Abstract
Hippocampal neurons must maintain control over cytosolic calcium levels, especially during development, as excitation and calcium flux is necessary for proper growth and function. But excessive calcium can lead to excitotoxic cell death. Previous work suggests that neonatal male and female hippocampal neurons regulate cytosolic calcium differently, thereby leading to differential susceptibility to excitotoxic damage. Hippocampal neurons are also exposed to gonadal hormones during development and express high levels of androgen receptors. Androgens have both neuroprotective and neurotoxic effects in adults and developing animals. The present study sought to examine the effect of androgen on cell survival after an excitatory stimulus in the developing hippocampus, and whether androgen mediated calcium regulation was the governing mechanism. We observed that glutamate did not induce robust or sexually dimorphic apoptosis in cultured hippocampal neurons at an early neonatal time point, but did five days later – only in males. Further, pretreatment with the androgen dihydrotestosterone (DHT) protected males from apoptosis during this time, but had no effect on females. Calcium imaging of sex specific cultures revealed that DHT decreased the peak of intracellular calcium induced by glutamate, but only in males. To determine a possible mechanism for this androgen neuroprotection and calcium regulation, we quantified three calcium regulatory proteins, plasma membrane calcium ATPase1 (PMCA1), sodium/calcium exchanger1 (NCX1), and the sarco/endoplasmic reticulum calcium ATPase 2 (SERCA2). Surprisingly, there was no sex difference in the level of any of the three proteins. Treatment with DHT significantly decreased PMCA1 and NCX1, but increased SERCA2 protein levels in very young animals but not at a later timepoint. Taken together, these data suggest a complex interaction of sex, hormones, calcium regulation and developmental age; however androgens acting during the first week of life are implicated in regulation of hippocampal cell death and may be an underlying mechanism for sexually dimorphic apoptosis.
Introduction
Gonadal hormones, acting during a critical perinatal period, can harness the developmental process of apoptosis to sculpt areas of the brain in a sexually dimorphic pattern (reviewed in Forger, 2006). Both the sexually dimorphic nucleus (SDN) and the anteroventral periventricular nucleus (AVPV) are larger in one sex than the other due mainly to hormonally-driven cell death (Davis et al., 1996; Krishnan et al., 2009; Waters and Simerly, 2009). Interestingly, the hormone estradiol drives apoptosis in opposite directions depending on the nucleus: decreasing cell death in the SDN but increasing it in the AVPV, suggesting that estradiol is not intrinsically neuroprotective or neurotoxic during development but instead is capable of both actions, though presumably via different mechanisms.
Research on the neuroprotective effects of gonadal hormones during normal development as well as during adult pathological states has focused mainly on estradiol, leaving neuroprotection due to androgens such as testosterone and dihydrotestosterone (DHT) underexplored. However, the same dichotomy of neuroprotection versus exacerbation of damage observed with estradiol has been demonstrated with androgens under a variety of conditions. Activation of the androgen receptor (AR) protects neurons against insults such as β-amyloid (Pike, 2001; Nguyen et al., 2005; 2010), oxidative stress (Ahlbom et al., 1999), serum deprivation (Hammond et al., 2001), and glutamate or kainate-induced death (Cardounel et al., 1999; Ramsden et al., 2003), but exacerbates neuron loss due to cerebral ischemia (Hawk et al., 1998; Cheng et al., 2007). Interestingly, in the adult ischemia model, androgens can be both neuroprotective and neurotoxic depending on hormone dose and other variables (Uchida et al., 2009; Fanaei et al., 2013). In the developing nervous system, androgens likewise protect against cell death in the spinal nucleus of the bulbocavernosus (Nordeen et al., 1985; Forger, 2006), but also exacerbate neuronal death induced by excitatory GABA in the hippocampus (Nunez and McCarthy, 2008). Although not classically considered a sexually dimorphic structure, the hippocampus is slightly but significantly larger in males, has sex specific hormonal regulation of dendritic spine density and regulates behaviors such as spatial navigation and stress responding which differ in males and females (Madeira and Lieberman, 1995; Isgor and Sengelaub, 1998; Shors et al., 2001; Isgor and Sengelaub, 2003; Leranth et al., 2003). Importantly, the hippocampus also expresses androgen receptors at very high levels throughout life (Sar et al., 1990; Kerr et al., 1995; Xiao and Jordan, 2002; Tabori et al., 2005) and is exquisitely sensitive to excitatory stimuli such as glutamate and excitatory GABA.
GABA is the major excitatory neurotransmitter during early postnatal development (Ben-Ari et al., 2012) but cedes that role to glutamate around the end of the first week of life (Ganguly et al., 2001), which is still a time of normal developmental cell death in the hippocampus (Ganguly et al., 2001; Nunez and McCarthy, 2007; Ben-Ari et al., 2012). Previous work from our lab on gonadal hormone neuroprotective/neurotoxic effects following excitotoxic insults found interactions of sex and hormones at different ages: estradiol is protective against glutamate excitotoxicity around the seventh day of life (Hilton et al., 2004; 2006), and DHT exacerbates excitatory GABA-induced cell death right after birth (Nunez and McCarthy, 2008). Further, sex alone seems to predetermine a neuron’s reaction to excitatory GABA right after birth, with males suffering more cell death than females (Nunez et al., 2003a; 2003b). However, the effect of androgens on neuronal survival following glutamate stimulation, and whether that effect is sex specific, is unknown at either developmental time point.
An important signal common to developmental cell death, excitotoxic cell death, and also androgen neuroprotection, is regulation of intracellular calcium. The developing brain is especially sensitive to calcium influx after an excitatory stimulus, so that only the appropriate level of neural/electrical activity allows for neuronal survival - a desirable outcome since only those neurons with appropriate connections are allowed to survive and thrive while others die off. In response to an excitatory stimulus, calcium can enter the cytosol from the extracellular milieu and/or be released from internal stores such as the endoplasmic reticulum (ER). Homeostatic mechanisms such as the plasma membrane calcium ATPase1 (PMCA1) and the sodium/calcium exchanger1 (NCX1) can help regulate cytosolic calcium by expelling it into the extracellular space, and the sarco/endoplasmic reticulum calcium ATPase 2 (SERCA2) can sequester cytosolic calcium within the neuron’s ER (Blaustein and Lederer, 1999; Bading, 2013). Calcium release from these internal stores appears to be especially important in regulating glutamate-induced cell death during early neonatal development (Hilton et al., 2006), with external calcium influx becoming more important later in life (Nunez and McCarthy, 2009). Interestingly, activation of the androgen receptor via DHT has been shown to increase the amount of calcium influx after glutamate in cultured hippocampal neurons by altering levels of SERCA2 mRNA (Foradori et al., 2007; Foradori and Handa, 2008). Therefore, the current study examined the effects of the androgen DHT on cell death and intracellular calcium levels following a mild glutamate stimulus in hippocampal cultures, as well as expression of the SERCA2, NCX1 and PMCA1 proteins, during early and late neonatal development - the time when glutamate starts to supersede GABA as the major excitatory neurotransmitter.
2. Experimental Procedures
2.1 Primary hippocampal cultures
Animal use and care procedures were approved by the University of Maryland, Baltimore Institutional Animal Care and Use Committee and followed National Institutes of Health Guidelines. All cell culture chemicals and solutions were purchased from Invitrogen (Carlsbad, CA) unless otherwise noted. Newborn rat pups (postnatal day 0; PND0) were obtained from Sprague Dawley breeding pairs (Harlan, Frederick, MD) at the University of Maryland, Baltimore breeding colony and hippocampi were dissected and cultured as described previously (Nunez et al., 2005; Hilton et al., 2006). Briefly, equal numbers of PND0 male and female hippocampi were dissected into a single sterile tube for mixed sex cultures, or into separate sterile tubes for sex specific cultures, that contained HBSS+ (88ml sterile H20, 10 ml Hank’s balanced salt solution (Ca++ and Mg++ -free) 10X, 1 ml HEPES buffer, 1.0 M, pH7.3, 1 ml antibiotic/antimytotic 100X liquid), then subjected to trypsin digestion and incubated for 20–30 minutes at 37°C. Supernatant was discarded and tissue washed twice with HBSS+, dissociated by tituration, plated on 25mm poly-L-lysine coated coverslips at a density of 400,000 cells per coverslip, and then placed in 100mm dishes containing 5 ml plating medium (86 ml MEM, 10 ml horse serum, 3 ml glucose (filter sterilized, 20%), 1 ml pyruvic acid, 100mM). Cell number and viability were determined by trypan blue exclusion and cells were allowed 4 hours to adhere to the coverslips in a 37°C, 5% CO2 incubator, then coverslips were removed from the plating dishes and placed into 35mm petri dishes filled with 3ml of Neurobasal+ media (1ml B-27 supplement, 1 ml antibiotic/antimycotic 100X liquid, 125 µl L-glutamine and filled to 50 ml with Neurobasal [glutamine and phenol red –free]). One-third of media was removed from each culture dish and replaced with fresh media on DIV4. For all experiments, cultures were treated with either DMSO (vehicle, <0.05% by volume) or 1nM DHT (Sigma) on day in vitro 0 (DIV0) and DIV1. Cultures for experiments examining an early neonatal timepoint were fixed and processed for Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) labeling on DIV2, and cultures for examining a late neonatal timepoint were fixed and processed for TUNEL labeling on DIV7.
