SUMMARY
Organisms with targeted genomic modifications are efficiently produced by gene editing in embryos using CRISPR/Cas9 RNA-guided DNA endonuclease. Here, to facilitate germline editing in rats, we used CRISPR/Cas9 to catalyze targeted genomic mutations in rat spermatogonial stem cell cultures. CRISPR/Cas9-modified spermatogonia regenerated spermatogenesis and displayed long-term sperm forming potential following transplantation into rat testes. Targeted germline mutations in Epsti1 and Erbb3 were vertically transmitted from recipients to exclusively generate “pure”, non-mosaic mutant progeny. Epsti1 mutant rats were produced with or without genetically selecting donor spermatogonia. Monoclonal enrichment of Erbb3-null germlines unmasked recessive spermatogenesis defects in culture that were buffered in recipients, yielding mutant progeny isogenic at targeted alleles. Thus, spermatogonial gene editing with CRISPR/Cas9 provided a platform to generate targeted germline mutations in rats, and to study spermatogenesis.
Keywords: germline editing, CRISPR/CAS9, spermatogonial stem cells, germline stem cells, mosaicism, gene targeting, gene editing, recessive screen, spermatogenesis, Epsti1, Erbb3
INTRODUCTION
CRISPR/Cas9 RNA-guided DNA endonuclease technology is being widely utilized to generate targeted genomic mutations in diverse cell types and organisms to study their biological processes (Harrison et al., 2014). Gene editing with CRISPR/Cas9 in mammals yields high rates of donor embryo-derived progeny harboring targeted gene mutations in rodents, pigs, goats and monkeys (Hai et al., 2014; Li et al., 2013; Ni et al., 2014; Niu et al., 2014; Wang et al., 2013; Yang et al., 2013). Mutant rodents can be produced in <1 month upon injection of constructs expressing gRNAs and Cas9 that direct cleavage of target gene sequences in donor zygotes (Li et al., 2013; Wang et al., 2013; Yang et al., 2013). CRISPR/Cas9 is so efficient, both alleles for multiple target genes can be disrupted in animals produced by co-injecting zygotes with respective gRNAs (Li et al., 2014; Ma et al., 2014; Ni et al., 2014; Wang et al., 2013; Yang et al., 2013).
CRISPR/Cas9 also efficiently catalyzes target allele mosaicism in animals, which reflects independent gene editing events made during early embryonic cleavage stages as the totipotent zygote undergoes pre-implantation development (Yen et al., 2014). CRISPR/Cas9 is typically delivered into mammalian zygotes on E0.5 to E1, which is ~5–6 days before the germline is specified in rodents. As an example, the mouse germline is specified within a small population of 10–20 proximal epiblast cells between E6 to E6.5 (Ohinata et al., 2005). Taken together, this explains why CRISPR/Cas9-target allele mosaicism is observed in tissues of first generation mutant animals, and why targeted alleles in somatic tissues can differ from those specified in the germline. Target allele heterogeneity in mosaic animals must be outcrossed to generate colonies of pure, non-mosaic germline mutants isogenic for a given targeted allele in all cells of their body (Jaenisch et al., 1981; Soriano and Jaenisch, 1986; Wilkie et al., 1986). Sorting out new mutant strains by experimentally outcrossing allelic mosaicism takes months in rodents, but can require years in some species due to longer life cycles and/or low fecundity (Niu et al., 2014; Pursel et al., 1989).
Target allele mosaicism is also generated when host embryos are reconstituted with pluripotent stem cells genetically modified using CRISPR/Cas9 (Wang et al., 2013). As with the early embryo, CRISPR/Cas9 holds potential to distinctly modify multiple target alleles within a stem cell clone as it divides (Wang et al., 2013). In addition to this variation, reconstitution of wildtype blastocysts with pluripotent donor cells further generates “chimeric” animals with organ systems and germlines developing from mixtures of host and donor embryonic cells (Tarkowski, 1961). Mosaicism and chimerism was substantially reduced when clonally enriched donor cells modified with CRISPR/Cas9 were used to produce pigs and goats by somatic cell nuclear transfer into enucleated oocytes (i.e. cloning) (Ni et al., 2014). Reconstituting tetraploid embryos with pre-screened, clonally expanded pluripotent lines would also be predicted to minimize mosaicism and chimerism in epiblast-derived tissues (Nagy et al., 1990). Alternatively, direct germline editing in donor spermatogonial stem cells would avoid both the totipotent and pluripotent states of embryogenesis (Brinster and Avarbock, 1994), thus eliminating production of mosaic/chimeric mutant progeny. Here, we report efficient production of mutant rats by spermatogonial gene editing with CRISPR/Cas9.
