Abstract
Objective
Platelets contribute to thrombosis, and platelet toll-like receptors (TLRs) are central in pathogen detection, potentially mediating infection-induced vascular occlusion. Using a large community-based cohort study, we sought to examine if platelets express all known TLR transcripts and analyze their association with cardiovascular risk factors.
Approach and result
Messenger RNA (mRNA) levels for TLRs were measured in isolated platelets by high-throughput quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) in 1625 participants (mean age 66.6±9, 54% women) of the Framingham Heart Study. We measured circulating inflammatory and thrombotic markers (CRP, IL6, MCP1, ICAM1, TNFR1, and P-selectin), and analyzed TLRs and their association with sex and cardiovascular risk factors by multivariable logit regression model adjusted for confounding factors. Platelets expressed all ten TLR-transcripts, and all TLRs were co-expressed. Women had higher platelet TLR expression, which associated with different cardiovascular risk factors as compared to men. In women, TLR1, TLR3, TLR6, and TLR7 were associated with BMI, and TLR5, TLR7, and TLR10 were associated with total cholesterol to HDL ratio. In men, TLR1, TLR2, and TLR3 were associated with lipid and TLR8 with hypertension treatment. Similarly, TLR expression in men was more commonly associated with circulating inflammatory markers (TNFR1, ICAM1), whereas in women TLR expression was associated with P-selectin levels.
Conclusion
We report the first study to demonstrate that platelets express all TLR transcripts using a large community-based observational cohort. These transcripts are more abundant in women and have distinct associations with cardiovascular risk and inflammatory biomarkers that vary by sex.
Introduction
Platelets mediate hemostasis and thrombosis and are detrimental in myocardial infarction and thrombotic stroke1, 2. Platelets are small circulating cell fragments that have no nucleus but carry prepackaged mRNA and proteins from their bone marrow precursors, the megakaryocytes2. Although small in size (2-5 um in humans), platelets are abundant and highly diverse in function spanning beyond hemostasis and thrombosis. Recently, platelets have been described as having a key role in innate immunity1-7 with evident crosstalk between the cardiovascular and the immune systems.
Initial immune response to microbial pathogens is mediated by pathogen recognition receptors, specifically, toll-like receptors (TLRs)8. Toll-like receptors recognize molecular structures that are broadly shared among pathogens. These pathogen-associated molecular patterns can induce TLR activation and initiate immune responses. In humans, ten TLRs have been identified, of which only TLR10 has unknown function8. Toll-like receptors can be classified based on their pathogen-sensing and subcellular localization. TLR1, TLR2, TLR4, TLR5, and TLR6 are expressed on the cell surface and sense structural protein components of foreign invaders. TLR3, TLR7, TLR8 and TLR9 are located intracellularly in the endosome compartments and sense foreign nucleic acids8. Surface TLRs recognize membrane components of bacterial origin (TLR1-TLR6), as well as molecular components on fungus (TLR2, TLR4, TLR6), parasites, and in some cases structural components of viruses (TLR2, TLR4)8. Endosomal TLRs sense single stranded RNA (TLR7 and TLR8), double stranded RNA (TLR3), and double stranded DNA (TLR9). Host viral defense is predominantly activated by the endosomal TLRs, although bacterial, fungal and parasite RNA/DNA can also initiate responses through these receptors8.
Preclinical studies have shown that platelets have functional TLR27, TLR45, 6, TLR99, 10, and their activation can be prothrombotic. TLR7 is also functional in platelets. However, stimulation of TLRs leads to activation of the innate immune system without directly interfering with platelet pro-thrombotic properties 3. Platelet-TLR2 and -TLR4 also are known to interact with the immune system by engaging the neutrophil population during stimulation 5-7. Finally, a deep sequencing study of platelets from four individuals showed that platelets may carry the mRNA transcripts of TLR1-TLR911. It is unclear, however, if human platelets broadly express these diverse TLR transcripts, if individuals express them simultaneously, and if these pro-inflammatory transcripts are associated with vascular inflammation or cardiovascular risk.
Previously, we utilized platelet mRNA from the Framingham Heart Study (FHS) Offspring Cohort (visit 8) to establish if platelets express inflammatory mRNA. The expression of inflammatory platelet transcripts closely associated with body mass index (BMI), suggesting that peripheral blood transcripts may reflect or contribute to the pathogenesis of coronary heart disease 12. The objective of our study was to look broadly at platelet-mediated immunity and inflammation by assessing the presence of platelet-specific TLR1-TLR10 transcripts in a large, well-characterized observational cohort and to determine their association with inflammation and cardiovascular risk factors. These findings provide the first data suggesting that platelets narrowly or broadly are associated with immunity, inflammation, and cardiovascular risk factors in a large unique community-based cohort.
Materials and Methods
Materials and methods are available in the online-only Data Supplement
Results
Human platelets broadly express TLR transcripts
We utilized previously isolated platelet RNA collected from 1625 participants in the FHS Offspring Cohort (visit 8, Table 1) and performed additional high-throughput RT-PCR measuring the expression of all known TLR (TLR1-TLR10) transcripts.
Table 1. Characteristics of the Framingham Offspring Study Sample Participants.
| Variable | |
|---|---|
| Sample Size | n=1625 |
| Women, n (%) | 880 (54) |
| Age, years | 66.6±8.6 |
| Body mass index, kg/m2 | 28.1±5.1 |
| Overweight - 25≤BMI<30, n (%) | 679 (42) |
| Obese - BMI≥30, n (%) | 489 (30) |
| Diabetes, n (%) | 232 (14) |
| Total cholesterol/HDL ratio, mg/100 mL | 3.5±1.1 |
| Triglycerides, mg/100 mL | 117±68 |
| Systolic blood pressure, mmHg | 129±17 |
| Diastolic blood pressure, mmHg | 73±10 |
| Prevalent coronary heart disease, n (%) | 171 (11) |
| Smoker, n (%) | 129 (8) |
| Antihypertensive Treatment, n (%) | 815 (50) |
| Lipid Treatment, n (%) | 715 (44) |
| Aspirin (3 times a week), n (%) | 743 (46) |
| Current hormone replacement therapy, n (%) | 94 (6%) |
HDL-high density lipoprotein; n=number of participants. Values are expressed at n (%) or mean ±SD.
