Abstract
Introduction
The purpose of the present study was to investigate the traceability of adverse events (AEs) for branded and generic drugs with identical nonproprietary names and to consider potential implications for the traceability of AEs for branded and biosimilar biologics.
Methods
Adverse event reports in the Food and Drug Administration AE Reporting System (FAERS) were compared with those in a commercial insurance claims database (Truven Health MarketScan®) for 2 drugs (levetiracetam and enoxaparin sodium) with manufacturing or prescribing considerations potentially analogous to those of some biosimilars. Monthly rates of branded- and generic-attributed AEs were estimated pre- and post-generic entry. Post-entry branded-to-generic AE relative rate ratios were calculated.
Results
In FAERS, monthly AE rate ratios during the post-generic period showed a pattern in which AE rates for the branded products were greater than for the generic products. Differences in rates of brand- and generic-attributed AEs were statistically significant for both study drugs; the AE rate for the branded products peaked at approximately 10 times that of the generic levetiracetam products and approximately 4 times that of the generic enoxaparin sodium products. In contrast, monthly ratios for the MarketScan data were relatively constant over time.
Conclusion
Use of the same nonproprietary name for generic and branded products may contribute to poor traceability of AEs reported in the FAERS database due to the significant misattribution of AEs to branded products (when those AEs were in fact associated with patient use of generic products). To ensure accurate and robust safety surveillance and traceability for biosimilar products in the United States, improved product identification mechanisms, such as related but distinguishable nonproprietary names for biosimilars and reference biologics, should be considered.
Electronic supplementary material
The online version of this article (doi:10.1007/s12325-015-0193-5) contains supplementary material, which is available to authorized users.
Keywords: Adverse event attribution, Biologic, Biosimilar, Drug traceability, Nonproprietary name, Pharmacovigilance
Introduction
Biologic therapies are medicinal products made by or derived from the living cells of humans, animals, or microorganisms [1]. Examples of biologic therapies include vaccines, blood products, cytokines, and monoclonal antibodies. Biosimilars, or follow-on biologics, are biologic therapies that are approved for marketing on the basis of data demonstrating that they have physicochemical and functional characteristics comparable to those of a previously licensed biologic therapy (i.e., the reference biologic). The manufacturing process for biologics, including biosimilars, is complex and prevents biosimilars from being exact replicas of the reference biologic. Differences in manufacturing processes could lead to small differences in a biosimilar’s overall efficacy and safety profile, including immunogenicity [2–4], which may not be detected in the abbreviated clinical and non-clinical premarket studies required for regulatory approval of the biosimilar product [3, 5]. Differences between a biosimilar and its reference product may also emerge over time due to manufacturing changes [6]. Further, biosimilars that share the same reference product need not meet regulatory standards of similarity with respect to each other, and thus may have different clinical profiles [7]. For these reasons, precise post-market traceability of biosimilars and reference biologics to further develop the adverse event (AE) profiles of, and identify any unexpected safety signals associated with, each biosimilar product is a point of key interest to health care providers, drug manufacturers, regulators, and policymakers.
Whether biosimilars will or should be considered interchangeable with their reference biologics is a subject of debate [8–10]. The pathway to approval of a biosimilar therapy in the United States (US) is dictated by the Biologics Price Competition and Innovation Act of 2009 [7]. This legislation established a standard for demonstrating that a proposed product is “biosimilar to” the reference biologic as well as a separate standard for demonstrating that a biosimilar is “interchangeable” with the reference biologic [7]. In the European Union (EU), interchangeability is regulated at the Member State level [2]. The potential for reference biologics and biosimilars to be interchanged by prescribers or substituted for one another by pharmacists complicates traceability.
Experience with biosimilar therapies is relatively limited. The EU was the first jurisdiction to develop a robust regulatory pathway for the approval of biosimilars. It approved its first biosimilar, Omnitrope® (somatropin; Sandoz GmbH, Holzkirchen, Germany), in 2006. No biosimilar products have been approved in the US as of January, 2015, although a generic version of enoxaparin sodium, a product that is regulated as a biosimilar in the EU, was approved by the US Food and Drug Administration (FDA) via the standard generic drug (Abbreviated New Drug Application) pathway in 2010. Experience from biosimilars marketed in the EU emphasizes the need for accurate tracking and tracing of biologics, including biosimilars, so that their safety can be adequately monitored post-market [4, 11]. Misattribution of AEs to the reference biologic rather than the responsible biosimilar product (or vice versa) could result in an inaccurate safety profile for both products or impede the identification and attribution of safety signals.
