Abstract
Periosteal chondrosarcoma (PC) is a rare low-grade malignant cartilaginous tumor originating on the bone surface. Wide surgical resection is the recommended treatment. Prognosis is usually good if surgery is adequate. Metastasis is late and very rare. We present the clinical, radiographic, and pathological features of a PC accompanied with fibular cortical invasion in a 30-year-old woman. Wide resection was performed at presentation, but a whole-body positron emission tomography/computed tomography (PET/CT) examination 10 months after operation showed multiple bone metastases (MBM) especially in the spine, pelvis, bilateral femurs, and humeri without local recurrence. To the best of our knowledge, the present report is the first concerning a PC with so extensive postoperative MBM but without local recurrence.
Keywords: Fibula, Periosteal chondrosarcoma, Multiple bone metastases, Cortical invasion, MRI, PET/CT
Background
PC is a rare low-grade malignant cartilaginous tumor originating on the bone surface, accounting for less than 2% of all chondrosarcomas and about 0.2% of all bone tumors [1-3]. Wide surgical resection is the recommended treatment. Prognosis is usually good if surgery is adequate. Metastasis is late and very rare [4].
We report a grade 2 PC with cortical invasion in the left proximal fibula of a 30-year-old woman. MBM happened especially in the spine, pelvis, bilateral femurs, and humeri in the absence of local recurrence at 10 months after operation.
Case presentation
A 30-year-old woman presented with a 1-year history of a gradually enlarging surface lesion on the posterolateral aspect of left proximal shank. She denied the history of infection, major traumatic injury, and familial history of cancer. Physical examination revealed a hard, painless, motionless swelling on the posterolateral aspect of the left proximal fibula. The overlying skin and local temperature was normal. The left lower extremity demonstrated full range of motion without neurovascular deficits. All laboratory data were within normal limits.
Plain radiograph showed a mass with ‘popcorn’ and ‘smoke ring’ pattern of calcification on the posterolateral aspect of the left proximal fibula. The erosion of underlying cortex was not obvious (Figure 1A, B). A magnetic resonance imaging (MRI) of the knees was done to assess the extent and anatomical position of the mass. MRI showed a vaguely lobulated solid mass measuring 5.5 × 5.0 × 4.5 cm on the posterolateral aspect of the left proximal fibula. The mass exhibited homogeneous low signal intense on T1-weight image (Figure 2A) and high signal intense on T2-weight images (Figure 2B,C). Axial T1-weight MRI with fat suppression showed cortical invasion without marrow involvement (Figure 2D). A whole-body bone scan with technetium-99 m-methylenediphosphonate (Tc-MDP) showed strong accumulation of the radiopharmaceutical agent on the outside aspect of the left proximal fibula, without strong accumulation in other parts of the body (Figure 3A).
Figure 1.
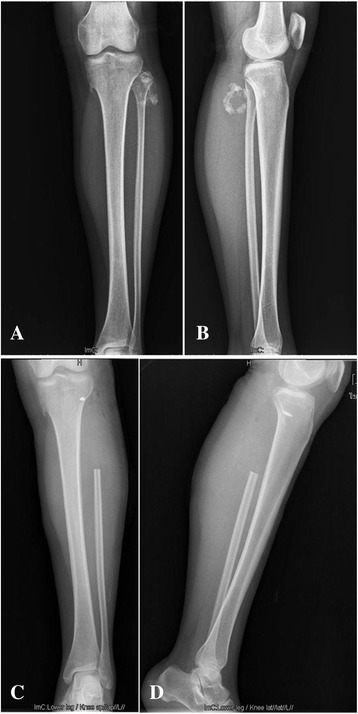
Radiographs of the patient’s left shank. A, B Anteroposterior and lateral views show a bone tumor with a ‘popcorn’ and ‘smoke ring’ pattern of calcification on the posterolateral aspect of the left proximal fibula, respectively. C, D Postoperative radiographs show the absence of the left proximal fibula and an anchor inserted the lateral tibial metaphysis.
Figure 2.
