Abstract
An inducible program of inflammatory gene expression is central to anti-microbial defenses. Signal-dependent activation of transcription factors, transcriptional co-regulators and chromatin modifying factors collaborate to control this response. Here we identify a long noncoding RNA that acts as a key regulator of this inflammatory response. Germline-encoded receptors such as the Toll-like receptors induce the expression of numerous lncRNAs. One of these, lincRNA-Cox2 mediates both the activation and repression of distinct classes of immune genes. Transcriptional repression of target genes is dependent on interactions of lincRNA-Cox2 with heterogeneous nuclear ribonucleoprotein A/B and A2/B1. Collectively, these studies unveil a central role of lincRNA-Cox2 as a broad acting regulatory component of the circuit that controls the inflammatory response.
The innate immune system coordinates host defenses through germline-encoded receptors, which recognize microbial products and activate signaling pathways that induce transcription of inflammatory genes (1). These surveillance receptors (e.g. Toll-like receptors) and their downstream pathways activate transcription factors (e.g. NFκB and Irf3) that act in concert with transcriptional co-regulators and chromatin modifying factors to regulate transcription (2–4). This complex transcriptional response leads to expression of hundreds of proteins involved in antimicrobial defense, cell migration, tissue repair, adaptive immunity and resolution of inflammation (2, 5). Recent studies have identified thousands of long non-coding RNAs (lncRNAs) in mammalian genomes (6–11). lncRNAs have emerged as major regulators of gene expression and are involved in various biological processes that include genomic imprinting, embryonic development, cell differentiation, tumor metastasis and regulation of the cell cycle [reviewed in (7)]. lncRNAs are differentially regulated in virus-infected cells (12) and in dendritic cells following lipopolysaccharide (LPS) stimulation (6). Very recently, a lncRNA called NeST, (nettoie Salmonella pas Theiler’s [cleanup Salmonella not Theiler’s]) was shown to control susceptibility to Theiler’s virus and Salmonella infection in mice through epigenetic regulation of the interferon-γ locus (13, 14). Although lncRNAs can be induced in innate immune cells (6, 12), it is not yet known whether lncRNAs act as regulators of gene expression in the innate immune response.
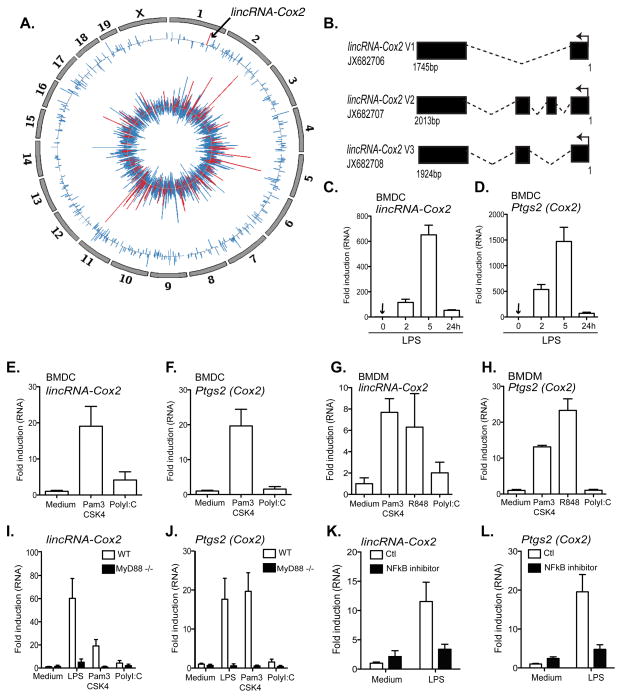
To identify lncRNAs that are transcribed during the innate immune response, we conducted whole-transcriptome analysis (RNA-seq) (8) of macrophages stimulated with the synthetic bacterial lipopeptide Pam3CSK4, a TLR2 ligand (Fig. 1A). The TLR2 ligand induced the transcription of numerous protein-coding genes involved in the immune response (Fig. 1A inner track) as well as 62 lncRNAs (Table S1, Fig. 1A outer track). Consistent with previous studies, overall changes in lncRNA expression were less than that observed for protein coding genes. The most significantly induced lncRNAs tended to occur in chromosomal regions where there was also increased expression of immune genes, suggesting that these genes are co-regulated. lincRNA-Cox2 was amongst the most highly induced lncRNAs and is proximal to the prostaglandin-endoperoxide synthase 2 (Ptgs2/Cox2) gene (Fig. 1A). In addition to lincRNA-Cox2, two additional lncRNAs, lncRNA-Ehd1 and lncRNA-Lyn were also induced following TLR2 and TLR4 stimulation (Fig. S1A–B).
