Abstract
Outcome of systemic peripheral T-cell lymphomas (PTCL) is unsatisfactory and no controlled clinical study guides the therapy. Phase II studies suggest to consolidate response achieved after front-line treatment with stem cell transplant (SCT). We retrospectively evaluate the impact of front-line SCT consolidation in a single Center cohort of 209 patients treated during the last two decades. Median age was 49 years (range 15-85) with a prevalence of male sex (61%), advanced stage (68%) while IPI was >2 in 44%. Primary treatment was MACOP-B (39%) CHO(E)P (39%), intensive regimens (18%) or others (4%). Complete response to primary treatment (i.e. before SCT) was 60% (5% partial remission). Forty-four patients further proceeded to SCT while 92 did not receive consolidation. Outcome of primary responders was good, with a 3-year overall survival of 74% (82% in ALCL ALK+ and 69% for the other histologies). By multivariate analysis a better overall survival was significantly associated with IPI<2 (P=0.001), primary response (P=0.000), and ALCL ALK+ (P=0.012). The multivariate analysis performed on responders, showed that only IPI was predictive of a better survival while ALCL ALK+ and undergoing SCT were not. Response to primary treatment rather than post-remission programs is the crucial determinant of PTCL outcome.
Introduction
Systemic peripheral T-cell lymphomas (PTCL) are a rare and heterogeneous group of non-Hodgkin lymphomas characterized by an aggressive clinical course and poor treatment response.
Several retrospective studies have shown that long-term survival of most PTCL do not exceed 30–40%, with systemic anaplastic large cell lymphoma (ALCL) expressing the anaplastic large cell lymphoma kinase (ALK) protein being the exception[1–4]. The optimal treatment of these lymphomas is still a matter of debate as conventional therapy based on CHOP/CHOP-like regimens provided unsatisfactory outcome[5, 6]. Attempts to improve response and survival with early treatment intensification led to similarly disappointing results[7–11]. Several retrospective studies [12–15] and few small-sized phase II trials[16–20] suggested that consolidation of the first response with high dose therapy (HDT) followed by autologous stem cell transplant (SCT) is a feasible option and may guarantee better disease control, with long-term overall survival (OS) up to 60–70%. This concept has been tested in a large phase II trial enrolling 160 histologically proved patients that reported an encouraging 5-year OS and progression free survival (PFS) as good as 51% and 44%, respectively[21]. Despite these interesting results, no comparative trial is available and it is not clear whether HDT plus autologous SCT should be considered a standard treatment in all PTCL subgroups. In addition, the role of allogeneic SCT as front-line treatment for these patients have been recently reported in a phase II clinical study[22].
In this study, we retrospectively evaluated the outcome of PTCL patients treated in the last two decades at our Center with the aim to evaluate the role of post-remission consolidation with SCT.
Subjects and Methods
Patients
Eligible for this retrospective analysis were untreated patients with systemic PTCL diagnosed between January 1990 and December 2012 at “Papa Giovanni XXIII” Hospital (formerly “Ospedali Riuniti”) of Bergamo. Patients treated with a palliative intent, those without pathological material suitable for revision and primary cutaneous T-cell lymphomas were excluded from the study. The diagnostic slides were independently reviewed by two hematopathologists and classified according to the 2008 WHO classification[23]. When necessary, immunostains were performed i.e. ALK in ALCL. Clinical information was gathered from the electronic charts. When necessary, the paper charts were reviewed. Patient’s written informed consent for the retrospective use of clinical data gathered from the electronic charts was obtained in all cases and this procedure was approved from Hospital’s Ethic Committee (“Comitato Etico della Provincia di Bergamo”). The study was approved and conducted in accordance with the Italian laws and the Declaration of Helsinki.
Treatments and outcome evaluation
Treatments included standard CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), CHOP with etoposide (CHOEP), MACOP-B[24] or intensive therapy including both clinical trials[16, 22] and intensive acute lymphoblastic leukemia-like schemes[25]. Patients with stage II–IV PTCL (excluding ALCL ALK positive after 2003), age <60 years with no medical contraindications were considered eligible to first-line SCT consolidation if a clinical response is achieved after a full course of treatment. Patients proceeding to autologous SCT received an additional stem cell mobilizing cycle (MAD or high-dose cyclophosphamide) followed by HDT according to BEAM (BCNU, etoposide, cytosine arabinoside, and melphalan) plus autologous SCT. Front-line allogeneic SCT was performed within a clinical trial[22]. Treatment response was defined according to the 1999 International Working Group criteria[26] and was recorded after the primary treatment (i.e. CHOP, CHOEP, MACOP-B or intensive therapy) and after SCT consolidation when applicable.
