Abstract
Autism spectrum disorders (ASDs) are highly heritable and characterised by deficits in social interaction and communication, as well as restricted and repetitive behaviours. Although a number of highly penetrant ASD gene variants have been identified, there is growing evidence to support a causal role for combinatorial effects arising from the contributions of multiple loci. By examining synaptic and circadian neurological phenotypes resulting from the dosage variants of unique human:fly orthologues in Drosophila, we observe numerous synergistic interactions between pairs of informatically-identified candidate genes whose orthologues are jointly affected by large de novo copy number variants (CNVs). These CNVs were found in the genomes of individuals with autism, including a patient carrying a 22q11.2 deletion. We first demonstrate that dosage alterations of the unique Drosophila orthologues of candidate genes from de novo CNVs that harbour only a single candidate gene display neurological defects similar to those previously reported in Drosophila models of ASD-associated variants. We then considered pairwise dosage changes within the set of orthologues of candidate genes that were affected by the same single human de novo CNV. For three of four CNVs with complete orthologous relationships, we observed significant synergistic effects following the simultaneous dosage change of gene pairs drawn from a single CNV. The phenotypic variation observed at the Drosophila synapse that results from these interacting genetic variants supports a concordant phenotypic outcome across all interacting gene pairs following the direction of human gene copy number change. We observe both specificity and transitivity between interactors, both within and between CNV candidate gene sets, supporting shared and distinct genetic aetiologies. We then show that different interactions affect divergent synaptic processes, demonstrating distinct molecular aetiologies. Our study illustrates mechanisms through which synergistic effects resulting from large structural variation can contribute to human disease.
Author Summary
Autism spectrum disorders (ASDs), which are characterised by poor social interaction and repetitive behaviours, are in part caused by genetic variation. A number of genes that vary in copy number in ASD patients have been identified, many of which were known to function at the neuronal synapse. We theorised that in some cases the dosage change of multiple genes simultaneously, rather than singularly, may lead to faulty neuronal development, and contribute to ASD. To test this, we asked whether alterations in these candidate genes would cause neuronal synapse and sleep/rest changes using the fruit fly Drosophila, and validated this model using single-gene models. We considered the simultaneous change of pairs of genes that were jointly affected by a large human copy number variant (CNVs), which are structural changes in the genome. In three of four CNVs, mutations in subsets of genes synergistically interacted to cause neuronal changes comparable to the single gene candidates. We also observed that the changes in synapse size followed the direction of the human gene copy number change. Finally, we show that different interactions affect the development of the synapse through different mechanisms, allowing us to identify distinct molecular alterations that illuminate the etiological heterogeneity of ASD.
Introduction
Autism spectrum disorders (ASDs) comprise a large group of complex neurodevelopmental diseases that are influenced by genetic and environmental factors [1]. They are characterised by altered cognitive function including poor social and verbal interaction capability, and repetitive and stereotyped verbal and non-verbal behaviours [1]. ASDs are highly heritable (∼90% monozygotic twin studies); however, the genetic cause has been identified in less than 30% of cases, while the increase in risk between di-zygotic twins is comparable to that of first degree siblings [2], suggesting that ASD-causative alleles are likely to be both numerous and rare [3].
Recently, large numbers of autistic individuals, with unaffected family members, have been shown to possess de novo copy number variants (CNVs) [2,4–6]. In addition, many rare variant studies have identified pathways or processes that are commonly contributed to by significant proportions of those genes found to be disrupted [7–9]. Two additional striking findings from a recent study into the genes affected by 192 de novo CNVs identified in individuals with ASD have also been identified [9]. Firstly, many of these CNVs affect genes that appear to operate in the same functional pathway/network and, secondly, a significant proportion of individual CNVs (33%) simultaneously affect multiple genes whose proteins interact within that functional pathway [9]. This raises the possibility that it is the combined effect of these genes’ copy number change that causally contributes to these patients’ autistic phenotypes. Combinatorial effects have also been observed beyond de novo variants, where an increased risk of ASD resulting from multiple distinct and inherited CNVs has been reported [10]. However, while the contribution from combinatorial effects of genetic variation has been proposed by computational and statistical analyses, these hypotheses have yet to be validated in vivo. Here, we use Drosophila as an in vivo system to examine genetic interactions that may contribute to neurological phenotypes like ASD.
Understanding the interactions between genes implicated in autism requires a tractable, high-throughput in vivo system. This is particularly important as patient genotypes possess variants affecting many genes, thus generating an exponential number of potential interactions. To this end, the fruit fly Drosophila melanogaster offers a versatile tool in which neurodevelopment and behaviour can be studied in isogenised genetic backgrounds, and under controlled environmental conditions [11–13]. To detect single and combinatorial gene dosage effects in the fly, we examine two neurological phenotypes, namely (1) abnormalities in larval neuromuscular junction (NMJ) bouton number and (2) circadian defects apparent through abnormalities in adult sleep rest cycles. The NMJ offers a sensitive in vivo system to identify interactions that alter synaptic growth and maturation [14] and has proved a valuable tool for studying genes associated with neurodevelopmental disorders including autism spectrum disorders, intellectual disability and neuropsychiatric diseases [15–19]. For example, mutations in Neurexin IV, the Drosophila orthologue of the autism gene CTNAP2, have been shown to decrease NMJ bouton number and the abundance of glutamate receptors that oppose the active zones. Circadian rhythm activity defects have been previously reported in Drosophila neurodevelopmental models, including fragile X syndrome and Angelman syndrome, and can be an indicator and causative factor of neurodevelopmental and neurodegenerative disorders in humans [20–23]. Recent studies in Drosophila have also identified sleep abnormalities in mutations of the candidate ASD gene cullin 3 (CUL3) [24–26]. Furthermore, sleep and circadian abnormalities are both significantly associated with ASD: Sleep disturbance is experienced by up to 80% of individuals with ASD, and while more strongly associated with ASD than other neurodevelopmental disorders it is not associated with intellectual disability, which is however frequently comorbid with ASD [22].
In this study, we modelled the effects of gene dosage changes on Drosophila neurological readouts using gene sets derived from multigenic de novo CNVs that had been identified in patients with autism [5,27–29]. We focussed our attention on the unique Drosophila orthologues of genes affected by these CNVs whose protein products had previously been found to participate in an ASD-associated interaction network, and which had a role in neural functioning [9]. To do this, we first considered those CNVs that changed only a single gene in the ASD-associated network, and show that the dosage alterations in the Drosophila orthologue yields neurological defects similar to those previously reported in Drosophila neurodevelopmental disease models [30,31]. We next looked at CNV gene sets that affected multiple genes in the ASD-associated network. Amongst these genes, no heterozygous mutation in a single gene led to significant synaptic defects in the fly. However, pairwise crosses between heterozygously-mutated genes yielded neurological defects comparable to the monogeneic models. We observe that (i) pairwise combinatorial dosage effects amongst these genes are not additive, but clearly synergistic, and (ii) that when the direction of copy change of the orthologues in individuals with ASD is considered, the observed effect at the Drosophila synapse supports a model of convergent phenotypic outcome between distinct synergistically-interacting gene pairs. No effects were observed among gene pairs that included neuronally-expressed Drosophila genes whose orthologues were affected by these CNVs but that were not part of the ASD-associated network. We show that the combinations of genes drawn from these CNVs that interact are specific, supporting distinct molecular aetiologies underlying ASD. We also show that these specific interactions affect different molecular processes at the Drosophila synapse, supporting the role of distinct molecular ASD related aetiologies. In total, we identified synergistically-interacting orthologous pairs among 3/4 of the CNVs considered, demonstrating novel synergistic interactions that may contribute to the aetiology of autism.
Results
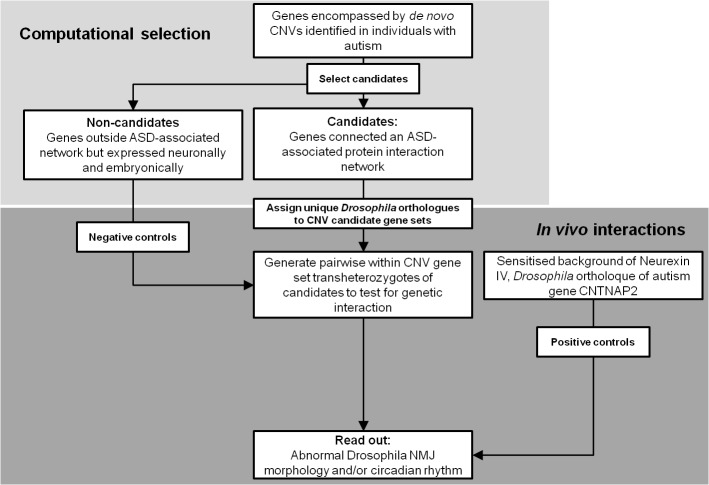
Previous studies applying network analyses to rare ASD associated genetic variants have proposed that these variants may genetically interact to exert their phenotypic influence in a patient [9,10]. To investigate the proposition of gene-gene interactions in these ASD cases, we used the fruit fly Drosophila. In particular, we modelled the effects of combinatorial heterozygous dosage changes of pairs of candidate genes, in the fly, and looked for synaptic and circadian defects. A schematic of our method is also set out in Fig. 1. Candidate genes were defined as those genes that had both (1) been identified to be previously affected in individuals with ASD by de novo CNVs, and additionally (2) those contributing to a large network of interacting proteins with roles in neural functioning, herein termed as an “ASD-associated network” [9]. Firstly, two CNVs were identified that affected only a single gene within the ASD-associated network: Specifically, 1 CNV affected CTNND2 while another CNV affected NOTCH1 (Table 1). These were brought forward as ‘monogenic’ candidates. Four additional de novo human CNVs were identified that each overlapped multiple ASD-associated network candidate genes, and where every candidate gene possessed a unique Drosophila orthologue. These CNVs gene sets were also taken forward for in vivo study (Table 1). In addition, from each of these 4 CNVs, two control genes were randomly selected and taken forward. These were genes that again possessed a unique Drosophila orthologue, and which were expressed in both the larval and adult nervous system (Table 1). Of the final 6 CNV gene sets taken forward for in vivo modelling, 2 sets were monogenic while 4 sets were polygenic. 4 were derived from copy number losses while 2 were derived from copy number gains. Table 1 details the CNVs, directionality, human genes and corresponding Drosophila ortholouges for all experiments.
