Abstract
Objective
Studies of mice with mild Marfan syndrome (MFS) have correlated the development of thoracic aortic aneurysm (TAA) with improper stimulation of non-canonical (Erk-mediated) TGFβ signaling by the angiotensin type I receptor (AT1r). This correlation was largely based on comparable TAA modifications by either systemic TGFβ neutralization or AT1r antagonism. However, subsequent investigations have called into question some key aspects of this mechanism of arterial disease in MFS. To resolve these controversial points, here we made a head-to-head comparison of the therapeutic benefits of TGFβ neutralization and AT1r antagonism in mice with progressively severe MFS (Fbn1mgR/mgR mice).
Approach and Results
Aneurysm growth, media degeneration, aortic levels of phosphorylated Erk and Smad proteins and the average survival of Fbn1mgR/mgR mice were compared after a ∼3 month long treatment with placebo and either the AT1r antagonist losartan or the TGFβ neutralizing antibody 1D11. In contrast to the beneficial effect of losartan, TGFβ neutralization either exacerbated or mitigated TAA formation depending on whether treatment was initiated before (post-natal day 16; P16) or after (P45) aneurysm formation, respectively. Biochemical evidence related aneurysm growth with Erk-mediated AT1r signaling, and medial degeneration with TGFβ hyperactivity that was in part AT1r-dependent. Importantly, P16-initiated treatment with losartan combined with P45-initiated administration of 1D11 prevented death of Fbn1mgR/mgR mice from ruptured TAA.
Conclusions
By demonstrating that promiscuous AT1r and TGFβ drive partially overlapping processes of arterial disease in MFS mice, our study argues for a therapeutic strategy against TAA that targets both signaling pathways while sparing the early protective role of TGFβ.
Keywords: aortic aneurysm, AT1 receptor, losartan, Marfan syndrome, TGFβ signaling
Introduction
Aortic aneurysms are life-threatening pathologies characterized by progressive vessel dilation associated with smooth muscle cell (SMC) dysfunction, localized inflammatory infiltrates and destructive (maladaptive) extracellular matrix (ECM) remodeling that together predispose the aortic wall to tear (dissection) and rupture.1,2 In contrast to the strong association of abdominal aortic aneurysm (AAA) with advanced age and a collection of environmental risk factors, thoracic aortic aneurysm (TAA) shows high heritability often accounted for by mutations in components of the ECM, cytoskeleton and TGFβ signaling cascade.1,2 Studies of mouse models of TAA and AAA have implicated the angiotensin II type I receptor (AT1r) in the etiology of both types of aneurysm, in addition to raising a controversy about TGFβ's role in arterial disease.3 On the one hand, inhibition of TGFβ signaling with a pan-TGFβ neutralizing antibody (TGFβ-Nab) prevented TAA formation in a mouse model of mild Marfan syndrome (MFS).4 On the other hand, TGFβ-Nab administration exacerbated the pathology of angiotensin-induced AAA and TAA in mice and conversely, TGFβ overexpression stabilized the expansion of experimental AAA in rats.5,6
MFS is a relatively common disease of connective tissue caused by mutations that alter the structure or impair the expression of the ECM component and TGFβ modulator fibrillin-1.1 Two mouse models representing distinct degrees of MFS severity have been instrumental in delineating the pathogenic mechanisms responsible for cardiovascular and musculoskeletal abnormalities. The first mouse model (Fbn1C1039G/+ mouse) produces equal amounts of normal and abnormal fibrillin-1 and replicates the less commonly observed form of mild MFS.7 Chung et al.8 have reported that by 6 months of age more than 90% of Fbn1C1039G/+ mice developed TAA of variable severity, but only ∼5% of them died of ruptured aortic aneurysm by 8 months of age. The second mouse model (Fbn1mgR/mgR mice) produces ∼20% of the normal amount of fibrillin-1 and replicates the more frequently diagnosed form of early onset, progressively severe MFS.9 In contrast to Fbn1C1039G/+ mice, ruptured TAA is a fully penetrant manifestation that leads to death of nearly all Fbn1mgR/mgR mice within the first year of life (average survival: 2.5 months of age).9
Prior analyses of Fbn1C1039G/+ mice have shown that either systemic AT1r antagonism or TGFβ neutralization normalize aneurysm growth along with the levels of phosphorylated (p)-Smad2 and p-Erk1/2.4 Even though AT1r and TGFβ can both activate Smad2 and Erk1/2 proteins,3 this finding was interpreted as indirect evidence of AT1r-dependent stimulation of canonical (Smad-mediated) and non-canonical (Erk-mediated) TGFβ signaling.4 Subsequent experiments have suggested a prominent role of the non-canonical Erk1/2 pathway in TGFβ-promoted arterial disease in Fbn1C1039G/+ mice.10 By contrast, studies of Fbn1mgR/mgR mice have implied that mechanisms other than improper AT1r activation stimulate promiscuous TGFβ signaling, as losartan administration mitigated but did not prevent ruptured TAA in this animal model of progressively severe MFS.11,12 While our study was being completed, Li et al.13 have reported that genetic disruption of TGFβ receptor II (Tgfbr2) in post-natal SMCs of Fbn1C1039G/+ mice at 4 weeks of age increased the rate and degree of TAA and aortic dissection.
