Abstract
Mitochondrial dysfunction is implicated in disease and in age-related infertility. Mitochondrial replacement therapies (MRT) in oocytes or zygotes such as pronuclear (PNT), spindle (ST) or polar body (PBT) transfer could prevent second generation transmission of mitochondrial DNA (mtDNA) defects. PNT, associated with high levels of mtDNA carryover in mice but low levels in human embryos, carries ethical issues secondary to donor embryo destruction. ST, developed in primates, supports normal development to adults and low mtDNA carryover. PBT in mice, coupled with PN or ST, may increase the yield of reconstructed embryos with low mtDNA carryover. MRT also offers replacement of the deficient cytoplasm in oocytes from older patients, with the expectation of high pregnancy rates following in vitro fertilization.
Keywords: mitochondria, mitochondrial DNA, mitochondrial replacement therapy, female infertility
Mitochondrial DNA and its role in pathologies
Mitochondria are cytoplasmic organelles with their own genome that play a major role in energy generation by oxidative phosphorylation (OXPHOS; see Glossary). The enzymatic machinery involved in OXPHOS requires both nuclear and mitochondrial DNA (mtDNA) participation and while the latter encodes only 37 genes, there are large numbers of mitochondria per cell, particularly in the oocyte. MtDNA in eukaryotes derives evolutionarily from bacteria and may play roles far beyond those described here [1]. Mutations in mtDNA, either alone or in conjunction with certain nuclear DNA mutations, can result in serious disorders that are often difficult to diagnose and for which there are currently no cures. The frequency of either inherited or acquired (somatic) mtDNA mutation is surprisingly high, reflecting vulnerability to replication errors and susceptibility to damaging reactive molecules confounded by limited DNA repair mechanisms. Diseases caused by mtDNA mutations were first described in 1988 [2–5]. Since then over 700 mutations, both germline and somatic, have been identified, some of which have been associated with human disorders including myopathies, neurodegenerative diseases, diabetes, cancer and infertility (http://www.mitomap.org/bin/view.pl/MITOMAP/WebHome).
Transmission of germline mtDNA mutations that potentially cause disease in the next generation has been reported at a frequency of 1 in 200 in newborns [6]. Symptoms can begin at any age and severity of disease relates to the specific mutation and its penetrance, the percentage of mtDNA with the mutation per cell (the heteroplasmy level), modulation by nuclear genome background, and other parameters (Box 1). Second generation transmission of mtDNA-based disease can be circumvented, at least in preclinical animal and human in vitro studies, predicated on the knowledge that mtDNA is exclusively inherited from the egg (i.e. maternally), and that safe, efficient mitochondrial replacement therapies (MRTs) are in place. MRTs intervene at the oocyte or one-cell embryonic stage (zygote) and are accomplished by extracting the nuclear DNA from the patient’s egg or embryo, leaving behind cytoplasm (cytoplast) with mutated mtDNA, followed by transplantation into a donor cytoplast containing wild type mtDNA and cytoplasm.
Box 1. Mitochondrial DNA heteroplasmy threshold for disease.
Mitochondrial production of ATP by OXPHOS occurs in virtually every cell in the body and the number of mitochondria per cell varies between types dependent on their energy requirements. Therefore, deficits in mitochondrial function are likely to be experienced differently throughout the body, with the potential for multi-tissue/organ involvement. This leads to a very complex situation when cause and effect relationships are sought. In heteroplasmic mtDNA disease not only are there differences in threshold levels between tissues in the same carrier, but between siblings in an affected family or between families affected with the same mutation. Consequently it is impossible to establish a single, uniform threshold for disease and its transmission. However, in the monkey and human studies summarized here, the mtDNA carryover levels following MRT were consistently at or below 2%, a value almost certainly below the threshold for disease. For an extensive review of these issues, see [2]. In the context of initial clinical trials for MRT, patient selection could be restricted to families carrying homoplasmic or high heteroplasmy mtDNA mutations, who have already given birth to an affected child with early disease onset.
Techniques for mitochondrial replacement therapies (MRT)
Alternatives to germline gene therapy have been described for couples at risk of transmitting mtDNA-based disorders, including prenatal and preimplantation genetic diagnosis (PGD). While potentially useful for low heteroplasmic conditions, however, these alternatives are inappropriate for homoplasmic conditions where the patient mutant load is 100% [7]. Novel approaches for circumventing mtDNA-based disease transmission that involve germline gene therapy are described here including pronuclei transfer (PNT), spindle transfer (ST) and polar body transfer (PBT).
Pronuclei transfer (PNT)
The zygote stage in mammals is characterized by the presence of two pronuclei (PN), each clearly visible and containing a haploid chromosomal complement of nuclear DNA from either sperm or oocyte (Figure 1). Transfer of both PN from one zygote to another was first accomplished in the early 1980s, demonstrating that manipulated mouse zygotes could develop into live offspring [8]. More recently, PNT in the mouse has been used to model MRT [9]. However, the efficacy of PNT in mice has been adversely affected by high mtDNA carryover levels in the pups; approximately 24% [10–12]. This is presumably due to the inevitable co-transfer of a small amount of cytoplasm containing mitochondria and mtDNA (Figure 1). As an example, in the most recent report [12] an average heteroplasmy level of 24% was associated with biopsied tissues from 7 pups and a similar level was sustained in the F2 generation. This could reflect the large size of PN and uneven mitochondrial distribution [13, 14]. Thus, isolation of PN, even if encapsulated in small karyoplasts, may result in the co-transfer of unacceptable numbers of mitochondria. Although most common inherited human mtDNA diseases are typically associated with high mutated mtDNA thresholds (Box 1) [15], these results in mice do not bode well for MRT in humans. Nevertheless, the feasibility of PNT in the human was reported in 2010 using abnormal zygotes with either one or >2 PN, which are normally discarded during routine in vitro fertilization (IVF) [16]. Zygotes containing 2PN were created by transfer of one PN from a poly PN zygote into a 1PN zygote. Noted that male and female PN in the human zygote cannot readily be differentiated by visual observation, only half of the reconstructed zygotes would contain both a male and female PN. Reconstructed zygotes (n=36) were then placed in culture and 8 developed beyond the 8-cell stage. Three, or 8%, of these embryos developed to the blastocyst stage with low mtDNA carryover (<2%). Despite the low yield of blastocysts, the authors concluded that this approach carries potential for MRT. However, even with this proof of principle, it is currently impossible to evaluate the safety and efficacy of PNT in normal human zygotes based on this preliminary report, and further investigation in nonhuman primates is warranted before reaching a final conclusion on PNT viability as a MRT technique in humans.