Fig 1. Drosophila screening strategy to detect interactions between the orthologues of genes simultaneously affected by a de novo CNVs identified in individuals with ASD (see Methods).
Table 1. Human CNVs, candidate (bold) and control genes, and their respective Drosophila orthologues.
| CNV Identity | Chr | Start | End | CNV Type | Human Candidate genes | Drosophila Orthologues of candidate genes | Figure |
|---|---|---|---|---|---|---|---|
| 11079_chr3_loss_197208363_l | 3 | 197208363 | 198838422 | Loss | Phosphate Cytidylyltransferase 1, Choline, Alpha (PCYT1A) | fsn | 3 |
| Discs large 1 (DLG1) | Discs large (dlg) | 3 | |||||
| p21-activated kinase 2 (PAK2) | p21-activated kinases (Pak) | 3 | |||||
| LOC220729 | CG5359 | 3 | |||||
| 12289_chr5_loss_11403621_l | 5 | 11403621 | 11493124 | Loss | catenin delta 2 (CTNND2) | p10 catenin (p120ctn) | 2 |
| 1946_301_chr9_gain_138505259 | 9 | 138505259 | 139336068 | Gain | Notch1 | Notch (N) | 2 |
| 12235_chr9_gain_129907917_l | 9 | 129907917 | 130512360 | Gain | Dynamin-1 (DNM1) | dynamin/shibire (shib) | 5 |
| Prostaglandin E Synthase 2 (PTGES2) | su(p) | 5 | |||||
| SWI5 Recombination Repair Homolog (SWI5) | CG14104 | 5 | |||||
| alpha-Spectrin (SPTAN1) | alpha-Spectrin (a-spec) | 5 | |||||
| 12239_chr22_loss_17249508_l | 22 | 17249508 | 18693261 | Loss | T-box 1 (TBX1) | optomotor-blind-related-gene-1 (orbg-1) | 4 |
| Guanine nucleotide-binding protein subunit beta-like protein 1 (GNB1L) | CG13192 | 4 | |||||
| histone cell cycle regulator (HIRA) | hira | 4 | |||||
| solute carrier family 25 (SLC25A1) | sea | 4 | |||||
| Zinc finger, DHHC-type containing 8 (ZDHHC8) | CG34449 | 4 | |||||
| George syndrome critical region gene 8 (DGCR8) | partner of drosha (pasha) | 4 | |||||
| Septin 5 (SEPT5) | Septin 4 (Sept4) | 4 | |||||
| 12691.p1_chr16_loss_68529466_s | 16 | 68529466 | 71494580 | Loss | VAC14 | CG5608 | S1 |
| PH domain and leucine rich repeat protein phosphatase-like (PHLPPL) | PH domain leucine-rich repeat protein phosphatase | S1 | |||||
| Splicing factor 3B subunit 3 (SF3B3) | CG13900 | S1 | |||||
| Calbindin 2 (CALB2) | Calbindin 53E (cbn) | S1 | |||||
| tyrosine aminotransferase (TAT) | CG5608 | S1 | |||||
| C-type lectin domain family 18, member C (CLEC18C) | CG3626 | S1 | |||||
| AP1G1 adaptor-related protein complex 1, gamma 1 subunit (AP1G1) | AP-1γ | S1 | |||||
| Dihydroorotate dehydrogenase (DHODH) | Dihydroorotate dehydrogenase (dhod) | S1 |
CNVs were selected from previous studies that identified de novo or rare CNVs in the genomes of individuals with ASD (see Methods). CNVs post-fixed “_1” were taken from the study by Levy et al. [28], CNVs post-fixed “_s” were taken from the study by Sanders et al.[29], while the CNV 1946_301_chr9_gain_138505259 was taken from the AGP study [5]. The candidate and control genes were among those genes affected by the given CNV: The protein products of the candidate genes interacted in a previously identified network of interacting proteins associated with neural functioning, while the control genes’ protein products are expressed embryonically and neuronally.
Modelling ASD genes in the fly with NMJ and circadian phenotypes
Singular and combinatorial effects resulting from the simultaneous dosage change of ASD-candidate genes were investigated by identifying changes in neuromuscular junction (NMJ) bouton number, and circadian rhythms (specifically alterations in the light/dark bias towards sleep and rest). As a complex disease with behavioural deficits relating to alterations in the human brain, ASD may not be wholly modelled in Drosophila. However, by enabling the rapid screening of multiple target genes the fly is a powerful model to test gene-gene interactions in vivo. It thus offers a tractable method to better understand the gene-gene interactions postulated to occur as a result from these large de novo CNVs. We believed bouton number, and circadian rhythms to be relevant because phenotypes because: (i) The fly NMJ, a tractable and highly characterised glutamatergic synapse, has been successfully used to detect synaptic defects in models of ASD, neuropsychiatric disease and intellectual disability [15,31]; (ii) circadian rhythm defects are associated with ASD and several fly ASD models [21,32,33].
Drosophila models of monogenic forms of ASD yield neurological phenotypes
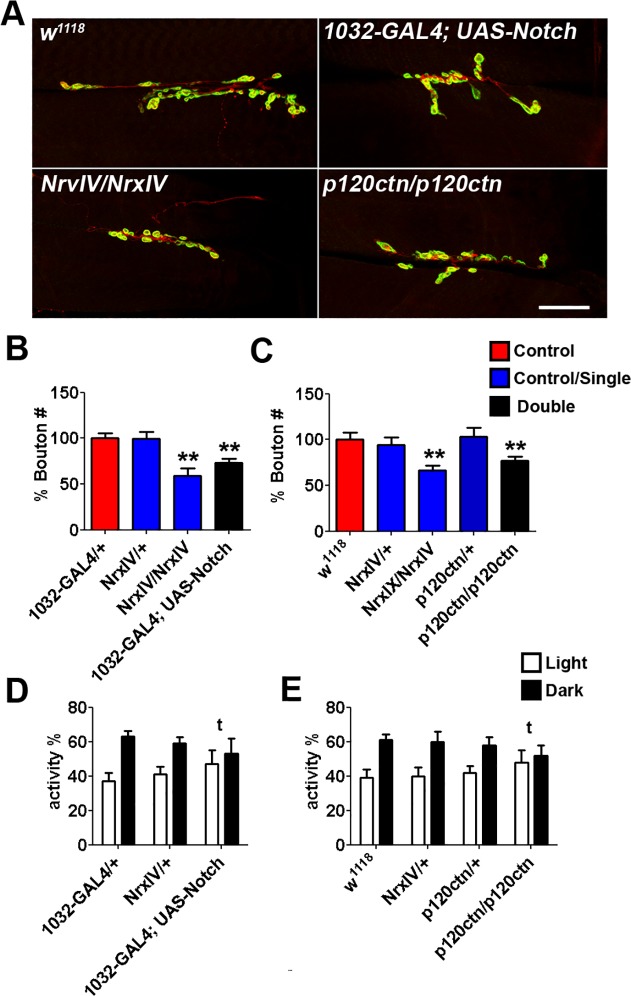
Two of the six CNV gene sets considered contained only one candidate gene. One monogenic gene set is derived from a loss CNVs that affected the orthologue of the Drosophila gene p120 catenin (p120ctn) with roles in cell adhesion and signal transduction, while the other CNV contained the evolutionarily conserved signalling molecule Notch, whose human orthologue was found to be copy number increased (Table 1). Mutants for neurexin IV (using Nrx-IV 4304), the orthologue of the autism gene CNTNAP2, and w 1118 were used as positive and wild type controls, respectively. All Drosophila stocks were isogenised to the w 1118 wild type background for 7 generations for this study. As previously described, we found that NrxIV homozygous null mutants display reduced bouton numbers, while heterozygote nulls have no observable difference when compared to wild type (Fig. 2A) [34].
Fig 2. Bouton number at the Drosophila NMJ following the overexpression and mutation of the Drosophila unique orthologues of candidate genes identified from human autism-associated copy number variants (CNVs).
A. Representative pictures of NMJs from NrxIV/NrxIV (using Nrx-IV 4304), Notch overexpessing (1032-GAL4, UAS-Notch), and p120ctn/p120ctn (using p120ctn 308) 3rd instar larvae; Scale bar = 20μm. B. Homozygous disruption of NrxIV, the orthologue of the autism gene CTNAP2, provided a positive control and displayed a reduced NMJ bouton number as described previously. Heterozygous mutants of NrxIV yielded no bouton number reduction. Overexpression of Notch (1032-GAL4; UAS-Notch-full), whose human orthologue is duplicated in the de novo gain CNV_946_301_chr9_gain_138505259 (Table 1), gave reduced bouton numbers (n>20, Kruskal-Wallis test, ** P<0.01). C. Homozygous disruption of p120ctn, that is affected by the de novo loss CNVs 12289_chr5_loss_1140362 (Table 1), yields reduced bouton numbers. (n>20, Kruskal-Wallis test, ** P<0.01). Heterozygous mutants of p120ctn have no significant change on NMJ morphology. D. and E. Circadian sleep/rest rhythm analysis of candidate genes from the CNV sets. 1032-GAL4, UAS-Notch, and p120ctn/p120ctn flies lost the dark bias, displaying no significant difference between light/dark sleeping patterns (t, representing the crosses where no light/dark sleep/rest bias was observed). Light/dark sleeping bias was measured using student’s t-tests.
The first monogenic CNV gene set we analysed was Drosophila Notch, the orthologue of human Notch1, derived from a human Chromosome 9 copy number gain CNV (Table 1). To investigate the increased expression of Drosophila Notch, we overexpressed Drosophila Notch (using UAS-Notch-Full) driven by the ubiquitous expression GAL4 driver 1032-Gal4 (Fig. 2B). While larvae overexpressing Notch had no overt effect on early larval survival, we observed reduced NMJ bouton numbers (n>20, Kruskal-Wallis test, ** P<0.01; Fig. 2B) showing that dosage increase in this gene yields synaptic phenotypes in Drosophila.
