Abstract
Innate immune mechanisms leading to liver injury following chronic alcohol ingestion are poorly understood. Natural killer T (NKT) cells, enriched in the liver and comprised of at least two distinct subsets, type I and type II, recognize different lipid antigens presented by CD1d molecules. We have investigated whether differential activation of NKT cell subsets orchestrates inflammatory events leading to alcoholic liver disease (ALD). We found that following chronic plus binge feeding of Lieber-DeCarli liquid diet in male C57BL/6 mice, type I but not type II NKT cells are activated leading to recruitment of inflammatory Gr-1highCD11b+ cells into liver. A central finding is that liver injury following alcohol feeding is dependent upon type I NKT cells. Thus liver injury is significantly inhibited in Jα18−/− mice deficient in type I NKT cells as well as following their inactivation by sulfatide-mediated activation of type II NKT cells. Furthermore we have identified a novel pathway involving all-trans retinoic acid (ATRA) and its receptor RARγ signaling that inhibits type I NKT cells and consequently ALD. A semi-quantitative PCR analysis of hepatic gene expression of some of the key proinflammatory molecules shared in human disease indicated that their upregulation in ALD is dependent upon type I NKT cells.
Conclusion
Type I but not type II NKT cells become activated following alcohol feeding. Type I NKT cells-induced inflammation and neutrophil recruitment results in liver tissue damage while type II NKT cells protect from injury in ALD. Inhibition of type I NKT cells by retinoids or by sulfatide prevents ALD. Since the CD1d pathway is highly conserved between mice and humans, NKT cell subsets might be targeted for potential therapeutic intervention in ALD.
Keywords: CD1d, ATRA, glycolipids, steatohe patitis, Retinoic acid receptor
Introduction
Alcoholic liver disease (ALD) comprising hepatic steatosis may progress to hepatitis, fibrosis and eventually liver cirrhosis, is one of the leading causes of death in the United States (1, 2). A detailed understanding of cellular and molecular mechanisms underlying liver tissue damage following alcohol ingestion is important in finding novel therapeutic approaches for ALD. The liver is enriched with several innate immune cells, including natural killer T (NKT) cells. How interactions among these cells provide tolerance to gut-derived antigens or metabolic products, e.g. following alcohol ingestion, and at the same time immunity against pathogens are not understood. NKT cells share the cell surface receptors of NK cells (NK1.1+ (mouse) or CD161+/CD56+ (human)) and in addition express a T cell receptor (TCR), enabling them to recognize lipid antigens presented by CD1d molecules (3, 4). Activation of NKT cells has a profound influence on the outcome of the immune response against tumors, infectious organisms and autoimmune diseases (5–8). Recently NKT cells have been suggested to play an important role in a number of experimental liver diseases as well as in human sickle cell disease and non-alcoholic steatohepatitis (9–15).
NKT cells can be divided into two categories: one using a semi-invariant TCR (iNKT or type I) and the other expressing a relatively more diverse TCR repertoire (type II NKT). The semi-invariant TCR is encoded predominantly by a germline Vα gene (Vα14/Jα18 in mice and Vα24/JαQ in humans) and a limited number of non-germline Vβ chain genes (Vβ8.2/7/2 in mice and Vβ11 in human) (3, 4). A major subset of type II NKT cells is reactive to a self-glycolipid, sulfatide, and their TCR repertoire is oligoclonal with predominant usage of Vα3/Vα1-Jα7/Jα9 and Vβ8.1/Vβ3.1-Jβ2.7 gene segments (16, 17). While the type I NKT TCR binds to CD1d in a parallel configuration mainly involving the α-chain, a type II NKT TCR contacts its ligands primarily via its β chain (18, 19). Thus type I and type II NKT cell subsets display distinct modes of antigen recognition. Notably, sulfatide-reactive type II NKT cells have been shown to be regulatory, as their activation results in protection from autoimmune and inflammatory diseases (8, 9, 11, 17, 20).
Here we have addressed some of the key questions related to the role of NKT cell subsets during the inflammatory cascade in ALD. We have used αGalCer/CD1d- and sulfatide/CD1d-tetramers to track type I and type II NKT cells respectively during ALD and found that type I but not type II NKT cells are activated, leading to recruitment of CD11b+Gr-1+ cells into liver. Accumulation of neutrophils as well as liver damage is significantly reduced in Jα18−/− mice lacking type I NKT cells. We have also identified a novel pathway of inhibition of type I NKT cells by all-trans retinoic acid (ATRA). A clinically relevant retinoic receptor γ (RARγ) agonist, Tazarotene, found to be the most efficient in blocking type I NKT cells. A number of crucial inflammatory genes also shared in human ALD are upregulated following alcohol feeding and are dependent upon the presence of type I NKT cells. Notably inhibition of type I NKT cells by retinoids or following sulfatide-mediated activation of type II NKT cells ameliorates ALD. Collectively these studies for the first time provide a crucial NKT-based immune mechanism involved in causing hepatic damage following ethanol ingestion and have important implications for potentially novel intervention in ALD.
Experimental procedures
Murine model of chronic plus binge ethanol feeding
C57BL/6J (B6) male mice (7–10 wk) were purchased from The Jackson Laboratory (Bar Harbor, ME). CD1d−/− and Jα18−/− BL/6 mice, originally generated in the laboratories of Drs. Luc Van Kaer (Vanderbilt Univ., Nashville, TN) and M. Taniguchi (Chiba Univ., Chiba, Japan), were provided by Dr. Mitch Kronenberg (La Jolla Institute for Allergy and Immunology, La Jolla, CA). All mice were bred and maintained in specific pathogen free-conditions in the Torrey Pines Institute for Molecular Studies (TPIMS) animal facility. Treatment of animals was in compliance with federal and institutional guidelines and approved by the TPIMS Animal Care and Use Committee.
