Abstract
Importance
With cure rates of childhood acute lymphoblastic leukemia (ALL) exceeding 85%, there is compelling need to mitigate treatment toxicities that can compromise quality of life. Peripheral neuropathy is the major dose-limiting toxicity of the microtubule inhibitor vincristine, an anticancer agent given to every child with ALL.
Objective
Identify genetic germline variants associated with the occurrence or severity of vincristine-induced peripheral neuropathy in children with ALL.
Design, Setting and Participants
All patients had been enrolled in one of two prospective clinical trials for childhood ALL that included treatment with 36–39 doses of vincristine. Genome-wide single nucleotide polymorphism (SNP) analysis and vincristine-induced peripheral neuropathy were assessed in all patients from whom DNA was available (n=321 patients); 222 patients (median age at 6.0 years, range 0.1–18.8 years) enrolled between 1994–1998 on the St. Jude Children’s Research Hospital protocol Total XIIIB (St. Jude cohort) with toxicity followed through January 2001, and 99 patients (median age 11.4 years, range 3.0–23.8 years) enrolled between 2007–2010 on the Children’s Oncology Group protocol AALL0433 (COG cohort) with toxicity followed through May 2011. Human leukemia cells and induced pluripotent stem cell neurons were used to assess the effects of lower CEP72 expression on vincristine sensitivity.
Exposures
Treatment with vincristine at a dosage of 1.5 or 2.0 mg/m2 as a component of protocol directed chemotherapy for childhood ALL.
Main Outcomes and Measures
Vincristine-induced peripheral neuropathy was assessed at each clinic visit using the National Cancer Institute Common Terminology Criteria for Adverse Events and prospectively graded as mild (grade 1), moderate (grade 2), serious/disabling (grade 3), or life-threatening (grade 4).
Results
Grade 2–4 vincristine-induced neuropathy during continuation therapy occurred in 28.8% of patients (n=64 of 222) in the St. Jude cohort and in 22.2% of patients (n=22 of 99) in the COG cohort. A SNP in the promoter region of the CEP72 gene, which encodes a centrosomal protein involved in microtubule formation, had a significant association with vincristine neuropathy (meta p =6.3 × 10−9). This SNP had a minor allele frequency of 37% (235/642), with 50 of 321 patients (16%, 95% CI 11.6%–19.5%) homozygous for the risk allele (TT at rs924607). Among patients with the high-risk CEP72 genotype (TT at rs924607), 28 of 50 patients (56%, 95% CI 41.2–70.0) developed at least one episode of grade 2–4 neuropathy, a higher rate than in patients with the CEP72 CC or CT genotype (58 of 271 patients; 21.4%, 95% CI 16.9–26.7); p=2.4×10−6. The severity (grade) of neuropathy was greater (2.4-fold by Poisson regression (p<0.0001), 2.7-fold based on mean grade of neuropathy (1.23 [95% CI 0.74 – 1.72] versus 0.45 [95% CI 0.3 – 0.6]; t test p=0.004)) in patients homozygous for the CEP72 risk allele (TT genotype), compared to patients with the CC or CT genotype. The CEP72 promoter SNP was shown to create a binding site for a transcriptional repressor causing lower mRNA expression, and reducing CEP72 expression in human neurons and leukemia cells increased their sensitivity to vincristine.
Conclusions
In this preliminary study of children with ALL, an inherited polymorphism in the promoter region of CEP72 was associated with increased risk and severity of vincristine-related peripheral neuropathy. If replicated in additional populations, this finding may provide a basis for safer dosing of this widely prescribed anticancer agent.
Introduction
Cancer remains the leading cause of death by disease in US children, despite major advances in the last 20 years. Acute lymphoblastic leukemia (ALL) is the most common childhood cancer, and as cure rates have surpassed 85%1,2, it becomes increasingly important to mitigate acute and chronic toxicities of treatment that adversely affect quality of life and longevity. Vincristine is one of the most widely used and effective anticancer agents for treating leukemias, lymphomas, brain tumors and solid tumors in both adults and children1,2 and is administered multiple times as part of treatment protocols for every child with ALL and for children with neuroblastoma, Wilm’s tumor, rhabdomyosarcoma, Ewing sarcoma, and retinoblastoma3
Vincristine exerts its cytotoxic effects by interfering with microtubule formation and mitotic spindle dynamics, leading to mitotic arrest and cell death4–6. The dose-limiting toxicity of vincristine is peripheral neuropathy7,8, characterized by neuropathic pain and sensory and motor dysfunction (e.g., impaired manual dexterity, balance and deep tendon reflexes, and altered gait). A substantial percentage of patients develop neuropathy that causes considerable morbidity and often disrupts curative treatment9. Currently, there are no reliable means of identifying patients at high risk of vincristine-induced neuropathy nor strategies to mitigate this drug toxicity. Although prior studies have documented racial differences in the incidence of vincristine neuropathy, candidate gene studies have failed to identify consistent genetic variants associated with an increased risk of vincristine-induced neuropathy9–12.
To determine whether there are genetic polymorphisms associated with vincristine-induced neuropathy, we performed a genome-wide association study (GWAS) in two independent cohorts of children receiving protocol-defined treatment for acute lymphoblastic leukemia (ALL).
Methods
Patients and treatment regimens
Two independent patient cohorts were treated either by St. Jude Children’s Research Hospital or the Children’s Oncology Group (COG) according to contemporary IRB-approved ALL protocols that prospectively assessed and graded vincristine neuropathy according to National Cancer Institute (NCI) criteria. We analyzed all consenting patients who had germline DNA available for genome-wide SNP analyses in May 2011. The first cohort included children with newly-diagnosed ALL enrolled and treated on the St. Jude Total XIIIB protocol 13–15(provided in online Supplement). The second cohort included patients with relapsed ALL enrolled on the COG AALL0433 protocol (http://clinicaltrials.gov/show/NCT00381680). Written informed consent was obtained from patients or their guardians and assent from patients (as appropriate).