RESULTS
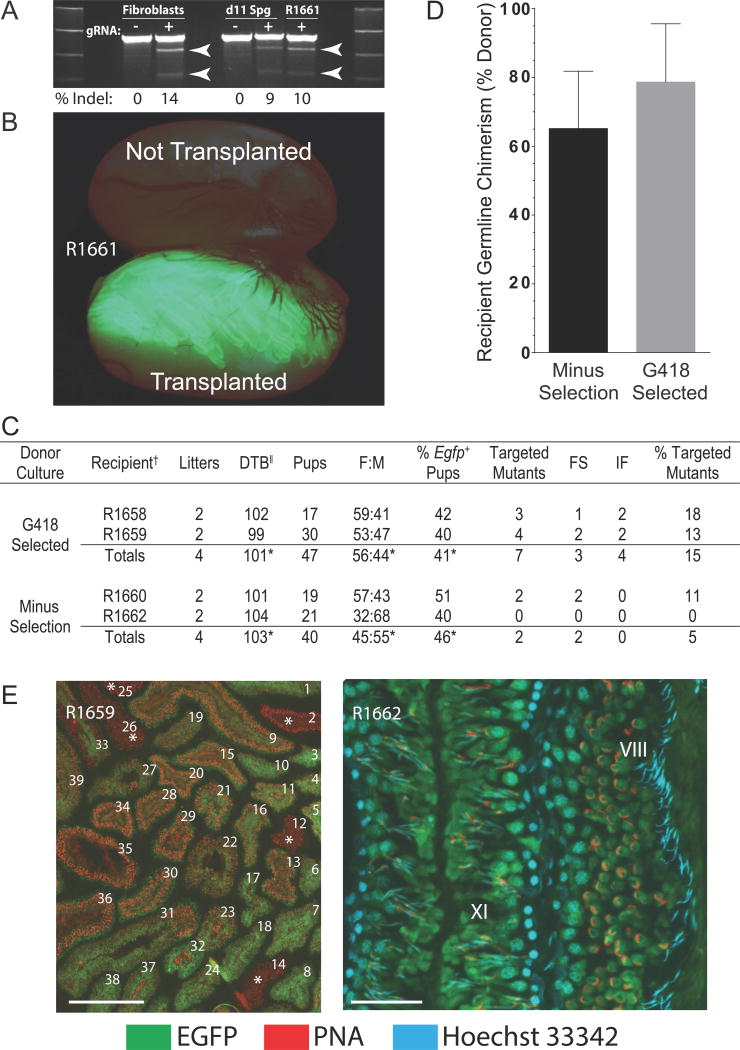
Spermatogonial gene editing at Epsti1 loci was conducted using CRISPR/Cas9 as proof-of-concept for efficient gene targeting in rats. Epsti1 encodes Epithelial Stromal Interaction, and Epsti1 variants in men are associated with alterations in sperm function and family size (Kosova et al., 2012). Guide RNAs demonstrating highest specificity to exon 2 of rat Epsti1 in silico were first tested for activity in rat fibroblast cultures (Figure 1A). A plasmid encoding an effective Epsti1 gRNA and Cas9 (pgEpsti1-300) was transfected (Neon electroporation) into rat spermatogonia hemizygous for a germline-specific Egfp marker (Cronkhite et al., 2005), with and without a second plasmid encoding a selectable marker (pNeoΔtk) (Hamra et al., 2005). Spermatogonia from each transfection, with and without pNeoΔtk were plated onto fibroblast feeder layers and selected with and without G418 in growth medium (d3 to d9). Spermatogonia were then sub-cultured for 2 passages (d11 and d23) (Chapman et al., 2011) and transplanted into rat seminiferous tubules (d34) (Figure 1B). Predicted cleavage of targeted Epsti1 alleles in donor germlines by the Surveyor assay was similar in spermatogonial cultures harvested at d11 post-transfection (~9% Indels) and in the total population of flow sorted EGFP+ spermatogenic cells produced in recipient R1661 by d56 post-transplantation (~10% Indels) (Figure 1A). This provided evidence that CRISPR/Cas9-dependent modifications to Epsti1 were maintained in stem cells during sub-culture and persisted during spermatogenesis in recipient rats.
Figure 1. CRISPR/Cas9-Mediated Gene Targeting in Donor Stem Spermatogonia.
(A) CRISPR/Cas9 cleavage of Epsti1 in rat Fibroblast and Spermatogonial (Sg d11) cultures on d11 post-transfection, and in flow sorted EGFP+ spermatogenic cells (R1661) on d56 after transplantation into the right testis of recipient R1661: transfection without (−) and with (+) Epsti1 gRNA. Arrows: predicted ~461 bp + ~226 bp Surveyor products.
(B) Spermatogenesis (green fluorescence) in testis of R1661 on d56 after transplantation that developed from donor EGFP+ rat spermatogonial cultures containing CRISPR/Cas9- catalyzed Epsti1 mutations.
(C) Targeted mutagenesis of Epsti1 exon 2 in rats by CRISPR/Cas9 spermatogonial gene editing. Donor spermatogonia were hemizygous for tgGCS-EGFP. FS, frame shift mutation; IF, mutation in frame, DTB, days from transplantation to first litter containing Epsti1 mutant animals. *Average value, †R(n), Recipient identifier, ||Litters born 99 to 128 d post-transplantation, % of total F1 progeny/recipient. See also Figure S1.
(D) Donor germline chimerism (%) in wildtype recipient rat testes. Ratio of seminiferous tubules containing EGFP+ elongating spermatids/total tubules containing elongating spermatids plotted/donor culture condition (Minus Selection and G418 Selected). See also Table S1 and Table S2.
(E) Acrosome (PNA) & nuclear (Hoechst 33342) labeling marking donor-derived elongating spermatids (EGFP+) in tubule cross sections from recipient R1659. Left, 39 of 39 tubules contained elongating spermatids (EGFP+ or EGFP−). Five EGFP− tubules are marked with an asterisk. Scale 500 μm. Right, higher magnification image of donor spermatogenesis at stages VIII and XI in the rat seminiferous epithelial cycle [See: (Hess, 1990)]. Scale 100 μm. See also Figure S2.
Based on robust colonization by stably modified donor spermatogonia in R1661 (Figure 1A and B), four remaining recipient rats (n=2 transplanted with G418 selected cells; n=2 transplanted with unselected cells) were paired with wildtype females at ~65 days post-transplantation until each pair produced 2 litters (n=8 total litters) (Figure 1C). F1 progeny harbored ~10% total Epsti1 mutants (n=9/87 F1 pups) within an estimated 87% donor derived progeny (38 EGFP+/87 F1 pups = 43.6% × 2; hemizygous marker) (Figure 1C). Spermatogonia selected in G418-containing medium yielded ~3x more mutant progeny than spermatogonia from unselected cultures (5% mutants, Minus Selection; 15% mutants, G418 Selected) (Figure 1C). 56% percent of Epsti1 mutations in progeny were frame shifts (5 of 9) (Figure 1C and S1A). Remaining Epsti1 variants (4 of 9) were in-frame deletions (Figure 1C and S1A). Thus, cultures of CRISPR/Cas9 mutagenized spermatogonial stem cells were successfully applied to produce pure Epsti1 mutant rats ~100 days post-transplantation (101.5±2.1 days; ±SD, n=4 recipients) (Figure 1C).
