Abstract
Wide surgical resection is the most effective treatment for the vast majority of chest wall tumors. This study evaluated the clinical success of chest wall reconstruction using a Prolene mesh and bone cement prosthetic sandwich. The records of all patients undergoing chest wall resection and reconstruction were reviewed. Surgical indications, the location and size of the chest wall defect, diaphragm resection, pulmonary performance, postoperative complications, and survival of each patient were recorded. From 1998 to 2008, 43 patients (27 male, 16 female; mean age of 48 years) underwent surgery in our department to treat malignant chest wall tumors: chondrosarcoma (23), osteosarcoma (8), spindle cell sarcoma (6), Ewing's sarcoma (2), and others (4). Nine sternectomies and 34 antero-lateral and postero-lateral chest wall resections were performed. Postoperatively, nine patients experienced respiratory complications, and one patient died because of respiratory failure. The overall 4-year survival rate was 60 %. Chest wall reconstruction using a Prolene mesh and bone cement prosthetic sandwich is a safe and effective surgical procedure for major chest wall defects.
Keywords: Chest wall reconstruction, Malignant chest wall tumors, Bone cement
Introduction
Malignant chest wall tumors are relatively rare, representing roughly 5 % of all thoracic neoplasms and 1 to 2 % of all primary tumors [1]. Martini et al. [2] reported 317 patients with chest wall lesions treated at their facility, and 83 patients (26 %) had primary chest wall tumors, 163 patients (51 %) had primary lung or breast cancer, and 71 patients (22 %) had metastatic lesions. Pairolero reported 100 consecutive patients undergoing chest wall resection; 44 % of all tumors were primary neoplasms [3].
Wide surgical resection is the most effective treatment for the majority of chest wall tumors. Improvements in reconstructive techniques and care of the perioperative patients have led to lesser morbidity and mortality rates for chest wall resection [4]. Keys to successful management include accurate diagnosis, wide surgical resection, and appropriate reconstruction of large chest wall defects [4]. Reconstruction of chest wall defects involves consideration of many factors [2]. While the size and location of the defect are important, medical history and local conditions of the wound may drastically alter a reconstruction. If full-thickness reconstruction is required, both the structural stability of the thorax and the soft tissue coverage must be considered [2]. When structural integrity is necessary to prevent chest wall collapse, silicone, Teflon, acrylic materials, or a Prolene mesh and bone cement sandwich have been utilized [4–6]. Our clinic commonly uses the bone cement sandwich with Prolene or Marlex mesh. Daigeler and associates [7] have observed that pulmonary function was only moderately reduced and was not significantly affected by the size or location of the resection. They concluded that reconstruction of the thoracic wall provides excellent stability to maintain pulmonary function; however, postoperative pain and sensation disorders are considerable [7]. A variety of synthetic materials can be used to reconstruct a chest wall defect [1–4, 6].
We intend to document the role of the Prolene mesh and bone cement sandwich prosthesis for chest wall reconstruction in this clinic by reviewing 10 years of medical cases, considering the pathological findings, surgical treatment, and survival rates.
Patients and Method
A retrospective review of patients with a diagnosis of primary or metastatic chest wall tumors and surgical treatment in our department between 1998 and 2008 was conducted. All charts and radiological images had been reviewed before surgery by a consulting team of thoracic surgeons, plastic surgeons, anesthesiologists, and other specialists. In the diagnosis work-up, CT scan was used in patients with pulmonary invasion and MRI indicated in patients with vascular or soft tissue invasion. In all cases, tissue diagnosis was obtained by core needle aspiration or incisional biopsy. In all cases, pulmonary function tests were measured prior to surgery. All surgical procedures were performed under general anesthesia. A double-lumen endobronchial tube was inserted if lung involvement was expected. A thoracic surgical team performed the en bloc resection of the chest wall. Resection was performed with at least a 2-cm-free margin. Extent of resection was considered sufficient when absence of neoplastic involvement was demonstrated at frozen sections of the margins. Reconstruction for stabilization of the chest wall utilized a sandwich of two layers of Prolene mesh with bone cement prosthesis placement. Histology was not affective in methods and reconstruction was performed for all patients. In a survey of seroma in the site of insertion, sonography and CT scan were done. At discharge, each case was reviewed for wound healing, and the patient was referred for oncological therapy or other follow-up treatment. Data including gender, age at the time of surgery, surgical procedures performed, anatomical defects, reconstruction techniques, total length of stay in the ICU, in-hospital postoperative morbidity, in-hospital mortality, and survival history were collected from the charts and entered into a computerized database. Wound healing was assessed, and clinical and pain measurements were done with visual analog score. Follow-up data were obtained by telephone calls directly to the patients and information provided by physicians at the clinic. Statistical analysis of the data was carried out with SPSS 17. Descriptive statistics were mean and standard deviation for the quantitative variables and frequencies for the qualitative variables.