After standard normalization to housekeeping genes 12, all TLR transcripts were identified in platelets but at variable levels of expression (Figure 1). To maintain relevant levels of percent distribution, TLR transcripts with dCT values smaller than 25 (i.e. higher expression levels) were utilized throughout this study (Figure 1). TLR9, TLR5 and TLR10 were expressed in the greatest number of participants, followed by TLR2 and TLR4; the transcripts with the lowest percent of expression were TLR6, TLR7 and TLR8 (Figure 1).
Figure 1. Percent of people expressing platelet TLRs with Ct<25.
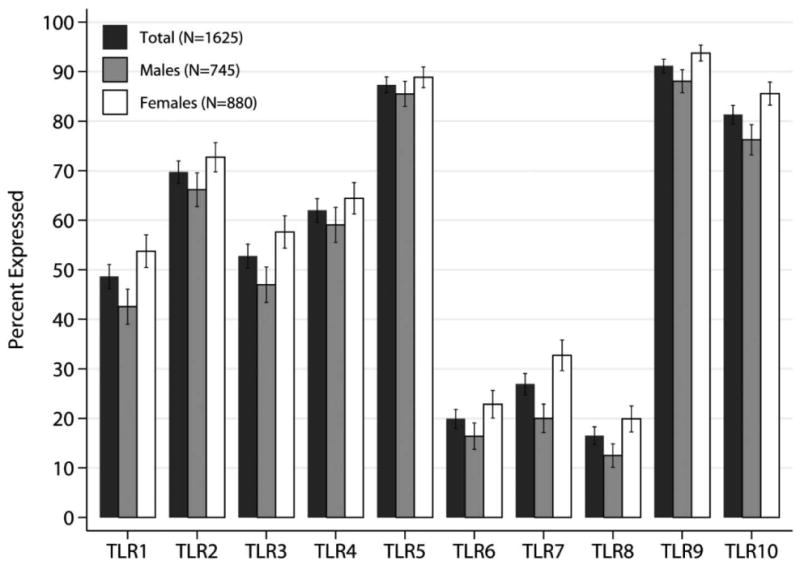
Bar graph plots of percent of individuals with each TLRs expressed in platelets from the total analyzed FHS sample population (n=1625, Visit 8; black bar), men (gray) and women (white) and confidence intervals (expressed as deviations). As shown in table 2, platelets of women were more likely to express all TLRs.
Co-expression of platelet TLRs
To assess the correlation between TLR transcript co-expression in platelets, we used a linear regression model correlating TLR mRNA levels with a correlation coefficient less than 0.5000. Grouping TLRs based on subcellular localization, transcripts of surface TLRs were highly co-expressed with the highest correlation coefficient between TLR4 and TLR2, followed by TLR4 and TLR1; TLR2 and TLR1; TLR6 and TLR1; TLR6 and TLR4 (Figure 2). Intracellular or endosomal TLR expression was strongly correlated only between TLR7 and TLR8. High association between co-expression of surface and intracellular TLR combinations were also observed, with the highest co-expression between TLR1 and TLR7 followed by TLR6 and TLR7 (Figure 2, Supplemental Table I).
Figure 2. Correlations between TLRs grouped based on cellular localization.
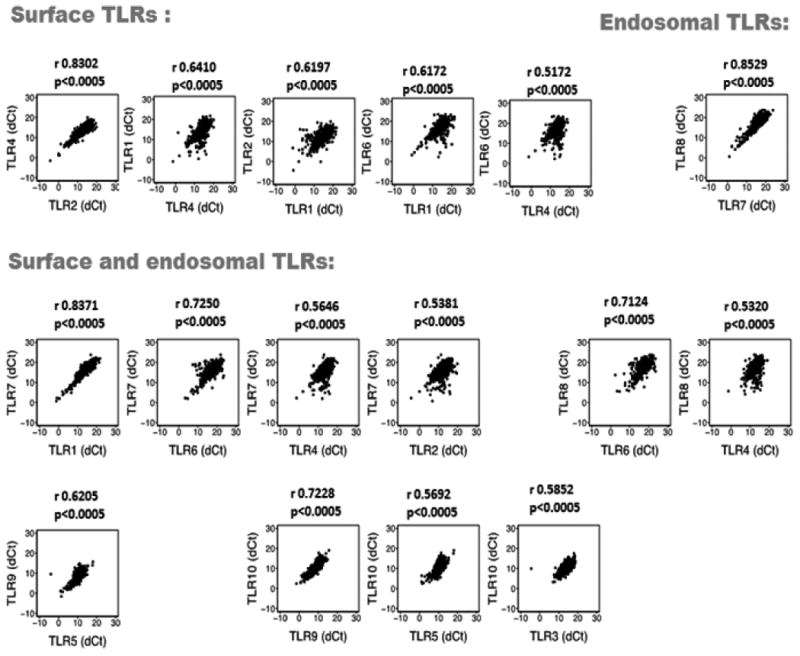
Scatter plots of TLRs in the platelets of people from the FHS (n=1625, Visit 8). Pearson correlation coefficient (r) was calculated between the normalized values of the transcript of each TLR pairs.
Association of Sex on TLR expression
Expression of all platelet TLRs was associated with sex. Platelets derived from women were more likely to have a higher percent of TLR transcript expressed as compared to men (Table 2). The most notable association of TLR distribution with sex was observed in the expression of TLR7, TLR9, TLR10 and TLR8 (in this order, Table 2, Figure 1); women had significantly higher transcript expression. Despite the higher distribution of TLR transcripts in women, the relative proportion among platelet TLRs was similar in men and women (Figure 1).