Following acclimatization with a nutritionally adequate Lieber-DeCarli liquid diet for a week, male B6 mice (6–8 wk) were given free access to alcohol or dextrin maltose-containing diet (Bio-Serv, NJ) (21, 22). Precautions were taken to prepare the diet fresh every day using autoclaved water in sterilized feeders and feed was changed daily. Mice in the ethanol group were fed liquid diet containing 5% ethanol for 4–5 weeks (chronic ethanol feeding) or for 10 days followed by (on day 11) a single dose of gavaged ethanol (5 g/kg body weight) (chronic plus binge ethanol feeding). Mice in the control group were gavaged isocaloric dextrin maltose. Following gavage of the high dose of ethanol, mice were euthanized 8–9 hr after gavage at which time maximum liver damage occurs as exemplified by liver histology or ALT/AST serum levels (21).
Additional materials and methods are described in the Supporting Information.
Results
Enrichment and activation of type I but not type II NKT cells in liver following chronic plus binge feeding of alcohol
To determine the number and phenotype of NKT cell subsets during ALD, groups of B6 and type I NKT-deficient Jα18−/− mice were subjected to chronic or chronic plus binge ethanol feeding and NKT cells were analyzed in liver MNCs following staining with αGalCer/CD1d- (type I), sulfatide/CD1d- (type II) tetramers, or by αGalCer/CD1d-tetramer- NK1.1+TCRβ+ cells. Data presented in Figure 1a show that although type I NKT cell numbers are increased during the chronic phase, their accumulation is significantly greater following chronic plus binge feeding. Most of the increase in NK1.1+TCRβ+CD4+ T cells was accounted for by an increase in type I NKT cells. In contrast there was no change in NK1.1+TCRβ+CD4+ T cells in either groups of Jα18−/− mice, which are predominantly type II NKT cells. Furthermore type I but not type II NKT cells were activated and secreted IFN-γ as determined by the expression of CD69 and intracytoplasmic staining of tetramer+ cells (Fig. 1b and 1c). Since liquid diet-derived products may influence NKT cells, we determined whether IFN-γ secretion by type I NKT cells occurs following only binge feeding of ethanol. As shown in Figure 1D, around 14–20% αGalCer/CD1d-tetramer+ cells secrete IFN-γ following binge feeding whereas most of them are IFN-γ+ following αGalCer administration. Notably, serum ALT levels did not change in these mice following binge alcohol only (data not shown). Collectively these data indicate that type I NKT but not type II NKT cells are enriched and become activated in liver rapidly following alcohol ingestion.
Figure 1. Enrichment and activation of Type I NKT cells but not type II NKT cells following chronic plus binge alcohol feeding.
(A) A representative summary of flow cytometric analysis of liver MNCs derived from groups of B6 or Jα18−/− mice placed on chronic or chronic plus binge alcohol feeding. Bar graphs depicting the % staining with αGalCer/CD1d tetramer+, NK1.1+TCRβ+ or Gr1highCD11b+ cells are shown. The gate (shown in Supplement Figure 1) included all of the mononuclear cells and excludes the dead cells and red blood cells. **** P<0.0001, ***P<0.001, **P <0.01, n.s., non-significant. (B) Relative expression of CD69 (MFI) and intracellular IFN-γ staining in αGalCer/CD1d-tetramer positive (+) following chronic plus binge ethanol feeding in B6 mice from Figure A is shown. *P<0.05. (C) Expression of CD69 on αGalCer/CD1d-tetramer- and sulfatide/CD1d-tetramer+ cells in liver MNCs from B6 or Jα18−/− mice as in 1B are shown. (D) Intracellular IFN-γ staining in αGalCer/CD1d-tetramer+ cells at indicated times following ethanol binge only and no liquid diet. *P<0.05.
Since sterile activation of type I NKT cells leads to infiltration of Gr-1highCD11b+ cells in liver injury (9, 11), we determined whether these cells are also enriched in liver in a type I NKT-dependent fashion following alcohol feeding. Data in Figure 1A shows that neutrophils are significantly increased in ALD and that this increase is dependent upon the presence of type I NKT cells.
Attenuation of alcoholic liver disease in mice deficient in type I NKT cells
Since type I but not type II NKT cells become activated following alcohol intake, we next determined their role in mediating liver damage. Groups of B6 and Jα18−/− mice were fed either chronic or chronic plus binge liquid ethanol diet or isocaloric matched liquid diet and examined liver for damage by assessing serum ALT levels and H&E staining of liver sections. Data presented in Figure 2A show that while ALT levels in B6 mice following only chronic alcohol feeding were not altered, a significant increase occurred following chronic plus binge alcohol feeding. Accompanying this a pronounced hepatic steatosis was found in B6 mice following chronic plus binge feeding but not following chronic exposure to alcohol (Fig. 2b). In contrast serum ALT levels as well as hepatic steatosis following chronic plus binge feeding of alcohol was significantly inhibited in Jα18−/− mice. Consistent with the pathogenic role of type I NKT, CD1d−/− mice lacking both NKT cell subsets are protected from ALD (Supplement Fig. 2). Collectively these data suggest that type 1 NKT cells play a key pathogenic role in ALD.