The St. Jude Total XIIIB protocol was divided into 3 phases: 8 weeks of remission induction therapy, 2 weeks of consolidation therapy, and 120 weeks of continuation therapy (including a re-induction phase at weeks 16–21). Vincristine was administered as a component of combination chemotherapy during the induction and continuation phases of therapy at a dosage of 1.5 mg/m2 with a maximum dose of 2 mg. Altogether, St. Jude patients were scheduled to receive a total of 36 vincristine doses. Consecutive patients were enrolled between August 1994 and July 1998, and toxicity was followed through 2001.
The COG AALL0433 protocol was a cooperative group wide, Phase III randomized study of intensive therapy for patients with recurrent childhood B-precursor ALL, including late bone marrow or combined relapse (≥36 months from diagnosis), or early (< 18 months from diagnosis) isolated extramedullary relapse in the central nervous system or testes (http://clinicaltrials.gov/show/NCT00381680). COG AALL0433 treatment was divided into induction, intensification (with a re-induction phase at weeks 28–32 or 29–33) and continuation phases. Vincristine was administered as a component of combination chemotherapy during the induction, intensification, re-induction and continuation phases of therapy; a total of 39 doses of vincristine were scheduled to be given per this protocol. Patients were randomly assigned to receive a vincristine dosage of 1.5 mg/m2 with a maximum dose of 2 mg (Group A) or 2 mg/m2 with a maximum dose of 2.5 mg (Group B). Group B treatment and the vincristine dose randomization were permanently suspended by COG in 2010, due to the high incidence of peripheral neuropathy, most prominently in patients ≥13 years of age. Patients were enrolled between March 2007 and September 2010 at the time of the suspension of Group B treatment, and toxicity was followed through May 2011.
As part of required clinical trial adverse event monitoring, children in both cohorts were prospectively assessed for the presence of peripheral neuropathy via physical examination by the treating oncologist at each clinic visit during treatment16,17. Children in the St. Jude cohort were assessed for toxicity weekly throughout all phases of therapy. Children in the COG cohort were assessed for toxicity weekly throughout the first year of therapy, and monthly thereafter. For these analyses children on St. Jude Total XIIIB were graded according to NCI Common Terminology Criteria for Adverse Events (CTCAE) version 1.0 and those in the COG cohort according to a modified “Balis” scale for neuropathy, which is a modification of the NCI CTCAE version 2.0. (eTables 1, 2). Both scales classify neuropathy events as mild (grade 1), moderate (grade 2), serious/disabling (grade 3), or life-threatening (grade 4)18. Those with Grades 2, 3 or 4 motor and/or sensory neuropathy were considered as neuropathy cases in our analyses. There were no neuropathy related deaths (Grade 5). Follow-up for the St. Jude cohort continued through January 2001; follow-up for the COG cohort continued through May 2011.
Genotyping
Germline DNA (500 ng) was extracted from normal peripheral blood leukocytes, digested with restriction enzymes, amplified, labeled, and hybridized to the Affymetrix GeneChip Human Mapping 500K array (532552 SNPs) or the SNP 6.0 array (906600 SNPs) (Affymetrix, Santa Clara, CA). SNPs were excluded if genotyping call rates were less than 95% and if the minor allele frequency less than 1%. (See Supplemental material for additional details). SNP imputation was carried out using Mach/1.0.1519. The total number of SNPs that were imputed was 21764463 (See supplemental material for additional details).
Race was genetically determined following a method previously described20. Briefly, the genetic ancestry estimates were determined by applying STRUCTURE (version 2.2.3) using HapMap project data as references (See Supplemental material for additional details). SNP genotype data for HapMap cell were downloaded from release 22 on the International HapMap Project website (http://www.HapMap.org).
Cellular analyses
The human pre-B leukemia cell line NALM6, the human T-lineage leukemia cell line CEM and the neuroblastoma cell line SH-SY5Y were cultured in RPMI-1640 medium containing 2mM glutamine and 10% (vol/vol) FBS at 37°C with 5% CO2. iCell neurons derived from human induced pluripotent stem cells were obtained from Cellular Dynamics International (Madison, WI) and maintained per manufacturer’s protocol. Primary ALL cells were isolated by applying a Ficoll-Hypaque gradient to bone marrow aspirates obtained at diagnosis (with a median of 97% blast cells). CEP72 expression was impaired by using short hairpin RNA (shRNA). In vitro drug sensitivity of primary ALL cells and of Nalm6 and CEM cell lines was determined using the MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-tetrazoliumbromide) assay, as previously reported21. The drug concentration lethal to 50 percent of the leukemia cells (LC50) was used as the measure of sensitivity to vincristine. Vincristine-induced neurotoxicity in iCell neurons was determined by individual cell measurements of total neurite outgrowth, number of processes and number of branches in 1000 cells for each replicate experiment. Luciferase assays to measure CEP72 expression were performed as described in the supplemental methods. Gene expression of HapMap cells measured by the Affymetrix GeneChip Human Exon 1.0 ST Array was downloaded from the website (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE7761.)