Surgical Technique
After completion of the resection, the skeletal chest wall defect was closed by use of bone cement–Prolene prosthesis. After resection of the chest wall, the vertical and horizontal size of the defect was measured (Fig. 1). Bone cement was mixed with solution in a receiver out of the body during hardening to prevent heat injury to adjacent structures. The bone cement was then distributed on the two-layer Prolene mesh and modeled to the resection margins of the chest wall defect. The size of defect or resection was measured with a sterile ruler. The edge of the two-layer Prolene mesh was sutured with nonresorbable interrupted sutures under tension into the chest wall defect (Fig. 2). The underlying lung was ventilated with positive end-expiratory pressure and normal tidal volumes to restore the natural shape of the chest wall. Soft tissue coverage was then performed with soft tissue, and the cosmetic appearance of the chest wall reconstruction with a two-layer Prolene mesh is shown in Fig. 3. Prophylactic antibiotics were given for 48 h postoperatively. All sutures were cut in 10–12 days.
Fig. 1.
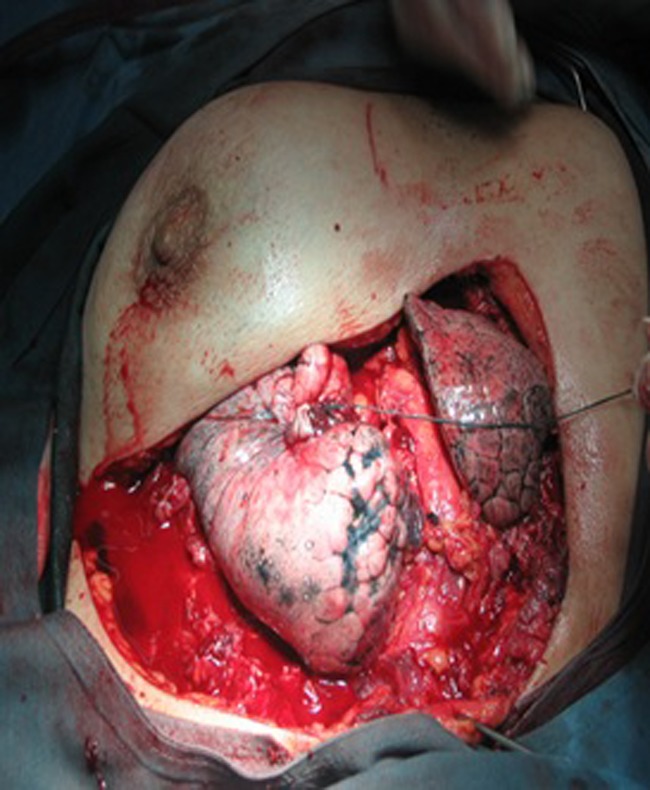
Vertical and transverse measurements of the defect were performed with a sterilized ruler in a 45-year-old female with chest wall cancer; extensive resections of four ribs and muscle of the chest wall were done
Fig. 2.
