Abstract
Background
To determine the MTD of Seneca Valley Virus (NTX-010) in children with relapsed/refractory solid tumors. Patients (≥ 3 to ≤ 21 years) with neuroblastoma, rhabdomyosarcoma, or rare tumors with neuroendocrine features were eligible.
Procedure
Part A (single dose of NTX-010) enrolled 13 patients at 3 dose levels [1×109 viral particles (vp)/kg (n=6), 1×1010 vp/kg (n=3), 1× 1011 vp/kg (n=4)]. Diagnoses included neuroblastoma (n=9), rhabdomyosarcoma (n=2), carcinoid tumor (n=1), and adrenocorticocarcinoma (n=1). Part B added cyclophosphamide (CTX) (oral CTX (25 mg/m2/day) days 1-14 and IV CTX (750 mg/m2) days 8 and 29) to two doses of NTX-010 (1×1011 vp/kg, days 8 and 29). Nine patients enrolled to Part B. Diagnoses included neuroblastoma (n=3), rhabdomyosarcoma (n=1), Wilms tumor (n=3), and adrenocorticocarcinoma (n=2).
Results
Twelve patients on Part A were evaluable for toxicity. There was a single DLT (grade 3 pain) at dose level 1. Additional grade ≥3 related adverse events (AEs) included leukopenia (n=1), neutropenia (n=3), lymphopenia (n=3), and tumor pain (n=1). No DLTs occurred on part B. Other grade ≥3 related AEs on Part B included: leukopenia (n=3), nausea (n=1), emesis (n=1), anemia (n=1), neutropenia (n=4), platelets (n=1), alanine aminotransferase (n=1) and lymphopenia (n=2). All patients cleared NTX-010 from blood and stool by 3 weeks with 17/18 patients developing neutralizing antibodies.
Conclusion
NTX-010 is feasible and tolerable at the dose levels tested in pediatric patients with relapsed/refractory solid tumors either alone or in combination with cyclophosphamide. However, despite the addition of cyclophosphamide, neutralizing antibodies appeared to limit applicability.
Keywords: Seneca Valley Virus, oncolytic, pediatric, solid tumors, relapsed
INTRODUCTION
Seneca Valley Virus (NTX-010) is an oncolytic RNA virus (family Picornaviridae) that replicates through an RNA intermediate, never goes through a DNA phase during replication, lacks reverse transcriptase activity, and does not integrate into the host genome.1 Picornaviruses relevant to human disease include rhinoviruses and enteroviruses. To date, there is no evidence that NTX-010 or serologically related viruses cause harmful disease in any species and exposure to NTX-010 does not appear to be prevalent in the human population.2 NTX-010 has been shown to induce cytotoxicity in tumors expressing neuroendocrine features (e.g. synaptophysin, chromogranin A and NSE) and in several in vitro and in vivo models of pediatric cancers including neuroblastoma, rhabdomyosarcoma and medulloblastoma.3,4
A phase I study of NTX-010 in adults with advanced solid tumors expressing neuroendocrine features was recently completed.5 Thirty patients (age range, 32-78 years) received a single infusion of NTX-010 at one of 5 dose levels ranging from 107 to 1011 viral particles (vp)/kg. NTX-010 was well tolerated at all dose levels with no dose-limiting toxicity (DLT) observed. All patients cleared virus from their blood, stool, urine and sputum and they all developed neutralizing antibodies within 2-weeks of NTX-010 administration.
One potential limitation to some oncolytic virotherapy is development of neutralizing antibodies (NA) and recruitment of host inflammatory cells (e.g. T-regulatory cells) that may be inhibitory toward an anti-tumor response.6,7 Historically, patients who are immunosuppressed have been observed to have greater response to oncolytic virotherapy (OV), which suggests that targeting the adaptive anti-viral immune response may enhance the anti-tumor effect by delaying development of NA and/or suppressing recruitment of inhibitory anti-inflammatory cells.8 One such approach to limiting the recruitment of inhibitory anti-inflammatory cells such as T-regulatory cells is to combine immunosuppressive therapy (e.g. cyclophosphamide) with OV. This approach was reported by Cerullo and colleagues using an oncolytic adenovirus in adult patients with metastatic tumors treated with oncolytic adenovirus alone or in combination with cyclophosphamide, showing greater anti-tumor efficacy when cyclophosphamide was added.9
Based on some encouraging tumor response reported in the above adult phase I NTX-010 study for patients with small cell lung cancer (SCLC) and carcinoid tumors, a phase II trial in SCLC was developed as well as our investigation of NTX-010 in children with solid tumors expressing neuroendocrine features. We report the results of a phase I trial of NTX-010 alone, as a single infusion in Part A, and with 2 doses of NTX-010 in combination with cyclophosphamide to mitigate development of neutralizing antibodies in Part B, for children with relapsed or refractory neuroblastoma, rhabdomyosarcoma or rare tumors with neuroendocrine features (NCT01048892). This is the first experience with this agent in children and the first cooperative group trial of an oncolytic virus in children. The primary objectives were to estimate the maximum tolerated dose (MTD) and/or recommended phase II dose of NTX-010 administered as a single infusion (Part A) and as two consecutive infusions, 3-weeks apart, in combination with low dose metronomic and intravenous cyclophosphamide (Part B).
