Abstract
Cardiac contractility is the hallmark of cardiac function and is a predictor of healthy or diseased cardiac muscle. Despite advancements over the last two decades, the techniques and tools available to cardiovascular scientists are limited in their utility to accurately and reliably measure the amplitude and frequency of cardiomyocyte contractions. Isometric force measurements in the past have entailed cumbersome attachment of isolated and permeabilized cardiomyocytes to a force transducer followed by measurements of sarcomere lengths under conditions of submaximal and maximal Ca2+ activation. These techniques have the inherent disadvantages of being labor intensive and costly. We have engineered a micro-machined cantilever sensor with an embedded deflection-sensing element that, in preliminary experiments, has demonstrated to reliably measure cardiac cell contractions in real-time. Here, we describe this new bioengineering tool with applicability in the cardiovascular research field to effectively and reliably measure cardiac cell contractility in a quantitative manner. We measured contractility in both primary neonatal rat heart cardiomyocyte monolayers that demonstrated a beat frequency of 3 Hz as well as human embryonic stem cell-derived cardiomyocytes with a contractile frequency of about 1 Hz. We also employed the β-adrenergic agonist isoproterenol (100 nmol l−1) and observed that our cantilever demonstrated high sensitivity in detecting subtle changes in both chronotropic and inotropic responses of monolayers. This report describes the utility of our micro-device in both basic cardiovascular research as well as in small molecule drug discovery to monitor cardiac cell contractions.
I. INTRODUCTION
Cardiac myocytes have the intrinsic ability to contract. The force of this contraction is responsive to both external and internal stimuli. Cardiac contractility is finely regulated by the expression, phosphorylation, and function of calcium regulatory proteins, such as sarcoplasmic reticular Ca2+ ATPase, ryanodine receptor type-2, phospholambam, and the sodium–calcium exchanger (NCX), all of which contribute to changes in the magnitude or timing of the calcium transient.1,2 In addition, post-translational modifications of myofilament proteins can modify the transduction of the calcium-dependent contractile response.3–6 Thus, an accurate analysis of cardiac contractility by either bio-molecular or bioengineering tools should provide relevant information on the excitation-contraction coupling of the heart. The information obtained would facilitate the proper handling and treatment of many cardiovascular diseases. Furthermore, the study of cardiomyocyte contractility would help unveil fundamental processes underlying heart function in health and disease. The relevance of all the above outlined cardiac parameters has created a dire need for both the development and analysis of tools that will accelerate research in cardiac biology and disease.
The current approaches available to cardiovascular scientists are limited in their applicability to measure contractile force directly. Initial studies that evaluated cardiac contractility entailed chemical permeabilization of the cardiac myocytes that has the inherent drawback of swelling of the myofilament lattice and the resultant leak of soluble proteins.7,8 The goal of other studies was to assess cardiac contractility in intact cardiac cells using a carbon fiber system for cell attachment and force measurement.9–11 However the carbon fiber technology relies on expensive instrumentation, unique expertise and is labor intensive. In more recent years, IonOptix technology has been used to measure sarcomere length and cell edge displacement using complex Fourier transformation software.12,13 Ion-Optix measurements of cardiac contractility rely primarily on using a video-based cell geometry system to quantify sarcomere dynamics.14 In addition, myocytes have to be field stimulated which adds another layer of complexity to measure native or intrinsic contractions. Scanning ion conductance microscopy15 has been used to measure cellular mechanics including cell volume, membrane potentials, cellular contraction, and single ion-channel currents. Optical mapping techniques that simultaneously measure action potential and calcium wave propagation as a surrogate of cardiac force generation have been developed by various laboratories but these technologies do not per se measure contractile force.16 Atomic force microscopy (AFM) has also been used17–19 for myocyte contractility studies.
Overall cardiac contractility assessment methods that are currently available to researchers need improvement and refinement for reliable measurements. In this report, we have developed a method that offers direct measurement of cardiac contractility in both spontaneously beating and pacing cardiac cell monolayers. The cantilever has the advantage that it can operate in opaque liquid environments. A highly sensitive polyimide cantilever sensor capable of detecting minor differences in force in intact, non-permeabilized cells is gently brought into contact with myocytes in a monolayer and is able to measure the contractile force and frequency. The cantilever includes a force sensor embedded inside the cantilever (Fig. 1). The cantilevers that we have fabricated offer high compliance, sensitivity, robustness, and durability and have the capability of measuring, in a fairly rapid manner (<1 min), cardiac contractility (force and frequency) in cultured cardiomyocyte monolayers without the need for any manipulations.