In the original study of Fbn1C1039G/+ mice, losartan and TGFβ-Nab dosing occurred for vastly different periods of time and treatment efficacy was assessed at different ages.4 To correct these disparities, here we employed the same treatment protocol to compare and contrast the impact of TGFβ vs. AT1r inhibition on TAA progression and survival of Fbn1mgR/mgR mice. Similar to prior studies with Fbn1C1039G/+ mice,4,10 we also examined the relative levels of p-Erk1/2 and p-Smad2 as surrogate molecular readouts of treatment efficacy. The results of our experiments expose the complexity associated with TGFβ inhibition in the diseased aorta, reconcile the existing controversy concerning TGFβ's role in aortic aneurysms, exclude a strict dependence of TGFβ over-activation on AT1r signaling, and correlate promiscuous AT1r and TGFβ activity with partially overlapping processes of arterial disease. Together our findings substantially revise the current view of TAA pathogenesis in MFS, in addition to suggesting that targeting both AT1r and TGFβ signaling is a more effective therapeutic strategy than solely blocking AT1r activity.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement
Results
Mutant aortas exhibit distinct temporal profiles of p-Erk1/2 and p-Smad2 accumulation
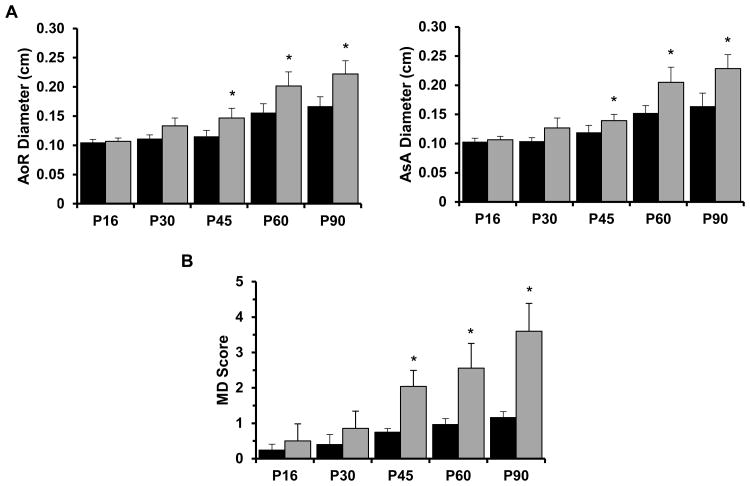
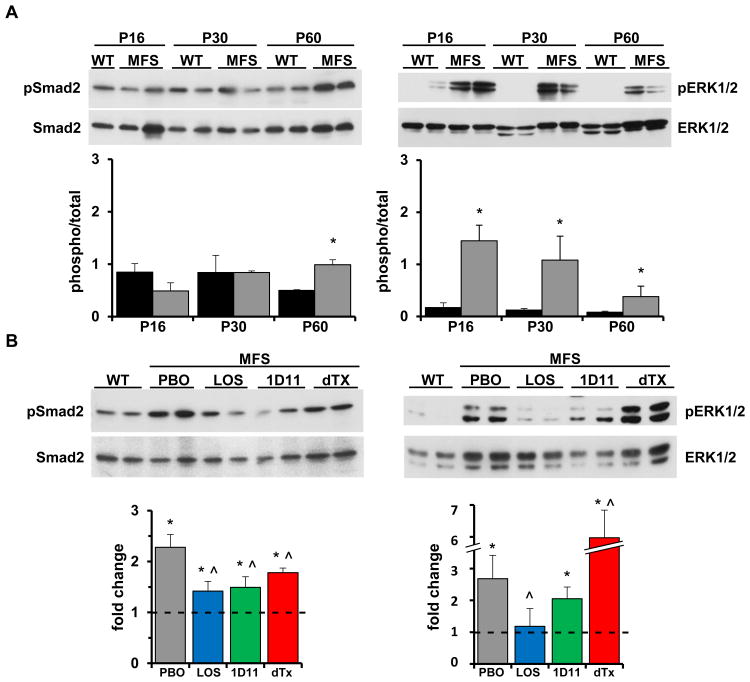
We first established the natural history of TAA formation in Fbn1mgR/mgR mice as the baseline for subsequently comparing the efficacy of different drug treatments. To this end, aneurysm growth, p-Erk1/2 and p-Smad2 accumulation and media degeneration (i.e., elastic fiber fragmentation and aortic wall thickening, histopathological markers of TGFβ-driven tissue proteolysis and fibrosis respectively) were monitored at selected time intervals during the first 3 months of life, when about half of Fbn1mgR/mgR mice die from complications of arterial disease.9 Echocardiographic measurements revealed a statistically significant enlargement of both the root and proximal ascending segment of the mutant aorta beginning at P45 that steadily increased thereafter relative to the WT counterparts (Fig. 1A). Histomorphometric analyses of aortic tissue sections identified P45 as the first time-point in which media degeneration was statistically significant (Fig. 1B). Immunoblot assays of protein extracts from the aortic arches of mutant and WT mice showed substantially higher than normal levels of p-Erk1/2 as early as P16 followed by a steep decline at P60, a stage when abnormal p-Smad2 accumulation in the dilating mutant aorta was first noted to be statistically significant (Fig. 2A). Abnormal activation of Erk and Smad proteins at discrete stages of TAA progression in Fbn1mgR/mgR mice was in line with our previous observation that over-activation of the stress response p-38 MAPK protein preceded abnormal p-Smad2 accumulation in the aortas of Fbn1-/- mice (a validated animal model of neonatal lethal MFS).14 Based on these findings, P60 and P90 were selected as the most informative ages in which to evaluate the effects of the different drug treatments on p-Erk1/2 and p-Smad2 accumulation and on aneurysm growth and media degeneration, respectively.