Statistical analysis
The long-term outcome was assessed in terms of overall survival (OS), progression-free survival (PFS) and disease-free survival (DFS) defined according to Cheson criteria[26]. The OS was defined as the time from the start of treatment to death for any cause. The PFS was defined as the time from the start of treatment to disease progression or death for any cause. The DFS was defined as the time from documentation of complete response to relapse or death for any cause. All the variables were analyzed by the Kaplan-Meier method. Patients were censored at the date of last contact, follow-up was updated in September 2013 and all living patients had been observed at least once in the previous 3 months. Differences in survival between groups were identified by generalized log-rank analysis. Multivariate analysis for survival was performed by Cox regression model; proportional hazards assumption was tested. A chi-squared test for independence was used to compare patient’s characteristics according to SCT consolidation.
Results
Clinical characteristics
Two-hundred and nine patients were included in the retrospective analysis. The baseline clinical characteristics are summarized in Table 1. Median age at diagnosis was 49 years with a male predominance (61%). In the majority of the cases (68%), patients had an advanced stage disease with an intermediate-high or high International Prognostic Index (IPI)[27] in 44%. There was a prevalence of ALCL (51%), particularly ALK positive (34%). These latter patients were characterized by lower median age than other histologies (42.6 vs 57.1 years, P = <0.0001) and showed lower IPI and PIT scores (P = 0.0017 and 0.0392, respectively). Primary treatment was MACOP-B in most of the cases until 2003 and CHOP or CHOEP later on while 18% of the patients received intensive treatments (Table 2).
Table 1. Patients Characteristics.
| All N (%) | Non-ALCL ALK+ N (%) | ALCL ALK+ N (%) | P | |
|---|---|---|---|---|
| All patients | 209 (100) | 139 (66) | 70 (34) | |
| Age | ||||
| Median (range) | 49.3 (15–85) | 57.1 (19–85) | 42.6 (15–82) | <0.0001 |
| ≥60 | 69 (33) | 60 (43) | 9 (13) | <0.0001 |
| Gender | ||||
| Male | 128 (61) | 80 (58) | 48 (69) | 0.1228 |
| Histology | ||||
| PTCL-NOS | 67 (32) | 67 (48) | ||
| ALCL ALK positive | 70 (34) | - | 70 (100) | |
| ALCL ALK negative | 36 (17) | 36 (26) | - | |
| AITL | 21 (10) | 21 (15) | - | |
| EATL | 10 (5) | 10 (7) | - | |
| Others | 5 (2) | 5 (4) | - | |
| Ann Arbor Stage | ||||
| III–IV | 143 (68) | 102 (73) | 41 (59) | 0.0297 |
| ECOG PS | ||||
| ≥2 | 87 (42) | 54 (39) | 33 (47) | 0.2464 |
| Serum LDH Level | ||||
| > ULN | 92 (44) | 64 (46) | 28 (40) | 0.3585 |
| Extranodal Sites | ||||
| >1 | 68 (33) | 54 (39) | 14 (20) | 0.0057 |
| Bone marrow involvement | ||||
| Yes | 22 (12)* | 18 (15)** | 4 (7)*** | 0.1153 |
| IPI | ||||
| >2 | 91 (44) | 71 (51) | 20 (29) | 0.0017 |
| PIT | ||||
| >2 | 26 (15)* | 22 (18)** | 4 (7)*** | 0.0392 |
Legend:
* N = 179;
** N = 120;
*** N = 59;
PTCL-NOS: Peripheral T-Cell Lymphoma Not Otherwise Specified; ALCL: Anaplastic Large-Cell Lymphoma; ALK: Anaplastic Large Cell Lymphoma Kinase; EATL: Enteropathy-Associated T-cell Lymphoma; AITL: Angioimmunoblastic T-cell Lymphoma; Others: includes hepatosplenic T-cell lymphoma (N = 3) and extranodal T/NK-cell lymphoma nasal type (N = 2);IPI: International Prognostic Index; PIT: Prognostic Index for PTCL-NOS
Table 2. Treatments and Outcome.
| All N (%) | Non-ALCL ALK+ N (%) | ALCL ALK+ N (%) | P | |
|---|---|---|---|---|
| All patients | 209 (100) | 139 (67) | 70 (33) | |
| Primary treatment | ||||
| CHO(E)P | 82 (39) | 65 (47) | 17 (24) | 0.0001 |
| MACOP-B | 81 (39) | 39 (28) | 42 (60) | |
| Intensive regimens | 37 (18) | 29 (21) | 8 (12) | |
| Others | 9 (4) | 6 (4) | 3 (4) | |
| Response to primary treatment | ||||
| CR | 126 (60) | 79 (57) | 47 (67) | 0.1300 |
| PR | 10 (5) | 6 (4) | 4 (6) | |
| SD/PD | 45 (22) | 30 (22) | 15 (21) | |
| Early death | 28 (13) | 24 (17) | 4 (6) | |
Legend: Others: includes MetAspDex regimen, ACVBP or ACOD; CR: Complete Remission; PR: Partial Remission; SD: Stable Disease; PD: Progressive Disease; SCT: Stem Cell Transplant. Early death indicates patients deceasing <6 months from diagnosis.