Next, we considered the monogenic CNV gene set corresponding to the loss of the Drosophila orthologue p120ctn. The previously described null mutant p120ctn 308 was isogenised to analyse hemizygous p120ctn loss [35]. However, p120ctn heterozygous null mutants displayed no significant change in NMJ (Fig. 2C) although homozygous p120ctn null mutants were found to display a significantly reduced bouton number (n>20, Kruskal-Wallis test, ** P<0.01, Fig. 2C). We note that, unlike in vertebrates, Drosophila p120ctn homozygous null mutants are viable [35].
We next looked for circadian rhythm defects in the monogenic CNV gene set orthologues Notch and p120ctn mutants. Notch overexpression larvae were reared at 16°C, and were transferred to 25°C during pupation, so to mitigate gross developmental defects. We analysed sleep/rest periods (measured as a contiguous 5 minute periods of inactivity) as a surrogate for looking at gross defects in rhythmicity. While all negative control and single mutants displayed normal light/dark differences in sleeping patterns (i.e more sleep/rest periods during the dark 12hrs; Fig. 2D, E), both p120ctn homozygous nulls (Fig. 2E) and the Notch (Fig. 2D) overexpressing flies all lost the dark bias and displayed no significant difference between light/dark sleeping patterns.
Taking these monogenic models together, we show that dosage change in Drosophila of the orthologues of known ASD diseases genes (NrxIV), and of ASD-candidate genes subject to de novo copy number increase (notch) and decrease (P120ctn) in human, all yield abnormalities at the NMJ, and in circadian rhythms (notch and p120ctn) (Fig. 2). We also find that despite differences in the direction of dosage change in Drosophila that are consistent with the copy change observed for these 3 genes in individuals with ASD, the bouton count at the NMJ is reduced in all models, supporting a convergent phenotypic outcome in both Drosophila and human.
Drosophila models of polygenic causes of ASD are driven by genetic interactions
We next considered the four CNVs that each affected multiple genes within the ASD-associated network. For each, we asked whether the dosage change of their Drosophila orthologues singularly or in pairwise combination yielded NMJ synaptic or circadian abnormalities. The number of ASD-associated network candidate genes in each of the five CNVs with multiple candidate genes ranged from 2–6, with a mean of ∼4. The four CNVs consisted of three loss CNVs (11079_chr3_loss_197208363_l with two candidate genes; 12239_chr22_loss_17249508_l with five candidates; 12691.p1_chr16_loss_68529466_s with six candidates) and one gain CNVs (12235_chr9_gain_129907917_l with two candidates) (Table 1)
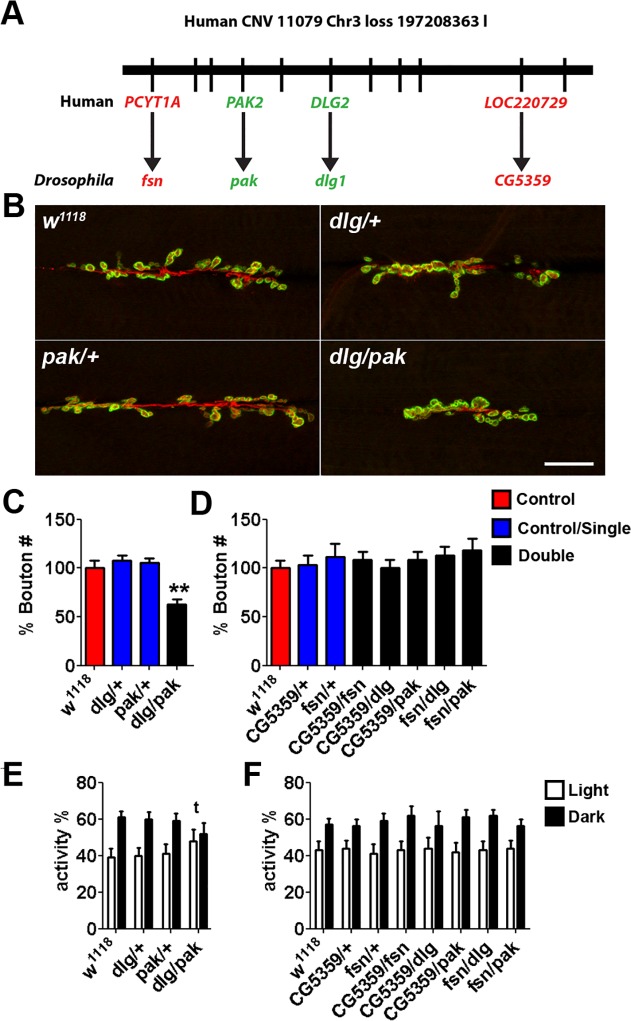
The first multiple candidate gene CNV studied, human de novo loss CNV 11079_chr3_197208363 (Fig. 3A), contained two candidates: the septate junction protein discs large (dlg) and p21-activated kinase (pak), a serine/threonine-protein kinase [36], which has been previously shown to control the synaptic Dlg localisation. Isogenised transheterozygotes of the mutants dlg (dlg 1) and pak (pak 6) were used and bouton number analysed for synaptic alterations. Single dlg and pak heterozygous mutants alone displayed no significant change in NMJ morphology when compared to controls (Fig. 3B, C), whilst homozygous mutants are lethal, as previously reported [37,38]. However, dlg/pak transheterozygotes (although the correct full geneotype is w 1118, dlg 1; +/+; pak 6/+ for this example, all transheterozygtes will be represented in the ‘gene/gene’ format going forward, for simplicity) displayed significant bouton number reductions (n>20 Kruskal-Wallis test, ** P<0.01; Fig. 3C). For additional controls, Fsn (using Fsn KG08128) and CG5359 (using CG5359 e03976), which were selected from genes found within CNV 11079_chr3_197208363 but did not participate in the ASD-associated network, were crossed to dlg and pak heterozygotes but no significant NMJ morphology changes were observed (Fig. 3D). To look for circadian behavioural phenotypes, day/night sleep patterns of adult flies were again analysed. Wild type flies and all negative controls (transheterozygote crosses with Fsn KG08128 and CG5359 e03976; Fig. 3E, F) and single mutants displayed normal light/dark differences in sleeping patterns, with more sleeping periods in the dark. However, dlg/pak flies lost the dark bias (Fig. 3E), displaying no significant difference between light/dark sleeping patterns. Thus, dlg/pak flies demonstrated synergistic effects, displaying both reduced NMJ bouton number and circadian rhythm defects only in the transheterozygotes.
Fig 3. Synergistic interaction in Drosophila between Dlg and Pak, the orthologues of ASD-candidate genes from a de novo loss CNV 11079_chr3_197208363.
A. The Locus of the CNV with mapped Drosophila orthologues (Candidates, green; controls, red). B. Representative pictures of NMJs from dlg/+ (using dlg 1), pak/+ (using pak 6), and dlg/pak 3rd instar larvae; Scale bar = 20μm. C. Synaptic alterations were characterised by NMJ bouton number. Individual heterozygous mutants of candidate gene orthologues dlg and pak (dlg/+ and pak/+) gave no significant change in NMJ morphology over w 1118 controls. However, dlg/pak transheterozygotes have reduced bouton numbers. (n>20, Kruskal-Wallis test, ** P<0.01). D. Non-candidate gene controls fsn (using Fsn KG08128) and CG5359 (using CG5359 e03976) selected from genes found within CNV gave no significant NMJ phenotype singularly or when crossed to form transheterozygotes with dlg or pak. E. and F. Circadian rhythm analysis of candidate genes. All negative control F. and single mutants displayed normal light/dark differences in sleeping patterns. However, transheterozygote dlg/pak flies lost the dark bias, and displayed no significant difference between light/dark sleeping patterns (t).
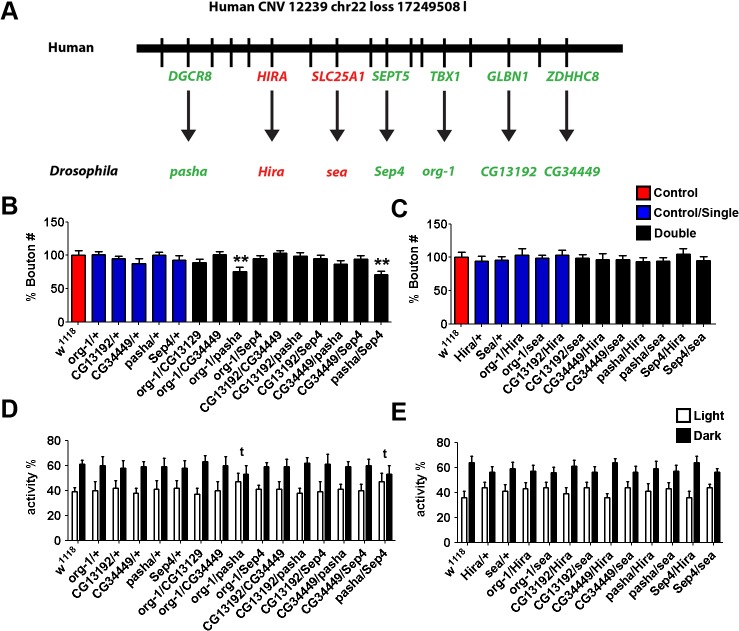
Analysis of a second human de novo loss (12239_chr22_loss_17249508_l; Table 1; Fig. 4A), covering the recurrent 22q11.2 microduplication critical region [39], found no evidence of abnormalities in NMJ bouton count nor circadian cycle in the single heterozygote mutants of any of the 7 genes examined (5 candidates and 2 controls; Fig. 4). However, the two transheterozygous combinations of partner of drosha (pasha; using pasha LL03360) [40] with optomotor-blind-related-gene-1 (org-1, using org-1MB01466) [41] and that of pasha with Septin4 (Sep4, using Sep4 NP7170) were both found to have reduced bouton numbers (Fig. 4B; n>20, Kruskal-Wallis test, ** P<0.01, *p<0.05). These relations, however, were not transitive as the combination of org-1 and Sep4 (org-1/Sep4) did not yield these phenotypes. Similarly, only the org-1/pasha and pasha/Sep4 transheterozygote flies also lost the dark bias, displaying no significant difference between light/dark sleeping patterns while org-1/Sep4 did not (Fig. 4D). No significant NMJ morphology or sleep/rest changes were seen when negative controls hira (using hira 185b) and sea (using sea EP3364) were crossed to form transheterozygotes with the candidates (Fig. 4C, E). Thus, again, we observe synergistic combinatorial effects, with both NMJ bouton number and circadian rhythm defects apparent only in the transheterozygotes for this second multigenic loss CNV gene set. However, a final multigenic loss CNV gene set, 12691.p1_chr16_loss_68529466_s, failed to yield any significant NMJ bouton number or circadian defects amongst single or pairwise heterozygotes (Table 1; S1 Fig.).