Figure 1. TAA progression in mice with severe MFS.
(A) Quantified diameters of the aortic root (AoR) and proximal ascending aorta (AsA), and (B) combined scores of wall thickness and elastic lamella integrity (MD) in WT (black) Fbn1mgR/mgR (grey) mice of the indicated ages (n≥ 5 per time-point and genotype); asterisks indicate statistically significant differences between individual sets of WT and MFS samples (p<0.05).
Figure 2. TAA markers in mice with severe MFS at baseline and after drug treatment.
(A) Representative immunoblots of Smad2 and Erk1/2 proteins in WT and Fbn1mgR/mgR (MFS) aortas of the indicated ages with the bar graphs below them summarizing the relative ratios between phosphorylated and total proteins in WT (black) and MFS (grey) samples (n≥5 per genotype and time-point); asterisks indicate statistically significant differences between individual sets of WT and MFS samples (p<0.05). (B) Representative immunoblots of Smad2 and Erk1/2 proteins in the aortas of Fbn1mgR/mgR mice treated as indicated (dTX, double treatment) with the bar graphs below them summarizing the fold change of phosphorylated vs. total proteins in drug-treated mutant specimens relative to WT counterparts (n= 5 per genotype and treatment); WT values arbitrarily expressed as 1 are highlighted by the dotted line across mutant bar graphs. Asterisks and the symbol ((x0005E)) indicate statistically significant differences between MFS and WT samples and between drug- and placebo-treated MFS specimens, respectively (p< 0.05, ANOVA p < 0.001).
AT1r and TGFβ inhibition exert distinct stage-related effects on TAA progression
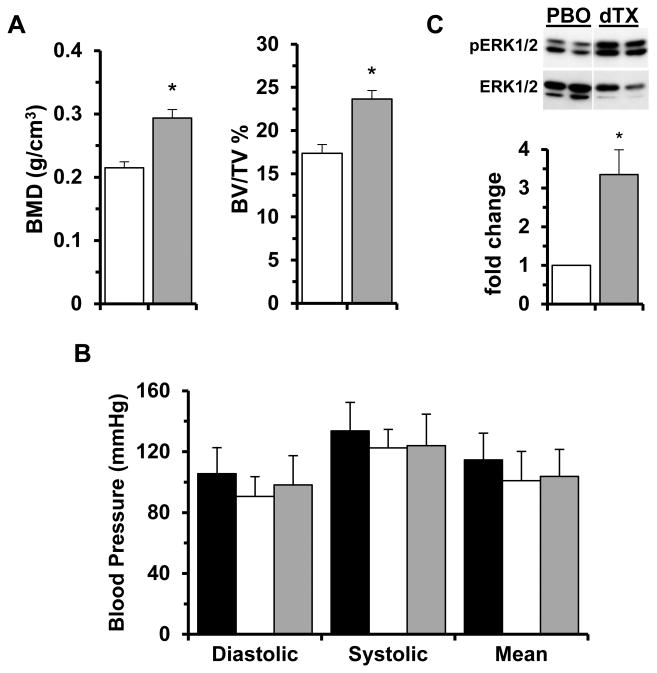
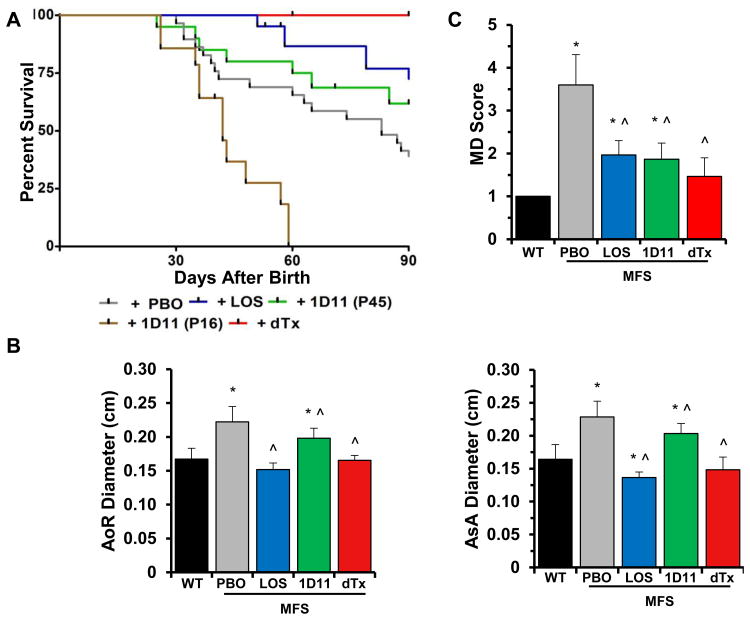
Fbn1mgR/mgR mice were treated with either losartan or TGFβ-Nab for a period of 74 days starting at P16. The mouse monoclonal TGFβ-Nab 1D11 was used in place of the rabbit polyclonal antibody employed in the original study of Fbn1C1039G/+ mice 4 so as to avoid that immunoreactivity to the neutralizing antibody might reduce its efficacy. The dose and dosing regimen of 1D11 was chosen to maximal efficacy for the neutralization of circulating TGFβ in mice, a value previously estimated to be ∼115 ng/ml in Fbn1C1039G/+ mice.15 Studies conducted in murine models of TGFβ-mediated disease have demonstrated that a subcutaneous dose of 5 mg/kg administered 3 times per week is highly efficacious.16,17 We therefore used a 2-fold higher dose (10 mg/kg) thrice per week so as to assure maximal efficacy. At such a dose and dosing regimen, the steady-state plasma levels of 1D11 were estimated to range between 400 and 200 μg/ml.16 Since the Ki for 1D11 neutralization of TGFβ1 in vitro is 0.3 μg/ml,16 the circulating levels of 1D11 in the current study were estimated to range between 1200- and 800-fold over the Ki for 1D11. In accordance with these considerations and the reported 1D11-induced modifications of osteoblast and osteoclast activities,18-20 our treatment protocol led to a statistically significant increase of bone mass and quality in both WT and Fbn1mgR/mgR mice (Fig. 3A and data not shown). To ensure that modifications of TAA pathology in 1D11-treated Fbn1mgR/mgR mice were not a consequence of vasopressor qualities of TGFβ inhibition, we also measured blood pressure in WT mice treated with 1D11 and