Response and survival in the whole cohort
Overall response rate to primary treatment (i.e. before SCT consolidation) was 65% with a complete response (CR) achieved in 60% of patients (Table 2). Of note, 13% of the patients died early during treatment (<6 months), with a similar incidence among the three treatment categories. Treatment response was slightly better in ALCL ALK positive patients than the others, however the result was not statistically significant (P = 0.130). Consolidation of first response with SCT was performed in 44 patients (21%). In most of these cases (N = 41/44, 93%) the stem cell source was autologous while an allogeneic SCT was performed only in 3 cases. Six of the seven patients undergoing autologous SCT in partial remission (PR) achieved the CR. Of the three patients undergoing allogeneic SCT, one deceased by pulmonary aspergillosis shortly after engraftment, one deceased by grade IV cutaneous and intestinal acute graft versus host disease (GVHD) at day 64 and the last patient is alive and well after nearly 5 years.
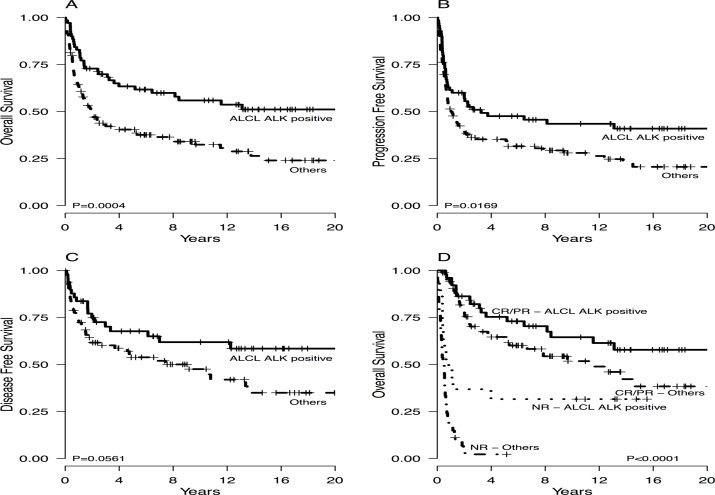
Overall survival, PFS and DFS of patients with ALCL ALK positive versus other histologies are presented in Fig. 1, while the impact of the different histology on clinical outcome is shown in S1 Fig.
Fig 1. Overall survival (A), progression-free survival (B) and disease free survival (C) in the whole cohort according to histologic subgroup.
Overall survival according to primary treatment response (E). ALCL: anaplastic large cell lymphoma; ALK: anaplastic large cell lymphoma kinase; CR: complete remission; PR: partial remission; NR: no response, including stable and progressive disease.
It was not possible to evaluate the impact of specific histology on outcome due to the relatively small sample size. Interestingly, the 5 patients with Enteropathy-Associated T-cell Lymphoma (EATL) who performed autologous SCT remained all disease free after a median follow-up of 5.61 (0.63–7.93) years.
By univariate analysis, an inferior clinical outcome was related to IPI >2 [Hazard Ratio (HR) 2.90, P<0.001], Prognostic Index for PTCL-unspecified (PIT)[28] >2 (HR 3.29, P<0.001), the male sex (HR 1.60, P = 0.016), the lack of a clinical response to primary treatment (HR 6.61, P<0.001), PTCL not otherwise specified (NOS) histology compared with ALCL ALK positive (HR 2.54, P<0.001) and MACOP-B treatment (HR 0.59, P = 0.040) while year of treatment >2003 (HR 1.32, P = 0.153) did not show any impact. By multivariate analysis IPI, ALCL ALK positive histology and primary treatment response were independent predictors of outcome (Table 3).
Table 3. Multivariate analysis for overall survival of the whole cohort.
| Hazard Ratio | CI 95% | P | 3-year OS | 10-year OS | |
|---|---|---|---|---|---|
| Age | |||||
| < 60 | 1.00 | 59% | 47% | ||
| ≥60 | 1.20 | 0.77–1.88 | 0.4251 | 38% | 26% |
| IPI | |||||
| ≤ 2 | 1.00 | 68% | 56% | ||
| > 2 | 1.82 | 1.21–2.74 | 0.0041 | 32% | 21% |
| Primary treatment response | |||||
| NR/EXITUS | 1.00 | 12% | 11% | ||
| CR/PR | 0.14 | 0.09–0.22 | <0.0001 | 74% | 57% |
| Histology | |||||
| ALCL ALK positive | 1.00 | 70% | 56% | ||
| Others | 2.78 | 1.68–4.59 | <0.0001 | 43% | 33% |
| Chemotherapy scheme | |||||
| CHO(E)P | 1.00 | 42% | 28% | ||
| MACOP-B | 1.00 | 0.57–1.76 | 0.9998 | 62% | 50% |
| Intensified | 0.78 | 0.46–1.34 | 0.3760 | 51% | 45% |
| Year of treatment | |||||
| ≤ 2003 | 1.00 | 57% | 44% | ||
| > 2003 | 0.77 | 0.48–1.25 | 0.2906 | 44% | - |
Response and survival according to primary treatment response
Overall survival according to primary treatment response shows that patients responding (CR/PR) to primary treatment fares well, with a global 3-year OS of 74% that was 82% in ALCL ALK positive patients and 69% for the other histologies (Fig. 1D). We next analyzed the effect of consolidation with autologous SCT in our cohort of responsive patients. For this purpose, we excluded the three patients receiving allogeneic SCT, and analyzed our patient population in two different cohort according to histology i.e. ALCL ALK positive and others. With the exception of age, the presenting clinical characteristic of non-ALCL ALK positive patient who received or not an autologous SCT after achieving a clinical response did not significantly differ (Table 4).