Figure 1. lincRNA-Cox2 expression is induced by TLR ligands in a MyD88 and NFκB dependent manner.
A, The Circos plot shows genome-wide differential expression (RNA-seq) between untreated bone marrow derived macrophages (BMDM) and BMDMs stimulated with Pam3CSK4 (TLR1/2) (5 h). The inner track shows log2 fold-change values for protein coding genes that are classified into immune genes (red, see methods) and other genes (blue). The outer track shows log2 fold-change value for all lncRNAs. lincRNA-Cox2 is highlighted in red on Chromosome 1 (arrow). B, lincRNA-Cox2 encodes three splice variants. C–H, qRT-PCR was performed on bone marrow derived dendritic cells (BMDC) (C–F) or bone marrow derived macrophages (BMDM). Elevated levels of lincRNA-Cox2 and Ptgs2 were observed following LPS (TLR4) (C–D), Pam3CSK4 (TLR2) (E–H), R848 (TLR7/8) (G–H) but not with Poly I:C (TLR3) (E–H) stimulation. I–J. Induction of lincRNA-Cox2 and Ptgs2 were found to be dependent on MyD88 following qRT-PCR on BMDMs obtained from wild type (WT) or MyD88 KO mice. K–L, BMDMs treated for 30min with an NFκB inhibitor (1 μg/ml), followed by stimulation with LPS (100 ng/ml) resulted in reduced expression levels of lincRNA-Cox2 (K) and Ptgs2 (L) as examined by qRT-PCR. Data represents mean ±SD from three independent experiments.
A previous study demonstrated that lincRNA-Cox2 was induced in dendritic cells following stimulation with lipopolysaccharide (LPS) (6). However, it is not known whether lincRNA-Cox2 regulates the inflammatory response that is associated with TLR signaling. Using PCR amplification, we identified three splice variants of lincRNA-Cox2 (Fig. 1B, accession numbers JX682706, JX682707, JX682708). Variant 1 was the most abundant transcript and contains exons 1 and 4, which are common to all splice variants. Consequently, we designed primers for quantitative PCR (qPCR) and shRNA that targeted these regions. Using qPCR, we confirmed that LPS induced similar temporal patterns of expression of both lincRNA-Cox2 and its neighboring Ptgs2 (Cox2) gene in bone marrow-derived dendritic cells (BMDCs, Fig. 1C–D). Unlike Pam3CSK4 (TLR2), Poly I:C, a synthetic double stranded RNA (TLR3) did not induce lincRNA-Cox2 or Ptgs2 (Cox2) significantly in BMDCs (Fig. 1E–F). LPS, Pam3CSK4 and R848 (which activates TLR7/8) also induced the expression of lincRNA-Cox2 and Ptgs2 (Cox2) in macrophages (Fig. 1G–H). Both Listeria-infected macrophages (in vitro) and isolated splenocytes from Listeria-infected mice (24 hr post infection) had elevated levels of lincRNA-Cox2 (Fig. S1C–D). Induction of lincRNA-Cox2 and its neighboring gene Ptgs2 (Cox2) was dependent on the TLR signaling adaptor protein MyD88 (Fig. I–J) and on activation of the transcription factor NFκB (Fig. 1K–L).
We next examined the protein-coding capacity of lincRNA-Cox2 by assessing its association with polysomes within cells. Macrophage cells were treated with cycloheximide to trap ribosomes on RNA molecules and either left untreated or pretreated with EDTA (which disrupts all RNA-protein interactions) or with harringtonine (which specifically disrupts translation). Cell lysates were then fractionated by sucrose density gradients and ultracentrifugation and RNA analysed in all fractions by qPCR (15–17). Using this approach we compared Gapdh, a well-translated mRNA with lincRNA-Cox2 and another lincRNA, lncRNA-Eps, that has previously been shown to be non-coding (18). As expected Gapdh RNA sedimented with a high velocity through the sucrose gradient since it was associated with ribosomes. In contrast, lincRNA-Cox2 and lincRNA-Eps both remained in lighter fractions (Fig. S2). Treatment with EDTA or specific disruption of translation by harringtonine resulted in a shift of Gapdh from the higher velocity to the lower velocity fractions indicating that this mRNA was actively translated. In contrast, the profile of lincRNA-Cox2 or lincRNA-Eps were largely unaffected by harringtonine treatment.
