Abstract
Objectives
(1) To assess whether there is evidence of an association between the number of peri-implant tissue complications and patient characteristics such as gender, diabetes status, smoking status, and bite force; (2) To assess whether there is evidence of an association between the number of peri-implant tissue complications and location of the implant, surgical technique used, bone graft status and sinus lift status.
Materials and Methods
This randomized controlled clinical trial included a total of 176 implants (Osseospeed, Dentsply) in 67 participants with 88 fixed dental prostheses. Information was obtained from health histories, a baseline exam, surgical notes, and postoperative exams. The data were analyzed using Fisher's exact and Mann-Whitney tests, and generalized estimating equations logistic regression with a significance level set at 0.05.
Results
All 176 implants survived within a recall period of three years but 11 implants demonstrated peri-implant tissue complications. Ten sites showed dehiscence and one case exhibited vertical bone loss. There was a statistically significant association between surgical technique used (1-stage or 2-stage) and the presence of soft tissue complications (p = 0.005), where 2-stage surgery was associated with a higher frequency of peri-implant soft tissue complications. A correlation, although not statistically significant (p=0.077) was noted, between peri-implant tissue complications and bone grafting, suggesting a possible role for this factor as well.
Conclusions
Participants who did not require any second stage surgery at the implant sites experienced fewer complications. Therefore, additional surgical procedures should be performed judiciously considering their possible effects on peri-implant tissue health.
Keywords: implant failure, implant success, soft tissue complications, bone loss, bone graft
Introduction
Technological advances are often proposed in the field of dentistry. In the past, restoration of completely and partially edentulous areas was primarily achieved through removable prosthetic devices. The advent of dental implants enabled dentists to replace missing teeth with “permanently” affixed prostheses. Implant-borne prostheses have success rates of approximately 98%, and they also ensure the structural integrity of adjacent natural teeth (Buser et al., 1990). Dental implants provide a functional and esthetic replacement for missing teeth (Misch et al., 2008). However, implants are designed to osseointegrate with the bone. They do not interact with the surrounding tissues as a natural tooth does with soft tissue (Weber and Cochran, 1998). Changes to the peri-implant tissues, such as gingival recession, will generally occur at some level, but the evaluation of all aspects of bone and soft tissue health is important to achieve the highest level of success with each patient (Oates et al., 2002).
One of the earliest and most widely accepted ways to define implant success is the low percentage of the technical complications and treatment failures. These complications have included, but were not limited to, the absence of persistent pain, paresthesia, infection, and violation of the mandibular canal (Albrektsson et al., 1986). The International Congress of Oral Implantologists (ICOI) developed the “Pisa Implant Quality of Health Scale”, ranking dental implants into four groups from optimum health to clinical failure or absolute failure based on levels of sensitivity, mobility, bone loss, probing depth, and presence of exudates (Misch et al., 2008). Survival is typically identified within the first 10 weeks of placement, but early failures can occur later, after the first three to five months following surgery, and during the healing phase. Late failures are possible after successful integration of the surrounding tissue during the maintenance period (Buser et al., 1990).
Many factors must be measured and quantified to assess the health of a dental implant. Radiographs are used for examining the potential changes in bone levels. Peri-implant bone loss is often a primary method of defining implant success. When profound bone loss occurs, soft tissue complications often follow (Chappuis et al., 2013). Soft tissue is examined utilizing many of the same parameters used in periodontal examinations, such as the presence or extent of the keratinized mucosa, probing pocket depths, attachment levels, and distance between the implant shoulder and mucosal margin (Misch et al., 2008). Keratinized mucosa is the attached gingiva and is measured from the mucogingival junction to free gingival margin (Bragger et al., 1997). The significance of the effect from most of these variables on soft tissue complications along the peri-implant regions remains controversial. The protocol for evaluating the peri-implant site is not standardized or agreed upon by researchers or clinicians.
The literature and studies present many contradictory measurement processes to quantify soft tissue complications. In one of the earliest classification methods for implant success, gingival and periodontal parameters were excluded as factors because of the uncertainty of their impact on success (Albrektsson et al., 1986). The ICOI believed that periodontal tissue examinations should be used for implant evaluation (Misch et al., 2008). However, numerous reports suggest that certain parameters must be analyzed and approached differently for implants than for the natural dentition. For the natural dentition, increased pocket depths indicate gingivitis or periodontal disease, but increased probing depths of peri-implant sites are not necessarily associated with destruction of bone (Weber & Cochran, 1998). Healthy implant sites often bleed upon probing, suggesting that peri-implant tissue elicits different responses to the same stimuli compared with periodontal tissue (Bragger et al., 1997). Many clinicians believe that certain tissue types should be selected for a greater chance of success. However, the presence of a sufficient band of keratinized mucosa (>2 mm) has not revealed a significant difference in plaque index, inflammation, and pocket depth compared with a deficient (<2 mm) band of keratinized mucosa (Kim et al., 2009).