Figure 2. Alcoholic liver injury is blocked in type I NKT cell-deficient mice.
(A) Serum ALT levels from chronic, or chronic plus binge alcohol- or isocaloric liquid diet-fed B6 or Jα18−/− mice on day 11 after the start of the liquid diet as in Figure 1 are shown. ****P<0.0001, n.s., non-significant. (B) Representative liver histology sections stained with H & E from mice in Figure 2A are shown. These data are representative of 4 different experiments.
Increase in key inflammatory genes in liver following alcohol ingestion is dependent upon type I NKT cells
Recently microarrays and quantitative PCR analysis of hepatic gene expression profiling in human ALD as well as in animal models have suggested potential common pathways involved in alcohol-mediated liver damage (reviewed in (1, 23, 24)). We determined whether an increase in proinflammatory genes is dependent upon type I NKT cells using real-time PCR analysis of liver tissues in WT or Jα18−/− alcohol-fed mice. Figures 3A and 3B shows a significant increase in cytokines, including OPN, IL-1β, IL-6 and TNFα as well as in chemokine/receptors related to NKT/neutrophils such as CXCL2 (MIP-2), CCL4 (MIP-1β) and CXCR1 following chronic plus binge alcohol diet. Interestingly, other Th1-related genes, including IFN-γ, IL-12, T-bet, GATA-3 and CXCR3 though altered following chronic plus binge alcohol feeding were not significantly different between B6 and Jα18−/− mice (data not shown). Similarly other genes related to fibrogenesis such as TGF-β and IL-5 but not IL-4 were also significantly upregulated in B6 but not in Jα18−/− mice following alcohol feeding (Fig. 3c). These data suggest that induction of a proinflammatory cascade involving IL-1/IL-6 pathway as well as OPN in ALD is dependent upon the presence of type I NKT cells.
Figure 3. Type I NKT dependent modulation of important inflammatory genes following chronic plus binge alcohol feeding.
A semi-quantitative Real-time PCR analysis of the indicated genes from liver tissues derived from B6 or Jα18−/− male mice or B6 mice treated with sulfatide or ATRA following chronic plus binge ethanol- or isocaloric liquid-diet is shown. These data are representative of three individual experiments. **** P<.0001, *** P<0.001, ** P<0.01, * P<0.05, n.s., non-significant.
Direct or indirect inhibition of type I NKT cells by ATRA or sulfatide inhibits ALD
Since ATRA levels are altered following chronic alcohol ingestion (25) we determined whether ATRA influences the function of type I NKT cells. Groups of B6 mice were administered ATRA for 5 days, and proliferation as well as cytokine secretion in type I NKT cells in response to an in vitro challenge with αGalCer was examined. Figure 4A and supplement Figure 3 show that the proliferative and cytokine secretion responses of type I NKT cells are significantly inhibited following ATRA treatment. Since ATRA can influence other cells or pathways, in vitro assays using freshly derived type I NKT cells or a type I NKT hybridoma (Hy1.2) were used to determine the specificity of its inhibitory effect. Figure 4B shows that ATRA (~18 mg/ml) inhibits both polyclonal (splenocytes) as well as monoclonal (Hy1.2) type I NKT cell responses. It is noteworthy to mention that IL-2 secretion by Hy1.2 treated with ATRA in the absence of antigen-presenting cells also inhibited. In contrast, response of conventional class II MHC (I-Au)-restricted MBP-reactive CD4+ T cells is not inhibited in the presence of ATRA at the concentration examined (Fig. 4b). Similarly type II NKT cells are also not inhibited in these assays (data not shown). These data clearly indicated that the function of type I NKT cells is specifically inhibited by ATRA.
Figure 4. Inhibition of type I NKT cells leads to amelioration of ALD.
(A) Proliferative and cytokine secretion profiles of type I NKT cells in response to an in vitro challenge with titrated concentrations of αGalCer in splenocytes derived from groups of B6 mice administered i.p. with ATRA (0.3 mg/animal/day for 5 days) or DMSO as a control are shown. These data are representative of 2 individual experiments.
(B) In vitro inhibition of proliferation or IL-2 secretion by type I NKT cells (splenocytes, or Hy1.2) or conventional MHC class II I-Au-restricted CD4+ T cells in response to an optimum concentration of αGalCer (10 ng/ml) or MBP Ac1-9 (20 μg/ml) and in the presence or absence of indicated concentrations of ATRA is shown.
(C) Serum ALT levels and H & E staining of liver sections from chronic plus binge alcohol- or isocaloric control diet-fed B6 mice are shown. For treatment groups B6 mice were either injected with DMSO or vehicle (Control) or with sulfatide 20 micrograms/mouse (d1 & d10) or 0.3 milligrams/animal ATRA or Tazarotene (d6 through d10) *P<0.05. Liver histology sections are shown at 200X magnification. These data are representative of 2 independent experiments.
Next we determined whether inhibition of type I NKT cells by ATRA also attenuates ALD. Groups of male B6 mice were administered ATRA and fed a chronic plus binge alcohol diet, and liver damage was assessed by serum ALT and hepatic steatosis. Data in Figure 4C clearly show that administration of ATRA significantly protected mice from ALD. Lack of further inhibition of liver damage by ATRA in Jα18−/− mice suggests it mainly attenuates ALD by inhibiting type I NKT cells (data not shown).