Table 4. Clinical Characteristics of Patients Responsive to Primary Treatment.
| Non-ALCL ALK+ | ALCL ALK+ | |||||
|---|---|---|---|---|---|---|
| With SCT N (%) | Without SCT N (%) | P | With SCT N (%) | Without SCT N (%) | P | |
| All patients | 26 (32) | 56 (68) | 15 (29) | 36 (71) | ||
| Age | ||||||
| Median (range) | 45.3 (19–62) | 63.9 (22–85) | 0.0002 | 31.4 (20–59) | 44.1 (17–79) | 0.0265 |
| ≥60 | 2 (8) | 32 (57) | <0.0001 | 0 (0) | 5 (14) | 0.1286 |
| Sex | ||||||
| Male | 16 (62) | 29 (52) | 0.4089 | 9 (60) | 24 (67) | 0.6499 |
| Histology | ||||||
| PTCL-NOS | 7 (27) | 28 (50) | 0.0209 | 0 | 0 | - |
| ALCL ALK positive | 0 | 0 | 15 (100) | 36 (100) | ||
| ALCL ALK negative | 8 (31) | 15 (27) | 0 | 0 | ||
| AITL | 4 (15) | 11 (20) | 0 | 0 | ||
| EATL | 5 (19) | 1 (1.5) | 0 | 0 | ||
| Others | 2 (8) | 1 (1.5) | 0 | 0 | ||
| Ann Arbor Stage | ||||||
| III–IV | 14 (54) | 39 (70) | 0.1638 | 12 (80) | 16 (44) | 0.0201 |
| IPI | ||||||
| >2 | 6 (23) | 23 (41) | 0.1127 | 6 (40) | 6 (17) | 0.0735 |
| PIT | ||||||
| >2 | 2 (9) | 3 (6) | 0.6185 | 2 (13) | 0 (0) | 0.0519 |
| Relapse/progression | ||||||
| Yes | 9 (35) | 22 (39) | 0.6849 | 2 (13) | 13 (36) | 0.1038 |
| Primary treatment response | ||||||
| CR | 21 (81) | 55 (98) | 0.0048 | 13 (87) | 34 (94) | 0.3465 |
| PR | 5 (19) | 1 (2) | 2 (13) | 2 (6) | ||
| Final response (after autologous SCT) | ||||||
| CR | 25 (96) | - | - | 15 (100) | - | - |
| PR | 1 (4) | - | - | 0 | - | - |
Legend: PTCL-NOS: Peripheral T-Cell Lymphoma Not Otherwise Specified; ALCL: Anaplastic Large-Cell Lymphoma; ALK: Anaplastic Large Cell Lymphoma Kinase; EATL: Enteropathy-Associated T-cell Lymphoma; AITL: Angioimmunoblastic T-cell Lymphoma; Others: includes hepatosplenic T-cell lymphoma (N = 3) and extranodal T/NK-cell lymphoma nasal type (N = 2); SCT: Stem Cell Transplant
Conversely, transplanted patients with ALCL ALK positive were at higher risk according to stage, PIT, and probably IPI. Of note, autologous SCT converted a PR in CR in 6 out of 7 cases. By multivariate analysis, only IPI >2 was associated with an inferior survival (HR 2.37, P = 0.018), while the clinical benefit related to SCT consolidation and ALCL ALK positive histology was not confirmed (Table 5). When excluding ALCL ALK positive patients from the analysis, the results did not change and receiving SCT was not associated to better prognosis (Table 6).