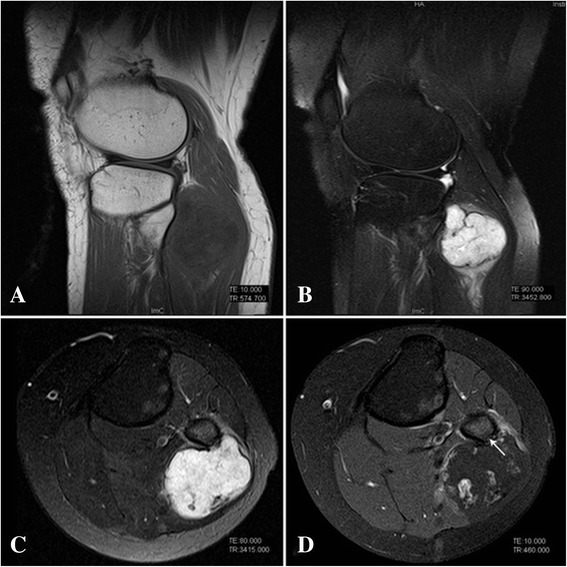
MRI of tumor. A Sagittal T1-weight image shows a homogeneous hypo-intensity mass on the posterolateral aspect of the left proximal fibula. B, C Sagittal and axial T2-weight images show a hyper-intensity mass (5.5 × 5.0 × 4.5 cm) with a multilobular configuration. D Axial T1-weighted MRI with fat suppression shows the contrast enhancement around the tumor and cortical invasion (arrow).
Figure 3.
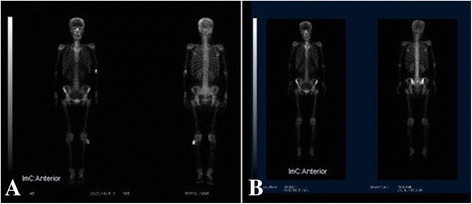
The whole-body bone scan of the patient. Bone scan shows strong accumulation of the radiopharmaceutical agent on the outside aspect of the left proximal fibula preoperatively (A) and in bilateral iliac regions postoperatively (B) without strong accumulation in other parts of the body.
Considering the above factors, a primary diagnosis of periosteal malignancy was made, including PC, periosteal osteosarcoma, and parosteal osteosarcoma. Among them, the most likely diagnosis was PC.
A wide resection was performed. The intraoperative result of frozen section examination indicated a PC. According to the pathologic result, an en-bloc resection of the tumor and the left proximal fibula was performed. The thickness of the excised normal soft tissues surrounding the tumor and the length of excised fibula was defined 1.0 and 8.5 cm, respectively. At surgery, the common peroneal nerve was protected carefully and the lateral collateral ligament was reattached to the lateral tibial metaphysis with an anchor.
Postoperative pathological examination confirmed the diagnosis of PC. Hematoxylin and eosin (H&E) staining indicated a vaguely lobulated neoplastic hyaline cartilage separated by fibrous bands and focal myxoid change (Figure 4A). The tumor cells enlarged and presented moderate grade of atypia, including multiple and enlarged nuclei. Mitoses were rare (Figure 4B). At high magnification, the tumor cells were seen in the Volkman canal (Figure 4C). The resection margins were clear. According to the above features and the grading system used for conventional intramedullary chondrosarcomas [1], the histopathological diagnosis of a PC (grade 2) with cortical invasion was rendered.
Figure 4.
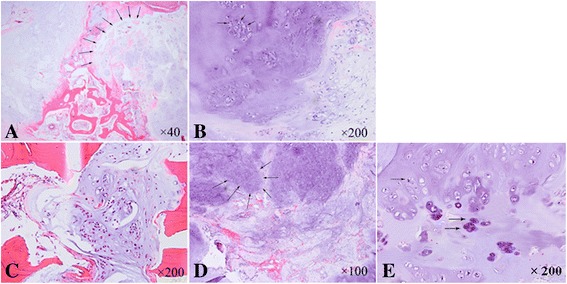
Histological appearance of tumor (H&E). A Microscopic appearance of the tissue specimen dissected from the fibula lesion shows a vaguely lobulated neoplastic hyaline cartilage separated by fibrous bands and focal myxoid change (×40). B High-power photomicrograph shows the enlarged tumor cells with moderate grade of atypia and exhibits multiple, enlarged grotesque nuclei. Mitoses can be seen (×200). C The tumor cells were seen in the Volkman canal (×200). D, E High-power photomicrograph of the tissue specimen from the needle biopsy of ilium shows a cartilaginous lesion with hypercellularity and cytologic atypia (×100, ×200, respectively).
The patient has been followed up for 16 months. Postoperative recovery was uneventful in the initial follow-up (Figure 1C, D). The patient, who was in a bad physical and mental state, visited our bone tumor clinic with a pain in pelvic region at 10 months after operation. On physical examination, no clinical results were found except the deep tenderness of sacral region and spine. A whole-body bone scan demonstrated high uptake especially in the bilateral iliac regions (Figure 3B).