Histological analysis of recipient testes at d178 post-transplantation revealed robust spermatogenic potential of donor stem cells under each culture condition tested (Figure 1D and E). Most seminiferous tubules in recipients contained EGFP+ elongating spermatids (65.5±14%, Minus Selection; 79±17%, G418 Selected; ±SD, n=4 testes from 2 rats/culture condition, 117–176 tubules scored/testis) (Figure 1D and Table S1). Crosses between recipient male and wildtype female rats produced normal sized litters (10.9±3.4 pups/litter, ±SD, n=8) (Harlan.com) with testes ~half normal weight (Table S2), but ~3 times heavier than reported for non-transplanted, busulfan-treated testes (Hamra et al., 2002). EGFP+ germ cells flow sorted from recipient rat testes (Figure S2) revealed 5% (1/20 amplicons) and 36% (8/22 amplicons) distinct mutant Epsti1 alleles derived from respective unselected and G418-selected spermatogonia (Figure S1B). Thus, intra-recipient donor haplotype frequencies were consistent with germline transmission rates for Epsti1 mutations (Figure 1C and Table S1).
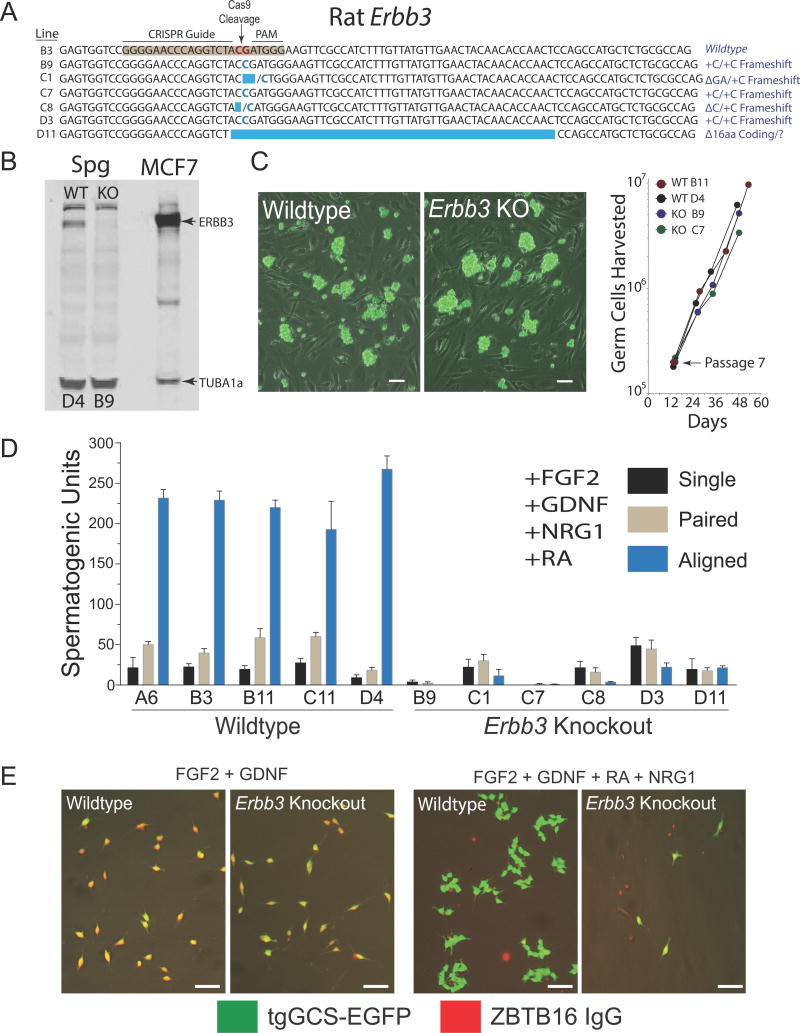
Although non-mosaic mutant rat strains were efficiently generated by spermatogonial gene editing with CRISPR/Cas9, the targeted Epsti1 alleles varied considerably between strains (Figure S1). This appeared as random repair of cleaved template in distinct donor stem cells. Monoclonal enrichment of donor spermatogonial stem cells in culture following genome editing with CRISPR/Cas9 would theoretically reduce variation of targeted alleles transmitted to progeny by recipients. Clonal enrichment of CRISPR/Cas9-modified stem spermatogonia in culture prior to transplantation would also facilitate studying the effects of recessive mutations on spermatogenesis (particularly in cases of embryonic lethality). As an example, Erbb3 is critical for embryogenesis in mice (Erickson et al., 1997), and encodes a receptor tyrosine kinase activated by the polypeptide ligand NRG1 (Carraway et al., 1994). Furthermore, NRG1, GDNF and serum were required for clonal development of differentiating spermatogenic cells in vitro on laminin (Hamra et al., 2007). To define a germline receptor for NRG1 in rats, we analyzed CRISPR/Cas9-targeted Erbb3 mutations in spermatogonial stem cell lines derived from individually picked colonies (Figure 2A).
Figure 2. Monoclonal Enrichment of Erbb3-Deficient Stem Spermatogonia.
(A) CRISPR/Cas9-targeted Erbb3-mutations (exon 2) in clonally expanded rat spermatogonial lines.
(B) Relative abundance of ERBB3 (upper arrowhead) and TUBA1a (lower arrowhead) in wildtype (colony D4; WT) and Erbb3-deficient (colony B9; KO) spermatogonial lines.
(C) Left: Cultures of clonally expanded Wildtype (colony D4) and Erbb3 Knockout (colony B9) spermatogonia expressing tgGCS-EGFP. Scale, 50 μm. Right: Growth rates of clonally expanded Wildtype (WT) and Erbb3 Knockout (KO) spermatogonial lines. See also Figure S3.
(D) NRG1-dependent development of differentiating spermatogenic cells from Wildtype and Erbb3 Knockout spermatogonial lines.
(E) Spermatogenic cells from colonies D4 (Wildtype) and B9 (Erbb3 Knockout) analyzed in panel D. tgGCS-EGFP (green), ZBTB16 antibody (Red). Scale, 40 μm.
Six of 26 picked colonies analyzed (~23%) were enriched with targeted Erbb3 mutations after clonal expansion on fibroblast feeder layers (Figure 2A and Figure S3A). Interestingly, all 6 mutant colonies were classified as harboring isogenic (3 of 6 colonies) or heterogeneous (3 of 6 colonies) biallelic targeted mutations (Figure S3A). Mutant colonies with isogenic targeted alleles (i.e. B9, C7, D3) were initially falsely classified as wildtype germlines by the Surveyor assay (Figure S3B), but subsequently defined as biallelic Erbb3 mutant germlines by sequencing (Figure S3B). This is consistent with the Surveyor assay becoming less accurate at estimating percent modified alleles as target allele variation decreases (Guschin et al., 2010).
