Abstract
Background
Increased bone marrow hemangioblast numbers, alterations in erythroid/myeloid lineages, increased reticulin, and greater circulating bone marrow progenitor cells are present in patients with pulmonary arterial hypertension (PAH). The data suggest that myeloid progenitors contribute to the pathogenesis of PAH, but there is little data on prevalence of pulmonary vascular disease among different forms of myeloid diseases. We hypothesized that there would be a higher prevalence of pulmonary vascular disease in myeloproliferative neoplasms that have high circulating progenitor cells, such as myelofibrosis and chronic myelogenous leukemia (CML), as compared to those with low circulating progenitors, as in aplastic anemia.
Methods
Patients with myelofibrosis, CML and aplastic anemia who underwent echocardiographic evaluation of cardiac function in preparation for bone marrow transplantation at the Cleveland Clinic between 1997–2012 were identified using electronic medical records for demographic data, blood cell counts, and pulmonary function tests. All echocardiograms were uniformly analyzed in a blinded fashion by an advanced sonographer and cardiologist for measures of right and left ventricular function and estimation of pulmonary vascular disease.
Results
Gender and race distribution between disease groups were similar. Myelofibrosis [N=19] and aplastic anemia [N=30] had increased right ventricle (RV) wall thickness compared to CML [N=82] [RV Thickness (cm): aplastic anemia 0.7 ± 0.1, CML 0.5 ± 0.1 and myelofibrosis 0.7 ± 0.1; p = 0.02]. Patients with myelofibrosis had higher levels of estimated RV systolic pressure as compared to the other groups [RVSP (mmHg): aplastic anemia 29.9 ± 1.5, CML 26.2 ± 1.1 and myelofibrosis 36.7 ± 3.7; p < 0.01].
Conclusion
The findings suggest an important role for myeloid progenitors in maintenance of pulmonary-vascular health, in which abnormal myeloproliferative progenitors are associated with right ventricle pathology.
Introduction
Pulmonary arterial hypertension (PAH) is a disease characterized by increased pulmonary vascular resistance and elevated right ventricular pressure, leading to permanent changes in the pulmonary vasculature, right ventricular failure, and death. The association between myeloproliferative neoplasms and pulmonary hypertension has been suggested by case reports and small series, but the prevalence of pulmonary vascular abnormalities in myeloid diseases is unknown [1, 2]. Patients with myeloproliferative diseases are at risk of developing pulmonary hypertension [3]. Likewise, patients with PAH are prone to develop overt myelofibrosis (MF) and/or thrombocytopenia. Popat et al. found myelofibrosis uniformly in all PAH patients who underwent bone marrow biopsy [4]. More recently, we have shown that reticulin fibrosis is present in patients with idiopathic and familial PAH and even non-affected family members of PAH patients with normal blood counts [5].
Myeloproliferative neoplasms are characterized by a common stem cell derived clonal proliferation, but are phenotypically diverse due to differences in genetic rearrangements or mutations. They usually exhibit terminal myeloid cell expansion in the peripheral blood [6]. An increased number of circulating hematopoietic progenitors has been described in myeloproliferative neoplasms, with the highest numbers observed in myelofibrosis [7–9]. Similar to myeloproliferative neoplasms, bone marrow-derived proangiogenic precursors are increased in the circulation of PAH patients as compared to healthy controls [10]. In addition, there is increased proliferation of hemangioblasts in the bone marrow, alterations in erythroid/myeloid lineages as well as increased reticulin fibrosis in the bone marrow of PAH patients. The relationship of the number of circulating progenitor cells to the severity of PAH, suggests a possible role for these cells in fueling the angioproliferative vascular remodeling in PAH [5]. Moreover, transplantation of bone marrow CD133+ stem cells from PAH patients into immunodeficient mice recapitulates key features of the diseases, including endothelial cell injury, in situ thrombi and right ventricular hypertrophy [11]. All of this supports a possible causal link between the myeloid abnormalities and PAH. In fact, there are case reports identifying pulmonary hypertension resolution with treatment of the myeloproliferative disease [3, 12, 13]. Conversely, pulmonary hypertension has been reported to be a complication of stem cell transplantation in 40 patients in the literature [14].