2.2 TUNEL labeling
Cultured cells were administered a total of three treatments of either saline or 10 µM glutamate starting on DIV1 for Experiment 1 and DIV6 for Experiment 2, with a second treatment 4 hours later, and a third treatment on the following day (DIV 2 for Experiment 1; DIV 7 for Experiment 2) for a final glutamate concentration of 30 nM. Four hours following the third treatment, cultures were processed for TUNEL reactivity, as previously described (Hilton et al., 2006; Nunez and McCarthy, 2008). Briefly, cells were fixed in 4% paraformaldehyde for 25 minutes at RT, washed for 5 min in PBS 3×, permeabilized by immersion in 0.25% TritonX-100 in PBS, and stained using the Promega DeadEnd Colorimetric TUNEL system (Madison, WI, USA), with TUNEL+ cells visualized using diaminobenzidine (DAB). Cells were lightly counter-stained with cresyl violet, dehydrated and mounted on slides. For each coverslip, the number of TUNEL+ and total number of cells were counted in two non-overlapping fields at 40× magnification per quadrant (eight fields total) using the MicrobrightField Neurolucida program (version 2.01, Colchester, VT, USA). The mean percent of TUNEL+ cells per coverslip was used in statistical analyses.
2.3 Calcium imaging
On DIV 7, sex-specific cultures were subjected to calcium imaging according to previously established protocols (Hilton et al., 2005; Nunez et al., 2005; Hilton et al., 2006). Hippocampal cells were incubated for 30 minutes at 34°C with the cell permeant fluorescent indicator Fura-2-AM (3µM; Molecular Probes, Eugene, OR USA) in less than 0.5% DMSO, then transferred to a tissue chamber mounted on a microscope stage, and perfused with physiological salt solution (PSS) at 32–34°C for 30 min to remove extracellular dye and allow for esterification of Fura-2-AM. The imaging system consisted of a Zeiss Axiovert 100 inverted microscope with Till Photonics Polychrome II Monochromator (Applied Scientific instrumentation, Eugene, OR. USA), a Hamamatsu CCD video camera and image intensifier. Image acquisition and analysis was performed with Metamorph Metafluor Imaging System, version 5.0 (Univ. Imaging Corporation, Downington, PA, USA). Cells in field of view using a 40× objective were characterized as glia or neurons morphologically. Specifically, the somas of pyramidal neurons are triangular in shape with rounded and clearly distinct edges. Glial cells appear amorphous, with flat and non-distinct edges. Individual neurons were selected and traced using the Metafluor program. Baseline measurement of resting calcium levels for individual cells was obtained over a 5min period while the cells were perfused with PSS. A 50 sec pulse of 10µM glutamate was administered in the perfusate; data were acquired for an additional 5–7 minutes following the glutamate stimulus until baseline was re-established. For antagonist experiments, specific antagonists were then administered in the perfusate for 3 minutes, followed immediately by concurrent application with the antagonist and glutamate (Hilton et al., 2006). The following antagonists were purchased from Sigma: nonselective metabotropic glutamate receptor antagonist LY341495 – (2S)-2-amino-2-[(1S,2S)-2-carboxycycloprop-1-yl)]-3-(xanth-9-yl) propanoic acid (Kingston et al., 1998); the L-type voltage-sensitive calcium channel blocker, diltiazem; and the NMDA receptor blocker, MK801. The ratio of the background corrected Fura-2 emission intensity (520 nm) at two excitation wavelengths (340 nm/380 nm) was recorded to obtain a relative calcium measurement. The following parameters were analyzed: 1) average baseline calcium; 2) peak calcium following glutamate application; 3) time to attain the peak (rise time); and 4) time to re-establish baseline following glutamate application (decay time). Data were taken from at least three separate culture runs per experiment.
2.4 Western Blot Analysis
All reagents and hormones used in Western Blot Analysis were purchased from Sigma unless otherwise noted. On the day of birth (PND0) and PND1, male and female rat pups were injected with DHT benzoate s.c. (100 µg/0.1 ml) or sesame oil vehicle, a dose routinely used by this laboratory to induce sexual differentiation (Zhang et al., 2008). On PND2 or PND7, animals were sacrificed and levels of SERCA2, PMCA1 and NCX1 protein were determined by Western Blot as previously described (Amateau and McCarthy, 2002). Specifically, hippocampi were microdissected and flash frozen in isopentane and stored at −80°C until homogenization with ice-cold lysis buffer containing 50 mM Tris-HCL, 1% Na-deoxycholate, 0.25% NP-40, 150 mM NaCl, 1mM EDTA and a protease inhibitor cocktail (1 µg/ml of aprotinin, leupeptin and pepstatin; 1mM phenylmethylsulfonyl fluoride). Homogenized samples were then centrifuged at 3000g for 30 min at −10 o C, the supernatant collected and total protein concentration determined by Bradford assay. Ten micrograms of total protein from each animal were electrophoresed on a SDS-PAGE gel and transferred to a polyvinylidenedifluoride membrane. Membranes were washed with 0.1M TBS, blocked for 1 h at RT in 0.1M TBS containing 5% nonfat dry milk, then incubated with primary antibodies: rabbit PMCA1 (1:2000, Affinity Bioreagents), mouse NCX1 (1:1000, Swant) or rabbit SERCA2 (1:2000, Bethyl Laboratories) for 3 h at RT in TBS containing 0.05% Tween-20 (TBS-T). Membranes were incubated in appropriate goat anti-rabbit or rabbit anti-mouse HRP-linked secondary antibody (1:3000, Cell Signaling Technology, Beverly, MA USA) for 30 min at RT, then rinsed with TBS-T. Immunoreactive bands were detected using an enhanced chemiluminesence kit (ECL kit, New England Biolabs, Beverly, MA USA) and exposed to film (Hyperfilm-ECL, Amersham Pharmacia Biotech, Arlington Heights, IL USA). Proteins were detected as specific bands (SERCA2 at 110 kDA; PMCA1 at 130 kDA; NCX1 at 116 kDA), and the integrative grayscale pixel area density (IAD, an arbitrary unit) was captured with a CCD camera and analyzed on a Macintosh computer using NIH Image. A Ponceau S stain was used to verify equal protein loading or to calculate IAD/PonceauS ratio to normalize data, since it is not altered by hormone treatments (Olesen and Auger, 2005; Speert et al., 2007; Romero-Calvo et al., 2010). Hippocampi from at least five animals per group were analyzed for all proteins.
2.5 Statistical Analyses
Three-way analyses of variance (sex X hormone X glutamate) were performed on the percent TUNEL-positive cells/all cells in sex specific cultures. A two-way ANOVA (hormone X glutamate) was performed on the percent TUNEL-positive cells/all cells in mixed sex culture at DIV2. Two-way ANOVAs (sex X hormone/antagonist) were performed on baseline calcium, peak intracellular calcium above baseline, rise time, and decay time, as well as on IAD or IAD/PonceauS ratio for western blots. A Tukey test was used when post-hoc analysis was required. Analyses were performed using SigmaPlot/SigmaStat (Systat Sofware, Inc. version 12.3); for all ANOVAs and post-hoc tests, a level of p<0.05 was considered statistically significant.