Biallelic mutant spermatogonial lines derived from picked colonies were deficient in ERBB3 (Figure 2B) and proliferated at similar rates over multiple passages in culture compared to clonally expanded wildtype lines (Figure 2C). Unlike wildtype lines, 6 of 6 clonally enriched Erbb3-deficient germlines were severely compromised in their ability to support development of ZBTB16− spermatogenic colonies in culture on laminin in a serum-free, spermatogonial differentiation medium supplemented with NRG1, GDNF, FGF2 and all-trans-retinoic acid (Figure 2D, E). All wildtype germlines picked from the same transfection effectively developed into syncytia containing 8–32 differentiating spermatogenic cells negative for ZBTB16 labeling (tgGCS-EGFP+, ZBTB16−; n=5 lines) (Figure 2D, E). ZBTB16 (or PLZF) is a marker for type A spermatogonia in mammalian testes, and is critical for spermatogonial stem cell self-renewal in mice (Buaas et al., 2004; Costoya et al., 2004). In contrast to their in vitro phenotypes, Erbb3-deficient spermatogonial lines (B9, C7) robustly regenerated spermatogenesis in recipient rats when analyzed 2.5–3.5 months post-transplantation (Figure 3A; Figure S4A). Thus, the ability of Erbb3-deficient germlines to regenerate spermatogenesis in recipient testes did not reflect NRG1’s requirement for clonal development of differentiating spermatogenic cells in culture (Figure 2D, E).
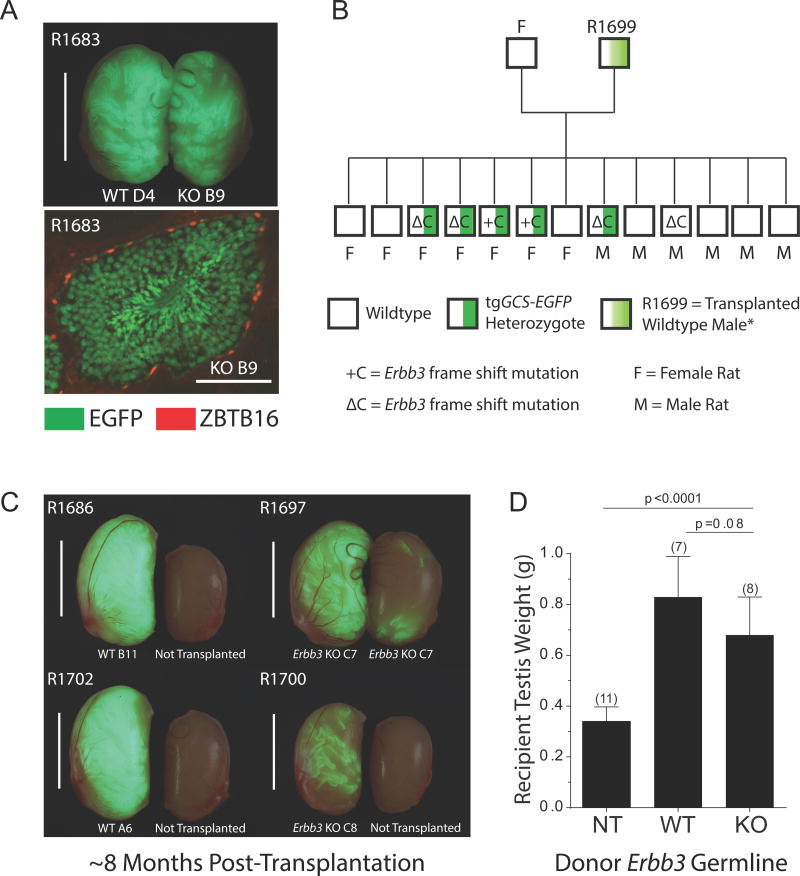
Figure 3. Transmission of Isogenic CRISPR/Cas9-Targeted Alleles to Rat Progeny.
(A) Top: Spermatogenesis (green) derived from WT colony D4 and Erbb3 KO colony B9 in testes from R1683 at ~3.5 mo post-transplantation. Scale, 1 cm. Bottom: Seminiferous tubule cross section from R1683 illustrating spermatogenesis derived from colony B9 marked by EGFP (green). ZBTB16+ spermatogonia (red nuclei). Scale, 100 μm. See also Figure S4.
(B) Genotypes of rat progeny derived from clonally expanded spermatogonial line C8. See also Figure S3. tgGCS-EGFP = hemizygous marker.
(C) Recipient rat testes ~7.8 mo post-transplantation. Clonally expanded lines were transplanted into the right testis of R1686, R1702 and R1700 (EGFP+), but contralateral left testes were not transplanted. Note: right and left testes of R1697 were transplanted. Scale, 1 cm.
(D) Mean testis weights from recipient rats analyzed ~7.8 mo after transplanting with wildtype (WT) spermatogonial lines (D4, n=2; B11, n=3; A6, n=2) and Erbb3-deficient (KO) spermatogonial lines (B9, n=2; C7, n=3; C8, n=3); NT, untransplanted testes, n=11 (error bars, ±SD; p values, multiple t-tests).
To assess the sperm-forming potential of Erbb3-deficient spermatogonial lines longer term, recipients of clonally expanded lines C7 (R1697) and C8 (R1699) were paired with wildtype females at d207 post-transplantation (~6.8 months; both testes transplanted/recipient). Recipient of line C8 was highly enriched with +C and ΔC frame shift mutations (Figure S3A, B), and fathered progeny inheriting each Erbb3 germline mutation (~46% of progeny were +C or ΔC targeted alleles; 6 of 13 pups) (Figure 3B; Figure S3C). All Egfp+ pups (5 of 5) harbored one of the two targeted alleles (Figure 3B; Figure S3C, D), consistent with colony C8 representing a homogeneous biallelic mutant germline (Figure S3A, B). Similarly, recipient of isogenic line C7 (enriched with +C frame shift mutations; Figure S3A, B) fathered a litter of 10 pups, 4 of which inherited the +C mutation in Erbb3 (Figure S3C). Again, all Egfp+ pups (3 of 3) harbored the targeted Erbb3 allele (Figure S3C, D), which would be expected in progeny derived from a monoclonal donor germline harboring two targeted Erbb3 alleles.
