Abstract
Background
In rhesus macaques LH secretion appears to be regulated by two distinct GnRH neuronal populations, which can be distinguished by their unique anatomical locations and because they express different molecular forms of GnRH (GnRH-I and GnRH-II).
Study Design
The effect of estradiol on GnRH gene expression was examined.
Results
Estradiol inhibited GnRH-I neurons but stimulated GnRH-II neurons, suggesting that GnRH-II neurons play the dominant role in mediating estradiol positive feedback and triggering the mid-cycle preovulatory LH surge.
Conclusions
Selective silencing of GnRH-II neurons in women could serve as a novel contraceptive, by blocking ovulation while leaving the rest of the reproductive axis relatively unperturbed.
Keywords: Estradiol, Gonadotropin-releasing hormone, Luteinizing hormone, Rhesus macaque
1. Introduction
Throughout most of the menstrual cycle luteinizing hormone (LH) has a pulsatile pattern of release from the pituitary gland. During the late follicular phase, however, the LH release pattern becomes characterized by a marked surge, which serves as the primary trigger for ovulation. It has generally been assumed that both the pulsatile and surge modes of LH release are regulated by the same population of hypothalamic neurons that produce gonadotropin-releasing hormone (GnRH), although there has never been a satisfactory explanation for how estrogen can inhibit these neurons during the follicular phase and yet stimulate them at the time of the preovulatory surge.
Recent findings from the rhesus macaque suggest that the pulsatile and surge modes of LH may actually be controlled by two distinct GnRH neuronal populations [1]. Importantly, these two populations can be readily distinguished because they have different anatomical locations in the hypothalamus and because they express slightly different molecular forms of the neuropeptide (i.e., GnRH-I and GnRH-II) [2–4]. Furthermore, there is increasing evidence that estrogen may differentially affect GnRH gene expression in these neurons, inhibiting GnRH-I and stimulating GnRH-II [1,5]. The goal of this study was to provide additional support for the hypothesis that estradiol’s positive feedback to the hypothalamus is mediated specifically through GnRH-II neurons.
2. Materials and methods
The study used rhesus macaques (Macaca mulatta), and was approved by the OHSU Institutional Animal Care and Use Committee. The animals were cared for by the Division of Comparative Medicine at the Oregon National Primate Research Center (ONPRC) in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals. In Experiment 1, postmortem RNA was obtained from a juvenile and an adult male as well as from an estradiol-treated ovariectomized adult female, and used for real-time PCR [6] detection of GnRH-I and GnRH-II in the pituitary gland and other brain regions. The following specific rhesus macaque primers were used for GnRH-I and GnRH-II, as well as for β-actin, a house-keeping gene: GnRH-I forward primer (F): TGTGTGTGGAGGGCTGCTC; GnRH-I reverse primer (R): GTCACTCCTTCTGGCCCAATAG; GnRH-II (F): TGAAGGAGCCATCTCATCCAC; GnRH-II (R): TGTCTTCCAGGTGAGCCAGG; β-actin (F):CATTGCTCCTCCTGAGCGCAAG; β-actin (R):GGGCCGGACTCGTCATACTCC. In experiment 2, hypothalamic RNA was obtained from ovariectomized animals that had been sacrificed at various time points after estradiol benzoate (EB) injection (a single animal per time point); this hormonal manipulation reliably induces a surge of LH approximately 48 hours later [7]. Quantitative real-time PCR [6] was performed using the following specific primers and probes for GnRH-I, GnRH-II, and two house-keeping genes, GAPDH and RPL13A: GnRH-I forward primer (F): TGTGTGTGGAGGGCTGCTC; GnRH-I reverse primer (R): GTCACTCCTTCTGGCCCAATAG; GnRH-I probe: 6FAM-CCAGCCACGTTCTCCCCTCCAAG-MGBNFQ; GnRH-II (F): TGAAGGAGCCATCTCATCCAC; GnRH-II (R): TGTCTTCCAGGTGAGCCAGG; GnRH-II probe: 6FAM-TGGTCCCATGGCTGGTACCC-MGBNFQ; GAPDH (F): AAGGGCATCCTGGGCTACA; GAPDH (R): GAAGAGTGGGTGTCGCTGTTG; GAPDH probe: 6FAM-TGAGCACCAGTGGTCTCCTCCGACT-MGBNFQ; RPL13A (F): TCACGAGGTTGGCTGGAAGT; RPL13A (R): GATCTTGGCTTTCTCCTTCCTCTT; RPL13A probe: 6FAM-CCAGGCAGTGACAGCCACCTTGG-MGBNFQ. The expression of GnRH-I and GnRH-II was normalized against the two house-keeping genes [6,8] and depicted relative to the temporal pattern of LH and estradiol in the plasma.