To further delineate the role of bone marrow derived hematopoietic stem cells in the development of pulmonary hypertension; patients with myeloproliferative neoplasms were identified and compared to patients with aplastic anemia, a hematological disease reflecting a deficiency of hematopoietic stem cells resulting in pancytopenia and bone marrow aplasia. We hypothesized that right ventricular and pulmonary vascular abnormalities would be associated with the elevated circulating progenitor cells in myeloproliferative processes as compared to myeloprogenitor-deficient aplastic anemia.
Methods
Study Population and Data Collection
The study protocol was approved by the Cleveland Clinic institutional review board. Patients who underwent allogenic stem cell transplantation for aplastic anemia (AA), myelofibrosis (MF) or chronic myelogenous leukemia (CML) from 1997 to 2012 at the Taussig Cancer Institute, Cleveland Clinic, were included in the study. This population was selected as patients who are considered for transplant undergo screening echocardiogram at the Cleveland Clinic. Data were collected from the bone marrow transplant registry and the medical records. We identified 131 patients who were eligible for the study. We reviewed demographics, blood counts and chemistry values, pulmonary function tests and echocardiograms. Two-dimensional (2D) echocardiograms performed from 1–3 months prior to transplant were analyzed by an advanced sonographer and reviewed by an experienced cardiologist, blinded to the patient diagnosis.
Statistical Analysis
All analyses were performed using JMP Pro, version 9.0 (SAS Institute). Descriptive measures for quantitative variables consist of means with appropriately derived standard errors in the form mean ± SEM. Comparisons of disease groups were performed using ANOVA. When ANOVA was significant, for post-hoc analysis Tukey’s HSD was performed for pairwise comparison.
Results
The baseline characteristics of the population [N=131] subgrouped by each disease are shown in Table 1. Myelofibrosis patients were older than patients with CML and aplastic anemia [p < 0.001]. The gender and race distribution between disease groups was not significantly different (p > 0.1). As expected, blood cell counts were significantly different among groups [Table 1]. Patients with aplastic anemia had lower cell counts compared to the other groups.
Table 1.
Baseline Characteristics of Disease Groups
| CML (N=82) |
Myelofibrosis (N=19) |
Aplastic Anemia (N=30) |
p-value | |
|---|---|---|---|---|
|
| ||||
| Age (years) | 42+1 | 56+1 | 35±3 | < 0.001 |
|
| ||||
| Race (Caucasian/African-American/Asian/Hispanic/Other) | 68/9/1/2/2 | 19/0/0/0/0 | 27/1/0/0/2 | 0.5 |
|
| ||||
| Gender (Female/Male) | 41/41 | 8/11 | 16/14 | 0.7 |
|
| ||||
| Hemoglobin (g/dL) | 11.8±0.2 | 9.3±0.4 | 9.1±0.3 | <0.001 |
|
| ||||
| WBC (k/μL) | 14.4±2.6 | 21.1±5.3 | 0.8±0.2 | 0.005 |
|
| ||||
| RBC (m/μL) | 3.8±0.1 | 3.3±0.2 | 3.0±0.1 | <0.001 |
|
| ||||
| Hematocrit (%) | 35.6±0.6 | 29.1±1.3 | 25.7±0.9 | <0.001 |
|
| ||||
| MCV (fL) | 94.8±1.0 | 88.6±1.8 | 86.2±1.5 | <0.001 |
|
| ||||
| MCH (pG) | 31.2±0.4 | 28.3±0.8 | 29.2±1.2 | 0.02 |
|
| ||||
| MCHC (g/dL) | 33.2±0.1 | 31.9±0.4 | 35.3±0.1 | <0.001 |
|
| ||||
| RDW (%) | 16.7±0.4 | 19.8±0.7 | 16.3±0.7 | <0.001 |
|
| ||||
| Platelet Count (k/μL) | 269±28 | 264±65 | 18±2 | <0.001 |
|
| ||||
| FEV1 (%) | 91±2 | 87±3 | 94±3 | 0.2 |
|
| ||||
| FVC (%) | 97±2 | 89±3 | 98±3 | 0.03 |
|
| ||||
| FEV1/FVC | 78±1 | 78±1 | 79±1 | 0.7 |
|
| ||||
| DLCO (%) | 86±2 | 73±3 | 64±4 | <0.001 |
|
| ||||
| DLCOc (%)* | 91±3 | 84±4 | 82±4 | 0.2 |
DLCOc, Diffusion of carbon monoxide corrected for hemoglobin concentration.