Analysis of recipient testes at d239 post-transplantation (7.8 months) revealed donor-derived spermatogenesis from lines C7 and C8 (Figure S4B), but at visibly reduced levels of colonization compared to wildtype germlines (Figure 3C). Neither recipient testis weights (Figure 3D), nor the percent seminiferous tubules containing both EGFP+ type A spermatogonia (ZBTB16+) and spermatids (PNA+) were significantly different between recipients of wildtype and Erbb3 mutant germlines (Figure S4B). Donor-derived spermatids were present in >90% of seminiferous tubule sections colonized by EGFP+ spermatogenic cells, independent of the clonal line transplanted (n=3 wildtype lines; n=3 biallelic mutant lines) (Figure S4B).
DISCUSSION
Here, we demonstrate how CRISPR/Cas9-mediated germline editing in donor spermatogonia can be used to produce “pure”, non-mosaic mutant animals, and to study the effects of gene mutations on spermatogenesis. In doing so, we provide proof-of-concept for targeted mutagenesis directly in fully functional rat donor germlines. This included polyclonal and monoclonal enrichment of rat spermatogonial lines harboring heritable, CRISPR/Cas9-targeted alleles. High colonization efficiency (Hamra et al., 2005; Wu et al., 2009), transfection efficiency and sperm forming potential of donor germlines translated into robust mutant rat production by spermatogonial gene editing with CRISPR/Cas9.
An estimated 15–30% of donor cell-derive progeny was obtained by breeding recipients transplanted with G418-selected donor spermatogonia (200,000/testis) carrying targeted Epsti1 alleles (Figure 1C). Similarly, ~43% of pups (10/23, n=2 litters, 1 litter/donor strain) were derived from monoclonally enriched donor spermatogonial lines (60,000/testis) harboring Erbb3-null mutations (Figure 3B; Figure S3). Actual transmission rates from targeted germlines would be contingent on how mutated alleles effect sperm development or function, and on rates at which biallelic null mutations were generated and maintained per transfected sperm forming spermatogonium. In the two examples reported here, even if Epsti1 or Erbb3 was found essential for male fertility, the phenotypically diploid nature of clonally derived spermatids imparted by ring canals (Braun et al., 1989; Wilkie et al., 1991) would enable spermatogonial stem cells containing at least one functional copy of the targeted allele (wildtype or in-frame mutant) to vertically transmit recessive loss-of-function donor haplotypes.
Here, monoclonally enriched spermatogonial lines harboring biallelic, CRISPR/Cas9-targeted Erbb3-null mutations were also applied to study spermatogenesis during culture in vitro and in recipient rat testes (Figures 2 and 3). Notably, the Erbb3-deficient germlines were analyzed in vitro using highly simplified, serum-free culture media that effectively promoted spermatogonial stem cell renewal (SG Medium) or differentiation (SD Medium) (Figure 2). Unknown factors in recipient testes, which were apparently absent or inactive in vitro, were sufficient to support spermatogonial differentiation on the Erbb3-deficient background. Looking ahead, the ability to “multiplex” with CRISPR/Cas9 holds potential to study such redundant or polygenic processes contributing to genetic robustness of spermatogenesis (Archambeault and Matzuk, 2014). Thus, recessive genetic assays in fully functional rat spermatogonial stem cells were established by these studies, providing an experimental platform to biochemically and genetically define in vitro spermatogenesis-stimulating factors, like NRG1 and ERBB3.
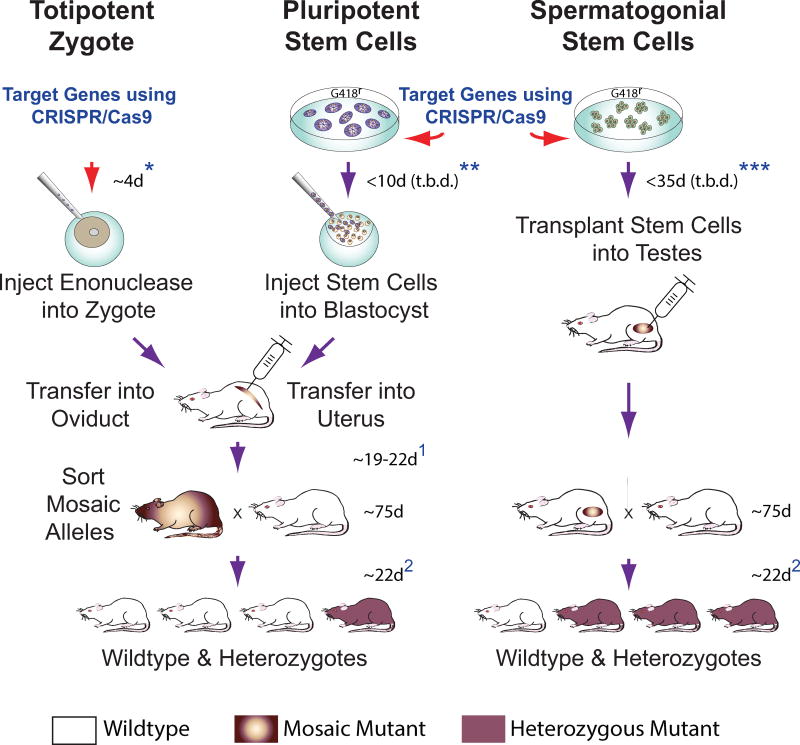
Based on germline transmission rates of targeted Epsti1 alleles we obtained by breeding recipient rats (Figure 1), transplanting spermatogonial stem cells into seminiferous tubules more directly after delivering CRISPR/Cas9 constructs holds clear potential to expedite production of pure mutant rat strains by up to an additional month (Figure 4). Spermatogonial gene editing using CRISPR/Cas9 technology can readily be adopted in rodents on a scalable level (Izsvak et al., 2010; Kanatsu-Shinohara et al., 2005; Nagano et al., 2001a), and could be established to streamline pure mutant animal production in other applied species important for science, industry, conservation and medicine (Arregui et al., 2013; Hermann et al., 2012; Nagano et al., 2001b; Zeng et al., 2013). Indeed, proof-of-concept for correcting genetic disease in mice by spermatogonial gene editing with CRISPR/Cas9 was reported during revision of this manuscript (Wu et al., 2014).