Table 2.
Association of sex with the expression of TLR Transcripts (Ct<25) in FHS Community Cohort (Visit 8, n=1625, 745 males, 880 females).
| OR (95%CI)* | p-value | |
|---|---|---|
| TLR1 | 1.66 (1.35, 2.05) | 1.3e-06 |
| TLR2 | 1.52 (1.22, 1.91) | 2.5e-04 |
| TLR3 | 1.68 (1.36, 2.06) | 9.9e-07 |
| TLR4 | 1.30 (1.05, 1.60) | 1.7e-02 |
| TLR5 | 1.37 (1.01, 1.87) | 4.5e-02 |
| TLR6 | 1.68 (1.30, 2.18) | 6.6e-05 |
| TLR7 | 2.21 (1.75, 2.80) | 4.8e-11 |
| TLR8 | 2.00 (1.51, 2.64) | 1.2e-06 |
| TLR9 | 2.07 (1.43, 3.00) | 1.1e-04 |
| TLR10 | 2.07 (1.58, 2.70) | 9.8e-08 |
Odds ratios (OR) from logistic regression models predicting TLR expression in women compared to men adjusting for clinical confounders listed in Table 1.
Interestingly, in a subpopulation with normal BMI, only the transcripts of TLR6, TLR7 and TLR8 are significantly increased in women when compared to men (Supplemental Table IIA, Demographics in Supplemental Table IIB).
Cardiovascular Risk Factors and platelet-TLR expression
Consistent with previous studies, higher mean BMI was most consistently associated with higher TLR expression (Table 3, Supplemental Table III)12 in the entire cohort. Additionally, aspirin intake (presence vs. absence of ingestion ≥3 times per week) was associated with decreased expression of TLR1, TLR3, and TLR4 in platelets (Table 3). Lipid treatment was associated with higher platelet-TLR1, TLR2, TLR3 and TLR9 (Table 3).
Table 3. Statistically significant (p<0.05) associations with CVD measures and TLR gene expression (Ct<25) in FHS Community Cohort (N=1625).
| BMI OR (95%CI)* |
Diastolic BP OR (95%CI)* |
Aspirin OR (95%CI)* |
Anti-Lipid Rx OR (95%CI)* |
|
|---|---|---|---|---|
|
| ||||
| TLR1 | 1.02 (1.00, 1.04) | --- | 0.80 (0.65, 0.99) | 1.31 (1.05, 1.63) |
| TLR2 | 1.03 (1.01, 1.05) | --- | --- | 1.44 (1.13, 1.84) |
| TLR3 | 1.02 (1.00, 1.04) | --- | 0.81 (0.66, 0.99) | 1.25 (1.01, 1.56) |
| TLR4 | --- | --- | 0.78 (0.63, 0.96) | --- |
| TLR5 | --- | --- | --- | --- |
| TLR6 | 1.03 (1.01, 1.05) | 0.98 (0.97, 1.00) | --- | --- |
| TLR7 | 1.03 (1.01, 1.06) | --- | --- | --- |
| TLR8 | 1.04 (1.01, 1.06) | --- | --- | --- |
| TLR9 | --- | --- | --- | 1.49 (1.00, 2.21) |
| TLR10 | --- | --- | --- | --- |
Odds ratios (OR) from logistic regression models associated with TLR expression adjusting for clinical confounders listed in table 1. ORs are expressed per one unit increase of continuous measures and presence vs. absence of use for medications. Only statistically significant associations are listed in this table. Full model results available in Supplemental Table III. BMI-body mass index, BP- blood pressure, lipid Rx-lipid lowering treatment, aspirin taken ≥3 times a week.
Since we observed a marginal sex effect in expression levels we decided to address correlates stratified by sex. Indeed, our data revealed differences in associations of TLRs and cardiovascular risk factors between men and women. Table 4 and Supplemental Table IV show that BMI was associated with greater expression of platelet TLR1, TLR3, TLR6, TLR7 only in women. Additionally, only in women, total cholesterol to HDL ratio was associated with higher expression of platelet TLR5, TLR7 and TLR10 while lipid treatment was associated with greater TLR expression only in men.
Table 4. Statistically significant (p<0.05) associations with CVD parameters and TLR gene expression (Ct<25) in FHS Community Cohort (N=1625, Visit 8) stratified by Sex.
| Cholesterol:HDL OR (95%CI)* |
BMI OR (95%CI)* |
SBP OR (95%CI)* |
DBP OR (95%CI)* |
Lipid Rx OR (95%CI)* |
Hypertensive Rx OR (95%CI)* |
|
|---|---|---|---|---|---|---|
| Females (N=880) | ||||||
|
| ||||||
| TLR1 | --- | 1.03 (1.00, 1.06) | --- | --- | --- | --- |
| TLR3 | --- | 1.03 (1.00, 1.06) | --- | --- | --- | --- |
| TLR5 | 1.49 (1.08, 2.06) | --- | --- | --- | --- | --- |
| TLR6 | --- | 1.03 (1.00, 1.06) | --- | --- | --- | --- |
| TLR7 | 1.22 (1.01, 1.46) | 1.04 (1.01, 1.07) | --- | --- | --- | --- |
| TLR8 | --- | 1.03 (1.00, 1.06) | --- | --- | --- | --- |
| TLR10 | 1.33 (1.00, 1.77) | --- | --- | --- | --- | |
|
| ||||||
| Males (N=745) | ||||||
|
| ||||||
| TLR1 | --- | --- | --- | --- | 1.58 (1.13, 2.21) | --- |
| TLR2 | --- | --- | --- | --- | 1.50 (1.05, 2.13) | --- |
| TLR3 | --- | --- | --- | --- | 1.42 (1.02, 1.98) | --- |
| TLR4 | --- | --- | --- | --- | --- | --- |
| TLR6 | --- | --- | 1.01 (1.00, 1.03) | 0.97 (0.95, 1.00) | --- | --- |
| TLR7 | --- | --- | --- | --- | --- | --- |
| TLR8 | --- | 1.05 (1.01, 1.09) | --- | --- | --- | 1.78 (1.08, 2.92) |
Odds ratios (OR) from logistic regression models predicting TLR expression adjusting for clinical confounders listed in table 1. ORs are expressed per one unit increase of continuous measures and presence vs. absence of use for medications. Full model results available in Supplemental Table IV. Note: Cholesterol:HDL is total cholesterol to HDL ratio, BMI-body mass index, SBP-systolic blood pressure, DBP-diastolic blood pressure, lipid Rx-lipid lowering treatment, hypertensive Rx-hypertensive treatment.