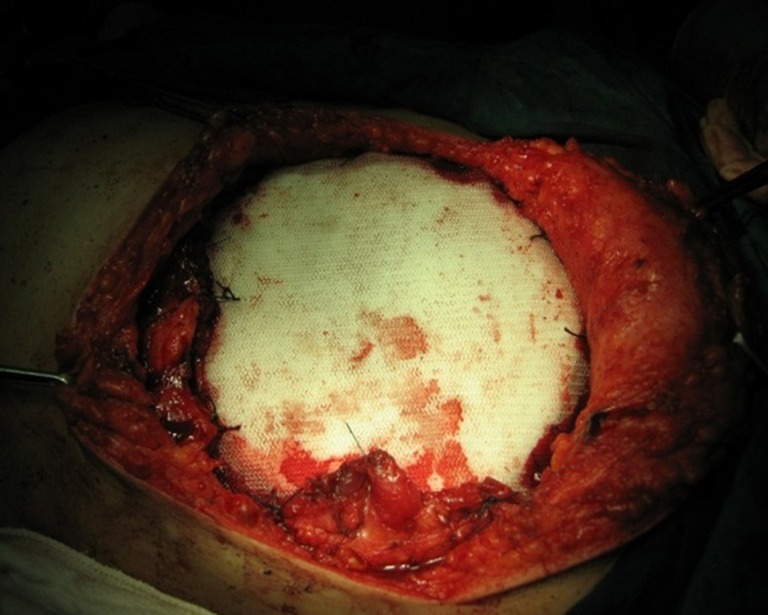
Reconstruction of the chest wall with bone cement and two layers of Prolene mesh was performed in a 45-year-old female with chest wall cancer; four ribs, the latissimus dorsi and stratus muscles were resected
Fig. 3.
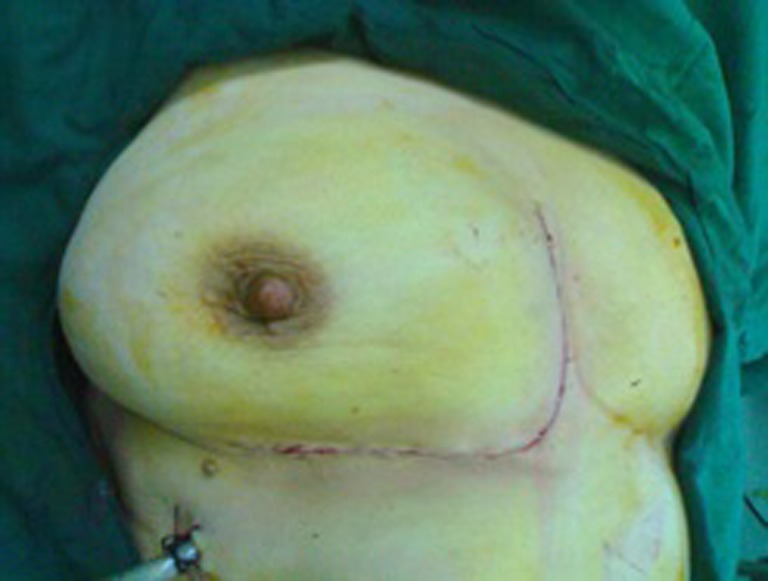
The postoperative cosmetic appearance of the chest wall reconstruction of the two-layer Prolene mesh in a 45-year-old female with chest wall cancer was good. The sandwich of bone cement and two layers of Prolene mesh was fixed to the chest wall defect
Results
From 1998 to 2008, 43 patients with primary and metastatic chest wall tumors were diagnosed and treated [27 males (62.8 %) and 16 females (37.2 %)]. The age of the patients ranged from 12 to 75 years, with a mean age of 48 years. Types of sarcomas diagnosed included: chondrosarcoma (n = 23), osteosarcoma (n = 8), spindle cell sarcoma (n = 6), Ewing's sarcoma (n = 2), and malignant fibrous histiocytoma (n = 2). One patient had a fibrosarcoma secondary to local radiotherapy for mediastinal lymphoma, and another case had a chest wall metastasis from adenocarcinoma of the colon. The histological types are depicted in Table 1.
Table 1.