Although type II NKT cells are not activated during ALD (Fig. 1), activation of these cells by the self-glycolipid sulfatide results in a powerful immune regulatory pathway involving anergy induction in type I NKT cells, tolerization of liver DCs and inhibition of conventional T cells (26). Next we determined whether sulfatide-activated type II NKT-mediated regulation of type I NKT cells also blocks ALD. As shown in Figure 4C, sulfatide-treated mice are significantly protected from alcohol-induced liver injury.
Next we determined whether upregulation of type I NKT-dependent proinflammatory genes is also blocked in ATRA or sulfatide-treated mice. Figure 3 shows that increase in OPN, IL-1 and IL-6 as well as CXCR1, CXCL2 and CCL4 is significantly suppressed following ATRA or sulfatide -treatment. However other genes that are not dependent upon type I NKT cells, such as IFN-γ, TRAIL and GATA3 are not inhibited following either ATRA or sulfatide administration. These data suggest that inhibition of type I NKT cells by ATRA or sulfatide leads to protection from ALD by suppression of alcohol-induced proinflammatory pathways in liver.
Consistent with the inhibition of type I NKT cells we found a significant reduction in the number of αGalCer/CD1d-tetramer+ cells accumulating in liver in groups of chronic plus binge alcohol-fed mice treated with sulfatide, ATRA or Tazarotene in comparison to the control group (Supplement Figure 4).
RARγ signaling pathway is involved in inhibition of type I NKT cells by ATRA and protection from ALD
ATRA plays a fundamental role in immune responses (27) and its effects are mediated by its binding to the retinoic acid receptors (RARs) (28). We determined the involvement of different RARs in the ATRA-mediated inhibition of type I NKT cells using analogs of ATRA as well as specific agonists of RARα, RARβ and RARγ. Figure 5A shows dose titration curves of inhibition of either polyclonal (splenic) or monoclonal (Hy1.2) type I NKT cells by several different agonists. A summary of the data and a dose at which 50% inhibition of proliferation or cytokine secretion of type I NKT cells is shown in Figure 5B and supplement Table 2. It is clear that RARγ agonists (TTNPB & CD1530) are most efficient in comparison to RARα or RARβ agonists in inhibiting type I NKT cells. Also ATRA as well as its analog Tretinoin are able to inhibit type I NKT cells. As shown previously, the PPARγ antagonist also inhibited type I NKT cells. However, Retinol, rexinoids (9-cis RA and 13-cis RA), PPARα, PPARβ, PPARγ agonists as well as pan PPAR agonist did not inhibit significantly.
Figure 5. RARγ agonists are most efficient in inhibiting type I NKT cells both in vitro and in vivo.
(A) Inhibition of a proliferative response in type I NKT cells in splenocytes (Left panels) or IL-2 secretion by Hy1.2 (Right panels) at an optimum concentration of αGalCer (10 ng/ml) and in the absence or presence of a titrated concentration of ATRA, other indicated retinoids is shown. These data are representative of three different experiments. (B) A summary of data presented in Figure 5A showing relative inhibition of type I NKT cells in response to αGalCer (10 ng/ml) at optimum concentrations (5–7μg/ml) of different retinoids, including ATRA, its clinical form Tretinoin, and agonists for RARα (AM580), RARβ2 (AC261066) RARγ (CD1530) is shown. (C) Inhibition of type I NKT cells in vitro in response to an optimum concentration of αGalCer (10 ng/ml) and in the presence of titrated doses of ATRA or Tazarotene is shown. These data are representative of 2 independent experiments. (D) Inhibition of a proliferative response of type I NKT cells following i.p. administration of ATRA, Tazarotene (0.3 mg/animal/day for 5 days), sulfatide or DMSO. These data are representative of two independent experiments.
Since RARγ agonists inhibit type I NKT cells at almost 10-fold lower concentration than ATRA, we next examined whether a RARγ agonist can inhibit ALD. To examine this we chose a clinically relevant RARγ agonist, Tazarotene, used for the topical treatment of psoriasis (29). Figure 5C shows that in both in vitro and in vivo assays Tazarotene is more potent (almost 10 fold) than ATRA in inhibiting type I NKT cells. Furthermore as shown in Figure 3C, Tazarotene also significantly blunts liver disease as indicated by ALT levels and hepatosteatosis. Collectively these data suggest that inhibition of type I NKT cells by ATRA specifically involves the RARγ signaling pathway.
Relatively higher constitutive expression of RARγ on type I NKT cells
Next we determined whether a difference in the expression of RARs on type I vs. type II or conventional CD4+ T cells could explain specific inhibition of type I NKT cells by ATRA. We used quantitative PCR to examine the expression of RARs in these cells. Figure 6 clearly show that RARγ expression in sorted αGalCer/CD1d-tetramer+ cells as well as in Hy1.2 is several fold higher in comparison to type II NKT cells (Hy19.3) or sorted conventional CD4+ T cells. Notably RARβ is not expressed in NKT or conventional T cells. In contrast RARα expression is similar to or even lower in comparison to its expression in type II NKT or conventional CD4+ T cells. These data suggest that increased expression of RARγ in type I NKT cells correlates with the relatively effective inhibition of type I NKT cells following RARγ–signaling.
Figure 6. Constitutively high expression of RARγ in type I NKT cells.