3. Results
3.1 Sex differences in glutamate-induced cell death and DHT neuroprotection are not present on DIV2, but are present on DIV7
On DIV2 (equivalent to PND2), primary cultures of mixed-sex hippocampal neurons treated with a mild glutamate stimulus did not show an increased amount of TUNEL+ cells over cultures treated with saline vehicle (main effect of glutamate F[1,26] = 0.247, p > 0.6). Pretreatment with DHT similarly had no effect on the number of TUNEL+ cells (main effect of DHT F[1,26] = 0.247, p > 0.58) compared to baseline levels of cell death (Fig. 1A), which were consistent with previous studies from our lab using hippocampal neurons and not significantly different between groups. Because a mixed sex culture could be masking sex-specific effects of glutamate, sex specific cultures were also examined on DIV2. Interestingly, there were similar levels of cell death induced by glutamate compared to the mixed sex culture, which in this case reached significance (main effect of glutamate F[1,17] = 4.969, p = 0.04); however, there was no significant effect of sex, DHT treatment, or three way interaction (F[1,17] = 1.09, p = 0.31), suggesting that the effects of glutamate were not different between males and females, and DHT was not significantly neuroprotective (Fig. 1B). At a later age, DIV7, the total number of cells present and the baseline amount of cell death was again consistent with previous studies from our lab using hippocampal neurons and not significantly different between groups; however, the same mild glutamate stimulus now significantly increased the number of TUNEL+ cells over baseline only in cells derived from males (3 way interaction F[1,24] = 4.654, p = 0.04; Tukey’s, p<0.05). Similarly, pretreatment with DHT significantly protected against glutamate-induced cell death, but only in male cells (Tukey’s, p<0.05). At this age, neurons derived from females did not respond to either manipulation, as there was no increase in cell death after glutamate, nor any protective or damaging effects of DHT (Fig. 1C).
Figure 1. Cell death in response to glutamate.
(A.) Glutamate, with or without DHT pretreatment, does not significantly alter levels of TUNEL+ cell death in DIV2 mixed-sex hippocampal cultures. (B.) Glutamate, with or without DHT pretreatment, does significantly increase TUNEL+ cells in DIV2 sex specific cultures in both sexes (p=0.04). (C.) On DIV7, males exhibit a higher amount of cell death after a mild glutamate challenge than females. Pretreatment with DHT protects against this cell death in males, but has no effect in females. For display purposes, data from glutamate-treated groups are expressed as percent change from the sex and hormone matched saline-treated control group: %TUNEL from glutamate −%TUNEL from saline / %TUNEL from saline), * p<0.05 compared to all other groups.
3.2 On DIV7, DHT decreases intracellular calcium flux in response to glutamate, but only in males
Because glutamate significantly increased cell death in a sexually dimorphic fashion on DIV7, we chose to examine calcium regulation in sex-specific DIV7 cultures. Baseline calcium amounts were not different between males and females (main effect of sex F[1,73] = 0.134, p > 0.7) and were not impacted by DHT (main effect of hormone F[1,73] = 1.16, p > 0.3). Peak amount of calcium above baseline after glutamate application was also not different between sexes (main effect of sex F[1,73] = 1.59, p > 0.2; Fig. 2B). Pretreatment with DHT, however, significantly attenuated the calcium peak, but only in neurons derived from males (interaction F[1,73] = 5.44 p < 0.03; Tukey’s, p<0.03; Fig. 2B). The rise time and decay time of the calcium were not different between sexes, after vehicle or after DHT administration (data not shown). In order to determine the route of calcium entry into the cytosol, several antagonists were applied. Diltiazem (100 µM), which blocks the L-type voltage sensitive calcium channels, LY341495 (10 µM), which blocks metabotropic glutamate receptors and thus release of Ca++ from intracellular stores, and MK801 (10 µM), which antagonizes NMDA receptors. Each blocked a significant, though partial, amount of the calcium peak (Fig. 3A – 3C; LY341495 F(1, 59) = 4.307, p=0.04; MK801 F(1, 62)= 9.381, p=0.003; diltiazem F(1, 73) = 7.103, p=0.01). Applying a combination of all calcium blockers almost completely abolished the calcium peak following glutamate application (F(1, 67) = 85.138, p<0.001; Fig. 3D). Interestingly, there was no sex difference in the actions of any blocker alone or in combination, although there was a trend for MK801 treatment to block more calcium influx in females than in males (Tukey’s p = 0.064). Previous work from our lab suggests that intracellular stores of calcium are particularly important for glutamate-mediated cell death in the hippocampus prior to the maturation of NMDA and AMPA receptors, after which point extracellular calcium sources dominate (Hilton et al., 2006; Nunez and McCarthy, 2009). Further, work from others suggests androgens can alter mRNA levels of SERCA2 in the rat hippocampus (Foradori et al., 2007), which helps mediate calcium flow between the cytosol and these internal stores. Therefore, we hypothesized that the mechanism by which DHT decreases glutamate-induced calcium influx at this late neonatal age is by decreasing expression of SERCA2 protein, so that less calcium is released into the cytosol from the internal stores after a mild glutamate stimulus.
Figure 2. Androgen modulation of glutamate-induced intracellular calcium flux.
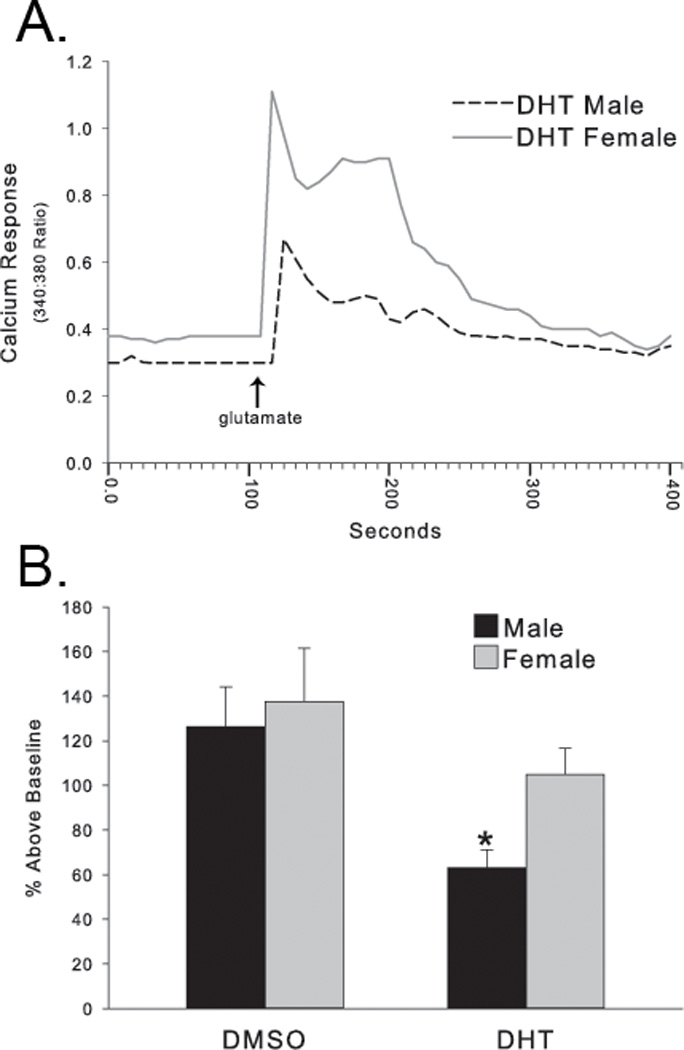
(A) Representative individual calcium responses induced by glutamate from a male (dashed line) or female (gray line) hippocampal neuron treated with DHT. (B) DHT significantly decreased the calcium peak induced by glutamate application, but only in males. DHT-treated females were not significantly different than DMSO-treated cells derived from either sex. Data are expressed as % change from baseline, * p<0.03 compared to DMSO-treated female.
Figure 3. Intracellular versus extracellular sources of calcium.