3. Results
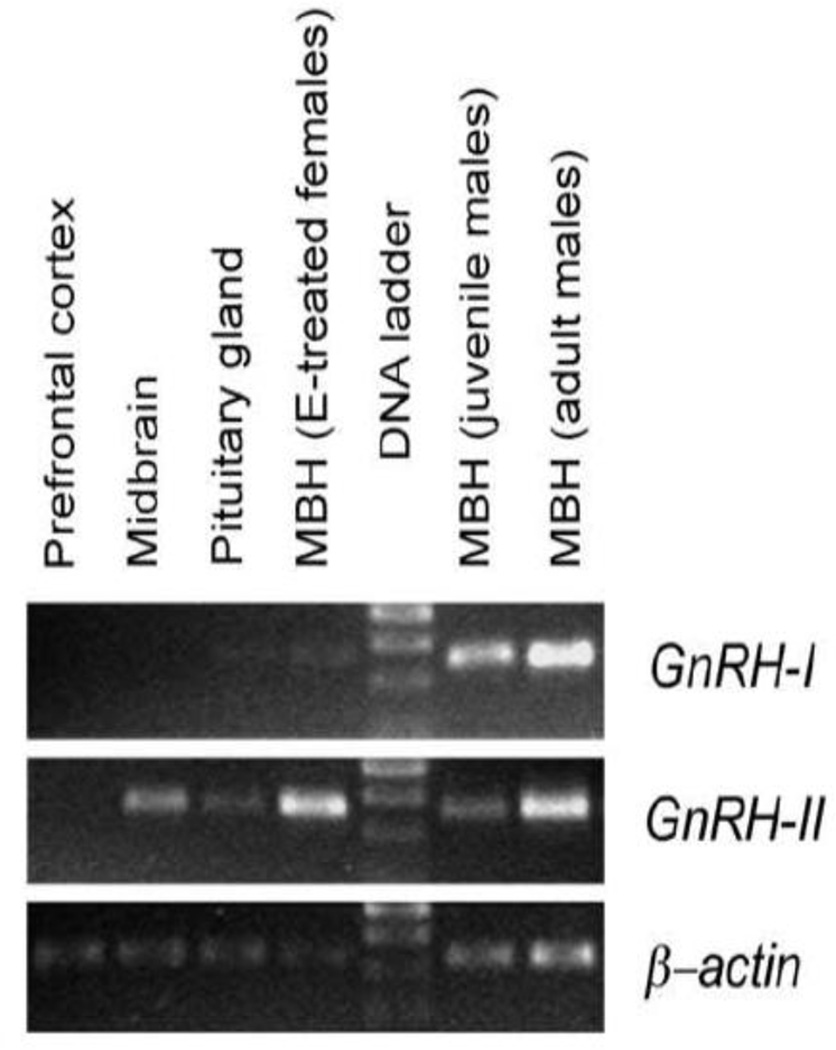
The preliminary data from this study indicate that GnRH-I mRNA was exclusively expressed in the medial basal hypothalamus (MBH), and not in any of the other brain regions examined or in the pituitary gland (Fig. 1). Furthermore, high GnRH-I mRNA levels were evident before and after puberty, but the level was much lower in estradiol-treated ovariectomized animals. GnRH-II mRNA was expressed in the midbrain and MBH, as previously reported [3], and also in the pituitary gland; importantly, GnRH-II mRNA levels appeared to be much higher after puberty and, in contrast to GnRH-I, appeared to be stimulated by estradiol.
