Abstract
Breast cancer is the most common cancer in women worldwide. The majority of deaths attributed to breast cancer are a result of metastatic disease, and 30% of early breast cancers (EBC) will develop distant disease. The 5-year survival of patients with metastatic disease is estimated at 23%. Breast cancer subtypes continue to be stratified histologically on oestrogen, progesterone and human epidermal growth factor-2 (HER2) receptor expression. HER2-positive breast cancers represent 25% of all breast cancer diagnoses. The therapies available for metastatic breast cancer (MBC) are expanding, in particular within the field of HER2-positive disease, with the approval of trastuzumab, pertuzumab, lapatinib and trastuzumab emtansine (TDM-1). Recently, TDM-1 has been shown to improve progression-free survival in HER2 MBC when compared to capecitabine and lapatinib in clinical studies. Its main toxicities are deranged liver function tests and thrombocytopenia. There have also been cases of acute liver failure. Therefore, its use in acute hepatic dysfunction, to our knowledge, has been neither studied nor reported. We report a patient with progressive HER2-positive MBC who had previously responded to multiple HER2-targeted therapies that presented with acute hepatic dysfunction. She was treated with dose-reduced TDM-1 safely, with clear evidence of rapid biochemical, clinical and radiological response. This allowed dose escalation of TDM-1, and the patient maintains an ongoing response.
Key Words: Human epidermal growth factor-2, Metastatic breast cancer, Hepatic dysfunction, Trastuzumab emtansine
Introduction
Breast cancer is the most common cancer in women worldwide [1]. One in 9 women will develop the disease in her lifetime [2]. In 2013, 230,000 women were diagnosed with breast cancer in the United States of America and 39,000 women died as a result of their breast cancer [2, 3]. Early breast cancer (EBC) mortality rates have declined by 30% over the past two decades, and 5-year overall survival rates approach 90% [4, 5]. The majority of breast cancer-related deaths are due to complications associated with metastatic disease [6]. Six percent of breast cancers will be metastatic at diagnosis, and approximately 30% of EBC patients will develop metastatic disease [6]. In contrast to EBC patients, overall survival of patients with metastatic breast cancer (MBC) is approximately 22 months [5].
Breast cancer is a heterogeneous disease with molecular signatures that vary between patients and within individual cancers [7, 8]. The molecular diversity is likely to account for varying prognosis and treatment responses in breast cancer patients [9]. Despite these complexities, breast cancer is still traditionally classified by oestrogen, progesterone and human epidermal growth factor-2 (HER2) receptor expression [7]. HER2-overexpressing cancers represent 25% of breast cancers [4].
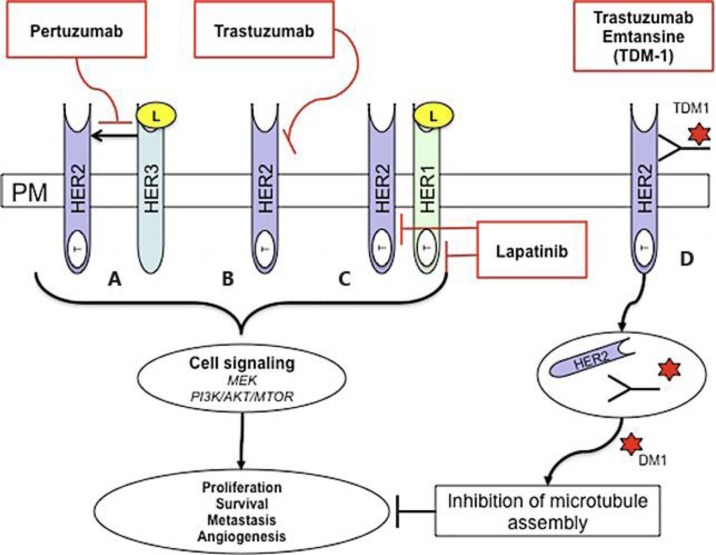
The treatment of HER2-positive MBC requires the analysis of histological, radiological and clinical information [10]. In addition to traditional chemotherapy regimes, hormonal manipulation and bone-protective therapies, there is an ever-expanding portfolio of HER2-targeted therapies for the treatment of MBC [7]. These include trastuzumab, lapatinib, pertuzumab and, most recently, trastuzumab emtansine (TDM-1; fig. 1). Since the introduction of trastuzumab for the management of MBC, the 2-year survival rates in the metastatic setting have doubled [11]. This highlights the impact of HER2-targeted therapies in the management of MBC.
