipocyte population would not be expected, and enhancement of fat graft retention may instead occur through a paracrine route.
To ascertain whether this was the case, single-cell gene analysis of transplanted ASCs was performed, evaluating expression levels for various transcripts out to 10 days following implantation. Further analysis was limited by progressive loss of viable, retrievable ASCs beyond this time point, as demonstrated by our bioluminescent and flow cytometric data. Interestingly, although ASCs have the potential to differentiate into many cell types, our study suggested that ASCs transplanted within fat grafts do not undergo significant adipogenic differentiation, as expression of genes associated with adipogenesis (e.g. PPARγ and LPL) was either minimal or undetectable. Instead, our data supported potential ASC involvement in the process of graft revascularization, with detectable expression of several angiogenic factors noted from ASCs. Through single cell gene analysis and qRT-PCR of pooled specimens, we found that ASCs produce growth factors and cytokines that may be involved in cell survival and new vessel formation(27–29). Although not all angiogenesis-associated genes tested were expressed by ASCs at each time point following implantation, our data supported a possible inductive role of ASCs in graft revascularization, mediated by factors such as VEGFA and FGF2 (30).
Of note, ASCs implanted with our fat grafts underwent prior in vitro culture following harvest for GFP and luciferase labeling. This deviates from the clinical setting in which freshly isolated cells would be immediately recombined with fat for re-injection, raising an important limitation with our study. Prior in vitro culture was necessary, however, to ensure adequate labeling of all ASCs prior to implantation and to facilitate efficient retrieval for analysis post-implantation.
Nonetheless, the paracrine activity of ASCs in promoting graft vascularization we observed is supported by findings of Suga et al. in which ASCs injected into mouse fat pads led to proliferation of nearby endothelial cells and increased production of growth factors including VEGF(32). Furthermore, Lu et al. found a graft retention rate of 74.1% in mice that underwent CAL with ASCs previously transfected with VEGF, a rate higher than that observed with conventional CAL(5). Finally, like VEGF, ASCs have also been shown to secrete FGF2 following administration to diabetic rat wounds, and this resulted in enhanced angiogenesis and accelerated wound healing.(33)
Despite this growing evidence for a paracrine effect of ASCs, though, it has been suggested that ASCs may instead improve vascularity through direct incorporation into the vasculature. Studies in the cardiovascular literature support ASC differentiation into endothelial cells(34). However, such differentiation is only weakly supported by CAL research. Reports by both Zhu et al. and Lu et al. showed that at the time of graft explantation at either 6 or 9-months post-transfer, labeled ASCs were only rarely detected in vessel walls(5, 6). And while our data did show expression of some known endothelial cell surface makers such as CD105, CD248, and CD90 by ASCs extracted from grafts, significant contribution to new vessel formation would not be expected given the low retention of viable cells observed from our bioluminescent imaging and flow cytometric quantification studies.
Lastly, we observed relative downregulation of several growth factors from in vitro cultured ASCs to ASCs extracted on day 1 post-grafting, with the notable exception of VEGFA. A more harsh, hypoxic in vivo environment compared to in vitro culture may have contributed to some of these observations. Nonetheless, we noted, a trend toward increased expression of angiogenic factors in vivo with time by ASCs, and this correlated with progressively greater CD31 staining in fat grafts at post-graft days 5 and 10, suggestive of new vessel formation. And as Eto et al. described an initial period of cell death followed by an increase in viable adipocyte area seven days after implantation of fat grafts, this stabilization and repair/regeneration of dead tissue may be enhanced by reestablishment of graft vascularity, a process facilitated by ASC expression of angiogenic factors in the early post-grafting period (35).
Conclusions
Cell-assisted lipotransfer has been shown to enhance fat grafting by increasing graft retention and reproducibility. In vivo, ASCs express multiple genes that act to promote angiogenesis. This expression also corresponds with increased graft vascularity. Viable ASC retention is limited, however, with the majority of cells lasting less than 1 week post-grafting at the site of implantation. Together, these data suggest that while ASCs undergo transcriptional changes during a limited period following grafting, they may adopt a more dynamic, pro-angiogenic profile to support the survival of co-injected adipose tissue. With such promising results, future studies will be needed to determine the optimal ASC: lipoaspirate ratio to enhance graft retention and to establish approved methods for safe, efficient ASC isolation and supplementation in clinical practice.