Statistical analysis
Analyses were conducted using R (http://www.r-project.org/) and SAS (SAS Institute, Cary, NC). Weighted logistic regression model fitting for recurrent events was used to test the associations between SNP genotypes and the phenotype of peripheral neuropathy (i.e., occurrence of grade 2 or higher vincristine-induced neuropathy during continuation therapy), including cumulative vincristine dosage and genetically determined ancestry as covariates, as previously described.15 See supplemental materials for additional statistical details. After filtering out SNPs with a call rate <95% (SNP call rate is the percentage of patients who have a confident genotype call for each SNP according to Affymetrix quality control criteria) and minor allele frequency <1%; the remaining SNPs (484,623 typed SNPs and 1,091,393 imputed SNPs) were assessed in a GWAS for their association with vincristine-induced neuropathy, in St. Jude and COG cohorts separately using weighted logistics regression models, then combined by pooling the p values from the two cohorts via Fisher’s method. All p-values were computed using two-sided tests; statistical significance was determined at the genome-wide significance level 5×10−8
Post-GWAS analyses focusing on the CEP72 SNP rs924607 were performed as follows: the relationship between the time to first episode and genotype was analyzed using cumulative incidence function estimates22 and Gray’s test23, Cox regression and accelerated failure time regression22, with cumulative dose and genetic ancestry as covariates. The relationship between the cumulative vincristine dosage at the first episode of toxicity and genotype was analyzed by Cox regression with genetically determined ancestry as a covariate. The relationship between severity (grade) of toxicity at the first episode and genotype was analyzed by Poisson regression model with cumulative dose and genetic ancestry as covariates. Student’s t test was used to examine the differences in luciferase reporter activity. A linear regression model was used to test the association between vincristine sensitivity (LC50) and the SNP genotype in primary ALL cells. In post-GWAS analyses and laboratory experiments, all tests were two-sided and significance was determined at the 0.05 level.
Results
Patient population
Genome-wide SNP analysis and vincristine-induced neuropathy were assessed in 321 patients enrolled on two ALL treatment protocols. Of 247 consecutive patients enrolled on the St. Jude Total XIIIB protocol, 222 patients with newly diagnosed ALL (median age at diagnosis 6.0 years, range 0.1–18.8 years) had germline DNA available for analysis and were included in this study (eFigure 1). Of the 137 patients who were enrolled in the COG AALL0433 protocol, 99 patients with relapsed ALL (median age at diagnosis 11.4 years, range 3.0–23.8 years) had germline DNA available for analysis and were included in this study (eFigure 2).
The demographic and clinical characteristics of these 321 patients are summarized in Table1 and for each cohort in eTables 3 and 4.
Table 1.
Characteristics of patients in the combined cohort of St. Jude and COG patients assessed for vincristine neuropathy.
| Characteristicsa | Vincristine Neuropathy
|
P valued | ||||
|---|---|---|---|---|---|---|
| No. Patients (%)
|
No. Episodes of Vincristine Neuropathy
|
|||||
| Total Patients (n=321)b | None to Grade 1 | 1 episode of Grade 2–4 | 2 or more episodes of Grade 2–4 | Episodes of Grade 2–4c | ||
|
Age at diagnosis, y
| ||||||
| < 1 | 8 (2.5) | 7 | 1 | 0 | 1 | 0.03 |
| 1–10 | 192 (59.8) | 137 | 34 | 21 | 103 | |
| > 10 | 121 (37.7) | 91 | 16 | 14 | 84 | |
|
| ||||||
|
Sex
| ||||||
| Male | 187 (58.3) | 142 | 29 | 16 | 88 | 0.02 |
| Female | 134 (41.7) | 93 | 22 | 19 | 100 | |
|
| ||||||
| Genetically-determined Ancestry | ||||||
|
| ||||||
| European | 209 (65.1) | 156 | 29 | 24 | 123 | 0.002 |
| African | 43 (13.4) | 33 | 8 | 2 | 15 | |
| Asian | 2 (0.6) | 1 | 1 | 0 | 1 | |
| Hispanic | 44 (13.7) | 30 | 7 | 7 | 36 | |
| Other | 23 (7.2) | 15 | 6 | 2 | 13 | |
|
| ||||||
| Cumulative vincristine dosage (mg/m2), median [range] | 47 [2–120] | 46 [2–120] | 51 [8–92] | 49 [10–120] | 52 [22–120] | 0.28 |
Data are presented as Number (%) of patients unless otherwise indicated.
Cohort includes patients treated on St. Jude Total XIIIB (n=222) and on COG AALL0433 (n=99).
Number of events.
p values are from univariate weighted logistic regression using each episode as an observation.
Vincristine-associated Neurotoxicity
Grade 2–4 vincristine-induced peripheral neuropathy during continuation therapy occurred in 28.8% (n=64 of 222) and 22.2% (n=22 of 99) of patients on the St Jude and COG protocols, respectively. Genetically-determined ancestry and vincristine cumulative dosage were the demographic or clinical characteristics significantly related to the occurrence of vincristine-induced neuropathy in each of the cohorts, as summarized in eTables 3 and 4. Within the COG trial, the rate of vincristine-induced neuropathy was higher in the cohort of patients randomized to the higher dosage of vincristine (2.0 mg/m2 with a maximum dose of 2.5 mg) compared to those randomized to the conventional dosage (1.5 mg/m2 with a maximum dose of 2 mg); 19 of 48 patients (39.6%) vs 3 of 51 patients (5.9%) p<0.0001, leading to early closure of the higher-dose treatment group of the COG protocol.