We also examined lincRNA-Cox2 for the presence of potential open reading frames (ORFs). Most of the ORFs identified were found to have poor Kozak strength suggesting that it is unlikely that this RNA sequence is translated, (Fig. S3A–B). Collectively, these studies indicate that lincRNA-Cox2 is unlikely to encode a protein product.
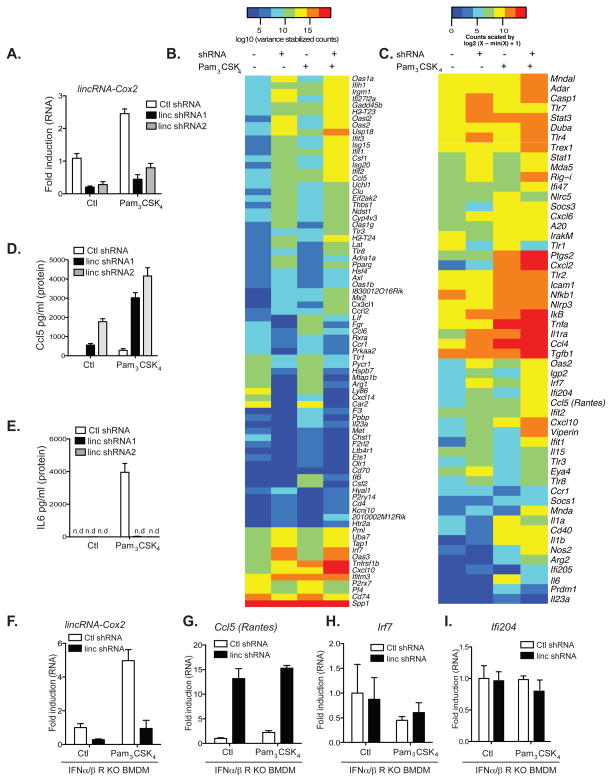
To define the functional role of lincRNA-Cox2 in inflammatory gene expression, we generated macrophage cell lines in which lincRNA-Cox2 expression was silenced by shRNA (Fig. 2A). Silencing of lincRNA-Cox2 did not alter expression of its neighboring gene Ptgs2 (Cox2) (Fig. S4). To identify potential targets of lincRNA-Cox2, we conducted RNA-seq in both control and lincRNA-Cox2 silenced cells before and after stimulation with Pam3CSK4. Silencing of lincRNA-Cox2 increased the expression of 787 genes by 3-fold or greater in unstimulated cells (Table S2). A GO enrichment analysis indicated that genes related to the immune response were significantly overrepresented in these up-regulated genes (Table S3, Fig. 2B). A number of chemokines (Ccl5, Cx3cl1), chemokine receptors (Ccrl) and interferon-stimulated genes (ISGs) (Irf7, Oas1a, Oas1l, Oas2, Ifi204 and Isg15) were up-regulated when lincRNA-Cox2 was silenced in unstimulated cells (Fig. 2B). In the same cells stimulated with Pam3CSK4, silencing of lincRNA-Cox2 resulted in attenuated expression of 713 genes by 3-fold or greater (Table S4). Examples of these genes include Tlr1, Il6 and Il23a.
Figure 2. lincRNA-Cox2 is a major regulator of immune genes.
A, qRT-PCR was carried out on BMDMs stably expressing lentiviral shRNA specific to lincRNA-Cox2 (shRNA) or a control shRNA. Expression of lincRNA-Cox2 was measured. B, RNA-seq was performed on lincRNA-Cox2 knockdown or control (ctl shRNA) BMDMs that were either stimulated with Pam3CSK4 or unstimulated. The heat map shows mRNA levels for genes annotated in GO as immune genes. These genes are among the top 50 up-regulated immune genes in unstimulated cells when lincRNA-Cox2 was silenced or the top 50 down-regulated immune genes in stimulated cells when lincRNA-Cox2 was silenced. The 100 genes were ranked by absolute log2 fold-change values and the top 80 differentially expressed genes displayed. C, Heatmap representation of differentially regulated genes from a custom designed gene codeset performed on RNA extracted from Ctl or lincRNA-Cox2 knockdown cells stimulated with Pam3CSK4 for 5 h. D–E, Cells were stimulated with Pam3CSK4 for 24 h, increased Ccl5 (Rantes) (D) and reduced IL6 (E) cytokine levels were identified in lincRNA-Cox2 knockdown cells by elisa (n.d means not detected). F–I, lincRNA-Cox2 was silenced in interferon α/β receptor (IFN α/β R) KO cells. Expression levels of lincRNA-Cox2 (F), Ccl5 (Rantes) (G), Irf7 (H) and Ifi204 (I) were measured using qRT-PCR to define direct targets of lincRNA-Cox2.
