Abstract
Objective
To determine the benefits of combination treatment with mechanical support and targeted intra-arterial infusion of peripheral blood stem cells (PBSCs) mobilized by granulocyte-colony stimulating factor (G-CSF) via the medial circumflex femoral artery on the progression of osteonecrosis of the femoral head (ONFH).
Methods
55 patients (89 hips) with early and intermediate stage ONFH were recruited and randomly assigned to combination treatment or mechanical support treatment (control group). All hips received mechanical support treatment (porous tantalum rod implantation). Then, Hips in the combination treatment group were performed targeted intra-arterial infusion of PBSCs. At each follow-up, Harris hip score (HHS) and Association Research Circulation Osseous classification (ARCO) were used to evaluate the symptoms and progression of osteonecrosis. Total hip arthroplasty (THA) was assessed as an endpoint at each follow-up.
Results
At 36 months, 9 of the 41 hips (21.95%) in the control group progressed to clinical failure and underwent THA whereas only 3 of the 48 hips (6.25%) in the combination treatment group required THA(p=0.031). Kaplan-Meier survival analysis showed a significant difference in the survival time between the two groups (Log-rank test; p=0.025). Compared to the control group, combination treatment significantly improved the HHS at 36 months (p=0.003). At the final follow-up examination, radiological progression was noted in 13 of 41 hips (31.71%) for the control group, while only in 4 of 48 hips (8.33%) for the combination treatment group (p=0.005). The overall collapse rates were 15.15% (5 of 33 hips) and 8.11% (3 of 37 hips) in the control and combination treatment groups, respectively.
Conclusions
Targeted intra-arterial infusion of PBSCs is capable of enhancing the efficacy of biomechanical support in the treatment of ONFH. This clinical trial confirmed that the combination treatment might be a safe and feasible choice for the treatment of early or intermediate stages of ONFH.
Keywords: Osteonecrosis of the femoral head, Peripheral blood stem cells, Medial circumflex femoral artery, Intra-arterial delivery, Granulocyte-colony stimulating factor
Introduction
Osteonecrosis of the femoral head (ONFH) is a progressive pathological process [1], which usually impairs hip joint function [2]. Since ONFH mainly affects young patients [3], and most frequently progresses to femoral head collapse and secondary symptomatic hip arthritis [2], any intervention for ONFH should be considered in order to preserve joint. Despite the variety of etiologies, the common final outcome for ONFH is the disruption of the blood supply to the femoral head [4–5]. Bone ischemia as a result of decreased blood flow to the femoral head will trigger the death of bone cells within the involved femoral head [6]. Although the reparative reaction can be observed around necrotic area, it is insufficient due to the lack of progenitor cells [7–9]. The insufficient repair process followed cell death can not attain the previous structural integrity of necrotic region of the femoral head, leading to biomechanical failure of the bone structure. Due to the impairment of the repair capacity and biomechanical properties of the necrotic femoral head, the optimal joint-preserving procedure for ONFH should provide cues for the biologic repair in the necrotic lesion site and sufficient biomechanical support to subchondral plate of the necrotic femoral head.
Transplantation of bone marrow-derived mesenchymal stromal cells (MSCs) has been introduced to augment the biologic repair process in the osteonecrosis [7, 9–15]. The satisfactory outcomes of MSCs transplantation in patients with ONFH have been reported [7, 9–15], whereas the invasiveness of bone marrow cells collection may leave pain or complications at the removal site and subjective problems from the bone marrow aspiration. Granulocyte-colony stimulating factor (G-CSF) is a well established mobilizer for mobilization of bone marrow MSC into peripheral circulation [16–18]. Mobilization of MSCs using G-CSF and subsequent implantation of collected peripheral blood stem cells (PBSCs) have been evaluated as an alternative, less invasive approach for the treatment of ONFH [19–20]. Favorable results have brought attention to the application of G-CSF based PBSCs transplantation into patients with ONFH. In most studies of PBSCs transplantation, CD34 has been served as a marker of MSCs for transplantation [17–26]. The efficacy and safety of G-CSF and CD34+ cell transplantation into patients with myocardial infarction and ischaemia have been demonstrated [17–26]. However, the feasibility and safety of G-CSF and transplantation of PBSCs mobilized with G-CSF in the patients with ONFH have not been assessed. In addition, the beneficial effect of sufficient biomechanical support to the subchondral bone has been confirmed [27–33]. However, no study has been done to compare clinical effectiveness of biomechanical support plus G-CSF based PBSC transplantation with that of PBSC transplantation or biomechanical support alone. Therefore, the present study was designed to test the feasibility and safety of G-CSF based PBSC transplantation in patients with ONFH, and to assess clinical benefits of combination treatment with biomechanical support and transplantation of PBSCs mobilized by G-CSF.
Subjects and Methods
Study design
The study is a single centre, parallel group, assessor blinded, and randomized controlled clinical trial, which was conducted at The First Affiliated Hospital of Zhejiang Chinese Medical University. The study protocol has been approved by the Ethics Committee of the hospital (approval code: 2013-X-063). Patients with ONFH were recruited and those eligible for the study were enrolled in the clinical trial. Before enrollment, written informed consent was obtained from each patient after disclosing the procedure and risks. The involved hips of patients were randomly allocated to either sufficient biomechanical support to the subchondral bone (control group) or to biomechanical support plus G-CSF based PBSCs transplantation (combination treatment group).
Vascularized or nonvascularized bone graft was applied to afford biomechanical support against collapse [34–39]. However, this procedure is associated with donor site morbidity, failure and other potential complications [31]. Alternatively, insertion of a porous tantalum rod has been developed recently [27, 29, 31–33]. The porous tantalum rod is deemed to be a reasonable mechanical substitute for a fibular graft [31]. Thus, nowadays, porous tantalum rod implantation, which is derived from the theory of free vascularized fibular grafting, represents an encouraging treatment to supply additional biomechanical support against collapse, and has achieved beneficial clinical outcomes for the treatment of early and intermediate stages of ONFH [27, 29, 31–33]. Targeted intra-arterial infusion via medial circumflex femoral artery has been described as a minimally invasive strategy of MSCs instillation into the osteonecrotic lesion area [13]. For these reasons, porous tantalum rod implantation was used as representative treatment of biomechanical support in this study and PBSCs mobilized by G-CSF were infused through medial circumflex femoral artery. All hips underwent the operation of porous tantalum rod implantation. On day 7 after the operation, hips in the combination treatment group received targeted intra-arterial delivery of PBSCs. Hips in the control group were not given any placebo.
We detected the efficacy and safety of G-CSF-based PBSCs therapy on the basis of clinical symptoms, which were gauged with Harris hip score (HHS), radiological progression of osteonecrosis, conversion to total hip arthroplasty (THA), which was defined as clinical failure, development of G-CSF-related adverse effects and specific complications regarding MSCs transplantation. The outcomes were assessed by investigators who were blinded to the allocated treatment.
Study population
In this clinical trial, the inclusion criteria for participation were that patients were aged 18 to 65 years and had ONFH stages I to III A in compliance with the Association Research Circulation Osseous (ARCO) classification [40]. If patients with any of the following circumstances would be excluded from the study: current or previous inflammatory arthritis or other disease of hip joint, skeletal immaturity, serious cardiovascular disease, hepatic or renal disease, a history of hip joint surgery, still on corticosteroid therapy, pregnant or lactating women, malignant disease, serious current infection or haematological disease, and mental health disorders.