Figure 1. Schematic representation of pronuclear transfer (PNT) procedure with zygote stage embryos.
Male and female pronuclei, both highly visible but similar in appearance, are removed from patient (lower left) and donor (upper left) zygotes by aspiration into karyoplasts using a micropipette. Note that a patient karyoplast contains cytoplasm with mitochondria and mtDNA, referred to as carryover. Patient karyoplasts containing PN are then placed in contact with the mtDNA donor cytoplast and the two entities are fused together, producing a reconstructed embryo that is ready for culture or transfer into the patient. Due to carryover of patient mitochondria, the reconstructed zygote may still contain unacceptable amounts of mutated mtDNA.
Spindle transfer (ST)
In 2009, ST was pioneered in the rhesus monkey demonstrating that MRT could be accomplished at the unfertilized oocyte stage (Figure 2) where efficacy and safety was demonstrated by live births, normal growth curves of offspring and low levels of mtDNA carryover [17, 18]. A step-by-step technical description of the ST protocol has been published [19, 20], supporting independent replication and verification [12, 21]. Briefly, Chinese and Indian-origin rhesus females carrying different wild-type mtDNA haplotypes were identified based on sequence polymorphism. Unlike zygotes, the distribution of mitochondria in oocytes is uniform, allowing ST without significant mtDNA carryover from the nuclear donor oocyte. Meiotic spindles surrounded by a small volume of cytoplasm and membrane were extracted into karyoplasts containing only 1.5% the volume of cytoplasts. Karyoplasts from Chinese-origin rhesus macaques were then fused to donor cytoplasts from Indian-origin macaques and vise-versa to produce reconstructed oocytes with donor mtDNA. Fertilization by intra-cytoplasmic sperm injection (ICSI) was followed by embryo culture to the blastocyst stage. Blastocyst development and quality was comparable to controls, and two embryonic stem cell (ESC) lines were established from eight ST embryos; a derivation efficiency similar to controls. Fifteen ST blastocysts were transferred into 9 females, resulting in three pregnancies and four live births. These efficiencies were also similar to results with intact, non-manipulated embryos. Longitudinal studies of these ST monkeys documented normal growth curves based on body weight and relatively constant mtDNA carryover levels at or below 2% (Figure 3). Additionally, normal blood chemistries, ATP levels and membrane potential in skin fibroblasts were reported [22].
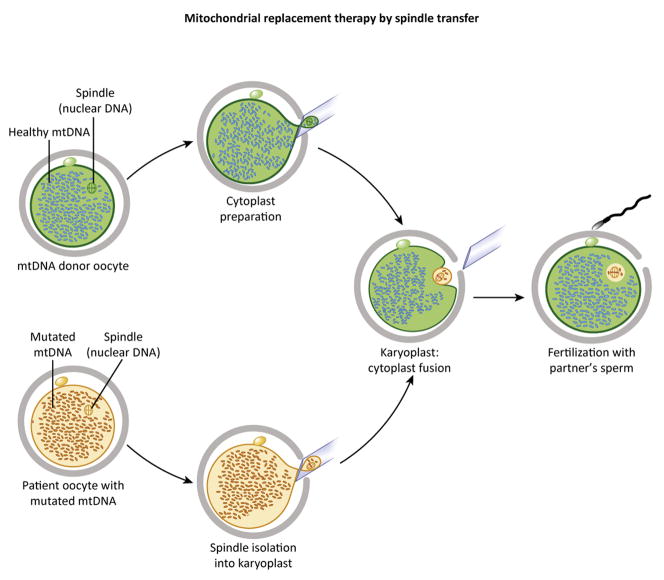
Figure 2. Individual steps in spindle transfer (ST).
The donor and mutated mtDNA-carrying unfertilized oocytes depicted on the left contain female chromosomes assembled into spindles. Spindles from both oocytes are extracted into karyoplasts. Due to the small spindle size, karyoplasts carry fewer mitochondria and less mutated mtDNA. Karyoplasts from patient oocytes are then fused to donor cytoplasts and fertilized with partner’s sperm (right) creating mtDNA-mutation free embryos.