MATERIALS AND METHODS
Patient Eligibility
Patients ≥3 years and ≤21 years with measurable or evaluable refractory incurable disease and histologic confirmation of neuroblastoma, rhabdomyosarcoma, Wilms tumor, retinoblastoma, adrenocortical carcinoma or carcinoid tumor were eligible. Other eligibility criteria included Karnofsky or Lansky score >50%; recovery from acute toxic effects of prior therapy; >3 weeks since myelosuppressive chemotherapy; >3 half-lives of the antibody since last monoclonal antibody administration; >2 weeks since local palliative irradiation; >6 weeks since treatment with therapeutic doses of MIBG; >6 weeks since total-body irradiation; >3 months since transplant or stem cell infusion; >42 days since immunotherapy; adequate bone marrow function (peripheral absolute neutrophil count (ANC) >1,000/mm3, platelet count >100,000/mm3 (transfusion independent), hemoglobin >8.0 g/dl); adequate renal function (age-adjusted normal serum creatinine or a glomular filtration rate >70 ml/ min/ 1.73 m2); adequate liver function (bilirubin <1.5 × institutional upper limit of normal for age, ALT <110U/L, serum albumin >2 g/dL) and adequate pulmonary function (oxygen saturation >92% on room air).
Patients were excluded if pregnant or breast feeding. Patients were also excluded if: requiring steroids and not on a stable or decreasing dose for the prior 7 days; receiving another investigational agent/anti-cancer agent; viral immunizations administered <7 days from enrollment; chronic diarrhea (> 7days), urinary incontinence during day/night, or uncontrolled infection. Patients with a primary CNS tumor or known metastatic CNS disease; known pulmonary tumors or metastases >5 cm, as evaluated by chest CT; having an indwelling urinary catheter or not completely toilet trained; or known pregnant member in their household.
The institutional review boards and institutional biosafety committees of each participating institution approved the protocol prior to patient enrollment and continuing approval was maintained throughout the trial. Patients or their legal guardian signed informed consent and assent, as appropriate and in accordance with local institutional guidelines prior to any study related procedures.
Drug Administration and Study Design
NTX-010 was supplied by Neotropix (Malvern, PA) and distributed by Theradex® (Princeton, NJ) in 1.5ml cryovials with 1×1013 viral particles (vp)/mL in aqueous buffer. Bio-safety level 2 precautions were used in preparation of NTX-010 in a certified Biosafety Cabinet by pharmacy personnel. Dilution of the virus was performed immediately prior to use and added to an IV bag (100 mL of 0.9% normal saline) within 2 hours from completion of thaw. NTX-010 was administered, without premedication, by intravenous infusion over 1 hour so that the infusion was completed within 2 hours the initial dilution.
The starting dose of NTX-010 for Part A was 1×109 vp/kg (dose level 1), administered as a single dose and 2 logs lower than the highest dose safely tolerated by adults.5 Patients were followed for at least 28 days or until viral clearance, whichever was longer, before the dose was escalated using a standard 3+3 phase 1 design. Part A included 4 dose levels (1×109 vp/kg to 1×1012 vp/kg), each increasing by 1-log.
Part A of the study was amended to include low dose metronomic and intravenous cyclophosphamide as immunomodulatory therapy since 10/11 patients in Part A developed neutralizing antibodies to NTX-010. Part B of the study used a single dose level of NTX-010 (dose level 3, 1×1011 vp/kg, maximum dose 12×1012 vp), which was identified in Part A as safe and tolerable. Patients initiated oral cyclophosphamide (CTX) [25mg/m2/day (maximum dose 50mg/day)] days 1-14 followed by a single intravenous 1-hour infusion of CTX (750mg/m2, maximum dose 1000mg) on day 8, 1 hour prior to the NTX-010 infusion. In absence of dose limiting toxicity or clinical evidence of disease progression, the regimen would be repeated with oral CTX beginning again on day 22 and IV CTX and NTX-010 on day 29, respectively (Figure 1). Three to 6 evaluable patients would be enrolled to Part B with no intrapatient dose escalation allowed in this study.
Figure 1.
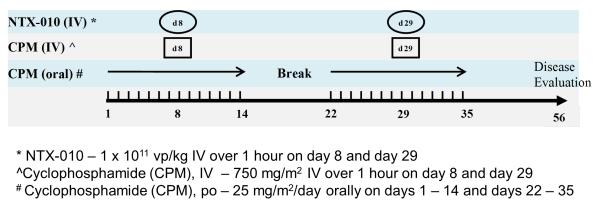
Study Schema for Part B of study: NTX-010 in Combination with oral and IV CTX
Toxicities were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Hematologic dose limiting toxicities (DLT) were defined as grade 4 neutropenia >7 days; or, a platelet count <25,000/m3 on 2 separate days or requiring a platelet transfusion on 2 separate days, within a 7 day period. Nonhematologic DLT was defined as any grade 3 or 4 non-hematologic toxicity attributable to NTX-010 with the exclusion of grade 3 nausea and vomiting <3 days duration, grade 3 liver enzyme elevation including ALT/AST/GGT that returned to grade <1 or baseline by day 29, grade 3 fever, grade 3 infection, grade 3 hypophosphatemia, hypokalemia, hypocalcemia or hypomagnesemia responsive to oral supplementation or grade 3 tumor pain that improved with analgesics. The study was amended shortly after opening to exclude grade 3 tumor pain that improved with analgesics as a DLT.