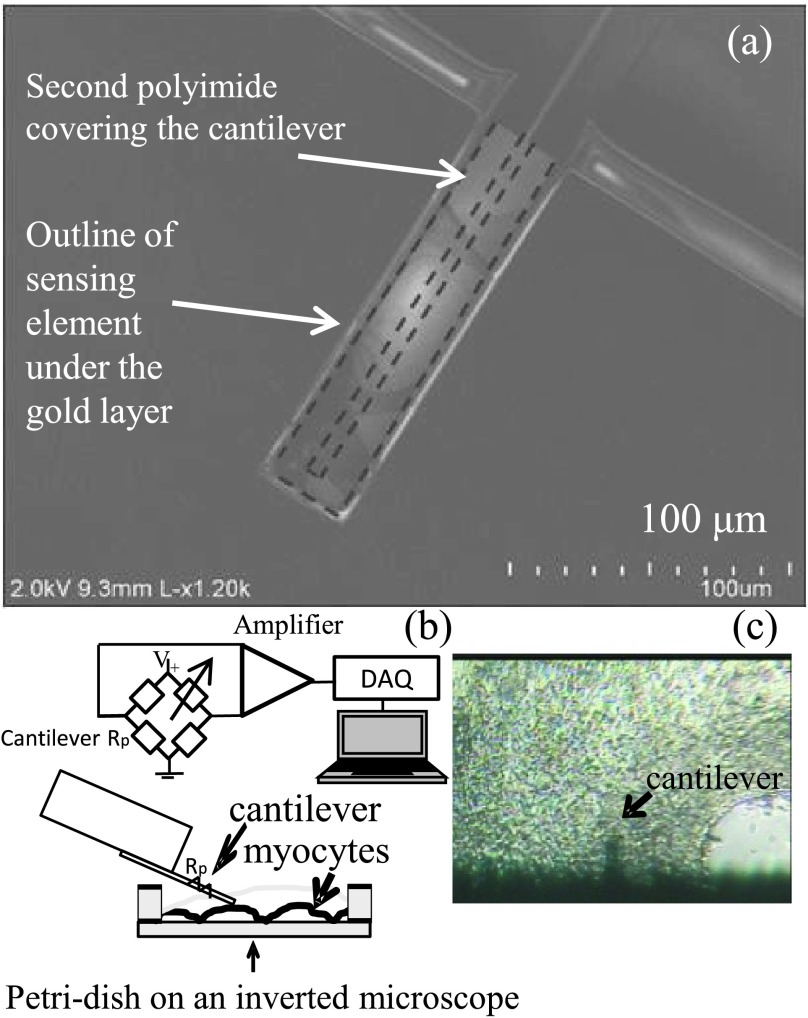
FIG. 1.
(a) Scanning electron micrograph of the cantilever depicting the location of the embedded sensing element (in dashed lines) sandwiched between two polyimide layers and the gold layer. (b) Schematic representation of the cantilever controlled by motorized micro-manipulator inside media and resting on the cell monolayer surface. The electrical interface is shown. Rp represents the cantilever’s sensing element in the bridge circuit arrangement shown. The output of the bridge connects to an amplifier, which is connected to a DAQ card and to a computer for data storage and analysis. (c) Phase contrast photomicrograph of the cantilever in contact with the cellular membrane of UM22-2 cardiomyocyte monolayer. Mag. 200x.
II. EXPERIMENTAL METHODS
A. Microcantilever
The specialized polyimide cantilever used in the present study20 and has an embedded sensing mechanism, eliminating the need for the laser feedback normally used in AFM technology (Fig. 1(a)). These cantilevers are 40 μm wide, 150 μm long, and 2 μm thick. There are two polyimide layers with thickness 1.5 μm and 0.5 μm, respectively. The sensing element is a thin film of Cr/Au with 2 nm/10 nm thickness and is sandwiched between the polyimide layers. The micro-cantilever was fabricated by oxidizing a silicon wafer, coating the wafer with 1.5 μm of polyimide and patterning. The 2 nm/10 nm thick Cr/Au sensing element is then deposited and patterned. The Ti/Au/Ti 20 nm/500 nm/2 nm pads are deposited. To form the top layer, a 0.5 μm layer of polyimide is deposited and patterned. Finally, to form the cantilever, the wafer is etched, while the front side and the handles are protected.
The cantilever’s spring constant, k, is ∼0.059 N/m and its nominal resistance is between 160 and 170 Ω. The polyimide cantilever is wired to establish electrical contact and then insulated with the inert polydimethylsiloxane (PDMS) material to insulate the electrical contacts. The cantilevers are glued on glass slides and thoroughly cleaned by sequential immersion for 10 min in methanol, acetone, isopropanol-2, and distilled water and finally for 5 min in phosphate buffered saline (PBS) before the start of measurements. The cantilever was also calibrated and initialized by measuring the change in resistance with distance travelled in the Z-axis before actual measurements with cells. This calibration was accomplished by moving the motorized stage at predefined intervals and recording the change in resistance of the cantilever with the stage’s movement. This measurement allowed us to later derive a simple relationship between the change in resistance and the movement of the cantilever in the Z-axis at the instant the cantilever comes in contact with the cell monolayer surface, which was used to estimate the contractile force as described below. The relationship between the change in resistance and the movement of the cantilever is linear.21–24
The fine movement of the cantilever is controlled with a motorized micromanipulator with 25 × 25 × 25 mm3 range and 0.1 μm minimal increment step (KT-LS28-MV, Zaber Technologies). LabVIEW software was used to control the movement and record the output of the sensor.