In addition to distinct brand names and batch numbers, another method to facilitate differentiation and traceability of biosimilars and reference biologics in the marketplace would be to assign each biosimilar a nonproprietary name that is related to, but distinguishable from, the reference biologic’s nonproprietary name. Several publications have advised that using identical nonproprietary names may complicate, and in some cases thwart, post-market traceability of biologic and biosimilar products [8, 11–14], including a study that found that AEs reported in the FDA AE Reporting System (FAERS) often are attributed to the branded product when a patient likely received a generic product with the same nonproprietary name [14]. In the present study, we aimed to further investigate the traceability of AEs attributed to branded and generic drugs. We selected 2 drugs, levetiracetam and enoxaparin sodium, because they have manufacturing or prescribing considerations potentially analogous to those of some biosimilars. We compared branded-to-generic AE rates as reported in FAERS [15] to those reported in an insurance claims database [16] that has more strict requirements for product identification.
Methods
Study Drugs
We selected the antiepileptic drug levetiracetam (reference products Keppra® and Keppra XR®; UCB Inc., Smyrna, GA, USA) [17] and the low molecular weight heparin (LMWH) enoxaparin sodium (reference product Lovenox®; Sanofi-Aventis US LLC, Bridgewater, NJ, USA) [18]. Although FDA has rated these products as therapeutically equivalent to their respective reference products (unlike biosimilars, which will likely not be interchangeable with their reference biologics in some cases), for purposes of this study, these products were considered potentially analogous in certain respects to biosimilars. Levetiracetam is considered a narrow therapeutic index (NTI) drug by certain insurers, including Medicaid [19], and the European Medicines Agency considers the follow-on version of enoxaparin sodium to be a biosimilar, because of the potential for considerable heterogeneity in the structure–effect relationship [20]. In the absence of approved biosimilars in the US, the intent here was to use variability in pharmacokinetics and structural complexity of these small-molecule products as appropriate surrogates for the clinical variability and heterogeneity expected with respect to biosimilars and their reference biologics (see online-only appendix for further details on study drug selection). From the perspective of AE monitoring, we would expect more careful tracking for small-molecule drugs with these characteristics.
Data Descriptions
FAERS
Food and Drug Administration maintains an electronic information database, FAERS, to assist its post-market safety surveillance program for all approved small-molecule drugs and therapeutic biologic products [15, 21]. FAERS data were used to estimate monthly reported AEs attributed to the branded or generic products of interest. Minimum data elements for a FAERS submission are an identifiable patient, an identifiable reporter, a reaction or event, and a suspect drug [21].
Truven Health MarketScan® Commercial Claims and Encounters and Medicare Supplemental Database
The MarketScan database includes enrollment history and claims for medical (provider and institutional) and pharmacy services for a large sample of >100 million unique de-identified patients [16]. It reflects real-world practice by capturing the full continuum of care across all settings, including physician office visits; hospital stays; and retail, mail order, and specialty pharmacies. This database was used to estimate the AEs attributed to the branded or generic products of interest as well as the number of monthly branded and generic prescriptions for these products. Compared with FAERS, the MarketScan database has more strict requirements for product identification. For example, drugs are identified in the MarketScan database via National Drug Codes (NDCs), which identify the labeler, product, and trade package size of each drug. As such, information such as the specific strength, dosage form, and formulation of a drug can be linked to each drug claim of a patient. NDCs are not a mandatory part of AE reports submitted to FAERS.
National Prescription Audit™ From IMS Health Incorporated
The study was dependent upon availability of prescribing data for the drugs studied: national monthly prescription counts [22] and MarketScan administrative claims data [16]. The number of branded and generic prescriptions filled by month was obtained from the National Prescription Audit (NPA) by IMS Health Incorporated, which represents and captures over 70% of all prescription activities in the US and covers all products, classes, and manufacturers [22]. We assumed that the unknown number of prescriptions contributing to AEs in FAERS is roughly proportional to the branded and generic drugs’ market shares in the IMS data.
AEs in the FAERS and MarketScan Data
Adverse events for each drug were identified 1 year before generic entry into the market and for up to 2 years after generic entry (Fig. 1). With the date of final FDA regulatory approval as a proxy for entry into the market, 38 generic products for levetiracetam and 3 generic products for enoxaparin sodium were on the US market during the study period (i.e., the first 2 years after first generic entry) (see Table S1, online-only supplemental information). Quarterly FAERS data were downloaded from the FAERS website for the period between 2007 Q1 and 2012 Q3; events from the US were identified and potential duplicate reports were removed as per FAERS guidelines [23]. The FAERS drug name variable was used to identify reports associated with the study drugs. All drug name values for reports remaining in this stage of the sample selection were reviewed, and reports corresponding to the study drugs (both the branded and generic names) were identified. Only those reports where one of these drugs was identified as the primary suspect were included. Potential misspellings of study drug names were reviewed independently by two individuals to minimize exclusion of AE reports in which a study drug name was probably misspelled.
Fig. 1.