Paraffin blocks (or unstained slides) of tumor tissue obtained at initial diagnosis or relapse were submitted for central review to assess markers of neuroendocrine expression and evaluation of potential expression of cell surface determinants of tropism. Samples were also obtained for pre-therapy NTX-010 titers (blood and stool), NTX-010 antibody titers, and T-regulatory cell analysis.
Following NTX-010 administration, CBCs were obtained twice weekly and history, physical examinations, and other laboratory studies were obtained weekly for 28 days or until viral clearance, whichever was longer. Disease evaluations were obtained at baseline, end of course 1 for Part A and 4 weeks after the 2nd infusion of NTX-010 (~day 56) for Part B, followed by 8 weeks, 16 weeks and then every 12 weeks. Tumor response was reported using a modified Response Evaluation Criteria in Solid Tumors (RECIST). Patients came off study either at 12-months post-documented viral clearance, becoming lost to follow-up, enrollment onto another therapeutic clinical trial or initiation of another anti-cancer treatment after viral clearance, withdrawal of consent or death.
Viral Clearance and NTX-010 Neutralizing Antibody Formation
Blood and stool specimens were collected at baseline then twice weekly (3-4 days apart) for the first 3 weeks post-NTX-010 infusion to monitor for viral clearance. Blood and stool specimens were then collected weekly until clearance was achieved. Viral detection was performed using a real-time polymerase chain reaction assay. Clearance from blood or stool was defined as two consecutive samples without detectable virus. Peripheral blood (3mL) was sent for evaluation of neutralizing antibodies at baseline and 2-weeks after NTX-010 administration in Part A and prior to, 14 (+2) and 20 (+1) days after the initial NTX-010 infusion in Part B.
Biologic Assays
Available tumor tissue (archival) was submitted for evaluation of markers of neuroendocrine expression including synaptophysin, chromogranin A, NSE, neural cell adhesion molecule, insulin-like growth factor 2, Bcl-2 and gastrin releasing peptide. Tumor tissue was also evaluated for type I interferon signaling pathway members and interferon stimulated genes. Hematoxylin and eosin stained slides were reviewed by a pathologist for tumor versus normal tissue content and cellularity. Of the 23 samples collected from 19 patients, 7 patients were excluded due to inadequate tissue/poor quality RNA. Consecutive slides were then macro-dissected and RNA was extracted using the AllPrep DNA/RNA FFPE Kit (Qiagen). Additional normal controls for adult muscle and adrenal tissue were obtained from Agilent Technologies. RNA yield and purity was determined using a NanoDrop ND-2000 spectrophotometer. Extracted RNA was further assessed on an Agilent 210 Bioanalyzer to estimate the fraction of RNA between 300-700 base pairs. Gene expression was assayed using the nCounter® GX Human Immunology CodeSet and nCounter® Analysis System (nanoString). Count data was normalized by the geometric mean of exogenous positive control probes, background corrected by the mean of the negative control probes, and sample content was normalized to a panel of housekeeping genes using the NanoStringNorm suite of tools for the R environment.10 Reported p-values for select genes were calculated by two-sided Mann-Whitney-Wilcoxon test. Competitive gene set testing to correct for inter-gene correlation was performed using CAMERA.11
For the evaluation of T-regulatory cells (CD4+/Foxp3+/CD25-high/CD127-low),12 blood samples (5mL) were collected at baseline, 14 (+2) days and 21 days after the initial NTX-010 infusion in Part B. Peripheral blood mononuclear cells (PBMC) were isolated and stained to detect surface expression of CD4, CD25 and CD127 followed by fixation, permeabilization and staining to detect intracellular Foxp3. Multi-parameter flow cytometry was used for quantitative analysis of T-regulatory cells.
RESULTS
The study opened for enrollment September 2009 through the Children’s Oncology Group Phase I Consortium and closed February 2013. Twenty-two patients enrolled to the study (Table I) with 4 patients determined to be in-evaluable for DLT assessment (withdrawal of consent prior to NTX-010 administration, n=2; did not receive 2nd planned NTX-010 infusion on Part B due to clinical evidence of disease progression, n=2). Due to limited supply of NTX-010 for this study and the decision to amend the trial to include the addition of oral and IV CTX as a strategy to modulate T-regulatory cells, only 3 of the initially planned 4 dose levels were completed on Part A. Patients enrolled to Part B of the study were treated at dose level 3 with no planned dose escalation based on NTX-010 being a replicating virus and the available supply of NTX-010 for this study.
Table I.
Patient Characteristics for Eligible Patients (n=22)
| Characteristic | Number (%) |
|---|---|
|
| |
| Age (years) | |
| Median (Range) | 8.8 (4.8-18.3) |
|
| |
| Sex | |
|
| |
| Male | 14 (63.6) |
| Female | 8 (36.4) |
|
| |
| Race | |
|
| |
| White | 17 (77.3) |
| Asian | 1 (4.5) |
| Black or African American | 4 (18.2) |
|
| |
| Ethnicity | |
|
| |
| Non-Hispanic | 22 (100.0) |
| Hispanic | 0(0) |
|
| |
| Diagnosis | |
|
| |
| Carcinoid tumor | 1 (4.5) |
|
| |
| Adrenal cortical carcinoma | 3 (13.6) |
|
| |
| Embryonal rhabdomyosarcoma | 1 (4.5) |
|
| |
| Alveolar rhabdomyosarcoma | 2 (9.1) |
|
| |
| Wilms’ tumor | 3 (13.6) |
|
| |
| Ganglioneuroblastoma | 1 (4.5) |
|
| |
| Neuroblastoma | 11 (50.0) |
|
| |
| Prior Therapy | |
|
| |
| Prior Chemotherapy Regimens, Median (Range) | 3 (1-6) |
|
| |
| Number of Patients with Prior Radiation Therapy | 15(68.2) |
Toxicity
A single DLT occurred in a patient enrolled onto Part A. This patient initially reported grade 2 pain (right shoulder) which recovered but then developed grade 3 pain (low back/pelvis) requiring analgesics and meeting criteria for DLT. Both the right shoulder and low back/pelvis region showed evidence of disease at the end of therapy evaluation and correlated to the areas of pain reported. The study was subsequently amended during Part A to exclude grade 3 tumor pain that improved with analgesics as a DLT. There were no further DLTs reported on either Part A or Part B of the study. As well, we observed no relationship between the dose of NTX-010 and toxicity reported.