observed no detectable effects (Fig. 3B). Survival curves of Fbn1mgR/mgR mice in the various placebo treatments (n≥11 per treatment) were grouped together (total n=54) since the distinct placebo arms behaved in nearly identical manners (Fig. 4A). As reported previously,11,12 systemic administration of losartan from P16 onward significantly improved the survival of Fbn1mgR/mgR mice by attenuating aneurysm growth and to a lesser extent, media degeneration (Fig. 4). Additionally, losartan treatment resulted in greater reduction of p-Erk1/2 than p-Smad2 levels (Fig. 2B). Together these findings suggested a causal relationship between aneurysm growth and AT1r-induced p-Erk1/2 accumulation, as well as a partial contribution of AT1r to Smad2 over-activation.
Figure 3. Effects of drug treatments in WT mice.
(A) Bone mineral density (BMD) and bone volume over total volume (BV/TV) in 3-month-old WT mice treated with either placebo (PBO; white bar) or 1D11 (grey bar) from P45 onward (n=5 per treatment; p<0.05). (B) Diastolic, systolic and mean blood pressure measured in 4-week-old WT mice at baseline (black) and treated with either placebo (white) or 1D11 (grey) (n = 10 per treatment; p<0.05). (C) Representative Erk1/2 immunoblots of whole protein extracts from the aortic arches of 2-month-old WT mice treated with either placebo (PBO) or losartan and 1D11 (dTx) with the bar graphs (white and grey, respectively) below summarizing the fold changes of phosphorylated vs. total proteins in the two samples (n= 5 per treatment). Asterisks indicate statistically significant differences between individual samples from drug- and placebo-treated WT mice (p<0.05).
Figure 4. Treatment-induced TAA modifications in MFS mice.
(A) Kaplan-Meier survival curves of MFS mice under indicated drug treatments (n≤15 per treatment); differences between individual experimental and control samples were all statistically significant (p < 0.05). (B) Diameters of the aortic root (AoR) and proximal ascending aorta (AsA), and (C) severity scores of media degeneration (MD) in 3-month-old WT and MFS mice treated as indicated (n≤ 5 per genotype and treatment); asterisks and the symbol (*) indicate statistically significant differences between MFS and WT mice and between drug- and placebo-treated mutant mice (p<0.05, ANOVA p<0.01). The severity score of MD in WT aortas is arbitrarily expressed as 1.
In contrast to losartan, administration of 1D11 from P16 onward exacerbated arterial disease in Fbn1mgR/mgR mice thereby causing death from ruptured TAA significantly earlier than in placebo-treated mutant mice (Fig. 4A). Echocardiographic and histomorphometric analyses of 1D11-treated Fbn1mgR/mgR mice that survived to P45 suggested an accelerated TAA progression, as evidenced by greater aneurysm growth and medial degeneration relative to placebo-treated mutant animals (Fig. 5). Since TGFβ neutralization had no negative effects on either aortic tissue morphology or fitness of WT littermates, we concluded that baseline TGFβ signaling is necessary to stabilize the fibrillin-1 deficient vessel under recurring hemodynamic load during the early stages of aneurysm formation. Furthermore, the beneficial effect of losartan treatment on TAA formation implied that the protective function of TGFβ is exerted independently of AT1r activity.