We confirmed these findings using a non-enzymatic RNA profiling technology that employs bar-coded fluorescent probes to simultaneously analyze mRNA expression levels of differentially regulated genes (nCounter, Nanostring). In unstimulated cells, silencing of lincRNA-Cox2 led to a marked increase in expression of Irf7, Ccl5 (Rantes), and ISGs (Ifi204, Ifi205 and Viperin) relative to control cells (Fig. 2C, columns 1–2), while the Pam3CSK4-induced expression of Tlr1 and Il6 was attenuated (Fig. 2C, columns 3–4). We confirmed these findings in three independent shRNA lines by measuring protein levels for Ccl5 (Rantes) and IL6 (Fig. 2D and E, Fig. S5). In contrast to these genes, the TLR2 dependent induction of IL1β was unaffected in cells lacking lincRNA-Cox2 (Fig. S6). We also observed reduced IL6 production in macrophages stimulated with Pam2CSK4 and R848, ligands of TLR2/6 and TLR7, respectively (Fig. S7). We next silenced lincRNA-Cox2 in macrophages lacking the type I interferon α/β receptor (IFN α/βR KO) in order to distinguish between direct and indirect (IFN-dependent) targets of lincRNA-Cox2 (Fig. 2F–I, Fig. S8). Non-enzymatic RNA profiling of gene expression in these cells indicated that while Ccl5 was regulated by lincRNA-Cox2 in a manner that was independent of type IFN signaling, regulation of Irf7 and Ifi204 was secondary to type I IFN signaling. Despite the elevated expression of IFN pathway components, silencing of lincRNA-Cox2 did not render these cells permissive to TLR2 induced TBK1 activation, a measure of the Irf3 signaling pathway (Fig. S9). Finally, since shRNA silencing of lincRNA-Cox2 led to decreased expression of Tlr1, it was important to eliminate the possibility that we inadvertently impaired Pam3CSK4 signaling via the TLR1/TLR2 heterodimer. To test this directly, we restored expression of mTLR1 in lincRNA-Cox2 silenced cells and confirmed that the differential regulation of Ccl5 and IL6 expression was not due to impaired expression of TLR1. Restoration of TLR1 did not alter the effects of lincRNA-Cox2 silencing on Ccl5 or IL6 protein levels (Fig. S10). Taken together, these data indicate that lincRNA-Cox2 regulates distinct classes of immune genes both basally and following TLR-stimulation.
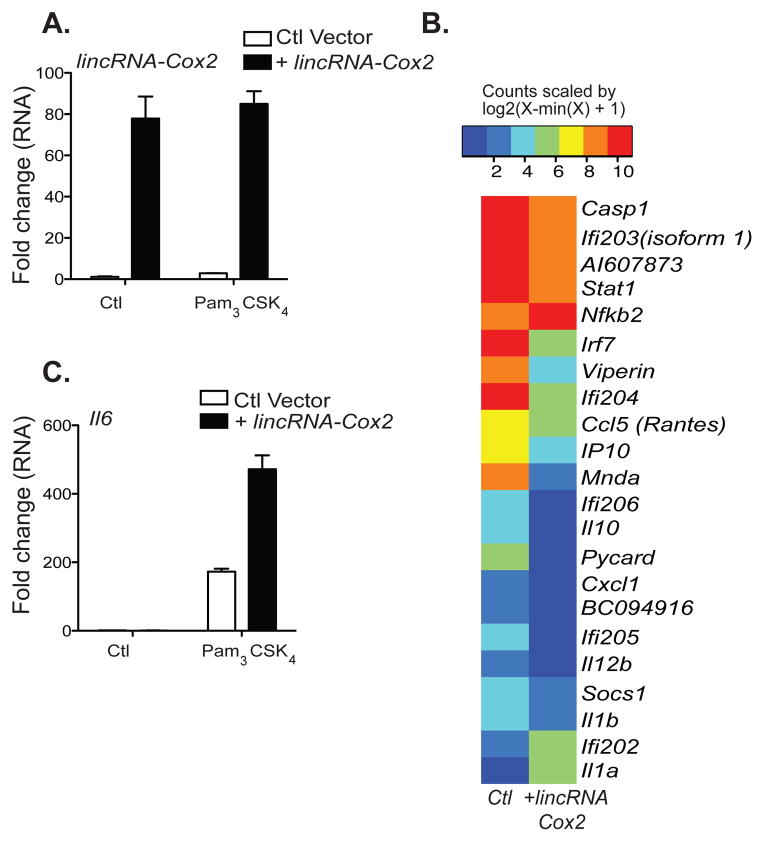
We next conducted ‘gain-of-function’ studies by generating macrophages that ectopically expressed lincRNA-Cox2 (Fig. 3A). As expected, over-expression of lincRNA-Cox2 mirrored the effects of silencing the lncRNA. Macrophages that ectopically expressed lincRNA-Cox2 had decreased levels of Ccl5, Irf5, Irf7, Stat1, Viperin, and three members of the PYHIN protein family (Ifi202, Ifi204, Mnda) (Fig. 3B). Il6 was not detected when lincRNA-Cox2 was over-expressed in unstimulated cells. However, Il6 levels were significantly enhanced when lincRNA-Cox2 was over-expressed in Pam3CSK4 stimulated cells (Fig. 3C). These results further demonstrate that lincRNA-Cox2 represses Ccl5, while simultaneously enhancing the expression of TLR-induced Il6.