Relative expression of RARα, RARβ and RARγ genes using quantitative RT-PCR assay in a polyclonal population of αGalCer/CD1d-tetramer+ cells or monoclonal populations of Hy1.2 (type I NKT), Hy19.3 (type II NKT) and Hy172.10 (MBP-reactive CD4+) cells is shown. These data are representative of three independent experiments. *** P<0.001, ** P<0.01, n.s., non-significant
Suppression of CCL4-induced liver fibrosis following sulfatide or ATRA-mediated inhibition of type I NKT cells
Since fibrosis is not one of the strong features in liver damage following alcohol feeding, we investigated whether this is altered in a relevant model of fibrosis in the absence of or following inhibition of type I NKT cells. Groups of B6 mice were administered sulfatide, ATRA or DMSO as shown in Figure 5, and liver fibrosis was induced following chronic CCL4 injections. In parallel, chronic CCL4 injections were also given into age-matched Jα18−/− mice. Data in Figure 7A and 7B show that CCL4-induced liver fibrosis as assessed by Sirius Red staining is significantly reduced in Jα18−/− mice as well as in mice treated with sulfatide or ATRA in comparison to control mice. Accordingly, increase in fibrogenesis related genes, TGF-β and collagen α-1 was blunted in the absence of functional type I NKT cells (Fig. 7c). Interestingly however serum ALT levels in Jα18−/− mice or in mice treated with sulfatide or ATRA were not significantly inhibited. These data suggest that inhibition of type I NKT cells or their absence results in the inhibition of liver fibrosis.
Figure 7. Treatment with ATRA or sulfatide significantly attenuates CCL4-induced liver fibrosis.
(A) Representative images of Sirius Red staining of liver sections from groups of male B6 or Jα18−/− mice after 12 weeks of CCL4 injections are shown. Treatment of B6 mice with sulfatide, ATRA or DMSO is same as in Figure 4. Original magnification X 100 is shown. (B) Serum ALT levels and hepatic fibrosis as assessed by morphometric analysis of the Sirius Red-stained area in the liver from different groups of mice are shown. (C) A semi-quantitative Real-time PCR analysis of the indicated genes from liver tissues as above in A. These data are representative of two independent experiments. ****P <0.0001, ***P<0.001, **P<0.01, n.s., non-significant.
Discussion
Our data here demonstrate that innate-like type I NKT but not type II NKT cells play an important pathogenic role in mediating liver damage following alcohol feeding. Accordingly type I but not type II NKT cells accumulate into liver rapidly, become activated and secrete cytokines/chemokines for the induction of an inflammatory cascade. Thus, in the absence of type I NKT cells or following their sulfatide- or ATRA-mediated inhibition, ALD is significantly diminished. Furthermore hepatic gene expression data indicate that one of the key inflammatory events following their activation is the involvement of IL-1/IL-6/osteopontin and neutrophil recruitment into liver. Notably, relatively higher expression of RARγ in type I NKT cells led to identification of a clinically relevant RARγ agonist, Tazarotene that inhibits type I NKT cells and blocks ALD. Collectively, our data suggest that while type I NKT cells are pathogenic, a major subset of type II NKT cells can be protective in ALD and that the inhibition of type I NKT cells using sulfatide or Tazarotene or their analogs can be examined in clinical studies for a potential novel intervention in ALD.
It is interesting that the greater increase in activated type I NKT cells resulting in liver disease requires chronic plus binge alcohol feeding. It seems that the number and activation of type I NKT cells as well as recruitment of neutrophils into liver during the chronic phase remain insufficient to cause injury as reflected by serum ALT and hepatosteatosis. Thus a second acute exposure with excess alcohol (chronic plus binge) is required for the maximum levels of activated type I NKT cell accumulation and inflammation in liver leading to the clinical signs of ALD. Consistent with the earlier indications(21), clinical manifestation of ALD requires chronic and multiple insults in which type I NKT cells become fully activated. Consistently accumulation of activated type I NKT cells secreting proinflammatory cytokines is a hallmark of liver injury following ischemic, alcoholic, diet or toxin-induced injuries in experimental animals as well as in humans (9, 11–15). Type I NKT cells become activated either following self or microbial lipid presentation by CD1d or indirectly following TLR stimulation and IL-12 (30–32) (33). Since alcohol feeding may result in leaking of the gut membranes there is a possibility that activation of type I NKT cells may involve gut microbial lipid presentation or TLR signaling as has been shown to be involved in ALD (34). Nevertheless our data indicate that type I NKT cells play a key pathogenic role in ALD, as recently proposed for inflammatory liver diseases (35). It is noteworthy that despite the relatively low number of type I NKT cells in humans, these cells have been shown to become activated and accumulate in patients with severe asthma, sickle cell disease as well as in NASH (14, 36, 37). Collectively a careful study of type I NKT cell profiling in different stages of human disease will provide important insight about their role in ALD.
On the other hand type II NKT cells are regulatory and, by cross-regulating type I NKT cells following activation with sulfatide, limit liver damage. Since type II NKT cells in liver are not activated following alcohol feeding, it is likely that the triggering requirement for the two NKT cell subsets is different, including presentation of different lipid antigens. Consistently some antigens like αGalCer or iGB3 can only activate type I but not type II NKT cells (4). Recent insights from the crystal structure of a sulfatide-reactive type II NKT cell TCR/CD1d/sulfatide complex suggest the presence of a distinct recognition motif for TCR recognition between the type I and type II NKT cell subsets (18, 19). Collectively our data suggest that type II NKT cells are not involved in mediating liver damage in ALD but may play a regulatory role following their activation by self-lipids. Sulfatide-mediated activation of type II NKT cells induces a novel immune regulatory pathway resulting in: (i) inactivation of type I NKT cells; (ii) tolerization of conventional DC; (iii) tolerization of microglia in the CNS; and (iv) inhibition of the effector functions of MHC-restricted CD4+ T cells (7, 11, 26, 35). Therefore sulfatide or its mimetic can be potentially used for the intervention in ALD.