Histopathological findings and method of diagnosis
| Histological type | Gender | Method of diagnosis | ||
|---|---|---|---|---|
| Male | Female | Core needle biopsy | Open biopsy | |
| Chondrosarcoma | 16 (69.5 %) | 7 (30.5 %) | 4 (17.4 %) | 19 (82.6 %) |
| Osteosarcoma | 6 (75 %) | 2 (25 %) | 0 | 8 (100 %) |
| Ewing sarcoma | 2 (100 %) | 0 | 0 | 2 (100 %) |
| Radio-induced sarcoma | 0 | 1 100 %) | 0 | 1 (100 %) |
| Metastasis adenocarcinoma carcinoma of colon | 1 (100 %) | 0 | 0 | 1 (100 %) |
| Spindle cell sarcoma | 0 | 6 (100 %) | 0 | 6 (100 %) |
| Malignant fibrous histiocytoma | 2 (100 %) | 0 | 2 (100 %) | 0 |
The size of anterior defects was 5–15 cm and the posterior, 10 cm. We performed 12 postero-lateral chest wall resections (27.9 %) with a mean number of 2.4 ribs resected (range, 2–5), 22 antero-lateral chest wall resections (51.2 %) with a mean number of 3.5 ribs resected (range, 2–6), and 9 sternectomies (21.9 %). Partial sternectomy was performed in five cases and total sternectomy in four. In four cases with lesions at the middle third and upper third of the sternum, resection of the body with the manubrium and the xiphoid process was performed; in two cases with upper third lesion, the medial ends of the clavicles were resected, and in two patients, the medial ends of the clavicles were preserved. Chest wall resections were extended to the lung in two patients and the diaphragm in three patients. In all cases of chest wall reconstruction, a sandwich of two layers of Prolene mesh with bone cement was used. Thirty-five patients (81.4 %) were extubated at the end of surgery; eight patients(18.6 %) were referred to ICU with intubation—seven patients (three with one sternectomy and four with extensive antero-lateral chest wall resections) were extubated in the intensive care unit within the first 48 h postoperatively, and one case required prolonged mechanical ventilation. Only one patient died postoperatively.
The measurement of pulmonary function showed that 30 % of patients had a restrictive pattern, 35 % had an obstructive pattern, and 35 % had both patterns. However, there was any impact of pulmonary function test in our decision making. The most common postoperative complication was atelectasia and seroma (Table 2). In pain measurement, the visual analog score was in the range of 6 to 8. Mean hospital stay was 12 days (range, 7 to 30 days). Information about survival was obtained for all the patients. Three patients died because of distant metastasis at 12, 22, and 30 months. Tracheal erosion and perforation by prosthesis occurred in one 45-year-old man with chondrosarcoma and partial sternectomy, after three postoperative years. Tracheal repair was performed, and the prosthesis around the trachea was resected. In the osteosarcoma group, all patients received chemotherapy postoperatively; four patients developed multiorgan metastasis within 2 years postsurgery and died of that disorder. All patients with Ewing's sarcoma were treated with induction chemotherapy and surgery. Two patients in this group died: a 12-year-old patient died after 18 months, and a 14-year-old patient died after 10 months of surgery from metastases. In a follow-up period (range, 24–60 months), we had a local recurrence in two patients with high-grade chondrosarcoma 12 and 36 months after surgery, and both patients underwent a new resection and radiotherapy. All cases of chondrosarcoma were alive at the time of review (range, 24–60 months). Patients with chondrosarcoma had better survival rates than patients with other chest wall tumors. In the chondrosarcoma group (n = 23), 90 % had a 5-year survival rate whereas the 5-year survival rate for patients with primary osteosarcoma (n = 8) was 30 %. In the osteosarcoma group, all patients received a combined treatment with induction (n = 3) and/or adjuvant (n = 7) chemotherapy. All patients with Ewing's sarcoma were treated with induction chemotherapy and surgery.
Table 2.