Table II summarizes the non-dose limiting hematologic toxicities and table III non-dose limiting non-hematologic toxicities observed in the 18 evaluable patients in Part A and B. The table includes any grade toxicity that occurred post-NTX-010 administration but prior to documented viral clearance (toxicity across cycle 1) as well as any toxicity occurring after documented viral clearance and prior to the patient coming off study (toxicity across post documented viral clearance). The non-dose limiting non-hematologic toxicities reported occurred in at least 10% of the evaluable patients and at least possibly attributable to NTX-010.
Table II.
Non-Dose Limiting Hematologic Toxicities Observed in Evaluable Patients
| Part A NTX-010 dose level 1 (1×109 vp/kg; n=6) | ||||||||
| Toxicity Type |
Maximum grade of toxicity across cycle
1 (Total, 6 cycles) |
Maximum grade of toxicity across post
documented viral clearance cycles (Total, 4 cycles) |
||||||
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Anemia | 1 | 2 | ||||||
| Lymphocyte count decreased | 2 | |||||||
| Neutrophil count decreased | 2 | 1 | 1 | 1 | ||||
| Platelet count decreased | 2 | 1 | ||||||
| White blood cell decreased | 3 | 1 | ||||||
| Part A NTX-010 dose level 2 (1×1010 vp/kg; n=3) | ||||||||
| Toxicity Type |
Maximum grade of toxicity across
cycle 1 (Total, 3 cycles) |
Maximum grade of toxicity across post
documented viral clearance cycles (Total, 5 cycles) |
||||||
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Anemia | 1 | 1 | ||||||
| Lymphocyte count decreased | 1 | 1 | 2 | |||||
| Neutrophil count decreased | 1 | 1 | ||||||
| Platelet count decreased | 3 | 1 | ||||||
| White blood cell decreased | 1 | 2 | ||||||
| Part A NTX-010 dose level 3 (1×1011vp/kg; n=3) | ||||||||
| Toxicity Type |
Maximum grade of toxicity across
cycle 1 (Total, 3 cycles) |
Maximum grade of toxicity across post
documented viral clearance cycles (Total, 3 cycles) |
||||||
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Anemia | 1 | 1 | ||||||
| Lymphocyte count decreased | 2 | 1 | ||||||
| Neutrophil count decreased | 1 | 1 | ||||||
| Platelet count decreased | 1 | 1 | ||||||
| White blood cell decreased | 2 | 1 | ||||||
| Part B NTX-010 dose level 1 (1×1011vp/kg plus cyclophosphamide; n=6) | ||||||||
| Toxicity Type |
Maximum grade of toxicity across
cycle 1 (Total, 6 cycles) |
Maximum grade of toxicity across post
documented viral clearance cycles (Total, 4 cycles) |
||||||
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Anemia | 2 | 2 | ||||||
| Lymphocyte count decreased | 2 | 2 | 2 | |||||
| Neutrophil count decreased | 1 | 4 | ||||||
| Platelet count decreased | 1 | 2 | 1 | |||||
| White blood cell decreased | 2 | 3 | ||||||
Table III.