The electrical signal from the cantilever was measured using two parameters. For higher frequencies (>1.5 Hz), we increased acquisition speeds by using a Wheatstone bridge type of circuit connected to a low noise amplifier, and a National Instruments digital acquisition (DAQ) card to record the data into a computer (Fig. 1(b)). This electronic circuitry was very useful for neonatal rat cardiomyocytes that typically have high contractility frequencies. For smaller frequencies (<1.5 Hz), such as the ones observed in human stem cell derived cardiomyocytes, we used a micro-Ohm meter (Agilent Technologies, HP-34420A) connected to a computer to directly record the resistance changes. The micro-Ohm meter provides better signal to noise ratio but is not capable of operating at high frequencies. Further, we obtained validation of our results on contractility measurements by utilizing the IonOptix Contractility System in independent experiments.
B. Cultured cardiomyocyte monolayers
We employed two different cellular models of cardiac myocytes to measure contractility to validate the versatility of our bioengineering device. First, we utilized primary cultures of neonatal rat cardiomyocyte monolayers.25 We also measured contractility in monolayers of human embryonic stem cell-derived cardiomyocytes (hESC-CMs) that have been used to generate specific cardiac myocyte lineages26,27 and approved on the NIH stem cell registry.28,29 The human cardiac cell line used was a control or healthy stem cell derived cardiomyocyte, UM22-2. The plating and differentiation of the hESC-CM cell line used in the present study have been extensively described.21,22 After complete differentiation, cell monolayers were maintained in RPMI 1640 basal medium (Invitrogen) plus B27 complete supplement (Invitrogen) and the medium was changed every 2 to 3 days. Both rat primary cardiac myocytes and hESC-CMs were maintained as monolayers in a humidified incubator with 5% CO2. The cells were seeded on Matrigel coated round coverslips for measurements.
C. Monolayer contractility measurement
On the day of the measurements, the hESC-CMs or rat cardiomyocyte monolayers plated on glass coverslips inside 6-well tissue culture (TC) plates were switched to Hank’s balanced salt solution (HBSS) (Sigma) (a defined balanced and buffered salt solution to avoid inaccuracies and discrepancies in measurement due to undefined growth factors present in serum) and placed inside a 35 mm TC dish. This TC dish was placed on the heated (37 °C) stage of an inverted Nikon TE2000 microscope. The stage was motorized and controlled remotely and also the myocytes were perfused as needed.
The micro-cantilevers were attached to a Zaber micro-manipulator that rested on top of a motorized stage. Prior to the start of the experiments, cardiomyocyte monolayers were selected on the basis of showing forceful contractions under an inverted microscope. If a monolayer area was deemed suitable for recording, then its position was recorded using the motorized stage controller. After optically focusing on the monolayer, the tip of the cantilever was brought slowly in contact with the cells. The motorized stage was used to finely position the cantilever over the cells establishing a 10 μm contact so that about 10 μm × 40 μm of the cantilever would be in contact with the monolayer. We ensured same amount of contact by first calibrating the cantilever on an area of the slide without cells and by bringing the cantilever in contact with the glass coverslip and recording the resistance change. The cantilever is slowly brought in contact with the cells (once there is contact, there is an immediate change in the resistance of the cantilever sensor) and cantilever is then moved 10 μm past the initial contact point.
Simultaneously, the resistance of the cantilever was continuously monitored and plotted using LabVIEW so that any detectable change in resistance would indicate a contact between the cantilever and the cells. The change in resistance or voltage of the deflection-sensing element of the micro-cantilever was measured and recorded with software loaded on a computer.
Typically, we collected data for 10-12 contractions for each monolayer under baseline conditions in the absence of any drug. In some experiments, we also superfused the monolayers with the non-selective beta-adrenergic agonist isoproterenol at a final concentration of 100 nM to investigate chronotropic and inotropic responses.