Inflammatory circulating biomarkers and platelet-TLR expression
To assess the relationship between general vascular inflammation and platelet immune gene expression, circulating cardiovascular inflammatory biomarkers were correlated with platelet-TLR expression (Table 5a, Table 5b, and Supplemental Table V-VII). Interestingly, there were sex-dependent and sex-independent associations. TLR3, TLR7, and TLR10 were associated with elevated CRP levels in sex-independent fashion. Similarly, in both sexes, an increase in ICAM1 levels, a risk factor for coronary events13, was associated with higher levels of TLR2, TLR4, TLR5, TLR6 and TLR10 transcripts. IL6 was associated with TLR2, TLR3 and TLR4. MCP1, a cytokine predicting adverse cardiovascular events14, 15 was associated with greater expression of only TLR1 in both men and women (Supplemental Table V).
Table 5A. Associations with measured serum biomarkers and TLR gene expression (Ct<25) in FHS Community Cohort (N=880, Visit 8) -- Women.
| CRP OR (95%CI) |
ICAM1 OR (95%CI) |
IL6 OR (95%CI) |
MCP1 OR (95%CI) |
TNFR OR (95%CI) |
P-selectin OR (95%CI) |
|
|---|---|---|---|---|---|---|
| TLR1 | --- | --- | --- |
1.64 (1.03, 2.60) p=3.7e-02 |
1.86 (1.20, 2.90) p=5.6e-03 |
--- |
| TLR2 | --- |
1.97 (1.07, 3.62) p=2.9e-02 |
1.47 (1.08, 2.00) p=1.5e-02 |
1.77 (1.05, 2.99) p=3.3e-02 |
--- | 1.83 (1.11, 3.01) p=1.7e-02 |
| TLR3 |
1.19 (1.01, 1.41) p=3.3e-02 |
--- |
1.37 (1.04, 1.80) p=2.6e-02 |
--- | --- | 1.60 (1.02, 2.51) p=4.0e-02 |
| TLR4 | --- |
2.12 (1.20, 3.74) p=9.4e-03 |
1.43 (1.08, 1.91) p=1.4e-02 |
2.29 (1.40, 3.74) p=9.9e-04 |
2.07 (1.29, 3.30) p=2.4e-03 |
2.29 (1.43, 3.66) p=5.2e-04 |
| TLR5 | 1.33 (1.03, 1.71) p=3.1e-02 |
4.06 (1.68, 9.84) p=1.9e-03 |
--- | --- | --- | 2.19 (1.08, 4.42) p=3.0e-02 |
| TLR6 | --- |
2.12 (1.13, 3.97) p=1.9e-02 |
--- | --- |
2.28 (1.38, 3.79) p=1.4e-03 |
1.70 (1.00, 2.89) p=4.8e-02 |
| TLR7 |
1.20 (1.01, 1.43) p=3.5e-02 |
--- | --- | --- |
1.74 (1.10, 2.75) p=1.8e-02 |
1.86 (1.15, 2.99) p=1.1e-02 |
| TLR8 | --- | --- | --- | --- |
1.86 (1.10, 3.17) p=2.2e-02 |
--- |
| TLR9 | 1.52 (1.08, 2.14) p=1.7e-02 |
--- | --- | --- | --- | 3.16 (1.26, 7.90) p=1.4e-02 |
| TLR10 |
1.31 (1.04, 1.64) p=2.2e-02 |
2.32 (1.07, 5.02) p=3.2e-02 |
1.75 (1.17, 2.61) p=6.5e-03 |
--- | --- | --- |
Odds ratios (OR) from logistic regression models predicting TLR expression adjusting for clinical confounders listed in Table 1 using the log-transformed assay levels. ORs are expressed per one unit increase of continuous measures. Associations in bold font are common for (both) men and women; associations in purple are predictive only in women. Abbreviations are as follows: CRP-C-reactive protein; ICAM1-intracellular cell adhesion molecule 1; IL6-intralukin 6; MCP1-monocyte chemoattractant protein 1; TNFR- soluble tumor necrosis factor alpha receptor 1; P-selectin- soluble platelet selectin. Full results are available in Supplemental Table VI.