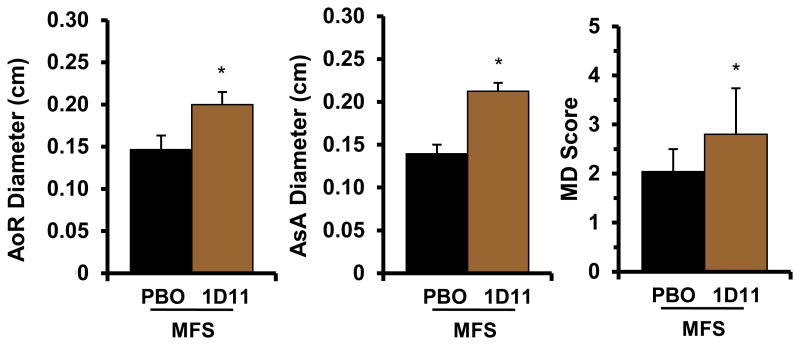
Figure 5. Phenotypic consequences of early TGFβ neutralization in MFS mice.
Quantified diameters of the aortic root (AoR) and ascending aorta (AsA) and severity scores of media degeneration (MD) in 45-day-old Fbn1mgR/mgR mice treated with placebo (black) or 1D11 (brown) from P16 onward (n ≥ 5 per treatment); asterisks indicate statistically significant differences between drug- and placebo-treated mutant mice (p<0.05)
Next, 1D11 treatment was initiated at a stage (P45) when significant TAA pathology was first detected (Fig. 1), prior to significant p-Smad2 accumulation (Fig. 2A) and after stabilization of systemic pressure and cardiac output.21 In contrast to the P16-initiated treatment, TGFβ neutralization started at P45 had a statistically significant positive impact on the survival of Fbn1mgR/mgR mice by slowing down disease progression from that time point onward (Fig. 4A). This result implied that elevated TGFβ signaling plays a detrimental role at later stages of TAA formation. Two important differences were noted between P16-initiated treatment with losartan and P45-initiated treatment with 1D11. Whereas AT1r antagonism mostly restricted aneurysm growth, TGFβ neutralization largely delayed media degeneration (Fig. 4B and C); and whereas losartan normalized p-Erk1/2 levels, 1D11 reduced them by only ∼25% relative to placebo-treated mutant animals (Fig. 2B). We interpreted these differences as additional evidence that aneurysm growth is predominantly dependent on Erk-mediated AT1r signaling (as opposed to Erk-mediated TGFβ signaling) 10 and that AT1r activity is partially (as opposed to solely) 4 responsible for TGFβ-driven media degeneration in MFS mice.
Combined AT1r and TGFβ antagonism prevents TAA formation and death of MFS mice
In light of the above findings, we decided to treat Fbn1mgR/mgR mice with losartan from P16 onward together with 1D11 from P45 onward. This combined drug treatment normalized both aneurysm growth and medial degeneration (Fig. 4B and C), thereby dramatically improving the survival of Fbn1mgR/mgR mice relative to either the P16-initiated losartan treatment or the P45-inititated 1D11 treatment (Fig. 4A). Indeed, only one of the sixteen Fbn1mgR/mgR mice subjected to the double drug treatment died prematurely and from TAA-unrelated complications. The greater therapeutic benefit of combining losartan and 1D11 vs. administering losartan alone indicated that additional AT1r-independent signaling pathways participate in augmenting TGFβ activity (Fig. 4). Along the same lines, paradoxical Erk1/2 hyperactivity in the aorta of Fbn1mgR/mgR mice treated with both agents suggested compensatory activation of other signaling pathways (either irrelevant or supportive of normal function) in the absence of both AT1r and TGFβ signaling (Fig. 2B). The finding that p-Erk1/2 levels were also significantly elevated in WT mice treated with the two drugs indirectly supported this conclusion (Fig. 3C). Irrespective of this last point, the remarkable therapeutic benefit of the combined drug treatment validated the notion that promiscuous AT1r and TGFβ signaling drive partially overlapping disease processes in the aorta of mice with severe MFS.
Discussion
While there is an emerging consensus that altered aortic mechanobiology represents the common trigger of inherited TAAs,22 the identity of and interactions among downstream signaling pathways that connect different mutant genes to tissue dysfunction remain poorly defined. In the case of MFS, it is widely believed that TGFβ hyperactivity is the main driver of TAA progression and that AT1r is the principle stimulator of pathogenic non-canonical (Erk-mediated) TGFβ signaling.1 This belief rests on the original observation that either AT1r antagonism or TGFβ neutralization prevented aneurysm growth and medial degeneration in MFS mice that seldom manifest dissection and rupture of the dilating vessel wall.4,10 As subsequent investigations have raised several questions about different key aspects of this disease model,3 we evaluated how inhibition of either AT1r or TGFβ activity influences TAA progression in mice with lethal MFS. This first head-to-head comparison of the two drug regimens was performed by monitoring the same disease markers as in the original study of Fbn1C1039G/+ mice, 4 and with the additional advantage of including mouse survival as a more informative clinical end-point of TAA progression. Our major finding is that, contrary to the current view, promiscuous AT1r and TGFβ activity in the MFS aorta are principally responsible for driving aneurysm growth and media degeneration through p-Erk1/2 and p-Smad2/3 signaling, respectively. We also found that TGFβ exerts opposite contextual effects on TAA pathology that broadly correlate with the early and late stages of disease progression. Both of these findings have important implications for TAA treatment in MFS.