Sample size
To detect a change of 7 points (standard deviation 9) in the Harris hip score with a two side 5% significance level and 90% power, based on the result of preliminary clinical test, we needed 35 hips per group. The change of 7 points is the lower limit of the range (7 to 10 points) suggested to show a minimum clinically important difference [61]. If we allowed for an anticipated dropout rate of 10%, the total sample size was 77 hips.
Enrollment and Randomization
Patients with ONFH were recruited at the First Affiliated Hospital of Zhejiang Chinese Medical University. We obtained written informed consent from each eligible patient. Hips were randomly divided into the control or combination treatment group.
Randomization assignments of hips were generated by using a computer generated, randomized number sequence. Treatment assignment of each hip was determined based on the random number sequence, and kept the assessor who collected and analyzed outcome data blinded.
Interventions
All patients were subjected to a uniform surgical procedure with epidural anesthesia. A porous tantalum rod was inserted into the necrotic area of femoral head to support the subchondral bone. Full details of the procedure can be found in the supplemental informations (Figure S1).
On day 7 after the operation, hips in the combination treatment group received targeted intra-arterial delivery of PBSCs. Before cell infusion, PBSCs were harvested from the patients by apheresis technique using COBE spectra apheresis system (COBE BCT Inc, Lakewood, CO, USA) after subcutaneous injections of G-CSF at a dosage of 10 μg/kg for 4 days to mobilize PBSCs (Figure S2). The collection process was carried out according to the mononuclear cell collection method which was recommended in the manual [41]. The number of CD34+ cells in the cell suspension was calculated. Then, the PBSCs were infused into the femoral head as previously described [13].
Result Assessments
Data were collected and assessed by two experienced orthopaedic surgeons who were blinded to the group assignment. Each hip of patients suffering from bilateral hip involvement was examined, respectively.
Primary Results
The close correlation between the clinical symptoms of ONFH and the activities of daily life of patients required us to record function of affected hip using standardized parameters. Harris hip scoring (HHS) system has been widely used to measure the hip function [9–10, 13]. The HHS ranges from 0 to 100, with lower scores indicating the more severe symptom [13]. In the present studies, clinical failure was defined as that deterioration on clinical symptoms was severe enough to demand THA, and conversion to THA was designed as the endpoint to evaluate the efficacy of the treatment. Correspondingly, the hips which did not require THA were regarded as survived hips.
Secondary Results
Radiographs and MRI were performed to determine ARCO stages of ONFH at the time of each assessment. Radiological progression was decided according to the development of the ARCO stage. The worsening of one or more grade on ARCO stages was considered to be radiological progression of ONFH. The progression of the ARCO stage I or II to ARCO stage III or IV on radiological appearance was defined as radiological collapse [42].
Any adverse reactions from G-CSF based PBSCs transplantation such as angina, bone pain, headache, fever, injection site tenderness, spleen thrombosis, dizziness and tumor induction, and any other complications were recorded during the course of the trial.
We collected data for these results at baseline (right before porous tantalum rod implantation), and in follow-up clinics at 3, 6, 12, 18, 24, 30 and 36 months after porous tantalum rod implantation.
Statistical Analysis
Continuous data are presented as mean ± SD. The chi-square test was used for comparison of categorical variables. The significance of differences between continuous variables was analyzed with the paired t test. The clinical survival was compared between the two groups with Kaplan-Meier survival analysis. The log rank test was used as a test of significance. Differences were considered significant when p<0.05 was obtained. All data were analyzed by SPSS statistical software (version 15.0; SPSS, Chicago, USA).
Results
In present study, we enlisted seventy five patients (110 hips) who were accorded with inclusion criteria. The flow of these patients through the trial was showed in Figure 1. 20 patients (21 hips) declined to participate in the trial with preference for alternative treatment. Of these patients, 14 patients preferred to receive a porous tantalum rod implantation for concerns about side effects of PBSCs implantation and the other six patients chose the other conservative treatments. Finally, a total of 55 patients (89 hips), 30 males and 25 females, were enrolled and randomly assigned: 30 patients (including 48 hips) to combination treatment and 25 patients (including 41 hips) to porous tantalum rod implantation alone. Table 1 summarizes the demographic characteristics of participants, etiological factor, ARCO stage of ONFH, and Harris hip score at onset of treatment. Baseline parameters were well balanced among treatment groups. At the primary endpoint of 36 months, all patients stay within entire follow-up studies.
Fig. 1.
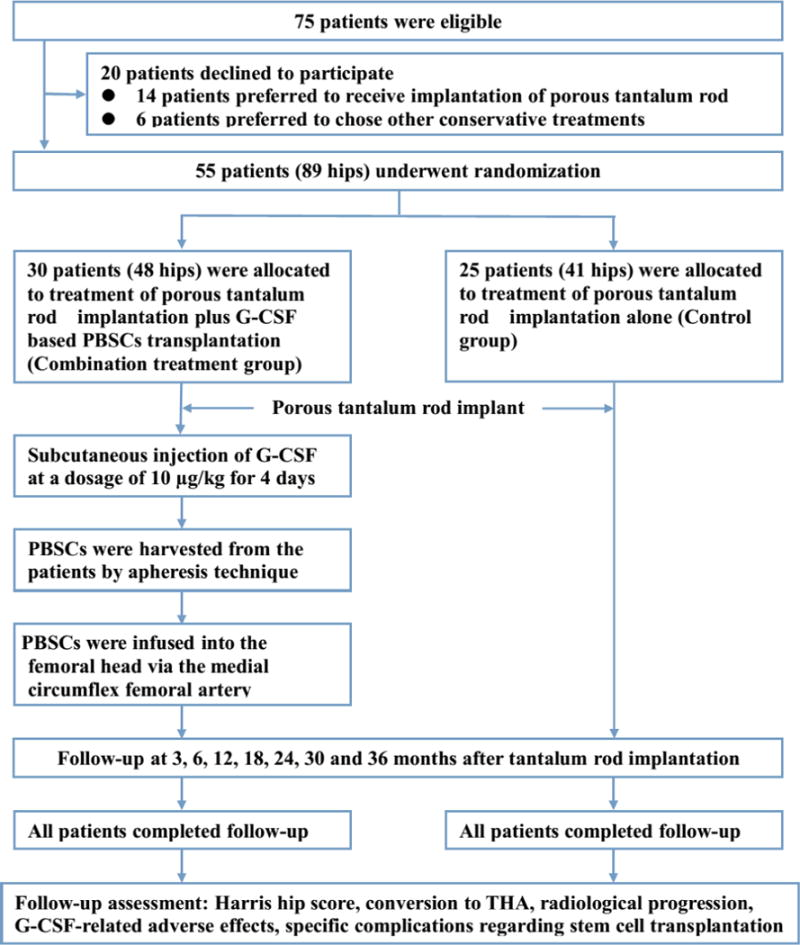
Patient flow chart.
Table 1.
Baseline characteristics of study participants and osteonecrosis.
| Characteristics | Combination treatment | Controls | P value |
|---|---|---|---|
| Characteristics of study participants | |||
| Number of patients | 30 | 25 | N/A |
| Age (years)* | 34.60 (11.50) | 36.12 (11.34) | 0.732 |
| Male:female | 17:13 | 13:12 | 0.729 |
| Characteristics of osteonecrosis | |||
| Number of hips | 48 | 41 | N/A |
| Etiologic Factors – hips (%) | |||
| Alcohol | 18 (37.50) | 14 (34.15) | |
| Steroids | 16 (33.33) | 15 (36.59) | 0.986 |
| Idiopathic | 14 (29.17) | 12 (29.26) | |
| Harris hip score | 62.70 (11.12) | 64.56 (8.55) | 0.461 |
| ARCO stage – hips (%) | |||
| I | 8 (16.67) | 10 (24.39) | |
| II | 29 (60.42) | 23 (56.10) | 0.420 |
| IIIA | 11 (22.91) | 8 (19.51) |
Data are number of patients or affected hips unless indicated otherwise.