Table 5. Multivariate analysis for overall survival of the responders*.
| Variables | Hazard Ratio | CI 95% | P | 3-year OS | 10-year OS |
|---|---|---|---|---|---|
| Age | |||||
| < 60 | 1.00 | 79% | 61% | ||
| ≥60 | 1.22 | 0.58–2.58 | 0.6013 | 63% | 47% |
| IPI | |||||
| ≤ 2 | 1.00 | 80% | 64% | ||
| > 2 | 2.14 | 1.14–4.02 | 0.0174 | 64% | 41% |
| 1st line autologous SCT consolidation | |||||
| Performed | 0.66 | 0.33–1.33 | 0.2443 | 82% | 65% |
| Histology | |||||
| ALCL ALK positive | 1.00 | 82% | 64% | ||
| Others | 1.65 | 0.88–3.10 | 0.1208 | 70% | 41% |
| Chemotherapy scheme | |||||
| CHO(E)P | 1.00 | 69% | 46% | ||
| MACOP-B | 1.18 | 0.60–2.31 | 0.6300 | 74% | 58% |
| Intensified | 0.69 | 0.25–1.93 | 0.4819 | 88% | 82% |
Legend:
* excluding 3 patients treated with allogeneic SCT
Table 6. Multivariate analysis for overall survival of the responders* (excluding ALCL ALK+).
| Variables | Hazard Ratio | CI 95% | P | 3-year OS | 10-year OS |
|---|---|---|---|---|---|
| Age | |||||
| < 60 | 1.00 | 79% | 61% | ||
| ≥60 | 1.59 | 0.70–3.61 | 0.2685 | 57% | 37% |
| IPI | |||||
| ≤ 2 | 1.00 | 78% | 66% | ||
| > 2 | 2.65 | 1.29–5.43 | 0.0077 | 55% | 25% |
| 1st line autologous SCT consolidation | |||||
| Performed | 0.97 | 0.41–2.27 | 0.9436 | 80% | 53% |
| Chemotherapy scheme | |||||
| CHO(E)P | 1.00 | 62% | 38% | ||
| MACOP-B | 0.77 | 0.35–1.72 | 0.5311 | 73% | 56% |
| Intensified | 0.68 | 0.24–1.93 | 0.4702 | 85% | 76% |
Legend:
* excluding 3 patients treated with allogeneic SCT
The clinical outcome of the 45 patients not responding to primary treatment was remarkably poor, with a 1-year OS of 19% (Fig. 1D) that was as low as 4% when excluding ALCL ALK positive patients. For these patients a salvage autologous SCT was performed in 7 cases (16%) and an allogeneic SCT in 6 (13%). Long-term survivors were only 6 patients (all with a diagnosis of ALCL ALK positive) who received either an allogeneic (N = 3) or autologous (N = 3) SCT. On the contrary, outcome of patients treated with chemotherapy only was very poor, with only one long-term survivor that, however, experienced multiple chemosensitive relapse.
Twenty-eight patients (13%) died early during treatment. The median age of this latter cohort of patients was 62.0 years (range 35–84), 21 patients (75%) showed an IPI >2 and the disease was symptomatic in 20 cases (71%). In most of the cases (89%) cause of death was infection, in large part associated to evidence of insufficient disease control.
Discussion
Over the last two decades, consolidation of first-response with HDT and autologous SCT has been explored in systemic PTCL with the intent to reduce the unacceptable high relapse rate of this group of lymphomas. The heterogeneity and relative rarity of these diseases was a great limit to perform informative trials and only few, small sized non-comparative prospective studies have been available until recently. Several studies have shown a long-term (≥3 years) OS ranging from 48 to 73% after HDT and autologous SCT[15–18, 20]. Recently, a large phase II trial including 160 histologically proven PTCL, with the exclusion of ALCL ALK positive, reported encouraging survival data, with a 5-year OS and PFS of 51% and 44%, using a dose dense CHOEP-14 induction therapy (CHOP-14 if age >60 years) and autologous SCT in responders[21]. More recently, allogeneic SCT has been as well explored in first-line setting[22].
In this study, we present the outcome of a large cohort of histologically confirmed PTCL patients derived from a single Institution and followed up for a prolonged period of time. To our knowledge, this is the first report that, though retrospectively, describes the outcome of responding patients according to subsequent consolidation strategy. Our results indicate that response to primary treatment is the key variable for the long term prognosis and consolidation with HDT and SCT may not be crucially important for most of the complete responders. However, in some patients such as those with EATL, first-line SCT looked particularly beneficial when performed in CR. Although not statistically significant due to the limited sample size, these data are in line with other recently published.[29] In addition, in some patients we could observe the ability of SCT consolidation in converting a partial response into a complete one.
Median age of our cohort was lower and the proportion of ALCL ALK positive patients was higher compared to other series.[4] This finding may reflect the pattern of referral as our Center is the tertiary care referral center, and is accordingly similar to those reported in other report, for example in a German series of 320 patients enrolled in clinical trials median age was 50 years and 60% of the patients had a diagnosis of ALCL (41% ALK positive)[9]. We included in the current analysis patients with ALK positive ALCL, that are not usually considered eligible for consolidation therapy, despite the severe prognosis of high risk disease[3]. Reason for this choice were the homogeneous good prognosis across histologies in responding patients, confirmed by multivariate analysis. Additionally, we could confirm that the results of the study did not change excluding ALCL ALK positive patients.