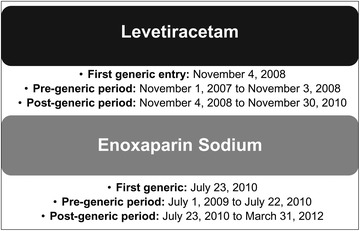
Generic entry dates and study periods for levetiracetam and enoxaparin sodium
In the FAERS database, AEs are coded using Medical Dictionary for Regulatory Activities (MedDRA) terms; MedDRA is a clinically validated international medical terminology dictionary used by regulatory authorities. The MarketScan database, in contrast, identifies medical conditions by International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. To identify the equivalent AEs in the MarketScan data, the MedDRA terms were mapped to ICD-9-CM codes using software provided by the Maintenance and Support Services Organization [MedDRA Desktop Browser 3.0.2b, MedDRA Maintenance and Support Services (MSSO), McLean, VA, USA] [24] and an ICD-9-CM code list. AEs identified in the FAERS database that did not have a suitable ICD-9-CM code or that had an ICD-9-CM code that was too broad (i.e., that would capture many AEs in MarketScan that may not be associated with the drug) were excluded. In addition, AEs identified in the FAERS data that were unlikely to be coded on a medical claim, such as nausea, headache, or common colds, were also excluded. To reduce noise further, only AEs that are listed in the drug prescribing information or those that were present in at least 1% of the AE reports for the given drug in the FAERS data were included. Similar MedDRA terms were grouped into AE categories (e.g., the following MedDRA terms were combined into an anemia category: anemia, hemorrhagic anemia, and iron deficiency anemia). Tables S2 and S3 (online-only supplemental information) include the AE categories and corresponding MedDRA terms and ICD-9-CM codes considered for the two study drugs.
Patient-level counts of branded- or generic-attributed AEs in FAERS were then summed to calculate total monthly FAERS branded- or generic-attributed AE counts; only one AE category per report was counted (i.e., a report containing multiple MedDRA terms from the same category was counted as one AE). To identify AEs in the MarketScan data, all medical claims among patients with claims for the drugs of interest were extracted. Among these, medical claims for the AE categories for each drug of interest (Tables S2 and S3, online) were identified via relevant ICD-9-CM codes. MarketScan medical claims for AEs during the periods of interest were attributed to a particular product at the patient level based on a patient’s continuous possession of either the branded or a generic drug. Specifically, claims for the branded and generic products of interest were identified via NDCs. See online-only appendix for additional details concerning the identification of AE claims and attribution.
Statistical Analysis
Food and Drug Administration AE Reporting System AE rates were calculated as the total monthly count of branded- or generic-attributed AEs reported in the FAERS database divided by the total number of branded or generic prescriptions filled that month in the IMS NPA database. The FAERS branded-to-generic ratio of AEs for each month was calculated as the brand-attributed rate of AEs divided by the generic-attributed rate of AEs. Likewise, MarketScan AE rates were calculated as the total monthly MarketScan count of branded- or generic-attributed AEs divided by the total MarketScan count of branded or generic prescriptions filled that month. The MarketScan branded-to-generic ratio of AEs for each month was calculated as the brand-attributed rate of AEs divided by the generic-attributed rate of AEs.
To investigate how AE reporting varied across time for the branded product both pre- and post-generic entry date, the monthly rates of brand- and generic-attributed AE rates in both the FAERS and MarketScan data, as well as an estimated monthly trend, were plotted. Likewise, to investigate whether the brand-to-generic AE rate ratios differ in FAERS data versus the MarketScan data, the monthly brand-to-generic AE rate ratios for the FAERS/IMS data and MarketScan data as well as the monthly estimated trend were also plotted.
To assess the changes in AE rates and rate ratios over time, the trends in the data were estimated. Exploratory data analysis revealed that a parametric model to estimate the trend (e.g., linear trend) may not provide a good fit to the data. Accordingly, a trend was fitted to the data using Locally Estimated Scatterplot Smoother (LOESS), a technique often used to estimate the trend in data over time when there is no parametric trend in the data [25]. A robust version of the algorithm resistant to the effects of outliers was used. 95% confidence intervals were constructed for the estimated trend from the standard error estimates of the residuals from the LOESS fit. Statistical analysis was carried out using R (version 3.0.0, R Foundation for Statistical Computing, Vienna, Austria).
Statement of Ethics
Ethical approval was not required, as the data analyzed were de-identified records from regulatory or insurance databases. This article does not contain any new studies with human or animal subjects performed by the authors.
Results
An overview of the number of AE reports and prescriptions for levetiracetam and enoxaparin sodium in both data sources is provided in Table 1.
Table 1.