Supplementary Material
Gene names and assay IDs for microfluidic single-cell gene expression analysis. Genes specifically relating to cell survival, stemness, and neovascularization were chosen, in addition to selected control, cell-cycle, and surface marker related probes.
Whisker plot representing raw qPCR cycle threshold for each gene across conditions. Individual dots represent single gene/cell qPCR reactions, with increased cycle threshold values corresponding to decreased mRNA content. Cycle threshold values of 40 were assigned to all reactions that failed to achieve detectable levels of amplification within 40 qPCR cycles. Cultured and day 1, 5, and 10 ASCs implanted with fat are colored in red, green, blue, and purple, respectively.
Footnotes
Data presented in this manuscript have not been previously presented at any meeting
Financial Disclosure and Products Page
M.T.L. was supported by NIH grants U01 HL099776, R01 DE021683-01, RC2 DE020771, the Oak Foundation, and Hagey Laboratory for Pediatric Regenerative Medicine, D.C.W. was supported by the ACS Franklin H. Martin Faculty Research Fellowship, the Hagey Laboratory for Pediatric Regenerative Medicine, and the Stanford University Child Health Research Institute Faculty Scholar Award.
References
- 1.Chung MT, Hyun JS, Lo DD, et al. Micro-computed tomography evaluation of human fat grafts in nude mice. Tissue Eng Part C Methods. 2013;19:227–232. doi: 10.1089/ten.tec.2012.0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gonzalez AM, Lobocki C, Kelly CP, Jackson IT. An alternative method for harvest and processing fat grafts: an in vitro study of cell viability and survival. Plast Reconstr Surg. 2007;120:285–294. doi: 10.1097/01.prs.0000264401.19469.ad. [DOI] [PubMed] [Google Scholar]
- 3.Tabit CJ, Slack GC, Fan K, Wan DC, Bradley JP. Fat grafting versus adipose-derived stem cell therapy: distinguishing indications, techniques, and outcomes. Aesthetic Plast Surg. 2012;36:704–713. doi: 10.1007/s00266-011-9835-4. [DOI] [PubMed] [Google Scholar]
- 4.Matsumoto DSK, Gonda K, Takaki Y, Shigeura T, Sato T, Aiba-Kojima E, Iizuka F, Inoue K, Suga H, Yoshimura K. Cell-assisted lipotransfer: Supportive use of human adipose-derived cells for soft tissue augmentation with lipoinjection. Tissue Eng. 2006;12:3375–3382. doi: 10.1089/ten.2006.12.3375. [DOI] [PubMed] [Google Scholar]
- 5.Lu F, LJ, Gao J, Ogawa R, Ou C, Yang B, Fu B. Improvement of the survival of human autologous fat transplantation by using VEGF-transfected adipos-derived stem cells. Plast Reconstr Surg. 2009;124:1437–1446. doi: 10.1097/PRS.0b013e3181babbb6. [DOI] [PubMed] [Google Scholar]
- 6.Zhu M, ZZ, Chen Y, Schreiber R, Ransom JT, Fraser JK, Hedrick MH, Pinkernell K, Kuo H. Supplementation of fat grafts with adipose-derived regenerative cells improves long term graft retention. Ann Plast Surg. 2010;64:222–228. doi: 10.1097/SAP.0b013e31819ae05c. [DOI] [PubMed] [Google Scholar]
- 7.Koh KS, OT, Kim H, Chung IW, Lee KW, Lee HB, Park EJ, Jung JS, Shin IS, Ra JC, Choi JW. Clinical application of human adipose tissue-derived mesenchymal stem cells in progressive hemifacial atrophy (Parry-Romberg Disease) with microfat grafting techniques using 3-dimensional computed tomography and 3-dimensional camera. Ann Plast Surg. 2012;69:331–337. doi: 10.1097/SAP.0b013e31826239f0. [DOI] [PubMed] [Google Scholar]
- 8.Tanikawa DYS, AM, Bueno DF, Passos-Bueno MR, Alonso N. Fat grafts supplemented with adipose-derived stromal cells in the rehabilitation of patients with craniofacial microsomia. Plast Reconstr Surg. 2013;132:141–152. doi: 10.1097/PRS.0b013e3182910a82. [DOI] [PubMed] [Google Scholar]
- 9.Yoshimura K, SK, Aoi N, Kurita M, Inoue K, Suga H, Eto H, Kato H, Hirohi T, Harii K. Cell-assisted lipotransfer for facial lipoatrophy: Efficacy of clinical use of adipose-derived stem cells. Dermatol Surg. 2008;34:1178–1185. doi: 10.1111/j.1524-4725.2008.34256.x. [DOI] [PubMed] [Google Scholar]