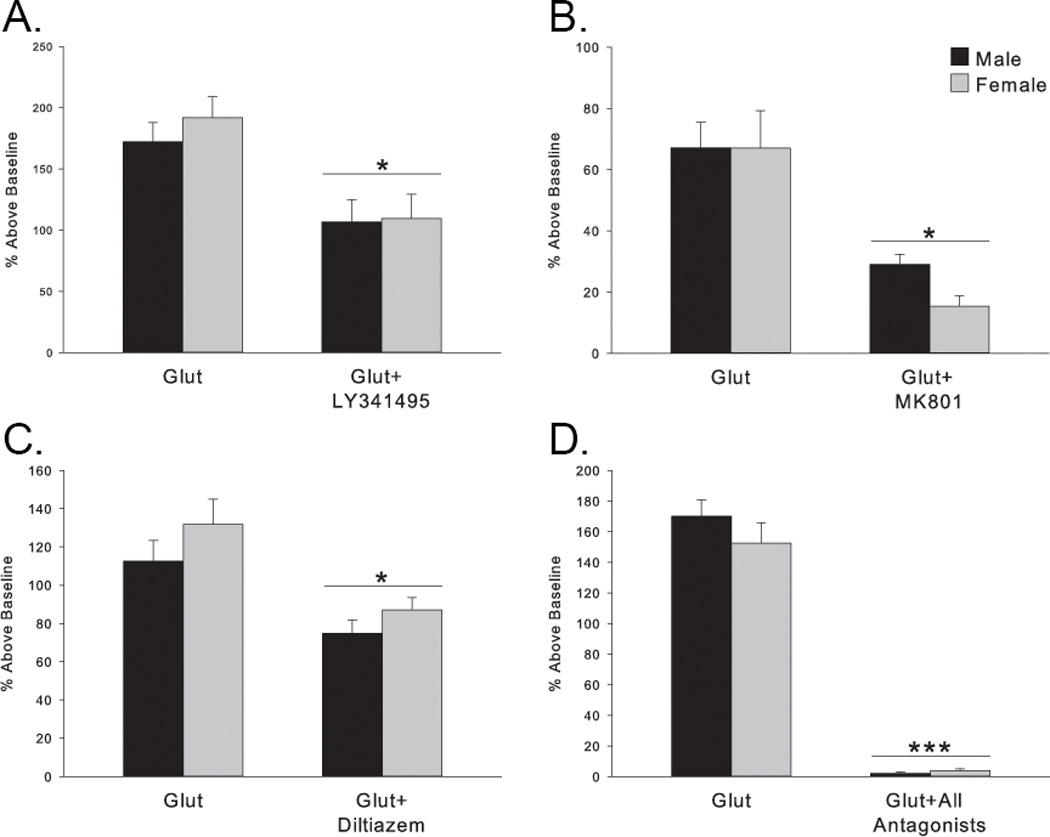
The glutamate-induced calcium peak in both sexes is significantly, but moderately, attenuated by blocking entry from either internal or external stores of calcium. (A) LY341495 at this concentration is a nonspecific metabotropic glutamate receptor antagonist and blocks calcium release from internal stores. (*p<0.04 compared to glutamate alone groups) (B) MK801 blocks NMDA receptors. (*p=0.003 compared to glutamate alone groups) (C) diltiazem blocks L-type voltage sensitive calcium channels (*p=0.01 compared to glutamate alone groups) (D) The combination of all three antagonists almost completely abolished the calcium peak following glutamate. (*p<0.001 compared to glutamate alone groups)
3.3 DHT pretreatment increases SERCA2 protein and decreases NCX1 and PMCA1 protein in PND2, but not PND7, hippocampus
Levels of SERCA2 protein were not significantly different in vivo between males and females at PND2 (main effect of sex F(1,22)=1.884, p>0.18), but were increased by DHT treatment to the same degree in both sexes (main effect of hormone F(1,22)=6.066, p<0.03; Fig. 4A). In contrast, SERCA2 protein levels were not significantly altered by either sex or DHT pretreatment at PND7 (sex: F(1,21)=0.954, p=0.34; DHT: F(1,21) = 0.421, p>0.52; Fig. 4B). Therefore, suppression of SERCA2 protein level on PND7 does not explain the sexually dimorphic effect of DHT on the calcium peak following glutamate on DIV7. In agreement with other studies (Foradori et al., 2007), however, DHT does increase SERCA2 protein in DIV2 tissue; surprisingly, DHT had this effect equally in both males and females (Fig. 4A).
Figure 4. Androgen modulation of SERCA2.
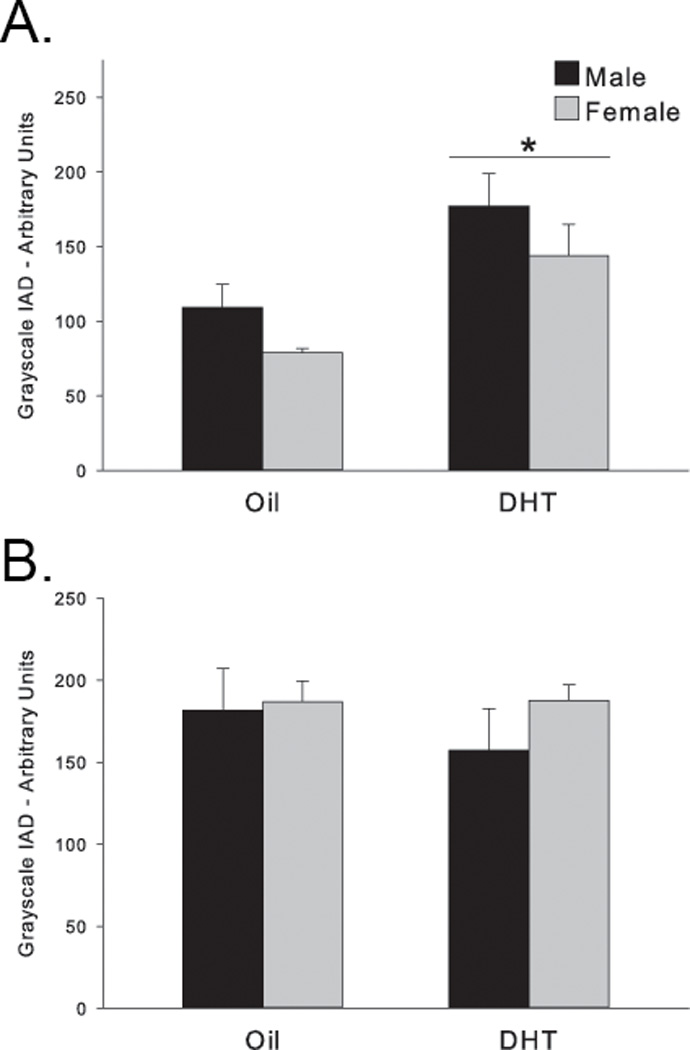
SERCA2 protein was increased equally in both sexes by DHT pretreatment on (A) PND2 but not on (B) PND7.
*p<0.03 compared to oil-treated animals.
The SERCA2 protein regulates the relationship of calcium sequestration/release between the cytosol and ER and thus helps to maintain cytosolic calcium homeostasis, a job that is supported by other regulatory proteins including PMCA1 and NCX1, which can extrude calcium out into the extracellular matrix. Therefore, we hypothesized that this external route may instead be the mechanism by which DHT exerts its effects on glutamate-induced changes in cytosolic calcium. Interestingly, in contrast to SERCA2 at PND2, DHT treatment significantly decreased both PMCA1 (F(1,20) =22.58, p<0.001; Fig. 5A) and NCX1 protein levels (F(1,20)=5.482, p <0.03; Fig. 5B) with no difference between the sexes (main effect of sex: PMCA1 p=0.42; NCX1 p=0.9). At PND7, neither PMCA1 nor NCX1 protein levels were significantly affected by sex (PMCA1: p=0.46; NCX1: p=0.27) or by DHT pretreatment (PMCA1: p=0.68; NCX1: p=0.42) in consensus with the SERCA2 results. Therefore, all three calcium regulatory proteins were altered by DHT at an early neonatal timepoint (PND2), but not a later neonatal timepoint (PND7) and sensitivity to androgen modulation was not influenced by sex.
Figure 5. Androgen modulation of PMCA1 and NCX1.