Possible explanations for the discrepancy between our and earlier results of systemic TGFβ neutralization with Fbn1C1039G/+ mice include the potentially greater neutralizing activity of monoclonal antibody 1D11 and the different experimental designs and MFS models employed in the two studies. Ongoing dosing experiments with Fbn1mgR/mgR mice seem to exclude the possibility that differences in the amount and frequency of TGFβ-Nab administration played a major role in causing such dramatically diverse vascular phenotypes. Similarly, the alternative explanation of differences between the two mouse models of MFS is not supported by ongoing investigations indicating that chronic (7-month-long) treatment of Fbn1C1039G/+ mice with 1D11 (administered at a dose comparable to that of the present study and to mutant mice of the same genetic background as in the original report) 4 is associated with an appreciable trend towards disrupting (rather than preserving) aortic tissue architecture (data not shown). We therefore argue that the observed discrepancy is more likely to reflect the stratification of additional TAA-related events in mice with progressively severe MFS relative to those afflicted with a milder form of the disease. In support of this postulate, we note that losartan treatment was recently shown to decrease the rate of aneurysm growth in a large cohort of adult MFS patients without modifying critical end-points of disease, such as vessel wall dissection. 23 Along the same lines, a randomized clinical trial of MFS children and young adults has subsequently demonstrated no significant differences in the rate of aortic root dilation between patient groups subjected to either losartan or the β-blocker atenolol monotherapy. 24
The dimorphic effects of TGFβ activity during TAA formation are yet another example of the contextual activity of these multifunctional signaling molecules in several physiological and disease processes.25 We believe that the beneficial role of TGFβ signaling during the early stage of TAA formation in MFS mice reflects the physiological response of a structurally compromised aortic wall to increasing hemodynamic stress. We rest our belief on the finding that P16-initiated TGFβ neutralization had a negative impact on TAA formation in Fbn1mgR/mgR but not in WT mice. Accordingly, we propose that the initial consequence of fibrillin-1 deficiency is to impair rather than enhance TGFβ signaling, as currently thought.1,4,10 The pathogenic action of TGFβ hyperactivity in advanced stages of TAA is conceivably accounted for by the establishment of a negative TGFβ-centered feedback loop that is likely to involve, among others, the immuno-inflammatory response to locally generated signals of ECM remodeling/repair.13 By documenting the greater therapeutic efficacy of the combined losartan/1D11 treatment vs. losartan alone, our analyses indicate that deleterious TGFβ signaling late in TAA formation is only partially dependent on AT1r action. Overall, our data demonstrate that TAA pathogenesis in MFS is significantly more complex than currently thought.1,4,10 Indeed, the paradoxical finding of Erk1/2 over-activation in doubly treated MFS mice exemplifies the complex dynamics of molecular interactions among pathogenic, productive, and correlative signaling events in response to chronic wall stress and pharmacological interventions.
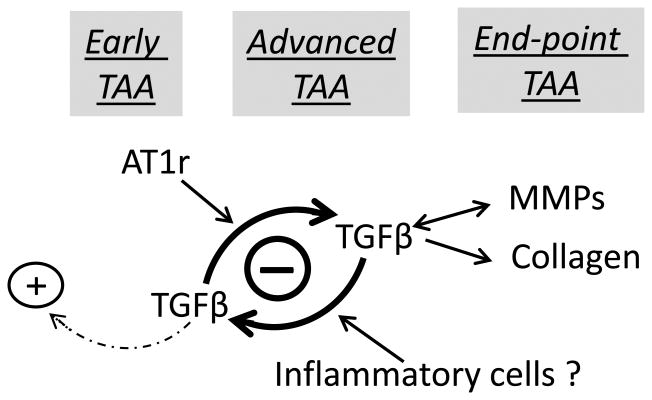
In closing, we propose a revised model of TAA pathogenesis in MFS that is based on the data presented here and related findings in the literature (Fig. 6).2, 3, 23 A fibrillin-1 deficient ECM triggers and communicates to resident cells signals of mechanical stress that are translated into biochemical responses aimed at maintaining physiological tone. Among others, the early vessel response to mechanical stress includes constitutive AT1r over-activation and protective TGFβ signaling. As arterial dysfunction exacerbates under recurring mechanical stress, promiscuous TGFβ signaling becomes the major driver of medial degeneration stimulated in part by constitutive AT1r activity, inflammatory cells and tissue remodeling enzymes. Together with TGFβ-induced collagen over-accumulation, this unopposed degenerative process further impairs aortic integrity and mechanics thereby leading to dissection and rupture of the vessel wall. Our model provides a unifying understanding of how genetically distinct lesions may promote TAA formation, as mutations in cytoskeletal proteins impair SMC contractility in response to hemodynamic load and mutations in components of the TGFβ signaling cascade are expected to perturb the development of a mechanically compliant aortic wall. 1, 2, 22 In this view, TGFβ hyperactivity activity in the MFS aorta represents a marker of unproductive tissue repair rather than the initial trigger of arterial disease.22 Our model also argues for a combinatorial treatment strategy against TAA that would target both promiscuous AT1r and TGFβ signaling without interfering with the early protective role of TGFβ activity in the structurally compromised aorta of MFS patients. However, a major obstacle against implementing such a promising treatment strategy is the lack of informative biomarkers of TAA severity that could rigorously determine the appropriate timing of TGFβ neutralization. Ongoing experiments are investigating new drug treatments that may solely target the detrimental action of TGFβ hyperactivity in the MFS aorta.