Figure 3. Differential gene expression following over expression of lincRNA-Cox2.
A, BMDM stably over-expressing lincRNA-Cox2 or a Ctl vector were generated. qRT-PCR was carried out and over-expression of lincRNA-Cox2 was confirmed. B, Heatmap representation of differentially regulated genes from a custom designed gene codeset performed on RNA extracted from Ctl or lincRNA-Cox2 over expressing BMDMs. C, qRT-PCR was carried out on Ctl or lincRNA-Cox2 over expressing cells stimulated with Pam3CSK4 for 5 h, increased Il6 expression levels were identified using qRT-PCR. Data represents mean ±SD from three independent experiments.
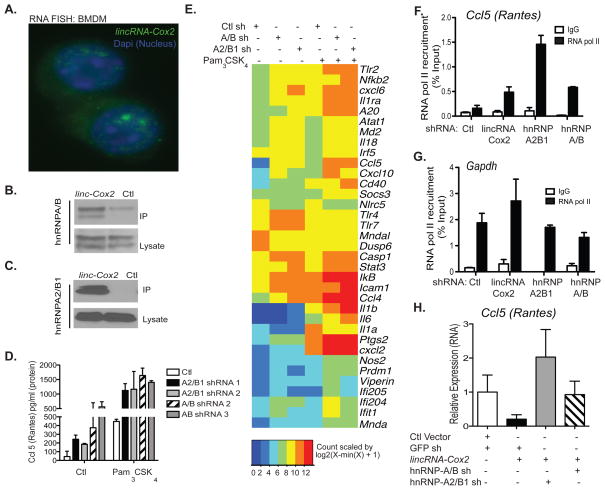
lncRNAs can be found in the nucleus, cytoplasm or in both compartments (19–21). We therefore examined the localization of lincRNA-Cox2 in macrophages using RNA fluorescence in situ hybridization (FISH), a methodology that allows simultaneous detection and localization of RNA transcripts in relation to cellular substructures. Significant amounts of lincRNA-Cox2 were clearly visible in both the nuclear and the cytosolic compartments of macrophages (Fig. 4A, Fig. S11). Analysis of RNA-seq data for different subcellular fractions of macrophages (22), confirmed that lincRNA-Cox2 was present in both the cytosol and nucleus (Fig. S12). Importantly RNA-FISH analysis demonstrated efficient targeting of lincRNA-Cox2 in the nuclear fractions of our shRNA silenced cells (Fig. S11C–F).
Figure 4. lincRNA-Cox2 is localized to both the cytosolic and nuclear compartments, interacts with hnRNP-A/B and A2/B1 to regulate immune genes.
A, BMDMs were labeled with a lincRNA-Cox2 probe using RNA FISH, and counterstained with DAPI (DNA). B–C, Biotinylated lincRNA-Cox2 or antisense RNA was incubated with nuclear extracts and interaction with endogenous hnRNP-A/B (B) or hnRNP-A2/B1 (C) assessed following IP/western (top panels). Expression levels of hnRNP- A/B (B) or hnRNP-A2/B1 (C) (lower panels) in input lysates were also examined. D, Cell lines with shRNA targeting hnRNP-A/B or hnRNP-A2/B1 were stimulated with Pam3CSK4, elevated Ccl5 (Rantes) production was identified in these cells by elisa. E, Heatmap representation of differentially regulated genes of Ctl, hnRNP-A/B or hnRNP-A2/B1 expressing BMDMs stimulated with Pam3CSK4 (100nM) for 5 h. F–G, Silencing of lincRNA-Cox2, hnRNP-A/B or hnRNP-A2/B1 promotes recruitment of RNA Pol II to the Ccl5 promoter as determined by CHIP analysis. H, hnRNP-A/B or hnRNP-A2/B1 was knocked down using lentiviral shRNA in lincRNA-Cox2 over-expressing BMDMs, Ccl5 expression levels were measured using qRT-PCR.