Meta-analysis of genome-wide association results
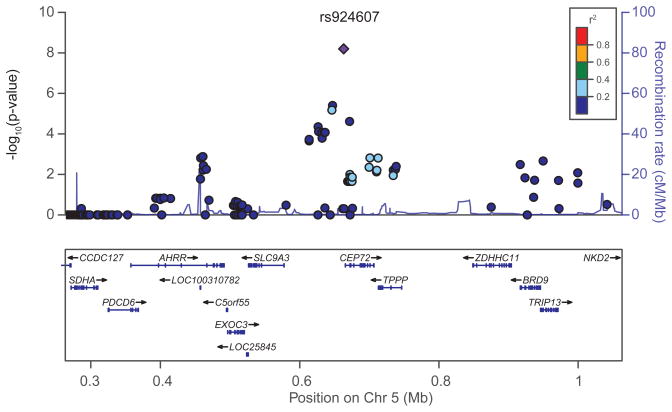
Assessing SNPs that had a p-value <0.05 in both cohorts and concordant effects in both populations yielded 5,051 typed SNPs and 10,195 imputed SNPs. The Manhattan plot (eFigurea) depicts p-values of SNPs from the two patient cohorts assessed via a meta-analysis. The SNPs with the lowest p-values for their association with vincristine-induced neuropathy in the two cohorts are summarized in Table 2 and in eTable 5. In a multivariate analysis that included genetically defined ancestry and cumulative dosage of vincristine, only rs924607 remained genome-wide significantly (p=4.7×10−8) related to the development of vincristine-induced neuropathy (Table 2 and eTable 5). Rs924607 is in the promoter region of the CEP72 gene in chromosome 5 (Figure 1).
Table 2.
SNPs associated with the incidence of vincristine-induced neuropathy (Grades 2–4).a
| SNP | Chr | Position | Nearest gene |
Allele major/ minor |
St Jude Total XIIIB
|
COG AALL0433
|
Meta-analysis (univariate) |
Meta-analysisc (multivariate) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MAF (%) |
OR | 95% CI | P-value | MAF (%) |
OR | 95% CI | P-value | P-value | P-value | |||||
|
|
|
|||||||||||||
| rs924607b | 5 | 663093 | CEP72 | C/T | 36.7 | 2.43 | 1.70–3.49 | 1.25×10−6 | 36.4 | 4.1 | 1.86–9.01 | 0.0004 | 6.33×10−9 | 4.68×10−8 |
| rs17032980 | 2 | 67156247 | ETAA1 | A/G | 26.6 | 3.17 | 1.95–5.17 | 3.67×10−6 | 19.2 | 10.4 | 2.97–36.15 | 0.0002 | 9.77×10−9 | 9.01×10−7 |
| rs12786200 | 11 | 91618666 | MTNR1B | C/T | 22.7 | 0.23 | 0.13–0.40 | 1.58×10−7 | 20.7 | 0.24 | 0.08–0.76 | 0.02 | 2.56×10−8 | 6.30×10−7 |
| rs4463516 b | 9 | 32857481 | TMEM215 | C/G | 33.6 | 2.89 | 1.90–4.39 | 6.83×10−7 | 24.2 | 4.94 | 1.65–14.79 | 0.004 | 3.02×10−8 | 1.19×10−7 |
| rs7818688 b | 8 | 96093258 | NDUFAF6 | C/A | 12.6 | 4.26 | 2.45–7.42 | 3.05×10−7 | 14.1 | 4.59 | 1.35–15.59 | 0.01 | 4.46×10−8 | 5.03×10−7 |
MAF = minor allele frequency, OR = odds ratio, CI = confidence interval of OR, Chr = chromosome
SNPs were selected based on their univariate meta-analysis p-values and the SNPs with the lowest p-values are shown. These SNPs had the lowest p-values among the 5051 typed SNPs and 10195 imputed SNPs with a p-value <0.05 in each cohort separately and with concordant direction (effect) in the two cohorts. When there were multiple SNPs in LD only the one with the lowest p-value is represented
imputed SNP
including genetically defined ancestry and cumulative VCR dosage as covariates
Figure 1.
Regional association plot for the rs924607 locus on chromosome 5 (blue diamond). Each SNP is plotted with respect to its chromosomal location (x axis) and the corresponding meta P value for association with vincristine neuropathy (left y axis). The blue line depicts the recombination rate (right y axis) at the corresponding region of chromosome 5 (x axis). SNPs are colored to indicate extent of LD with the indexed SNP (pairwise r2 values from HapMap CEU). The NCBI Build 37 corresponding to the 1000 Genomes Project March 2012 reference was used.
Twenty of 32 patients (62%) with the TT genotype in the St. Jude cohort and 8 of 18 patients (44%) with the TT genotype in the COG cohort developed at least one episode of vincristine-induced neuropathy. In total, 28 out of 50 patients (56%) with the TT genotype developed at least one episode of grade 2–4 vincristine-induced neuropathy (Table 3), compared to 58 of 271 patients (21.4%) with the CC or CT genotype (p=2.4×10−6). The allelic odds ratio for the development of neuropathy for rs924607 was 2.43 (95% CI: 1.70–3.49) and 4.1 (95% CI: 1.86–9.01) in the St. Jude and COG cohorts, respectively. As summarized in eTable 6, the frequency of the CEP72 risk allele (T) differed by ancestry, with a lower frequency in African ancestry patients and in the Hapmap cells from individuals of African ancestry (Yoruba in Ibadan, Nigeria; African ancestry in Southwest USA; Luhva in Webuve, Kenya; Maasai in Kinyama, Kenya,), compared to cells from individuals of European ancestry.
Table 3. Vincristine-induced peripheral neuropathy and rs924607 genotype.