Fig 4. Synergistic interactions in Drosophila between org-1, pasha and Sept4, the orthologues of ASD-candidate genes from a de novo loss CNV 12239_chr22_loss_17249508_l.
A. The Locus of the CNV with mapped Drosophila orthologues (candidates, green; control, red). B. Synaptic alterations were characterised by NMJ bouton number. Individual heterozygous mutants of 5 candidate gene orthologues (org-1, CG13192, CG34449, pasha, Sep4; blue bars) gave no significant change in NMJ morphology over w 1118 controls (red bar). org-1/pasha and pasha/Sep4 transheterozygotes display reduced bouton. (n>20, Kruskal-Wallis test, ** P<0.01). The mutants pasha LL03360, org-1MB01466 and Sep4 NP7170 were used respectively. C. Non-candidate gene controls Hira (using Hira 185) and Sea (using sea EP3364) selected from genes found within CNV gave no significant NMJ phenotype singularly or when crossed to form transheterozygotes with candidate genes. D. and E. Circadian rhythm analysis of candidate genes. All negative control and single mutants displayed normal light/dark differences in sleeping patterns. However, org-1/pasha and pasha/Sep4 transheterozygotes lost the dark bias, and displayed no significant difference between light/dark sleeping patterns (t).
A Drosophila model of a human gain CNVs supports convergent aetiologies following copy number change in ASD
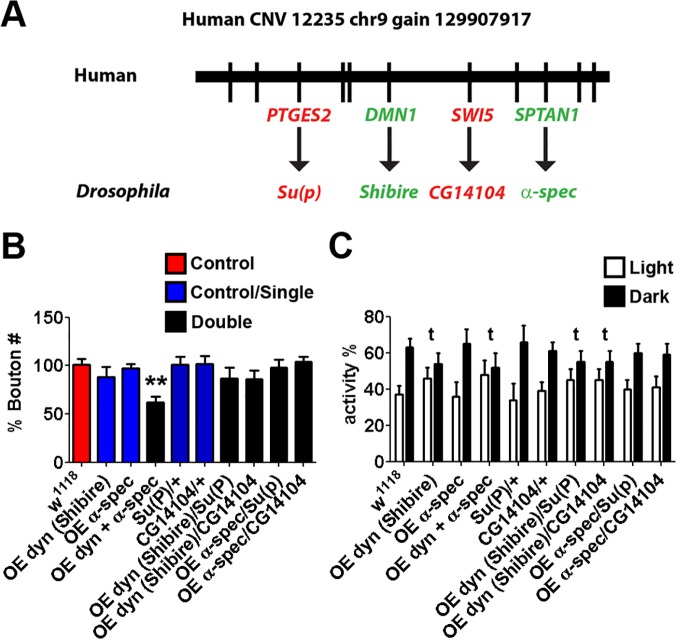
We next analysed a gene set derived from a copy number gain (12235_chr9_gain_129907917_l, Fig. 5A), by generating constructs for overexpression, and by employing the UAS-GAL4 over-expression system. The two ASD-associated network genes, dynamin (Shibire) and alpha spectrin, when over-expressed together display a decreased NMJ bouton number (Fig. 5B) and lost the dark bias to sleep (Fig. 5C). The observed decrease in bouton number following pairwise over-expression of these candidate genes duplicated in humans with ASD is consistent with the bouton number decrease also observed among the pairwise disruptions of candidate genes found to be deleted in humans with ASD. Although the dynamin (Shibire) over-expresser alone also showed a loss of dark sleep bias in this case, individually-driven genes displayed no significant change in NMJ morphology over w 1118 controls. No significant NMJ morphology changes are seen when non-ASD-network controls from the CNV gene set Su(P) (using Su(P) EY13245) and CG14104 (using CG14104 f07593) are crossed into the overexpressing backgrounds (Fig. 5B for NMJ analysis and Fig. 5C for sleep/rest analysis).
Fig 5. Synergistic interactions in Drosophila between shibire and alpha spectrin, the orthologues of ASD-candidate genes from a de novo gain CNV 12235_chr9_gain_129907917_l.
A. The Locus of the CNV with mapped Drosophila orthologues (Target, green; control, red). B. Synaptic alterations were characterised by NMJ type IB bouton number. Individual heterozygous mutants of candidate gene orthologues gave no significant change in NMJ morphology over w 1118 controls. However, Shibire and alpha-spectrin double over expressers display reduced bouton numbers (using 1032-GAL4; UAS-Dynamin/UAS-alpha-spectrin; n>20, Kruskal-Wallis test, * P<0.05). Non-candidate gene controls Su(P) (using Su(P) EY13245) and CG14104 (using CG14104 f07593) selected from genes found within CNV gave no significant NMJ phenotype singularly or when crossed to form transheterozygotes with candidate genes. C. Circadian rhythm analysis of candidate genes. Negative controls and candidate gene orthologue overexpression of alpha-spectrin displayed normal light/dark differences in sleeping patterns singularly or when crossed. However, Shibire overexpression, and co-overexpression with alpha-spectrin lost the dark bias, and displayed no significant difference between light/dark sleeping patterns (t).
Taking all the polygenic models together, with one exception (dynamin (Shibire) dark bias; Fig. 5B), we show that only particular pairwise combinations of dosage change generate interactions that yield neurological phenotypes comparable to those observed in the monogenic models (Figs. 2–5). As with the mongenic CNV gene sets examined, among the 3 CNV gene sets that demonstrate pairwise interactions, we observe directionality effects in NMJ bouton count that are consistent with a convergent phenotypic outcome. Finally, singularly or in pairwise combinations, we observed no phenotypes for any model involving non-ASD-associated network genes.
Specific subsets of the candidates modify the Neurxin IV background
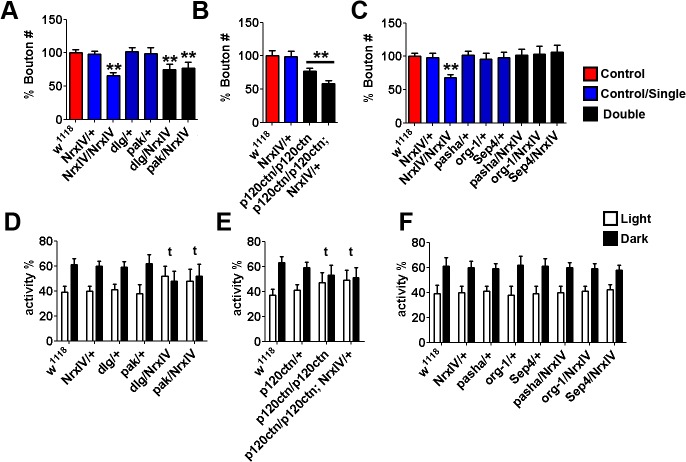
Understanding the functional relationships between genes underlying ASD will help elucidate the processes that lead to neurological dysfunction and ultimately may pinpoint common mechanisms that lead to the disorder. To test the relationship between our candidate genes and a known ASD candidate we crossed subsets of our candidates with neurexin IV, the orthologue of the autism gene CNTNAP2. From our candidate list we selected dlg, pak and p120ctn which have functional roles in cell adhesion processes that may involve neurexin IV [35]. We crossed heterozygous dlg (dlg 1), pak (pak 6) and p120ctn homozygotes (p120ctn 308/p120ctn 308) to a sensitised background of NrxIV (Nrx-IV 4304/+) and analysed bouton number. In all three cases, the transheterozgotes of each of dlg, pak and p120ctn/p120ctn in combination with NrxIV (Nrx-IV 4304/+) synergistically yielded reduced bouton number and displayed a loss in the dark bias to sleep suggesting that these proteins may act in the same pathway (Fig. 6A, B for NMJ analysis and Fig. 6D, E for sleep/rest analysis). It is worth noting that p120ctn/p120ctn flies in combination with NrxIV had a significantly reduced survival.
Fig 6. Selective genetic interactions observed between the Drosophila orthologues of ASD candidate genes and Neurexin IV.
A sensitised background of Neurexin IV (NrxIV/+), the orthologue of the autism gene CTNAP2, was used to look for interactions between NrxIV and the ASD candidate gene orthologues dlg, pak, p120ctn, pasha, and org-1. A. dlg/+, pak/+ and NrxIV/+ heterozygous mutants have no significant change in NMJ morphology over w 1118 controls. However, dlg/NrxIV and pak/NrxIV crosses both displayed reduced bouton numbers (n>20 Kruskal-Wallis test, ** P<0.01). B. Significant NMJ morphology changes are also seen for the NrxIV cross with the homozygous p120ctn mutant cross, when compared to the homozygous p120ctn mutant cross alone (n>20 Kruskal-Wallis test, ** P<0.01). C. No NMJ morphology changes were observed when pasha and org-1, both from human de novo loss 12239_chr22_loss_17249508_l, were crossed to NrxIV/+. D. to F. Circadian rhythm analyses of models in Panels A, B and C support observed genetic interactions: dlg/NrxIV, pak/NrxIV transheterozygotes and the NrxIV cross with the homozygous p120ctn mutant flies lost the dark bias, and displayed no significant difference between light/dark sleeping patterns (t), while pasha/NrxIV and org-1/NrxIV transheterozygotes displayed no abnormal circadian phenotype.