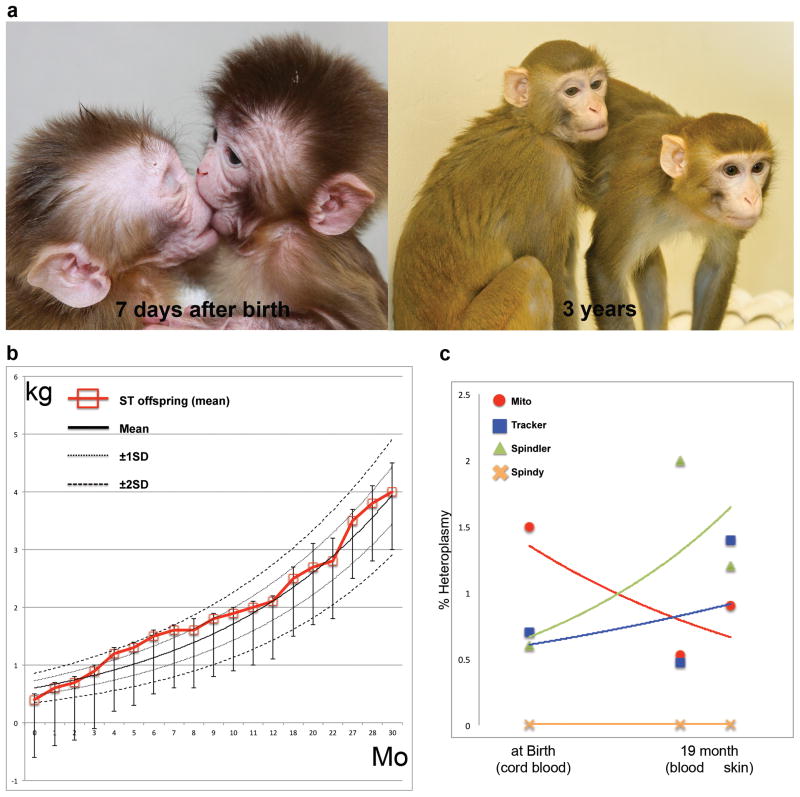
Figure 3. Transmitochondrial monkeys produced by spindle transfer.
A) Spindle transfer (ST) rhesus monkeys, Mito and Tracker, shortly after birth and at 3 years of age.
B) Growth curves based on weight measurements (kg) showing that spindle transfer (ST) offspring were in the normal range for the first 30 months (Mo) of their lives.
C) MtDNA carryover levels (% heteroplasmy) in four monkeys (Mito, Tracker, Spindler and Spindy), as measured in cord blood at birth or in blood and skin at 19 months. The levels were essentially at the limit of detection and did not change significantly over time. From [22], with permission.
These results in monkeys encouraged studies in humans where oocyte donors with different mtDNA haplotypes were recruited for mtDNA tracking purposes [22]. Of 106 oocytes donated for research, 65 were subjected to reciprocal ST and 33 served as controls. Fertilization levels in ST oocytes at 73% were similar to controls (75%), however, a significant portion of ST zygotes (52%) showed abnormal fertilization as determined by an irregular number of PN (most often the presence of three PN) [21, 22], an unexpected result much different from that seen in monkeys. This outcome was thought to reflect premature oocyte activation under suboptimal culture conditions. Among normally fertilized ST zygotes, blastocyst development (62%) and ESC isolation (38%) rates were comparable to controls. The availability of ESC lines that can be propagated indefinitely supported detailed genetic, cytogenetic and pluripotency evaluations, and mtDNA carryover levels were assessed and found to be undetectable. The feasibility of ST for human oocytes was independently examined in a different laboratory where ST oocytes were not fertilized but rather artificially activated to mimic normal development [21]. This approach was mandated presumably by ethical and legal concerns. Some parthenogenetic embryos progressed to blastocysts supporting ESC derivation. MtDNA heteroplasmy levels in ESCs and derived phenotypes were less than 1%. Although this study involved parthenote-derived embryos, the results are consistent with the conclusion that ST can be accomplished with minimal mtDNA carryover in humans.
Polar body transfer (PBT)
During meiosis, the mammalian oocyte undergoes two reductive divisions with uneven cytoplasmic segregation and the abstriction of two small bodies, both containing a complement of chromosomes. The first polar body (PB1) contains a diploid set of chromosomes, and the second (PB2) contains a haploid set. In the mouse, transfer of PB1 or PB2 into appropriate oocyte or zygotic cytoplasm supports normal completion of meiosis and full term development to viable offspring [23, 24]. PBT has been described recently in the context of MRT [12] (Figure 4 and 5). The rationale was that PBs that contain only a few mitochondria could be easily visualized and handled while their efficient utilization could double the yield of reconstructed oocytes and zygotes by ST and PNT. Using this approach with simultaneous PB1 transfer and ST from 27 donor oocytes, 43 reconstructed oocytes were produced. Simultaneous PB2 and PNT were also conducted to increase the yield from donor zygotes. Live births from reconstructed oocytes or embryos mirrored control rates, and mtDNA carryover in PB1 transfer, PB2 transfer and ST but not PNT pups was low or undetectable and remained so in the F2 generation. Birth rates were comparable to controls, and all living pups derived from reconstructed oocytes respired normally and fell in the weight range of non-manipulated controls. PBT has not been successfully replicated in other mammals (including primates) despite considerable efforts. In most species, PBs experience a brief lifetime due to apoptotic pressures that lead to DNA fragmentation and degradation. In humans, a substantial effort has focused on genetic analysis of PBs for disease assessment, however, their functional capacity by PBT remains to be determined. Of note, due to meiotic recombination, the genetic makeup of PB1 and PB2 is not identical to their homologous counterparts in the oocyte or zygote [25]. Therefore, offspring derived by PB1 transfer and ST from the same donor metaphase II (MII) oocyte would not be genetically identical twins, but rather share a sibling relationship. The same is true for PB2 transfer and PNT offspring. Efforts to isolate viable PBs following ovarian stimulation in patients could be counterproductive if substantial alterations in timing of oocyte harvest and PBT are required.