Many lncRNAs regulate transcription through their interactions with chromatin-modifying complexes or with heterogeneous nuclear ribonucleoproteins (hnRNP) (7, 23–25). To identify binding partners for lincRNA-Cox2, we incubated in vitro-transcribed biotinylated lincRNA-Cox2 as well as an antisense lincRNA-Cox2 control RNA with nuclear or cytosolic extracts and subjected RNA binding proteins to Mass Spectrometry for identification. hnRNP-A/B and hnRNP-A2/B1 were identified as specific binding partners for lincRNA-Cox2 in both the cytosolic and nuclear fractions (data not shown). The ability of hnRNP-A/B and hnRNP-A2/B1 to bind lincRNA-Cox2 was confirmed by western blot analysis (Fig. 4B and C). hnRNPs are involved in several RNA-related biological processes such as transcription, pre-mRNA processing, mature mRNA transport to the cytoplasm, and translation (26, 27). In addition, hnRNPs are known to form complexes with lncRNAs and are emerging as important mediators of lncRNA-induced transcriptional repression (24, 25). hnRNP-A/B has been linked to transcriptional repression of some genes (e.g. OPN and Sm-a-A) (28, 29) and hnRNPA2/B1 associates with hnRNP-A/B (30). Therefore, we hypothesized that lincRNA-Cox2 regulates transcription of immune genes by forming complexes with these hnRNP proteins. To test this hypothesis, we generated macrophages where expression of hnRNP-A/B and hnRNP-A2/B1 were silenced by shRNA (Fig. S13A–B). Knockdown of hnRNP-A/B and hnRNP-A2/B1 did not modulate the levels of lincRNA-Cox2 expression (Fig. S14), indicating that these hnRNPs did not regulate lincRNA-Cox2 levels. Similar to our lincRNA-Cox2 silencing studies (Fig. 2D), we detected a significant enhancement of Ccl5 protein in TLR2-stimulated cells and unstimulated cells that lacked hnRNP-A/B and hnRNP-A2/B1 (Fig. 4D). Multiplex RNA analysis also revealed elevated levels of Ccl5, Stat1, Tlr7, Icam1 and IκB RNA in hnRNP-silenced macrophages (Fig. 4E). There was considerable overlap between genes that were regulated by lincRNA-Cox2 and these two hnRNP proteins (Fig. S15, Table. S5). We also examined RNA polymerase II recruitment to the promoter of the Ccl5 and Irf7 promoters using RNA polymerase II Chromatin immunoprecipitation (ChIP). As expected, recruitment of RNA Pol II to the promoters of Ccl5 and Irf7 was increased when lincRNA-Cox2 or the hnRNPs were silenced (Fig. 4F-G, Fig. S16), indicating that lincRNA-Cox2, hnRNPA2/B1 and A/B negatively regulate transcription of these genes. In contrast to these genes, RNA Pol II displayed decreased recruitment to the promoter of Il6 only when lincRNA-Cox2 was knocked down (Fig. S16), an effect that was consistent with the hnRNPs having no effect on Il6 mRNA levels (Fig. 4E). Silencing of hnRNP-A2/B1 or hnRNP-A/B in cells that over-expressed lincRNA-Cox2 reversed the inhibitory effect of lincRNA-Cox2 on Ccl5 expression (Fig. 4H, Fig. S17). Taken together, these experiments confirm that hnRNP-A/B and A2/B1 form a complex with lincRNA-Cox2 in order to repress the transcription of immune genes.
In summary, these studies identify lincRNA-Cox2 as a critical component of the inflammatory response. The impact of this lncRNA on the TLR response was both dramatic and broad-acting with unprecedented effects not previously seen with other regulatory RNAs (e.g. microRNAs). The identification of hnRNP-A/B and A2/B1 as mediators of lincRNA-Cox2’s transcriptional repressive functions underscore the importance of hnRNPs in lncRNA function. Recently, lincRNA-p21, identified as a repressor in the p53-dependent transcriptional response, was shown to associate with hnRNP-K which facilitates the proper genomic localization of hnRNP-K at repressed genes to modulate p53 dependent apoptosis (23–25).