Most early implant studies have been focused on survival and success based on implant osseointegration. There remains a need to examine and standardize the effects of the peri-implant tissue on implant success. The objectives of this research study are as follows:
To assess whether there is evidence of an association between the number of peri-implant tissue complications and patient characteristics such as gender, diabetes status, smoking status, and bite force;
To assess whether there is evidence of an association between the number of peri-implant tissue complications and location of the implant, surgical technique used, bone graft status and sinus lift status.
Materials and Methods
Study Design
This randomized, controlled clinical trial was conducted to determine the performance and survival of implant-supported, three-unit fixed dental prostheses (FDPs) as a function of several design parameters. The study also proposed to determine implant survival in posterior areas of the mouth where increased load and oral hygiene are factors to be considered. This single-blinded pilot study included a total of 68 participants, who required a total of 89 FDPs to replace missing posterior teeth. The participants' prosthesis sites were randomly assigned to receive either a metal-ceramic or a ceramic-ceramic FDP. These sites were further subdivided according to the thickness of the veneer ceramic, the connector's radius of curvature, and the connector height. The overall thickness of the core plus veneer thickness was 2.0 mm. Thus, the ratio of the veneer thickness to core thickness was variable. The veneer thickness ranged from 0.5 mm to 1.5 mm.
Prosthetic materials
Implants
Commercially pure titanium, air blasted with titanium dioxide and fluoride particles (OsseoSpeed, ASTRA TECH Implant System, DENTSPLY Implants, Mölndal, Sweden)
Custom abutments
Custom-milled, gold-shaded, titanium abutments (ATLANTIS abutment, DENTSPLY Implants, Waltham, USA)
Metal-ceramic FDPs (MC)
Noble Pd-Au-Ag alloy (Capricorn, Ivoclar, Vivadent, Schaan, Liechtenstein) retainer and pontic substructure were veneered with a press-on leucite reinforced glass-ceramic veneer (IPS InLine POM, Ivoclar Vivadent).
Ceramic-ceramic FDPs (CC)
An yttria-stabilized zirconia core (IPS e.max ZirCAD, Ivoclar Vivadent, Schaan, Liechtenstein) was veneered with a pressable fluorapatite glass ceramic (IPS ZirPress, Ivoclar Vivadent, Schaan, Liechtenstein).
Study Population
Participants were recruited through broadcast e-mail, flyers and newspaper advertisements. Participants were selected based on the following criteria:
Age between 21 and 75 years
No contraindications to dental treatment
Good overall dental health (no active caries and no periodontal disease)
Periodontal pocket depths less than 4 mm
Missing at least three posterior teeth.
Natural teeth opposing the edentulous area and
A full complement of teeth or restored teeth in all other areas of the mouth
Adequate bone height and width in areas of proposed implants
Adequate interocclusal distance to accommodate each prosthesis
Good oral hygiene and compliance with oral hygiene instructions as determined by the amount of plaque present on tooth surfaces
Compliance with appointments and willingness to pay $2625 for a 3-unit implant-supported FDP
Study Intervention
A total of 68 enrolled participants (29 males and 39 females) were randomly assigned to receive either a metal-ceramic or a ceramic-ceramic three-unit FDP (supported by two implants). No participant was selected to receive more than two posterior FDPs. Groups were further subdivided according to design parameters. For participant allocation, a computer-generated random number table was formulated to facilitate assignment to a specific group by the principal investigator. Participants were treated at the University of Florida College of Dentistry (UFCD) between 2008 and 2012. The UFCD Institutional Review Board (IRB) approved the research protocol (No. 95-2007) for treating human subjects. All subjects were required to sign an informed consent form prior to initiating the study, which was obtained by either the principal investigator or the study coordinator. The following baseline information was collected:
General medical history and physical examination data to include gender, smoking status and diabetes status
Maximum bite force (measured by a gnathodynamometer)(Gibbs, et al. 1986)