Fig. 1.
HER2-targeted therapies. Human epidermal growth factor (EGF) HER1, HER2, HER3 and HER4 are receptor tyrosine kinases within the plasma membrane (PM) that are involved in signal transduction pathways that modulate cellular processes. They consist of an extracellular ligand binding domain and intracellular tyrosine kinase domain (T) (except for HER3). The ligand (L) binds the receptor extracellular domain, homo-dimerisation and hetro-dimerisation leads to tyrosine kinase domain phosphorylation and activation of downstream signaling pathways. These include the MEK and PI3K/AKT/MTOR pathway that regulate proliferation, survival, metastasis and angiogenesis. HER2 is an orphan receptor and does not need ligand binding for activation. HER2 is also the preferred dimerisation partner for other HER family member proteins. The humanized monoclonal antibody pertuzumab binds HER2 at a region distinct from that of trastuzumab and prevents HER2 dimerisation (A). Similarly, the humanized monoclonal antibody trastuzumab binds HER2, leading to inhibition of downstream signaling in a mechanism that is not fully understood (B). Lapatinib is a reversible small-molecule tyrosine kinase inhibitor that has dual activity against the tyrosine kinase ATP-binding pocket of HER1 and HER2 (C). The antibody-drug conjugate TDM-1 combines trastuzumab linked to the microtubulin-disruptive agent emtansine (DM1). Trastuzumab binds HER2-overexpressing cells and is internalized via endocytosis. Emtansine is released, causing inhibition of microtubule assembly and regulation of cellular processes (D).
TDM-1 is currently available for the treatment of HER2-positive MBC in patients who have progressive disease and have previously been treated with a taxane and trastuzumab. TDM-1 demonstrated activity against trastuzumab-resistant breast cancer models in pre-clinical studies [12]. Phase II clinical studies have confirmed TDM-1 activity in various settings of HER2-positive MBC [13, 14, 15]. Recently, the phase III EMILIA trial compared TDM-1 to capecitabine and lapatinib in patients with HER2-positive MBC previously treated with anthracycline, taxane and trastuzumab [16]. TDM-1 improved progression-free survival (PFS) when compared to capecitabine and lapatinib (9.6 vs. 6.4 months, p < 0.0001) [16]. Ongoing phase III trials will further evaluate the use of TDM-1 in HER2-positive breast cancer [4].
There have been reports of drug-induced liver failure associated with the use of TDM-1. Toxicities reported from the EMILIA trial included thrombocytopenia (12.9%, grade 3), increased alanine transaminase (2.9%, grade 3) and increased aspartate transaminase (4.3%, grade 3) [16]. Reports suggest that TDM-1 has not been studied in patients with serum transaminases >2.5 × ULN (grade 2) or total bilirubin >1.5 × ULN (grade 2) prior to initiation of treatment [17]. Therefore, experience of TDM-1 use within the context of hepatic dysfunction is limited.
To our knowledge, this is the first report describing the use of dose-reduced TDM-1 in acute hepatic dysfunction. Dose-reduced TDM-1 was safe and active allowing a rapid dose escalation.
Case Presentation
A 59-year-old woman with HER2-positive MBC presented with EBC in 2008. Investigations demonstrated this to be a 35-mm grade 3 invasive ductal carcinoma that was oestrogen receptor positive, progesterone receptor negative and HER2 receptor positive. There was also biopsy-proven left axillary disease but no distant metastasis on further imaging. She therefore proceeded to neo-adjuvant chemotherapy.
The patient received 4 cycles of epirubicin and cyclophosphamide followed by 4 cycles of accelerated paclitaxel. The HER2-targeted antibody trastuzumab was added to her treatment regime along with the initiation of the accelerated paclitaxel component. On completion of her neo-adjuvant chemotherapy in May 2009, she underwent a mastectomy and axillary node clearance. Post-operative histological analysis demonstrated complete pathological response to treatment with fibrosis within the breast and axillary lymph nodes, consistent with previous sites of the disease. In the post-operative period, she underwent adjuvant radiotherapy, continued with trastuzumab for 1 year and commenced adjuvant letrozole.