Mean (standard deviation).
Treatment Details
The lead operating surgeon in all porous tantalum rod implantations was the same senior orthopaedic surgeon. The porous tantalum rod has a 10-mm-diameter cylindrical shape with length options of 70 to 130 mm (in 5-mm increments), and threads designed to engage lateral cortex of the femur (25 mm long and 14 mm diameter).
The numbers of monouclear cells and CD34+ cells in peripheral blood reached a peak after 4-days subcutaneous injection of C-GSF. Concentration of peripheral blood monouclear cells and CD34+ cells in the circulation were 6.59±1.63×109/L and 4.57±1.56×107/L, respectively.
The targeted intra-arterial infusion of PBSCs in all patients was performed by the same senior interventional radiologist. The average original volume of cell suspension harvested from each patient was 60±15.74 mlduring apheresis. We have administered average of 2.47±0.5×108 monouclear cells collected from peripheral blood to each hip with ONFH which contained 1.71±0.7×106 CD34+ cells.
Primary Outcomes
Intention to treat analysis showed the evidence of a difference in hip function and the rate of THA between treatment groups at 36 months of follow-up in favor of the combination treatment group (Table 2). At 36 months, the mean HHS of hips in the combination treatment group was statistically higher than that of hips in the control group (p=0.003) (Table 2). Temporal trends for HHS based on per protocol showed that HHS in the combination treatment group increased from baseline levels to the peak levels at 24 months and then declined at months of 30 and 36, whereas HHS increased in the control group from the baseline levels to the peak levels at 18 months and then declined at 24, 30 and 36 months. Overall, the combination treatment group achieved greater improvement in the pain and joint symptoms of hips with respect to the HHS compared to the control group (p<0.05) (Figure 2).
Table 2.
Outcome data for 55 trial participants at 36 months after porous tantalum rod implantiaon
| Outcome measure | Combination treatment (n=48) | Controls (n=41) | P value |
|---|---|---|---|
| Primary outcome | |||
| Harris hip score* | 88.10 (3.25) | 78.47 (8.68) | 0.003 |
| Conversion to THA (%) | 3 (6.25) | 9 (21.95) | 0.031 |
| Secondary outcome | |||
| Radiological progression (%) | 4 (8.33) | 13 (31.71) | 0.005 |
| Radiological collapse (%) | 3 (8.11) | 5 (15.15) | 0.545 |
| Complication (%) | 1 (2.08) | 1 (2.44) | 1.00 |
Data are number of affected hips unless indicated otherwise.
Mean (standard deviation).
Fig. 2.
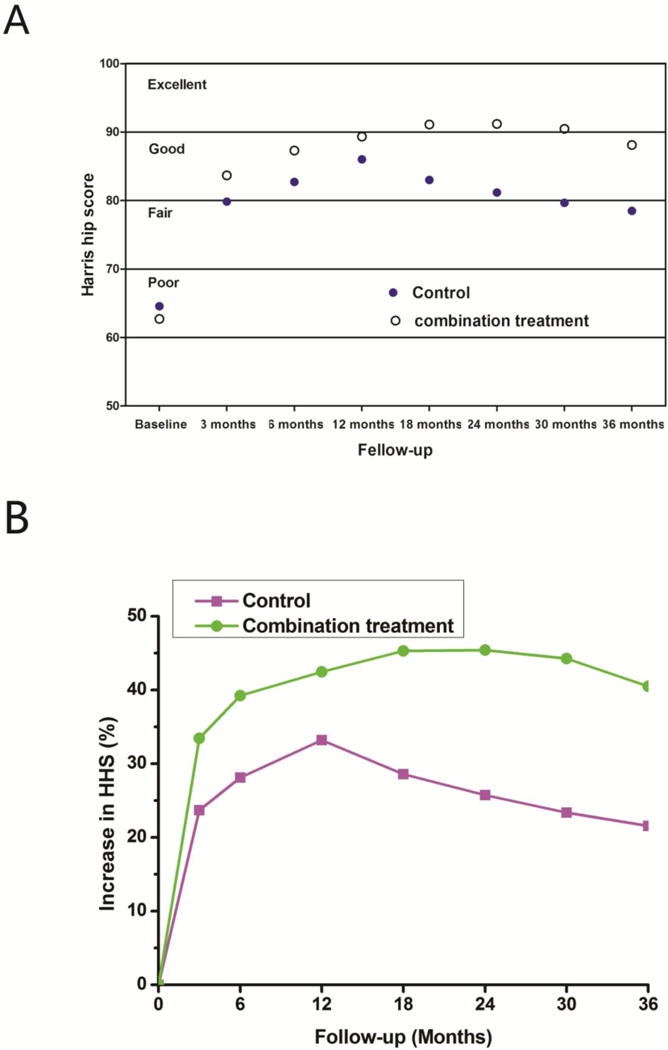
Temporal trends in Harris hip score (A) and the percent increase of Harris hip score (B) in each treatment group over the entire follow-up period.
Over the 36-month follow-up period, qualitative difference to the conclusion was made in the numbers of hips that progressed to clinical failure between two groups (p=0.031) (Table 2). At 36 months, 45 of 48 hips (93.75%) in the combination treatment group had a satisfactory clinical result, which was defined as no need of additional surgery compared to 32 of 41 hips (78.05%) (no additional surgery is needed) in the control group (p=0.031) (Figure S3). After 36 months of follow-up, 3 of 48 hips (6.25%) in the combination treatment group were found to be clinical failure requiring THA, including 1 hip between 24 and 30 months, and 2 hips between the 30 and 36 months follow-up (Table 2 and 3). In contrast, 9 of 41 hips (21.95%) in the control group underwent THA, including 1 case between 12 and 18 months, 2 cases between 18 and 24 months, 3 cases between 24 and 30 months, and 3 cases between 30 and 36 months follow-up (Table 2 and 3). Over the entire follow-up period, combination treatment made more contributions in delaying THA than porous tantalum rod implantion treatment (Figure S4). Kaplan-Meier survival analysis demonstrated a significant difference in the survival time between two groups at 36 months (log rank test; p=0.025). The combination treatment group showed a significantly higher probability of survival versus the control group (Figure 3). The average survival times were 35.88 months in the combination treatment group and 33.51 months for the control group (Table 3).
Table 3.
Number of clinnical failure hips at follow-up and average survival times of hip for each treatment group
| Follow-up period (months)
|
Average survival time (months) | |||||||
|---|---|---|---|---|---|---|---|---|
| 3 | 6 | 12 | 18 | 24 | 30 | 36 | ||
| Combination treatment | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 35.88 |
| Controls | 0 | 0 | 0 | 3 | 3 | 2 | 1 | 33.51 |
Fig. 3.