Table 5B. Associations with measured serum biomarkers and TLR gene expression (Ct<25) in FHS Community Cohort (N=745, Visit 8) -- Men.
| CRP OR (95%CI) |
ICAM1 OR (95%CI) |
IL6 OR (95%CI) |
MCP1 OR (95%CI) |
TNFR OR (95%CI) |
P-selectin OR (95%CI) |
|
|---|---|---|---|---|---|---|
| TLR1 | 1.37 (1.14, 1.66) p=9.4e-04 | 1.95 (1.14, 3.33) p=1.4e-02 | 1.79 (1.33, 2.39) p=1.0e-04 |
2.38 (1.44, 3.92) p=7.1e-04 |
2.89 (1.79, 4.68) p=1.6e-05 |
--- |
| TLR2 | 1.22 (1.01, 1.48) p=4.1e-02 |
2.57 (1.43, 4.61) p=1.6e-03 |
1.47 (1.08, 2.00) p=1.3e-02 |
--- | 3.90 (2.29, 6.64) p=5.5e-07 |
--- |
| TLR3 |
1.20 (1.00, 1.44) p=4.8e-02 |
--- |
1.52 (1.14, 2.03) p=3.9e-03 |
2.54 (1.55, 4.16) p=2.2e-04 |
2.42 (1.51, 3.87) p=2.3e-04 |
--- |
| TLR4 | 1.27 (1.06, 1.53) p=1.1e-02 |
2.92 (1.66, 5.16) p=2.2e-04 |
1.48 (1.10, 1.98) p=9.3e-03 |
--- |
3.74 (2.26, 6.20) p=2.9e-07 |
--- |
| TLR5 | --- |
2.32 (1.08, 5.02) p=3.2e-02 |
--- | 2.32 (1.18, 4.56) p=1.5e-02 |
2.88 (1.43, 5.79) p=2.9e-03 |
--- |
| TLR6 | --- |
2.43 (1.23, 4.81) p=1.1e-02 |
1.42 (0.98, 2.06) p=6.5e-02 |
--- |
3.14 (1.73, 5.71) p=1.7e-04 |
--- |
| TLR7 |
1.38 (1.09, 1.74) p=6.5e-03 |
2.70 (1.43, 5.10) p=2.2e-03 |
1.36 (0.96, 1.92) p=8.7e-02 |
2.28 (1.23, 4.21) p=8.6e-03 |
2.66 (1.52, 4.67) p=6.6e-04 |
--- |
| TLR8 | --- | --- | 1.60 (1.05, 2.44) p=2.8e-02 |
--- |
2.66 (1.36, 5.20) p=4.2e-03 |
--- |
| TLR9 | --- | 2.55 (1.11, 5.85) p=2.8e-02 |
--- | 2.18 (1.05, 4.55) p=3.7e-02 |
6.34 (2.79, 14.41) p=1.0e-05 |
--- |
| TLR10 |
1.31 (1.06, 1.62) p=1.4e-02 |
2.80 (1.45, 5.40) p=2.1e-03 |
--- | --- | 2.57 (1.45, 4.54) p=1.2e-03 |
--- |
Odds ratios (OR) from logistic regression models predicting TLR expression adjusting for clinical confounders listed in table 1 using the log-transformed assay levels. ORs are expressed per unit of continuous measures. Associations in bold font are common for (both) men and women; associations in blue are predictive only in men; Abbreviations are as follows: CRP-C-reactive protein; ICAM1-intracellular cell adhesion molecule 1; IL6-intralukin 6; MCP1-monocyte chemoattractant protein 1; TNFR- soluble tumor necrosis factor alpha receptor 1; P-selectin- soluble platelet selectin. Full results are available in Supplemental Table VII.
Once stratified by sex, however, distinct associations between plasma biomarkers and platelet TLR transcripts were evident. In women, TLR expression (except TLR1, TLR8, and TLR10) was associated with higher mean plasma P-selectin levels. None of the platelet-TLRs in men were associated with P-selectin. In men, higher levels in the cardiovascular inflammatory mediators TNFR1 and ICAM1 were associated with specific TLRs (TNFR1 with TLR2, TLR3, TLR5, TLR9 and TLR10 and ICAM1 with TLR1, TLR6, TLR7 and TLR9). For IL6, there was an association with higher levels of TLR1, TLR6, TLR7 and TLR9 in men. MCP1 levels were associated with higher levels of TLR3, TLR5, TLR7 and TLR9 in men which was not observed in women. In women high IL6 levels were associated with TLR10 expression and high MCP1 was correlated with TLR2 and TLR10. These data suggest that in addition to the common inflammatory profile correlated with platelet TLRs, platelet TLRs derived from women associate with pro-thrombotic P-selectin and in men TLR expression associates with the inflammatory mediators TNFR1 and ICAM1.
Correlation between TLRs and expression of transcripts coding for proteins involved in their downstream signaling pathways (MyD88) or endosomal translocation (UNCB93B1)
In cells, all TLRs with the exception of TLR3 (no data currently available for TLR10) can signal by engaging the myeloid differentiation primary response gene 88 (MyD88) adapter-protein pathway and eventually initiate nuclear events8. As seen in Table 6, presence of MyD88 transcript in platelets was associated with higher expression of all TLRs with the exception of TLR10. These data are consistent with co-expression of TLRs and their downstream signaling pathways.
Table 6. Co-expression of MYD88 and UNC93B1 (dCt values*) and TLR gene expression (Ct<25) in FHS Community Cohort (N=1625, Visit 8).
| MyD88 OR (95%CI)* |
UNC93B1 OR (95%CI)* |
|
|---|---|---|
| TLR1 | 1.38 (1.31, 1.45) p=6.0e-36 |
1.40 (1.33, 1.48) p=3.1e-33 |
| TLR2 | 1.07 (1.02, 1.11) p=2.9e-03 |
1.37 (1.30, 1.45) p=5.3e-30 |
| TLR3 | 1.10 (1.06, 1.15) p=5.7e-07 |
1.53 (1.44, 1.63) p=4.2e-44 |
| TLR4 | 1.05 (1.01, 1.10) p=7.3e-03 |
1.34 (1.27, 1.41) p=3.5e-28 |
| TLR5 | 0.88 (0.83, 0.92) p=9.1e-08 |
1.38 (1.29, 1.47) p=2.3e-23 |
| TLR6 | 1.28 (1.22, 1.34) p=4.4e-23 |
1.45 (1.36, 1.56) p=2.2e-25 |
| TLR7 | 1.54 (1.46, 1.64) p=3.7e-46 |
1.46 (1.37, 1.56) p=1.0e-29 |
| TLR8 | 1.57 (1.47, 1.68) p=1.9e-41 |
1.55 (1.43, 1.68) p=3.0e-27 |
| TLR9 | 0.88 (0.83, 0.93) p=4.9e-06 |
1.41 (1.32, 1.52) p=2.3e-22 |
| TLR10 | 0.97 (0.92, 1.01) p=1.2e-01 |
1.61 (1.50, 1.72) p=9.9e-41 |
Odds ratios (OR) from logistic regression models predicting TLR expression adjusting for clinical confounders listed in table 1. MyD88 (Myeloid differentiation primary response gene 88) and UNC93B1 (UNC-93 homolog B1) dCt values were inverted so that OR>1 indicate increased expression in those expressing TLR and OR<1 indicated decreased expression in those expressing TLRs.