III. EXPERIMENTAL RESULTS
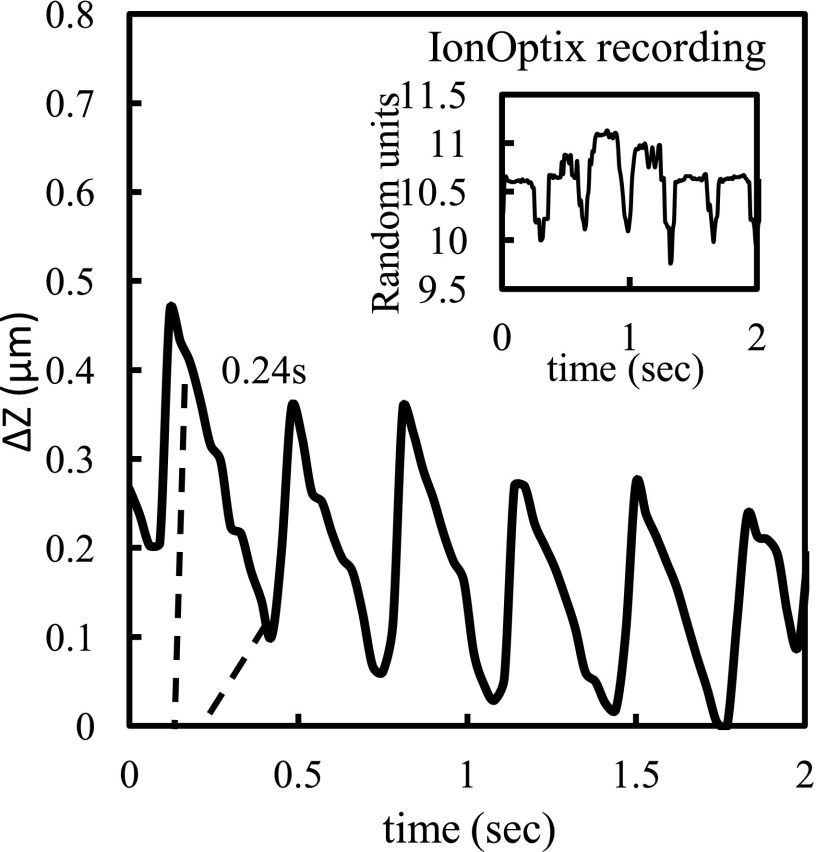
We first measured the contractile force in rat monolayers, as illustrated in Fig. 2. We estimated the distance traveled in micrometers (μm) by the cantilever by converting the reading from voltage or resistance using a simple calibration described above in Sec. II C. The beat frequency of the rat monolayer was 2.97 ± 0.06 Hz in agreement with values reported earlier. The frequency was similar to the one recorded with the IonOptix system, and as expected, the time between the crest and the trough of each contraction was 0.24 s (Figure 2). The values obtained of the cantilever displacement in μm from the change in resistance were then used to estimate the force by multiplying the displacement with the spring constant of the cantilever. Subsequently, the force per unit area was approximated from an estimation of the contact area (10 μm × 40 μm) of the cantilever with the cells (where N is the number of samples) (N = 4) 38.6 ± 2.2 N/m2. The slight drift in the recording is due to the inherent noise of the bridge circuit and the amplifier. This drift is not critical because we are looking at the relative signal change.
FIG. 2.
Contractility force measurements in a neonatal rat cardiomyocyte monolayer. The Y-axis is the cantilever displacement measured from a change in resistance and the X-axis represents the time elapsed in seconds. The broken vertical lines mark the time elapsed between the crest and the trough of a single contraction. The inset shows the contractile frequency recorded independently using the IonOptix system in the same monolayer.
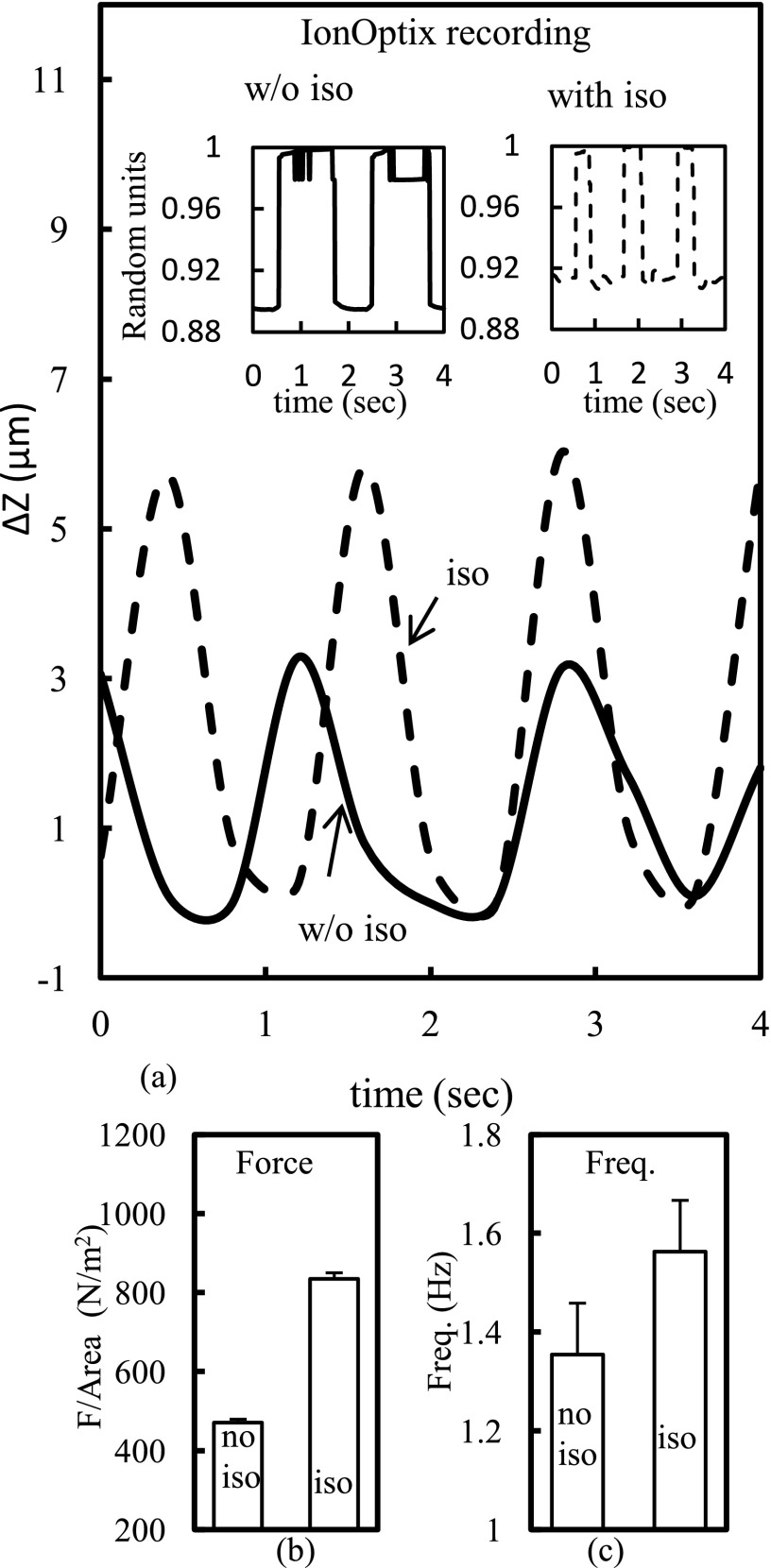
After measurements with the rat monolayers, we extended our studies to human cells. We utilized monolayers of hESC-CMs designated as UM22-2 in the stem cell registry. The UM22-2 contractility measurements are shown in Fig. 3. The Y-axis is the cantilever displacement and the X-axis represents the time. The solid-line represents the cantilever recording in monolayers without isoproterenol, while dashed-line represents the recording with isoproterenol at a final concentration of over 100 nM. In 4 monolayers, treatment of the cultures with the β-agonist increased the beat frequency from 1.35 ± 0.1 Hz to 1.56 ± 0.1 Hz showing a positive inotropic effect. Isoproterenol also decreased the time between the crest and the trough, making the drop sharper, from 0.8 s to 0.6 s. As previously, we estimated the basal force generated per unit area as 471 ± 8.2 N/m2 (N = 4) for measurements without isoproterenol addition, a nd the force generated per unit area as 835 ± 15 N/m2 (N = 4) after addition of isoproterenol. Thus, contractile force almost doubled after treatment of cultured monolayers with isoproterenol. Compared with the rat monolayers, the force of