Surgical complications
| Atelectasia | 4 (9.3 %) |
| Pneumonia | 2 (4.6 %) |
| Acute respiratory failure | 2 (4.6 %) |
| Atrial fibrillation | 2 (4.6 %) |
| Wound infection | 3 (7 %) |
| Seroma | 4 (9.3 %) |
| Hematoma | 2 (4.6 %) |
| Prolonged air leak | 1 (2.3 %) |
Discussion
Malignant primary chest wall tumors are relatively uncommon, representing roughly 5 % of all thoracic neoplasms and 1 to 2 % of all primary tumors [1]. Mansour et al. [6] reported 27 % of all chest wall resections were for primary chest wall tumors; Martini [2] reported 26 % (83 of 317 chest wall resections) were primary tumors; however, Pairolero cited that among 100 consecutive patients undergoing chest wall resection, 44 % of them were primary neoplasms [3].
The most common origin of primary chest wall tumors is bone or cartilage, as in our series [8, 9]. The ribs are a more frequent location of primary chest wall tumors than the sternum [8, 9], as we confirmed in our study. Mansour et al. reported 200 chest wall resections; 75 patients (38 %) had lung cancer whereas 53 (27 %) had sarcoma and 43 (22 %) had breast cancer [6]. In most studies, sarcomas were the most frequent metastatic tumors.
Chest wall resection and reconstruction surgery is a complicated treatment modality for thoracic surgeons in part due to significant morbidity and mortality factors. With improvements in thoracic surgery and reconstruction techniques, anesthesiology, ICU care, and antibiotics, extensive chest wall resections now have more acceptable morbidity and mortality rates [1–4, 6–8]. The importance of radical surgery in primary chest wall tumors is well documented [10, 11]. After wide resection of the chest wall, reconstruction plays a crucial role in determining postoperative morbidity and mortality [11–13]. Defects smaller than 5 cm in size in any location and defects up to 10 cm in size located posteriorly do not generally require reconstruction, while larger defects and most defects in the anterior region do require reconstruction. The most commonly used materials for a non-rigid prosthetic are Prolene mesh, Marlex mesh, and PTFE [8, 11, 14]. Stability and integrity are necessary after resection to prevent chest wall collapse, and various materials, including bone cement sandwich, silicone, Teflon, or acrylic materials have been used [15]. During respiration, uncoordinated motion of the chest wall after major resection is seen in a flail chest [14] and is associated with pulmonary insufficiency. Rigid prosthetic devices prevent paradoxical motion of the chest wall. Nagayasu and colleagues performed chest wall reconstruction using a 2-mm DualMesh in 11 patients and demonstrated that chest wall reconstruction using DualMesh had acceptable durability and biocompatibility, even after long-term follow-up [16].
In our clinic, we have commonly used a non-rigid device using a sandwich of Prolene or Marlex mesh with bone cement. Others [3, 4, 6, 13] have used this same approach, and Daigeler and associates [7] have observed that pulmonary function was only moderately reduced and was not significantly affected by the size or location of the resection. They concluded that reconstruction of the thoracic wall with this device provides excellent stability to maintain pulmonary function, but with postoperative pain and sensation disorders [11–13]. As in our study, some patients complained of pain after reconstruction.
Complications after chest wall resection are common and range from 46 to 69 % [8, 9]. Respiratory complications, including pneumonia, acute respiratory distress syndrome, and atelectasia, are the most common problems and have been reported to be 24 % of reported cases [1–4, 6–8]. In a report by Michael J. Weyant [12], a total of 114 complications occurred to 87 patients (33.2 %) during the postoperative period. The most common complications were respiratory, occurring in 29 patients (11 %), and included respiratory failure in eight patients (3.1 %), pneumonitis in five patients (1.9 %), pneumonia in seven patients (2.7 %), atelectasia requiring bronchoscopy in eight patients (3.1 %), and aspiration in one patient (0.4 %). Respiratory complications in our study occurred in nine patients.
Wound complications such as infection, dehiscence, and hematoma are reported to occur in 8 to 20 % of the patient group [8, 9, 17]. In our study, wound complications occurred in 21 % of the patients. Seroma was the most common complication after dual-mesh reconstruction of the bony chest wall [16]. Deschamps et al. reported 64 patients (32.5 %) with reconstruction using a polypropylene mesh and 133 patients (67.5 %) with reconstruction using a polytetrafluoroethylene. Eight deaths (operative mortality rate, 4.1 %) occurred, and 41 patients (46.2 %) experienced complications such as seromas in 14 patients (7.1 %). In addition, wound infections occurred in nine patients (4.6 %; five patients with polypropylene mesh and four patients with polytetrafluoroethylene) [18]. Weyant reported that wound complications occurred in 19 patients (7 %) including wound dehiscence in three patients (1.1 %) and flap hematoma requiring reoperation in three patients. Wound infections occurred in 13 patients (4.7 %); of that group, the removal of the prosthesis was required in eight patients (3.8 %) [12]. In our study, wound complications occurred in nine patients, and in one case, the prosthesis was removed.