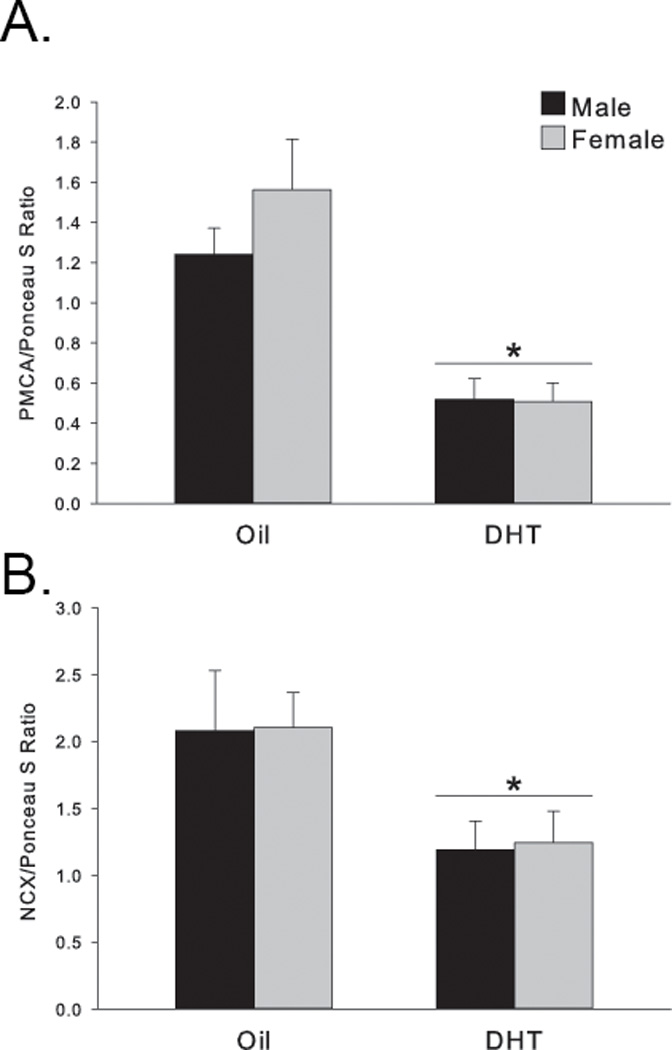
DHT pretreatment significantly decreased (A) PMCA1 (*p<0.001 compared to oil-treated animals) and (B) NCX1 protein levels equally in both sexes on PND2 (*p<0.03 compared to oil-treated animals).
4. Discussion
Gonadal hormones, including androgens, can have either neuroprotective or neurotoxic effects on the brain depending on factors such as the nature of the insult and the sex of the individual. The current study suggests that another point of divergence in androgen neuroprotection vs exacerbation is also the age/maturational stage of the developing neurons. Specifically, the androgen DHT is protective against glutamate-induced hippocampal cell death in males, only at a late neonatal time point (DIV7). Endogenous hippocampal androgens are still elevated at this time but falling rapidly, after which levels stabilize briefly between PND12 to PND30 and then decline to very low adult levels (Konkle and McCarthy, 2011). Our observed neonatal androgen neuroprotection is consistent with other studies showing androgen neuroprotection against glutamate excitotoxicity in adult or aged animals (Rosario et al., 2004; 2006) as well as in male only or mixed sex cultures (Mizoguchi et al., 1992; Ramsden et al., 2003) examined at later time points - after glutamate has assumed its role as the major excitatory neurotransmitter and calcium influx is predominantly from the extracellular milieu via NMDA receptors (Nunez and McCarthy, 2009). In the early neonatal timepoint examined here, DHT did not significantly protect nor exacerbate the damage induced by glutamate in sex specific cultures. Previous work from our lab demonstrated that DHT exacerbates damage from excitatory GABA during this time (Nunez and McCarthy, 2008) in a sexually dimorphic fashion, with males suffering worse damage than females. Thus, it is possible that DHT effects are limited to the primary excitatory neurotransmitter present: GABA at the early timepoint, and glutamate during late neonatal development. Interestingly, glutamate did not significantly induce cell death above baseline in mixed sex cultures at DIV2. Others have also shown that glutamate does not induce large amounts of toxicity in the rodent brain during the first week of life (Campochiaro and Coyle, 1978; Marks et al., 1996); although, again, most of these studies have been performed in unidentified (presumably mixed sex) cultures or in male animals.
The sexually dimorphic effects of glutamate and DHT at the late neonatal time point provide insight into a possible mechanism for hormonal sculpting of the sexually dimorphic brain. The same level of glutamate stimulation that killed neurons in males at this age had no effect on neurons derived from the female hippocampus, suggesting a fundamental difference exists between male and female hippocampal neurons resulting in an increased vulnerability of male neurons and/or protection of female neurons. We predict there is a developmental program between day 2 and day 7 of life that is inherently different between male and female neurons which affects their response to a mild glutamate challenge at DIV7. One week of age is the midpoint of when excitatory GABA shifts to adopt the inhibitory role it plays in the adult brain. However, we have demonstrated that this shift happens earlier in females than in males, such that on PND7, female hippocampal neurons have already switched to glutamate as the major excitatory neurotransmitter (Auger et al., 2001; Nunez and McCarthy, 2007). While at first pass this would seem to increase females’ sensitivity to excitotoxic glutamate, instead it may reflect a more mature and stable network that is resistant to perturbation. Regardless, this is further evidence that male neurons are more vulnerable than female neurons (male neurons respond more, and to smaller insults, than female neurons), an observation that is supported both experimentally and clinically. For example, hypoxic/ischemic birth injury (H/I) affects males more than females. Clinically, newborn males who survive an H/I suffer more severe brain damage, and experience worse cognitive outcomes than females following a similar insult (Hindmarsh et al., 2000; Lauterbach et al., 2001; Johnston and Hagberg, 2007). One of the most robust sex-specific patterns in humans is the observation that almost all sychological/neurological developmental disorders occur more frequently in boys than in girls (Zup and Forger, 2002); another pattern that suggests the developing male brain has a heightened vulnerability to developmental perturbations. Since gonadal hormones are the primary (though not only) agent responsible for shaping a male-typical brain, and one apparent consequence of being male is a vulnerability to developmental perturbation, then gonadal hormones presumably interact with basic developmental processes to result in this vulnerability perhaps by increasing the amount of cell death in response to subtle developmental perturbations. However, levels of testosterone and DHT within the hippocampus are equivalent in males and females during the first week of life (Konkle and McCarthy, 2011), suggesting that acute differences in hippocampal androgens are not driving the sex difference in vulnerability during this time period. Hippocampal androgen levels do exhibit a sex difference prenatally (E19-E21; Konkle and McCarthy, 2011), perhaps acting as a first step in a cascade which substantially precedes their ultimate sexually dimorphic organizational effects on cell death seen at later postnatal time points.
One part of this cascade initiated by differential prenatal androgen exposure may be expression of calcium regulatory proteins such as SERCA2. Acute administration of DHT to neurons on DIV11 increases SERCA2 mRNA (Foradori et al., 2007), whereas the current data show an increase in SERCA2 protein on PND2, which is gone by PND7. Interestingly, Foradori and colleagues (2007) generated mixed-sex primary hippocampal cultures from E17–18 embryos, and the cells were kept in culture for 11–14 days. Since there is a surge in plasma testosterone around E19, and then again around the day of birth (Weisz and Ward, 1980), it is possible that neurons removed before this hormone surge may react differently to subsequent acute hormone application than neurons that did experience in vivo hormone levels prior to being removed for culture. This theory is not mutually exclusive with the hypothesis that the levels of SERCA2 decrease at PND7 and then increase again 7 days later in the dynamically changing hippocampus, although it would be difficult to envision a hormonal causal link since androgen levels do not change drastically during the second week of life in either sex (Konkle and McCarthy, 2011).
The intracellular mechanism of androgen action responsible for neuronal changes, whether protective or not, is largely unknown. For example, DHT can regulate adult hippocampal spines by an androgen receptor (AR)-independent mechanism (MacLusky et al., 2006). Androgen neuroprotection, however, appears to be carried out mainly via activation of AR (Cardounel et al., 1999; Hammond et al., 2001; Nguyen et al., 2005; 2010). Indeed, Fordori and colleagues (2007) have shown that DHT regulation of intracellular calcium dynamics, as well as an increase in SERCA2 mRNA, is AR dependent in hippocampal neurons. Therefore the currently demonstrated effects of DHT are likely to be mediated by AR, although that has not been specifically tested. The most commonly used AR antagonist, flutamide, can have both antagonist and agonist properties (Yeh et al., 1999; Lee et al., 2002; MacLusky et al., 2004). Animals with a Tfm mutation (testicular feminization mutation) have been used to bypass the problems with pharmacological AR manipulations. The mutation does not render all ARs completely nonfunctional, however, and large doses of DHT can stimulate the AR receptor even in Tfm rats (Yarbrough et al., 1990; Langley et al., 1998). Therefore, further studies with converging evidence will be needed to more fully answer this question.