Participants were evaluated for bone height and width through computerized tomography. Some participants were treated by an oral surgeon if bone augmentation was required prior to placement of implants. Healing time was controlled by the extent of bone augmentation and usually ranged from four to six months. The surgical technique used, bone graft status and sinus lift status were recorded for future evaluation. Implant surgical technique refers to the number of implant surgeries required prior to exposing the implant to the oral environment. If the implant was deemed stable within the bone, a single-stage surgery was performed where the healing abutment was exposed through the gingival mucosal. However if there was a need for simultaneous bone grafting as a result of not having enough buccal or lingual bone thickness, then bone grafting was performed and a two-stage surgery was warranted. Depending on the initial stability of the implant, two-stage surgery was occasionally required to expose the implant to the oral environment. This allows passive healing of the implant prior to exposing the healing abutment to the oral environment. Two-stage surgery was achieved by performing a crestal incision using a 15c (Integra® Miltex®, York, PA) blade. No tissue was removed and if conditions were suitable, a thin band of keratinized mucosa was split in half (buccal and lingual). Sutures were placed if they were needed. Two implants were placed using a surgical guide. Final impressions were made after eight weeks of healing using the open-tray technique. Final impressions were made with vinylpolysiloxane impression material (Aquasil, Dentsply Caulk). Type IV stone (Die-Keen, Whip Mix Corp) and soft tissue material (Gingitech, Ivoclar Vivadent, Schaan, Liechtenstein) were poured in the impressions. Mounted casts were prepared for abutment fabrication, allowing an interocclusal clearance of 2 mm for the FDP material. Custom abutments were prepared to allow for accurate representation of occlusion. For ceramic-ceramic FDPs, core substructures were milled from pre-sintered blanks after they were designed by a CAD system (InEOS, Sirona). For metal-ceramic FDPs, wax patterns for metal substructures were prepared, measured to exact dimensions, invested, burned out, and the metal was cast into the mold cavity. Framework substructures were inserted intra-orally along with the custom abutments to determine the accuracy of mounting.
An occlusal record was confirmed using acrylic resin (Duralay resin, Reliance Dental Mfg. Co., Worth, IL). Corresponding veneer porcelains were applied and fired according to the manufacturers' instructions. Completed FDPs were seated and evaluated prior to cementation. Occlusal adjustments were made with an ultrafine-grit diamond bur (Brassler, USA). Occlusion was adjusted to ensure light contact with a strip of 8-μm-thick shim stock (Shimstock Occlusion Foil, Almore, USA). Adjusted FDPs were polished with abrasive wheels and diamond paste. FDPs were cemented with a resin cement (RelyX Unicem Self-Adhesive Universal Resin Cement, 3M ESPE).
Post-cementation Analysis
Participants were recalled at six months and annually for five years after cementation. Participants were asked to report any unusual occurrence related to their prosthesis as well as their implants. Periapical radiographs were made of both implants and an overall evaluation was made of the prostheses. Implant survival and peri-implant tissue complications such as gingival recession were evaluated during recall exams. Implant survival was judged using the ICOI criteria, which specifies Class 1 as successful and Class IV as failure (Table 1). Peri-implant status and complications were classified as (1) Type 1 – gingival recession with exposure of the abutment margin; (2) Type 2 – gingival recession that includes bone loss and exposure of implant threads; and (3) Type 3 – dehiscence of bone without gingival recession. Adverse conditions around the peri-implant site were referred to a specialist for proper hard- or soft-tissue treatment. Prosthesis fractures were examined clinically and details of the fracture were recorded through impressions of the prostheses (Anusavice, 2012).
Table 1. ICOI Implant Quality Scale.
| Quality Scale | Clinical Condition |
|---|---|
| I. Success |
|
| II. Satisfactory |
|
| III. Compromised |
|
| IV. Failure |
|
Statistical Methods
The R statistical software package (Vienna, Austria; V.2.15.0) was used to conduct all statistical testing. Fisher's exact and Mann-Whitney tests were used to compare patients with any soft-tissue complication to those without on all patient factors (gender, bite force, smoking and diabetes). Generalized estimating equations logistic regression was used to compare implants with complications to those without on all implant factors (location, load time, stages, bone graft and sinus lift). For these analyses, we took complications (yes or no) as the outcome variable, we considered patient a repeated factor to account for the multiple observations on some patients, and we assumed an exchangeable working correlation. The Kaplan-Meier method was used to assess survival of the implants. Because of the small number of events (11 soft-tissue complications), we did not attempt to evaluate the effects of any factors in a multivariable context.