Innate immune responses have the capacity to both combat infectious microbes and drive pathological inflammation, which contributes to diseases such as atherosclerosis, autoimmunity and cancer. A multitude of regulatory checkpoints control TLR signaling and inflammatory responses. We propose a model whereby TLR signaling induces lncRNAs, such as lincRNA-Cox2, that serve as repressors and activators of genes through their physical interactions with various regulatory complexes. As such, lncRNAs represent a novel component of the innate immune response that can restrain and promote aspects of inflammatory signaling. Further characterization of these regulatory networks is likely to reveal novel drug targets and opportunities for therapeutic intervention in infectious and inflammatory diseases.
Supplementary Material
Table S1. lncRNAs induced following TLR2 stimulation
Table S2. Upregulated genes following TLR2 stimulation of lincRNA-Cox2 knockdown cells
Table S3. GO enrichment analysis of the top 800 upregulated genes
Table S4. Downregulated genes following TLR2 stimulation of lincRNA-Cox2 knockdown cells
Table S5. Common genes affected by lincRNA-Cox2, hnRNP-A/B or hnRNPA2/B1 knockdown in macrophages
Acknowledgments
This work is supported by a HRB/Marie Curie Fellowship (MCPD/2010/04 to S.C) and by NIH grants (AI067497 to K.A.F). The authors would like to thank Adam Wespiser for generating heatmaps, Nirmal Singh for his helpful advise on carrying out RNA-seq and Lynda Stuart, Neal Silverman, Douglas Golenbock and Egil Lien for critical reading of this manuscript.
Footnotes
Materials and Methods, Figs S1–S15, Tables S1–5.
Author contributions
S.C, D.R.C and K.A.F designed the research, S.C, M.A, P.G, E.R, L.H, M.B, B.M, M.H.B, J.L, M.M conducted experiments, analyzed data. D.A and D.R.C collected and processed all RNA-seq data. S.C, D.R.C and K.A.F wrote the manuscript. D.R.C, L.A.J.O’N and K.A.F supervised the project.
References
- 1.Kawai T, Akira S. Toll-like receptor and RIG-I-like receptor signaling. Ann N Y Acad Sci. 2008 Nov;1143:1. doi: 10.1196/annals.1443.020. [DOI] [PubMed] [Google Scholar]
- 2.Medzhitov R, Horng T. Transcriptional control of the inflammatory response. Nature reviews Immunology. 2009 Oct;9:692. doi: 10.1038/nri2634. [DOI] [PubMed] [Google Scholar]
- 3.Hargreaves DC, Horng T, Medzhitov R. Control of inducible gene expression by signal-dependent transcriptional elongation. Cell. 2009 Jul 10;138:129. doi: 10.1016/j.cell.2009.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foster SL, Medzhitov R. Gene-specific control of the TLR-induced inflammatory response. Clinical immunology. 2009 Jan;130:7. doi: 10.1016/j.clim.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murray PJ, Smale ST. Restraint of inflammatory signaling by interdependent strata of negative regulatory pathways. Nature immunology. 2012 Sep 18;13:916. doi: 10.1038/ni.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guttman M, et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009 Mar 12;458:223. doi: 10.1038/nature07672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rinn JL, Chang HY. Genome regulation by long noncoding RNAs. Annual review of biochemistry. 2012;81:145. doi: 10.1146/annurev-biochem-051410-092902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods. 2008 Jul;5:621. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- 9.Guttman M, et al. Ab initio reconstruction of cell type-specific transcriptomes in mouse reveals the conserved multi-exonic structure of lincRNAs. Nat Biotechnol. 2010 May;28:503. doi: 10.1038/nbt.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guttman M, Rinn JL. Modular regulatory principles of large non-coding RNAs. Nature. 2012 Feb 16;482:339. doi: 10.1038/nature10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Birney E, et al. Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature. 2007 Jun 14;447:799. doi: 10.1038/nature05874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peng X, et al. Unique signatures of long noncoding RNA expression in response to virus infection and altered innate immune signaling. MBio. 2010;1 doi: 10.1128/mBio.00206-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collier SP, Collins PL, Williams CL, Boothby MR, Aune TM. Cutting Edge: Influence of Tmevpg1, a Long Intergenic Noncoding RNA, on the Expression of Ifng by Th1 Cells. J Immunol. 