The number of patients (n) for each rs924607 genotype and at each grade of vincristine-induced neuropathy are presented for the St. Jude Total XIIIB cohort (a), for the COG AALL0433 cohort (b), and for the combined cohort (c). Patients who experienced more than one episode of neuropathy at different grades were included at the highest grade reported.
| a. St Jude Total XIIIB
| |||||||
|---|---|---|---|---|---|---|---|
| Patients | Grade 0 (n) | Grade 1 (n) | Grade 2 (n) | Grade 3 (n) | Grade 4 (n) | Total (n) | % with Grade 2–4 Vincristine Neuropathy |
| CC genotype | 65 | 0 | 17 | 8 | 1 | 91 | 28.6 |
| CT genotype | 81 | 0 | 9 | 9 | 0 | 99 | 18.2 |
| TT genotype | 12 | 0 | 13 | 7 | 0 | 32 | 62.5 |
| Total | 158 | 0 | 39 | 24 | 1 | 222 | |
| b. COG AALL0433
| |||||||
|---|---|---|---|---|---|---|---|
| Patients | Grade 0 (n) | Grade 1 (n) | Grade 2 (n) | Grade 3 (n) | Grade 4 (n) | Total (n) | % with Grade 2–4 Vincristine Neuropathy |
| CC genotype | 37 | 1 | 4 | 3 | 0 | 45 | 15.6 |
| CT genotype | 28 | 1 | 4 | 3 | 0 | 36 | 19.4 |
| TT genotype | 9 | 1 | 3 | 5 | 0 | 18 | 44.4 |
| Total | 74 | 3 | 11 | 11 | 0 | 99 | |
| c. Combined cohort of St Jude Total XIIIB and COG AALL0433
| |||||||
|---|---|---|---|---|---|---|---|
| Patients | Grade 0 (n) | Grade 1 (n) | Grade 2 (n) | Grade 3 (n) | Grade 4 (n) | Total (n) | % with Grade 2–4 Vincristine Neuropathy |
| CC genotype | 102 | 1 | 21 | 11 | 1 | 136 | 24.3 |
| CT genotype | 109 | 1 | 13 | 12 | 0 | 135 | 18.5 |
| TT genotype | 21 | 1 | 16 | 12 | 0 | 50 | 56.0 |
| Total | 232 | 3 | 50 | 35 | 1 | 321 | |
Because rs924607 is an imputed SNP, we validated the genotype in a subset of 30 patients, randomly chosen from patients to represent the three diplotypes for CEP72. This documented complete concordance between the imputed genotypes and those obtained by direct genotyping. (eFigure 4)
Association of CEP72 rs924607 genotype with development, onset time, and severity of vincristine-induced peripheral neuropathy
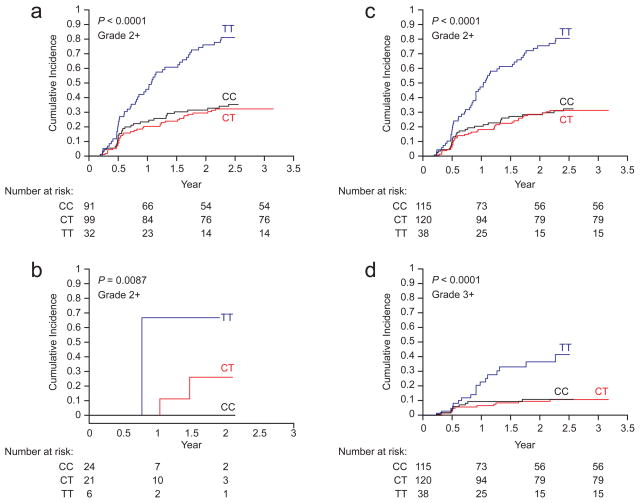
The cumulative incidence of all neuropathy episodes (grade 2–4) differed significantly by CEP72 rs924607 genotype (diplotype) in both patient cohorts treated with 1.5 mg/m2 of vincristine, with p-values in the St. Jude, the COG, and the combined cohorts of p<0.0001, p=0.0087 and p<0.0001 respectively (Gray’s test; Figure 2a–c). Likewise, the cumulative incidence of severe neuropathy episodes (grades 3–4) was also significantly higher in patients homozygous for the CEP72 risk allele (Figure 2d; p<0.0001). Simply based on binomial proportions, 21 of 38 (55.3% [95% CI 39.7%–69.9%]) patients with the CEP72 TT genotype experienced grade 2–4 neuropathy over the monitoring period compared to 46 of 235 (19.6% [95% CI 14.8%–25.3%]) patients with the CC/CT genotype (p<0.001). The cumulative incidence of the first neuropathy episode (grade 2–4) was 60.8% (95% CI 43.9%–77.6%) among patients with the CEP72 TT genotype compared to 23.4% (95% CI 17.4%–29.4%) among patients with the CC/CT genotype (p<0.001). The risk of developing neuropathy was significantly higher in patients with the CEP72 TT genotype (TT vs. CT/CC hazard ratio of 3.58, 95% CI 2.1, 6.1 for TT compared to the CT/CC patients) in a Cox regression model adjusting for cumulative vincristine dosage and genetically determined ancestry (eTable 7). This CEP72 SNP was not significantly associated with the incidence of vincristine-induced neuropathy among patients randomized to treatment with the higher (experimental) dosage of vincristine (eFigure 5).
Figure 2.
Cumulative incidence of vincristine-induced neuropathy among patients with different CEP72 genotypes. The cumulative incidence of vincristine-induced neuropathy (grades 2–4) differed by CEP72 promoter SNP (rs924607) genotype in the St Jude cohort (a) (p< 0.0001), the COG cohort group A (b) (p =0.0087) and in the combined St Jude and COG group A cohort (c) (p <0.0001). Likewise, the cumulative incidence of severe neuropathy (grades 3–4) was also significantly higher in patients homozygous for the CEP72 risk allele (d). P values were computed based on weighted logistic regression adjusting for ancestry and accounting for multiple episodes of neuropathy. The number of patients valuable and at risk of neuropathy is provided for each CEP72 genotype (diplotype). The x-axis represents time since the start of continuation therapy for ALL.