Figure 4. CRISPR/Cas9 Gene Targeting in early Embryos and Spermatogonia.
(Left) Zygotes are injected with CRISPR/Cas9 constructs and transferred into surrogate female oviducts to support donor embryo development into mutant progeny. Mosaic mutant progeny born ~19–22 d post-transfer (depending on cleavage stage transferred)1 display variation in targeted alleles in various tissues, and must be crossed to wildtype stock to generate pure heterozygous mutants isogenic at respective targeted alleles in all cells of their body.
(Center) Rat Pluripotent Stem Cell cultures could prospectively be genetically modified using CRISPR/Cas9. Rat ES cells with targeted mutations have been selected in culture prior to blastocyst injection (Tong et al., 2011). Injected blastocysts are transferred into uteri of surrogate females to produce mosaic/chimeric mutant animals ~19 d post-transfer1. Mosaic/chimeric animals are crossed to wildtype stock to establish pure heterozygous mutants.
(Right) Spermatogonial Stem Cells can be genetically modified in culture using CRISPR/Cas9. Modified spermatogonia are injected into recipient rat testes to produce mutant spermatozoa that transmit targeted genomic modifications to heterozygous mutant progeny. Timelines for each approach listed above must consider ~75 d for rat breeder pairs to reach reproductive age, 21–23 d rat gestation time, plus a 4–5 d estrus cycle in rats (Lohmiller and Swing, 2006)2.
*Includes additional 4 d to establish pseudo-pregnant female recipients by paring with vasectomized males; does not include time needed to prepare vasectomized male rats.
**Remains to be determined (t.b.d) using CRISPR/Cas9; estimate based on rat ES cell lines selected following transfection with classical DNA targeting constructs (Tong et al., 2011); includes additional 4 d to establish pseudo-pregnant female recipients.
***Current study; minimum time required following delivery of CRISPR/CAS9 constructs to spermatogonia (with or without genetic selection) prior to transplantation was not studied and remains to be determined (t.b.d); includes 12 d to prepare recipient males.
EXPERIMENTAL PROCEDURES
Spermatogonial Gene Editing with CRISPR/Cas9
Spermatogonial lines were derived from freshly isolated laminin-binding spermatogonia using individual heterozygous SD-Tg(ROSA-EGFP)2-4Reh rats (Hamra et al., 2005). SD-Tg(ROSA-EGFP)2-4Reh Sprague Dawley rats are referred to as tgGCS-EGFP rats because they exhibit germ cell specific expression of enhanced green fluorescent protein (EGFP) (Cronkhite et al., 2005). Spermatogonial lines were propagated on feeder layers of irradiated mouse embryonic fibroblasts (MEFs) as previously detailed using Spermatogonial Culture (SG) Medium containing 6 ng/ml bFGF (PGF0023, Life Technologies, Inc.) and 6 ng/ml GDNF (512-GF, R&D Systems, Inc.) (Chapman et al., 2011; Wu et al., 2009). To generate Epsti1 mutants, spermatogonia were harvested at passage 8 and co-transfected in suspension with plasmids pNeoΔtk and/or pX330 (Cong et al., 2013) using the Neon Transfection System set for 2 pulses at 1100 V, 20 ms. pX330 co-expressed Cas9 and gRNAs 5′-tgatagcaccgaacgagacc-3′ (pgEpsti1-330; cloned into BbsI sites). pNeoΔtk was generated from parental plasmid, pKO1904 (Stratagene, Inc.) by excising its thymidine kinase cassette, and retaining the neomycin phosphotransferase open reading frame under control of the PGK1 promoter. Cultures not undergoing G418 selection were transfected using 10 μg pgEpsti1-330/106 spermatogonia; cultures undergoing G418 selection were co-transfected using 3 μg pgEpsti1-330 + 7 μg pNeoΔtk/106 spermatogonia. Transfected spermatogonia for each respective condition were plated onto fresh MEFs for 3 days (d3), selected in SG medium containing 0 or 65 μg/ml G418 for 6 days (d9) prior to harvesting and plating on fresh MEFs in SG medium on d11 (Chapman et al., 2011). To generate clonally enriched Erbb3-deficient germlines, spermatogonial stem cells from passage 6 were similarly transfected with pgErbb3-330 (gRNA 5′-ggggaacccaggtctacgat-3′) and diluted post-transfection by plating an equivalent of ~7.5×104 to 1.5×105 cells/9.5 cm2 in SG medium to promote picking individual colonies for derivation of clonally enriched spermatogonial lines, as described in detail (Chapman et al., 2011; Ivics et al., 2011; Izsvak et al., 2010). Individually picked colonies required 81±20 and 89±21 days to expand wildtype (n=5, ±SD) and Erbb3-deficient (n=6, ±SD) spermatogonial lines to ~2×105 cells, respectively.
Supplementary Material
Acknowledgments
We thank Robert E. Hammer, Thomas M. Wilkie, David R. Corey and David J. Mangelsdorf for critically evaluating this manuscript. This work was supported by National Institutes of Health grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development: R01HD053889 and R01HD061575; National Center for Research Resources: R24RR032326; and Office of the Director: R24OD011108.
Footnotes
Supplemental Information for this article includes five figures, two tables and standard Experimental Procedures.