contraction increased over one order of magnitude, while the frequency was reduced by about three times in the human UM-22-2 cardiomyocyte monolayer. The contractile frequency recorded independently with the IonOptix system with and without isoproterenol yielded similar results. The optical measurements using the IonOptix software were performed on the coverslip in close proximity to the location before or right after the cantilever measurements and closely match the cantilever recording (shown in in-set of Fig. 3(a)).
FIG. 3.
(a) Sample raw data of contractile force measurements in human embryonic stem cell derived UM22-2 cardiomyocytes. (Inset) IonOptix recording. (b) Force per area and (c) bar graphs showing frequency of the contractile beat in the presence and absence of isoproterenol (100 nM).
IV. DISCUSSION
We present a versatile method to measure the contractile force in cultures of cardiac myocytes using a micro-machined cantilever with an embedded sensing mechanism. The system is readily adaptable to function with a conventional inverted microscope and thus can be widely used in cardiovascular laboratories to study cardiac biology. We demonstrate that neonatal rat cardiomyocyte monolayers had a contractility beat frequency of ∼3 Hz and that the human cardiac cell lines demonstrated a beat frequency between 0.41 and 0.75 Hz. We have also demonstrated that our cantilever is sensitive enough to effectively detect subtle changes in both chronotropic and inotropic responses to β-adrenergic stimulation. These initial observations reinforce our contention that our cantilevers have the potential to indirectly depict changes in Ca2+ fluxes since these translate to increases or decreases in force generation in cardiac myocytes. Our micro-machined polyimide cantilevers with an embedded deflection-sensing element offer unlimited possibilities to monitor cardiac contractility in a real-time fashion.
However, one inherent limitation of the present study is that we measured cardiac contractility in monolayers of rat and human cardiac cells. Since single cell cardiac contractility is critical for both basic and drug discovery research, we envision that with improvements of cantilever sensitivity, acquisition speed, and refinements in the software, our cantilever will also be beneficial to monitor single cell cardiac contractions. The cantilever sensitivity to the contractile force changes can be improved by reducing the spring constant of the cantilever, k. This can be optimally achieved by reducing the thickness or the width and increasing the length of the cantilever. The current sampling time of 30 ms, that is dependent on the DAQ hardware and LabVIEW software, can be improved for faster measurements of single cell contractions by using a higher sampling rate in the DAQ card with an optimized program.