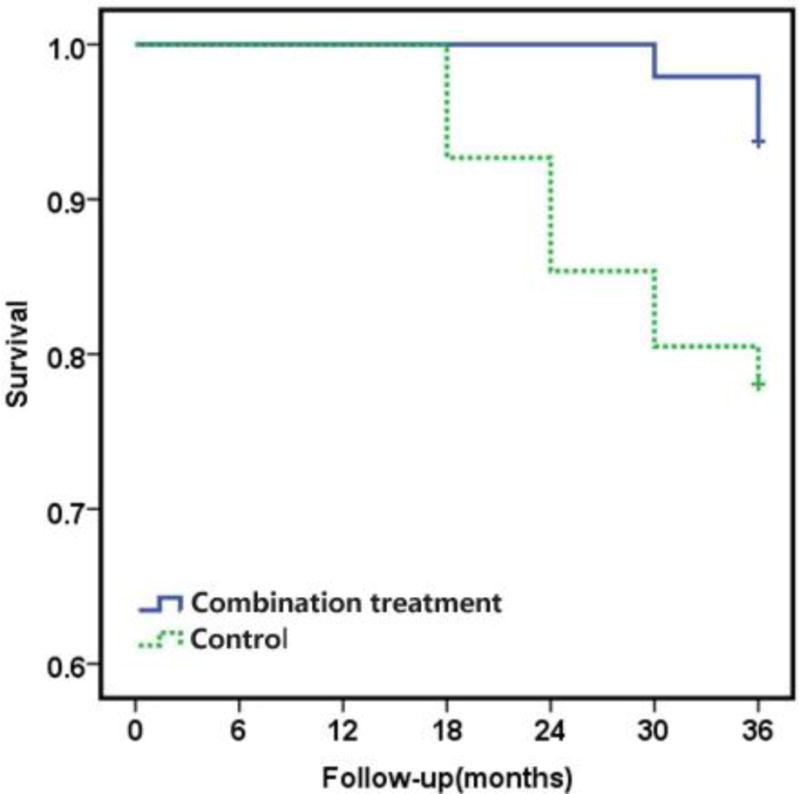
Survivorship curves for the hips in each treatment group, with the convertion to THA as the end point. Kaplan-Meier survivorship analysis showed a significant difference between the two groups in the distributions of the time to THA (Log-rank test; p=0.025).
Secondary Outcomes
The radiological progression as a secondary outcome measurement at 36 months is documented in Table 2. At the final follow-up examination, radiological progression was noted in 13 of 41 hips for the control group, while only observed in 4 of 48 hips for the combination treatment group (Table 2). The rate of radiological progression in the combination treatment group was statistically lower than that in the control group (Figure S5), demonstrating that combination treatment can better prevented hips from progressing to higher stages of osteonecrosis versus biomechanical support alone (Figure 4). The radiological status of the hips at follow-up is documented in Table 4. The number of hips that had the radiological progression was increased with time in two groups (Table 4). However, it was different in the time of progressing to higher osteonecrotic stages between the two groups (Table 4).
Fig. 4.
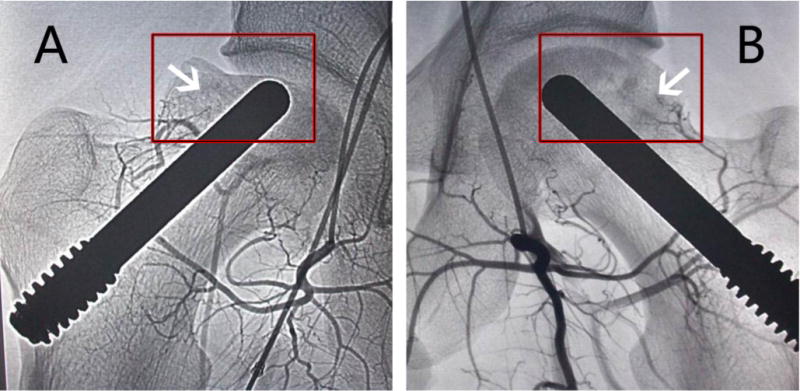
The photographs were taken by digital substraction angiography at 36 months. (A) Blood vessel regeneration was not found in the hip of the control group (arrow), and the femoral head collapsed (in box); (B) Blood vessel regeneration was observed in the hip of the combination treatment group (arrow), new blood vessels were developing sufficiently to reach the femoral head region (arrow), and the femoral head remained intact and round (in box).
Table 4.
ARCO stage of the hips at follow-up
| At onset | Follow-up period (months)
|
|||||
|---|---|---|---|---|---|---|
| 12 | 18 | 24 | 30 | 36 | ||
| Combination treatment | ||||||
| I | 8 | 9 | 11 | 11 | 10 | 9 |
| II | 29 | 28 | 26 | 25 | 25 | 24 |
| III | 11 | 11 | 10 | 10 | 10 | 11 |
| IV | 0 | 0 | 1 | 2 | 3 | 4 |
| Controls | ||||||
| I | 10 | 11 | 12 | 11 | 9 | 6 |
| II | 23 | 22 | 20 | 20 | 21 | 22 |
| III | 8 | 8 | 8 | 8 | 8 | 7 |
| IV | 0 | 0 | 1 | 2 | 3 | 6 |
During this 36 month follow-up period, three hips in the combination treatment group progressed to the subchondral fracture stage of osteonecrosis at 24, 30 and 36 months of follow-up, respectively (Table 2 and 5). In contrast, we observed the presence of subchondral fracture in five hips of the control group, including 3 hips at 18, 24 and 36 months, respectively and the other two hips at 36 months (Table 2 and 5). At a follow-up of three years, the overall collapse rate was 15.15% (5 of 33 hips) in the control group and 8.11% (3 of 37 hips) in the combination treatment group (Table 2). The changes of the collapse rate for each group over the entire follow-up period are presented in Figure S6. Although no evidence indicating significant difference on the outcome measurements of radiological collapse between two groups (p=0.545), it was worse in hips of the control group than that in hips of the combination treatment group (Table 2 and 5). The mean time to collapse was 30 months for hips in the cell infusion group and 28.8 momths for hips in the control group (Table 5).
Table 5.
Number of hips progressed to collapse and the mean time to collapse
| Follow-up period (months)
|
Mean time to collapse (months) | |||||||
|---|---|---|---|---|---|---|---|---|
| 3 | 6 | 12 | 18 | 24 | 30 | 36 | ||
| Combination treatment | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 30 |
| Controls | 0 | 0 | 0 | 1 | 1 | 1 | 2 | 28.8 |
Overall complications rates did not differ between treatment groups (Table 2). One patient in the control group experienced a postoperative infection. This patient was successfully treated with antibiotics. The porous tantalum rod was found to be displaced in one patient of the combination treatment group. This patient was given conservative treatment. No any adverse reactions from G-CSF based PBSCs transplantation were observed during or after the treatment.
Discussion
In this randomized clinical trial we observed the significant differences in hip function and the rate of THA between patients having combination treatment with porous tantalum rod implantation and targeted intra-arterial delivery of PBSCs versus porous tantalum rod implantation alone for ONFH. Compared to the control group, combination treatment is associated with increased HHS, suggesting that combination treatment may works more effectively in alleviating pain and ameliorating the function, activity and motion of the affected hip. The improvements in average HHS from baseline were observed in both groups at each follow-up visit, but this did not last throughout the entire follow-up period (Figure 2). The average HHS began to decline from 18 months for the control group and 30 months for the combination treatment group (Figure 2), suggesting that there is a much more rapid deterioration of hips in the control group. In addition, this finding suggests that the disease progression may be controlled better if the infusion of PBSCs is repeated every 30 months. The increase in HHS observed in the combination treatment group is associated with reduction in the risk of conversion to THA compared to the control group, revealing that combination treatment may play a more important role in avoiding THA than porous tantlalum rod implantation alone. A difference in the time to THA was also evident in this study. THA occurred sooner in hips of the control group than that in hips of the combination treatment group, suggesting that combination treatment has significant effect on delaying THA as compared to porous tantalum rod implantation alone. Overall, we have shown that combination treatment contributes more to decreasing hip pain and other joint symptoms, ameliorating the function of the affected hip and delaying or avoiding THA than porous tantalum rod implantation alone.