Non-Dose Limiting Non-Hematologic toxicities related to protocol therapy and observed in more than 10 percent* of total evaluable patients in each Part
| Part A NTX-010 dose level 1 (1×109 vp/kg; n=6) | ||||||||
| Toxicity Type |
Maximum grade of toxicity across
cycle 1 (total, 6 cycles) |
Maximum grade of toxicity across
post documented viral clearance cycles (Total, 4 cycles) |
||||||
|
Grade
1 |
Grade
2 |
Grade
3 |
Grade
4 |
Grade
1 |
Grade
2 |
Grade
3 |
Grade
4 |
|
| Alanine aminotransferase increased | 4 | |||||||
| Anorexia | 2 | |||||||
| Aspartate aminotransferase increased | 1 | |||||||
| Back pain | 1 | |||||||
| Chills | 1 | |||||||
| Diarrhea | 1 | |||||||
| Fatigue | 2 | |||||||
| Fever | 1 | |||||||
| Headache | 2 | |||||||
| Hypercalcemia | 1 | |||||||
| Hyponatremia | ||||||||
| Part A NTX-010 dose level 2 (1×1010 vp/kg; n=3) | ||||||||
| Toxicity Type |
Maximum grade of toxicity across
cycle 1 (total, 3 cycles) |
Maximum grade of toxicity across
post documented viral clearance cycles (Total, 5 cycles) |
||||||
|
Grade
1 |
Grade
2 |
Grade
3 |
Grade
4 |
Grade
1 |
Grade
2 |
Grade
3 |
Grade
4 |
|
| Alanine aminotransferase increased | 2 | |||||||
| Anorexia | 2 | |||||||
| Aspartate aminotransferase increased | 1 | |||||||
| Back pain | 1 | |||||||
| Chills | 1 | |||||||
| Diarrhea | 1 | |||||||
| Fatigue | 1 | |||||||
| Fever | 2 | |||||||
| Headache | 1 | |||||||
| Hypercalcemia | 1 | 1 | ||||||
| Hyponatremia | 1 | |||||||
| Part A NTX-010 dose level 3 (1×1011vp/kg; n=3) | ||||||||
| Toxicity Type |
Maximum grade of toxicity across
cycle 1 (total, 3 cycles) |
Maximum grade of toxicity across
post documented viral clearance cycles (Total, 3 cycles) |
||||||
|
Grade
1 |
Grade
2 |
Grade
3 |
Grade
4 |
Grade
1 |
Grade
2 |
Grade
3 |
Grade
4 |
|
| Alanine aminotransferase increased | 1 | |||||||
| Anorexia | ||||||||
| Aspartate aminotransferase increased | ||||||||
| Back pain | ||||||||
| Chills | ||||||||
| Diarrhea | ||||||||
| Fatigue | 1 | |||||||
| Fever | 1 | 1 | 1 | |||||
| Headache | ||||||||
| Hypercalcemia | ||||||||
| Hyponatremia | 1 | |||||||
| Part B NTX-010 dose level 1 (1×1011vp/kg plus cyclophosphamide; n=6) | ||||||||
| Toxicity Type |
Maximum grade of toxicity across
cycle 1 (total, 6 cycles) |
Maximum grade of toxicity across
post documented viral clearance cycles (Total, 4 cycles) |
||||||
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Abdominal pain | 1 | |||||||
| Alanine aminotransferase increased | 1 | |||||||
| Aspartate aminotransferase increased | 1 | |||||||
| Blood bilirubin increased | 1 | |||||||
| Diarrhea | 1 | |||||||
| Fatigue | 1 | |||||||
| Hyperglycemia | 1 | |||||||
| Hypoalbuminemia | 1 | |||||||
| Hypocalcemia | 1 | |||||||
| Hyponatremia | 1 | |||||||
| Nausea | 1 | |||||||
| Vomiting | 1 | |||||||
Toxicities that occurred in more than 10 % of patients as determined in the first cycle of protocol therapy.
Responses
There were no objective responses (complete or partial response) observed at the time of disease re-evaluation with NTX-010 as a single infusion in Part A or following the combination of NTX-010 and CTX in Part B. Stable disease at the time of disease re-evaluation was observed in six of twelve evaluable patients on Part A [neuroblastoma (n=4), carcinoid tumor (n=1) and alveolar rhabdomyosarcoma (n=1)] and four of six evaluable patients on Part B [neuroblastoma (n=2) and Wilms tumor (n=2)]. Since patients only received one or two infusions before removal from protocol therapy or receipt of other anti-cancer therapy the duration of stable disease could not be assessed.
Viral Clearance and NTX-010 Neutralizing Antibody Formation
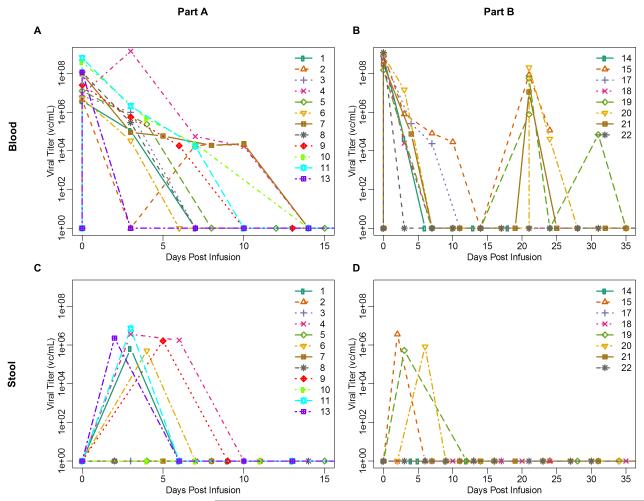
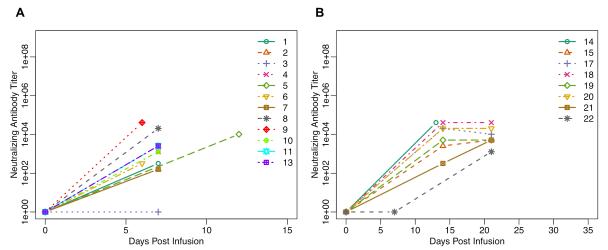
All 12 evaluable patients in Part A cleared NTX-010 from the blood within 3 weeks of viral infusion (median 1.5 weeks) (Figure 2A). Clearance from the stool occurred by 1 week post-NTX-010 infusion in six of the 12 patients who had evidence of NTX-010 in the stool specimens submitted over a 4 week period post NTX-010 (Figure 2C). Neutralizing antibodies, collected during week 2 post-NTX-010 infusion, were identified in 11 of the 12 evaluable patients in Part A with a median titer of 1:1,280 (range, 160 - 40,960) (Figure 3A).
Figure 2.