We next crossed pasha, sep4, and org-1 heteozygotes with neurexin IV to see if modification of the NMJ and dark sleep bias was a common feature when alleles were present in the sensitised NrxIV (Nrx-IV 4304/+) background. In these cases no significant changes to the NMJ phenotypes (Fig. 6C) or sleep/rest rhythms (Fig. 6F) were observed suggesting that pasha, Sep4 and org-1 are acting on non-converging pathways.
Genetic interactions subsets cause differential synaptic defects
To better understand how these interacting and non-interacting gene pairs exert common circadian and synaptic phenotypic effects, we next looked for molecular defects at the synapse. Single homozygous mutations of Drosophila ASD gene orthologues display defects in synapse development [18,42]. Examples of these defects include alterations in glutamate receptors abundances, active zone numbers, and presynaptic and postsynaptic structural defects at the larval NMJ [15,17,18,21,30,42]. To investigate whether gene dosage changes from the transheterozygote subsets above cause molecular synaptic defects, we looked for alterations in active zone localisation and glutamate receptors abundance at the Drosophila larval NMJ. Drosophila active zones are identified by staining with the protein bruchpilot (BRP, Fig. 7A), which is positioned presynaptically and opposite to the postsynaptic neurotransmitter receptors. We measured BRP foci and normalised them to bouton area from transheterozygotes of NrxIV, dlg, pak, pasha, Sep4 and org-1. We found that all transheterozygous crosses between nrxIV, dlg and pak, which we have shown to genetically interact, displayed a reduction in BRP localisation at the synapse (Fig. 7B, C). However, this was not observed for the genetically-interacting transheterozygotes pasha/Sep4 or pasha/org-1 or single heterozygous mutants and controls. We next explored whether dosage changes in our candidate genes might lead to the destabilisation of the clustering of the postsynaptic glutamate receptors, by studying the levels of GluRIIA at the synapse. Again, alterations in glutamate receptor subtypes have been discovered in single homozygous mutations of Drosophila ASD gene orthologues [42,43]. In this case, we found that out of all single and transheterozygote crosses, only pasha/Sep4 displayed a reduction in the levels of GluRIIA (Fig. 7D, E). Taken together, our findings demonstrate that distinct molecular developmental alterations are associated with the different genetically interacting gene combinations, supporting the idea that distinct molecular aetiologies may contribute to ASD by converging on common phenotypic outcomes (Fig. 7F).
Fig 7. Different genetic interactions effect distinct synaptic defects suggesting that distinct molecular aetiologies underlie ASD.
A. The Drosophila NMJ contains presynaptic active zones (labelled by Bruchpilot, BRP) and postsynaptic glutamate receptor (labelled by GluRIIA). B. Representative images of BRP staining demonstrate a reduced number of active zone (BRP) puncta in the transheterozygotes dlg/pak, NrxIV/dlg and NrxIV/pak as compared to control (w 1118), C. The number of active zone (BRP) puncta (normalised to bouton size) were significantly reduced in dlg/pak, NrxIV/Dlg and NrxIV/Pak transheterozygotes. D. The fluorescence of the post-synaptic glutamate receptors were scored and normalised to bouton size HRP levels. Pasha/Sep4 transheterozygotes were the only genotype to demonstrate decreased glutamate receptor abundance. E. Representative images of GluRIIA staining demonstrating the reduced fluorescence in the transheterozygote Pasha/Sep4 when compared to control. F. A schematic showing the sub-types of genetic interactions supporting distinct molecular aetiologies underlying ASD that converge to yield defects at the synapse.
Discussion
In this study we have developed an in vivo model system in Drosophila to determine how genes can synergistically interact within ASD associated de novo CNVs. Specifically, we have shown that (i) of the 4 human CNVs containing 2, 2, 5 and 6 network-identified candidate genes respectively (from a combined total of 114 copy-changed protein-coding genes), pairwise interactions between Drosophila orthologues yielding changes in the neuromuscular junction (NMJ) bouton number and circadian rhythms were observed for 3 CNVs; (ii) that the interactions observed are synergistic, as opposed to additive, in nature, and (iii) that the synaptic bouton counts observed following the simultaneous dosage change of all 5 pairs of interacting CNV candidate genes’ orthologues within Drosophila support a convergent phenotypic outcome arising from these genes’ dosage change for the individuals with ASD within whom they were identified (Figs. 2–5, S2). We show that the combinations of genes drawn from these CNVs that interact are specific, both within a CNV (Fig. 4) and between CNVs (Fig. 6), supporting distinct aetiologies underlying ASD. Finally, we go on to show these specific interactions act through different molecular aetiologies, supporting the role of distinct molecular aetiologies in ASD (Fig. 7).
The synergistic, as opposed to additive, nature of the pairwise genetic interactions that we observe in Drosophila has important consequences for identifying the genetic causes of ASD, and (i) the conserved orthology of the interactors, (ii) the human orthologues’ participation in an ASD-relevant network constructed from known mammalian interactions, and (iii) the concordance between the direction of dosage change and phenotype all support the inter-species relevance of our findings. Although there are over 100 ASD candidate genes currently identified, at least 70% of the genetic causes remain to be explained [9,44]. The presence of multiple genetic variants in many patients [29,45] suggests that inherited variants might lead to ASD through the combinatorial effects of distinct deleterious variants which affect a shared biological pathway (Fig. 6) [10]. Where variants that act additively to cause ASD in a proband are inherited from each parent, those variants individually may cause detectable ASD-relevant traits in the parents [10,46,47]. However, if combinations of variants act only synergistically to cause ASD, there would be no expectation of ASD-relevant traits in either parent. Importantly, if sub-threshold ASD traits affect fecundity then variants that are only deleterious in combination may rise to a higher frequency in the population. Our results in Drosophila show that only particular combinations of dosage variants act together to yield an abnormal phenotype (Fig. 4 and Fig. 6). Identifying those variants that contribute to ASD only in combination with other specific variants, amongst a background of large amounts of non-contributing genetic variation, will be challenging because the variety of gene variant combinations is extremely large, and allele frequencies are likely very rare.
The genes participating in the pairwise genetic interactions identified by our screen were discs large (dlg: human orthologue (h.o) DLG1), p21-activated kinase (pak: h.o. PAK2), p20 catenin (p120ctn: h.o. CTNND2), Notch (N: h.o Notch 1), shibire/dynamin (shi/dynamin: h.o. DNM1), alpha-Spectrin (α-spec: h.o. SPTAN1), optomotor-blind-related-gene-1 (org-1: h.o. TBX1), partner of drosha (pasha: h.o. DGCR8) and Septin 4 (Sep4: h.o. SEPT5). An examination of CNVs listed in the Database of Genomic Variants (DGV) [48] reveals that most of these genes are found to be individually dosage changed in the same direction in apparently healthy individuals (DLG1, 7 CNVs; PAK2, 1 CNV; DNM1, 1 CNV; SPTAN, 1 CNV; SEPT5, 2 CNVs; TBX1, 9 CNVs; DGCR8 5 CNVs). However, only one of these CNVs might simultaneously change two genes that our study demonstrate genetically-interact in the fly (variant nsv828939; [49]) and CNVs strongly implicated in ASD have previously been reported in apparently healthy individuals [47,50]
Many of the interacting genes have known functions in the nervous system. For example the localisation of the septate junction and neuronal adhesion protein Dlg at the NMJ has been shown to be regulated by Pak serine/threonine-protein kinase activity [36]. In addition, it is interesting to point out that p21-activated kinase (PAK) has been shown to interact with the protein SHANK3 in rat, whose disruption can also cause ASD, with mutant Shank3 altering actin dynamics driven by PAK signalling [51]. Destabilisation of the actin filaments at the NMJ leads to defective NMDAR-mediated synaptic current in neurons. PAK inhibitors have also been shown to rescue fragile X syndrome phenotypes in Fmr1 KO mice [52], suggesting an important role for Pak serine/threonine-protein kinase activity in ASD and ID. The gene alpha-spectrin, which we show genetically interacts with the dynamin protein shabire [53], is known to cross link actin, and has been shown to be important for the localisation of Dlg at the synapse [54]. The phenotypes resulting from the combination of these genes’ variants suggests an important role for the control of synapse integrity via actin stabilisation in ASD [55]. This again is supported up by a particular enrichment for genes directly and indirectly associated with both cell adhesion and cytoskeletal associated cell membrane proteins in our interacting genes (5 out of 9; discs large, p120 catenin, Notch, alpha-spectrin, pak), several of which have been identified to have properties in the neuron [54,56–59]. Many studies have linked neurodevelopmental disorders, including ASD, to mutations in synaptic adhesion proteins, including the neurexins and neuroligins, and mutations in these in Drosophila have yielded both behavioural and larval NMJ defects [30,31,60]. We show specific interactions between P120ctn, dlg and pak with Drosophila neurexin IV, which has been shown to be involved in the maturation of the Drosophila NMJ. [34,61,62]. Notably, the ASD-network orthologues (namely org-1, pasha and sep4) that contribute to the interactions modelling the CNV 12239_chr22_loss_17249508_l that covers the 22q11.2 microdeletion critical region, did not yield phenotypes in the sensitised NrxIV background (Fig. 6) suggesting that these intracellular genes may be exerting phenotypic effects through an alternative process. While other (non-ASD network) genes in this 22q11.2 critical region have received interest in effecting the many associated phenotypes, our study suggests that interactions between the human genes TBX1, DGCR8 and SEPT5 may play a significant causal role [39].