The radiological progression can also be used to gauge the efficacy of treatment for ONFH. Compared to porous tantalum rod implantation alone, the reduction in the rate of radiological progression seems to be closely related to combination treatment at 36 months. This finding suggests that combination treatment is superior to porous tantalum rod implantation alone in delaying the progression of ONFH. The percentage of hips progressed to collapse in the combination treatment group was lower than that in the control group. However, we observed no evidence of a significant statistical difference in terms of collapse rate between two groups in this study, implying that combination treatment have no significant impact on reducing the probability of collapse compared to porous tantalum rod implantation alone. The explanation for this phenomenon may be related to the limited number of participants enrolled and short term follow-up in current trial.
Femoral head collapse and THA conversion rates after porous tantalum rod implantation have been well documented in the literature. In current study, the collapse rate in the control group was 15.15%, which is consistent with previous reports[31, 43], in which the collapse rate was 13.63% and 16.3%, respectively. However, the collapse rate of the combination treatment group, which was 8.11%, is conspicuously lower than previous reports[31, 43]. This study was designed to assess THA as an endpoint. 64% of the untreated hips required THA at the end of 2-years [44], compared with our result of 6.25% in the combination treatment group and 21.95% in the control group at the end of 3-years. Liu [45] found that the rate of hips underwent THA after porous tantalum rod implantation were 22.34%. In present study, THA conversion rate of the control group is very close to the literature, yet the combination treatment group showed superior result compared to the literature.
The major purpose of the treatment for ONFH is to preserve the femoral head and prevent destruction of the hip joint. Early intervention is the key for the success of joint-preserving procedures [15]. To date, the therapy recommendations of ONFH remain controversial, and the exact indications have not yet been established [46]. In order to prevent the collapse of femoral head which is caused by biomechanical changes of subchondral bone, complex surgical techniques with subchondral biomechanical support following removal of structurally compromised necrotic bone have been performed, including non-vascularized or vascularized autologous bone grafting, and promising clinical data have been achieved [34–39]. However, previous studies of the bone grafting have reported the disadvantages include donor site morbidity, long operative time, as well as blood loss [34, 43, 47]. Alternatively, the use of porous tantalum rods has been recommended to supply additional mechanical support against the fracture of subchondral bone [27, 29, 31–33]. The use of porous tantalum rod is a relatively easy, minimally invasive and reproducible procedure that can provide satisfactory functional improvement at both pre- and post-collapse stages of ONFH [33]. Thus, in present studies we applied porous tantalum rod to support the subchondral bone. Porous tantalum rod is characterized with excellent biocompatibility, similar elastic modulus to subchondral bone [48–49] and high volumetric porosity [31, 33]. Pores are fully interconnected that allows a relatively free flow of nutrients to the interior of the porous tantalum rod [33, 50]. Furthermore, the microtexture of the material (dodecahedron) permits uninhibited vascularization and rapid bony ingrowth [33]. Thus, porous tantalum rod was proposed as a reasonable mechanical substitute for a bone graft [31]. However, porous tantalum rod can only provide provisional structural support until the ingrowth of new bone [45], and the histological study has shown that the absence of new bone tissue growing into porous tantalum rod in necrotic lesions make this approach less desirable [51]. Decrease of osteogenic progenitors in femoral head is one of the common features of ONFH [7]. Reduction in the number and function of circulating endothelial progenitor cells has also been observed in patients with ONFH [52]. Hence, lack of osteogenesis and revascularization may contribute to the insufficient ingrowth of new bone into porous tantalum rod. Ttreatment modalities should stimulate and guide bone remodeling to sufficient creeping substitution to preserve the integrity of the femoral head [7]. Recent studies explored the potential of MSCs in the treatment of ONFH, and promising clinical data on the application of MSCs to treat ONFH have been reported [7, 9–15]. However, in these studies, the investigators chose to use the concentrated bone marrow cells or isolated bone marrow derived progenitor cells. PBSCs transplantation has advantages in terms of easy collect and enrich CD34+ cells, more cost-effective, and lower risk for contamination with tumor cells in comparison to the conventional bone marrow cell transplantation [53–57]. Therefore, we combined the PBSCs transplantation with mechanical support and designed this randomized clinical trial to sufficiently validate efficiency of the combination treatment in comparison with biomechanical support alone. Since PBSCs transplantation can promote new bone ingrowth into the tantalum rod and dead bone repaired by stimulating and guiding bone remodeling, the combination treatment obtained a better effect in the treatment of ONFH than biomechanical support alone.
The therapeutic efficiency of PBSCs may be closely related to the fate of PBSCs implanted into femoral head. We did not examine the fate of PBSCs after transplantation, but there have been reports demonstrating that MSCs can migrate into the target tissues and participate in repairing injured tissues via blood circulation after ischemia [58–59]. 60% of the MSCs remained in the femoral head 24 h after implantation as shown by the radionuclide labeling [10]. Li et al [60] found that the amounts of MSCs in the necrotic femoral head were higher than that in the normal femoral head, liver and lungs in the rabbit model of ONFH after intravenous transplantation. They then concluded that femoral head ischemia or necrosis is able to attract MSCs to migrate into and survive in injured femoral heads. Similarly, Yan et al[15] demonstrated that MSCs could survive, expand and differentiate into osteoblast in the osteonecrotic region of ONFH after the transplantation, and hypoxia can stimulate MSCs to secrete angiogenic factors resulting in increased angiogenesis. From these studies, we can deduce that these cells including PBSCs have the capacity to respond actively to their environment, migrate into injured tissues, survive and differentiate into osteoblast in the osteonecrotic region, and secrete angiogenic factors. The capacity may contribute to the bone healing process. Recently, many papers have been published using PBSCs to treat patients with myocardial infarction and peripheral limb ischemia [17–26]. For these diseases, the use of PBSCs in neovascularization is the same as that for ONFH. However, PBSCs also have the responsibility of myogenesis in myocardial infarction and peripheral limb ischemia, whereas PBSCs bear the task of osteogenesis in ONFH.
To our knowledge this is the first report of clinical outcome study of a combinational treatment for ONFH that utilizes tantalum rod implantation in combination with targeted intra-arterial infusion of PBSCs. The study is focusing on the feasibility and effectiveness of the treatment modality to alleviate hip pain, and on the progression of the disease.
This study was a parallel group, prospective randomized controlled clinical trial. Randomization was done by using a computer generated, randomised number sequence. This ensures that the distribution of confounders across treatment groups is well balanced. Even though recruitment of patients was accomplished only in one center, variety of patients with diverse age, sex, etiological factors, and osteonecrotic stages involved probably reflects the wider population of patients in China. We obtained complete follow-up data from each patient at the primary endpoint. Thus, the trial was adequately powered to declare that this combined therapy has better efficacy than treatment with porous tantalum rod implantation alone.
The key limitation of this trial is that the patients themselves were not blinded to their treatment, but the researchers are blinded in terms of treatment allocation and measurement of the clinical outcomes. The outcome data of this trial have shown that combined therapy overmatched porous tantalum rod implantation alone in relieving symptoms of ONFH, ameliorating the function of the affected hip and delaying or avoiding THA, although it is associated with limitations, such as low power to detect if the combined therapy is superior in preventing the collapse of the femoral head than porous tantalum rod implantation alone. Other limitations include short-term follow-up and not being able to detect whether the combined therapy has better efficacy than the treatment of targeted intra-arterial infusion of PBSCs alone. Therefore, further observation and follow-up is necessary. We did not have enough data to fully examine the effects of the combined therapy among patients from different etiologies and osteonecrotic stages. We will continue to review the patient data in this trial over the coming years. In addition, we do not have evidence for survival of grafted PBSCs, and new bone ingrowth into porous tantalum rod at the present time. Further investigations are required.