The role of allogeneic transplantation as a front line consolidation treatment option could not be addressed by this study and is the matter of investigation of a different ad hoc designed clinical trial. Therefore, while waiting for additional data, allogeneic SCT consolidation should be used with caution in patients responding to a first line treatment.
The primary determinant of outcome resulted to be treatment response, and despite several clinical trials have been addressing the unsatisfactory outcome of PTCL in the last two decades, 6 to 8 cycles of chemotherapy with CHOP with or without etoposide (CHOEP) still remains the standard[30, 31]. Several novel molecules are currently facing the clinical ground and there is high expectation from the combination of standard chemotherapy with newer biological agents[32]. Despite the rapid growth of clinical trials in PTCL driven by new drugs, the role of consolidation with HDT and autologous SCT remains not addressed, yet.
Conversely, since primary response is such a key factor for outcome, there is an urgent need to develop strategies aimed at the early identification of poor responders. In this respect, the role of interim 18F-fluorodeoxyglucose—positron emission tomography (FDG—PET) remains controversial but in general, differently from Hodgkin disease[33], it does not provide informative data as to chemosensitivity of this group of patients[34–36].
In conclusion, our study underlines the crucial importance of increasing the quality of response to primary treatment and the need of new drugs or innovative treatment strategies to achieve such a goal. Among these latter treatment strategies, the role of a consolidation of first remissions with autologous stem cell transplantation should always be considered[37] but its benefit remains to be proven by well conducted comparative, prospective, clinical trials.
Supporting Information
PTCL-NOS: Peripheral T-Cell Lymphoma Not Otherwise Specified; ALCL: Anaplastic Large-Cell Lymphoma; ALK: Anaplastic Large Cell Lymphoma Kinase; EATL: Enteropathy-Associated T-cell Lymphoma; AITL: Angioimmunoblastic T-cell Lymphoma; Others: includes hepatosplenic T-cell lymphoma and extranodal T/NK-cell lymphoma nasal type.
(TIFF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported in part by grants from Associazione Matteo Riboldi (www.associazionematteoriboldi.org), Associazione Paolo Belli - Associazione Italiana Lotta alla Leucemia (AIL) sezione di Bergamo (www.ailbergamo.it) and the Associazione Italiana per la Ricerca sul Cancro (www.airc.it) - “AIRC 5 per Mille” –IIC Project, Innate Immunity in Cancer (IIC), Molecular Targeting and Cellular Therapy (# 9962). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Delabie J, Holte H, Vose JM, Ullrich F, Jaffe ES, Savage KJ, et al. Enteropathy-associated T-cell lymphoma: clinical and histological findings from the international peripheral T-cell lymphoma project. Blood 2011;118(1):148–55. 10.1182/blood-2011-02-335216 [DOI] [PubMed] [Google Scholar]
- 2. Weisenburger DD, Savage KJ, Harris NL, Gascoyne RD, Jaffe ES, MacLennan KA, et al. Peripheral T-cell lymphoma, not otherwise specified: a report of 340 cases from the International Peripheral T-cell Lymphoma Project. Blood 2011;117(12):3402–8. 10.1182/blood-2010-09-310342 [DOI] [PubMed] [Google Scholar]
- 3. Savage KJ, Harris NL, Vose JM, Ullrich F, Jaffe ES, Connors JM, et al. ALK- anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. Blood 2008;111(12):5496–504. 10.1182/blood-2008-01-134270 [DOI] [PubMed] [Google Scholar]
- 4. Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol 2008;26(25):4124–30. 10.1200/JCO.2008.16.4558 [DOI] [PubMed] [Google Scholar]
- 5. Jerkeman M, Anderson H, Cavallin-Stahl E, Dictor M, Hagberg H, Johnson A, et al. CHOP versus MACOP-B in aggressive lymphoma—a Nordic Lymphoma Group randomised trial. Ann Oncol 1999;10(9):1079–86. [DOI] [PubMed] [Google Scholar]
- 6. Gisselbrecht C, Gaulard P, Lepage E, Coiffier B, Briere J, Haioun C, et al. Prognostic significance of T-cell phenotype in aggressive non-Hodgkin's lymphomas. Groupe d'Etudes des Lymphomes de l'Adulte (GELA). Blood 1998;92(1):76–82. [PubMed] [Google Scholar]