It is clear from our data that increase in proinflmmatory cytokines, including OPN, IL-1 and IL-6 as well as MIP-2 in ALD leading to recruitment of neutrophils (Fig. 1, 3) in liver is dependent upon type I NKT cells. Notably antibody-mediated depletion of hepatic neutrophils in this model markedly attenuates liver injury suggesting their pathogenic role (21). Furthermore blockade of type I activation either directly by retinoids, such as ATRA or Tazarotene, or indirectly following sulfatide inhibits the inflammatory cascade and liver damage. (Fig. 4, 8). Notably, high expression of OPN in ALD subjects correlates with the disease severity (1, 23, 38, 39) and OPN stimulates hepatic stellate cells (HSC) in both ALD and NASH (40). Collectively, these data suggest that type I NKT cell interaction with other innate cells in liver, including, e.g., Kupffer cells, is decisive in the recruitment of neutrophils leading to hepatocytes injury following alcohol consumption.
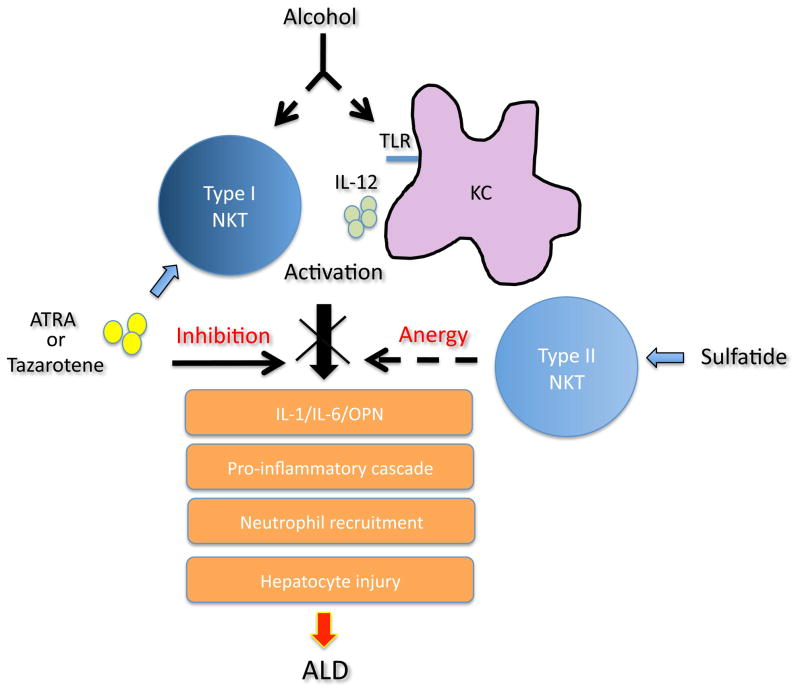
Figure 8. A model depicting role of NKT cell subsets in ALD.
Chronic alcohol feeding leads to activation of Kuppfer cells and type I NKT cells and their interactions induce a proinflammatory cascade involving IL-1β/IL-6 and OPN as well as recruitment of neutrophils eventually resulting in hepatosteatosis and liver disease. Inhibition of type I NKT cells by ATRA via RARγ signaling or by sulfatide-mediated activation of type II NKT cells blocks ALD.
Ethanol ingestion also depletes liver retinyl esters and alters physiological levels of ATRA, a biologically active form of vitamin A that support many biological functions including modulation of immune responses (41). Our data suggest that ATRA via RARγ signaling specifically inhibits type I NKT cells and protects mice from ALD (Fig. 4). Furthermore inhibition of type I NKT cells also attenuates chronic CCL4-induced liver fibrosis (Fig. 7). Thus while type I NKT cells may be protective from acute liver injury (42, 43) they potentiate chronic CCL4-induced fibrosis. Consistent with our data ATRA has been shown to attenuate CCL4-induced fibrosis by suppressing cytokines in liver (44). Our data also indicate that type I NKT cells express relatively higher levels of RARγ (Fig. 6). It is interesting that though conventional CD4+ T cells express both RARα and RARγ, the effect of ATRA on adaptive immunity is predominantly mediated via RARα (27). Thus our data suggest that unwanted effects on adaptive immune response as well as toxicity of ATRA should be mitigated by development of RARγ agonists for immune intervention. Since CD1d-dependent antigen recognition pathway is highly conserved from mice to humans, and several key features of NKT cell subsets are shared between them our data suggest that activation of type II NKT cells by sulfatide and/or inhibition of type I NKT cells by ATRA or RARγ agonists should be examined for intervention of ALD in humans.
Supplementary Material
Acknowledgments
Grant support: This work was supported by grants from the National Institutes of Health, USA, R01 CA100660 and R01 AA020864 (VK).
Authors would like to thank other members of Kumar’s laboratory and Drs. Randle Ware and Marc Hertz for a critical reading of the manuscript.