Conclusions
In the present studies, benefits of porous tantalum rod implantation in combination with targeted intra-arterial infusion of PBSCs were compared with porous tantalum rod implantation alone. This work reveals that combination treatment is superior regarding clinical outcome compared to biomechanical support alone. Although we do not have histological evidence for better repair of bone tissue after infusion of PBSCs, we are optimistic that infusion of PBSCs is capable of enhancing the efficacy of biomechanical support in the treatment of ONFH. The favorable effects and the absence of adverse events suggest that combination treatment is a feasible intervention to slow and possibly reverse the effects of early and intermediate stages of ONFH. This study could not determine the long-term effect of combination treatment. We will continue to review the patients enrolled in this study over the coming years, as well as expand study samples to examine the therapeutic effects of combination treatment in different stages of ONFH. In a word, the combination treatment has provided opportunities for combining mechanical and biological concepts to treat ONFH.
Supplementary Material
Acknowledgments
The authors appreciate the patients and their families. Roles of authors: Study design: Hongting Jin and Peijian Tong. Study conduct: Qiang Mao and Peijian Tong. Data collection: Weidong Wang and Luwei Xiao. Data analysis: Weidong Wang and Luwei Xiao. Data interpretation: Taotao Xu and Shanxing Zhang. Drafting manuscript: Qiang Mao, Weidong Wang and Di Chen. Revising manuscript content: Qiang Mao, Weidong Wang and Di Chen.
Footnotes
This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi: [10.1002/jbmr.2390]
Additional Supporting Information may be found in the online version of this article.
Disclosures
The authors state that they have no conflicts of interest.
Supplemental Information
Supplemental surgical procedure and Figure S1, 2, 3, 4, 5, 6 and 7 can be found in supplemental data for review and online publication only.
Contributor Information
Qiang Mao, Email: peijiantongzy@126.com.
Weidong Wang, Email: wangrz2006@163.com.
Taotao Xu, Email: nacle1990829@163.com.
Shanxing Zhang, Email: zhangshanxing1984@163.com.
Luwei Xiao, Email: xlw@139.com.
Di Chen, Email: di_chen@rush.edu.
References
- 1.Wen Q, Ma L, Chen YP, Yang L, Luo W, Wang XN. Treatment of avascular necrosis of the femoral head by hepatocyte growth factor-transgenic bone marrow stromal stem cells. Gene Ther. 2008;15(23):1523–1535. doi: 10.1038/gt.2008.110. [DOI] [PubMed] [Google Scholar]
- 2.Fan M, Peng J, Qin L, Lu S. Experimental animal models of osteonecrosis. Rheumatol Int. 2011;31(8):983–994. doi: 10.1007/s00296-011-1819-9. [DOI] [PubMed] [Google Scholar]
- 3.Lieberman JR, Engstrom SM, Meneghini RM, SooHoo NF. Which factors influence preservation of the osteonecrotic femoral head? Clin Orthop Relat Res. 2012;470(2):525–534. doi: 10.1007/s11999-011-2050-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aaron RK. Osteonecrosis: etiology, pathophysiology and diagnosis. In: Callaghan JJ, Rosenberg AG, Rubash HE, editors. The adult hip. Lippincott-Raven; Philadelphia: 1998. pp. 451–466. [Google Scholar]
- 5.Lieberman JR1, Berry DJ, Mont MA, Aaron RK, Callaghan JJ, Rajadhyaksha AD, Urbaniak JR. Osteonecrosis of the hip: management in the 21st century. Instr Course Lect. 2003;52:337–355. [PubMed] [Google Scholar]
- 6.Cardozo JB, Andrade DM, Santiago MB. The use of bisphosphonate in the treatment of avascular necrosis: a systematic review. Clin Rheumatol. 2008;27(6):685–688. doi: 10.1007/s10067-008-0861-9. [DOI] [PubMed] [Google Scholar]
- 7.Hernigou P, Zilber S, Filippini P, Rouard H, Methieu G, Poignard A. Bone marrow injection in hip osteonecrosis. Tech Orthop. 2008;23:18–25. [Google Scholar]
- 8.Li W, Sakai T, Nishii T, Nakamura N, Takao M, Yoshikawa H, Sugano N. Distribution of TRAP-postive cells and expression of HIF-1alpha, VEGF, and FGF-2 in the reparative reaction in patients with osteonecrosis of the femoral head. J Orthop Res. 2009;27(5):694–700. doi: 10.1002/jor.20802. [DOI] [PubMed] [Google Scholar]
- 9.Zhao D, Cui D, Wang B, Tian F, Guo L, Yang L, Liu B, Yu X. Treatment of early stage osteonecrosis of the femoral head with autologous implantation of bone marrow-derived and cultured mesenchymal stem cells. Bone. 2012;50(1):325–330. doi: 10.1016/j.bone.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Gangji V, De Maertelaer V, Hauzeur JP. Autologous bone marrow cell implantation in the treatment of non-traumatic osteonecrosis of the femoral head: Five year follow-up of a prospective controlled study. Bone. 2011;49(5):1005–1009. doi: 10.1016/j.bone.2011.07.032. [DOI] [PubMed] [Google Scholar]
- 11.Xie XH1, Wang XL, He YX, Liu Z, Sheng H, Zhang G, Qin L. Promotion of bone repair by implantation of cryopreserved bone marrow-derived mononuclear cells in a rabbit model of steroid-associated osteonecrosis. Arthritis Rheum. 2012;64(5):1562–1571. doi: 10.1002/art.34525. [DOI] [PubMed] [Google Scholar]
- 12.Cui Q, Botchwey EA. Treatment of precollapse osteonecrosis using stem cells and growth factors. Clin Orthop Relat Res. 2011;469(9):2665–2669. doi: 10.1007/s11999-010-1738-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mao Q, Jin H, Liao F, Xiao L, Chen D, Tong P. The efficacy of targeted intraarterial delivery of concentrated autologous bone marrow containing mononuclear cells in the treatment of osteonecrosis of the femoral head: A five year follow-up study. Bone. 2013;57(2):509–16. doi: 10.1016/j.bone.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rackwitz L, Eden L, Reppenhagen S, Reichert JC, Jakob F, Walles H, Pullig O, Tuan RS, Rudert M, Nöth U. Stem cell- and growth factor-based regenerative therapies for avascular necrosis of the femoral head. Stem Cell Res Ther. 2012;3(1):7. doi: 10.1186/scrt98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan Z, Hang D, Guo C, Chen Z. Fate of mesenchymal stem cells transplanted to osteonecrosis of femoral head. J Orthop Res. 2009;27(4):442–446. doi: 10.1002/jor.20759. [DOI] [PubMed] [Google Scholar]