2012 Jul 30; doi: 10.4049/jimmunol.1200774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomez JA, et al. The NeST long ncRNA controls microbial susceptibility and epigenetic activation of the interferon-gamma locus. Cell. 2013 Feb 14;152:743. doi: 10.1016/j.cell.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ingolia NT, Brar GA, Rouskin S, McGeachy AM, Weissman JS. The ribosome profiling strategy for monitoring translation in vivo by deep sequencing of ribosome-protected mRNA fragments. Nature protocols. 2012 Aug;7:1534. doi: 10.1038/nprot.2012.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ingolia NT, Ghaemmaghami S, Newman JR, Weissman JS. Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science. 2009 Apr 10;324:218. doi: 10.1126/science.1168978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ingolia NT, Lareau LF, Weissman JS. Ribosome profiling of mouse embryonic stem cells reveals the complexity and dynamics of mammalian proteomes. Cell. 2011 Nov 11;147:789. doi: 10.1016/j.cell.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu W, Yuan B, Flygare J, Lodish HF. Long noncoding RNA-mediated anti-apoptotic activity in murine erythroid terminal differentiation. Genes & development. 2011 Dec 15;25:2573. doi: 10.1101/gad.178780.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng J, et al. Transcriptional maps of 10 human chromosomes at 5-nucleotide resolution. Science. 2005 May 20;308:1149. doi: 10.1126/science.1108625. [DOI] [PubMed] [Google Scholar]
- 20.Wu Q, et al. Poly A- transcripts expressed in HeLa cells. PLoS One. 2008;3:e2803. doi: 10.1371/journal.pone.0002803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Louro R, Smirnova AS, Verjovski-Almeida S. Long intronic noncoding RNA transcription: expression noise or expression choice? Genomics. 2009 Apr;93:291. doi: 10.1016/j.ygeno.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Bhatt DM, et al. Transcript dynamics of proinflammatory genes revealed by sequence analysis of subcellular RNA fractions. Cell. 2012 Jul 20;150:279. doi: 10.1016/j.cell.2012.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khalil AM, et al. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Natl Acad Sci U S A. 2009 Jul 14;106:11667. doi: 10.1073/pnas.0904715106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huarte M, et al. A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell. 2010 Aug 6;142:409. doi: 10.1016/j.cell.2010.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hasegawa Y, et al. The matrix protein hnRNP U is required for chromosomal localization of Xist RNA. Developmental cell. 2010 Sep 14;19:469. doi: 10.1016/j.devcel.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 26.Dreyfuss G, Hentze M, Lamond AI. From transcript to protein. Cell. 1996 Jun 28;85:963. doi: 10.1016/s0092-8674(00)81298-2. [DOI] [PubMed] [Google Scholar]
- 27.Krecic AM, Swanson MS. hnRNP complexes: composition, structure, and function. Curr Opin Cell Biol. 1999 Jun;11:363. doi: 10.1016/S0955-0674(99)80051-9. [DOI] [PubMed] [Google Scholar]
- 28.Gao C, et al. S-nitrosylation of heterogeneous nuclear ribonucleoprotein A/B regulates osteopontin transcription in endotoxin-stimulated murine macrophages. J Biol Chem. 2004 Mar 19;279:11236. doi: 10.1074/jbc.M313385200. [DOI] [PubMed] [Google Scholar]
- 29.Gao C, Guo H, Mi Z, Wai PY, Kuo PC. Transcriptional regulatory functions of heterogeneous nuclear ribonucleoprotein-U and -A/B in endotoxin-mediated macrophage expression of osteopontin. J Immunol. 2005 Jul 1;175:523. doi: 10.4049/jimmunol.175.1.523. [DOI] [PubMed] [Google Scholar]
- 30.Raju CS, et al. In cultured oligodendrocytes the A/B-type hnRNP CBF-A accompanies MBP mRNA bound to mRNA trafficking sequences. Molecular biology of the cell. 2008 Jul;19:3008. doi: 10.1091/mbc.E07-10-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. lncRNAs induced following TLR2 stimulation
Table S2. Upregulated genes following TLR2 stimulation of lincRNA-Cox2 knockdown cells
Table S3. GO enrichment analysis of the top 800 upregulated genes
Table S4. Downregulated genes following TLR2 stimulation of lincRNA-Cox2 knockdown cells
Table S5. Common genes affected by lincRNA-Cox2, hnRNP-A/B or hnRNPA2/B1 knockdown in macrophages