UNC93B1 is a protein associated with the translocation of TLR3, TLR7 and TLR9 from the endoplasmic reticulum to the endolysosomes16. Platelets lack proper endosomal structures but contain lysosomes. In our study, UNC93B1 in platelets strongly associated with presence of all of TLRs (Table 6).
Discussion
Platelets play an intricate role in thrombosis, myocardial infarction, and thrombotic stroke and have functional TLR2, TLR4, TLR9 and TLR73, 6, 7, 10. TLR1, TLR2, and TLR4 are expressed in human atherosclerotic lesions17 and TLR4 polymorphism (Asp299Gly abolishing signaling) is associated with reduced atherosclerosis18, atherosclerosis progression19, and with decreased risk of acute coronary events17. Using the FHS Offspring cohort, we sought to examine if platelets demonstrate specific immunity or broadly express TLRs and if presence of TLRs in platelets is associated with vascular inflammation and/or cardiovascular risk factors. We report that platelets expressed all TLRs, but at variable levels. Further, platelet TLRs were found at higher percent in women and the TLRs varied in their association with different cardiovascular risk factors and circulating inflammatory biomarkers by sex.
The ultimate function of TLRs is to initiate the initial immune response to infections. Platelets and platelet-TLRs are instrumental in the initial response to infection and ablation of platelets before viral infection3 or LPS-induced septic shock20 leads to decreased survival in mice. Interestingly, men are generally more susceptible to a vast onset of bacterial, viral, or parasite infections than women 21-23 and have lower survival rates23. In the case of hospital-acquired pneumonia, or HIV, however, women experience worse clinical outcomes24, 25. Here we show that not only are women more likely to have higher levels of platelet-TLRs, but these TLRs associate with different inflammatory patterns. In addition, even in a sub-cohort with normal BMI, women continued to have higher expression of TLR6, TLR7 and TLR8. It is possible that platelets, as the most abundant blood component (after red blood cells), are contributing to the sex-mediated response during infection and consequently contributing to morbidity and mortality.
It has long been established that cardiovascular outcome varies by sex. Diabetes and hypertension are stronger contributors to the outcome of myocardial infarction in women than in men26, 27. Platelets play a key role in myocardial infarction by adhering to the raptured atherosclerotic plaque and thereby contribute to the pathogenesis of the event. As previously discussed, some platelet TLRs have been described as pro-thrombotic and this may contribute to clot burden in the setting of MI.
Using a large community-based cohort study, we report significant associations between cardiovascular risk factors, inflammatory circulating cytokines, and platelet transcripts that vary by sex. The variation by sex is particularly interesting as only TLR7 and TLR8 are located on the X chromosome; the remaining TLRs are on autosomal chromosomes (TLR1, TLR2, TLR3, TLR6 and TLR10 on chromosome 4, TLR4 on chromosome 9, TLR5 on chromosome 1)28. In our study, cardiovascular risk factors including BMI and total cholesterol to HDL ratio were associated with TLR transcripts predominantly in women. Blood pressure, hypertensive treatment, and lipid-lowering treatment associated with platelet TLR expression only in men. Furthermore, these observations are interesting as sex-mediated differences in platelet-TLR signatures may translate into modifications of therapies that may lead to more effective, sex-targeted, clinical outcome.
Circulating inflammatory biomarkers also had varied in their patterns of association with TLR expression by sex. Interestingly, differences in cytokine profiles between men and women have been described during infection21, 29 but have not been stratified during cardiovascular events. In our study, plasma P-selectin, a pro-thrombotic biomarker, was associated with TLR expression only in women. In men, inflammatory biomarkers predicting cardiovascular risk, such as TNFR1, ICAM1 and MCP1, were associated with a greater number of TLRs as compared to women.
Cardiovascular, sex-dependent differences have been described with TLR genetic variants. TLR4 (Asp229Gly) polymorphism, for instance, is associated with an increased risk of myocardial infarction in men but not in women30. These findings suggest that TLR signature in men and women may be differentially regulated in platelets depending on the inflammatory status and may lead to differences in cardiovascular risk.
Downstream signaling of all TLRs, with the exception of TLR3 (TLR10 signaling is unknown), involve physical engagement of the MyD88 adopter protein and initiate distinct inflammatory responses in nucleated cells. In platelets, MyD88 is able to mediate granular release and potentiate platelet aggregation in a TLR4-, and TLR9-dependent manner 9, 14. The current study suggests that TLR signaling in platelets functions in a conventional manner and requires MyD88. The fact that TLR3 associates with Myd88 is not surprising as TLR3 is co-expressed with other TLRs in platelets. Further, UNC93B1 is an ER protein necessary for the trafficking of endosomal TLRs from the ER to the endosomes or lysosomes16. Thus, the association between the UNC93B1 transcript and other TLRs, although unexpected, is consistent with co-expression between endosomal and surface receptors.