Abbreviations
- ALD
alcoholic liver disease
- NKT
natural killer T cells
- MNC
mononuclear cells
- ATRA
all-trans retinoic acid
- RAR
retinoic acid receptor
- αGalCer
α Galactosylceramide
- ALT
alanine transaminase
- AST
aspartate transaminase
- MBP
myelin basic protein
- OPN
osteopontin
References
- 1.Altamirano J, Bataller R. Alcoholic liver disease: pathogenesis and new targets for therapy. Nat Rev Gastroenterol Hepatol. 2011;8:491–501. doi: 10.1038/nrgastro.2011.134. [DOI] [PubMed] [Google Scholar]
- 2.Szabo G, Bala S. Alcoholic liver disease and the gut-liver axis. World J Gastroenterol. 2010;16:1321–1329. doi: 10.3748/wjg.v16.i11.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 4.Godfrey DI, Stankovic S, Baxter AG. Raising the NKT cell family. Nat Immunol. 2010;11:197–206. doi: 10.1038/ni.1841. [DOI] [PubMed] [Google Scholar]
- 5.Brennan PJ, Brigl M, Brenner MB. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat Rev Immunol. 2013;13:101–117. doi: 10.1038/nri3369. [DOI] [PubMed] [Google Scholar]
- 6.Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d-dependent NKT cells. J Clin Invest. 2004;114:1379–1388. doi: 10.1172/JCI23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar V, Delovitch TL. Different subsets of natural killer T cells may vary in their roles in health and disease. Immunology. 2014 doi: 10.1111/imm.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rhost S, Sedimbi S, Kadri N, Cardell SL. Immunomodulatory type II natural killer T lymphocytes in health and disease. Scand J Immunol. 2012;76:246–255. doi: 10.1111/j.1365-3083.2012.02750.x. [DOI] [PubMed] [Google Scholar]
- 9.Arrenberg P, Maricic I, Kumar V. Sulfatide-mediated activation of type II natural killer T cells prevents hepatic ischemic reperfusion injury in mice. Gastroenterology. 2011;140:646–655. doi: 10.1053/j.gastro.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng ZB, Liu Y, Liu C, Xiang X, Wang J, Cheng Z, Shah SV, et al. Immature myeloid cells induced by a high-fat diet contribute to liver inflammation. Hepatology. 2009;50:1412–1420. doi: 10.1002/hep.23148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halder RC, Aguilera C, Maricic I, Kumar V. Type II NKT cell-mediated anergy induction in type I NKT cells prevents inflammatory liver disease. J Clin Invest. 2007;117:2302–2312. doi: 10.1172/JCI31602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishikawa S, Ikejima K, Yamagata H, Aoyama T, Kon K, Arai K, Takeda K, et al. CD1d-restricted natural killer T cells contribute to hepatic inflammation and fibrogenesis in mice. J Hepatol. 2011;54:1195–1204. doi: 10.1016/j.jhep.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 13.Syn WK, Agboola KM, Swiderska M, Michelotti GA, Liaskou E, Pang H, Xie G, et al. NKT-associated hedgehog and osteopontin drive fibrogenesis in non-alcoholic fatty liver disease. Gut. 2012 doi: 10.1136/gutjnl-2011-301857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wallace KL, Marshall MA, Ramos SI, Lannigan JA, Field JJ, Strieter RM, Linden J. NKT cells mediate pulmonary inflammation and dysfunction in murine sickle cell disease through production of IFN-gamma and CXCR3 chemokines. Blood. 2009;114:667–676. doi: 10.1182/blood-2009-02-205492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu SJ, Yang YH, Tsuneyama K, Leung PS, Illarionov P, Gershwin ME, Chuang YH. Innate immunity and primary biliary cirrhosis: activated invariant natural killer T cells exacerbate murine autoimmune cholangitis and fibrosis. Hepatology. 2011;53:915–925. doi: 10.1002/hep.24113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arrenberg P, Halder R, Dai Y, Maricic I, Kumar V. Oligoclonality and innate-like features in the TCR repertoire of type II NKT cells reactive to a beta-linked self-glycolipid. Proc Natl Acad Sci U S A. 2010;107:10984–10989. doi: 10.1073/pnas.1000576107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jahng A, Maricic I, Aguilera C, Cardell S, Halder RC, Kumar V. Prevention of autoimmunity by targeting a distinct, noninvariant CD1d-reactive T cell population reactive to sulfatide. J Exp Med. 2004;199:947–957. doi: 10.1084/jem.20031389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Girardi E, Maricic I, Wang J, Mac TT, Iyer P, Kumar V, Zajonc DM. Type II natural killer T cells use features of both innate-like and conventional T cells to recognize sulfatide self antigens. Nat Immunol. 2012;13:851–856. doi: 10.1038/ni.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel O, Pellicci DG, Gras S, Sandoval-Romero ML, Uldrich AP, Mallevaey T, Clarke AJ, et al. Recognition of CD1d-sulfatide mediated by a type II natural killer T cell antigen receptor. Nat Immunol. 2012;13:857–863. doi: 10.1038/ni.2372. [DOI] [PubMed] [Google Scholar]
- 20.Subramanian L, Blumenfeld H, Tohn R, Ly D, Aguilera C, Maricic I, Mansson JE, et al. NKT cells stimulated by long fatty acyl chain sulfatides significantly reduce the incidence of type 1 diabetes in nonobese diabetic mice [corrected] PLoS One. 2012;7:e37771. doi: 10.1371/journal.pone.0037771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bertola A, Park O, Gao B. Chronic plus binge ethanol feeding synergistically induces neutrophil infiltration and liver injury in mice: a critical role for E-selectin. Hepatology. 2013;58:1814–1823. doi: 10.1002/hep.26419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lieber CS, DeCarli LM. The feeding of alcohol in liquid diets: two decades of applications and 1982 update. Alcohol Clin Exp Res. 1982;6:523–531. doi: 10.1111/j.1530-0277.1982.tb05017.x. [DOI] [PubMed] [Google Scholar]