In March 2010, she relapsed with biopsy-proven skin recurrence that was negative for the oestrogen and progesterone receptor and positive for HER2. Imaging demonstrated no distant metastatic disease, and she was commenced on capecitabine and lapatinib (first-line treatment for MBC). Her disease remained stable until September 2011, when there was clinical and radiological progression within the left axilla and chest wall soft tissue. Therefore, her treatment was changed to vinorelbine and trastuzumab (second-line treatment for MBC), and repeat imaging in March 2012 demonstrated no measurable disease. At this juncture, the patient stopped vinorelbine treatment and continued maintenance trastuzumab treatment alone. The response to treatment was short-lived, and her chest wall disease recurred in June 2012, when vinorelbine was again added to trastuzumab leading to stabilisation of her disease.
In January 2013, follow-up imaging demonstrated a solitary liver metastasis alongside progression of the chest wall disease. In light of her progressive disease, treatment was changed to docetaxel with ongoing maintenance trastuzumab (third-line treatment for MBC). Interval imaging demonstrated response to treatment and subsequently she underwent radiofrequency ablation (RFA) to her isolated liver metastasis with complete remission and continued maintenance trastuzumab alone.
The patient's chest wall disease progressed clinically in September 2013, and she was commenced on vertical dual blockade with lapatinib and trastuzumab given in the absence of chemotherapy (fourth-line treatment for MBC). Clinical response to treatment was seen in the chest wall disease within the first three cycles of treatment. Interval imaging in January 2014 showed recurrence at the site of the previous RFA, and the patient underwent further RFA to the area in March 2014. Her chest wall disease remained under good control and she continued on lapatinib and trastuzumab.
In April 2014, the patient showed further signs of progression with the development of jaundice and abdominal discomfort. This was accompanied by an acute deterioration in her liver function tests (LFTs) (fig. 2). The patient was admitted to the hospital for further investigations. Ultrasound of the patient's abdomen showed significant progression of her liver metastasis. This was confirmed by computerised tomography imaging demonstrating a dominant lesion (7 cm) that had regrown in segment V of the liver causing intrahepatic biliary dilatation. Over the next 48 h, her LFTs worsened (fig. 2). It was felt that percutaneous or endoscopic intervention to relieve the intrahepatic biliary dilatation would be technically difficult, and without immediate systemic treatment to gain disease control, her LFTs would continue to deteriorate.
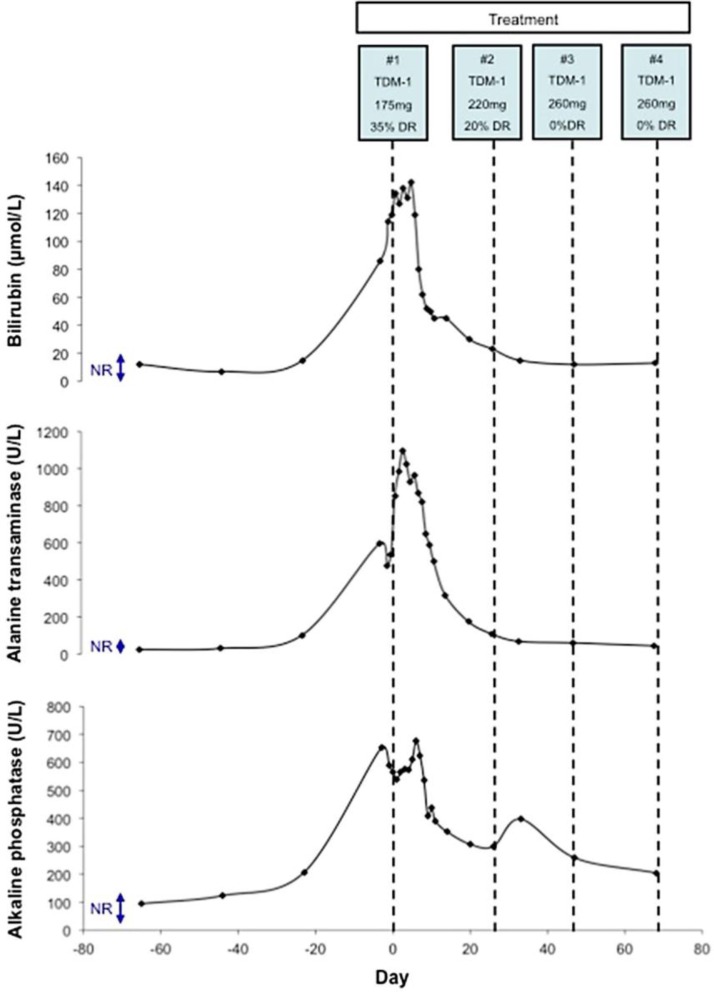
Fig. 2.