By fitting an accelerated failure time model22 with CEP72 genotype, cumulative vincristine dosage and genetically-determined ancestry as explanatory variables, the estimated ratio of median time to the first episode of neuropathy in TT patients compared to patients with a CT or CC genotype was approximately 20% shorter in TT patients within any race and at any cumulative vincristine dosage level (p-value = 6.19×10−6 for the regression coefficient of the SNP) (eTable 8). Without adjusting for covariates, among those who developed grade 2–4 neuropathy, the average time to develop neuropathy was 225 days (95% CI: 169–281 days) in patients with the CEP72 genotype TT compared to 307 days in patients with the CT/CC genotype (95% CI: 244–370 days).
In unadjusted analysis comparing the average grade of neuropathy observed at the first episode (assigning grade 0 to those who did not develop neuropathy) in patients treated with 1.5 mg/m2 vincristine, the mean grade of neuropathy in white patients with the CEP72 high-risk genotype (TT) was significantly higher (2.7-fold) than the mean grade of neuropathy in patients with the CEP72 CC or CT genotypes (1.23 [95% CI 0.74 – 1.72] versus 0.45 [95% CI 0.3 – 0.6]; t test p=0.004). The numbers of patients in other ancestry groups were too small to make meaningful subgroup comparisons. Using the Poisson regression model to assess the severity (grade) of neuropathy at first episode, with genetically defined ancestry and cumulative vincristine dosage as covariates in the combined cohort, the average grade (by CTCAE) of neuropathy was 2.4-fold greater (95% CI 1.59–3.69) in patients homozygous for the CEP72 risk allele (TT) compared to all other patients (p<0.0001; eTable 9).
Rs924607 and CEP72 mRNA expression
Because rs924607 is located in the promoter region of CEP72, we examined CEP72 mRNA expression levels for association with rs924607 genotype in Hapmap samples (CEU), using the publicly available gene expression data set24. Patients who were homozygous for the variant T-allele had significantly lower expression of CEP72 mRNA compared to those who were heterozygous or homozygous for the wild-type C-allele (p=0.025, eFigure 6a). The effect of rs924607 genotype on CEP72 expression was confirmed using a luciferase reporter assay in two different human cell lines, documenting significantly lower expression with the T-allele compared to the C-allele in both SH-SY5Y neuroblastoma cells (eFigure 6b) and in Nalm6 leukemia cells (eFigure 6c). The rs924607 T variant creates a consensus binding site for the NKX-6.3 transcription factor (repressor) (eFigures 6d and 6e, eTable 10; eFigure 6f, 6g, 6h), markedly enhancing NKX-6.3 binding to the risk allele, leading to lower CEP72 mRNA expression.
Low CEP72 expression and vincristine-induced toxicity
The relationship between CEP72 expression and vincristine-induced neurotoxicity was assessed in neurons derived from human induced pluripotent stem cells (iPSC), revealing significantly greater sensitivity to vincristine when CEP72 expression was reduced (eFigure 7a, 7b).
Likewise, reducing expression of CEP72 in two human ALL cells lines, Nalm6 (B-lineage) (eFigure 7c) and CEM (T-lineage) (eFigure 7e), was associated with increased sensitivity to vincristine (eFigure 7d, 7f). Moreover, primary leukemia cells from newly diagnosed patients who were homozygous for the CEP72 risk allele (TT) (mean LC50 = 0.9 μM, 95% CI −0.5 – 2.3 μM) were more sensitive to vincristine when compared to patients with either the CT (mean LC50 = 2.6 μM, 95% CI 0.6 – 4.7 μM) or CC (mean LC50 = 11.9 μM, 95% CI 3.0 – 20.7 μM) CEP72 genotype (p=0.016, eFigure 7g).
Discussion
We found that an inherited variant in the promoter region of the CEP72 gene was associated with a higher incidence and severity of vincristine-related peripheral neuropathy in children with ALL, with the cumulative incidence of neuropathy significantly higher and the average grade of neuropathy significantly greater in patients homozygous for the CEP72 risk allele (TT) compared to all other patients. The CEP72 gene encodes a centrosomal protein that is essential for microtubule formation, and vincristine exerts its pharmacologic effects by inhibiting microtubule formation. We found that the CEP72 variant associated with vincristine neuropathy (T allele at rs924607) creates a binding site for a transcriptional repressor, leading to lower CEP72 mRNA expression, and we showed that reducing the expression of CEP72 in human iPSC neurons or leukemia cells increased their sensitivity to vincristine. Because vincristine-induced toxicities can be influenced by cumulative dosage of vincristine and differ by race, we included both cumulative dosage and ancestry as covariates in our analyses, revealing that the CEP72 rs924607SNP was the only SNP reaching genome-wide significance (<5×10−8) in its association with vincristine-induced neuropathy. The frequency of the risk allele (T) was lower in African-American patients compared to the other racial groups, consistent with its lower frequency in African ancestry samples in the Hapmap collection. This finding is also consistent with the reported lower incidence of vincristine-induced neuropathy in black patients25. We found that children who inherited two copies of the CEP72 risk allele (homozygous for T at rs924607) had a significantly higher incidence of vincristine-induced peripheral neuropathy (60.8% [95% CI 43.9%–77.6% in TT patients compared to 23.4% [95% CI 17.4%–29.4] in CC/CT patients). We also found that CEP72 TT patients had increased severity (grade) of toxicity at first episode (2.4 fold higher grade of neuropathy; 95% CI 1.6–3.7 for TT compared to CT/CC patients) at any given cumulative dosage of vincristine.
Vincristine is widely used to treat leukemia, lymphoma, various solid tumors and brain tumors in children and adults26. Vincristine-induced peripheral neuropathy, characterized by sensory and motor dysfunction, causes extensive morbidity and often disrupts treatment. In children with ALL, the reported incidence of vincristine-induced neuropathy differs across treatment protocols, due in part to the grade of toxicity considered27,28, the VCR dosage and number of doses given29, and the types of symptoms captured9. In the current study, patients received uniform treatment according to prospective clinical trials, with neuropathy assessed by NCI criteria to prospectively identify all patients who developed grades 2–4 neuropathy during continuation therapy with vincristine. Although a genetic component of vincristine-induced neuropathy would be consistent with previously reported racial differences in the incidence of vincristine-induced neuropathy25, previous candidate gene studies have not yielded reproducible findings. We therefore took an agnostic genome-wide approach to identify genetic variants influencing vincristine-induced peripheral neuropathy, revealing the previously unrecognized association with CEP72 genotype.