- 23.Seth D, Haber PS, Syn WK, Diehl AM, Day CP. Pathogenesis of alcohol-induced liver disease: classical concepts and recent advances. J Gastroenterol Hepatol. 2011;26:1089–1105. doi: 10.1111/j.1440-1746.2011.06756.x. [DOI] [PubMed] [Google Scholar]
- 24.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–1585. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kane MA, Folias AE, Wang C, Napoli JL. Ethanol elevates physiological all-trans-retinoic acid levels in select loci through altering retinoid metabolism in multiple loci: a potential mechanism of ethanol toxicity. FASEB J. 2010;24:823–832. doi: 10.1096/fj.09-141572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maricic I, Halder R, Bischof F, Kumar V. Dendritic Cells and Anergic Type I NKT Cells Play a Crucial Role in Sulfatide-Mediated Immune Regulation in Experimental Autoimmune Encephalomyelitis. J Immunol. 2014 doi: 10.4049/jimmunol.1302898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hall JA, Grainger JR, Spencer SP, Belkaid Y. The role of retinoic acid in tolerance and immunity. Immunity. 2011;35:13–22. doi: 10.1016/j.immuni.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10:940–954. [PubMed] [Google Scholar]
- 29.Weigle N, McBane S. Psoriasis. Am Fam Physician. 2013;87:626–633. [PubMed] [Google Scholar]
- 30.Brennan PJ, Tatituri RV, Brigl M, Kim EY, Tuli A, Sanderson JP, Gadola SD, et al. Invariant natural killer T cells recognize lipid self antigen induced by microbial danger signals. Nat Immunol. 2011 doi: 10.1038/ni.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gapin L, Godfrey DI, Rossjohn J. Natural Killer T cell obsession with self-antigens. Curr Opin Immunol. 2013;25:168–173. doi: 10.1016/j.coi.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rossjohn J, Pellicci DG, Patel O, Gapin L, Godfrey DI. Recognition of CD1d-restricted antigens by natural killer T cells. Nat Rev Immunol. 2012;12:845–857. doi: 10.1038/nri3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kinjo Y, Kitano N, Kronenberg M. The role of invariant natural killer T cells in microbial immunity. J Infect Chemother. 2013;19:560–570. doi: 10.1007/s10156-013-0638-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hritz I, Mandrekar P, Velayudham A, Catalano D, Dolganiuc A, Kodys K, Kurt-Jones E, et al. The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology. 2008;48:1224–1231. doi: 10.1002/hep.22470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar V. NKT-cell subsets: Promoters and protectors in inflammatory liver disease. J Hepatol. 2013;59:618–620. doi: 10.1016/j.jhep.2013.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akbari O, Faul JL, Hoyte EG, Berry GJ, Wahlstrom J, Kronenberg M, DeKruyff RH, et al. CD4+ invariant T-cell-receptor+ natural killer T cells in bronchial asthma. N Engl J Med. 2006;354:1117–1129. doi: 10.1056/NEJMoa053614. [DOI] [PubMed] [Google Scholar]
- 37.Syn WK, Agboola KM, Swiderska M, Michelotti GA, Liaskou E, Pang H, Xie G, et al. NKT-associated hedgehog and osteopontin drive fibrogenesis in non-alcoholic fatty liver disease. Gut. 2012;61:1323–1329. doi: 10.1136/gutjnl-2011-301857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Apte UM, Banerjee A, McRee R, Wellberg E, Ramaiah SK. Role of osteopontin in hepatic neutrophil infiltration during alcoholic steatohepatitis. Toxicol Appl Pharmacol. 2005;207:25–38. doi: 10.1016/j.taap.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 39.Seth D, Gorrell MD, Cordoba S, McCaughan GW, Haber PS. Intrahepatic gene expression in human alcoholic hepatitis. J Hepatol. 2006;45:306–320. doi: 10.1016/j.jhep.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 40.Syn WK, Choi SS, Liaskou E, Karaca GF, Agboola KM, Oo YH, Mi Z, et al. Osteopontin is induced by hedgehog pathway activation and promotes fibrosis progression in nonalcoholic steatohepatitis. Hepatology. 2011;53:106–115. doi: 10.1002/hep.23998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol. 2008;8:685–698. doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lisbonne M, L’Helgoualc’h A, Nauwelaers G, Turlin B, Lucas C, Herbelin A, Piquet-Pellorce C, et al. Invariant natural killer T-cell-deficient mice display increased CCl(4) -induced hepatitis associated with CXCL1 over-expression and neutrophil infiltration. Eur J Immunol. 2011;41:1720–1732. doi: 10.1002/eji.201041006. [DOI] [PubMed] [Google Scholar]
- 43.Park O, Jeong WI, Wang L, Wang H, Lian ZX, Gershwin ME, Gao B. Diverse roles of invariant natural killer T cells in liver injury and fibrosis induced by carbon tetrachloride. Hepatology. 2009;49:1683–1694. doi: 10.1002/hep.22813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hisamori S, Tabata C, Kadokawa Y, Okoshi K, Tabata R, Mori A, Nagayama S, et al. All-trans-retinoic acid ameliorates carbon tetrachloride-induced liver fibrosis in mice through modulating cytokine production. Liver Int. 2008;28:1217–1225. doi: 10.1111/j.1478-3231.2008.01745.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.