AUTHOR CONTRIBUTIONS
F.K.H, C.O., M.N. and J.H. designed the experiments and performed data analysis. K.C., G.M., P.J., J.C., A.W. and F.K.H. conducted the experiments. F.K.H. wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Archambeault DR, Matzuk MM. Disrupting the male germ line to find infertility and contraception targets. Annales d’endocrinologie. 2014;75:101–108. doi: 10.1016/j.ando.2014.04.006. [DOI] [PubMed] [Google Scholar]
- Arregui L, Dobrinski I, Roldan ER. Germ cell survival and differentiation after xenotransplantation of testis tissue from three endangered species: Iberian lynx (Lynx pardinus), Cuvier. Reproduction, fertility, and development. 2013 doi: 10.1071/RD12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun RE, Behringer RR, Peschon JJ, Brinster RL, Palmiter RD. Genetically haploid spermatids are phenotypically diploid. Nature. 1989;337:373–376. doi: 10.1038/337373a0. [DOI] [PubMed] [Google Scholar]
- Brinster RL, Avarbock MR. Germline transmission of donor haplotype following spermatogonial transplantation. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:11303–11307. doi: 10.1073/pnas.91.24.11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buaas FW, Kirsh AL, Sharma M, McLean DJ, Morris JL, Griswold MD, de Rooij DG, Braun RE. Plzf is required in adult male germ cells for stem cell self-renewal. Nature genetics. 2004;36:647–652. doi: 10.1038/ng1366. [DOI] [PubMed] [Google Scholar]
- Carraway KL, 3rd, Sliwkowski MX, Akita R, Platko JV, Guy PM, Nuijens A, Diamonti AJ, Vandlen RL, Cantley LC, Cerione RA. The erbB3 gene product is a receptor for heregulin. The Journal of biological chemistry. 1994;269:14303–14306. [PubMed] [Google Scholar]
- Chapman KM, Saidley-Alsaadi D, Syvyk AE, Shirley JR, Thompson LM, Hamra FK. Spermatogonial Stem Cell Mediated Gene Transfer. In: Pease S, Saunders T, editors. Transgenic Technology. Vol. 1. Springer Press; 2011. [Google Scholar]
- Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costoya JA, Hobbs RM, Barna M, Cattoretti G, Manova K, Sukhwani M, Orwig KE, Wolgemuth DJ, Pandolfi PP. Essential role of Plzf in maintenance of spermatogonial stem cells. Nature genetics. 2004;36:653–659. doi: 10.1038/ng1367. [DOI] [PubMed] [Google Scholar]
- Cronkhite JT, Norlander C, Furth JK, Levan G, Garbers DL, Hammer RE. Male and female germline specific expression of an EGFP reporter gene in a unique strain of transgenic rats. Developmental biology. 2005;284:171–183. doi: 10.1016/j.ydbio.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Erickson SL, O’Shea KS, Ghaboosi N, Loverro L, Frantz G, Bauer M, Lu LH, Moore MW. ErbB3 is required for normal cerebellar and cardiac development: a comparison with ErbB2-and heregulin-deficient mice. Development. 1997;124:4999–5011. doi: 10.1242/dev.124.24.4999. [DOI] [PubMed] [Google Scholar]
- Guschin DY, Waite AJ, Katibah GE, Miller JC, Holmes MC, Rebar EJ. A rapid and general assay for monitoring endogenous gene modification. Methods in molecular biology. 2010;649:247–256. doi: 10.1007/978-1-60761-753-2_15. [DOI] [PubMed] [Google Scholar]
- Hai T, Teng F, Guo R, Li W, Zhou Q. One-step generation of knockout pigs by zygote injection of CRISPR/Cas system. Cell research. 2014;24:372–375. doi: 10.1038/cr.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamra FK, Chapman KM, Nguyen D, Garbers DL. Identification of neuregulin as a factor required for formation of aligned spermatogonia. The Journal of biological chemistry. 2007;282:721–730. doi: 10.1074/jbc.M608398200. [DOI] [PubMed] [Google Scholar]
- Hamra FK, Chapman KM, Nguyen DM, Williams-Stephens AA, Hammer RE, Garbers DL. Self renewal, expansion, and transfection of rat spermatogonial stem cells in culture. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:17430–17435. doi: 10.1073/pnas.0508780102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamra FK, Gatlin J, Chapman KM, Grellhesl DM, Garcia JV, Hammer RE, Garbers DL. Production of transgenic rats by lentiviral transduction of male germ-line stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:14931–14936. doi: 10.1073/pnas.222561399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison MM, Jenkins BV, O’Connor-Giles KM, Wildonger J. A CRISPR view of development. Genes & development. 2014;28:1859–1872. doi: 10.1101/gad.248252.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann BP, Sukhwani M, Winkler F, Pascarella JN, Peters KA, Sheng Y, Valli H, Rodriguez M, Ezzelarab M, Dargo G, et al. Spermatogonial stem cell transplantation into rhesus testes regenerates spermatogenesis producing functional sperm. Cell stem cell. 2012;11:715–726. doi: 10.1016/j.stem.2012.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess RA. Quantitative and qualitative characteristics of the stages and transitions in the cycle of the rat seminiferous epithelium: light microscopic observations of perfusion-fixed and plastic-embedded testes. Biology of reproduction. 1990;43:525–542. doi: 10.1095/biolreprod43.3.525. [DOI] [PubMed] [Google Scholar]
- Ivics Z, Izsvak Z, Medrano G, Chapman KM, Hamra FK. Sleeping Beauty transposon mutagenesis in rat spermatogonial stem cells. Nature protocols. 2011;6:1521–1535. doi: 10.1038/nprot.2011.378. [DOI] [PubMed] [Google Scholar]
- Izsvak Z, Frohlich J, Grabundzija I, Shirley JR, Powell HM, Chapman KM, Ivics Z, Hamra FK. Generating knockout rats by transposon mutagenesis in spermatogonial stem cells. Nature methods. 2010;7:443–445. doi: 10.1038/nmeth.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R, Jahner D, Nobis P, Simon I, Lohler J, Harbers K, Grotkopp D. Chromosomal position and activation of retroviral genomes inserted into the germ line of mice. Cell. 1981;24:519–529. doi: 10.1016/0092-8674(81)90343-3. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Toyokuni S, Shinohara T. Genetic selection of mouse male germline stem cells in vitro: offspring from single stem cells. Biology of reproduction. 2005;72:236–240. doi: 10.1095/biolreprod.104.035659. [DOI] [PubMed] [Google Scholar]