Figure 6. Proposed new model of TAA pathogenesis in MFS.
Schematic representation of the progressive stratification of signaling events driving arterial disease during the early, advanced and end-point stages of TAA in mice with severe MFS. The model focuses on AT1R and TGFβ signaling and assumes that recurring hemodynamic load is the primary trigger of aorta dysfunction. The (+) and (-) symbols respectively highlight the protective and detrimental roles of baseline and overactive TGFβ signaling; the dotted arrow signifies the unproductive response of baseline TGFβ signaling to wall stress; and the double-headed arrow indicate the reciprocal pathogenic relationship between TGFβ and MMP hyperactivity.
Supplementary Material
Significance.
Aortic aneurysms are common pathologies often associated with tear (dissection) and rupture of the vessel wall. Characterization of mouse models of TAA and AAA has identified AT1r as a common primary trigger of arterial disease, in addition to raising a controversy regarding TGFβ's role in aneurysm progression and its strict dependence on AT1r activity. Our report addresses these two key aspects of TAA pathogenesis by comparing the impact of AT1r and TGFβ inhibition on TAA formation in a validated mouse model of early onset, progressively severe MFS. The results of these pharmacological interventions demonstrate that TGFβ signaling exerts stage-specific dimorphic effects on arterial disease progression that are largely independent of AT1r action. Consistent with these observations, we documented the superior benefit of combining anti-AT1r and anti-TGFβ therapies over subjecting MFS mice to either drug regimen alone. These findings have important implications for TAA therapy in MFS patients.
Acknowledgments
We thank Y. Lu, L. Phillips and W. Zhang for assisting in statistical and histopathological analyses and K. Johnson for organizing the manuscript.
Sources of Funding: This work was supported by the National Institutes of Health (NIH) grants P01-AR49698 (F. Ramirez) and T32GM007280 (J. Cook) and funds from the National Marfan Foundation and the Elster Family Research Endowment (F. Ramirez).
Nonstandard Abbreviations and Acronyms
- AT1r
Angiotensin II type I receptor
- ECM
Extracellular matrix
- MFS
Marfan syndrome
- SMC
Smooth muscle cell
- TAA
Thoracic aortic aneurysm
- TGFβ-Nab
TGFβ-neutralizing ntibody
- Tgfbr2
TGFβ receptor II
- WT
Wild-type
Footnotes
Disclosures: Genzyme Corporation manufactured the monoclonal antibodies 1D11 and 13C4.
References
- 1.Lindsay ME, Dietz HC. Lessons on the pathogenesis of aneurysm from heritable conditions. Nature. 2011;473:308–316. doi: 10.1038/nature10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Milewicz DM, Guo DC, Tran-Fadulu V, Lafont AL, Papke CL, Inamoto S, Kwartler CS, Pannu H. Genetic basis of thoracic aneurysms and dissections: focus on smooth muscle cell contractile dysfunction. Annu Rev Genomics Hum Genet. 2008;9:283–302. doi: 10.1146/annurev.genom.8.080706.092303. [DOI] [PubMed] [Google Scholar]
- 3.Chen X, Lu H, Rateri DL, Cassis LA, Daugherty A. Conundrum of angiotensin II and TGF-β interactions in aortic aneurysms. Curr Opin Pharmacol. 2013;13:1–6. doi: 10.1016/j.coph.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Habashi JP, Judge DP, Holm TM, et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312:117–121. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dai J, Losy F, Guinault AM, Pages C, Anegon I, Desgranges P, Becquemin JP, Allaire E. Overexpression of transforming growth factor-β1 stabilizes already-formed aortic aneurysms: a first approach to induction of functional healing by endovascular gene therapy. Circulation. 2005;112:1008–1015. doi: 10.1161/CIRCULATIONAHA.104.523357. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Ait-Oufella H, Herbin O, Bonnin P, Ramkhelawon B, Taleb S, Huang J, Offenstadt G, Combadiere C, Renia L, Johnson JL, Tharaux PL, Tedgui A, Mallat Z. TGF-β activity protects against inflammatory aortic aneurysm progression and complications in angiotensin II-infused mice. J Clin Invest. 2010;120:422–432. doi: 10.1172/JCI38136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Judge DP, Biery NJ, Keene DR, Geubtner J, Myers L, Huso DL, Sakai LY, Dietz HC. Evidence for a critical contribution of haploinsufficiency in the complex pathogenesis of Marfan syndrome. J Clin Invest. 2004;114:172–181. doi: 10.1172/JCI20641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung AWY, Yang HHC, Breemen van Breemen C. Imbalanced synthesis of cyclooxygenase-derived thromboxane A2 and prostacyclin compromises vasomotor function of the thoracic aorta in Marfan syndrome. Br J Pharmacol. 