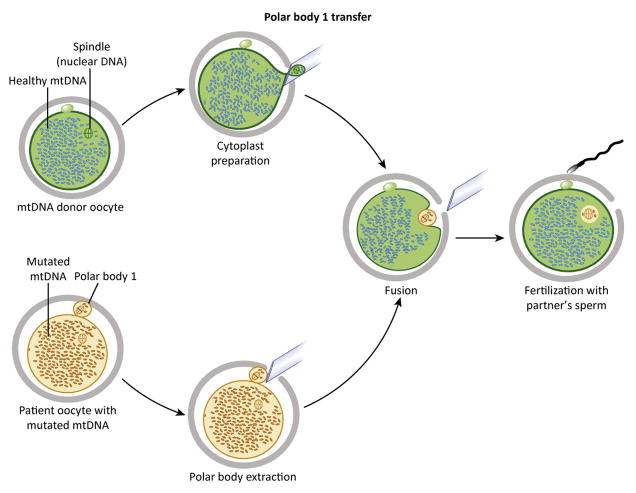
Figure 4. Transfer of polar body 1 (PB1).
The procedure is initiated by PB1 recovery from patient unfertilized metaphase II (MII) oocytes and is followed by fusion with donor MII cytoplasts from which the spindle has been removed (upper). Reconstructed MII oocytes are then fertilized and transferred into a recipient.
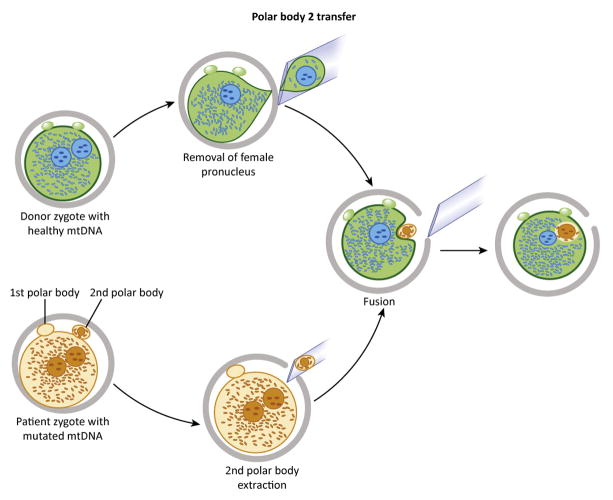
Figure 5. Polar body 2 (PB2) transfer.
PB2 is recovered from pronuclear-stage zygotes. Reconstructed zygotes are produced by transfer of PB2 into recipient zygotes from which the female pronuclei have been removed (upper).
MRT for infertility patients
Advanced female age (over 35) is a major factor responsible for infertility, embryonic and fetal loss (miscarriages) and birth defects such as Down syndrome. While direct patient treatment options are limited to low temperature storage of oocytes recovered at earlier ages, normal pregnancies and live birth rates can also be realized when oocytes or embryos donated by younger women are substituted. Parenthetically, oocyte cryopreservation supports acceptable IVF outcomes as determined by the American Society of Reproductive Medicine and long term cryostorage of oocytes collected from young patients is realistic based on mouse and cattle studies. Obviously, in the second scenario cited above and absent cryostored oocytes, the female patient’s DNA does not contribute to the resulting fetus. Several findings implicate declining oocyte quality as the main factor in this type of infertility. Impaired cytoplasmic maturation could explain increases in meiotic and mitotic abnormalities and aneuploidy, while cytoplasmic deficiencies in specific transcripts and proteins could impact embryonic preimplantation development [26–28]. A mitochondrial component is probable based on observed associations of increased maternal age with increased rates of mtDNA mutations in eggs [29]; decreased metabolic activity in embryos [30]; reduced ATP production [31], and; altered mitochondrial calcium homeostasis [32]. Moreover, age related increases in mtDNA deletions have been described in cumulus cells that surround and metabolically support oocytes [33], and cytoplasmic deficiencies in general and mitochondrial dysfunction in particular can contribute to nuclear genome instability in aged oocytes, resulting in embryo aneuploidy. For example, spindle abnormalities have been attributed to insufficient energy supplies, and/or shifts in redox regulation [34, 35].
In 1998–99, cytoplasmic transfer, or the augmentation of patient eggs with a small volume (1–5%) of donor cytoplasm was used by several IVF clinics in an effort to overcome repeated IVF failures in selected patients. The procedure essentially involved co-injection of donor cytoplasm with sperm, as an extension of ICSI [36]. Several pregnancies were established before the US Food and Drug Administration (FDA), for regulatory purposes, insisted that an investigational new drug application be filed before the success or failure of this approach could be determined. Due to the current trend toward delayed childbearing in the Western world, age related infertility is commonplace [37]. In the absence of cryopreserved oocytes recovered earlier in life, and limitations of existing ART applications confined to donated embryos or eggs, MRT would provide efficient and complete replacement of the entire cytoplasmic component while retaining the female partner’s nuclear DNA [38, 39].
Ethics and regulation
Somatic gene therapy has generally met with ethical acceptance since it results in genetic changes that are not passed on to the next generation. However, germline gene therapy involves permanent correction of mutated genes in the germ cells, resulting in passage of the alteration to subsequent generations. Concerns over human germline gene therapy are largely related to safety and efficacy limitations associated with current nuclear gene transfer approaches involving recombinant DNA. However, MRT, as discussed here, is based on utilization of natural human mtDNA provided by healthy egg donors. Another advantage of MRT is that transmission of any mtDNA mutations (point mutations or deletions) can be treated since the entire mtDNA complement is replaced. In MRT, the presence of donor mtDNA adds a third source, albeit a very limited one, of DNA that would be passed to both genders in the F1 generation. However, F2 passage of donor mtDNA would be restricted to the female lineage only, because male children do not pass their mtDNA to offspring. While it is widely thought that mtDNA does not encode components that are prime determinants of genetic identity or kinship [40], recent evidence suggests that this conclusion may require refinement [1]. Nevertheless MRT, although under consideration, is not currently permitted in the UK or the USA.