- Kosova G, Scott NM, Niederberger C, Prins GS, Ober C. Genome-wide association study identifies candidate genes for male fertility traits in humans. American journal of human genetics. 2012;90:950–961. doi: 10.1016/j.ajhg.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Qiu Z, Shao Y, Chen Y, Guan Y, Liu M, Li Y, Gao N, Wang L, Lu X, et al. Heritable gene targeting in the mouse and rat using a CRISPR-Cas system. Nature biotechnology. 2013;31:681–683. doi: 10.1038/nbt.2661. [DOI] [PubMed] [Google Scholar]
- Li P, Estrada JL, Burlak C, Montgomery J, Butler JR, Santos RM, Wang ZY, Paris LL, Blankenship RL, Downey SM, et al. Efficient generation of genetically distinct pigs in a single pregnancy using multiplexed single-guide RNA and carbohydrate selection. Xenotransplantation. 2014 doi: 10.1111/xen.12131. [DOI] [PubMed] [Google Scholar]
- Lohmiller JJ, Swing SP. The Laboratory Rat: Chapter 6 - Reprodution and Breeding. 2. Elsevier Academic Press; 2006. [Google Scholar]
- Ma Y, Shen B, Zhang X, Lu Y, Chen W, Ma J, Huang X, Zhang L. Heritable multiplex genetic engineering in rats using CRISPR/Cas9. PloS one. 2014;9:e89413. doi: 10.1371/journal.pone.0089413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano M, Brinster CJ, Orwig KE, Ryu BY, Avarbock MR, Brinster RL. Transgenic mice produced by retroviral transduction of male germ-line stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2001a;98:13090–13095. doi: 10.1073/pnas.231473498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano M, McCarrey JR, Brinster RL. Primate spermatogonial stem cells colonize mouse testes. Biology of reproduction. 2001b;64:1409–1416. doi: 10.1095/biolreprod64.5.1409. [DOI] [PubMed] [Google Scholar]
- Nagy A, Gocza E, Diaz EM, Prideaux VR, Ivanyi E, Markkula M, Rossant J. Embryonic stem cells alone are able to support fetal development in the mouse. Development. 1990;110:815–821. doi: 10.1242/dev.110.3.815. [DOI] [PubMed] [Google Scholar]
- Ni W, Qiao J, Hu S, Zhao X, Regouski M, Yang M, Polejaeva IA, Chen C. Efficient Gene Knockout in Goats Using CRISPR/Cas9 System. PloS one. 2014;9:e106718. doi: 10.1371/journal.pone.0106718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu Y, Shen B, Cui Y, Chen Y, Wang J, Wang L, Kang Y, Zhao X, Si W, Li W, et al. Generation of gene-modified cynomolgus monkey via Cas9/RNA-mediated gene targeting in one-cell embryos. Cell. 2014;156:836–843. doi: 10.1016/j.cell.2014.01.027. [DOI] [PubMed] [Google Scholar]
- Ohinata Y, Payer B, O’Carroll D, Ancelin K, Ono Y, Sano M, Barton SC, Obukhanych T, Nussenzweig M, Tarakhovsky A, et al. Blimp1 is a critical determinant of the germ cell lineage in mice. Nature. 2005;436:207–213. doi: 10.1038/nature03813. [DOI] [PubMed] [Google Scholar]
- Pursel VG, Pinkert CA, Miller KF, Bolt DJ, Campbell RG, Palmiter RD, Brinster RL, Hammer RE. Genetic engineering of livestock. Science. 1989;244:1281–1288. doi: 10.1126/science.2499927. [DOI] [PubMed] [Google Scholar]
- Soriano P, Jaenisch R. Retroviruses as probes for mammalian development: allocation of cells to the somatic and germ cell lineages. Cell. 1986;46:19–29. doi: 10.1016/0092-8674(86)90856-1. [DOI] [PubMed] [Google Scholar]
- Tarkowski AK. Mouse chimaeras developed from fused eggs. Nature. 1961;190:857–860. doi: 10.1038/190857a0. [DOI] [PubMed] [Google Scholar]
- Tong C, Huang G, Ashton C, Li P, Ying QL. Generating gene knockout rats by homologous recombination in embryonic stem cells. Nature protocols. 2011;6:827–844. doi: 10.1038/nprot.2011.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Yang H, Shivalila CS, Dawlaty MM, Cheng AW, Zhang F, Jaenisch R. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell. 2013;153:910–918. doi: 10.1016/j.cell.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie TM, Braun RE, Ehrman WJ, Palmiter RD, Hammer RE. Germ-line intrachromosomal recombination restores fertility in transgenic MyK-103 male mice. Genes & development. 1991;5:38–48. doi: 10.1101/gad.5.1.38. [DOI] [PubMed] [Google Scholar]
- Wilkie TM, Brinster RL, Palmiter RD. Germline and somatic mosaicism in transgenic mice. Developmental biology. 1986;118:9–18. doi: 10.1016/0012-1606(86)90068-0. [DOI] [PubMed] [Google Scholar]
- Wu Y, Zhou H, Fan X, Zhang Y, Zhang M, Wang Y, Xie Z, Bai M, Yin Q, Liang D, et al. Correction of a genetic disease by CRISPR-Cas9-mediated gene editing in mouse spermatogonial stem cells. Cell research. 2014 doi: 10.1038/cr.2014.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Falciatori I, Molyneux LA, Richardson TE, Chapman KM, Hamra FK. Spermatogonial culture medium: an effective and efficient nutrient mixture for culturing rat spermatogonial stem cells. Biology of reproduction. 2009;81:77–86. doi: 10.1095/biolreprod.108.072645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Wang H, Shivalila CS, Cheng AW, Shi L, Jaenisch R. One-step generation of mice carrying reporter and conditional alleles by CRISPR/Cas-mediated genome engineering. Cell. 2013;154:1370–1379. doi: 10.1016/j.cell.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen ST, Zhang M, Deng JM, Usman SJ, Smith CN, Parker-Thornburg J, Swinton PG, Martin JF, Behringer RR. Somatic mosaicism and allele complexity induced by CRISPR/Cas9 RNA injections in mouse zygotes. Developmental biology. 2014;393:3–9. doi: 10.1016/j.ydbio.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng W, Tang L, Bondareva A, Honaramooz A, Tanco V, Dores C, Megee S, Modelski M, Rodriguez-Sosa JR, Paczkowski M, et al. Viral transduction of male germline stem cells results in transgene transmission after germ cell transplantation in pigs. Biology of reproduction. 2013;88:27. doi: 10.1095/biolreprod.112.104422. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.