Overview of adverse event (AE) reports and prescriptions for levetiracetam and enoxaparin sodium
| Levetiracetam | Enoxaparin sodium | |
|---|---|---|
| Number of AEs | ||
| FAERS | 1061 | 385 |
| Claims data | 93,006 | 175,837 |
| Number of drug prescriptions | ||
| IMS NPA | 16,247,074 | 8,143,747 |
| Claims data | 900,571 | 537,516 |
FAERS Food and Drug Administration AE Reporting System, NPA National Prescription Audit
AE Rates for Levetiracetam
For the branded levetiracetam product in the FAERS dataset, the monthly trend in the AE rate was fairly constant during the period before generic entry and steadily increased over the two-year post-generic entry period (Fig. 2a). For the generic levetiracetam products in the FAERS dataset, the monthly AE rate steadily decreased over time and stabilized approximately 1 year after entry (Fig. 2a). In contrast to the FAERS data, the monthly AE rates for the branded levetiracetam product in the MarketScan claims data were fairly constant for the entire 3-year study period, and the AE rates of the branded and generic levetiracetam products were relatively constant during the 2-year post-generic entry period (Fig. 2b). Figure 3 compares the ratio of the monthly brand AE rate to the monthly generic AE rate for levetiracetam during the post-generic period for the two datasets. Although the ratios were nearly constant in the claims dataset, the ratio increased over time in the FAERS dataset; that is, the monthly AE rate for the branded levetiracetam product, was on average almost 10 times that of the generic products toward the end of the study period. This difference in the brand-to-generic AE rate ratios between the FAERS and claims data was statistically significant.
Fig. 2.
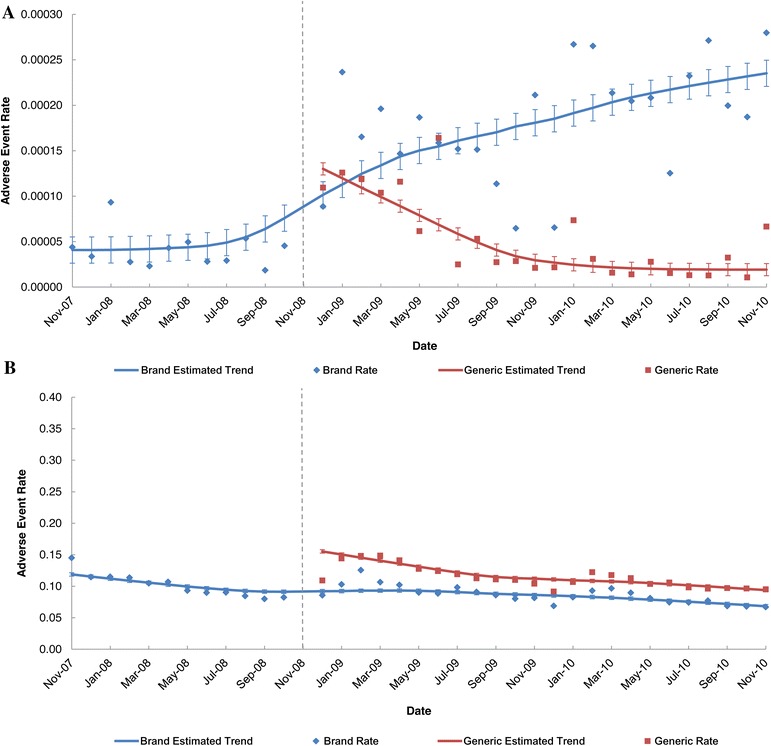
Levetiracetam: monthly brand- and generic-attributed adverse event rate and 95% confidence intervals during the pre- and post-generic entry periods. AE rates were calculated as the total monthly count of branded- or generic-attributed AEs divided by the total number of branded or generic prescriptions filled that month. a FAERS. b Truven Health MarketScan® database. The dotted vertical line represents introduction of generic levetiracetam; data points to the left of the line represent the pre-generic period and data points to the right of the line represent the post-generic period. FAERS Food and Drug Administration Adverse Event Reporting System
Fig. 3.
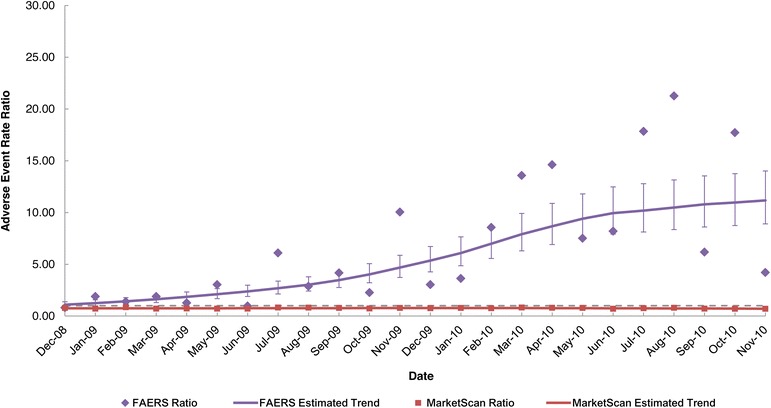
FAERS and MarketScan® adverse event rate ratios and 95% confidence intervals for levetiracetam during the post-generic entry period. FAERS Food and Drug Administration Adverse Event Reporting System
AE Rates for Enoxaparin Sodium
For enoxaparin sodium, the trends were similar to those observed for levetiracetam but less pronounced (in Figs. 4a, b, 5). In the FAERS data, the monthly AE rates for the branded product increased over time after generic entry, whereas the monthly AE rates for the generic products decreased and then stabilized over time (Fig. 4a). In the MarketScan data, the monthly AE rates were nearly constant for the branded enoxaparin sodium product over the three-year study period, and the monthly AE rates of the generic enoxaparin sodium products were relatively constant during the post-generic period (Fig. 4b). The monthly AE rates ratios for the FAERS data indicate that the ratio increased and then stabilized to around 4 (i.e., the AE rate for the branded enoxaparin sodium product was 4 times that of the generic products on average) (Fig. 5). In contrast, the monthly ratios for the claims data were almost constant over time. These differences were statistically significant.