- 16.Elliott MJ, De Coppi P, Speggiorin S, Roebuck D, Butler CR, Samuel E, Crowley C, McLaren C, Fierens A, Vondrys D, Cochrane L, Jephson C, Janes S, Beaumont NJ, Cogan T, Bader A, Seifalian AM, Hsuan JJ, Lowdell MW, Birchall MA. Stem-cell-based, tissue engineered tracheal replacement in a child: a 2-year follow-up study. Lancet. 2012;380(9846):994–1000. doi: 10.1016/S0140-6736(12)60737-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang HJ, Kim HS, Zhang SY, Park KW, Cho HJ, Koo BK, Kim YJ, Soo Lee D, Sohn DW, Han KS, Oh BH, Lee MM, Park YB. Effects of intracoronary infusion of peripheral blood stem-cells mobilised with granulocyte-colony stimulating factor on left ventricular systolic function and restenosis after coronary stenting in myocardial infarction: the MAGIC cell randomised clinical trial. Lancet. 2004;363(9411):751–756. doi: 10.1016/S0140-6736(04)15689-4. [DOI] [PubMed] [Google Scholar]
- 18.Hsieh MM, Kang EM, Fitzhugh CD, Link MB, Bolan CD, Kurlander R, Childs RW, Rodgers GP, Powell JD, Tisdale JF. Allogeneic hematopoietic stem-cell transplantation for sickle cell disease. N Engl J Med. 2009;361(24):2309–2317. doi: 10.1056/NEJMoa0904971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song HJ, Lan BS, Cheng B, Zhang KF, Yan HW, Wang WZ, Gao ZQ. Peripheral blood stem cell transplantation for ischemic femoral head necrosis. Transplant Proc. 2010;42(5):1862–1864. doi: 10.1016/j.transproceed.2010.02.077. [DOI] [PubMed] [Google Scholar]
- 20.Song HJ, Lan BS, Cheng B, Zhang KF, Yan HW, Wang WZ, Gao ZQ. Treatment of early avascular necrosis of femoral head by small intestinal submucosal matrix with peripheral blood stem cells. Transplant Proc. 2011;43(5):2027–2032. doi: 10.1016/j.transproceed.2010.12.060. [DOI] [PubMed] [Google Scholar]
- 21.Tse HF, Kwong YL, Chan JKF, Lo G, Ho CL, Lau CP. Angiogenesis in ischaemic myocardium by intramyocardial autologous bone marrow mononuclear cell implantation. Lancet. 2003;361(9351):47–49. doi: 10.1016/S0140-6736(03)12111-3. [DOI] [PubMed] [Google Scholar]
- 22.Kajiguchi M, Kondo T, Izawa H, Kobayashi M, Yamamoto K, Shintani S, Numaguchi Y, Naoe T, Takamatsu J, Komori K, Murohara T. Safety and efficacy of autologous progenitor cell transplantation for therapeutic angiogenesis in patients with critical limb ischemia. Circ J. 2007;71(2):196–201. doi: 10.1253/circj.71.196. [DOI] [PubMed] [Google Scholar]
- 23.Nevskaya T, Ananieva L, Bykovskaia S, Eremin I, Karandashov E, Khrennikov J, Mach E, Zaprjagaeva M, Guseva N, Nassonov E. Autologous progenitor cell implantation as a novel therapeutic intervention for ischaemic digits in systemic sclerosis. Rheumatology (Oxford) 2009;48(1):61–64. doi: 10.1093/rheumatology/ken407. [DOI] [PubMed] [Google Scholar]
- 24.Iwasaki H, Fukushima K, Kawamoto A, Umetani K, Oyamada A, Hayashi S, Matsumoto T, Ishikawa M, Shibata T, Nishimura H, Hirai H, Mifune Y, Horii M, Sugimura K, Suehiro S, Asahara T. Synchrotron radiation coronary microangiography for morphometric and physiological evaluation of myocardial neovascularization induced by endothelial progenitor cell transplantation. Arterioscler Thromb Vasc Biol. 2007;27(6):1326–1333. doi: 10.1161/ATVBAHA.106.137141. [DOI] [PubMed] [Google Scholar]
- 25.Wen Y, Meng L, Ding Y, Ouyang J. Autologous transplantation of blood-derived stem progenitor cells for ischaemic heart disease. Int J Clin Pract. 2011;65(8):858–65. doi: 10.1111/j.1742-1241.2011.02715.x. [DOI] [PubMed] [Google Scholar]
- 26.Dubsky M, Jirkovska A, Bem R, Fejfarova V, Pagacova L, Sixta B, Varga M, Langkramer S, Sykova E, Jude EB. Both autologous bone marrow mononuclear cell and peripheral blood progenitor cell therapies similarly improve ischaemia in patients with diabetic foot in comparison with control treatment. Diabetes Metab Res Rev. 2013;29(5):369–376. doi: 10.1002/dmrr.2399. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Li L, Shi ZJ, Wang J, Li ZH. Porous tantalum rod implant is an effective and safe choice for early-stage femoral head necrosis: a meta-analysis of clinical trials. Eur J Orthop Surg Traumatol. 2013;23(2):211–217. doi: 10.1007/s00590-012-0962-7. [DOI] [PubMed] [Google Scholar]
- 28.Yang S, Wu X, Xu W, Ye S, Liu X, Liu X. Structural augmentation with biomaterial loaded allograft threaded cage for the treatment of femoral head osteonecrosis. J Arthroplasty. 2010;25(8):1223–1230. doi: 10.1016/j.arth.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 29.Floerkemeier T, Thorey F, Daentzer D, Lerch M, Klages P, Windhagen H, von Lewinski G. Clinical and radiological outcome of the treatment of osteonecrosis of the femoral head using the osteonecrosis intervention implant. Int Orthop. 2011;35(4):489–495. doi: 10.1007/s00264-009-0940-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malizos KN, Papasoulis E, Dailiana ZH, Papatheodorou LK, Varitimidis SE. Early results of a novel technique using multiple small tantalum pegs for the treatment of osteonecrosis of the femoral head: a case series involving 26 hips. J Bone Joint Surg Br. 2012;94(2):173–178. doi: 10.1302/0301-620X.94B2.27287. [DOI] [PubMed] [Google Scholar]
- 31.Liu G, Wang J, Yang S, Xu W, Ye S, Xia T. Effect of a porous tantalum rod on early and intermediate stages of necrosis of the femoral head. Biomed Mater. 2010;5(6):065003. doi: 10.1088/1748-6041/5/6/065003. [DOI] [PubMed] [Google Scholar]
- 32.Floerkemeier T, Lutz A, Nackenhorst U, Thorey F, Waizy H, Windhagen H, von Lewinski G. Core decompression and osteonecrosis intervention rod in osteonecrosis of the femoral head: clinical outcome and finite element analysis. Int Orthop. 2011;35(10):1461–14666. doi: 10.1007/s00264-010-1138-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Varitimidis SE, Dimitroulias AP, Karachalios TS, Dailiana ZH, Malizos KN. Outcome after tantalum rod implantation for treatment of femoral head osteonecrosis: 26 hips followed for an average of 3 years. Acta Orthop. 2009;80(1):20–25. doi: 10.1080/17453670902804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen CC, Lin CL, Chen WC, Shih HN, Ueng SW, Lee MS. Vascularized iliac bone-grafting for osteonecrosis with segmental collapse of the femoral head. J Bone Joint Surg Am. 2009;91(10):2390–2394. doi: 10.2106/JBJS.H.01814. [DOI] [PubMed] [Google Scholar]
- 35.Seyler TM, Marker DR, Ulrich SD, Fatscher T, Mont MA. Nonvascularized bone grafting defers joint arthroplasty in hip osteonecrosis. Clin Orthop Relat Res. 2008;466(5):1125–1132. doi: 10.1007/s11999-008-0211-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoo MC, Kim KI, Hahn CS, Parvizi J. Long-term followup of vascularized fibular grafting for femoral head necrosis. Clin Orthop Relat Res. 2008;466(5):1133–1140. doi: 10.1007/s11999-008-0204-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuhan C, Hu CC, Chen DW, Ueng SW, Shih CH, Lee MS. Local cancellous bone grafting for osteonecrosis of the femoral head. Surg Innov. 2009;16(1):63–67. doi: 10.1177/1553350608330398. [DOI] [PubMed] [Google Scholar]