In order to further define and assess the extent and anatomical position of metastasis, PET/CT with 2-fluoro [fluorine-18]-2-deoxy-d-glucose (F-18 FDG) was recommended to be done. PET/CT showed focal F-18 FDG uptake especially in the spine, pelvis, bilateral femurs, and humeri, with a maximum SUV of 10.8, but with no signs of local recurrence and no focal F-18 FDG uptake in the brain, head, neck, chest, and abdomen except a small nodule in the liver which was suspected as a calcification (Figure 5). The histopathological result of the needle biopsy of the ilium confirmed the diagnosis of MBM of chondrosarcoma, and the grade of metastatic chondrosarcoma was similar to that of primary PC (Figure 4D, E).
Figure 5.
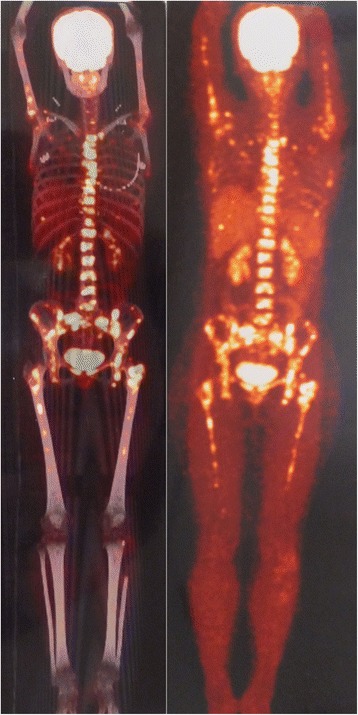
PET/CT of the patient. F-18 FDG PET/CT shows multiple bone metastases especially and symmetrically in the axial skeleton and proximal extremities including bilateral femurs and humeri, with a maximum SUV of 10.8, but with no signs of local recurrence and no focal F-18 FDG uptake in the brain, head, neck, chest, and abdomen except a small nodule in the liver which was suspected as a calcification.
Over the ensuing days, the patient received only the palliative therapy such as analgesic treatment without chemotherapy or radiotherapy. The patient died at 6 months after the definite diagnosis of MBM.
Discussion
The mean age of PC is about 32 years (range, 20 to 40 years) [2,3,5]. The location mainly exists in the limbs, including the distal femur, proximal tibia, and proximal humerus [2-5]. PC of the fibula is still rare. There have been only two case reports about PC of the fibula. Papagelopoulos et al. [6] reported a PC (grade 2) of the left distal fibula of a 31-year-old man measuring 2 cm in size. Robinson et al. [7] also reported a PC of the right distal fibula and tibia which induced the underling cortex erosion without marrow invasion of a 64-year-old woman. Lesions of PC are usually 4 to 20 cm in length [2,5]; in most cases, they exceed 5 cm in maximum diameter [5]. Robinson et al. [7] concluded that periosteal chondroid lesions greater than 3 cm in length should be considered malignant.
Chemotherapy and radiotherapy are not effective treatments for PC. Wide surgical resection with a sufficient margin remains the recommended therapy for this type of bone tumor. Prognosis is usually good if surgery is adequate [4,8,9]. The reported incidence of local recurrence varies from 13% to 28% and depends on the type of surgical treatment [6,10]. Even when local recurrence occur, the prognosis is still good if the wide resection can be performed again. Lung metastases, even regional lymph node metastases, have been reported in published literatures [6,11,12]. It is important to note that the majority of lung metastases have inadequate surgical treatment of the primary lesion and often accompany with local recurrence. Bone metastasis is even rare. There have been few studies about bone metastasis of chondrosarcoma. Ozaki et al. [13,14] reported two cases of chondrosarcoma with bone metastasis and found only five cases of chondrosarcoma with bony metastases by reviewing literatures. They also found all metastases mainly in the axial skeleton or proximal extremities, and there were only three cases of chondrosarcoma with bony metastases without local recurrence. There were also some literatures about bony metastases of extraskeletal myxoid chondrosarcoma and clear cell chondrosarcoma [15-17]. But, there have been no reports about bony metastases of PC.
The mechanism of bony metastases is not very clear. Ozaki et al. [14] believed that the metastasis was in the lumbar spine and may not have been associated with lung metastases because of the venous connections between the vertebral and other veins and the absence of a basement membrane in the capillaries of bone marrow. Matsumura et al. [18] described that PC microscopically invaded the underlying cortex through the Volkman canal in the cortical bone. Andrew et al. [19] reported that although a chondrosarcoma might metastasize late or not at all, it could invade and spread through veins. Death might also result from relentless local growth and invasion of adjacent vital structures. Therefore, considering the above literatures, we deduced that tumor cells had spread through the venules in the Volkman canal before surgery, which could not be tested by the present medical equipments. The microscopic cortical invasion through the Volkman canal and the spread of tumor cells through veins may be the most likely reason for bony metastases without local recurrence in the present case. Other cancers which had not been found before and/or after surgery may be another reason.