Fig. 1.
Differential expression of GnRH-I and GnRH-II mRNA in the rhesus macaque, as revealed by RT-PCR. MBH = medial basal hypothalamus.
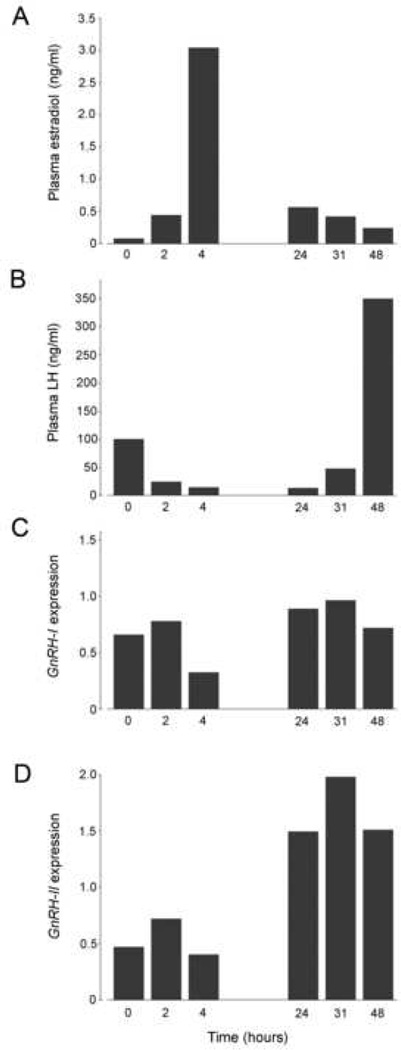
As expected, plasma estradiol levels increased markedly within 4 hours of EB injection, and gradually declined thereafter (Fig. 2A). Plasma LH levels showed a corresponding decrease within the first few hours of EB injection, reflecting the strong initial negative feedback influence of estradiol; an LH surge ensued 31–48 hours later (Fig. 2B). GnRH-I mRNA levels failed to show an increase corresponding to the time of the LH surge (Fig. 2C), whereas GnRH-II mRNA levels showed a marked increase following the estradiol peak and then remained elevated throughout the LH surge (Fig. 2D).
Fig. 2.
Differential effects of estrogen on GnRH-I and GnRH-II mRNA in female rhesus macaques, as revealed by quantitative real-time RT-PCR. Each time point includes data from a single animal.
4. Discussion
GnRH neurons represent the primary neuroendocrine link between the brain and the rest of the reproductive system, and traditionally it has been assumed that a single population of GnRH neurons controls both pusatile LH release as well as the preovulatory LH surge. This view has profoundly influenced our strategies for contraception and for the treatment of infertility in women. However, based on recent findings, including the preliminary results from this study, it is likely that this fundamental assumption is incorrect. These data show that: Primates express two distinct molecular forms of GnRH [2,3,9], both of which are highly effective at stimulating LH release [10]; GnRH-I and GnRH-II, are encoded on different chromosomes, and the neurons that secrete them have completely distinct locations in the hypothalamus [4]; and GnRH-I neurons respond to estrogen exclusively in a negative manner, while GnRH-II neurons respond to estrogen exclusively in a positive manner [1,5]. Moreover, the temporal association between EB administration, increased GnRH-II expression (but not GnRH-I), and the ensuing elevated plasma LH levels is consistent with the view that GnRH-II neurons play a leading role in triggering the preovulatory LH surge, while GnRH-I neurons play a permissive role.