Results
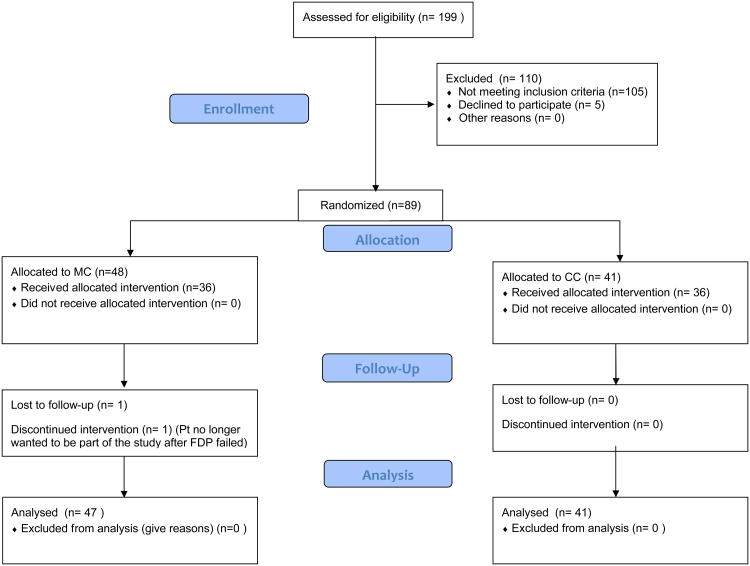
The consort diagram (Appendix, Figure 1) shows the patient population and describes how many subjects were recruited for the study. Of the 89 FDPs with a total of 178 implant bodies, 41 (46.1%) were ceramic-ceramic and 48 (53.9%) were metal-ceramic prostheses. The average follow-up time is 3.2 years with the median follow up time at 3.5 years (25th percentile=2.2 years; 75th percentile=4.0 years). Thirteen (14.6%) of the 89 cemented FDPs exhibited fractures, six (14.6%) of which were in ceramic-ceramic and seven (14.6%) in metal ceramic prostheses. With one patient opting out of the study, a total of 176 implants were recalled and examined in a population of 68 participants with 88 fixed dental prostheses. One patient was lost to implant follow-up after the patient withdrew from the study after fracturing her FDP. All but one of the 176 implants were classified as a Class I type failure according to the ICOI implant survival scale, signifying a 99% survival rate after an average follow up time of 3.2 years. One implant was classified as a Class III type failure with a 10 mm vertical bone loss at the third year recall. Eleven (6.3%) of the implant sites exhibited peri-implant tissue complications with 64% judged as Type I (7/11); 9% as Type 2 (1/11), with an example shown in Fig 2; and 27% as Type 3 (3/11). A life table (Fig 3) shows that the mean survival time for all patients is 250 weeks (95% CI=[243, 258]). Calculation for the mean is restricted to a maximum value of 263 weeks, which is the maximum follow up time among all the implants. So a mean equivalent to 250 indicates that in the first 263 weeks (or five years), the average patient would be free from soft-tissue complications for 250 weeks. Median survival time was not estimable for all survival analyses because 92% of implants were censored or survived to the end.
Figure 1.
CONSORT diagram detailing patient recruitment and retention.
Figure 2.
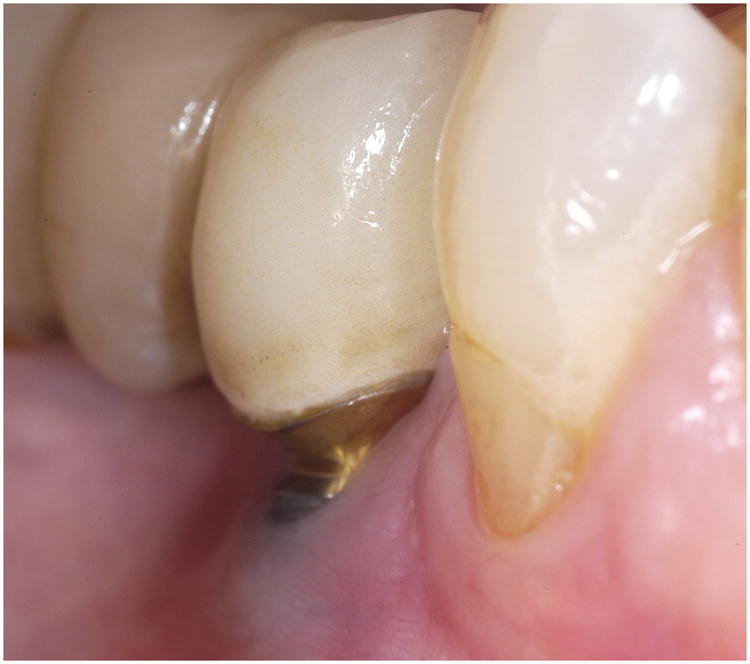
Representative Class 2 peri-implant complications with gingival tissue recession and bone loss exposing implant threads and abutment margins.
Figure 3.