NTX-010 Clearance: 2A) NTX-010 serum clearance from Part A of the study; 2B) NTX-010 serum clearance from Part B of the study; Patients 14 and 17 received a single dose of NTX-010 whereas patients 15 and 18-22 received 2 doses of NTX-010 2C) NTX-010 stool clearance from Part A of the study; 2D) NTX-010 stool clearance from Part B of the study; Patients 14 and 17 received a single dose of NTX-010 whereas patients 15 and 18-22 received 2 doses of NTX-010.
Figure 3.
Neutralizing Antibodies to NTX-010: 3A) Neutralizing antibodies to NTX-010 on Part A of the study with each color representing a single patient’s data; 3B) Neutralizing antibodies to NTX-010 on Part B of the study with each color representing a single patient’s data. Patients 14 and 17 received a single dose of NTX-010 whereas patients 15 and 18-22 received 2 doses of NTX-010.
All 6 evaluable patients in Part B cleared NTX-010 from the blood within 2 weeks of viral infusion (median 1 week) (Figure 2B). Clearance of NTX-010 from the stool also occurred within 2 weeks (range, 1-2) for 3/6 patients who had detectable virus in submitted specimens (Figure 2D). Virus was not detected in the stool of any patient after receiving a second infusion of NTX-010. Despite the addition of CTX, neutralizing antibodies were identified in all patients with a median titer of 1:3,840 (range, 10 – 40,960). Five of the 6 evaluable patients had evidence of neutralizing antibody at the 2-week time-point while a single patient remained without evidence of antibody until the 3-week assessment prior to their 2nd infusion of NTX-010 (Figure 3B).
Biologic Assays
Peripheral blood samples were collected from all 6 evaluable patients in Part B of the study for T-regulatory cell analysis. Three of the 6 patients did not have a CBC performed at the time of sample submission to calculate an absolute T-regulatory cell (ATR) count at baseline. Of the 3 patients who had available ATR count at baseline, 2 had a marginal decrease in ATR after completing 14 days of CTX (64.8 → 60.6; 282.2 → 246.24) and one patient had a rise in ATR (57.1 → 69.16). As well, there was no consistent pattern with respect to ATR count between the 14 and 21 day time point among the 6 patients (Supplementary Figure 1A).
Diagnostic tumor tissue was evaluated for expression of genes induced by the type I interferon response to assess whether any genes correlated with persistence of NTX-010 in serum as a surrogate for intratumoral replication. No significant difference was found between patients who cleared NTX-010 from the serum by day 7 post-infusion and those with remaining viral titers at day 7 in terms of interferon induced gene sets. However, higher baseline expression of TLR3, which detects the pathogen associated molecular pattern of dsRNA used as a replicative intermediate for picornaviruses, was detected in patients who cleared NTX-010 within the 1st week (Supplementary Figure 1B). This difference in baseline gene expression may contribute to an inability of certain patient tumors to detect and respond to NTX-010 infection through TLR3, although we did not see any difference in tumor response based on TLR3 gene expression.
DISCUSSION
Children with relapsed/refractory solid tumor malignancies continue to have very poor outcomes. Therefore new strategies are needed to improve survival. Oncolytic viruses which can selectively target and destroy malignant tumors is one strategy that has been evaluated in early phase adult trials with varying success,13-24 although development of neutralizing antibodies to some viruses may limit the efficacy of this strategy, particularly viruses administered intravenously.6,7 Oncolytic viruses can be administered either via intratumoral injection or intravenously. Intratumoral injection of an oncolytic virus can be a difficult procedure to perform in children, particularly when targeting tumors that reside deep in the abdomen such as many of the eligible diagnoses in this study (e.g. neuroblastoma, Wilms tumor, adrenocortical carcinoma). An advantage of NTX-010 as an oncolytic virus in pediatric patients is that it is administered intravenously and therefore can, not only reach deep residing tumors but metastatic disease as well. A potential disadvantage of intravenously administering oncolytic viruses is that they may become a direct target of the host immune system which can rapidly develop NA, potentially binding and inhibiting the virus from any oncolytic effect, as well as mounting a T-cell response against the virus (e.g. T-regulatory cells). Although the immune system can have a negative impact on oncolytic virotherapy, in cases of larger DNA oncolytic viruses (e.g., HSV-1, vaccinia), initiating a strong immune response may be advantageous to eliciting an anti-tumor response and become the desired effect.25-26
One approach to preventing the recruitment of inhibitory anti-inflammatory cells such as T-regulatory cells is to combine immunosuppressive therapy with oncolytic virotherapy. Cerullo and colleagues reported results of a controlled, nonrandomized retrospective study of adult patients with metastatic tumors progressing after conventional therapy, treated with oncolytic adenovirus (OA) alone or with the combination of OA and CTX.9 Patients who received OA plus metronomic CTX or metronomic and IV CTX, had a significantly lower number of T-regulatory cells after treatment (p=0.001 and p=0.032, respectively) as well as greater disease control. Disease control, defined as stable disease or greater, was reported in 22% of controls compared to 77% for patients treated with OA plus metronomic and IV CTX. Median progression-free survival was greatest in the OA plus metronomic and IV CTX group reporting 376 days and 53% progression free at 1-year compared to 63 days for controls.