- 10.Yoshimura K, SK, Aoi N, Kurita M, Hirohi T, Harii K. Cell-assisted lipotransfer for cosmetic breast augmentation: Supportive use of adipose-derived stem/stromal cells. Aesth Plast Surg. 2007;32:48–55. doi: 10.1007/s00266-007-9019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kolle ST, FNA, Mathiasen AB, Elberg JJ, Oliveri RS, Glovinski PV, Kastrup J, Kirchoff M, Rasmussen BS, Talman MM, Thomsen C, Dickmeiss E, Drzewiecki KT. Enrichment of autologous fat grafts with ex-vivo expanded adipose tissue-derived stem cells for graft survival: A randomised placebo-controlled trial. Lancet. 2013;382:1113–1120. doi: 10.1016/S0140-6736(13)61410-5. [DOI] [PubMed] [Google Scholar]
- 12.Zuk PA, ZM, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 13.Garza RM, Paik KJ, Chung MT, et al. Studies in fat grafting: Part III. Fat grafting irradiated tissue--improved skin quality and decreased fat graft retention. Plast Reconstr Surg. 2014;134:249–257. doi: 10.1097/PRS.0000000000000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung MT, Paik KJ, Atashroo DA, et al. Studies in Fat Grafting: Part I. Effects of Injection Technique on in vitro Fat Viability and in vivo Volume Retention. Plast Reconstr Surg. 2014 doi: 10.1097/PRS.0000000000000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atashroo D, Raphel J, Chung MT, et al. Studies in fat grafting: Part II. Effects of injection mechanics on material properties of fat. Plast Reconstr Surg. 2014;134:39–46. doi: 10.1097/PRS.0000000000000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glotzbach JP, Januszyk M, Vial IN, et al. An Information Theoretic, Microfluidic-Based Single Cell Analysis Permits Identification of Subpopulations among Putatively Homogeneous Stem Cells. PLoS One. 2011;6:e21211. doi: 10.1371/journal.pone.0021211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X, Seed B. A PCR primer bank for quantitative gene expression analysis. Nucleic Acids Res. 2003;31:e154. doi: 10.1093/nar/gng154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spandidos A, Wang X, Wang H, Dragnev S, Thurber T, Seed B. A comprehensive collection of experimentally validated primers for Polymerase Chain Reaction quantitation of murine transcript abundance. BMC Genomics. 2008;9:633. doi: 10.1186/1471-2164-9-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chung MT, Liu C, Hyun JS, et al. CD90 (Thy-1)-positive selection enhances osteogenic capacity of human adipose-derived stromal cells. Tissue engineering Part A. 2013;19:989–997. doi: 10.1089/ten.tea.2012.0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung MT, Zimmermann AS, Paik KJ, et al. Isolation of human adipose-derived stromal cells using laser-assisted liposuction and their therapeutic potential in regenerative medicine. Stem cells translational medicine. 2013;2:808–817. doi: 10.5966/sctm.2012-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Folgiero V, Migliano E, Tedesco M, et al. Purification and characterization of adipose-derived stem cells from patients with lipoaspirate transplant. Cell transplantation. 2010;19:1225–1235. doi: 10.3727/09638910X519265. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 23.Po A, Ferretti E, Miele E, et al. Hedgehog controls neural stem cells through p53-independent regulation of Nanog. The EMBO journal. 2010;29:2646–2658. doi: 10.1038/emboj.2010.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nauta A, Seidel C, Deveza L, et al. Adipose-derived stromal cells overexpressing vascular endothelial growth factor accelerate mouse excisional wound healing. Molecular therapy: the journal of the American Society of Gene Therapy. 2013;21:445–455. doi: 10.1038/mt.2012.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roca C, Adams RH. Regulation of vascular morphogenesis by Notch signaling. Genes & development. 2007;21:2511–2524. doi: 10.1101/gad.1589207. [DOI] [PubMed] [Google Scholar]