Alterations in active zone structures have been connoted in ASD [63]. Moreover, neuron specific knockdown of the Drosophila orthologues of the ASD genes CNTNAP2 and NRXN1, NrxIX and Nrx-1 (dnrx), have been shown to alter the levels of the active zone protein BRP [18]. BRP shows both sequence and functional homology with the mammalian ELKS/CAST proteins that are structural components of the vertebrate active zone [64,65]. Here we show that dosage changes created by transheretozygotes between NrxIV, dlg and pak lead to a reduction in BRP foci. Dlg is a postsynaptic anchoring protein which is required for the development and stability of the postsynaptic subsynaptic reticulum (SSR), whilst Pak is known to phosphorylate Dlg and control its abundance at the synapse [36]. NrxIV is predominantly presynaptic, but is required for the cell-cell contacts that influence synaptic development [66], and govern the interconnectivity between both neurons, glial cells and the pre- and postsynapse [30]. Dosage alterations in NrxIV with Dlg, Pak and p120 catenin may lead to alterations in adhesion protein interactions, causing the destabilisation of the synaptic architecture in both the pre- and postsynapse, ultimately leading to defective synaptic maturation. In the null mutant of the Drosophila orthologue of NRXN1, Nrx-1 (dnrx), GluRIIA subunit fluorescence and BRP active zone density were increased, although bouton numbers still remain reduced [62]. It has been suggested that interactions between Drosophila neurexins and neuroligins may synchronise GluRIIA, and presynaptic active zone neurexin and neuroligin may be involved in the link between GluRIIA expression and presynaptic active zone dynamics [30,62]. The interactions observed between P120ctn, NrxIV dlg and pak also result in synaptic maturation defects. Null mutants in pak and dlg have also been shown to lead to alterations in glutamate receptor subunits (GluRIIA) [36], however, here we did not see a significant interaction between the dlg/pak transheterozygotes, or the interactions with NrxIV. GluRIIA levels were affected in the pasha/Sep4 cross. Reductions in GluRIIA have been found to lead to a compensatory increase in active zone size [67]. We did not observe a change in active zone puncta in the pasha/Sep4 cross, suggesting that these compensatory mechanisms may be compromised in this case. It is also worth noting that, through changes in the mammalian target of rapamycin mTOR, altered eIF4E-dependent translation results in ASD-relevant phenotypes in mouse [68] and altered regulation of the synthesis of neuroligins. Mutations in Drosophila TOR and eIF4E alter levels of GluRIIA but do not alter the active zones [69]. Interestingly, the fragile X syndrome associated protein FMRP (fragile X syndrome has 30% co-morbidity with ASD) and the miRNA pathway are known to mechanistically interact [70] (Pasha, is part of the miRNA microprocessor complex), while the mRNA of the Sept4 human orthologue (SEPT5) is an FMRP target [10]. Both FMRP, which is known to pause ribosomal translocation [71], and Pasha are involved in translational repression [72,73]. In addition, both mutations in FMRP and the microRNA processing machinery affect the ratios of GluR subunits [43,74]. It may be that pasha/Sep4 deficit leads to the suboptimal translation of Sep4, which functions in complexes that associate with cellular membranes and actin filaments. This may lead to inefficient synaptic anchoring. Further analysis of this process, and those arising from the gene-gene interactions in this study, can now be performed. In summary, our in vivo model system may be well suited to rapidly evaluate how combinations of genes may contribute synergistically to the neurological defects that, in turn, may contribute to ASD.
Although our data strongly supports a significant causal role for synergistic effects underlying ASD, our current study design is unable to reliably estimate the extent as it was limited to (i) considering only pairwise interactions among sets of candidate genes, defined as those genes whose protein products were identified as participating in an ASD-associated interaction network [9], (ii) considering a limited number of neurological phenotypes studied in the model organism Drosophila [11] and (iii) our study considered only those 4 multigenic de novo CNVs identified in individuals with ASD in previous studies where each candidate gene possessed a unique Drosophila orthologue (see Methods). Given that each CNV in those previous studies affected on average 16 protein-coding genes (including non-network genes), we might only expect only 4 genes to possess unique human:Drosophila orthologues (see Methods), severely limiting the ability of this model to examine all combinations of affected genes. However, given that even 16 genes per CNV would generate 240 pairwise gene combinations, it is difficult to imagine the extent and nature of these interactions being examined in a less tractable model with a higher ratio of unique orthologues. While we employed NMJ analysis as a tractable system for studying synaptic function, and circadian analysis to provide a high throughput method for studying behavioural deficit, it would be interesting to expand the behavioural assays to include those which studied social interaction, such as the social space index [75], and also courtship analysis [76]. Nonetheless, the relevance of our findings in Drosophila to humans is supported by the consistent directional effects observed between the increased or decreased bouton counts, which correspond well with the direction of gene dosage change in the human CNV. Taken together with the fact no non-ASD-associated network gene examined yielded abnormal phenotypic effects, when disrupted singularly or in combination (Figs. 2–5), the development of an informatics-targeted Drosophila-screen presents a rapid approach for identifying disease-relevant candidate interactions.
Methods
Selecting Drosophila orthologues of genes affected by de novo CNVs identified in individuals with autism
We considered the four sets of CNVs we informatically examined previously: (1) 73 de novo CNVs from the Autism Genome Project study [5], (2) 28 de novo CNVs from the Marshall et al. study [27], (3) 94 de novo or rare CNVs from the Levy et al. study[28] and (4) 67 de novo or rare CNVs from the Sanders et al. study [29]. On average each CNV overlaps 16 genes with an s.d. of 23 showing wide variation. In order to reduce the combinatorial search space, we considered only those 210 genes whose protein products had been identified in a previous CNV study to participate in a large and highly-significant network of interacting proteins with roles in neural functioning (herein termed the ASD-associated network) [9]. This network provides an aetiological basis through which genetic interactions might be mediated. We downloaded the set of the unique human:Drosophila orthologues as determined by the InParanoid tool [77]. Although our study has strictly focused on unique (1:1) orthologues, we note that a much larger number of Drosophila orthologues could be identified by relaxing the requirement of only a unique human orthologue [78]. Nonetheless, examining the 95 de novo CNVs that harboured genes from the ASD-associated network [9], we identified 7 CNVs for which a unique fly orthologue could be identified for every CNV-overlapped network gene (Table 1; Figs. 2–6, S1, S2). In addition, we selected two non-network genes from each CNV with multiple candidate genes, whose unique fly orthologues were neuronally-expressed in the larval stage. Acknowledging the limited number of unique human: Drosophila orthologues, we were not seeking here to exhaustively ascertain combinatorial effects in Drosophila between all simultaneously copy number changed genes in individuals with autism but rather to investigate the informatically-proposed presence of such effects in vivo (see Discussion). All selected genes were completely overlapped by their respective genes.
Drosophila genetics
All Drosophila stocks were isogenised to the w 1118 wild type background for 7 generations. Where possible, previously described amorphic mutants were selected for analysis. Uncharacterised insertions were validated using deficiencies. Stocks were acquired for positive mutation hits from the Bloomington Drosophila Stock Center (BDSC, Indiana University) unless otherwise stated and contained the following insertions or lesions: p120ctn 308, dlg 1, pak 6, hts k06121, loco KG02176, aux 727, org-1 MB01466 and pasha LL03360, α-Spec rg41, Sep4 NP7170 (Drosophila Genetic Resource Center, Kyoto Institute of Technology), CG13192 EY07746, CG34449 d00976, CtBP 87De-10, CG8507G4779, httMB03997. w 1118; UAS-notch Full, UAS-alpha-spectrin and UAS-Dynactin (Shabire) were used for overexpression relating to gains. To generate these, the coding sequences were amplified using primers containing KpnI sites, subcloned into pUAST, and injected into embryos. The 1032-GAL4 ubiquitous driver was used for overexpression due to its moderate ubiquitous expression. NrxIV4304 (BDSC, Indiana University) was used as a positive control. Negative controls were randomly selected from genes that were not picked as candidates from the CNV set. All negative controls selected displayed both larval and adult neuronal expression (BDSC, Indiana University). w1118; Fsn KG08128, w 1118; cg5359 e03976, w 1118; Hira 185 w 1118; sea EP3364, w 1118; Su(P) EY13245, w 1118; CG14104 f07593, w 1118; nelf-a KG09483, w 1118; CG8507 G4779 and CG3321 c00226 were used for negative controls.
Drosophila larval NMJ analysis
All stocks were cultured on standard molasses/maize meal and agar medium in plastic vials or bottles at 25°C. Larvae were reared on apple juice plates supplemented with molasses/maize meal and yeast as previously described [79]. Larvae were selected for NMJ analysis at 5 days post egg laying. For analysis of bouton number was performed on the NMJ innervating muscles 6 and 7 from hemisegment A2 (1). Over 15 larvae were analysed for each genotype. For immunohistochemistry larvae were fixed for 20mins in 4% paraformaldehyde, or Bouin’s fixative for 30 minutes (GluRIIA). Primary antibodies used were anti-discs large (DLG, Developmental Studies Hybridoma Bank (DSHB), Iowa City, Iowa, USA),anti-Fas2 (DSHB), anti-GluRIIA (DSHB) and anti-BRP (DSHB), all used at 1/100. Secondary antibodies used were AlexaFluor 488 goat anti-rabbit and AlexaFluor 633 goat anti-mouse (Invitrogen) at 1/1000, and anti-HRP-TRITC (The Jackson Laboratory, Bar Harbor, Maine, USA). Z-stacks were taken using a laser-scanning confocal microscope (Leica TCS SP5 II confocal microscope) and analysis performed using ImageJ and Adobe Photoshop. For statistical analysis of the genetic interactions, ANOVA was performed between the control, the two single heterozygous mutations and the transheterozygotes.
GluRIIA and BRP fluorescence analysis
For GluRIIA and BRP analysis at the NMJ, synapses were analysed with optical sections of 0.2μm using a laser-scanning confocal microscope (Leica TCS SP5 II confocal microscope) All digital analysis performed using ImageJ. For BRP staining the number of puntcta was scored over the synapse and normalized to synapse area. For GluRIIA analysis the average fluorescence intensity was analysed over the whole synapse (marked by HRP staining) and then normalized to HRP intensity. No alterations in HRP levels were observed in any genotypes.