In a report by Gonfiotti [19], stability was obtained by a prosthetic material, rigid and non-rigid, and a muscular flap and a polytetrafluoroethylene patch as non-rigid material. In this report, no major septic and respiratory complications were reported, but two patients developed a seroma and were treated conservatively without consequences [19]. Also, postoperative hospital stay averaged 8.6 days (range, 5–14 days), and local recurrence was seen in two patients (5 %) at 15 and 26 months at which time surgery was performed to remove a desmoid tumor and a high-grade chondrosarcoma, respectively [19]. In our own study, local recurrence occurred in two patients with high-grade chondrosarcoma, and both patients underwent a new resection.
Regarding mortality, Weyant reported ten deaths (3.8 %). Seven of the ten deaths occurred due to respiratory complications [12]. In our study, two patients died as a result of respiratory and septic complications.
For chest wall tumors, 5-year survival metrics range from 46 to 66 % with a wide difference among the various histological types [11, 20]. Alessandro Gonfiotti reported 5-year survival was 61 % with a mean follow-up time of 48 months (range, 24–60 months). The overall 5- and 10-year survival in the malignant chest wall tumor group was 61 and 47 %, respectively [19]. In our study, the 5-year survival rate for the chondrosarcoma group (n = 23) was 90 %, while the 5-year survival rate for patients with primary osteosarcoma (n = 8) was 30 %.
Based on our study and the review of similar research, we conclude that in the treatment of malignant chest wall tumors, wide resection with tumor-free margins is necessary for long-term survival. The numerous medical advances and the availability of various prosthetic materials for chest wall reconstruction, including the Prolene mesh and bone cement sandwich, allow successful reconstruction to fix the chest wall.
References
- 1.Kroll S, Walsh G, Ryan B, King R. Risks and benefits of using Marlex mesh in chest wall reconstruction. Ann Plast Surg. 1993;31:303–306. doi: 10.1097/00000637-199310000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Martini N, McCormack P, Bains M. Chest wall tumors: clinical results of treatment. Vol 2. Philadelphia: WB Saunders; 1987. p. 285. [Google Scholar]
- 3.Pairolero P, Arnold P. Chest wall tumors. Experience with 100 consecutive patients. J Thorac Cardiovasc Surg. 1985;90(3):367–372. [PubMed] [Google Scholar]
- 4.Kilic D, Gungor A, Kavukcu S, Okten I, Ozdemir N, Akal M, et al. Comparison of mersilene mesh-methyl metacrylate sandwich and polytetrafluoroethylene grafts for chest wall reconstruction. J Invest Surg. 2006;19(6):353–360. doi: 10.1080/08941930600985694. [DOI] [PubMed] [Google Scholar]
- 5.Eschapasse H, Gaillard J, Fournial G, Berthoumieu F, Henry E, Hornus E, et al. Use of acrylic prosthesis for the repair of large defects of the chest wall (author's transl) Acta Chir Belg. 1977;76(3):281–285. [PubMed] [Google Scholar]
- 6.Mansour K, Thourani V, Losken A, Reeves J, Miller JJ, Carlson G, et al. Chest wall resections and reconstruction: a 25-year experience. Ann Thorac Surg. 2002;73(6):1720–1726. doi: 10.1016/S0003-4975(02)03527-0. [DOI] [PubMed] [Google Scholar]