In the UK an alteration in existing law is required with approval by Parliament, an activity that could occur shortly (http://www.hfea.gov.uk/9025.html). In the USA, the FDA has recently convened a meeting of the Cellular, Tissue, and Gene Therapy Advisory Committee discussing the current state of preclinical and clinical science regarding assisted reproductive methods and the design of early-phase clinical trials of MRT to prevent transmission of mtDNA diseases and for the treatment of female infertility (http://www.fda.gov/AdvisoryCommittees/Calendar/ucm380042.htm).
The FDA has also recognized ethical and social policy issues related to genetic modification of eggs and embryos that will likely affect regulatory decisions. Therefore, the National Academy of Sciences (NAS)/Institute of Medicine (IOM) (http://www8.nationalacademies.org/cp/projectview.aspx?key=IOM-HSP-14-25) has been asked to convene a consensus panel and address several questions: (i) Whether manipulation of mitochondrial content should be considered germline modification, or viewed differently from modification of nuclear DNA, from a social and ethical perspective; (ii) The implications of manipulating mitochondrial content both in children born to women as a result of participating in these studies and in descendants of any female children; (iii) Ethical issues in providing “consent” or “permission” to accept risks on behalf of a child who does not exist; and (iv) Ethical and social issues that arise if a child is born with genetic material from three individuals. The FDA will utilize this information when reviewing clinical trial applications.
In the UK, following a 6 month review of ethical issues, the Nuffield Council on Bioethics (http://nuffieldbioethics.org/project/mitochondrial-dna-disorders/) concluded that MRT techniques, if determined to be safe and effective, would be ethical for affected families provided such families are offered full information and support. Thus, it was envisioned that “avoidance of mtDNA based disease transmission could offer significant health and social benefits to individuals and families, who could potentially live their lives free from what can be very severe and debilitating disorders.” Additionally, it can be argued that medicine should respond to the reproductive health needs of patients and that germline gene therapy may be the only effective treatment for mtDNA based diseases. On the other side of this ethical argument there is the slippery slope fear of eventually creating designer children, that too much risk is involved and that the rights of the subsequent generation are violated.
The safety and effectiveness issues in MRT referenced above by the Nuffield Council could include, in the case of ST and PNT, oocyte exposure to the microfilament disruptor cytochalasin B to facilitate spindle or PN isolation and reduce lysis. In addition to cytochalasin B, PNT requires exposure of zygotes to nocodozole, a microtubule disruptor. These reagents are used briefly and in low concentrations before oocytes or embryos are extensively rinsed, and there does not appear to be any detrimental effects based on the results described in this review. A recent study in the mouse model corroborates this conclusion [12]. In addition, MRT relies on the use of inactivated Sendai virus extract to promote membrane fusion between isolated karyoplasts and donor cytoplasts. This extract is completely inactivated and free of Sendai genomic RNA [17], therefore viral infection or proliferation from this source is unlikely. Incomplete removal of chromosomal DNA during micromanipulation represents another potential confounding factor as the developmental competence of the reconstructed oocyte would be jeopardized. This can, and should, be assessed in clinical trials by PGD or prenatal diagnosis. Finally, safety concerns have been raised due to hypothetical nuclear-to-mitochondrial genome incompatibility resulting from “unmatched” MRT [41, 42].
Substantial differences exist in mtDNA sequences between individual families and between human populations. Since mitochondrial complexes implicated in OXPHOS include proteins encoded by both nuclear and mtDNA that must interact efficiently for normal energy production, a unique relationship must exist between nuclear and mtDNA, disruption of which could occur in MRT with the introduction of a foreign source of mtDNA. Indeed, a cautionary note to this effect appeared recently in which studies in model organisms from mice to fruit flies were reviewed [42].
For example, mouse models have been developed to document the effects of mtDNA mismatching between mouse subspecies that diverged genetically and phenotypically over one million years ago. Sophisticated breeding and backcrossing was employed to produce congenic mice, with exchanged mtDNA between laboratory Mus musculus domesticus and Mus musculus musculus or Mus musculus spretus. Under these extreme mismatch conditions, differences were described in the level of performance (running time to exhaustion) and in growth rate in male animals; females have not been tested typically because the estrus cycle affects physical performance [43]. Also, direct evidence of mtDNA involvement in cognitive functioning was established by tests for modified learning, exploration sensory development and the anatomy of the brain in mice produced by mtDNA exchange between Mus musculus domesticus and Mus musculus brevirostris [44]. However, the existence of morbidity or mortality was not reported in these animals possessing highly mismatched nuclear and mtDNA.
Clinical applications of MRT will be based on donor mtDNA from common human populations, rather than interspecies mitochondria. Therefore, preclinical studies in monkeys and mice involving intraspecies MRT are also relevant [12, 17, 22, 45]. Normal monkey and mouse offspring generated with donor mtDNA, although limited in number, are supportive of the conclusion that MRT does not introduce mismatch outcomes that result in morbidity or mortality. This is true for the overall health and development of ST monkeys from genetically distant mtDNA subpopulations of Macaca mulatta originated in India and China [17, 22] and for transmitochondrial mice generated using C57Bl/6 and NZB strains carrying distinct mtDNA haplotypes [12, 45].
Hence, although caution is always prudent, one could argue that the ability to have disease-free, biological children for most affected patients far outweighs the modest downsides suggested by existing mouse interspecies studies. Matching patient and cytoplast donors for mitochondrial haplotypes might also be considered if matching criteria were established.