Based on the encouraging results from the adult experience incorporating metronomic and IV CTX with oncolytic adenovirus9 and the relatively limited tumor response observed in patients enrolled to Part A of our phase I study where 10/11 patients developed NA 2-weeks post-infusion, the study was amended (Part B) to incorporate metronomic and IV CTX with NTX-010 at dose level 3 (1×1011vp/kg), the highest dose level reached during Part A. Similar to the adult experience when combining CTX with OV, we witnessed no affect in either viral clearance or development of NA compared to patients treated with NTX-010 alone. The effect on T-regulatory cells was not consistent across patients but was limited by the very small sample size.
This phase I study is the first pediatric trial investigating an oncolytic virus in children. All three dose levels investigated during Part A of the study, as well as the single dose level with the addition of CTX in Part B, were well tolerated with a single DLT observed at the initial dose level in Part A. Overall, NTX-010 was well tolerated when given as an intravenous infusion alone or in combination with CTX for pediatric patients with relapsed or refractory solid tumors. Despite the incorporation of immunosuppressive therapy into Part B of the study, all patients rapidly cleared virus from both blood and stool and developed NA within 3-weeks of NTX-010 infusion. The administration of NTX-010 alone or in combination with cyclophosphamide was found to be feasible in this pediatric phase I study. Future studies of NTX-010 will need to focus on strategies to minimize neutralizing antibodies and T-regulatory cells which may be impacting treatment response in pediatric neuroendocrine tumors.
Supplementary Material
Supplementary Figure 1. Biologic Assays: 1A) Absolute T-regulatory Cell Count (Total lymphocyte count × % T-regulatory cell count): Absolute T-Regulatory Cell (ATR) count on Part B of the study during cycle 1 with each line representing a single patient’s ATR count at the time points indicated; 1B) Expression of TLR3 at baseline between patients who cleared virus rapidly (black triangles) and those in whom replication was persistent (black squares).
Acknowledgments
We are forever grateful to all the patients and families who participated in this study. This study was supported by Peter Lanciano and Neotropix who developed NTX-010 and without; this study never would have been possible. We are also thankful for the pathological contributions of Drs. Carlos Manivel and Charles Rudin and the flow cytometric analyses of Dr. Julie Curtsinger.
Funding: Funding for this Phase I study was provided through the Phase I/Pilot Consortium 5UM1 CA097452-12 grant. Additional funding came from Cookies for Kids Cancer and American Recovery and Reinvestment Act (ARRA) Funding to support Accelerating Clinical Trials of Novel Oncology PathWays (ACTNOW).
Footnotes
Author Contributions Conception and design: Susan Blaney, Brenda Weigel, Michael Burke, Charlotte Ahern, Timothy Cripe, M. Brooke Bernhardt, John T. Poirier, Charles M. Rudin
Administrative support: Biljana Georgievska
Provision of study materials or patients: Michael Burke, Susan Blaney, Brenda Weigel, Timothy Cripe, M. Brooke Bernhardt
Collection and assembly of data: Michael Burke, Susan Blaney, Brenda Weigel, Charlotte Ahern
Data analysis and interpretation: Michael Burke, Susan Blaney, Brenda Weigel, Charlotte Ahern, Timothy Cripe, M. Brooke Bernhardt, John T. Poirier, Charles M. Rudin
Manuscript writing: Michael Burke, Susan Blaney, Brenda Weigel, Charlotte Ahern, Timothy Cripe, M. Brooke Bernhardt, John T. Poirier, Charles M. Rudin
Final approval of manuscript: Michael Burke, Susan Blaney, Brenda Weigel, Charlotte Ahern, Timothy Cripe, M. Brooke Bernhardt, John T. Poirier, Charles M. Rudin
Conflict of Interest Statement The author(s) indicate no potential conflicts of interest
References
- 1.Rueckert RR. Fields Virology. 2nd Edition Raven Press; New York, NY: 1990. Picornaviridae and their replication. [Google Scholar]
- 2.Reddy PS, Burroughs KD, Hales LM, et al. Seneca Valley virus, a systemically deliverable oncolytic picornavirus, and the treatment of neuroendocrine cancers. J Natl Cancer Inst. 2007;99:1623–33. doi: 10.1093/jnci/djm198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morton CL, Houghton PJ, Kolb EA, et al. Initial testing of the replication competent Seneca Valley virus (NTX-010) by the pediatric preclinical testing program. Pediatr Blood Cancer. 2010;55:295–303. doi: 10.1002/pbc.22535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu L, Baxter PA, Zhao X, et al. A single intravenous injection of oncolytic picornavirus SVV-001 eliminates medulloblastomas in primary tumor-based orthotopic xenograft mouse models. Neuro Oncol. 2011;13:14–27. doi: 10.1093/neuonc/noq148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rudin CM, Poirier JT, Senzer NN, et al. Phase I clinical study of Seneca Valley Virus (SVV-001), a replication-competent picornavirus, in advanced solid tumors with neuroendocrine features. Clin Cancer Res. 2011;17:888–95. doi: 10.1158/1078-0432.CCR-10-1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang L, Pang Y, Moses HL. TGF-beta and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends Immunol. 2010;31:220–7. doi: 10.1016/j.it.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melcher A, Parato K, Rooney CM, et al. Thunder and lightning: immunotherapy and oncolytic viruses collide. Mol Ther. 2011;19:1008–16. doi: 10.1038/mt.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelly E, Russell SJ. History of oncolytic viruses: genesis to genetic engineering. Mol Ther. 2007;15:651–9. doi: 10.1038/sj.mt.6300108. [DOI] [PubMed] [Google Scholar]