Our data on the beat frequency and force generated in rat and human cardiac monolayers are both in agreement and in disagreement with some of the values reported in the literature. The vertical displacement of the cantilever that is used to measure force generated from change in resistance gave us values of ∼0.8 μm in rat monolayers and ∼8-10 μm in the human monolayers. These values are in very close agreement with values reported in both rat ventricular myocytes and hESC-CMs using scanning ion conductance microscopy that uses an AFM type glass nanopipette as a sensitive probe.15 However, the magnitude of force generated in the cardiac myocyte preparations varies widely in the literature from the values obtained in our study. We obtained force values of 38.6 N/m2 (0.04 nN/μm2) in rat monolayers and basal value of 471 N/m2 (0.471 nN/μm2) in human cardiac monolayers. In comparison, other groups have reported values that are 1-2 orders of magnitude larger (5.5 nN/μm2) using novel approaches for real-time and high-resolution measurements of forces applied by cardiac cells using elastic micropatterned substrates30 as well as values that are several orders of magnitude higher (7 mN/mm2) as reported in rat trabecular muscle from right ventricles and values of 11 mN/mm2 in single human ventricular cells using force transducers.31,32
It is also important to mention that as compared to conventional AFMs, our device with an embedded sensing element does not require laser alignment and the operation is not affected by operation in the liquid tissue culture medium. The cantilever is mounted on a simple motorized stage that has range of motion of several tens of millimeters in the XYZ axis. The biocompatible polymer cantilever is very compliant and does not require a sharp tip on the cantilever, thus simplifying the preparation and allowing for preservation of cell integrity. The polymer cantilevers can be bent at angles greater than 90° without breaking thereby allowing it measure tens of microns of motion and achieve high force sensitivity. A high compliance adds a long lifetime to these sensors.
Myocardial contractility (inotropy) is a fundamental property of normal heart function. Analysis of cardiomyocyte mechanics has historically proven an excellent tool in providing relevant information on the excitation-contraction coupling of the heart.33 The study of cardiomyocyte contractility has helped to unveil the fundamental processes underlying heart function in health and disease.14,34 The relevance of this study has created a need for analysis tools in this area of research. Many inotropic factors modulate the contractile behavior of the heart, which can be studied in isolated cardiomyocytes.35,36 With the cantilever technique presented in this manuscript, we were able to measure the force and frequency of contraction of cardiomyocyte monolayers. Since this procedure does not require extended waiting time, it is immediate, highly reliable, and robust, and can be completed in <1 min from start-to-finish. Finally, compared to IonOptix measurement of sarcomere lengths as a surrogate for cardiac contractility, our device does not require pacing or sophisticated tools and expensive software to accomplish the goal.
We envision that with further refinements, our device can have important applications in both basic and drug discovery cardiovascular arenas. We need to emphasize that the current sensor works with monolayers of beating cardiac myocytes and we are in the process of replicating our results using single beating cardiac myocytes. Needless to mention that it is of paramount importance to assess cardiac inotropy early in the drug discovery process before a potential drug enters clinical trials. The ultimate goal of our future research and effort is to develop a novel diagnostic tool for rapidly monitoring cardiac force generation to facilitate both basic and drug discovery research in the field of cardiac biology and a device which may even find clinical diagnostic applications. The proposed methodology is relatively easy to understand and implement, yet provides a robust measurement of contractility without the need for expensive and sophisticated equipment.
Acknowledgments
We would like to thank Dr. Gary Smith, Dr. Andre M. Rocha, Dr. Weibin Zhu, and Proffesor Yogesh Gianchandani for their help and support with this project. This research was funded by NIH Grant No. GM084520 (Gaitas) and by the Leducq Foundation (Jalife).