Changes in LFTs with TDM-1 treatment. Serum bilirubin (μmol/l), alanine transaminase (U/l) and alkaline phosphatase (U/l) levels are shown. The normal ranges (NR) for bilirubin, alanine transaminase and alkaline phosphatase are <17 µmol/l, <40 U/l and 24–110 U/l, respectively. Each cycle of TDM-1 treatment is shown along with its cycle number, dose and any dose reduction instituted. Day 0 represents the administration of the first cycle of TDM-1.
The patient had previously responded well to sequential HER2-targeted therapies (trastuzumab and lapatinib) and it was felt that she would benefit from further treatment with HER2-directed therapy. In light of her progression on both trastuzumab and lapatinib, she was treated with TDM-1 (fifth-line treatment for HER2-positive MBC). There is limited experience of TDM-1 in acute liver dysfunction, so the patient was treated with a calculated dose reduction (125 mg, 35% dose reduction) and remained as an inpatient for close observation (fig. 2, fig. 3). Magnetic resonance cholangiopancreatography (MRCP) was performed at baseline and confirmed an 87-mm metastatic deposit in segment V of the liver (fig. 3). In addition, her cancer antigen 15–3 (CA15–3) demonstrated an increase in line with disease progression both clinically and on imaging (fig. 3).
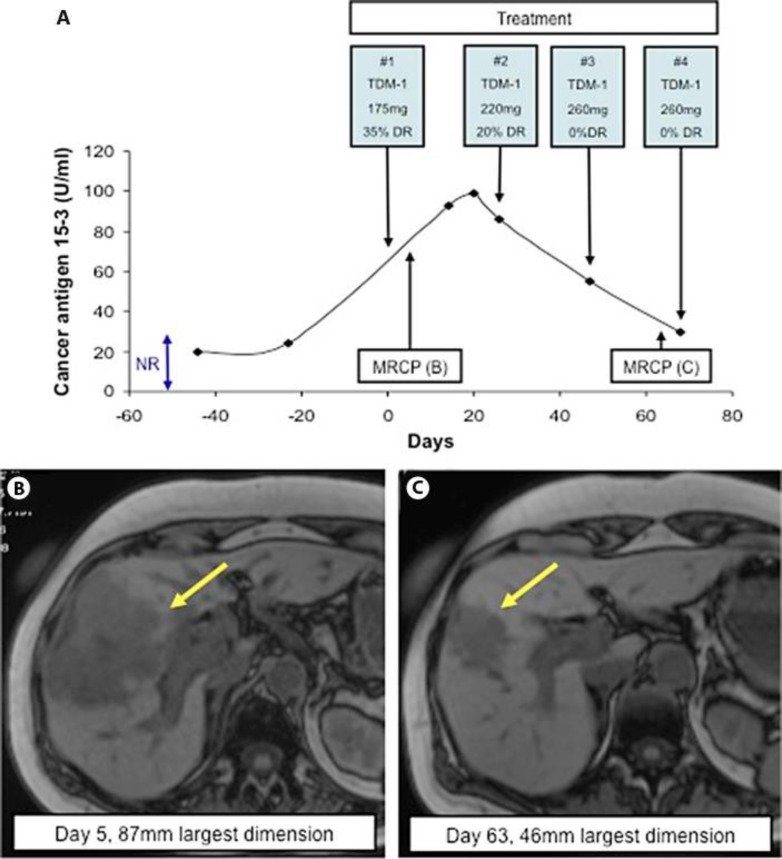
Fig. 3.
CA15–3 and radiological response to treatment with TDM-1. Changes in CA15–3 (U/ml) were recorded in response to treatment (A). The normal range (NR) for CA15–3 is <28 U/ml. Each cycle of TDM-1 treatment is shown along with its cycle number, dose and any dose reduction instituted. Day 0 represents the administration of the first cycle of TDM-1. MRCP was performed at baseline (B) and after administration of 3 cycles of TDM-1 (C) to monitor radiological response of the metastatic deposit (yellow arrows).