Intracellular calcium regulation is a critical regulator of excitotoxic damage, and the current study shows that pretreatment with DHT significantly attenuates the calcium peak following glutamate, only in males, thus suggesting a possible mechanism for androgen neuroprotection at this age and in this sex. There is precedent for the idea that neurons derived from males and females handle intracellular calcium differently; for example, depolarizing GABA causes a sexually dimorphic calcium response in DIV2 neurons (Nunez and McCarthy, 2008). The question remains, though, as to how DHT administration results in sexually dimorphic calcium attenuation and thus neuroprotection. One possible explanation is that the intracellular calcium peak following an excitatory stimulus originates from different stores of calcium in males and females. For example, DHT may alter calcium release from internal stores, a mechanism used by males, whereas intracellular calcium increases in females are via external routes. This does not seem likely, however, since blocking various routes of calcium entry all had an equally significant, but moderate effect on calcium flux in the two sexes. Further, there was no sex difference in the levels of the PMCA1, NCX1 or SERCA2 proteins, gateways for calcium regulation via both the external milieu and internal stores. The sexually dimorphic neuroprotection of DHT may be insult dependent: protective against glutamate-induced excitotoxicity, but not GABA-mediated excitotoxic damage. Therefore the change in SERCA2 protein seen on the second day of life could serve a protective function against GABA-induced damage, which is mediated by internal calcium changes, but not when the major role of excitatory neurotransmitter is ceded to glutamate. However, this also seems unlikely since DHT has been shown to exacerbate GABA-mediated damage, although increasing SERCA2 protein levels may be a defense against even more catastrophic damage, and did not protect against glutamate-mediated damage in sex specific cultures. A compensatory action of other PMCA and NCX family members is possible, since PMCA1–4 and NCX 1–3 are all found in the brain, although SERCA2 interacts in neurons specifically with NCX1 and possibly PMCA1 (Blaustein et al., 2002; Papa et al., 2003; Lencesova et al., 2004; Kenyon et al., 2010). A contribution from other SERCA family members is also possible, though SERCA1 is apparently not found in the brain and SERCA3 may be present in hippocampal cells, but is not affected by DHT treatment (Foradori et al., 2007). On the other hand, SERCA2 is a bidirectional gateway into the endoplasmic reticulum, so that an increased amount of SERCA2 protein could result in an increased amount of calcium stored in the ER following excitatory stimulation, which is ultimately shunted into the mitochondria (Goldberg and Barres, 2000). Indeed, an increase in SERCA2 protein with a concomitant decrease in PMCA1 and NCX1, as seen in PND2 animals, may lead to decreased calcium extrusion to the extracellular space, but sharply increased levels of sequestered ER calcium. Calcium release from the mitochondria is a critical step in the apoptotic process, and excess ER calcium can be shuttled into mitochondria and act to promote apoptosis (Goldberg and Barres, 2000; Jiao et al., 2005). Therefore, the androgen-mediated increase in SERCA2 and decrease in PMCA1 and NCX1 could explain the exacerbation of cell death seen following excitatory GABA during an early neonatal time point and may also “program” altered calcium dynamics later in life specifically in males resulting in short-term protection against glutamate-induced damage, but long-term heightening of vulnerability to the variety of experimental and clinical perturbations discussed previously.
In summary, we have demonstrated a sexually dimorphic neuroprotective effect of androgen on glutamate-mediated excitoxicity in neonatal hippocampal neurons. This neuroprotective effect is correlated with a decrease in the peak of intracellular calcium induced by glutamate, but the mechanism by which this is achieved remains unclear. More work is required to solve the intricate interaction between hormones, calcium regulation and age; the current study highlights the first week of life, a time of transition from excitatory GABA to excitatory glutamate, as an inflection point in the hormonally-driven mechanism of sculpting sexually dimorphic brain areas.
Androgens can be neuroprotective or neurotoxic.
Hippocampal neurons are differentially affected by glutamate depending on sex.
DHT decreases calcium influx and cell death after glutamate, but only in males.
Levels of calcium regulatory proteins do not differ by sex, but are altered by DHT.
These sex and androgen effects change during the first week of life.
Acknowledgements
We would like to thank Michael Taylor for excellent technical assistance. This work was supported by R01 NS050525 to MMM.
ABBREVIATIONS
- PMCA1
plasma membrane calcium ATPase 1
- SERCA2
sarco/endoplasmic reticulum calcium ATPase 2
- NCX1
sodium/calcium exchanger 1
- SDN
sexually dimorphic nucleus
- AVPV
anteroventral periventricular nucleus
- AR
androgen receptor
- DHT
dihydrotestosterone
- ER
endoplasmic reticulum
- PND
postnatal day
- DIV
day in vitro
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
- DAB
diaminobenzidine
- PSS
physiological salt solution
- IAD
integrative grayscale pixel area density
- H/I
hypoxic/ischemic birth injury
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahlbom E, Grandison L, Bonfoco E, Zhivotovsky B, Ceccatelli S. Androgen treatment of neonatal rats decreases susceptibility of cerebellar granule neurons to oxidative stress in vitro. Eur J Neurosci. 1999;11:1285–1291. doi: 10.1046/j.1460-9568.1999.00529.x. [DOI] [PubMed] [Google Scholar]
- Amateau SK, McCarthy MM. A novel mechanism of dendritic spine plasticity involving estradiol induction of prostaglandin-E2. J Neurosci. 2002;22:8586–8596. doi: 10.1523/JNEUROSCI.22-19-08586.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger AP, Perrot-Sinal TS, McCarthy MM. Excitatory versus inhibitory GABA as a divergence point in steroid-mediated sexual differentiation of the brain. Proc Natl Acad Sci USA. 2001;98:8059–8064. doi: 10.1073/pnas.131016298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bading H. Nuclear calcium signalling in the regulation of brain function. Nat Rev Neurosci. 2013;14:593–608. doi: 10.1038/nrn3531. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Woodin MA, Sernagor E, Cancedda L, Vinay L, Rivera C, Legendre P, Luhmann HJ, Bordey A, Wenner P, Fukuda A, van den Pol AN, Gaiarsa J-L, Cherubini E. Refuting the challenges of the developmental shift of polarity of GABA actions: GABA more exciting than ever! Front Cell Neurosci. 2012:6. doi: 10.3389/fncel.2012.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein MP, Juhaszova M, Golovina VA, Church PJ, Stanley EF. Na/Ca exchanger and PMCA localization in neurons and astrocytes: functional implications. Ann N Y Acad Sci. 2002;976:356–366. doi: 10.1111/j.1749-6632.2002.tb04762.x. [DOI] [PubMed] [Google Scholar]
- Blaustein MP, Lederer WJ. Sodium/calcium exchange: its physiological implications. Physiol Rev. 1999;79:763–854. doi: 10.1152/physrev.1999.79.3.763. [DOI] [PubMed] [Google Scholar]
- Campochiaro P, Coyle JT. Ontogenetic development of kainate neurotoxicity: correlates with glutamatergic innervation. Proc Natl Acad Sci USA. 1978;75:2025–2029. doi: 10.1073/pnas.75.4.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardounel A, Regelson W, Kalimi M. Dehydroepiandrosterone protects hippocampal neurons against neurotoxin-induced cell death: mechanism of action. ProcSocExpBiolMed. 1999;222:145–149. doi: 10.1046/j.1525-1373.1999.d01-124.x. [DOI] [PubMed] [Google Scholar]