REFERENCES
- 1.Ather S., Respress J. L., Li N., and Wehrens X. H., “Alterations in ryanodine receptors and related proteins in heart failure,” Biochim. Biophys. Acta, Mol. Basis Dis. 1832(12), 2425–2431 (2013). 10.1016/j.bbadis.2013.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldhaber J. I. and Philipson K. D., “Cardiac sodium-calcium exchange and efficient excitation-contraction coupling: Implications for heart disease,” in Sodium Calcium Exchange: A Growing Spectrum of Pathophysiological Implications (Springer, 2013), pp. 355-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu L., Eu J. P., Meissner G., and Stamler J. S., “Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation,” Science 279(5348), 234-237 (1998). 10.1126/science.279.5348.234 [DOI] [PubMed] [Google Scholar]
- 4.Adachi T., Weisbrod R. M., Pimentel D. R., Ying J., Sharov V. S., Schöneich C., and Cohen R. A., “S-glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide,” Nat. Med. 10(11), 1200-1207 (2004). 10.1038/nm1119 [DOI] [PubMed] [Google Scholar]
- 5.Lancel S., Zhang J., Evangelista A., Trucillo M. P., Tong X., Siwik D. A., Cohen R. A., and Colucci W. S., “Nitroxyl activates SERCA in cardiac myocytes via glutathiolation of cysteine 674,” Circ. Res. 104(6), 720-723 (2009). 10.1161/circresaha.108.188441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinberg S. F., “Oxidative stress and sarcomeric proteins,” Circ. Res. 112(2), 393-405 (2013). 10.1161/circresaha.111.300496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brady A. J., “Mechanical properties of isolated cardiac myocytes,” Physiol. Rev. 71(2), 413-428 (1991). [DOI] [PubMed] [Google Scholar]
- 8.Le Guennec J.-Y., Peineau N., Argibay J., Mongo K., and Garnier D., “A new method of attachment of isolated mammalian ventricular myocytes for tension recording: Length dependence of passive and active tension,” J. Mol. Cell. Cardiol. 22(10), 1083-1093 (1990). 10.1016/0022-2828(90)90072-a [DOI] [PubMed] [Google Scholar]
- 9.Yasuda S.-I., Sugiura S., Kobayakawa N., Fujita H., Yamashita H., Katoh K., Saeki Y., Kaneko H., Suda Y., and Nagai R., “A novel method to study contraction characteristics of a single cardiac myocyte using carbon fibers,” Am. J. Physiol.: Heart Circ. Physiol. 281(3), H1442-H1446 (2001). [DOI] [PubMed] [Google Scholar]
- 10.Iribe G., Helmes M., and Kohl P., “Force-length relations in isolated intact cardiomyocytes subjected to dynamic changes in mechanical load,” Am. J. Physiol.: Heart Circ. Physiol. 292(3), H1487-H1497 (2007). 10.1152/ajpheart.00909.2006 [DOI] [PubMed] [Google Scholar]
- 11.King N. M., Methawasin M., Nedrud J., Harrell N., Chung C. S., Helmes M., and Granzier H., “Mouse intact cardiac myocyte mechanics: Cross-bridge and titin-based stress in unactivated cells,” J. Gen. Physiol. 137(1), 81-91 (2011). 10.1085/jgp.201010499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim C. C., Yang H., Yang M., Wang C.-K., Shi J., Berg E. A., Pimentel D. R., Gwathmey J. K., Hajjar R. J., and Helmes M., “A novel mutant cardiac troponin C disrupts molecular motions critical for calcium binding affinity and cardiomyocyte contractility,” Biophys. J. 94(9), 3577-3589 (2008). 10.1529/biophysj.107.112896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harmer A., Abi-Gerges N., Morton M., Pullen G., Valentin J., and Pollard C., “Validation of an in vitro contractility assay using canine ventricular myocytes,” Toxicol. Appl. Pharmacol. 260(2), 162-172 (2012). 10.1016/j.taap.2012.02.007 [DOI] [PubMed] [Google Scholar]
- 14.Ren J. and Wold L. E., “Measurement of cardiac mechanical function in isolated ventricular myocytes from rats and mice by computerized video-based imaging,” Biol. Proced. Online 3(1), 43–53 (2001). 10.1251/bpo22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miragoli M., Moshkov A., Novak P., Shevchuk A., Nikolaev V. O., El-Hamamsy I., Potter C. M., Wright P., Kadir S. S. A., and Lyon A. R., “Scanning ion conductance microscopy: A convergent high-resolution technology for multi-parametric analysis of living cardiovascular cells,” J. R. Soc., Interface 8(60), 913-925 (2011). 10.1098/rsif.2010.0597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herron T. J., Lee P., and Jalife J., “Optical imaging of voltage and calcium in cardiac cells & tissues,” Circ. Res. 110(4), 609-623 (2012). 10.1161/circresaha.111.247494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J., Sun N., Bruce M. A., Wu J. C., and Butte M. J., “Atomic force mechanobiology of pluripotent stem cell-derived cardiomyocytes,” PloS one 7(5), e37559 (2012). 10.1371/journal.pone.0037559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang W.-T., Yu D., Lai Y.-C., Lin K.-Y., and Liau I., “Characterization of the mechanodynamic response of cardiomyocytes with atomic force microscopy,” Anal. Chem. 85(3), 1395-1400 (2013). 10.1021/ac3022532 [DOI] [PubMed] [Google Scholar]