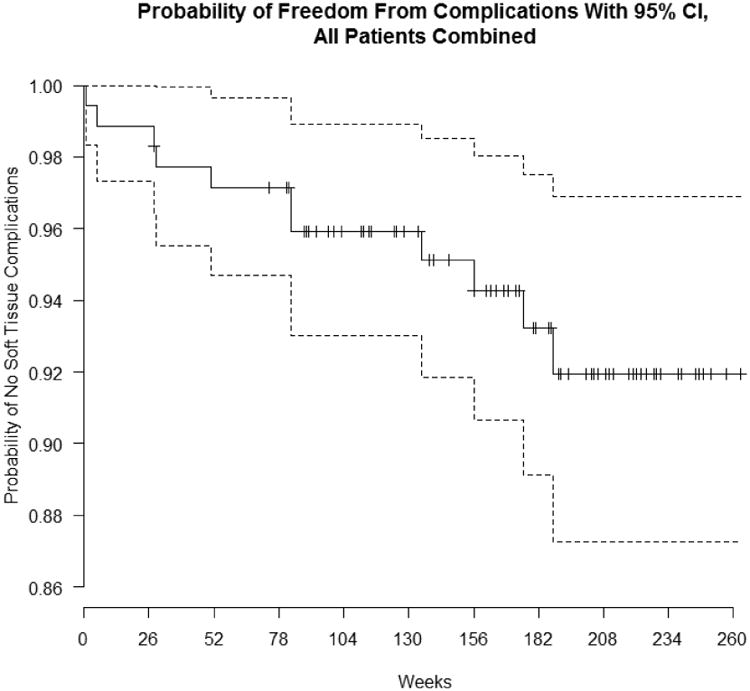
Life table showing the length of time implants are free of soft tissue complications.
No correlation was found between the occurrence of soft-tissue complications and patient characteristics (Table 2). A statistically significant difference was found between the occurrence of peri-implant tissue complications and implant surgical technique (p = 0.005), where two-stage surgeries were associated with more peri-implant tissue complications. A life table (Figure 4) shows the mean survival for implants with one stage surgery is 250 weeks (95% CI=[244, 256]), restricted to 256 weeks. The mean survival for implants with a two-stage surgery is 230 weeks (95% CI=[213, 247]), restricted to 256 weeks. Survival is significantly higher in the one stage surgery group (logrank p=.005). Nine of the eleven implants, which exhibited soft tissue complications, are implants, which had some form of bone grafting or bone augmentation performed prior to implant placement. Bone grafting in this study is a term used to encompass all methods of bone augmentation procedures that were performed to ensure sufficient bone at the site of placement. Bone grafting exhibited a marginal correlation with number of complications in the peri-implant tissue, although this was not statistically significant (p=0.077). No other variables related to the individual implants (site location of implant, implant load time and sinus lift) showed any statistical correlation with number of soft tissue complications (Table 3).
Table 2. Statistical analysis results for patient characteristics and their possible association with the occurrence of peri-implant tissue complications.
| Overall (n=67) | No soft tissue comp (n=56, 83.6%) | Soft tissue comp (n=11, 16.4%) | p-value† | |
|---|---|---|---|---|
| Gender | ||||
| Female | 39 (58.2%) | 30 (76.9%) | 9 (23.1%) | .104* |
| Male | 28 (41.8%) | 26 (92.9%) | 2 (7.1%) | |
| Bite force | ||||
| mean±SD; median [IQR] |
62.1±30.2; 54 [43.5, 75.5] |
64.4±32.3; 54 [45, 80.5] |
53.4±19.5; 52 [39.5, 61.8] |
.614** |
| Bite force by gender mean±SD; median [IQR] | ||||
| Female | 54.4±24.8; 48.5 [32, 72] |
57.4±27.6; 50 [33, 76.5] |
47.3±16.1; 45 [34.5, 55.5] |
.741** |
| Male | 70.3±33.8; 56 [50, 84] |
70.1±35.4; 55 [49, 83] |
71.5±21.9; 71.5 [NA] |
.465 |
| Smoker | ||||
| No | 59 (88.1%) | 49 (83.1%) | 10 (16.9%) | 1* |
| Yes | 8 (11.9%) | 7 (87.5%) | 1 (12.5%) | |
| Diabetes | ||||
| No | 58 (86.6%) | 49 (84.5%) | 9 (15.5%) | 1* |
| Yes | 9 (13.4%) | 7 (77.8%) | 2 (22.2%) | |
IQR: Interquartile range (the 25th and 75th percentiles)
p-values are the results of Fisher's exact test (*) or Mann-Whitney U test (**).
Figure 4. Life table showing probability of soft tissue complications based on either a one stage or two-stage surgery.