The current study has limitations, including the small sample size (n=321), the small number of patients homozygous for the CEP72 risk allele (~16% of patients, n=50), and our assessment of only children in the context of ALL chemotherapy. However, our clinical findings are corroborated by multiple lines of laboratory evidence in human iPSC neurons and leukemia cells, and if verified in additional patient populations, could lead to a new approach for identifying patients at high-risk of vincristine-related neuropathy. Because our data suggest that the leukemia cells of patients who are homozygous for the CEP72 risk allele are more sensitive to vincristine, it may be possible to treat these patients with a lower dosage of vincristine to decrease the risk or severity of neuropathy without compromising the antileukemic effects of vincristine, a possibility that merits assessment in future clinical trials.
Conclusion
In this preliminary study of children with ALL, an inherited polymorphism in the promoter region of CEP72 was associated with increased risk and severity of vincristine-related peripheral neuropathy. If replicated in additional populations, this finding may provide a basis for safer dosing of this widely prescribed anticancer agent.
Supplementary Material
Acknowledgments
Funding/Support
The study was supported in part by NIH Grants No. R37 CA36401 (W.E.E. and M.V.R.), U01 GM92666 (M.V.R., D.P., C.C., W.Y., S.P., M.L.L., M.D. and W.E.E.), UO1 GM61393 (M.E.D.), R01 CA136765 (M.E.D.) F32 CA165823 (H.E.W.), Comprehensive Cancer Center grant CA21765, U10 CA98543 (COG Chair’s grant), U10 CA98413 (COG Statistical Center), and U24 CA114766 (COG Specimen Banking) from the National Cancer Institute, and by the American Lebanese Syrian Associated Charities.
Footnotes
Additional Contributions
We thank the St. Jude Hartwell Center for Bioinformatics and Biotechnology and the Flow Cytometry & Cell Sorting Shared Resource of the St. Jude Children’s Research Hospital Comprehensive Cancer Center. We thank Kirsten Ness (PT, PhD) for her assistance with preparation of the online-only summary of NCI Common Toxicity Terminology for assessing neuropathy. We thank Jun Yang (PhD), Virginia Perez-Andreu (MD), Nancy Kornegay (MBA), Mark Wilkinson (BS), and Colton Smith (PhD) for their expertise and assistance in specific components of data analysis and Julie Groff (BA), Elizabeth Stevens and Joshua Stokes (BA) for preparation of Figures, and Caleb Simmons (BA) for the preparation of the manuscript.
Statement
William E. Evans had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Author Contributions
Conception and design of the study: William E. Evans; Mary V. Relling
Acquisition, analysis, or interpretation of data: Barthelemy Diouf; Kristine R. Crews; Glen Lew; Deqing Pei; Cheng Cheng; Ju Bao; Jie J. Zheng; Wenjian Yang; Yiping Fan; Heather E. Wheeler; Claudia Wing; Shannon M. Delaney; Masaaki Komatsu; Steven W. Paugh; Joseph Robert McCorkle; Xiaomin Lu; Naomi J. Winick; William L. Carroll; Mignon L. Loh; Stephen P. Hunger; Meenakshi Devidas; Ching-Hon Pui; M. Eileen Dolan; Mary V. Relling and William E. Evans
Drafting of the manuscript: Barthelemy Diouf, Kristine Crews, Ching-Hon Pui, Cheng Cheng, William E. Evans
Critical revision of the manuscript for important intellectual content: Barthelemy Diouf; Kristine R. Crews; Glen Lew; Deqing Pei; Cheng Cheng; Ju Bao; Jie J. Zheng; Wenjian Yang; Yiping Fan; Heather E. Wheeler; Claudia Wing; Shannon M. Delaney; Masaaki Komatsu; Steven W. Paugh; Joseph Robert McCorkle; Xiaomin Lu; Naomi J. Winick; William L. Carroll; Mignon L. Loh; Stephen P. Hunger; Meenakshi Devidas; Ching-Hon Pui; M. Eileen Dolan; Mary V. Relling and William E. Evans
Statistical analysis: Deqing Pei; Cheng Cheng;
Obtaining funding: William E. Evans, Mary V. Relling, Stephen P. Hunger.
Supervision: William E. Evans
Conflict of Interest Disclosures: The authors declare no competing financial interests.
Role of the Sponsors: None of the funding sources played any role in the design and conduct of the study; collection, management, analysis and interpretation of the data; or preparation, review, or approval of the manuscript.