- 19.Domke J., Parak W. J., George M., Gaub H. E., and Radmacher M., “Mapping the mechanical pulse of single cardiomyocytes with the atomic force microscope,” Eur. Biophys. J. 28(3), 179-186 (1999). 10.1007/s002490050198 [DOI] [PubMed] [Google Scholar]
- 20.Gaitas A., Malhotra R., and Pienta K., “A method to measure cellular adhesion utilizing a polymer micro-cantilever,” Appl. Phys. Lett. 103(12), 123702 (2013). 10.1063/1.4821946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarid D., Coratger R., Ajustron F., and Beauvillain J., “Scanning force microscopy-with applications to electric, magnetic and atomic forces,” Microsc., Microanal., Microstruct. 2(6), 649 (1991). 10.1051/mmm:0199100206064900 [DOI] [Google Scholar]
- 22.Thaysen J., Yalcinkaya A. D., Vestergaard R., Jensen S., Mortensen M., Vettiger P., and Menon A., “SU-8 based piezoresistive mechanical sensor,” Micro Electro Mechanical Systems, 2002. The Fifteenth IEEE International Conference on (IEEE, 2002), pp. 320-323. [Google Scholar]
- 23.Thaysen J., “Cantilever for bio-chemical sensing integrated in a microliquid handling system,” Ph.D. thesis (Technical University of Denmark, 2001). [Google Scholar]
- 24.Gaitas A., Li T., and Zhu W., “A probe with ultrathin film deflection sensor for scanning probe microscopy and material characterization,” Sens. Actuators, A 168(2), 229-232 (2011). 10.1016/j.sna.2011.04.023 [DOI] [Google Scholar]
- 25.Hou L., Deo M., Furspan P., Pandit S. V., Mironov S., Auerbach D. S., Gong Q., Zhou Z., Berenfeld O., and Jalife J., “A major role for HERG in determining frequency of reentry in neonatal rat ventricular myocyte monolayer,” Circ. Res. 107(12), 1503-1511 (2010). 10.1161/circresaha.110.232470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bizy A., Guerrero-Serna G., Hu B., Ponce-Balbuena D., Willis B. C., Zarzoso M., Ramirez R. J., Sener M. F., Mundada L. V., and Klos M., “Myosin light chain 2-based selection of human iPSC-derived early ventricular cardiac myocytes,” Stem Cell Res. 11(3), 1335-1347 (2013). 10.1016/j.scr.2013.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J., Klos M., Wilson G. F., Herman A. M., Lian X., Raval K. K., Barron M. R., Hou L., Soerens A. G., and Yu J., “Extracellular matrix promotes highly efficient cardiac differentiation of human pluripotent stem cells the matrix sandwich method,” Circ. Res. 111(9), 1125-1136 (2012). 10.1161/circresaha.112.273144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Um22-2 grants.nih.gov/stem_cells/registry/current.htm?id=587.
- 29.UM38-2 PGD; disease-specific mutation, grants.nih.gov/stem_cells/registry/current.htm?id=534.
- 30.Balaban N. Q., Schwarz U. S., Riveline D., Goichberg P., Tzur G., Sabanay I., Mahalu D., Safran S., Bershadsky A., and Addadi L., “Force and focal adhesion assembly: A close relationship studied using elastic micropatterned substrates,” Nat. Cell Biol. 3(5), 466-472 (2001). 10.1038/35074532 [DOI] [PubMed] [Google Scholar]
- 31.Shen X., Tan Z., Zhong X., Tian Y., Wang X., Yu B., Ramirez-Correa G., Murphy A., Gabrielson K., and Paolocci N., “Endocardial endothelium is a key determinant of force-frequency relationship in rat ventricular myocardium,” J. Appl. Physiol. 115(3), 383-393 (2013). 10.1152/japplphysiol.01415.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Witayavanitkul N., Mou Y. A., Kuster D. W., Khairallah R. J., Sarkey J., Govindan S., Chen X., Ge Y., Rajan S., and Wieczorek D. F., “Myocardial infarction-induced n-terminal fragment of cardiac myosin-binding protein C (cMyBP-C) impairs myofilament function in human myocardium,” J. Biol. Chem. 289(13), 8818-8827 (2014). 10.1074/jbc.m113.541128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Banyasz T., Lozinskiy I., Payne C. E., Edelmann S., Norton B., Chen B., Chen-Izu Y., Izu L. T., and Balke C. W., “Transformation of adult rat cardiac myocytes in primary culture,” Exp. Physiol. 93(3), 370-382 (2008). 10.1113/expphysiol.2007.040659 [DOI] [PubMed] [Google Scholar]
- 34.Maltsev V., Sabbah H., Tanimura M., Lesch M., Goldstein S., and Undrovinas A., “Relationship between action potential, contraction-relaxation pattern, and intracellular Ca2+ transient in cardiomyocytes of dogs with chronic heart failure,” Cell. Mol. Life Sci. 54(6), 597-605 (1998). 10.1007/s000180050187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delbridge L. and Roos K. P., “Optical methods to evaluate the contractile function of unloaded isolated cardiac myocytes,” J. Mol. Cell. Cardiol. 29(1), 11-25 (1997). 10.1006/jmcc.1996.0247 [DOI] [PubMed] [Google Scholar]
- 36.Mitcheson J. S., Hancox J. C., and Levi A. J., “Cultured adult cardiac myocytes future applications, culture methods, morphological and electrophysiological properties,” Cardiovasc. Res. 39(2), 280-300 (1998). 10.1016/s0008-6363(98)00128-x [DOI] [PubMed] [Google Scholar]