Drosophila sleep/wake circadian behavioural assays
All stocks and F1 crosses were cultured on standard molasses/maize meal and agar medium in plastic vials or bottles at 25°C within a light/dark cycle of 12 hrs light/ 12 hrs dark (12:12 LD). For overexpressions, flies were reared at 16°C and then switched during late pupation so to mitigate gross developmental defects. The flies were then transferred to 25°C within a light/dark cycle of 12 hrs light/12 hrs dark (12:12 LD). Flies selected for analysis were between 3 and 5 days old. Flies were the transferred to activity tubes containing 5% sucrose and 2% Bacto agar at one end and were continually synchronized and entrained using a light/dark cycle of 12 hrs light/12 hrs dark (12:12 LD) at 25°C in the circadian incubator for 3 days before data collection. The flies were then switched analysed for experimentation and data collection. Sleep/rest periods were identified as contiguous 5 minute periods of inactivity and were scored and averaged over 2 day period for both dark ‘day’ and ‘night’ cycles. The raw binary data is processed using DAM Filescan102X (Trikinetics, Inc.) and summed into 5 minute bins when analysing sleep/rest parameters. Data analysis was performed within Excel. Statistics were performed using student’s t-tests between ‘day’ and ‘night’ activity.
Supporting Information
A) NMJ morphology and B) circadian analysis for Drosophila orthologues of ASD candidate genes (Table 1). No NMJ or light/dark bias changes were observed in any of the single heterozygous mutants or pairwise crosses.
(TIF)
Green indicates an interaction, red no interaction.
(TIF)
Acknowledgments
We would like to thank all patients, their families and supporting organisations for making available the genotypic data examined in this study. We would like to thank Chris Ponting for helpful suggestions in the presentation of this work.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Medical Research Council, UK funding [SJG, JLL, CW] and the European Union's Seventh Framework Programme under grant agreement n°[241995], project GENCODYS [SJG, CW]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Veenstra-Vanderweele J, Christian SL, Cook EH Jr. (2004) Autism as a paradigmatic complex genetic disorder. Annu Rev Genomics Hum Genet 5: 379–405. [DOI] [PubMed] [Google Scholar]
- 2. Sandin S, Lichtenstein P, Kuja-Halkola R, Larsson H, Hultman CM, et al. (2014) The familial risk of autism. JAMA 311: 1770–1777. 10.1001/jama.2014.4144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bailey A, Le Couteur A, Gottesman I, Bolton P, Simonoff E, et al. (1995) Autism as a strongly genetic disorder: evidence from a British twin study. Psychol Med 25: 63–77. [DOI] [PubMed] [Google Scholar]
- 4. Kusenda M, Sebat J (2008) The role of rare structural variants in the genetics of autism spectrum disorders. Cytogenet Genome Res 123: 36–43. 10.1159/000184690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pinto D, Pagnamenta AT, Klei L, Anney R, Merico D, et al. (2010) Functional impact of global rare copy number variation in autism spectrum disorders. Nature 466: 368–372. 10.1038/nature09146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moreno-De-Luca D, Sanders SJ, Willsey AJ, Mulle JG, Lowe JK, et al. (2013) Using large clinical data sets to infer pathogenicity for rare copy number variants in autism cohorts. Mol Psychiatry 18: 1090–1095. 10.1038/mp.2012.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gilman SR, Iossifov I, Levy D, Ronemus M, Wigler M, et al. (2011) Rare de novo variants associated with autism implicate a large functional network of genes involved in formation and function of synapses. Neuron 70: 898–907. 10.1016/j.neuron.2011.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gai X, Xie HM, Perin JC, Takahashi N, Murphy K, et al. (2012) Rare structural variation of synapse and neurotransmission genes in autism. Mol Psychiatry 17: 402–411. 10.1038/mp.2011.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Noh HJ, Ponting CP, Boulding HC, Meader S, Betancur C, et al. (2013) Network topologies and convergent aetiologies arising from deletions and duplications observed in individuals with autism. PLoS Genet 9: e1003523 10.1371/journal.pgen.1003523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Steinberg J, Webber C (2013) The roles of FMRP-regulated genes in autism spectrum disorder: single- and multiple-hit genetic etiologies. Am J Hum Genet 93: 825–839. 10.1016/j.ajhg.2013.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Alphen B, van Swinderen B (2013) Drosophila strategies to study psychiatric disorders. Brain Res Bull 92: 1–11. 10.1016/j.brainresbull.2011.09.007 [DOI] [PubMed] [Google Scholar]
- 12. Greenspan RJ (2009) Selection, gene interaction, and flexible gene networks. Cold Spring Harb Symp Quant Biol 74: 131–138. 10.1101/sqb.2009.74.029 [DOI] [PubMed] [Google Scholar]
- 13. Mackay TF (2014) Epistasis and quantitative traits: using model organisms to study gene-gene interactions. Nat Rev Genet 15: 22–33. 10.1038/nrg3627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liebl FL, Werner KM, Sheng Q, Karr JE, McCabe BD, et al. (2006) Genome-wide P-element screen for Drosophila synaptogenesis mutants. J Neurobiol 66: 332–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gatto CL, Broadie K (2011) Drosophila modeling of heritable neurodevelopmental disorders. Curr Opin Neurobiol 21: 834–841. 10.1016/j.conb.2011.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lu Y, Wang F, Li Y, Ferris J, Lee JA, et al. (2009) The Drosophila homologue of the Angelman syndrome ubiquitin ligase regulates the formation of terminal dendritic branches. Hum Mol Genet 18: 454–462. 10.1093/hmg/ddn373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pan L, Zhang YQ, Woodruff E, Broadie K (2004) The Drosophila fragile X gene negatively regulates neuronal elaboration and synaptic differentiation. Curr Biol 14: 1863–1870. [DOI] [PubMed] [Google Scholar]
- 18. Zweier C, de Jong EK, Zweier M, Orrico A, Ousager LB, et al. (2009) CNTNAP2 and NRXN1 are mutated in autosomal-recessive Pitt-Hopkins-like mental retardation and determine the level of a common synaptic protein in Drosophila. Am J Hum Genet 85: 655–666. 10.1016/j.ajhg.2009.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Oortveld MA, Keerthikumar S, Oti M, Nijhof B, Fernandes AC, et al. (2013) Human intellectual disability genes form conserved functional modules in Drosophila. PLoS Genet 9: e1003911 10.1371/journal.pgen.1003911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Morales J, Hiesinger PR, Schroeder AJ, Kume K, Verstreken P, et al. (2002) Drosophila fragile X protein, DFXR, regulates neuronal morphology and function in the brain. Neuron 34: 961–972. [DOI] [PubMed] [Google Scholar]
- 21. Wu Y, Bolduc FV, Bell K, Tully T, Fang Y, et al. (2008) A Drosophila model for Angelman syndrome. Proc Natl Acad Sci U S A 105: 12399–12404. 10.1073/pnas.0805291105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Glickman G (2010) Circadian rhythms and sleep in children with autism. Neurosci Biobehav Rev 34: 755–768. 10.1016/j.neubiorev.2009.11.017 [DOI] [PubMed] [Google Scholar]
- 23. Bourgeron T (2007) The possible interplay of synaptic and clock genes in autism spectrum disorders. Cold Spring Harb Symp Quant Biol 72: 645–654. 10.1101/sqb.2007.72.020 [DOI] [PubMed] [Google Scholar]
- 24. Stavropoulos N, Young MW (2011) insomniac and Cullin-3 regulate sleep and wakefulness in Drosophila. Neuron 72: 964–976. 10.1016/j.neuron.2011.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. O'Roak BJ, Vives L, Girirajan S, Karakoc E, Krumm N, et al. (2012) Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature 485: 246–250. 10.1038/nature10989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grima B, Dognon A, Lamouroux A, Chelot E, Rouyer F (2012) CULLIN-3 controls TIMELESS oscillations in the Drosophila circadian clock. PLoS Biol 10: e1001367 10.1371/journal.pbio.1001367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marshall CR, Noor A, Vincent JB, Lionel AC, Feuk L, et al. (2008) Structural variation of chromosomes in autism spectrum disorder. Am J Hum Genet 82: 477–488. 10.1016/j.ajhg.2007.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Levy D, Ronemus M, Yamrom B, Lee YH, Leotta A, et al. (2011) Rare de novo and transmitted copy-number variation in autistic spectrum disorders. Neuron 70: 886–897. 10.1016/j.neuron.2011.05.015 [DOI] [PubMed] [Google Scholar]
- 29. Sanders SJ, Ercan-Sencicek AG, Hus V, Luo R, Murtha MT, et al. (2011) Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron 70: 863–885. 10.1016/j.neuron.2011.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Knight D, Xie W, Boulianne GL (2011) Neurexins and neuroligins: recent insights from invertebrates. Mol Neurobiol 44: 426–440. 10.1007/s12035-011-8213-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bottos A, Rissone A, Bussolino F, Arese M (2011) Neurexins and neuroligins: synapses look out of the nervous system. Cell Mol Life Sci 68: 2655–2666. 10.1007/s00018-011-0664-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tessier CR, Broadie K (2012) Molecular and genetic analysis of the Drosophila model of fragile X syndrome. Results Probl Cell Differ 54: 119–156. 10.1007/978-3-642-21649-7_7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cortesi F, Giannotti F, Ivanenko A, Johnson K (2010) Sleep in children with autistic spectrum disorder. Sleep Med 11: 659–664. 10.1016/j.sleep.2010.01.010 [DOI] [PubMed] [Google Scholar]
- 34. Sun M, Liu L, Zeng X, Xu M, Fang M, et al. (2009) Genetic interaction between Neurexin and CAKI/CMG is important for synaptic function in Drosophila neuromuscular junction. Neurosci Res 64: 362–371. 10.1016/j.neures.2009.04.009 [DOI] [PubMed] [Google Scholar]