A limitation of our study is that the cohort was comprised of middle-age to older adults of European descent. A polymorphism of TLR7-TLR8 for instance has been associated with differential outcomes depending on sex in Swedish vs. Chinese populations31. Whether our findings will generalize to other ages or races/ethnicities is uncertain. However, our study is particularly important in reference to previous observations that older women may have a worse outcome in the setting of cardiovascular disease as compared to men13. Our study was cross-sectional and observational; hence the temporality of the associations and whether they are causal cannot be determined. We cannot exclude residual confounding or false positive findings by virtue of multiple testing.
In conclusion, our large human expression data are the first to demonstrate that all TLR transcripts are present in platelets and these transcripts are more likely to be expressed at higher levels in women. Additionally, these data are the first to show that, depending on sex, platelet TLR transcripts associate with different cardiovascular risk factors and circulating inflammatory levels. Further study will be required to clarify whether platelet TLRs provide a link between infection, immunity and CVD that may be distinct between men and women.
Supplementary Material
Significance.
Cardiovascular disease and infection exhibit sex differences, but these variances are poorly understood and relatively understudied. Platelets are central in mediating thrombosis, cardiovascular outcomes and more recently have been described as instrumental in host survival during infections. Toll-like receptors (TLRs) have been described as crucial for initiating the immune response to foreign pathogens. There is a growing appreciation that infections are associated with an increased risk of cardiovascular diseases including myocardial infarction and stroke due to prothrombotic events that occlude blood vessels. In a large community-based study involving 1625 participants (Framingham Heart Study), we found that platelets express all TLR transcripts at variable levels and, surprisingly, women had higher levels of all known TLRs (1-10). Further, men and women have distinct associations with cardiovascular risk and inflammatory biomarkers. This is the first epidemiological study using a large cohort that describes associations of platelet TLRs with cardiovascular risk factors and inflammation.
Acknowledgments
MK and JEF designed, interpreted the results, and wrote this manuscript. EM conducted and provided the statistical analysis. EJB was involved in the collection of the FHS inflammatory biomarker data. EM and KT run the quantitative PCR. All authors edited this manuscript.
Sources of funding: This work was supported by N01-HC 25195, RFA-HL-12-008 (to J.E. Freedman), RFA-RM-12-013 (to J.E. Freedman), P01-HL085381 (to J.E. Freedman), and 1RO1 HL64753, R01 HL076784, and 1 R01 AG028321 (to E.J. Benjamin) from the National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH).
Abbreviations
- TLR
toll-like receptors
- FHS
Framingham Heart Study
- CRP
C-reactive protein
- ICAM1
intracellular cell adhesion molecule 1
- IL6
intralukin 6
- MCP1
monocyte chemoattractant protein 1
- TNFR1
soluble tumor necrosis factor alpha receptor 1
- P-selectin
soluble platelet selectin
- dCT
delta cycle threshold
Footnotes
Disclosures: None.
References
- 1.Rondina MT, Weyrich AS, Zimmerman GA. Platelets as cellular effectors of inflammation in vascular diseases. Circ Res. 2013;112:1506–1519. doi: 10.1161/CIRCRESAHA.113.300512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Semple JW, Italiano JE, Jr, Freedman J. Platelets and the immune continuum. Nat Rev Immunol. 2011;11:264–274. doi: 10.1038/nri2956. [DOI] [PubMed] [Google Scholar]
- 3.Koupenova M, Vitseva O, MacKay CR, Beaulieu LM, Benjamin EJ, Mick E, Kurt-Jones EA, Ravid K, Freedman JE. Platelet-tlr7 mediates host survival and platelet count during viral infection in the absence of platelet-dependent thrombosis. Blood. 2014;124:791–802. doi: 10.1182/blood-2013-11-536003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blair P, Rex S, Vitseva O, Beaulieu L, Tanriverdi K, Chakrabarti S, Hayashi C, Genco CA, Iafrati M, Freedman JE. Stimulation of toll-like receptor 2 in human platelets induces a thromboinflammatory response through activation of phosphoinositide 3-kinase. Circulation Research. 2009;104:346–354. doi: 10.1161/CIRCRESAHA.108.185785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andonegui G, Kerfoot SM, McNagny K, Ebbert KV, Patel KD, Kubes P. Platelets express functional toll-like receptor-4. Blood. 2005;106:2417–2423. doi: 10.1182/blood-2005-03-0916. [DOI] [PubMed] [Google Scholar]
- 6.Clark SR, Ma AC, Tavener SA, et al. Platelet tlr4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 7.Blair P, Rex S, Vitseva O, Beaulieu L, Tanriverdi K, Chakrabarti S, Hayashi C, Genco CA, Iafrati M, Freedman JE. Stimulation of toll-like receptor 2 in human platelets induces a thromboinflammatory response through activation of phosphoinositide 3-kinase. Circ Res. 2009;104:346–354. doi: 10.1161/CIRCRESAHA.108.185785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Panigrahi S, Ma Y, Hong L, Gao D, West XZ, Salomon RG, Byzova TV, Podrez EA. Engagement of platelet toll-like receptor 9 by novel endogenous ligands promotes platelet hyperreactivity and thrombosis. Circ Res. 2013;112:103–112. doi: 10.1161/CIRCRESAHA.112.274241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thon JN, Peters CG, Machlus KR, Aslam R, Rowley J, Macleod H, Devine MT, Fuchs TA, Weyrich AS, Semple JW, Flaumenhaft R, Italiano JE., Jr T granules in human platelets function in tlr9 organization and signaling. J Cell Biol. 2012;198:561–574. doi: 10.1083/jcb.201111136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rowley JW, Oler AJ, Tolley ND, Hunter BN, Low EN, Nix DA, Yost CC, Zimmerman GA, Weyrich AS. Genome-wide rna-seq analysis of human and mouse platelet transcriptomes. Blood. 