- 7. Kluin-Nelemans HC, van Marwijk Kooy M, Lugtenburg PJ, van Putten WL, Luten M, Oudejans J, et al. Intensified alemtuzumab-CHOP therapy for peripheral T-cell lymphoma. Ann Oncol 2011;22(7):1595–600. 10.1093/annonc/mdq635 [DOI] [PubMed] [Google Scholar]
- 8. Simon A, Peoch M, Casassus P, Deconinck E, Colombat P, Desablens B, et al. Upfront VIP-reinforced-ABVD (VIP-rABVD) is not superior to CHOP/21 in newly diagnosed peripheral T cell lymphoma. Results of the randomized phase III trial GOELAMS-LTP95. Br J Haematol 2010;151(2):159–66. 10.1111/j.1365-2141.2010.08329.x [DOI] [PubMed] [Google Scholar]
- 9. Schmitz N, Trumper L, Ziepert M, Nickelsen M, Ho AD, Metzner B, et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood 2010;116(18):3418–25. 10.1182/blood-2010-02-270785 [DOI] [PubMed] [Google Scholar]
- 10. Gallamini A, Zaja F, Patti C, Billio A, Specchia MR, Tucci A, et al. Alemtuzumab (Campath-1H) and CHOP chemotherapy as first-line treatment of peripheral T-cell lymphoma: results of a GITIL (Gruppo Italiano Terapie Innovative nei Linfomi) prospective multicenter trial. Blood 2007;110(7):2316–23. [DOI] [PubMed] [Google Scholar]
- 11. Escalon MP, Liu NS, Yang Y, Hess M, Walker PL, Smith TL, et al. Prognostic factors and treatment of patients with T-cell non-Hodgkin lymphoma: the M. D. Anderson Cancer Center experience. Cancer 2005;103(10):2091–8. [DOI] [PubMed] [Google Scholar]
- 12. Kyriakou C, Canals C, Goldstone A, Caballero D, Metzner B, Kobbe G, et al. High-dose therapy and autologous stem-cell transplantation in angioimmunoblastic lymphoma: complete remission at transplantation is the major determinant of Outcome-Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol 2008;26(2):218–24. 10.1200/JCO.2007.12.6219 [DOI] [PubMed] [Google Scholar]
- 13. Chen AI, McMillan A, Negrin RS, Horning SJ, Laport GG. Long-term results of autologous hematopoietic cell transplantation for peripheral T cell lymphoma: the Stanford experience. Biol Blood Marrow Transplant 2008;14(7):741–7. 10.1016/j.bbmt.2008.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rodriguez J, Caballero MD, Gutierrez A, Marin J, Lahuerta JJ, Sureda A, et al. High-dose chemotherapy and autologous stem cell transplantation in peripheral T-cell lymphoma: the GEL-TAMO experience. Ann Oncol 2003;14(12):1768–75. [DOI] [PubMed] [Google Scholar]
- 15. Smith SM, Burns LJ, van Besien K, Lerademacher J, He W, Fenske TS, et al. Hematopoietic cell transplantation for systemic mature T-cell non-hodgkin lymphoma. J Clin Oncol 2013;31(25):3100–9. 10.1200/JCO.2012.46.0188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Corradini P, Tarella C, Zallio F, Dodero A, Zanni M, Valagussa P, et al. Long-term follow-up of patients with peripheral T-cell lymphomas treated up-front with high-dose chemotherapy followed by autologous stem cell transplantation. Leukemia 2006;20(9):1533–8. [DOI] [PubMed] [Google Scholar]
- 17. Rodriguez J, Conde E, Gutierrez A, Arranz R, Leon A, Marin J, et al. Frontline autologous stem cell transplantation in high-risk peripheral T-cell lymphoma: a prospective study from The Gel-Tamo Study Group. Eur J Haematol 2007;79(1):32–8. [DOI] [PubMed] [Google Scholar]
- 18. Reimer P, Rudiger T, Geissinger E, Weissinger F, Nerl C, Schmitz N, et al. Autologous stem-cell transplantation as first-line therapy in peripheral T-cell lymphomas: results of a prospective multicenter study. J Clin Oncol 2009;27(1):106–13. 10.1200/JCO.2008.17.4870 [DOI] [PubMed] [Google Scholar]
- 19. Nickelsen M, Ziepert M, Zeynalova S, Glass B, Metzner B, Leithaeuser M, et al. High-dose CHOP plus etoposide (MegaCHOEP) in T-cell lymphoma: a comparative analysis of patients treated within trials of the German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL). Ann Oncol 2009;20(12):1977–84. 10.1093/annonc/mdp211 [DOI] [PubMed] [Google Scholar]
- 20. Mercadal S, Briones J, Xicoy B, Pedro C, Escoda L, Estany C, et al. Intensive chemotherapy (high-dose CHOP/ESHAP regimen) followed by autologous stem-cell transplantation in previously untreated patients with peripheral T-cell lymphoma. Ann Oncol 2008;19(5):958–63. 10.1093/annonc/mdn022 [DOI] [PubMed] [Google Scholar]