Fig. 4.
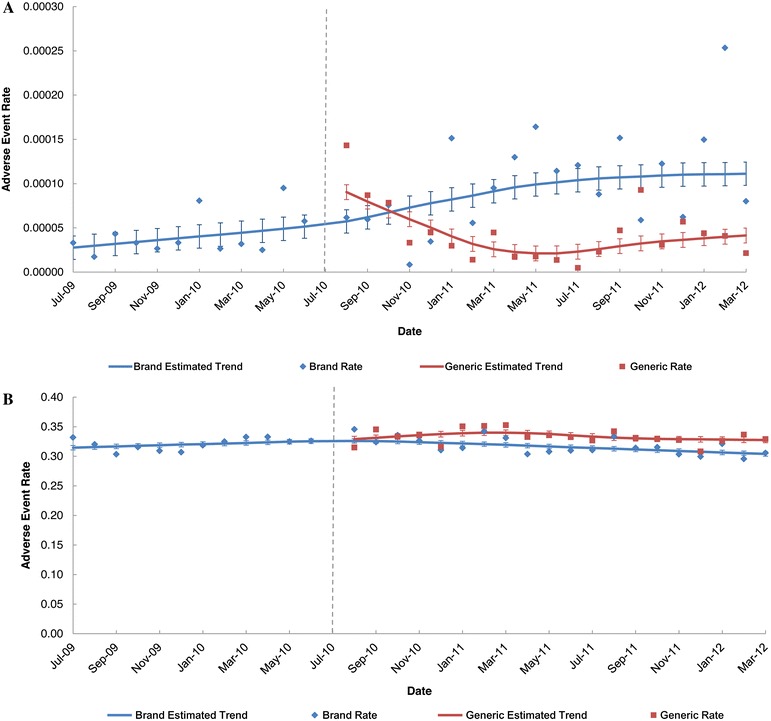
Enoxaparin sodium: monthly brand- and generic-attributed adverse event rate and 95% confidence intervals during the pre- and post-generic entry periods. AE rates were calculated as the total monthly count of branded- or generic-attributed AEs divided by the total number of branded or generic prescriptions filled that month. a FAERS. b MarketScan®. The dotted vertical line represents introduction of generic enoxaparin sodium; data points to the left of the line represent the pre-generic period and data points to the right of the line represent the post-generic period. FAERS Food and Drug Administration Adverse Event Reporting System
Fig. 5.
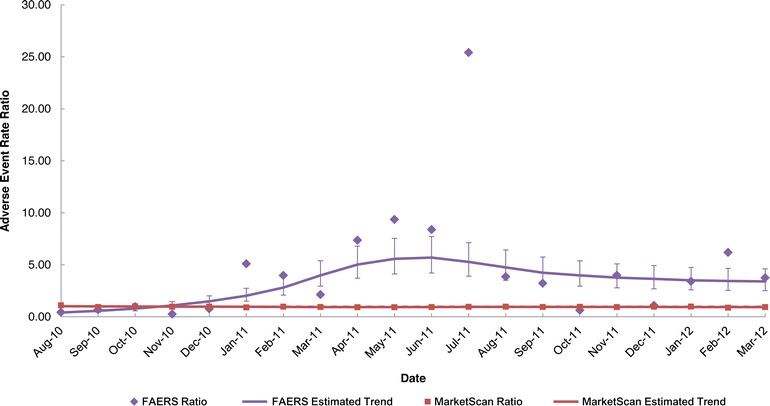
FAERS and MarketScan® adverse event rate ratios and 95% confidence intervals for enoxaparin sodium during the post-generic entry period. FAERS Food and Drug Administration Adverse Event Reporting System
Discussion
When comparing FAERS-derived branded- and generic-attributed AEs against an insurance claims data source that has more strict requirements for product identification, one would expect that the branded and generic AE rates would be similar in both databases, given that these products share the same active drug substance and FDA has deemed them therapeutically equivalent. Consistent with this expectation, branded AE rates in a nationally representative claims database were nearly equal to those of the generic products and remained constant after generic introduction. The observed increase in branded-to-generic AE ratio in the FAERS data, in contrast, indicates that AEs in the FAERS database were frequently misattributed to the branded product when a generic product was in fact associated with the AEs. Our study, thus, helps to confirm the findings of Lietzan et al. [14], namely that AEs associated with generic products are often misattributed to branded products in FAERS data. These results suggest that FAERS data may not provide reliable traceability for generic drugs and their branded reference products, which share the same nonproprietary name [14]. Moreover, a recent publication also evaluated potential misattribution AEs for enoxaparin generics in passive (i.e., FAERS) and active (i.e., claims data) surveillance [26]. The authors found that the number of AE reports processed by generic manufactures was much lower than expected based on market share and the number of events attributable to specific generic enoxaparin products was approximately ninefold lower than expected [26].