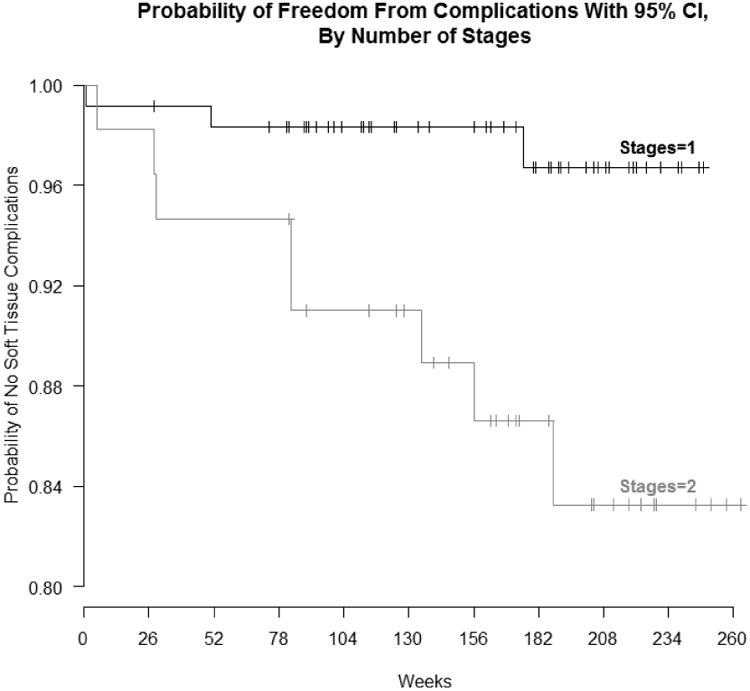
Table 3.
Statistical analysis results for implant characteristics and their association with the occurrence of complications.
| Overall (n=176) | No soft tissue comp (n=165, 93.8%) | Soft tissue comp (n=11, 6.2%) | p-value† | |
|---|---|---|---|---|
| Location (4-way categorization) | ||||
| Lower left | 52 (29.5%) | 49 (94.2%) | 3 (5.8%) | .840 |
| Lower right | 62 (35.2%) | 57 (91.9%) | 5 (8.1%) | |
| Upper left | 30 (17.0%) | 29 (96.7%) | 1 (3.3%) | |
| Upper right | 32 (18.2%) | 30 (93.8%) | 2 (6.2%) | |
| Location (2-way categorization) | ||||
| Lower | 114 (64.8%) | 106 (93.0%) | 8 (7.0%) | .564 |
| Upper | 62 (35.2%) | 59 (95.2%) | 3 (4.8%) | |
| Impant load time (weeks) | ||||
| mean±SD; median [IQR] |
36.5±18.1; 32 [26, 43] |
36.1±16.8; 32 [25, 44] |
43.4±33.2; 34 [27.5, 41.5] |
.129 |
| Stages | ||||
| 1 | 120 (68.2%) | 117 (97.5%) | 3 (2.5%) | .005 |
| 2 | 56 (31.8%) | 48 (85.7%) | 8 (14.3%) | |
| Bone graft | ||||
| No | 80 (45.5%) | 78 (97.5%) | 2 (2.5%) | .077 |
| Yes | 96 (54.5%) | 87 (90.6%) | 9 (9.4%) | |
| Sinus lift | ||||
| No | 150 (85.2%) | 141 (94.0%) | 9 (6.0%) | .751 |
| Yes | 26 (14.8%) | 24 (92.3%) | 2 (7.7%) | |
IQR: Interquartile range (the 25th and 75th percentiles)
p-values are the results of generalized estimating equations logistic regression to account for multiple observations on some patients.
Discussion
Many factors have been linked to increased implant success. Proper treatment planning involves promoting healthy tissue, proper surgical techniques, allowing an undisturbed healing phase, and fabricating a prosthetic design that helps maintain the integrity of the surrounding area (Buser, et al. 1990). The length of dental implants appears to have no effect on bone loss, but implants with greater diameters may contribute to better preservation of the crestal bone (Cecchinato et al., 2004). The presence of natural teeth adjacent to implants has also been shown to help maintain the proximal bone crest level and soft-tissue health (Chang and Wennstrom, 2010). The anatomical form of the interproximal papilla improves the esthetics of dental implants so the periodontium is better sustained when placed next to teeth (Lops et al., 2012). Location of implant placement in the maxilla or mandible has a direct link to success, and it is generally believed that thick gingiva can diminish the chances of recession, bone and papilla loss, and other defects (Lee et al., 2011). A thicker gingival biotype seems to provide an enhanced environment for implant placement since these tissues are less friable and more vascularized (Lee et al., 2011). This is important because, without an underlying periodontal ligament, implant sites do not have an additional blood supply for healing and maintenance. Generally a narrow keratinized mucosa occurs with recession and inflammation, but only weak correlations with peri-implant health have been reported.(Chappuis et al., 2013; Lin et al., 2013) Several studies also show that adequate oral hygiene can negate the impacts of keratinized mucosa.(Lin, et al. 2013)
We found no evidence that patient characteristics such as gender or medical status are associated with the occurrence of peri-implant complications. However, our findings suggest a statistically significant association between surgical technique and the occurrence of peri-implant complications. The additional second-stage surgery that was required to expose the implant bodies proved to be significant in contributing to peri-implant complications. A possible explanation for this could be the need to advance the flap to achieve primary closure accompanied by a possible loss of keratinized mucosa. This supports other findings, which recommend that implants be left undisturbed during healing (Balshi et al.; 2005, Morton et al.; 2004, Wang and Boyapati, 2006). We also found a marginal association (0.05 ≤ p ≤ 0.1) between bone graft surgeries and peri-implant complications. Since our effective sample size is relatively small (11 complications were observed), we consider this result as marginal association evidence of a likely effect of bone graft surgeries in the population.