- 35. Myster SH, Cavallo R, Anderson CT, Fox DT, Peifer M (2003) Drosophila p120catenin plays a supporting role in cell adhesion but is not an essential adherens junction component. J Cell Biol 160: 433–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Albin SD, Davis GW (2004) Coordinating structural and functional synapse development: postsynaptic p21-activated kinase independently specifies glutamate receptor abundance and postsynaptic morphology. J Neurosci 24: 6871–6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Harden N, Lee J, Loh HY, Ong YM, Tan I, et al. (1996) A Drosophila homolog of the Rac- and Cdc42-activated serine/threonine kinase PAK is a potential focal adhesion and focal complex protein that colocalizes with dynamic actin structures. Mol Cell Biol 16: 1896–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Perrimon N (1988) The maternal effect of lethal(1)discs-large-1: a recessive oncogene of Drosophila melanogaster. Dev Biol 127: 392–407. [DOI] [PubMed] [Google Scholar]
- 39. Jonas RK, Montojo CA, Bearden CE (2014) The 22q11.2 Deletion Syndrome as a Window into Complex Neuropsychiatric Disorders Over the Lifespan. Biol Psychiatry 75: 351–360. 10.1016/j.biopsych.2013.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Denli AM, Tops BB, Plasterk RH, Ketting RF, Hannon GJ (2004) Processing of primary microRNAs by the Microprocessor complex. Nature 432: 231–235. [DOI] [PubMed] [Google Scholar]
- 41. Porsch M, Hofmeyer K, Bausenwein BS, Grimm S, Weber BH, et al. (1998) Isolation of a Drosophila T-box gene closely related to human TBX1. Gene 212: 237–248. [DOI] [PubMed] [Google Scholar]
- 42. Chen YC, Lin YQ, Banerjee S, Venken K, Li J, et al. (2012) Drosophila neuroligin 2 is required presynaptically and postsynaptically for proper synaptic differentiation and synaptic transmission. J Neurosci 32: 16018–16030. 10.1523/JNEUROSCI.1685-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pan L, Broadie KS (2007) Drosophila fragile X mental retardation protein and metabotropic glutamate receptor A convergently regulate the synaptic ratio of ionotropic glutamate receptor subclasses. J Neurosci 27: 12378–12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Betancur C (2011) Etiological heterogeneity in autism spectrum disorders: more than 100 genetic and genomic disorders and still counting. Brain Res 1380: 42–77. 10.1016/j.brainres.2010.11.078 [DOI] [PubMed] [Google Scholar]
- 45. Klei L, Sanders SJ, Murtha MT, Hus V, Lowe JK, et al. (2012) Common genetic variants, acting additively, are a major source of risk for autism. Mol Autism 3: 9 10.1186/2040-2392-3-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bernier R, Gerdts J, Munson J, Dawson G, Estes A (2012) Evidence for broader autism phenotype characteristics in parents from multiple-incidence autism families. Autism Res 5: 13–20. 10.1002/aur.226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stefansson H, Meyer-Lindenberg A, Steinberg S, Magnusdottir B, Morgen K, et al. (2014) CNVs conferring risk of autism or schizophrenia affect cognition in controls. Nature 505: 361–366. 10.1038/nature12818 [DOI] [PubMed] [Google Scholar]
- 48. MacDonald JR, Ziman R, Yuen RK, Feuk L, Scherer SW (2014) The Database of Genomic Variants: a curated collection of structural variation in the human genome. Nucleic Acids Res 42: D986–992. 10.1093/nar/gkt958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Park H, Kim JI, Ju YS, Gokcumen O, Mills RE, et al. (2010) Discovery of common Asian copy number variants using integrated high-resolution array CGH and massively parallel DNA sequencing. Nat Genet 42: 400–405. 10.1038/ng.555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. McDonald-McGinn DM, Zackai EH (2008) Genetic counseling for the 22q11.2 deletion. Dev Disabil Res Rev 14: 69–74. 10.1002/ddrr.10 [DOI] [PubMed] [Google Scholar]
- 51. Duffney LJ, Wei J, Cheng J, Liu W, Smith KR, et al. (2013) Shank3 Deficiency Induces NMDA Receptor Hypofunction via an Actin-Dependent Mechanism. J Neurosci 33: 15767–15778. 10.1523/JNEUROSCI.1175-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dolan BM, Duron SG, Campbell DA, Vollrath B, Shankaranarayana Rao BS, et al. (2013) Rescue of fragile X syndrome phenotypes in Fmr1 KO mice by the small-molecule PAK inhibitor FRAX486. Proc Natl Acad Sci U S A 110: 5671–5676. 10.1073/pnas.1219383110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. van der Bliek AM, Meyerowitz EM (1991) Dynamin-like protein encoded by the Drosophila shibire gene associated with vesicular traffic. Nature 351: 411–414. [DOI] [PubMed] [Google Scholar]
- 54. Featherstone DE, Davis WS, Dubreuil RR, Broadie K (2001) Drosophila alpha- and beta-spectrin mutations disrupt presynaptic neurotransmitter release. J Neurosci 21: 4215–4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Melom JE, Littleton JT (2011) Synapse development in health and disease. Curr Opin Genet Dev 21: 256–261. 10.1016/j.gde.2011.01.002 [DOI] [PubMed] [Google Scholar]
- 56. Pielage J, Bulat V, Zuchero JB, Fetter RD, Davis GW (2011) Hts/Adducin controls synaptic elaboration and elimination. Neuron 69: 1114–1131. 10.1016/j.neuron.2011.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hagedorn EJ, Bayraktar JL, Kandachar VR, Bai T, Englert DM, et al. (2006) Drosophila melanogaster auxilin regulates the internalization of Delta to control activity of the Notch signaling pathway. J Cell Biol 173: 443–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Guan B, Hartmann B, Kho YH, Gorczyca M, Budnik V (1996) The Drosophila tumor suppressor gene, dlg, is involved in structural plasticity at a glutamatergic synapse. Curr Biol 6: 695–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. de Bivort BL, Guo HF, Zhong Y (2009) Notch signaling is required for activity-dependent synaptic plasticity at the Drosophila neuromuscular junction. J Neurogenet 23: 395–404. 10.3109/01677060902878481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sudhof TC (2008) Neuroligins and neurexins link synaptic function to cognitive disease. Nature 455: 903–911. 10.1038/nature07456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chen K, Gracheva EO, Yu SC, Sheng Q, Richmond J, et al. (2010) Neurexin in embryonic Drosophila neuromuscular junctions. PLoS One 5: e11115 10.1371/journal.pone.0011115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Li J, Ashley J, Budnik V, Bhat MA (2007) Crucial role of Drosophila neurexin in proper active zone apposition to postsynaptic densities, synaptic growth, and synaptic transmission. Neuron 55: 741–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Delorme R, Ey E, Toro R, Leboyer M, Gillberg C, et al. (2013) Progress toward treatments for synaptic defects in autism. Nat Med 19: 685–694. 10.1038/nm.3193 [DOI] [PubMed] [Google Scholar]
- 64. Wagh DA, Rasse TM, Asan E, Hofbauer A, Schwenkert I, et al. (2006) Bruchpilot, a protein with homology to ELKS/CAST, is required for structural integrity and function of synaptic active zones in Drosophila. Neuron 49: 833–844. [DOI] [PubMed] [Google Scholar]
- 65. Hida Y, Ohtsuka T (2010) CAST and ELKS proteins: structural and functional determinants of the presynaptic active zone. J Biochem 148: 131–137. 10.1093/jb/mvq065 [DOI] [PubMed] [Google Scholar]
- 66. Stork T, Thomas S, Rodrigues F, Silies M, Naffin E, et al. (2009) Drosophila Neurexin IV stabilizes neuron-glia interactions at the CNS midline by binding to Wrapper. Development 136: 1251–1261. 10.1242/dev.032847 [DOI] [PubMed] [Google Scholar]
- 67. Reiff DF, Thiel PR, Schuster CM (2002) Differential regulation of active zone density during long-term strengthening of Drosophila neuromuscular junctions. J Neurosci 22: 9399–9409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gkogkas CG, Khoutorsky A, Ran I, Rampakakis E, Nevarko T, et al. (2013) Autism-related deficits via dysregulated eIF4E-dependent translational control. Nature 493: 371–377. 10.1038/nature11628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Penney J, Tsurudome K, Liao EH, Elazzouzi F, Livingstone M, et al. (2012) TOR is required for the retrograde regulation of synaptic homeostasis at the Drosophila neuromuscular junction. Neuron 74: 166–178. 10.1016/j.neuron.2012.01.030 [DOI] [PubMed] [Google Scholar]
- 70. Im HI, Kenny PJ (2012) MicroRNAs in neuronal function and dysfunction. Trends Neurosci 35: 325–334. 10.1016/j.tins.2012.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Darnell JC, Van Driesche SJ, Zhang C, Hung KY, Mele A, et al. (2011) FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell 146: 247–261. 10.1016/j.cell.2011.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Edbauer D, Neilson JR, Foster KA, Wang CF, Seeburg DP, et al. (2010) Regulation of synaptic structure and function by FMRP-associated microRNAs miR-125b and miR-132. Neuron 65: 373–384. 10.1016/j.neuron.2010.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Schratt G (2009) microRNAs at the synapse. Nat Rev Neurosci 10: 842–849. 10.1038/nrn2763 [DOI] [PubMed] [Google Scholar]
- 74. Karr J, Vagin V, Chen K, Ganesan S, Olenkina O, et al. (2009) Regulation of glutamate receptor subunit availability by microRNAs. J Cell Biol 185: 685–697. 10.1083/jcb.200902062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Simon AF, Chou MT, Salazar ED, Nicholson T, Saini N, et al. (2012) A simple assay to study social behavior in Drosophila: measurement of social space within a group. Genes Brain Behav 11: 243–252. 10.1111/j.1601-183X.2011.00740.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Goodwin SF, O'Dell KM (2012) The best laid plans: analyzing courtship defects in Drosophila. Cold Spring Harb Protoc 2012: 1140–1145. 10.1101/pdb.prot071647 [DOI] [PubMed] [Google Scholar]
- 77. O'Brien KP, Remm M, Sonnhammer EL (2005) Inparanoid: a comprehensive database of eukaryotic orthologs. Nucleic Acids Res 33: D476–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Reiter LT, Potocki L, Chien S, Gribskov M, Bier E (2001) A systematic analysis of human disease-associated gene sequences in Drosophila melanogaster. Genome Res 11: 1114–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Grice SJ, Liu JL (2011) Survival motor neuron protein regulates stem cell division, proliferation, and differentiation in Drosophila. PLoS Genet 7: e1002030 10.1371/journal.pgen.1002030 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A) NMJ morphology and B) circadian analysis for Drosophila orthologues of ASD candidate genes (Table 1). No NMJ or light/dark bias changes were observed in any of the single heterozygous mutants or pairwise crosses.
(TIF)
Green indicates an interaction, red no interaction.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.