The nomenclature for biosimilars has been debated in the US and internationally; however, no formal policy has been announced by the FDA as of January, 2015. Assuming that biosimilars would be susceptible to FAERS traceability issues similar to those found in our study (i.e., misattribution of AEs where products share the same nonproprietary name), our results can provide guidance to policy makers responsible for determining optimal nomenclature for biosimilars. Our study suggests that use of distinguishable nonproprietary names for biosimilars and reference biologics has the potential to enhance drug traceability and pharmacovigilance. Enhanced traceability in FAERS data would be useful, because despite that claims databases have strict product identification requirements that facilitate accurate AE attribution, they are not ideal for detection of unexpected AEs. Our study suggests that distinguishable nonproprietary names for biosimilars may be one way to help promote the attribution of AEs to the correct product [13, 27], assisting the identification and tracking of safety information under the current system in the US.
In a prior study that investigated the traceability of products, including biosimilars, in the EU’s EudraVigilance database, Vermeer et al. [4] reported that the specific biologic was identifiable in 96% of AE reports involving suspected biologics for which a biosimilar was available in the EU. This identification was based on inclusion of either of the biologic’s brand name or the biologic’s nonproprietary name as well as the manufacturer name in reports. The Vermeer et al. [4] study, however, did not address the possibility that AEs may have been misattributed to the wrong brand name product or the wrong manufacturer. In the same study, 79% of Eudra Vigilance reports involving suspected biologics and 95% of Eudra Vigilance reports for biosimilars identified by product name lacked a batch number, which highlights the challenges associated with tracing AEs to the batch level [4].
In the AbbVie-sponsored study by Lietzan et al. [14], which compared total brand-attributed AEs in FAERS against total branded prescriptions filled, the number of brand-attributed AEs tended to remain the same or increase as the total number of branded scripts decreased for 6 of the 8 branded/generic comparisons studied. The single NTI drug studied failed to show a pattern of misattribution of AEs to the branded product after introduction of the generic product, and 1 drug did not have sufficient data post-generic entry to determine whether a reliable trend existed. For the remaining 6 products, the increase in the ratio of brand-attributed AEs to branded prescriptions suggested that many of the AEs experienced by patients receiving the generic products likely were incorrectly reported as associated with the branded drug [14]. The present study confirmed this pattern of misattribution in the FAERS database for the LMWH drug enoxaparin sodium and also the NTI antiepileptic drug levetiracetam. Further, the present study expanded on the findings of Lietzan et al. [14] by demonstrating that the pattern of misattribution observed in the FAERS database did not occur in the nationally representative MarketScan claims database, a finding that was also reported in the Grampp et al. [26] study of enoxaparin generics.
In contrast, a policy paper issued by the chief scientific officer of Hospira [28] argues that distinct brand names will ensure adequate traceability of biosimilars and their reference biologics. The commentary cites internal safety reporting data for two biosimilars, Retacrit™ (epoetin alfa; Hospira, Lake Forest, IL, USA) and Nivestim™ (filgrastim; Hospira, Lake Forest, IL, USA). It discusses Hospira’s post-market pharmacovigilance data from the EU, in which the suspect drug was identified by its brand name in >99% of post-market reports for Hospira’s erythropoietin biosimilar and >95% of reports for Hospira’s filgrastim biosimilar [28]. A potential bias of the Hospira study, however, is that results were based on reports received by the manufacturer and did not include other sources such as EudraVigilance [29]. When reporters notify the manufacturer of an AE, they are likely confident that the suspect drug is that manufacturer’s product; thus, this method is subject to selection bias for reporters who would not misattribute AEs. Moreover, Hospira’s study did not address how many AE reports associated with its biosimilars may have been misattributed to the brand product or to other biosimilars by brand name [28]. Considering these limitations, further evidence is needed to support Hospira’s proposition that distinct brand names will ensure effective pharmacovigilance.
Limitations
Similar to the drugs studied in the analysis by Lietzan et al. [14] and Grampp et al. [26], the generic products investigated in the present analysis have been rated by FDA as therapeutically equivalent to their branded reference products. AE misattribution, therefore, may be greater for these products compared with biologics that FDA does not deem interchangeable. With therapeutically equivalent products, AE reporters may perceive that it is less important to specify the particular product when reporting an AE or may not be aware that a substitution actually occurred. Likewise, it is possible that reporters may be more vigilant with regard to AE attribution for biosimilars, and misattribution rates could be lower with respect to biosimilars than observed in our study.