In addition, histological grading (grades 1 to 3) for chondrosarcomas is related to clinical behavior and prognosis [9,13]. Grade 1 chondrosarcomas rarely metastasize, while grade 3 chondrosarcmas develop metastases in 70% of patients [9]. Ozaki et al. [13] concluded that the median interval between surgery for the primary lesion and metastases was 10 months and the median survival time after metastasis of the patients who could not or did not undergo metastectomy was 5 months (range 0 to 10 months). On the other hand, the survival periods after metastasis of two patients who underwent metastectomy were 15 and 29 months. Papagelopoulos et al. [6] also found patients with grade 2 PC had a worse prognosis than patients with grade 1 PC in the 5-year metastasis-free survival (50% versus 94%).
The prognosis of most PC is usually good, but regular follow-up is still important for the early finding of local relapse or metastases. F-18 FDG PET/CT is a recommended examination for finding early metastases. Maybe, the patients with early local metastases will have the opportunity for metastectomy. For the minority of patients with late and multiple metastases, whether chemotherapy is applicable needs further study. New drugs and other treatments are also urgently needed.
Conclusions
In conclusion, we reported a rare case which has the following features: (1) the location was the proximal fibula, not the common location including distal femur, proximal tibia, and proximal humerus; (2) ‘smoke ring’ pattern of calcification was shown on lateral roentgenogram which has been rarely reported in published literatures; (3) cortical erosion was shown by MRI and histopathological examination which was reported only once; (4) the most important feature is that MBM was shown by F-18 FDG PET/CT especially and symmetrically in the axial skeleton and proximal extremities including bilateral femurs and humeri; and (5) the period from surgery to postoperative metastases and the survival span of our patient was 10 and 6 months, respectively, which was consistent with the previous reports.
Finally, we hope this report will increase the understanding of this rare bone tumor whose prognosis sometimes deviates from the good expectations.
Consent
Written informed consent was obtained from the patient’s husband for publication of this case report and any accompanying images. The report was approved by the Ethics Committee of our hospital.
Acknowledgements
We thank Dr. Wenli Zhang, the attending doctor in Department of Orthopedics at West China Hospital, for his review over the case.
Abbreviations
- CT
computed tomography
- F-18 FDG
2-fluoro [fluorine-18]-2-deoxy-d-glucose
- H&E
hematoxylin and eosin
- MBM
multiple bone metastases
- MRI
magnetic resonance imaging
- PC
periosteal chondrosarcoma
- PET
positron emission tomography
- Tc-MDP
technetium-99 m-methylene-diphosphonate
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
XWL, LM, SH, and CQT performed the operation. XWL, LM, GJC, SH, and CQT collected and reviewed the case data. XWL conducted a literature search and wrote the manuscript. XWL, LM, GJC, and SH edited and revised the manuscript. CQT was the guarantor, proofread, and critically revised the manuscript. All authors read and approved the final manuscript.
Contributor Information
Xiwei Liu, Email: liw721@163.com.
Li Min, Email: hxyyml@163.com.
Ganjun Chen, Email: blkcgj@126.com.
Song Hong, Email: h2014s@163.com.
Chongqi Tu, Email: chongqitu@163.com.