Implant complications occur for multiple reasons. Peri-implantitis is defined by the ICOI as bone loss surrounding the implant, bacterial infections around the implant, stress, an inflammatory process, and increased probing depths (Misch et al., 2008). Quantification of these parameters is not universally accepted, thus exposing the need for additional research. Studies show that bone loss at implant sites tends to be greater in females than in males (Chappuis et al., 2013). Other findings also indicate that hard and soft tissues experience the greatest change in the first post-surgical year (Chang and Wennstrom, 2010). Definitive changes after this period represent a post-surgery complication (Albrektsson et al., 1986). In addition, greater changes in peri-implant tissue occur in mandibular implants compared with maxillary implants (Oates et al., 2002).
Complications and potential failures can be corrected and avoided. The presence of keratinized mucosa is strongly correlated with plaque control, and increased plaque level can lead to complications (Chappuis et al., 2013). If proper compliance with hygiene and plaque control is achieved, sufficient keratinized mucosa may not be an important factor for selection (Wennstrom and Derks, 2012). When biological complications are recognized early and treated with intervention and antibiotic therapy to prevent hard- or soft-tissue loss, the complications can be eliminated and health can be restored (Chappuis et al., 2013). Soft-tissue complications can also be corrected with soft tissue grafts. These may not be successful at the time of implant placement but are effective in correcting esthetic and biological complications after the implant healing phase (Wennstrom and Derks, 2012).
Limitations
The relatively small number of complications observed in this study prevented us from exploring the effects of patient and implant factors in a multivariable context. Hence, confounding effects and resulting biases may be present. In addition, while our data provide evidence of a lack of association between many factors and complications, our sample size was too small to test conclusively whether associations truly do not exist.
Conclusion
The results of this study indicate that the Dentsply Osseospeed implant system provides successful integration for posterior sites at three years. This research also suggests that implants which need additional surgery may contribute to subsequent peri-implant complications. Proper treatment planning is crucial to ensure the clinical success of implants and their restorations and to minimize complications. Additional surgery should be performed with caution, soft tissue evaluation should be completed, and the potential soft-tissue effects on peri-implant health must be considered.
Clinical Significance.
The clinical implication of this research study is that secondary surgery should be considered with caution during implant placement and it should be performed only when other options have been exhausted, as it has been shown to have a direct adverse effect on the long-term peri-implant tissue health.
Acknowledgments
This study was supported by NIH-NIDCR grants K23DE18414 and DE06672, Ivoclar Vivadent, DENTSPLY Implants, and the UF Office of Research. We also acknowledge the surgical expertise of Drs. Emma Lewis and Luiz Gonzaga, the laboratory expertise of Robert Lee and Barry Nicholas, and the clinical assistance of Kelly Raulerson and Renita Jenkins. This trial can be accessed in clinicaltrials.gov, K23 D2007-46.
Footnotes
There are no conflicts of interest associated with this study.