Another potential limitation is that some of the AE reports actually attributable to branded products in the FAERS database in the present study could have been identified only by generic name. Such cases would lead to a lower branded AE rate relative to the generic AE rate. Evidence from Lietzan et al. [14], Grampp et al. [26], and the present study, however, suggests that AE misattribution is likely to be more common in the other direction; that is, AEs associated with generic drugs are more likely to be reported by brand name.
The temporal reporting of prescriptions filled and the occurrence of an AE is the only way to directly link an AE to a specific drug in claims data. Thus, it is possible that some of the AEs attributed to the respective drugs in the MarketScan database may have been unrelated to the study drugs. The impact of this occurrence is probably minimal, for there is no reason to suspect bias in favor of branded vs. generic drugs.
Our study examined only data in the 1 year pre- and 2 years post-generic entry; it is possible that longer term data could have yielded more robust findings. Finally, our study used an assumption that the unknown number of prescriptions contributing to AEs in FAERS was proportional to the branded and generic drug market share in IMS. This assumption was based on the fact that IMS is a national representative sampling of prescription drug sales and, thus, is the best source available for such a calculation.
Conclusion
Results of the present study may have implications for the traceability of biosimilars once they enter the US market. To ensure accurate and robust safety surveillance and traceability for biosimilar products in the US, improved product identification mechanisms, such as related but distinguishable nonproprietary names for biosimilars and reference biologics, should be considered.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This study, article processing charges and all data analyses were funded by AbbVie Inc. AbbVie participated in the study design, research, data collection, analysis and interpretation, and in the writing and reviewing of this publication. All authors had full access to all the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. Medical writing support was provided by Cathryn M. Carter, MS, of Arbor Communications, Inc; this support was funded by AbbVie.
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Conflict of interest
J. Chao is an employee of AbbVie and may hold stocks or shares.
M. Skup is an employee of AbbVie and may hold stocks or shares.
E. Alexander is an employee of AbbVie and may hold stocks or shares.
N. Tundia is an employee of AbbVie and may hold stocks or shares.
P. Mulani is an employee of AbbVie and may hold stocks or shares.
D. Macaulay is an employee of Analysis Group, and received payment from AbbVie for participating in this research.
E. Wu is an employee of Analysis Group, and received payment from AbbVie for participating in this research.
Compliance with ethics guidelines
Ethical approval was not required, as the data analyzed were de-identified records from regulatory or insurance databases. This article does not contain any new studies with human or animal subjects performed by the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
References
- 1.Questions and answers on biosimilar medicines (similar biological medicinal products). European Medicines Agency EMA/837805/2011, 27 September 2012. http://www.ema.europa.eu/docs/en_GB/document_library/Medicine_QA/2009/12/WC500020062.pdf. Accessed Jan 2015.
- 2.Guideline on similar biological medicinal products. European Medicines Agency CHMP/437/04, 30 October 2005. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003517.pdf. Accessed Jan 2015.
- 3.US Food and Drug Administration Guidance for industry: scientific considerations in demonstrating biosimilarity to a reference product. Rockville: FDA, 2012. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM291128.pdf. Accessed Jan 2015.
- 4.Vermeer NS, Straus SM, Mantel-Teeuwisse AK, et al. Traceability of biopharmaceuticals in spontaneous reporting systems: a cross-sectional study in the FDA Adverse Event Reporting System (FAERS) and EudraVigilance databases. Drug Saf. 2013;36:617–625. doi: 10.1007/s40264-013-0073-3. [DOI] [PubMed] [Google Scholar]
- 5.Schellekens H. Bioequivalence and the immunogenicity of biopharmaceuticals. Nat Rev Drug Discov. 2002;1:457–462. doi: 10.1038/nrd818. [DOI] [PubMed] [Google Scholar]
- 6.Ramanan S, Grampp G. Drift, evolution, and divergence in biologics and biosimilars manufacturing. BioDrugs. 2014;28:363–372. doi: 10.1007/s40259-014-0088-z. [DOI] [PubMed] [Google Scholar]
- 7.Biologics Price Competition and Innovation Act, 2009. Washington, DC: FDA, 2009. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/UCM216146.pdf. Accessed Jan 2015.