Over and above the micromanipulation techniques involved in MRT there is the need to synchronize donor and patient ovarian cycles. While this is done on a routine basis in IVF clinics, it is not without challenges. One approach to mitigation is low temperature storage of oocytes or karyoplasts. Vitrification of oocytes involving the use of high concentrations of cryoprotectant and extremely rapid temperature changes is now routine [46], and survival of the spindle complex in monkeys following vitrification has been described [22].
Concluding remarks
The need for MRT is apparent for families carrying mtDNA based disease and for older infertility patients without cryostored young oocytes and refractile to conventional IVF. So if the need exists, and the risk to benefit ratio is favorable, then the question becomes how we move towards implementation. Regulatory agencies in the US and UK are evaluating safety and efficacy issues based on animal models and human studies such as those described here. One of the most important unresolved issues in these discussions is what further useful research can be envisioned before moving forward with clinical trials? Apart from generating additional confirmatory results, perhaps in other species, our suggestions include revisiting the health and donor mtDNA status of the 30 or so children (now teenagers) produced by cytoplasmic transfer, under the assumption that a detectable mtDNA transfer occurred during the procedure. This is now underway at the St Barnabus Medical Center [47].
Male ST monkeys at the Oregon National Primate Research Center (ONPRC) have now reached sexual maturity and their fertility status could be tested in time-mated breeding colonies. Similarly, female ST monkeys could be bred, providing offspring for evaluating second generation transmission of donor mtDNA. In both cases, mtDNA heteroplasmy levels should be monitored over time in ST adults as well as infants, since selective amplification or segregation of carryover mtDNA is possible. Further insights into the feasibility and safety of MRT, especially PNT and PBT, in non-human primates could be considered, dependent upon the identification and study of appropriate mtDNA disease models. Finally, it should be possible to conduct additional physical and cognitive studies on reportedly normal F1 and F2 PB1, or ST mice with low levels of carryover mitochondria [12].
Looking much farther into the future, genome editing technologies are emerging that enable targeted correction of mutated genes in human cells [48]. These involve precise modification of gene sequences by adding, deleting or correcting specific genomic loci [48–51]. Such advances will likely allow selective correction of nuclear or mitochondrial gene defects in human gametes or preimplantation embryos, thereby ultimately preventing the passage of most genetic conditions.
Highlights.
Mitochondrial dysfunction is implicated in disease and infertility
Mitochondrial replacement therapy averts transmission of mtDNA defects to children
Efficacy, safety, regulation and ethical issues are considered
Acknowledgments
This work was supported by grants from National Institutes of Health R01-HD063276, R01-HD057121, R01-HD059946, R01-EY021214, P51-OD011092, a grant from the Leducq Foundation, and OHSU institutional funds.
Glossary
- Adenosine triphosphate (ATP)
An important biological molecule that contains high energy chemical bonds. Energy, for metabolic purposes, is released when one or more of the bonds in ATP are broken, yielding AMP or ADP (adenosine mono- or di-phosphate)
- Blastocyst
A young, spherically-shaped embryo typically formed 5–6 days after fertilization, and prior to implantation. The blastocyst contains an outer layer of cells called the trophectoderm and an inner cluster called the inner cell mass (ICM)
- Cumulus cells
An extensive, adherent cluster of cells surrounding the ovulated oocyte. These cells play a nurturing role during the maturation process but are shed during oocyte/embryo transport via the oviduct
- Cytoplast
Cell membrane enclosed substance of the cell excluding the nucleus formed after the cell enucleation or spindle removal procedure
- Germline
The line of cells terminating in gametes (eggs and sperm) used by sexually reproducing organisms to transmit their genes to offspring. Mutations in germ cell DNA will pass from generation to generation
- Heteroplasmy
A term used to describe the mixture of two or more different mitochondrial genomes within a cell. The ratio of mutated to healthy mtDNA determines the severity of mitochondrial DNA based diseases
- In vitro fertilization (IVF)
A process in which the egg is fertilized by sperm outside the body. Embryos produced by this technique are used in the treatment of families with fertility issues
- Intracytoplasmic sperm injection (ICSI)
A method of fertilizing an oocyte by direct injection insuring sperm incorporation and avoiding multiple sperm penetration
- Karyoplast
A cell spindle or nucleus, along with a small amount of cytoplasm, enclosed by cell membrane created during the nuclear transfer procedure
- Meiotic spindle
The oocytes’s spindle-shaped structure containing the nuclear genome, composed of microtubules attached to chromosomes. Spindles are important for chromosome alignment and segregation during reductive divisions
- Metaphase II oocyte
An egg arrested at the metaphase stage of meiosis, typically representative of a mature oocyte collected after ovarian stimulation during IVF
- Mitochondrial carryover
mutant or patient mitochondria (and hence mtDNA) that are transferred with a karyoplast during MRT. The number of mtDNA molecules carried over is a function of the amount of cytoplasm and the density of mitochondria in the spindle or pronuclear region
- Mitochondrial DNA (mtDNA) mismatch
detrimental interaction between the donor mitochondrial and patient nuclear genomes after MRT due to sequence differences in mtDNA