Lung Functions
Pulmonary function testing done prior to transplantation was compared among disease groups [Table 1]. Forced Vital Capacity (% FVC) was significantly lower in the myelofibrosis group [p = 0.03]. There was no difference in Force Expiratory Volume in 1 second (FEV1) and FEV1/FVC (all p > 0.05). Lung diffusion capacity (DLCO) was significantly higher in the CML and myelofibrosis groups compared to the aplastic anemia group, but adjusted for hemoglobin, differences were not significant [Table 1].
Echocardiographic Measures of Cardio-pulmonary Disease
A total of 131 individuals had echocardiograms obtained 1–3 months before bone marrow transplantation available for re-analyses. Left sided function was not different among the groups [LVEF (%): AA 57.1 ± 0.7, CML 58.3 ± 0.6 and MF 59.6 ± 0.8; p = 0.2] [Table 2]. However, left atrial systolic dimension (LASD) was significantly higher in MF patients [LASD (cm): AA 3.3 ± 0.2, CML 3.6 ± 0.1 and MF 4.2 ± 0.2; p < 0.01]. Left atrial biplane volume (LAV) was measured but was only available on 10 subjects [LAV (cm2): AA 82 ± 34, CML 54 ± 4 and MF 84; p = 0.6]. The right atrial pressure (RAP) and tricuspid annular plane systolic excursion (TAPSE) were not significantly different between groups at baseline [RAP (mmHg): AA 14.7 ± 1.0, CML 17.1 ± 0.8 and MF 18.9 ± 1.5; p=0.8 and TAPSE (cm): AA 2.0 ± 0.1 CML 2.4 ± 0.1 and MF 2.3±0.2; p = 0.7]. On the other hand, myelofibrosis patients had significantly higher right ventricular systolic pressure (RVSP) and right atrial area average than the other two groups of patients [RVSP (mmHg): AA 29.9 ± 1.5, CML 26.2 ± 1.1 and MF 36.7 ± 3.7; p < 0.01 and RAAA (cm2): AA 4.0 ± 1.0, CML 3.3 ± 0.8 and MF 5.3 ± 1.6; p = 0.04]. Right ventricular fractional shortening (RVFS) tended to be higher in myelofibrosis patients [RVFS (%): AA 32.7 ± 2.2, CML 34.2 ± 2.3 and MF 41.1 ± 3.2; p = 0.08] [Table 2]. Myelofibrosis and Aplastic Anemia patients had significantly thicker right ventricle (RV) compared to CML patients [RV Thickness (cm): AA 0.7 ± 0.1, CML 0.5 ± 0.1 and MF 0.7 ± 0.1; p = 0.02] [Figure 1]. In addition, interventricular septal diastolic thickness (IVSD) was different among groups [IVSD thickness (cm): AA 0.98 ± 0.04, CML 1.04 ± 0.02 and MF 1.18 ± 0.06; p = 0.005].
Table 2.
Differences in Echocardiographic Parameters among Myeloid Diseases
| Variable | CML | Myelofibrosis | Aplastic Anemia Anemia |
p-value |
|---|---|---|---|---|
|
| ||||
| Left Atrial Systolic Dimension (cm) | 3.6±0.1 | 4.2±0.2 | 3.3±0.2 | <0.01 |
|
| ||||
| Left Ventricular Ejection Fraction (%) | 58.3±0.6 | 59.6±0.8 | 57.1±0.7 | 0.2 |
|
| ||||
| Left Ventricular End Diastolic Dimension (cm) |
4.8±0.1 | 4.8±0.1 | 4.7±0.1 | 0.8 |
|
| ||||
| Interventricular Diastolic Septal Thickness (cm) |
1.04±0.02 | 1.18±0.06 | 0.98±0.04 | 0.005 |
|
| ||||
| Right Ventricular Systolic Pressure (mmHg) | 26.2±1.1 | 36.7±3.7 | 29.9±1.5 | <0.01 |
|
| ||||
| Right Ventricular Thickness (cm) | 0.5±0.1 | 0.7±0.1 | 0.7±0.1 | 0.02 |
|
| ||||
| Right Ventricular Fractional Shortening (%) |
34.2±2.3 | 41.1±3.2 | 32.7±2.2 | 0.08 |
|
| ||||
| Tricuspid Annular Plane Systolic Excursion (cm) |
2.4±0.1 | 2.3±0.2 | 2.0±0.1 | 0.7 |
|
| ||||
| Right Atrial Pressure (mmHg) | 17.1±0.8 | 18.9±1.5 | 14.7±1.0 | 0.8 |
|
| ||||
| Right Atrial Area Average (cm2) | 3.3±0.8 | 5.3±1.6 | 4.0±1.0 | 0.04 |
Figure 1.