References
- Albrektsson T, Zarb G, Worthington P, Eriksson AR. The long-term efficacy of currently used dental implants: A review and proposed criteria of success. The International journal of oral & maxillofacial implants. 1986;1:11–25. [PubMed] [Google Scholar]
- Anusavice KJ. Standardizing failure, success, and survival decisions in clinical studies of ceramic and metal-ceramic fixed dental prostheses. Dental Materials. 2012;28:102–111. doi: 10.1016/j.dental.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balshi SF, Allen FD, Wolfinger GJ, Balshi TJ. A resonance frequency analysis assessment of maxillary and mandibular immediately loaded implants. The International journal of oral & maxillofacial implants. 2005;20:584–594. [PubMed] [Google Scholar]
- Bragger U, Burgin WB, Hammerle CH, Lang NP. Associations between clinical parameters assessed around implants and teeth. Clinical Oral Implants Research. 1997;8:412–421. doi: 10.1034/j.1600-0501.1997.080508.x. [DOI] [PubMed] [Google Scholar]
- Buser D, Weber HP, Lang NP. Tissue integration of non-submerged implants. 1-year results of a prospective study with 100 iti hollow-cylinder and hollow-screw implants. Clinical Oral Implants Research. 1990;1:33–40. doi: 10.1034/j.1600-0501.1990.010105.x. [DOI] [PubMed] [Google Scholar]
- Cecchinato D, Olsson C, Lindhe J. Submerged or non-submerged healing of endosseous implants to be used in the rehabilitation of partially dentate patients. Journal of Clinical Periodontology. 2004;31:299–308. doi: 10.1111/j.1600-051X.2004.00527.x. [DOI] [PubMed] [Google Scholar]
- Chang M, Wennstrom JL. Peri-implant soft tissue and bone crest alterations at fixed dental prostheses: A 3-year prospective study. Clinical Oral Implants Research. 2010;21:527–534. doi: 10.1111/j.1600-0501.2009.01874.x. [DOI] [PubMed] [Google Scholar]
- Chappuis V, Buser R, Bragger U, Bornstein MM, Salvi GE, Buser D. Long-term outcomes of dental implants with a titanium plasma-sprayed surface: A 20-year prospective case series study in partially edentulous patients. Clin Implant Dent Relat Res. 2013;15:780–790. doi: 10.1111/cid.12056. [DOI] [PubMed] [Google Scholar]
- Gibbs CH, Mahan PE, Mauderli A, Lundeen HC, Walsh EK. Limits of human bite strength. J Prosthet Dent. 1986;56:226–229. doi: 10.1016/0022-3913(86)90480-4. [DOI] [PubMed] [Google Scholar]
- Kim BS, Kim YK, Yun PY, Yi YJ, Lee HJ, Kim SG, Son JS. Evaluation of peri-implant tissue response according to the presence of keratinized mucosa. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontics. 2009;107:e24–28. doi: 10.1016/j.tripleo.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Lee A, Fu JH, Wang HL. Soft tissue biotype affects implant success. Implant Dentistry. 2011;20:e38–47. doi: 10.1097/ID.0b013e3182181d3d. [DOI] [PubMed] [Google Scholar]
- Lin GH, Chan HL, Wang HL. The significance of keratinized mucosa on implant health: A systematic review. Journal of Periodontology. 2013;84:1755–1767. doi: 10.1902/jop.2013.120688. [DOI] [PubMed] [Google Scholar]
- Lops D, Romeo E, Chiapasco M, Procopio RM, Oteri G. Behaviour of soft tissues healing around single bone-level-implants placed immediately after tooth extraction a 1 year prospective cohort study. Clinical Oral Implants Research. 2012 doi: 10.1111/j.1600-0501.2012.02531.x. [DOI] [PubMed] [Google Scholar]
- Misch CE, Perel ML, Wang HL, Sammartino G, Galindo-Moreno P, Trisi P, Steigmann M, Rebaudi A, Palti A, Pikos MA, Schwartz-Arad D, Choukroun J, Gutierrez-Perez JL, Marenzi G, Valavanis DK. Implant success, survival, and failure: The international congress of oral implantologists (icoi) pisa consensus conference. Implant Dentistry. 2008;17:5–15. doi: 10.1097/ID.0b013e3181676059. [DOI] [PubMed] [Google Scholar]
- Morton D, Jaffin R, Weber HP. Immediate restoration and loading of dental implants: Clinical considerations and protocols. The International journal of oral & maxillofacial implants. 2004;19 Suppl:103–108. [PubMed] [Google Scholar]
- Oates TW, West J, Jones J, Kaiser D, Cochran DL. Long-term changes in soft tissue height on the facial surface of dental implants. Implant Dentistry. 2002;11:272–279. doi: 10.1097/00008505-200207000-00013. [DOI] [PubMed] [Google Scholar]
- Wang HL, Boyapati L. “Pass” principles for predictable bone regeneration. Implant Dentistry. 2006;15:8–17. doi: 10.1097/01.id.0000204762.39826.0f. [DOI] [PubMed] [Google Scholar]
- Weber HP, Cochran DL. The soft tissue response to osseointegrated dental implants. The Journal of prosthetic dentistry. 1998;79:79–89. doi: 10.1016/s0022-3913(98)70198-2. [DOI] [PubMed] [Google Scholar]
- Wennstrom JL, Derks J. Is there a need for keratinized mucosa around implants to maintain health and tissue stability? Clinical Oral Implants Research. 2012;23 Suppl 6:136–146. doi: 10.1111/j.1600-0501.2012.02540.x. [DOI] [PubMed] [Google Scholar]