References
- 1.Hatano H, Ogose A, Hotta T, Otsuka H, Takahashi HE. Periosteal chondrosarcoma invading the medullary cavity. Skeletal Radiol. 1997;26:375–8. doi: 10.1007/s002560050251. [DOI] [PubMed] [Google Scholar]
- 2.Weinberg J, Miller TT, Handelsman JE, Kahn LB, Godfried DH, Kenan S. Periosteal chondrosarcoma in a 9-year-old girl with osteochondromatosis. Skeletal Radiol. 2005;34:539–42. doi: 10.1007/s00256-005-0898-7. [DOI] [PubMed] [Google Scholar]
- 3.Kumta SM, Griffith JF, Chow LTC, Leung PC. Primary juxtacortical chondrosarcoma dedifferentiating after 20 years. Skeletal Radiol. 1998;27:569–73. doi: 10.1007/s002560050439. [DOI] [PubMed] [Google Scholar]
- 4.Fabbri N, Donati D, et al. Periosteal Chondrosarcoma. In: Picci P, et al., editors. Atlas of Musculoskeletal Tumors and Tumor like Lesions. Switzerland: Springer International Publishing; 2014. pp. 129–31. [Google Scholar]
- 5.Kenan S, Abdelwahab IF, Klein MJ, Hermann G, Lewis MM. Lesions of juxtacortical origin (surface lesions of bone) Skeletal Radiol. 1993;22:337–57. doi: 10.1007/BF00198395. [DOI] [PubMed] [Google Scholar]
- 6.Papagelopoulos PJ, Galanis EC, Mavrogenis AF, Savvidou OD, Bond JR, Unni KK, et al. Survivorship analysis in patients with periosteal chondrosarcoma. Clin Orthop Relat Res. 2006;448:199–207. doi: 10.1097/01.blo.0000194684.40624.a8. [DOI] [PubMed] [Google Scholar]
- 7.Robinson P, White LM, Sundaram M, Kandel R, Wunder J, McDonald DJ, et al. Periosteal chondroid tumors: radiologic evaluation with pathologic correlation. AJR Am J Roentgenol. 2001;177:1183–8. doi: 10.2214/ajr.177.5.1771183. [DOI] [PubMed] [Google Scholar]
- 8.Miura K, Kondo R, Kurai M, Isobe K, Ishii K. Recurrence of juxtacortical chondrosarcoma a rising on the rib. Gen Thorac Cardiovasc Surg. 2014. http://link.springer.com/article/10.1007/s11748-014-0431-9. Accessed 6 Jun 2014. [DOI] [PubMed]
- 9.Gelderblom H, Hogendoorn PC, Dijkstra SD, van Rijswijk CS, Krol AD, Taminiau AH, et al. The clinical approach towards chondrosarcoma. Oncologist. 2008;13:320–9. doi: 10.1634/theoncologist.2007-0237. [DOI] [PubMed] [Google Scholar]
- 10.Vanel D, De Paolis M, Monti C, Mercuri M, Picci P. Radiological features of 24 periosteal chondrosarcomas. Skeletal Radiol. 2001;30:208–12. doi: 10.1007/s002560100340. [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto K, Hukuda S, Ishizawa M, Saruhashi Y, Okabe H, Asano Y. Parosteal (juxtacotical) chondrosarcoma of the humerus associated with regional lymph node metastasis. A case report. Clin Orthop Relat Res. 1993;290:168–73. [PubMed] [Google Scholar]
- 12.Nojima T, Unni KK, McLeod RA, Pritchard DJ. Periosteal chondroma and periosteal chondrosarcoma. Am J Surg Pathol. 1985;9:666–77. doi: 10.1097/00000478-198509000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Ozaki T, Hillmann A, Lindner N, Blasius S, Winkelmann W. Metastasis of chondrosarcoma. J Cancer Res Clin Oncol. 1996;122:625–8. doi: 10.1007/BF01221195. [DOI] [PubMed] [Google Scholar]
- 14.Ozaki T, Hillmann A, Blasius TS, Winkelmann W. Skeletal metastases of intermediate grade chondrosarcoma without pulmonary involvement. A case report. Int Orthop. 1998;22:131–3. doi: 10.1007/s002640050224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takeda A, Tsuchiya H, Mori Y, Nonomura A, Tomita K. Extraskeletal myxoid chondrosarcoma with multiple skeletal metastases. J Orthop Sci. 2000;5:171–4. doi: 10.1007/s007760050145. [DOI] [PubMed] [Google Scholar]
- 16.Ehara S, Nishida J, Shiraishi H, Yoshioka H, Okada K, Sumiya H, et al. Skeletal recurrences and metastases of extraskeletal myxoid chondrosarcoma. Skeletal Radiol. 2007;36:823–7. doi: 10.1007/s00256-007-0303-9. [DOI] [PubMed] [Google Scholar]
- 17.Kawano T, Hamada J, Morioka M, Mihara Y, Ishimaru Y, Kaku M, et al. Skull metastasis from clear cell chondrosarcoma. Neurol Med Chir (Tokyo). 2005;45:367–70. doi: 10.2176/nmc.45.367. [DOI] [PubMed] [Google Scholar]
- 18.Matsumura T, Yamaguchi T, Hasegawa T, Kaya M, Wada T, Yamashita T. Periosteal chondrosarcoma with microscopic cortical invasion. J Orthop Sci. 2008;13:379–82. doi: 10.1007/s00776-008-1229-3. [DOI] [PubMed] [Google Scholar]
- 19.Andrew G. Huvos: chondrosarcoma and its variants. J Orthop Sci. 1996;1:90–7. doi: 10.1007/BF01234119. [DOI] [Google Scholar]