- Oxidative phosphorylation (OXPHOS)
The transfer of electrons through several protein complexes that occurs inside mitochondria supporting the production, storage and release of chemical energy in the form of ATP
- Parthenogenesis
The development of embryos from unfertilized eggs. In mammals, parthenogenesis can be induced artificially, and following diploidization, can result in development of preimplantation stage embryos
- Polar bodies
Small cells containing DNA formed during meiotic, reductive divisions of the oocyte. Polar body I is formed before fertilization and can be visualized readily in the ovulated oocyte while polar body II is formed after fertilization and can be seen in the zygote. Both polar bodies eventually disintegrate
- Preimplantation genetic diagnosis (PGD)
A reproductive technique used in the diagnosis of genetic abnormalities in early embryos. Typically, one or two cells (blastomeres) from an eight-cell embryo (3-days old) are removed for testing and only normal embryos at five-days of age are transferred to the patient
- Pronuclei
Membrane enclosed entities in the zygote that house the male and female chromosomal contributions. Their fusion completes fertilization and immediately precedes the mitotic division that results in the 2-cell embryo
- Zygote
The one-cell embryo formed soon after fertilization containing 2 pronuclei, one from the egg and one from the sperm
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hamilton G. The micromanagers. New Scientist. 2014:42–45. [Google Scholar]
- 2.Wallace DC, Chalkia D. Mitochondrial DNA genetics and the heteroplasmy conundrum in evolution and disease. Cold Spring Harbor perspectives in medicine. 2013;3:a021220. doi: 10.1101/cshperspect.a021220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chinnery PF, Hudson G. Mitochondrial genetics. British medical bulletin. 2013;106:135–159. doi: 10.1093/bmb/ldt017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wallace DC, et al. Mitochondrial DNA mutation associated with Leber’s hereditary optic neuropathy. Science. 1988;242:1427–1430. doi: 10.1126/science.3201231. [DOI] [PubMed] [Google Scholar]
- 5.Zeviani M, et al. Deletions of mitochondrial DNA in Kearns-Sayre syndrome. Neurology. 1988;38:1339–1346. doi: 10.1212/wnl.38.9.1339. [DOI] [PubMed] [Google Scholar]
- 6.Elliott HR, et al. Pathogenic mitochondrial DNA mutations are common in the general population. Am J Hum Genet. 2008;83:254–260. doi: 10.1016/j.ajhg.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitalipov S, et al. Limitations of preimplantation genetic diagnosis for mitochondrial DNA diseases. Cell reports. 2014;7:935–937. doi: 10.1016/j.celrep.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGrath J, Solter D. Nuclear transplantation in the mouse embryo by microsurgery and cell fusion. Science. 1983;220:1300–1302. doi: 10.1126/science.6857250. [DOI] [PubMed] [Google Scholar]
- 9.Sato A, et al. Gene therapy for progeny of mito-mice carrying pathogenic mtDNA by nuclear transplantation. Proc Natl Acad Sci U S A. 2005;102:16765–16770. doi: 10.1073/pnas.0506197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meirelles FV, Smith LC. Mitochondrial genotype segregation in a mouse heteroplasmic lineage produced by embryonic karyoplast transplantation. Genetics. 1997;145:445–451. doi: 10.1093/genetics/145.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meirelles FV, Smith LC. Mitochondrial genotype segregation during preimplantation development in mouse heteroplasmic embryos. Genetics. 1998;148:877–883. doi: 10.1093/genetics/148.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang T, et al. Polar body genome transfer for preventing the transmission of inherited mitochondrial diseases. Cell. 2014;157:1591–1604. doi: 10.1016/j.cell.2014.04.042. [DOI] [PubMed] [Google Scholar]
- 13.Sathananthan AH, Trounson AO. Mitochondrial morphology during preimplantational human embryogenesis. Hum Reprod. 2000;15(Suppl 2):148–159. doi: 10.1093/humrep/15.suppl_2.148. [DOI] [PubMed] [Google Scholar]
- 14.Van Blerkom J, et al. Differential mitochondrial distribution in human pronuclear embryos leads to disproportionate inheritance between blastomeres: relationship to microtubular organization, ATP content and competence. Hum Reprod. 2000;15:2621–2633. doi: 10.1093/humrep/15.12.2621. [DOI] [PubMed] [Google Scholar]
- 15.Samuels DC, et al. Preventing the transmission of pathogenic mitochondrial DNA mutations: Can we achieve long-term benefits from germ-line gene transfer? Hum Reprod. 2013;28:554–559. doi: 10.1093/humrep/des439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Craven L, et al. Pronuclear transfer in human embryos to prevent transmission of mitochondrial DNA disease. Nature. 2010;465:82–85. doi: 10.1038/nature08958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tachibana M, et al. Mitochondrial gene replacement in primate offspring and embryonic stem cells. Nature. 2009;461:367–372. doi: 10.1038/nature08368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee HS, et al. Rapid mitochondrial DNA segregation in primate preimplantation embryos precedes somatic and germline bottleneck. Cell reports. 2012;1:506–515. doi: 10.1016/j.celrep.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tachibana M, et al. Chromosome transfer in mature oocytes. Nat Protoc. 2010;5:1138–1147. doi: 10.1038/nprot.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tachibana M, et al. Chromosome transfer in mature oocytes. Fertil Steril. 2012;97:e16. doi: 10.1016/j.fertnstert.2012.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paull D, et al. Nuclear genome transfer in human oocytes eliminates mitochondrial DNA variants. Nature. 2013;493:632–637. doi: 10.1038/nature11800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tachibana M, et al. Towards germline gene therapy of inherited mitochondrial diseases. Nature. 2013;493:627–631. doi: 10.1038/nature11647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wakayama T, Yanagimachi R. The first polar body can be used for the production of normal offspring in mice. Biol Reprod. 1998;59:100–104. doi: 10.1095/biolreprod59.1.100. [DOI] [PubMed] [Google Scholar]