- 9.Cerullo V, Diaconu I, Kangasniemi L, et al. Immunological effects of low-dose cyclophosphamide in cancer patients treated with oncolytic adenovirus. Mol Ther. 2011;19:1737–46. doi: 10.1038/mt.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waggott D, Chu K, Yin S, et al. NanoStringNorm: an extensible R package for the pre-processing of NanoString mRNA and miRNA data. Bioinformatics. 2012;28:1546–8. doi: 10.1093/bioinformatics/bts188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu D, Smyth GK. Camera: a competitive gene set test accounting for inter-gene correlation. Nucleic Acids Res. 2012;40:e133. doi: 10.1093/nar/gks461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trzonkowski P, Szarynska M, Mysliwska J, et al. Ex vivo expansion of CD4(+)CD25(+) T regulatory cells for immunosuppressive therapy. Cytometry A. 2009;75:175–88. doi: 10.1002/cyto.a.20659. [DOI] [PubMed] [Google Scholar]
- 13.Russell SJ, Peng KW, Bell JC. Oncolytic virotherapy. Nat Biotechnol. 2012;30:658–70. doi: 10.1038/nbt.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirn D. Oncolytic virotherapy for cancer with the adenovirus dl1520 (Onyx-015): results of phase I and II trials. Expert Opin Biol Ther. 2001;1:525–38. doi: 10.1517/14712598.1.3.525. [DOI] [PubMed] [Google Scholar]
- 15.Laurie SA, Bell JC, Atkins HL, et al. A phase 1 clinical study of intravenous administration of PV701, an oncolytic virus, using two-step desensitization. Clin Cancer Res. 2006;12:2555–62. doi: 10.1158/1078-0432.CCR-05-2038. [DOI] [PubMed] [Google Scholar]
- 16.Freytag SO, Movsas B, Aref I, et al. Phase I trial of replication-competent adenovirus-mediated suicide gene therapy combined with IMRT for prostate cancer. Mol Ther. 2007;15:1016–23. doi: 10.1038/mt.sj.6300120. [DOI] [PubMed] [Google Scholar]
- 17.Lorence RM, Roberts MS, O’Neil JD, et al. Phase 1 clinical experience using intravenous administration of PV701, an oncolytic Newcastle disease virus. Curr Cancer Drug Targets. 2007;7:157–67. doi: 10.2174/156800907780058853. [DOI] [PubMed] [Google Scholar]
- 18.Park BH, Hwang T, Liu TC, et al. Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: a phase I trial. Lancet Oncol. 2008;9:533–42. doi: 10.1016/S1470-2045(08)70107-4. [DOI] [PubMed] [Google Scholar]
- 19.Aghi MK, Chiocca EA. Phase ib trial of oncolytic herpes virus G207 shows safety of multiple injections and documents viral replication. Mol Ther. 2009;17:8–9. doi: 10.1038/mt.2008.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li JL, Liu HL, Zhang XR, et al. A phase I trial of intratumoral administration of recombinant oncolytic adenovirus overexpressing HSP70 in advanced solid tumor patients. Gene Ther. 2009;16:376–82. doi: 10.1038/gt.2008.179. [DOI] [PubMed] [Google Scholar]
- 21.Pesonen S, Nokisalmi P, Escutenaire S, et al. Prolonged systemic circulation of chimeric oncolytic adenovirus Ad5/3-Cox2L-D24 in patients with metastatic and refractory solid tumors. Gene Ther. 2010;17:892–904. doi: 10.1038/gt.2010.17. [DOI] [PubMed] [Google Scholar]
- 22.Hwang TH, Moon A, Burke J, et al. A mechanistic proof-of-concept clinical trial with JX-594, a targeted multi-mechanistic oncolytic poxvirus, in patients with metastatic melanoma. Mol Ther. 2011;19:1913–22. doi: 10.1038/mt.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geletneky K, Huesing J, Rommelaere J, et al. Phase I/IIa study of intratumoral/intracerebral or intravenous/intracerebral administration of Parvovirus H-1 (ParvOryx) in patients with progressive primary or recurrent glioblastoma multiforme: ParvOryx01 protocol. BMC Cancer. 2012;12:99. doi: 10.1186/1471-2407-12-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pesonen S, Diaconu I, Cerullo V, et al. Integrin targeted oncolytic adenoviruses Ad5-D24-RGD and Ad5-RGD-D24-GMCSF for treatment of patients with advanced chemotherapy refractory solid tumors. Int J Cancer. 2012;130:1937–47. doi: 10.1002/ijc.26216. [DOI] [PubMed] [Google Scholar]
- 25.Sampath P, Li J, Hou W, et al. Crosstalk between immune cell and oncolytic vaccinia therapy enhances tumor trafficking and antitumor effects. Mol Ther. 2013;21:620–8. doi: 10.1038/mt.2012.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Workenhe ST, Simmons G, Pol JG, et al. Immunogenic HSV-mediated oncolysis shapes the antitumor immune response and contributes to therapeutic efficacy. Mol Ther. 2014;22:123–31. doi: 10.1038/mt.2013.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Biologic Assays: 1A) Absolute T-regulatory Cell Count (Total lymphocyte count × % T-regulatory cell count): Absolute T-Regulatory Cell (ATR) count on Part B of the study during cycle 1 with each line representing a single patient’s ATR count at the time points indicated; 1B) Expression of TLR3 at baseline between patients who cleared virus rapidly (black triangles) and those in whom replication was persistent (black squares).