- 26.Tigges U, Hyer EG, Scharf J, Stallcup WB. FGF2-dependent neovascularization of subcutaneous Matrigel plugs is initiated by bone marrow-derived pericytes and macrophages. Development. 2008;135:523–532. doi: 10.1242/dev.002071. [DOI] [PubMed] [Google Scholar]
- 27.Zhang J, Cao R, Zhang Y, Jia T, Cao Y, Wahlberg E. Differential roles of PDGFR-alpha and PDGFR-beta in angiogenesis and vessel stability. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2009;23:153–163. doi: 10.1096/fj.08-113860. [DOI] [PubMed] [Google Scholar]
- 28.Bagley RG, Honma N, Weber W, et al. Endosialin/TEM 1/CD248 is a pericyte marker of embryonic and tumor neovascularization. Microvascular research. 2008;76:180–188. doi: 10.1016/j.mvr.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 29.Yu J, Tu YK, Tang YB, Cheng NC. Stemness and transdifferentiation of adipose-derived stem cells using l-ascorbic acid 2-phosphate-induced cell sheet formation. Biomaterials. 2014;35:3516–3526. doi: 10.1016/j.biomaterials.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 30.Kolle ST, OR, Glovinski PV, Elberg JJ, Fischer-Nielsen A, Drzewiecki KT. Importance of mesenchymal stem cells in autologous fat grafting: A systematic review of existing studies. J Plast Surg Hand Surg. 2012;46:59–68. doi: 10.3109/2000656X.2012.668326. [DOI] [PubMed] [Google Scholar]
- 31.Philips BJ, GT, Valentin JE, Chung CW, Bliley JM, Pfeifer ME, Roy SB, Sohini B, Dreifuss S, Kelmendi-Doko A, Kling RE, Ravuri SK, Marra KG, Donnenberg VS, Donnenberg AD, Rubin PJ. Prevalence of endogenous CD34+ adipose stem cells predicts human fat graft retention in a xenograft model. Plast Reconstr Surg. 2013;132:845–858. doi: 10.1097/PRS.0b013e31829fe5b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suga H, GJP, Sorkin M, Longaker MT, Gurtner GC. Paracrine Mechanism of Angiogenesis in Adipose-Derived Stem Cell Transplantation. Ann Plast Surg. 2013;72:234–241. doi: 10.1097/SAP.0b013e318264fd6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nie C, Yang D, Xu J, Si Z, Jin X, Zhang J. Locally administered adipose-derived stem cells accelerate wound healing through differentiation and vasculogenesis. Cell Transplant. 2011;20:205–216. doi: 10.3727/096368910X520065. [DOI] [PubMed] [Google Scholar]
- 34.Planat-Benard V, SJ, Cousin B, Andre M, Nibbelnik M, Tamarat R, Clergue M, Manneville C, Saillan-Barreau C, Duriez M, Tedgui A, Levy B, Penicaud L, Casteilla L. Plasticity of human adipose lineage cells toward endothelial cells: Physiological and therapeutic perspectives. Circulation. 2004;109:656–663. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- 35.Eto H, Kato H, Suga H, et al. The fate of adipocytes after nonvascularized fat grafting: evidence of early death and replacement of adipocytes. Plast Reconstr Surg. 2012;129:1081–1092. doi: 10.1097/PRS.0b013e31824a2b19. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Gene names and assay IDs for microfluidic single-cell gene expression analysis. Genes specifically relating to cell survival, stemness, and neovascularization were chosen, in addition to selected control, cell-cycle, and surface marker related probes.
Whisker plot representing raw qPCR cycle threshold for each gene across conditions. Individual dots represent single gene/cell qPCR reactions, with increased cycle threshold values corresponding to decreased mRNA content. Cycle threshold values of 40 were assigned to all reactions that failed to achieve detectable levels of amplification within 40 qPCR cycles. Cultured and day 1, 5, and 10 ASCs implanted with fat are colored in red, green, blue, and purple, respectively.