By exploiting the differential characteristics of GnRH-I and GnRH-II neurons pharmacologically it should be possible to silence specific components of the reproductive axis, while leaving the rest unperturbed [1]. More studies are needed to fully characterize these differences, but in theory they could be used to selectively inhibit GnRH-II neurons (to block the preovulatory LH surge), while leaving the pulsatile activity of GnRH-I neurons unperturbed. Importantly, this highly-focused novel approach to contraception would be directed specifically at the ovulatory mechanism, without interfering with other aspects of the normal menstrual cycle. A more radical permanent contraceptive based on these findings would involve selectively ablation of the GnRH-II neuronal population in the hypothalamus. Moroever, because the pulsatile activity of the GnRH-I neurons would not be affected, the pituitary gland would remain sufficiently primed to respond appropriately to a bolus administration of exogenous GnRH; hence it should be still be possible to artificially induce a preovulatory LH surge and trigger ovulation, is so desired.
Acknowledgement
This work was supported by National Institutes of Health grant OD-011092.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure summary: The author has nothing to disclose.
References
- 1.Urbanski HF. Differential roles of GnRH-I and GnRH-II neurons in the control of the primate reproductive axis. Front Genomic Endocrinol. 2012;3:20. doi: 10.3389/fendo.2012.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lescheid DW, Terasawa E, Quanbeck C, Urbanski HF, Warby CM, Sherwood NM. A second form of gonadotropin-releasing hormone is present in the primate brain. Endocrinology. 1997;138:5618–5629. doi: 10.1210/endo.138.12.5592. [DOI] [PubMed] [Google Scholar]
- 3.Urbanski HF, White RB, Fernald RD, Kohama SG, Garyfallou VT, Densmore VS. Two distinct molecular forms of gonadotropin-releasing hormone in the brain of rhesus macaques. Endocrinology. 1999;140:1945–1948. doi: 10.1210/endo.140.4.6779. [DOI] [PubMed] [Google Scholar]
- 4.Latimer VS, Rodrigues SM, Garyfallou VT, Kohama G, White RB, Fernald RD, Urbanski HF. Two molecular forms of gonadotropin-releasing hormone (GnRH-I and GnRH-II) are expressed by two separate populations of cells in the rhesus macaque hypothalamus. Mol Brain Res. 2000;75:287–292. doi: 10.1016/s0169-328x(99)00316-2. [DOI] [PubMed] [Google Scholar]
- 5.Densmore VS, Urbanski HF. Effect of estradiol on hypothalamic GnRH-II gene expression in the female rhesus macaque. J Molec Endocrinol. 2004;33:145–153. doi: 10.1677/jme.0.0330145. [DOI] [PubMed] [Google Scholar]
- 6.Sorwell KG, Kohama SG, Urbanski HF. Perimenopausal regulation of steroidogenesis in the nonhuman primate. Neurobiol Aging. 2012;33:1487.e1–1487.e13. doi: 10.1016/j.neurobiolaging.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Witkin JW, Xiao E, Popilskis S, Ferin M, Silverman AJ. FOS expression in the gonadotropin-releasing hormone (GnRH) neuron does not increase during the ovarian steroid-induced GnRH surge in the rhesus monkey. Endocrinology. 1994;135:956–961. doi: 10.1210/endo.135.3.8070392. [DOI] [PubMed] [Google Scholar]
- 8.Noriega NC, Kohama SG, Urbanski HF. Microarray analysis of relative gene expression stability for selection of internal reference genes in the rhesus macaque brain. BMC Molecular Biology. 2010;11:47–71. doi: 10.1186/1471-2199-11-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White RB, Eisen JA, Kasten TL, Fernald RD. Second gene for gonadotropin-releasing hormone in humans. Proc Natl Acad Sci USA. 1998;95:305–309. doi: 10.1073/pnas.95.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Densmore VS, Urbanski HF. Relative effects of GnRH-I and GnRH-II on gonadotropin release. J Clin Endocrinol Metab. 2003;88:2126–2134. doi: 10.1210/jc.2002-021359. [DOI] [PubMed] [Google Scholar]