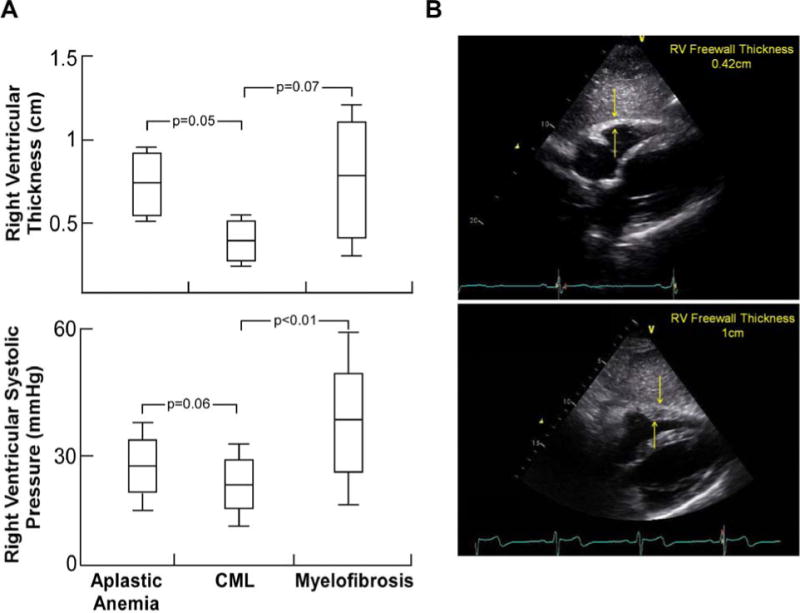
A. Right Ventricular Thickness was higher in patients with aplastic anemia and myelofibrosis as compared to patients with chronic myelogenous leukemia (CML) (ANOVA p = 0.02). Right Ventricular Systolic Pressure (RVSP) was highest in patients with myelofibrosis, with average levels that would qualify as pulmonary hypertension (mean RVSP 36.7). Aplastic anemia patients tended to have higher RVSP as compared to patients with CML, but levels were generally lower than what would qualify as pulmonary hypertension (ANOVA p < 0.01). P-values for comparison of mean between groups was obtained using Tukey’s HSD test.
B. RV Free wall thickness measured by echocardiography. Upper and lower panels showing normal and pathological thickening of RV wall respectively.
Discussion
The association of pulmonary hypertension with myelofibrosis and other myeloproliferative disorders has been reported in case series. However, the exact prevalence remains unknown and is likely to be underestimated. To our knowledge, this study represents the largest evaluation of cardiopulmonary function of patients with myeloid disease. Similar to prior reports [3, 15], significant right heart changes are found in patients with myelofibrosis, including elevated RVSP and right ventricle thickness. Unexpectedly, aplastic anemia patients also had thickened right ventricles, although RVSP was not elevated. Though the data from the aplastic anemia patients are limited, this is the first report describing RV thickness abnormality in this population. The findings suggest that myeloid progenitors are important to maintain right ventricle function, i.e. too few, or too many, circulating myeloid progenitors appear to be associated with alterations in the right heart size or structure. Just as important, the left ventricle was not affected across any of the myeloid diseases, indicating right heart and pulmonary vascular localized effects of myeloid elements.