The patient's LFTs worsened in the first 6 days after administration of her first cycle of TDM-1 (fig. 2). However, by day 6, her LFTs began to improve and this was maintained (fig. 2). Consistent with this, her clinical state was much improved, her jaundice and associated symptoms resolved and her CA15–3 stabilised. The patient received her second cycle of TDM-1 as an outpatient, and with rapid further improvement in her clinical state the dose was escalated (220 mg, 20% dose reduction). Her second cycle of treatment was accompanied by a continued improvement in her LFTs and associated drop in CA15–3 (fig. 2, fig. 3). She remained clinically well with no predictable toxicities associated with TDM-1 such as thrombocytopenia, worsening LFTs or lethargy.
In light of her ongoing improvement she received her third cycle of TDM-1 at a full dose (260 mg, 0% dose reduction). This was again well tolerated and both the patient's LFTs and CA15–3 continued to improve (fig. 2, fig. 3). In addition to her clinical and biochemical improvement, repeat MRCP after 3 cycles of treatment demonstrated radiological evidence of disease response (fig. 3). It demonstrated a reduction in the previously seen metastasis (from 87 to 46 mm) and resolution of the previously described intrahepatic dilatation. The patient continued on full dose TDM-1 on a three weekly basis as an outpatient. She continued to tolerate the treatment well with no predicable toxicities and with ongoing improvement in her LFTs and CA15–3.
Discussion
MBC remains incurable with an estimated 5-year survival of 23% [18]. However, there are an ever increasing number of therapies available for the treatment of MBC [7]. HER2-positive breast cancer represents 25% of all breast cancer diagnoses [19]. Over recent years, a number of therapies for HER2-positive MBC have been approved, including the monoclonal antibodies trastuzumab and pertuzumab, the dual tyrosine kinase inhibitor lapatinib, and most recently, the antibody-drug conjugate TDM-1 (fig. 1). The multiple treatment options available for HER2-positive MBC mean that its transformation to a chronic disease is becoming reality with improved patient outcomes [4]. The challenge to the oncology community is how to best use these treatments to ensure maximum benefit to the patient population.
TDM-1 has recently been approved for the use in HER2-positive MBC. TDM-1 is a unique antibody-drug conjugate combining the HER2-targeted antibody trastuzumab and the cytotoxic moiety emtansine [20]. Trastuzumab inhibits HER2 signaling, mediates antibody-dependent cell-mediated death and inhibits HER2 shedding [21]. In addition, the associated delivery of emtansine to the cell provides cytotoxic activity, including microtubule disruption, mitotic catastrophe and apoptosis [22].
The approval of a new therapy leads to questions regarding its clinical positioning. TDM-1 has demonstrated activity in HER2-positive MBC in the first and second line setting. In the first-line treatment of HER2-positive MBC, TDM-1 demonstrated improved PFS compared to the combination of docetaxel and trastuzumab (14.2 vs. 9.2 months) [14]. Similarly, TDM-1 demonstrated improved PFS compared to capecitabine and lapatinib (9.6 vs. 6.4 months) as second-line treatment of HER2-positive MBC [16]. Although no large randomised trials have investigated its efficacy beyond second-line treatment, TDM-1 has demonstrated activity as a single agent in HER2-positive MBC previously treated with multiple therapies [13, 15]. Ongoing studies will further evaluate the role of TDM-1 in the treatment of HER2-positive breast cancer [4]. In the case reported, we demonstrated clinical, biochemical and radiological response to TDM-1 in the fifth-line setting for HER2-positive MBC.
The most commonly reported toxicities associated with TDM-1 within the studies described are thrombocytopenia and raised LFTs. In addition, there have been cases of acute hepatic dysfunction [13, 14, 15, 16, 17]. In light of this, experience of TDM-1 in the context of acute hepatic dysfunction is limited and, to our knowledge, has not been reported. Here, we described the use of TDM-1 in a patient with acute hepatic dysfunction. Treatment with dose-reduced TDM-1 led to rapid disease control with clinical, biochemical and radiological response allowing a rapid dose escalation. In addition, the patient experienced no predictable toxicities reported with TDM-1.
The challenge to the oncology community remains to define the best treatment strategies for individual patients with HER2-positive MBC. This will involve decisions, aided by further clinical trials, on where to position each HER2-targeted therapy to gain maximum benefit. Finally, oncologists will need to determine at which point it is reasonable to re-challenge patients with targeted therapies to gain additional clinical benefit.