References
- 1.Pui CH, Evans WE. Acute lymphoblastic leukemia. The New England journal of medicine. 1998;339(9):605–615. doi: 10.1056/NEJM199808273390907. [DOI] [PubMed] [Google Scholar]
- 2.Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. The New England journal of medicine. 2006;354(2):166–178. doi: 10.1056/NEJMra052603. [DOI] [PubMed] [Google Scholar]
- 3.Pizzo PA, Poplack DG. Principles and Practice of Pediatric Oncology. 6 Wolters Kluwer/Lippincott Williams&Wilkins; 2011. [Google Scholar]
- 4.Jordan MA, Toso RJ, Thrower D, Wilson L. Mechanism of mitotic block and inhibition of cell proliferation by taxol at low concentrations. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(20):9552–9556. doi: 10.1073/pnas.90.20.9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nature reviews Cancer. 2004;4(4):253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 6.Toh HC, Sun L, Koh CH, Aw SE. Vinorelbine induces apoptosis and caspase-3 (CPP32) expression in leukemia and lymphoma cells: a comparison with vincristine. Leukemia & lymphoma. 1998;31(1–2):195–208. doi: 10.3109/10428199809057599. [DOI] [PubMed] [Google Scholar]
- 7.Bradley WG, Lassman LP, Pearce GW, Walton JN. The neuromyopathy of vincristine in man. Clinical, electrophysiological and pathological studies. Journal of the neurological sciences. 1970;10(2):107–131. doi: 10.1016/0022-510x(70)90013-4. [DOI] [PubMed] [Google Scholar]
- 8.Gidding CE, Meeuwsen-de Boer GJ, Koopmans P, Uges DR, Kamps WA, de Graaf SS. Vincristine pharmacokinetics after repetitive dosing in children. Cancer chemotherapy and pharmacology. 1999;44(3):203–209. doi: 10.1007/s002800050968. [DOI] [PubMed] [Google Scholar]
- 9.Egbelakin A, Ferguson MJ, MacGill EA, et al. Increased risk of vincristine neurotoxicity associated with low CYP3A5 expression genotype in children with acute lymphoblastic leukemia. Pediatric blood & cancer. 2011;56(3):361–367. doi: 10.1002/pbc.22845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aplenc R, Glatfelter W, Han P, et al. CYP3A genotypes and treatment response in paediatric acute lymphoblastic leukaemia. British journal of haematology. 2003;122(2):240–244. doi: 10.1046/j.1365-2141.2003.04430.x. [DOI] [PubMed] [Google Scholar]
- 11.Ross CJ, Visscher H, Rassekh SR, et al. Pharmacogenomics of serious adverse drug reactions in pediatric oncology. Journal of population therapeutics and clinical pharmacology = Journal de la therapeutique des populations et de la pharamcologie clinique. 2011;18:e134–151. [PubMed] [Google Scholar]
- 12.Plasschaert SL, Groninger E, Boezen M, et al. Influence of functional polymorphisms of the MDR1 gene on vincristine pharmacokinetics in childhood acute lymphoblastic leukemia. Clinical pharmacology and therapeutics. 2004;76(3):220–229. doi: 10.1016/j.clpt.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Pui CH, Sandlund JT, Pei D, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Total Therapy Study XIIIB at St Jude Children’s Research Hospital. Blood. 2004;104(9):2690–2696. doi: 10.1182/blood-2004-04-1616. [DOI] [PubMed] [Google Scholar]
- 14.Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. The New England journal of medicine. 2009;360(26):2730–2741. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kishi S, Cheng C, French D, et al. Ancestry and pharmacogenetics of antileukemic drug toxicity. Blood. 2007;109(10):4151–4157. doi: 10.1182/blood-2006-10-054528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13(3):176–181. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 17.Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309(22):2371–2381. doi: 10.1001/jama.2013.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cancer Therapy Evaluation Program (CTEP) [Accessed November 21, 2014];National Cancer Institute website. http://dctd.cancer.gov/ProgramPages/CTEP/
- 19.Li Y, Willer CJ, Ding J, Scheet P, Abecasis GR. MaCH: using sequence and genotype data to estimate haplotypes and unobserved genotypes. Genetic epidemiology. 2010;34(8):816–834. doi: 10.1002/gepi.20533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang JJ, Cheng C, Devidas M, et al. Ancestry and pharmacogenomics of relapse in acute lymphoblastic leukemia. Nature genetics. 2011;43(3):237–241. doi: 10.1038/ng.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holleman A, Cheok MH, den Boer ML, et al. Gene-expression patterns in drug-resistant acute lymphoblastic leukemia cells and response to treatment. N Engl J Med. 2004;351(6):533–542. doi: 10.1056/NEJMoa033513. [DOI] [PubMed] [Google Scholar]
- 22.Kalbfleisch JD, Prentice R. The Statistical Analysis of Failure Time Data. 2. Chapter 7 Wiley & Sons Inc; New York: 2002. [Google Scholar]
- 23.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Statist. 1988;16:1141–1154. [Google Scholar]
- 24.Huang RS, Duan S, Shukla SJ, et al. Identification of genetic variants contributing to cisplatin-induced cytotoxicity by use of a genomewide approach. American journal of human genetics. 2007;81(3):427–437. doi: 10.1086/519850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Renbarger JL, McCammack KC, Rouse CE, Hall SD. Effect of race on vincristine-associated neurotoxicity in pediatric acute lymphoblastic leukemia patients. Pediatric blood & cancer. 2008;50(4):769–771. doi: 10.1002/pbc.21435. [DOI] [PubMed] [Google Scholar]
- 26.Rowinsky EK, Donehower RC. The clinical pharmacology and use of antimicrotubule agents in cancer chemotherapeutics. Pharmacology & therapeutics. 1991;52(1):35–84. doi: 10.1016/0163-7258(91)90086-2. [DOI] [PubMed] [Google Scholar]
- 27.Aytac S, Yetgin S, Tavil B. Acute and long-term neurologic complications in children with acute lymphoblastic leukemia. The Turkish journal of pediatrics. 2006;48(1):1–7. [PubMed] [Google Scholar]
- 28.Crom WR, de Graaf SS, Synold T, et al. Pharmacokinetics of vincristine in children and adolescents with acute lymphocytic leukemia. The Journal of pediatrics. 1994;125(4):642–649. doi: 10.1016/s0022-3476(94)70027-3. [DOI] [PubMed] [Google Scholar]
- 29.Pal PK. Clinical and electrophysiological studies in vincristine induced neuropathy. Electromyography and clinical neurophysiology. 1999;39(6):323–330. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.