Myelofibrosis patients have higher numbers of circulating CD34+/CD133+ bone marrow progenitor cells than other myeloid diseases [16]. Levels of circulating CD34+ CD133+ bone marrow-derived proangiogenic precursors are similarly high in PAH patients [10]. Moreover, non-affected members had reticulin fibrosis and increased myeloid progenitor cells similar to affected members, suggesting that myeloproliferative disease precedes the development of PAH [5]. Interestingly, mice transplanted with CD34+ CD133+ bone marrow-derived progenitor cells from PAH patients develop pulmonary vascular endothelial injury, angioproliferative remodeling and right ventricular hypertrophy and failure [9]. Here, cardiac structure and function were evaluated in patients with myeloid diseases, which are known to have high, or low, levels of circulating progenitors. Although CML patients have high levels of circulating bone marrow progenitors, these individuals did not have increased RV thickness or elevated RVSP. Rather patients with aplastic anemia, who have the lowest progenitors, had increased RV thickness, similar to myelofibrosis patients, albeit with no elevation of RVSP. Based on these data, it is interesting to speculate that myeloid effects on the RV may be independent of pulmonary vascular effects.
Bone marrow derived progenitor cells have been reported to repair or regenerate somatic cells in either homeostasis or injury settings [17]. In autopsy study of gender-discordant bone marrow transplantation, a significant fraction of the transplant recipient’s cardiomyocytes was derived from the donor-bone marrow progenitor cells [18]. Important to the current findings, a prior report showed that allogeneic bone marrow transplantation improved cardiac function in an aplastic anemia patient [19]. Altogether, these reports and the finding of right ventricular changes in aplastic anemia in this study, indicate that hematopoietic progenitor cells are important to maintain RV homeostasis. Although others and we focus on the circulating progenitor cells, it is also possible that paracrine factors produced by the resident bone marrow cells may be supportive of right heart and pulmonary vascular health.
Interestingly, we compared post transplantation outcomes in the different disease groups, and found that ~75% of patients with aplastic anemia were still alive whereas ~65% of patients with myelofibrosis were deceased at the time of data collection (data not shown). There was no difference in outcomes based on the RV systolic pressure or RV thickness; however, as expected there was a difference in outcomes based on age of the patient. It is hard to draw conclusions without accounting for the different confounders for death related to factors including age, and post-transplantation complications. Further studies are needed to assess differences in outcomes among the different disease groups specifically in relation to RV function.
One shortcoming of this study is that evaluation of right heart pressures were not confirmed by right heart catheterization. This was not possible due to the retrospective nature of our study. In addition, to possibly overestimating the rate of PH in this population, secondary causes of PH cannot be excluded. For example, portal hypertension, which can be associated with myelofibrosis, high cardiac output state, chemotherapeutic agents, or extra-medullary hematopoiesis, are all known to be associated with pulmonary hypertension [20]. Whether these complications of myeloid diseases were present in the patients is unknown.
In conclusion, this study confirms the strong association between myelofibrosis and PAH, and reveals a new association between myeloid disease and RV abnormalities that is independent of changes in RVSP. The findings add to the growing evidence that myeloid progenitors are important in maintenance of right ventricular and pulmonary vascular homeostasis.