- 38.Wang BL, Sun W, Shi ZC, Zhang NF, Yue DB, Guo WS, Shi SH, Li ZR. Treatment of nontraumatic osteonecrosis of the femoral head using bone impaction grafting through a femoral neck window. Int Orthop. 2010;34(5):635–639. doi: 10.1007/s00264-009-0822-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shih LY, Wong YC, Shih HN. One-stage hip arthroplasty and bone grafting for bilateral femoral head osteonecrosis. Clin Orthop Relat Res. 2009;467(6):1522–1528. doi: 10.1007/s11999-008-0393-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mont MA, Marulanda GA, Jones LC, Saleh KJ, Gordon N, Hungerford DS, Steinberg ME. Systematic analysis of classification systems for osteonecrosis of the femoral head. J Bone Joint Surg Am. 2006;88(Suppl 3):16–26. doi: 10.2106/JBJS.F.00457. [DOI] [PubMed] [Google Scholar]
- 41.The COBE Spectra Apheresis System Operator’s Manual. Lakewood: COBE BCT; 1999. [Google Scholar]
- 42.Agarwala S, Shah S, Joshi VR. The use of alendronate in the treatment of avascular necrosis of the femoral head: follow-up to eight years. J Bone Joint Surg Br. 2009;91(8):1013–1018. doi: 10.1302/0301-620X.91B8.21518. [DOI] [PubMed] [Google Scholar]
- 43.Shuler MS, Rooks MD, Roberson JR. Poroustantalum implant in early osteonecrosis of the hip: preliminary report on operative, survival, and outcomes results. J Arthroplasty. 2007;22(1):26–31. doi: 10.1016/j.arth.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 44.Lai KA, Shen WJ, Yang CY, Shao CJ, Hsu JT, Lin RM. The use of alendronate to prevent early collapse of femoral head in patients with nontraumatic osteonecrosis: a randomized clinical study. J Bone Joint Surg Am. 2005;87(10):2155–2159. doi: 10.2106/JBJS.D.02959. [DOI] [PubMed] [Google Scholar]
- 45.Liu B, Sun W, Yue D, Li Z, Guo W. Combined tantalum implant with bone grafting for the treatment of osteonecrosis of the femoral head. J Invest Surg. 2013;26(3):158–162. doi: 10.3109/08941939.2012.718409. [DOI] [PubMed] [Google Scholar]
- 46.Alves EM, Angrisani AT, Santiago MB. The use of extracorporeal shock waves in the treatment of osteonecrosis of the femoral head: a systematic review. Clin Rheumatol. 2009;28(11):1247–1251. doi: 10.1007/s10067-009-1231-y. [DOI] [PubMed] [Google Scholar]
- 47.Veillette CJ, Mehdian H, Schemitsch EH, McKee MD. Survivorship analysis and radiographic outcome following tantalum rod insertion for osteonecrosis of the femoral head. J Bone Joint Surg Am. 2006;88(Suppl 3):48–55. doi: 10.2106/JBJS.F.00538. [DOI] [PubMed] [Google Scholar]
- 48.Tsao AK, Roberson JR, Christie MJ, Dore DD, Heck DA, Robertson DD, Poggie RA. Biomechanical and clinical evaluations of a porous tantalum implant for the treatment of early-stage osteonecrosis. J Bone Joint Surg Am. 2005;87(Suppl 2):22–27. doi: 10.2106/JBJS.E.00490. [DOI] [PubMed] [Google Scholar]
- 49.Tanzer M, Karabasz D, Krygier JJ, Cohen R, Bobyn JD. Bone augmentation around and within porous implants by local bisphosphonate elution. Clin Orthop Relat Res. 2005;441:30–39. doi: 10.1097/01.blo.0000194728.62996.2d. [DOI] [PubMed] [Google Scholar]
- 50.Shimko DA, Shimko VF, Sander EA, Dickson KF, Nauman EA. Effect of porosity on the fluid flow characteristics and mechanical properties of tantalum scaffolds. J Biomed Mater Res B Appl Biomater. 2005;73(2):315–324. doi: 10.1002/jbm.b.30229. [DOI] [PubMed] [Google Scholar]
- 51.Tanzer M, Bobyn JD, Krygier JJ, Karabasz D. Histopathologic retrieval analysis of clinically failed porous tantalum osteonecrosis implants. J Bone Joint Surg Am. 2008;90(6):1282–1289. doi: 10.2106/JBJS.F.00847. [DOI] [PubMed] [Google Scholar]
- 52.Feng Y, Yang SH, Xiao BJ, Xu WH, Ye SN, Xia T, Zheng D, Liu XZ, Liao YF. Decreased in the number and function of circulation endothel ial progenitor cells in patients with avas cular necrosis of the femoral head. Bone. 2010;46(1):32–40. doi: 10.1016/j.bone.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 53.Jansen J, Hanks S, Thompson JM. Transplantation of hematopoietic stem cells from the peripheral blood. J Cell Mol Med. 2005;9:37. doi: 10.1111/j.1582-4934.2005.tb00335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lie AK, To LB. Peripheral blood stem cells: transplantation and beyond. Oncologist. 1997;2(1):40–49. [PubMed] [Google Scholar]
- 55.To LB, Haylock DN, Simmons PJ, Juttner CA. The biology and clinical uses of blood stem cells. Blood. 1997;89(7):2233–2258. [PubMed] [Google Scholar]
- 56.Shyu WC, Lin SZ, Chiang MF, Su CY, Li H. Intracerebral peripheral blood stem cell (CD34) implantation induces neuroplasticity by enhancing β1 integrin-mediated angiogenesis in chronic stroke rats. J Neurosci. 2006;26(13):3444–3453. doi: 10.1523/JNEUROSCI.5165-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Georgantas RW, 3rd, Hildreth R, Morisot S, Alder J, Liu CG, Heimfeld S, Calin GA, Croce CM, Civin CI. CD34+ hematopoietic stem-progenitor cell microRNA expression and function: a circuit diagram of differentiation control. Proc Natl Acad Sci U S A. 2007;104(8):2750–2755. doi: 10.1073/pnas.0610983104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25(11):2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 59.Yagi H, Soto-Gutierrez A, Parekkadan B, Kitagawa Y, Tompkins RG, Kobayashi N, Yarmush ML. Mesenchymal stem cells: Mechanisms of immunomodulation and homing. Cell Transplant. 2010;19(6):667–679. doi: 10.3727/096368910X508762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li ZH, Liao W, Cui XL, Zhao Q, Liu M, Chen YH, Liu TS, Liu NL, Wang F, Yi Y, Shao NS. Intravenous transplantation of allogeneic bone marrow mesenchymal stem cells and its directional migration to the necrotic femoral head. Int J Med Sci. 2011;8(1):74–83. doi: 10.7150/ijms.8.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Costa ML, Achten J, Parsons NR, Edlin RP, Foguet P, Prakash U, Griffin DR, Young Adult Hip Arthroplasty Team Total hip arthroplasty versus resurfacing arthroplasty in the treatment of patients with arthritis of the hip joint: single centre, parallel group, assessor blinded, randomised controlled trial. BMJ. 2012;19(344):e2147. doi: 10.1136/bmj.e2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