- 21. d'Amore F, Relander T, Lauritzsen GF, Jantunen E, Hagberg H, Anderson H, et al. Up-front autologous stem-cell transplantation in peripheral T-cell lymphoma: NLG-T-01. J Clin Oncol 2012;30(25):3093–9. 10.1200/JCO.2011.40.2719 [DOI] [PubMed] [Google Scholar]
- 22. Corradini P, Vitolo U, Rambaldi A, Miceli R, Patriarca F, Gallamini A, et al. Intensified chemo-immunotherapy with or without stem cell transplantation in newly diagnosed patients with peripheral T-cell lymphoma. Leukemia 2014. [DOI] [PubMed] [Google Scholar]
- 23. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Fourth Edition ed Lyon, France: IARC Press; 2008. [Google Scholar]
- 24. Klimo P, Connors JM. MACOP-B chemotherapy for the treatment of diffuse large-cell lymphoma. Ann Intern Med 1985;102(5):596–602. [DOI] [PubMed] [Google Scholar]
- 25. Bassan R, Spinelli O, Oldani E, Intermesoli T, Tosi M, Peruta B, et al. Improved risk classification for risk-specific therapy based on the molecular study of minimal residual disease (MRD) in adult acute lymphoblastic leukemia (ALL). Blood 2009;113(18):4153–62. 10.1182/blood-2008-11-185132 [DOI] [PubMed] [Google Scholar]
- 26. Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol 1999;17(4):1244 [DOI] [PubMed] [Google Scholar]
- 27. A predictive model for aggressive non-Hodgkin's lymphoma. The International Non-Hodgkin's Lymphoma Prognostic Factors Project. N Engl J Med 1993;329(14):987–94. [DOI] [PubMed] [Google Scholar]
- 28. Gallamini A, Stelitano C, Calvi R, Bellei M, Mattei D, Vitolo U, et al. Peripheral T-cell lymphoma unspecified (PTCL-U): a new prognostic model from a retrospective multicentric clinical study. Blood 2004;103(7):2474–9. [DOI] [PubMed] [Google Scholar]
- 29. Jantunen E, Boumendil A, Finel H, Luan JJ, Johnson P, Rambaldi A, et al. Autologous stem cell transplantation for enteropathy-associated T-cell lymphoma: a retrospective study by the EBMT. Blood 2013;121(13):2529–2532. 10.1182/blood-2012-11-466839 [DOI] [PubMed] [Google Scholar]
- 30.Non-Hodgkin's Lymphomas. NCCN Clinial Practice Guidelines in Oncology 2013;Version 2.
- 31. Dearden CE, Johnson R, Pettengell R, Devereux S, Cwynarski K, Whittaker S, et al. Guidelines for the management of mature T-cell and NK-cell neoplasms (excluding cutaneous T-cell lymphoma). Br J Haematol 2011;153(4):451–85. 10.1111/j.1365-2141.2011.08651.x [DOI] [PubMed] [Google Scholar]
- 32. Savage KJ. Therapies for Peripheral T-Cell Lymphomas. ASH Education Program Book 2011;2011(1):515–524. [DOI] [PubMed] [Google Scholar]
- 33. Gallamini A, Rossi A, Patti C, Picardi M, Di Raimondo F, Cantonetti M, et al. Early Treatment Intensification in Advanced-Stage High-Risk Hodgkin Lymphoma (HL) Patients, with a Positive FDG-PET Scan After Two ABVD Courses—First Interim Analysis of the GITIL/FIL HD0607 Clinical Trial. ASH Annual Meeting Abstracts 2012;120(21):550-. [Google Scholar]
- 34. Cahu X, Bodet-Milin C, Brissot E, Maisonneuve H, Houot R, Morineau N, et al. 18F-fluorodeoxyglucose-positron emission tomography before, during and after treatment in mature T/NK lymphomas: a study from the GOELAMS group. Ann Oncol 2011;22(3):705–11. 10.1093/annonc/mdq415 [DOI] [PubMed] [Google Scholar]
- 35. Pellegrini C, Casadei B, Derenzini E, Broccoli A, Stefoni V, Gandolfi L, et al. The Role of Interim-PET and Final-PET in the Outcome of Peripheral T-Cell Lymphoma (PTCL) Treated At the Diagnosis with CHOP. ASH Annual Meeting Abstracts 2012;120(21):2721-. [Google Scholar]
- 36. Casulo C, Schoder H, Feeney J, Lim R, Maragulia J, Zelenetz AD, et al. 18F-fluorodeoxyglucose positron emission tomography in the staging and prognosis of T cell lymphoma. Leuk Lymphoma 2013;54(10):2163–7. 10.3109/10428194.2013.767901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lunning MA, Horwitz S. Treatment of peripheral T-cell lymphoma: are we data driven or driving the data? Curr Treat Options Oncol 2013;14(2):212–23. 10.1007/s11864-013-0232-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PTCL-NOS: Peripheral T-Cell Lymphoma Not Otherwise Specified; ALCL: Anaplastic Large-Cell Lymphoma; ALK: Anaplastic Large Cell Lymphoma Kinase; EATL: Enteropathy-Associated T-cell Lymphoma; AITL: Angioimmunoblastic T-cell Lymphoma; Others: includes hepatosplenic T-cell lymphoma and extranodal T/NK-cell lymphoma nasal type.
(TIFF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.