Acknowledgments
The study was supported in part by HL60917, HL115008 and R01HL103931. Kewal Asosingh is a Scholar of the International Society for Advancement of Cytometry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marvin KS, Spellberg RD. Pulmonary hypertension secondary to thrombocytosis in a patient with myeloid metaplasia. Chest. 1993;103(2):642–4. doi: 10.1378/chest.103.2.642. [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Manero G, et al. Pulmonary hypertension in patients with myelofibrosis secondary to myeloproliferative diseases. Am J Hematol. 1999;60(2):130–5. doi: 10.1002/(sici)1096-8652(199902)60:2<130::aid-ajh8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 3.Garypidou V, et al. Incidence of pulmonary hypertension in patients with chronic myeloproliferative disorders. Haematologica. 2004;89(2):245–6. [PubMed] [Google Scholar]
- 4.Popat U, et al. New onset of myelofibrosis in association with pulmonary arterial hypertension. Ann Intern Med. 2005;143(6):466–7. doi: 10.7326/0003-4819-143-6-200509200-00017. [DOI] [PubMed] [Google Scholar]
- 5.Farha S, et al. Hypoxia-inducible factors in human pulmonary arterial hypertension: a link to the intrinsic myeloid abnormalities. Blood. 2011;117(13):3485–93. doi: 10.1182/blood-2010-09-306357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foucar K. Myelodysplastic/myeloproliferative neoplasms. Am J Clin Pathol. 2009;132(2):281–9. doi: 10.1309/AJCPJ71PTVIKGEVT. [DOI] [PubMed] [Google Scholar]
- 7.Samuelson SJ, Popat U, Prchal JT. Marrow fibrosis does not account for circulating CD34+ cells in myelofibrosis with myeloid metaplasia. Haematologica. 2007;92(4):e47. doi: 10.3324/haematol.11163. [DOI] [PubMed] [Google Scholar]
- 8.Chervenick PA. Increase in circulating stem cells in patients with myelofibrosis. Blood. 1973;41(1):67–71. [PubMed] [Google Scholar]
- 9.Andreasson B, Swolin B, Kutti J. Patients with idiopathic myelofibrosis show increased CD34+ cell concentrations in peripheral blood compared to patients with polycythaemia vera and essential thrombocythaemia. European Journal of Haematology. 2002;68(4):189–93. doi: 10.1034/j.1600-0609.2002.01610.x. [DOI] [PubMed] [Google Scholar]
- 10.Asosingh K, et al. Circulating angiogenic precursors in idiopathic pulmonary arterial hypertension. Am J Pathol. 2008;172(3):615–27. doi: 10.2353/ajpath.2008.070705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asosingh K, et al. Pulmonary vascular disease in mice xenografted with human BM progenitors from patients with pulmonary arterial hypertension. Blood. 2012;120(6):1218–27. doi: 10.1182/blood-2012-03-419275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rossoff LJ, et al. Primary pulmonary hypertension in a patient with CD8/T-cell large granulocyte leukemia: amelioration by cladribine therapy. Chest. 1997;112(2):551–3. doi: 10.1378/chest.112.2.551. [DOI] [PubMed] [Google Scholar]
- 13.Steensma DP, et al. Low-dose, single-fraction, whole-lung radiotherapy for pulmonary hypertension associated with myelofibrosis with myeloid metaplasia. Br J Haematol. 2002;118(3):813–6. doi: 10.1046/j.1365-2141.2002.03695.x. [DOI] [PubMed] [Google Scholar]
- 14.Dandoy CE, et al. Pulmonary hypertension after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19(11):1546–56. doi: 10.1016/j.bbmt.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Cortelezzi A, et al. Pulmonary arterial hypertension in primary myelofibrosis is common and associated with an altered angiogenic status. Leukemia. 2008;22(3):646–9. doi: 10.1038/sj.leu.2404943. [DOI] [PubMed] [Google Scholar]
- 16.Massa M, et al. Circulating CD34+, CD133+, and vascular endothelial growth factor receptor 2-positive endothelial progenitor cells in myelofibrosis with myeloid metaplasia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23(24):5688–95. doi: 10.1200/JCO.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 17.Fuchs E, Segre JA. Stem cells: a new lease on life. Cell. 2000;100(1):143–55. doi: 10.1016/s0092-8674(00)81691-8. [DOI] [PubMed] [Google Scholar]
- 18.Deb A, et al. Bone marrow-derived cardiomyocytes are present in adult human heart: A study of gender-mismatched bone marrow transplantation patients. Circulation. 2003;107(9):1247–9. doi: 10.1161/01.cir.0000061910.39145.f0. [DOI] [PubMed] [Google Scholar]
- 19.Kunisaki Y, et al. Marked improvement of cardiac function early after non-myeloablative BMT in a heavily transfused patient with severe aplastic anemia and heart failure. Bone Marrow Transplant. 2007;40(6):593–5. doi: 10.1038/sj.bmt.1705764. [DOI] [PubMed] [Google Scholar]
- 20.Machado RF, Farber HW. Pulmonary hypertension associated with chronic hemolytic anemia and other blood disorders. Clin Chest Med. 2013;34(4):739–52. doi: 10.1016/j.ccm.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