- 8.Nowicki M. Basic facts about biosimilars. Kidney Blood Press Res. 2007;30:267–272. doi: 10.1159/000105133. [DOI] [PubMed] [Google Scholar]
- 9.Strand V, Cronstein B. Biosimilars: how similar? Intern Med J. 2014;44:218–223. doi: 10.1111/imj.12292. [DOI] [PubMed] [Google Scholar]
- 10.Danese S, Gomollon F, Governing Board and Operational Board of ECCO ECCO position statement: the use of biosimilar medicines in the treatment of inflammatory bowel disease (IBD) J Crohns Colitis. 2013;7:586–589. doi: 10.1016/j.crohns.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 11.Casadevall N, Edwards IR, Felix T, et al. Pharmacovigilance and biosimilars: considerations, needs and challenges. Expert Opin Biol Ther. 2013;13:1039–1047. doi: 10.1517/14712598.2013.783560. [DOI] [PubMed] [Google Scholar]
- 12.Zuñiga L, Calvo B. Biosimilars: pharmacovigilance and risk management. Pharmacoepidemiol Drug Saf. 2010;19:661–669. doi: 10.1002/pds.1948. [DOI] [PubMed] [Google Scholar]
- 13.Casadevall N, Felix T, Strober BE, Warnock DG. Similar names for similar biologics. BioDrugs. 2014;28:439–444. doi: 10.1007/s40259-014-0099-9. [DOI] [PubMed] [Google Scholar]
- 14.Lietzan EF, Sim LE, Alexander EA. Biosimilar naming: How do adverse event reporting data support the need for distinct nonproprietary names for biosimilars? Food and Drug Law Institute’s Food and Drug Policy Forum 2013;3:1–20. http://www.fdli.org/docs/default-document-library/lietzan-faers-bio-final-3-27-13.pdf. Accessed Jan 2015.
- 15.US Food and Drug Administration. FDA Adverse Event Reporting System (FAERS). http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/default.htm. Accessed Jan 2015.
- 16.Truven Health Analytics. Truven Health Analytics MarketScan® Research databases. http://truvenhealth.com/your-healthcare-focus/analytic-research/marketscan-research-databases. Accessed Jan 2015.
- 17.Keppra® (levetiracetam) prescribing information. UCB, Inc. Smyrna, GA. (IMS National Prescription Audit™). http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021035s089,021505s030lbl.pdf. Accessed Jan 2015.
- 18.Lovenox® (enoxaparin sodium) prescribing information. Sanofi Aventis US, LLC. Bridgewater, NJ. http://products.sanofi.us/lovenox/lovenox.html. Accessed Jan 2015.
- 19.Anthem HealthKeepers Plus. Medicaid Approved Formulary/Preferred Drug List (Condensed Version), Effective September 1, 2014. http://www.anthem.com/provider/va/f3/s3/t0/pw_ad089954.pdf. Accessed Jan 2015.
- 20.Draft Guideline on non-clinical and clinical development of similar biological medicinal products containing low molecular-weight-heparins. European Medicines Agency EMEA/CHMP/BMWP/118264/2007 Rev. 1. January 17, 2013. http://www.ema.europa.eu/ema/doc_index.jsp?curl=pages/includes/document/document_detail.jsp?webContentId=WC500138309&murl=menus/document_library/document_library.jsp&mid=0b01ac058009a3dc. Accessed Jan 2015.
- 21.US Food and Drug Administration. Specifications for preparing and submitting electronic ICSRs and ICSR attachments to FAERS. http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/FormsSubmissionRequirements/ElectronicSubmissions/UCM153588.pdf. Accessed Jan 2015.
- 22.IMS National Prescription Audit™. IMS Health. http://www.imshealth.com/deployedfiles/ims/Global/Content/Insights/Researchers/NPA_Data_Brief.pdf. Accessed Jan 2015.
- 23.US Food and Drug Administration. FDA Adverse Event Reporting System (FAERS): latest quarterly data files. http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/ucm082193.htm. Accessed Jan 2015.
- 24.MedDRA Maintenance and Support Services Organization (MSSO). http://www.meddra.org/about-meddra/organisation/msso. Accessed Jan 2015.
- 25.Cleveland WS, Devlin SJ. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc. 1979;74:829–836. doi: 10.1080/01621459.1979.10481038. [DOI] [Google Scholar]
- 26.Grampp G, Bonafede M, Felix T, et al. Active and passive surveillance of enoxaparin generics: a case study relevant to biosimilars. Expert Opin Drug Saf. 2015;14:349–360. doi: 10.1517/14740338.2015.1001364. [DOI] [PubMed] [Google Scholar]
- 27.Zelenetz AD, Ahmed I, Braud EL, et al. NCCN biosimilars white paper: regulatory, scientific, and patient safety perspectives. J Natl Compr Cancer Netw. 2011;9(supp 4):S1–S22. doi: 10.6004/jnccn.2011.0136. [DOI] [PubMed] [Google Scholar]
- 28.Ramachandra S. What’s in a name? The importance of biosimilar nonproprietary names for healthcare innovation. Hospira Policy Paper 2013. http://www.hpm.com/pdf/blog/What%27s%20In%20a%20Name%20-%20Hospira%20Policy%20Paper%20-%20Oct%202013.pdf. Accessed Jan 2015.
- 29.EudraVigilance. Pharmacovigilance in the European Economic Area. European Medicines Agency. https://eudravigilance.ema.europa.eu/highres.htm. Accessed Jan 2015.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