- 7.Daigeler A, Druecke D, Hakimi M, Duchna H, Geortz O, Homann H, et al. Reconstruction of the thoracic wall-long-term follow-up including pulmonary function tests. Langenbecks Arch Surg. 2009;394(4):705–715. doi: 10.1007/s00423-008-0400-9. [DOI] [PubMed] [Google Scholar]
- 8.Hsu P, Hsu H, Lee H, Hsieh C, Wu Y, Wang L, et al. Management of primary chest wall tumors: 14 years' clinical experience. J Chin Med Assoc. 2006;69(8):377–382. doi: 10.1016/S1726-4901(09)70276-X. [DOI] [PubMed] [Google Scholar]
- 9.Burt M. Primary malignant tumors of the chest wall. The Memorial Sloan-Kettering Cancer Center experience. Chest Surg Clin N Am. 1994;4(1):137–154. [PubMed] [Google Scholar]
- 10.McKenna RR, Mountain C, McMurtrey M, Larson D, Stiles Q. Current techniques for chest wall reconstruction: expanded possibilities for treatment. Ann Thorac Surg. 1988;46(5):508–512. doi: 10.1016/S0003-4975(10)64686-3. [DOI] [PubMed] [Google Scholar]
- 11.Incarbone M, Pastorino U. Surgical treatment of chest wall tumors. World J Surg. 2001;25(2):218–230. doi: 10.1007/s002680020022. [DOI] [PubMed] [Google Scholar]
- 12.Weyant M, Bains M, Venkatraman E, Downey R, Park B, Flores R, et al. Results of chest wall resection and reconstruction with and without rigid prosthesis. Ann Thorac Surg. 2006;81(1):279–285. doi: 10.1016/j.athoracsur.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Lardinois D, Muller M, Furrer M, Banic A, Gugger M, Krueger T, et al. Functional assessment of chest wall integrity after methylmethacrylate reconstruction. Ann Thorac Surg. 2000;69(3):919–923. doi: 10.1016/S0003-4975(99)01422-8. [DOI] [PubMed] [Google Scholar]
- 14.Athanassiadi K, Kalavrouziotis G, Rondogianni D, Loutsidis A, Hatzimichalis A, Bellenis I. Primary chest wall tumors: early and long-term results of surgical treatment. Eur J Cardiothorac Surg. 2001;19(5):589–593. doi: 10.1016/S1010-7940(01)00655-8. [DOI] [PubMed] [Google Scholar]
- 15.Miller D, Mansour K. Chest wall reconstruction. 7. Philadelphia: Lippincott Williams & Wilkins; 2010. p. 679. [Google Scholar]
- 16.Nagayasu T, Yamasaki N, Tagawa T, Tsuchiya T, Miyazaki T, Nanashima A, et al. Long-term results of chest wall reconstruction with DualMesh. Interact Cardiovasc Thorac Surg. 2010;11(5):581–584. doi: 10.1510/icvts.2010.242040. [DOI] [PubMed] [Google Scholar]
- 17.Chapelier A, Macchiarini P, Rietjens M, Lenot B, Margulis A, Petit J, et al. Chest wall reconstruction following resection of large primary malignant tumors. Eur J Cardiothorac Surg. 1994;8(7):351–356. doi: 10.1016/1010-7940(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 18.Deschamps C, Tirnaksiz BM, Darbandi R, Trastek VF, Allen MS, Miller DL, et al. Early and long-term results of prosthetic chest wall reconstruction. J Thorac Cardiovasc Surg. 1999;177(3):588–592. doi: 10.1016/S0022-5223(99)70339-9. [DOI] [PubMed] [Google Scholar]
- 19.Gonfiotti A, Santini P, Campanacci D, Innocenti M, Ferrarello S, Caldarella A, et al. Malignant primary chest-wall tumours: techniques of reconstruction and survival. Eur J Cardiothorac Surg. 2010;38(1):39–45. doi: 10.1016/j.ejcts.2009.12.046. [DOI] [PubMed] [Google Scholar]
- 20.Warzelhan J, Stoelben E, Imdahl A, Hasse J. Results in surgery for primary and metastatic chest wall tumors. Eur J Cardiothorac Surg. 2001;18(5):584–588. doi: 10.1016/S1010-7940(01)00638-8. [DOI] [PubMed] [Google Scholar]