- 24.Wakayama T, et al. Participation of the female pronucleus derived from the second polar body in full embryonic development of mice. J Reprod Fertil. 1997;110:263–266. doi: 10.1530/jrf.0.1100263. [DOI] [PubMed] [Google Scholar]
- 25.Daughtry B, Mitalipov S. Concise review: parthenote stem cells for regenerative medicine: genetic, epigenetic, and developmental features. Stem cells translational medicine. 2014;3:290–298. doi: 10.5966/sctm.2013-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Minami N, et al. Zygotic gene activation and maternal factors in mammals. J Reprod Dev. 2007;53:707–715. doi: 10.1262/jrd.19029. [DOI] [PubMed] [Google Scholar]
- 27.Schultz GA, Heyner S. Gene expression in pre-implantation mammalian embryos. Mutat Res. 1992;296:17–31. doi: 10.1016/0165-1110(92)90029-9. [DOI] [PubMed] [Google Scholar]
- 28.Tang F, et al. Maternal microRNAs are essential for mouse zygotic development. Genes Dev. 2007;21:644–648. doi: 10.1101/gad.418707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keefe DL, et al. Mitochondrial deoxyribonucleic acid deletions in oocytes and reproductive aging in women. Fertil Steril. 1995;64:577–583. [PubMed] [Google Scholar]
- 30.Wilding M, et al. Mitochondrial aggregation patterns and activity in human oocytes and preimplantation embryos. Hum Reprod. 2001;16:909–917. doi: 10.1093/humrep/16.5.909. [DOI] [PubMed] [Google Scholar]
- 31.Van Blerkom J, et al. ATP content of human oocytes and developmental potential and outcome after in-vitro fertilization and embryo transfer. Hum Reprod. 1995;10:415–424. doi: 10.1093/oxfordjournals.humrep.a135954. [DOI] [PubMed] [Google Scholar]
- 32.Van Blerkom J. Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion. 2011;11:797–813. doi: 10.1016/j.mito.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 33.Tsai HD, et al. Mitochondria DNA deletion and copy numbers of cumulus cells associated with in vitro fertilization outcomes. J Reprod Med. 2010;55:491–497. [PubMed] [Google Scholar]
- 34.Schon EA, et al. Chromosomal non-disjunction in human oocytes: is there a mitochondrial connection? Hum Reprod. 2000;Suppl 2:160–172. doi: 10.1093/humrep/15.suppl_2.160. [DOI] [PubMed] [Google Scholar]
- 35.Eichenlaub-Ritter U, et al. Age related changes in mitochondrial function and new approaches to study redox regulation in mammalian oocytes in response to age or maturation conditions. Mitochondrion. 2011;5:783–796. doi: 10.1016/j.mito.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 36.Cohen J, et al. Ooplasmic transfer in mature human oocytes. Mol Hum Reprod. 1998;4:269–280. doi: 10.1093/molehr/4.3.269. [DOI] [PubMed] [Google Scholar]
- 37.Bellieni CV. Neonatal risks from in vitro fertilization and delayed motherhood. World journal of clinical pediatrics. 2012;1:34–36. doi: 10.5409/wjcp.v1.i4.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Braveman FR. Pregnancy in patients of advanced maternal age. Anesthesiol Clin. 2006;24:637–646. doi: 10.1016/j.atc.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Habbema JD, et al. The effect of in vitro fertilization on birth rates in western countries. Hum Reprod. 2009;24:1414–1419. doi: 10.1093/humrep/dep004. [DOI] [PubMed] [Google Scholar]
- 40.Robertson JA. Reconstituting eggs: the ethics of cytoplasm donation. Fertil Steril. 1999;71:219–221. doi: 10.1016/s0015-0282(98)00466-x. [DOI] [PubMed] [Google Scholar]
- 41.Chinnery PF, et al. The challenges of mitochondrial replacement. PLoS Genet. 2014;10:e1004315. doi: 10.1371/journal.pgen.1004315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reinhardt K, et al. Medicine. Mitochondrial replacement, evolution, and the clinic. Science. 2013;341:1345–1346. doi: 10.1126/science.1237146. [DOI] [PubMed] [Google Scholar]
- 43.Nagao Y, et al. Decreased physical performance of congenic mice with mismatch between the nuclear and the mitochondrial genome. Genes & genetic systems. 1998;73:21–27. doi: 10.1266/ggs.73.21. [DOI] [PubMed] [Google Scholar]
- 44.Roubertoux PL, et al. Mitochondrial DNA modifies cognition in interaction with the nuclear genome and age in mice. Nat Genet. 2003;35:65–69. doi: 10.1038/ng1230. [DOI] [PubMed] [Google Scholar]
- 45.Jenuth JP, et al. Random genetic drift in the female germline explains the rapid segregation of mammalian mitochondrial DNA. Nat Genet. 1996;14:146–151. doi: 10.1038/ng1096-146. [DOI] [PubMed] [Google Scholar]
- 46.Rienzi L, et al. Consistent and predictable delivery rates after oocyte vitrification: an observational longitudinal cohort multicentric study. Hum Reprod. 2012;27:1606–1612. doi: 10.1093/humrep/des088. [DOI] [PubMed] [Google Scholar]
- 47.Connor S. Medical dilemma of ‘three-parent babies’: Fertility clinic investigates health of teenagers it helped to be conceived through controversial IVF technique. The Independent 2014 [Google Scholar]
- 48.Genovese P, et al. Targeted genome editing in human repopulating haematopoietic stem cells. Nature. 2014;510:235–240. doi: 10.1038/nature13420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yusa K, et al. Targeted gene correction of alpha1-antitrypsin deficiency in induced pluripotent stem cells. Nature. 2011;478:391–394. doi: 10.1038/nature10424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suzuki K, et al. Targeted gene correction minimally impacts whole-genome mutational load in human-disease-specific induced pluripotent stem cell clones. Cell Stem Cell. 2014;15:31–36. doi: 10.1016/j.stem.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwank G, et al. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell. 2013;13:653–658. doi: 10.1016/j.stem.2013.11.002. [DOI] [PubMed] [Google Scholar]