Disclosure Statement
Prof. Stephen R.D. Johnson has received honoraria from GlaxoSmithKline, Roche and Novartis, and research funding from AstraZeneca and Pfizer.
Acknowledgment
The authors have received support from the National Institute for Health Research Biomedical Research Centre at The Royal Marsden Hospital.
References
- 1.Hery C, et al. Quantification of changes in breast cancer incidence and mortality since 1990 in 35 countries with Caucasian-majority populations. Ann Oncol. 2008;19:1187–1194. doi: 10.1093/annonc/mdn025. [DOI] [PubMed] [Google Scholar]
- 2.Breast Cancer Statistics. http://www.breastcancer.org/symptoms/understand_bc/statistics (accessed December 19, 2014)
- 3.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 4.Tinoco G, et al. Treating breast cancer in the 21st century: emerging biological therapies. J Cancer. 2013;4:117–132. doi: 10.7150/jca.4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegel R, et al. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 6.O'Shaughnessy J. Extending survival with chemotherapy in metastatic breast cancer. Oncologist. 2005;10(suppl 3):20–29. doi: 10.1634/theoncologist.10-90003-20. [DOI] [PubMed] [Google Scholar]
- 7.Sharp A, Harper-Wynne C. Treatment of advanced breast cancer (ABC): the expanding landscape of targeted therapies. J Cancer Biol Res. 2014;2:1036. [Google Scholar]
- 8.Norum JH, Andersen K, Sorlie T. Lessons learned from the intrinsic subtypes of breast cancer in the quest for precision therapy. Br J Surg. 2014;101:925–938. doi: 10.1002/bjs.9562. [DOI] [PubMed] [Google Scholar]
- 9.Sorlie T, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li SG, Li L. Targeted therapy in HER2-positive breast cancer. Biomed Rep. 2013;1:499–505. doi: 10.3892/br.2013.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dawood S, et al. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J Clin Oncol. 2010;28:92–98. doi: 10.1200/JCO.2008.19.9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis Phillips GD, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008;68:9280–9290. doi: 10.1158/0008-5472.CAN-08-1776. [DOI] [PubMed] [Google Scholar]
- 13.Burris HA, 3rd, et al. Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer after prior HER2-directed therapy. J Clin Oncol. 2011;29:398–405. doi: 10.1200/JCO.2010.29.5865. [DOI] [PubMed] [Google Scholar]
- 14.Hurvitz SA, et al. Phase II randomized study of trastuzumab emtansine versus trastuzumab plus docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. 2013;31:1157–1163. doi: 10.1200/JCO.2012.44.9694. [DOI] [PubMed] [Google Scholar]
- 15.Krop IE, et al. A phase II study of trastuzumab emtansine in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer who were previously treated with trastuzumab, lapatinib, an anthracycline, a taxane, and capecitabine. J Clin Oncol. 2012;30:3234–3241. doi: 10.1200/JCO.2011.40.5902. [DOI] [PubMed] [Google Scholar]
- 16.Verma S, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kadcyla: summary of product characteristics. http://www.medicines.org.uk/emc/medicine/28568/SPC/Kadcyla+100+mg+%26 + 160+mg+Powder+for+Concentrate+for+Solution+for+Infusion/ (accessed December 19, 2014)
- 18.American Cancer Society: Cancer Facts and Figures. 2011. http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-029771.pdf (accessed December 19, 2014)
- 19.Slamon DJ, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 20.Barok M, Joensuu H, Isola J. Trastuzumab emtansine: mechanisms of action and drug resistance. Breast Cancer Res. 2014;16:209. doi: 10.1186/bcr3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Junttila TT, et al. Trastuzumab-DM1 (T-DM1) retains all the mechanisms of action of trastuzumab and efficiently inhibits growth of lapatinib insensitive breast cancer. Breast Cancer Res Treat. 2011;128:347–356. doi: 10.1007/s10549-010-1090-x. [DOI] [PubMed] [Google Scholar]
- 22.Barok M, et al. Trastuzumab-DM1 causes tumour growth inhibition by mitotic catastrophe in trastuzumab-resistant breast cancer cells in vivo. Breast Cancer Res. 2011;13:R46. doi: 10.1186/bcr2868. [DOI] [PMC free article] [PubMed] [Google Scholar]