2011;118:e101–111. doi: 10.1182/blood-2011-03-339705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freedman JE, Larson MG, Tanriverdi K, O'Donnell CJ, Morin K, Hakanson AS, Vasan RS, Johnson AD, Iafrati MD, Benjamin EJ. Relation of platelet and leukocyte inflammatory transcripts to body mass index in the framingham heart study. Circulation. 2010;122:119–129. doi: 10.1161/CIRCULATIONAHA.109.928192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roquer J, Campello AR, Gomis M. Sex differences in first-ever acute stroke. Stroke. 2003;34:1581–1585. doi: 10.1161/01.STR.0000078562.82918.F6. [DOI] [PubMed] [Google Scholar]
- 14.Zhang G, Han J, Welch EJ, Ye RD, Voyno-Yasenetskaya TA, Malik AB, Du X, Li Z. Lipopolysaccharide stimulates platelet secretion and potentiates platelet aggregation via tlr4/myd88 and the cgmp-dependent protein kinase pathway. J Immunol. 2009;182:7997–8004. doi: 10.4049/jimmunol.0802884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Lemos JA, Morrow DA, Blazing MA, Jarolim P, Wiviott SD, Sabatine MS, Califf RM, Braunwald E. Serial measurement of monocyte chemoattractant protein-1 after acute coronary syndromes: Results from the a to z trial. J Am Coll Cardiol. 2007;50:2117–2124. doi: 10.1016/j.jacc.2007.06.057. [DOI] [PubMed] [Google Scholar]
- 16.Blasius AL, Beutler B. Intracellular toll-like receptors. Immunity. 2010;32:305–315. doi: 10.1016/j.immuni.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 17.Ameziane N, Beillat T, Verpillat P, Chollet-Martin S, Aumont MC, Seknadji P, Lamotte M, Lebret D, Ollivier V, de Prost D. Association of the toll-like receptor 4 gene asp299gly polymorphism with acute coronary events. Arterioscler Thromb Vasc Biol. 2003;23:e61–64. doi: 10.1161/01.ATV.0000101191.92392.1D. [DOI] [PubMed] [Google Scholar]
- 18.Kiechl S, Lorenz E, Reindl M, Wiedermann CJ, Oberhollenzer F, Bonora E, Willeit J, Schwartz DA. Toll-like receptor 4 polymorphisms and atherogenesis. N Engl J Med. 2002;347:185–192. doi: 10.1056/NEJMoa012673. [DOI] [PubMed] [Google Scholar]
- 19.Netea MG, Hijmans A, van Wissen S, Smilde TJ, Trip MD, Kullberg BJ, de Boo T, Van der Meer JW, Kastelein JJ, Stalenhoef AF. Toll-like receptor-4 asp299gly polymorphism does not influence progression of atherosclerosis in patients with familial hypercholesterolaemia. Eur J Clin Invest. 2004;34:94–99. doi: 10.1111/j.1365-2362.2004.01303.x. [DOI] [PubMed] [Google Scholar]
- 20.Xiang B, Zhang G, Guo L, Li XA, Morris AJ, Daugherty A, Whiteheart SW, Smyth SS, Li Z. Platelets protect from septic shock by inhibiting macrophage-dependent inflammation via the cyclooxygenase 1 signalling pathway. Nat Commun. 2013;4:2657. doi: 10.1038/ncomms3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klingstrom J, Lindgren T, Ahlm C. Sex-dependent differences in plasma cytokine responses to hantavirus infection. Clin Vaccine Immunol. 2008;15:885–887. doi: 10.1128/CVI.00035-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klein SL. The effects of hormones on sex differences in infection: From genes to behavior. Neurosci Biobehav Rev. 2000;24:627–638. doi: 10.1016/s0149-7634(00)00027-0. [DOI] [PubMed] [Google Scholar]
- 23.Moore SL, Wilson K. Parasites as a viability cost of sexual selection in natural populations of mammals. Science. 2002;297:2015–2018. doi: 10.1126/science.1074196. [DOI] [PubMed] [Google Scholar]
- 24.Crabtree TD, Pelletier SJ, Gleason TG, Pruett TL, Sawyer RG. Gender-dependent differences in outcome after the treatment of infection in hospitalized patients. JAMA. 1999;282:2143–2148. doi: 10.1001/jama.282.22.2143. [DOI] [PubMed] [Google Scholar]
- 25.Cescon A, Patterson S, Chan K, Palmer AK, Margolese S, Burchell AN, Cooper C, Klein MB, Machouf N, Montaner JS, Tsoukas C, Hogg RS, Raboud JM, Loutfy MR. Gender differences in clinical outcomes among hiv-positive individuals on antiretroviral therapy in canada: A multisite cohort study. PLoS One. 2013;8:e83649. doi: 10.1371/journal.pone.0083649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barrett-Connor EL, Cohn BA, Wingard DL, Edelstein SL. Why is diabetes mellitus a stronger risk factor for fatal ischemic heart disease in women than in men? The rancho bernardo study. JAMA. 1991;265:627–631. [PubMed] [Google Scholar]
- 27.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the interheart study): Case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 28.Armant MA, Fenton MJ. Toll-like receptors: A family of pattern-recognition receptors in mammals. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-8-reviews3011. REVIEWS3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fish EN. The x-files in immunity: Sex-based differences predispose immune responses. Nat Rev Immunol. 2008;8:737–744. doi: 10.1038/nri2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edfeldt K, Bennet AM, Eriksson P, Frostegard J, Wiman B, Hamsten A, Hansson GK, de Faire U, Yan ZQ. Association of hypo-responsive toll-like receptor 4 variants with risk of myocardial infarction. Eur Heart J. 2004;25:1447–1453. doi: 10.1016/j.ehj.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Nilsson D, Andiappan AK, Hallden C, De Yun W, Sall T, Tim CF, Cardell LO. Toll-like receptor gene polymorphisms are associated with allergic rhinitis: A case control study. BMC Med Genet. 2012;13:66. doi: 10.1186/1471-2350-13-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