- Cheng J, Alkayed NJ, Hurn PD. Deleterious effects of dihydrotestosterone on cerebral ischemic injury. Journal of Cerebral Blood Flow & Metabolism. 2007;27:1553–1562. doi: 10.1038/sj.jcbfm.9600457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EC, Popper P, Gorski RA. The role of apoptosis in sexual differentiation of the rat sexually dimorphic nucleus of the preoptic area. Brain Res. 1996;734:10–18. [PubMed] [Google Scholar]
- Fanaei H, Sadeghipour HR, Karimian SM, Hassanzade G. Flutamide Enhances Neuroprotective Effects of Testosterone during Experimental Cerebral Ischemia in Male Rats. ISRN Neurol. 2013;2013:592398. doi: 10.1155/2013/592398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foradori CD, Handa RJ. Living or dying in three quarter time: Neonatal orchestration of hippocampal cell death pathways by androgens and excitatory GABA. Experimental Neurology. 2008;213:1–6. doi: 10.1016/j.expneurol.2008.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foradori CD, Werner SB, Sandau US, Clapp TR, Handa RJ. Activation of the androgen receptor alters the intracellular calcium response to glutamate in primary hippocampal neurons and modulates sarco/endoplasmic reticulum calcium ATPase 2 transcription. Neuroscience. 2007;149:155–164. doi: 10.1016/j.neuroscience.2007.06.054. [DOI] [PubMed] [Google Scholar]
- Forger NG. Cell death and sexual differentiation of the nervous system. Neuroscience. 2006;138:929–938. doi: 10.1016/j.neuroscience.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Ganguly K, Schinder AF, Wong ST, Poo M. GABA itself promotes the developmental switch of neuronal GABAergic responses from excitation to inhibition. Cell. 2001;105:521–532. doi: 10.1016/s0092-8674(01)00341-5. [DOI] [PubMed] [Google Scholar]
- Goldberg JL, Barres BA. The relationship between neuronal survival and regeneration. Annu Rev Neurosci. 2000;23:579–612. doi: 10.1146/annurev.neuro.23.1.579. [DOI] [PubMed] [Google Scholar]
- Hammond J, Le Q, Goodyer C, Gelfand M, Trifiro M, LeBlanc A. Testosterone-mediated neuroprotection through the androgen receptor in human primary neurons. J Neurochem. 2001;77:1319–1326. doi: 10.1046/j.1471-4159.2001.00345.x. [DOI] [PubMed] [Google Scholar]
- Hawk T, Zhang YQ, Rajakumar G, Day AL, Simpkins JW. Testosterone increases and estradiol decreases middle cerebral artery occlusion lesion size in male rats. Brain Res. 1998;796:296–298. doi: 10.1016/s0006-8993(98)00327-8. [DOI] [PubMed] [Google Scholar]
- Hilton GD, Bambrick LL, Thompson SM, McCarthy MM. Estradiol Modulation of Kainic Acid-Induced Calcium Elevation in Neonatal Hippocampal Neurons. Endocrinology. 2005;147:1246–1255. doi: 10.1210/en.2005-1258. [DOI] [PubMed] [Google Scholar]
- Hilton GD, Ndubuizu AN, McCarthy MM. Neuroprotective effects of estradiol in newborn female rat hippocampus. Developmental Brain Research. 2004;150:191–198. doi: 10.1016/j.devbrainres.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Hilton GD, Nunez JL, Bambrick L, Thompson SM, McCarthy MM. Glutamate-mediated excitotoxicity in neonatal hippocampal neurons is mediated by mGluR-induced release of Ca ++from intracellular stores and is prevented by estradiol. European Journal of Neuroscience. 2006;24:3008–3016. doi: 10.1111/j.1460-9568.2006.05189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindmarsh GJ, O'Callaghan MJ, Mohay HA, Rogers YM. Gender differences in cognitive abilities at 2 years in ELBW infants. Extremely low birth weight. Early Hum Dev. 2000;60:115–122. doi: 10.1016/s0378-3782(00)00105-5. [DOI] [PubMed] [Google Scholar]
- Isgor C, Sengelaub DR. Prenatal gonadal steroids affect adult spatial behavior, CA1 and CA3 pyramidal cell morphology in rats. Hormones and behavior. 1998;34:183–198. doi: 10.1006/hbeh.1998.1477. [DOI] [PubMed] [Google Scholar]
- Isgor C, Sengelaub DR. Effects of neonatal gonadal steroids on adult CA3 pyramidal neuron dendritic morphology and spatial memory in rats. J Neurobiol. 2003;55:179–190. doi: 10.1002/neu.10200. [DOI] [PubMed] [Google Scholar]
- Jiao J, Huang X, Feit-Leithman RA, Neve RL, Snider W, Dartt DA, Chen DF. Bcl-2 enhances Ca(2+) signaling to support the intrinsic regenerative capacity of CNS axons. EMBO J. 2005;24:1068–1078. doi: 10.1038/sj.emboj.7600589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston MV, Hagberg H. Sex and the pathogenesis of cerebral palsy. Dev Med Child Neurol. 2007;49:74–78. doi: 10.1017/s0012162207000199.x. [DOI] [PubMed] [Google Scholar]
- Kenyon KA, Bushong EA, Mauer AS, Strehler EE, Weinberg RJ, Burette AC. Cellular and subcellular localization of the neuron-specific plasma membrane calcium ATPase PMCA1a in the rat brain. J Comp Neurol. 2010;518:3169–3183. doi: 10.1002/cne.22409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JE, Allore RJ, Beck SG, Handa RJ. Distribution and hormonal regulation of androgen receptor (AR) and AR messenger ribonucleic acid in the rat hippocampus. Endocrinology. 1995;136:3213–3221. doi: 10.1210/endo.136.8.7628354. [DOI] [PubMed] [Google Scholar]
- Kingston AE, Ornstein PL, Wright RA, Johnson BG, Mayne NG, Burnett JP, Belagaje R, Wu S, Schoepp DD. LY341495 is a nanomolar potent and selective antagonist of group II metabotropic glutamate receptors. Neuropharmacology. 1998;37:1–12. doi: 10.1016/s0028-3908(97)00191-3. [DOI] [PubMed] [Google Scholar]
- Konkle ATM, McCarthy MM. Developmental Time Course of Estradiol, Testosterone, and Dihydrotestosterone Levels in Discrete Regions of Male and Female Rat Brain. Endocrinology. 2011;152:223–235. doi: 10.1210/en.2010-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan S, Intlekofer KA, Aggison LK, Petersen SL. Central role of TRAF-interacting protein in a new model of brain sexual differentiation. Proc Natl Acad Sci USA. 2009;106:16692–16697. doi: 10.1073/pnas.0906293106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley E, Kemppainen JA, Wilson EM. Intermolecular NH2-/Carboxyl-terminal Interactions in Androgen Receptor Dimerization Revealed by Mutations That Cause Androgen Insensitivity. Journal of Biological Chemistry. 1998;273:92–101. doi: 10.1074/jbc.273.1.92. [DOI] [PubMed] [Google Scholar]
- Lauterbach MD, Raz S, Sander CJ. Neonatal hypoxic risk in preterm birth infants: the influence of sex and severity of respiratory distress on cognitive recovery. Neuropsychology. 2001;15:411–420. [PubMed] [Google Scholar]
- Lee Y-F, Lin W-J, Huang J, Messing EM, Chan FL, Wilding G, Chang C. Activation of mitogen-activated protein kinase pathway by the antiandrogen hydroxyflutamide in androgen receptor-negative prostate cancer cells. Cancer Res. 2002;62:6039–6044. [PubMed] [Google Scholar]
- Lencesova L, O'Neill A, Resneck WG, Bloch RJ, Blaustein MP. Plasma membrane-cytoskeleton-endoplasmic reticulum complexes in neurons and astrocytes. J Biol Chem. 2004;279:2885–2893. doi: 10.1074/jbc.M310365200. [DOI] [PubMed] [Google Scholar]
- Leranth C, Petnehazy O, MacLusky NJ. Gonadal hormones affect spine synaptic density in the CA1 hippocampal subfield of male rats. J Neurosci. 2003;23:1588–1592. doi: 10.1523/JNEUROSCI.23-05-01588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLusky NJ, Hajszan T, Leranth C. Effects of Dehydroepiandrosterone and Flutamide on Hippocampal CA1 Spine Synapse Density in Male and Female Rats: Implications for the Role of Androgens in Maintenance of Hippocampal Structure. Endocrinology. 2004;145:4154–4161. doi: 10.1210/en.2004-0477. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Hajszan T, Prange-Kiel J, Leranth C. Androgen modulation of hippocampal synaptic plasticity. Neuroscience. 2006;138:957–965. doi: 10.1016/j.neuroscience.2005.12.054. [DOI] [PubMed] [Google Scholar]