2007;152:305–312. doi: 10.1038/sj.bjp.0707391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pereira L, Lee SY, Gayraud B, Andrikopoulos K, Shapiro SD, Bunton T, Jensen Biery N, Dietz HC, Sakai LY, Ramirez F. Pathogenetic sequence for aneurysm revealed in mice underexpressing fibrillin-1. Proc Natl Acad Sci USA. 1999;96:3819–3823. doi: 10.1073/pnas.96.7.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holm TM, Habashi JP, Doyle JJ, et al. Noncanonical TGFβ signaling contributes to aortic aneurysm progression in Marfan syndrome mice. Science. 2011;332:358–361. doi: 10.1126/science.1192149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nistala H, Lee-Arteaga S, Carta L, Cook JR, Smaldone S, Siciliano G, Rifkin AN, Dietz HC, Rifkin DB, Ramirez F. Differential effects of alendronate and losartan therapy on osteopenia and aortic aneurysm in mice with severe Marfan syndrome. Hum Mol Genet. 2010;19:4790–4798. doi: 10.1093/hmg/ddq409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiong W, Meisinger T, Knispel R, Worth JM, Baxter BT. MMP-2 regulates Erk1/2 phosphorylation and aortic dilation in Marfan syndrome. Circ Res. 2012;110:e92–e101. doi: 10.1161/CIRCRESAHA.112.268268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li W, Li Q, Jiao Y, Qin L, Ali R, Zhou J, Ferruzzi J, Kim RW, Geirsson A, Dietz HC, Offermanns S, Humphrey JD, Tellides G. Tgfbr2 disruption in postnatal smooth muscle impairs aortic wall homeostasis. J Clin Invest. 2014;124:755–767. doi: 10.1172/JCI69942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carta L, Smaldone S, Zilberberg L, Loch D, Dietz HC, Rifkin DB, Ramirez F. p38 MAPK is an early determinant of promiscuous Smad2/3 signaling in the aortas of fibrillin-1 (Fbn1)-null mice. J Biol Chem. 2009;284:5630–5636. doi: 10.1074/jbc.M806962200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matt P, Schoenhoff F, Habashi J, Holm T, Van Erp C, Loch D, Carlson OD, Griswold BF, Fu Q, De Backer J, Loeys B, Huso DL, McDonnell NB, Van Eyk JE, Dietz HC, GenTAC Consortium Circulating transforming growth factor-β in Marfan syndrome. Circulation. 2009;120:526–532. doi: 10.1161/CIRCULATIONAHA.108.841981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson CA, Hunter RB, Quigley LA, Girgenrath S, Weber WD, McCullough JA, Dinardo CJ, Keefe KA, Ceci L, Clayton NP, McVie-Wylie A, Cheng SH, Leonard JP, Wentworth BM. Inhibiting TGF-β activity improves respiratory function in mdx mice. Amer J Path. 2011;178:2611–2621. doi: 10.1016/j.ajpath.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu S, Song W, Boulanger JH, et al. Role of TGF-β in a mouse model of high turnover renal osteodystrophy. J Bone Min Res. 2014;29:1141–1157. doi: 10.1002/jbmr.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edwards JR, Nyman JS, Lwin ST, Moore MM, Esparaza J, O'Quinn EC, Hart AJ, Biswas S, Patil CA, Lonning S, Mahadevan-Jansen A, Mundy GR. Inhibition of TGF-β signaling by 1D11 antibody treatment increases bone mass and quality in in vivo. J Bone Miner Res. 2010;25:2419–2426. doi: 10.1002/jbmr.139. [DOI] [PubMed] [Google Scholar]
- 19.Biswas S, Nyman JS, Alvarez J, Chakrabarti A, Ayres A, Sterling J, Edwards J, Rana T, Johnson R, Perrien DS, Lonning S, Shyr Y, Matrisian LM, Mundy GR. Anti-transforming growth Factor β antibody treatment rescues bone loss and prevents breast cancer metastasis to bone. PLos One. 2011;6:e27090. doi: 10.1371/journal.pone.0027090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grafe I, Yang T, Alexander S, et al. Excessive transforming growth factor-β signaling is a common mechanism in osteogenesis imperfects. Nat Med. 2014;20:670–675. doi: 10.1038/nm.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wagenseil JE, Mecham RP. Vascular extracellular matrix and arterial mechanics. Physiol Rev. 2009;89:957–989. doi: 10.1152/physrev.00041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Humphrey JD, Milewicz DM, Tellides G, Schwartz MA. Cell Biology. Dysfunctional mechanosensing in aneurysms Science. 2014;244:477–479. doi: 10.1126/science.1253026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Groenink M, den Hartog AW, Franken R, Radonic T, de Waard V, Timmermans J, Scholte AJ, van Den Berg MP, Spijkerboer AM, Marquering HA, Zwinderman AH, Mulder BJ. Losartan reduces aortic dilation rate in adults with Marfan syndrome: a randomized controlled trial. Eur Heart J. 2013;34:3491–3500. doi: 10.1093/eurheartj/eht334. [DOI] [PubMed] [Google Scholar]
- 24.Lacro RV, Dietz HC, Sleeper LA, et al. Atenolol versus losartan in children and young adults with Marfan syndrome. N Engl J Med. 2014;371:2061–2071. doi: 10.1056/NEJMoa1404731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Massague' J. TGFβ signaling in context. Nat Rev Mol Cell Biol. 2012;13:616–630. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.