- Madeira MD, Lieberman AR. Sexual dimorphism in the mammalian limbic system. Prog Neurobiol. 1995;45:275–333. doi: 10.1016/0301-0082(94)00052-j. [DOI] [PubMed] [Google Scholar]
- Marks JD, Friedman JE, Haddad GG. Vulnerability of CA1 neurons to glutamate is developmentally regulated. Brain Res Dev Brain Res. 1996;97:194–206. doi: 10.1016/s0165-3806(96)00149-6. [DOI] [PubMed] [Google Scholar]
- Mizoguchi K, Kunishita T, Chui DH, Tabira T. Stress induces neuronal death in the hippocampus of castrated rats. Neurosci Lett. 1992;138:157–160. doi: 10.1016/0304-3940(92)90495-s. [DOI] [PubMed] [Google Scholar]
- Nguyen T-VV, Yao M, Pike CJ. Androgens activate mitogen-activated protein kinase signaling: Role in neuroprotection. J Neurochem. 2005;94:1639–1651. doi: 10.1111/j.1471-4159.2005.03318.x. [DOI] [PubMed] [Google Scholar]
- Nguyen TV, Jayaraman A, Quaglino A, Pike CJ. Androgens Selectively Protect Against Apoptosis in Hippocampal Neurones. Journal of Neuroendocrinology. 2010;22:1013–1022. doi: 10.1111/j.1365-2826.2010.02044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordeen EJ, Nordeen KW, Sengelaub DR, Arnold AP. Androgens prevent normally occurring cell death in a sexually dimorphic spinal nucleus. Science (New York, NY. 1985;229:671–673. doi: 10.1126/science.4023706. [DOI] [PubMed] [Google Scholar]
- Nunez JL, Alt JJ, McCarthy MM. A novel model for prenatal brain damage. II. Long-term deficits in hippocampal cell number and hippocampal-dependent behavior following neonatal GABAA receptor activation. Exp Neurol. 2003a;181:270–280. doi: 10.3201/eid0906.020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez JL, Alt JJ, McCarthy MM. A new model for prenatal brain damage. I. GABAA receptor activation induces cell death in developing rat hippocampus. Exp Neurol. 2003b;181:258–269. doi: 10.3201/eid0906.030118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez JL, Bambrick LL, Krueger BK, McCarthy MM. Prolongation and enhancement of gamma-aminobutyric acid receptor mediated excitation by chronic treatment with estradiol in developing rat hippocampal neurons. Eur J Neurosci. 2005;21:3251–3261. doi: 10.1111/j.1460-9568.2005.04175.x. [DOI] [PubMed] [Google Scholar]
- Nunez JL, McCarthy MM. Evidence for an extended duration of GABA-mediated excitation in the developing male versus female hippocampus. Devel Neurobio. 2007;67:1879–1890. doi: 10.1002/dneu.20567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez JL, McCarthy MM. Androgens predispose males to GABAA-mediated excitotoxicity in the developing hippocampus. Experimental Neurology. 2008;210:699–708. doi: 10.1016/j.expneurol.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez JL, McCarthy MM. Resting intracellular calcium concentration, depolarizing Gamma-Aminobutyric Acid and possible role of local estradiol synthesis in the developing male and female hippocampus. Neuroscience. 2009;158:623–634. doi: 10.1016/j.neuroscience.2008.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen KM, Auger AP. Sex differences in Fos protein expression in the neonatal rat brain. J Neuroendocrinol. 2005;17:255–261. doi: 10.1111/j.1365-2826.2005.01302.x. [DOI] [PubMed] [Google Scholar]
- Papa M, Canitano A, Boscia F, Castaldo P, Sellitti S, Porzig H, Taglialatela M, Annunziato L. Differential expression of the Na+-Ca2+ exchanger transcripts and proteins in rat brain regions. J Comp Neurol. 2003;461:31–48. doi: 10.1002/cne.10665. [DOI] [PubMed] [Google Scholar]
- Pike CJ. Testosterone attenuates beta-amyloid toxicity in cultured hippocampal neurons. Brain Res. 2001;919:160–165. doi: 10.1016/s0006-8993(01)03024-4. [DOI] [PubMed] [Google Scholar]
- Ramsden M, Shin TM, Pike CJ. Androgens modulate neuronal vulnerability to kainate lesion. Neuroscience. 2003;122:573–578. doi: 10.1016/j.neuroscience.2003.08.048. [DOI] [PubMed] [Google Scholar]
- Romero-Calvo I, Ocón B, Martínez-Moya P, Suárez MD, Zarzuelo A, Martínez-Augustin O, de Medina FS. Reversible Ponceau staining as a loading control alternative to actin in Western blots. Analytical Biochemistry. 2010;401:318–320. doi: 10.1016/j.ab.2010.02.036. [DOI] [PubMed] [Google Scholar]
- Rosario ER, Carroll JC, Oddo S, LaFerla FM, Pike CJ. Androgens Regulate the Development of Neuropathology in a Triple Transgenic Mouse Model of Alzheimer's Disease. J Neurosci. 2006;26:13384–13389. doi: 10.1523/JNEUROSCI.2514-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario ER, Chang L, Stanczyk FZ, Pike CJ. Age-related testosterone depletion and the development of Alzheimer disease. JAMA. 2004;292:1431–1432. doi: 10.1001/jama.292.12.1431-b. [DOI] [PubMed] [Google Scholar]
- Sar M, Lubahn DB, French FS, Wilson EM. Immunohistochemical localization of the androgen receptor in rat and human tissues. Endocrinology. 1990;127:3180–3186. doi: 10.1210/endo-127-6-3180. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Chua C, Falduto J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J Neurosci. 2001;21:6292–6297. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speert DB, Konkle ATM, zup SL, Schwarz JM, Shiroor C, Taylor ME, McCarthy MM. Focal Adhesion Kinase and Paxillin: Novel Regulators of Brain Sexual Differentiation? Endocrinology. 2007;148:3391–3401. doi: 10.1210/en.2006-0845. [DOI] [PubMed] [Google Scholar]
- Tabori NE, Stewart LS, Znamensky V, Romeo RD, Alves SE, McEwen BS, Milner TA. Ultrastructural evidence that androgen receptors are located at extranuclear sites in the rat hippocampal formation. Neuroscience. 2005;130:151–163. doi: 10.1016/j.neuroscience.2004.08.048. [DOI] [PubMed] [Google Scholar]
- Uchida M, Palmateer JM, Herson PS, DeVries AC, Cheng J, Hurn PD. Dose-dependent effects of androgens on outcome after focal cerebral ischemia in adult male mice. Journal of Cerebral Blood Flow & Metabolism. 2009;29:1454–1462. doi: 10.1038/jcbfm.2009.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters EM, Simerly RB. Estrogen Induces Caspase-Dependent Cell Death during Hypothalamic Development. J Neurosci. 2009;29:9714–9718. doi: 10.1523/JNEUROSCI.0135-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz J, Ward IL. Plasma testosterone and progesterone titers of pregnant rats, their male and female fetuses, and neonatal offspring. Endocrinology. 1980;106:306–316. doi: 10.1210/endo-106-1-306. [DOI] [PubMed] [Google Scholar]
- Xiao L, Jordan CL. Sex Differences, Laterality, and Hormonal Regulation of Androgen Receptor Immunoreactivity in Rat Hippocampus. Hormones and Behavior. 2002;42:327–336. doi: 10.1006/hbeh.2002.1822. [DOI] [PubMed] [Google Scholar]
- Yarbrough WG, Quarmby VE, Simental JA, Joseph DR, Sar M, Lubahn DB, Olsen KL, French FS, Wilson EM. A single base mutation in the androgen receptor gene causes androgen insensitivity in the testicular feminized rat. J Biol Chem. 1990;265:8893–8900. [PubMed] [Google Scholar]
- Yeh S, Kang HY, Miyamoto H, Nishimura K, Chang HC, Ting HJ, Rahman M, Lin HK, Fujimoto N, Hu YC, Mizokami A, Huang KE, Chang C. Differential induction of androgen receptor transactivation by different androgen receptor coactivators in human prostate cancer DU145 cells. Endocrine. 1999;11:195–202. doi: 10.1385/endo:11:2:195. [DOI] [PubMed] [Google Scholar]
- Zhang J-M, Konkle ATM, Zup SL, McCarthy MM. Impact of sex and hormones on new cells in the developing rat hippocampus: a novel source of sex dimorphism? Eur J Neurosci. 2008;27:791–800. doi: 10.1111/j.1460-9568.2008.06073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zup S, Forger N. In: Hormones and sexual differentiation. Ramachandran VS, editor. San Diego: Elsevier Science; 2002. [Google Scholar]



