Abstract
Malignant melanoma is a difficult cancer to treat due to the rapid development of resistance to drugs targeting single proteins. One response to this observation is to identify single pharmacological agents that due to a unique mechanism of action simultaneously target multiple key pathways involved in melanoma development. To develop a single nanoparticle-based agent targeting the PI3 kinase, STAT3 and MAP kinase signaling pathways, a natural product library was screened, identifying leelamine, as a potential inhibitor. To overcome the poor bioavailability of leelamine in animals and lethality when administered intravenously, a nanoliposomal-based delivery system has been developed called Nanolipolee-007, which stably loads 60% of the compound. The nanoparticle was as effective at killing melanoma cells as leelamine dissolved in DMSO and was 4.5-fold more effective at killing cultured melanoma cell survival compared to normal cells. Mechanistically, Nanolipolee-007 inhibited PI3K/Akt, STAT3 and MAPK signaling mediated through inhibition of cholesterol transport shutting down these key pathways. Nanolipolee-007 inhibited the growth of preexisting xenografted melanoma tumors by an average of 64% by decreasing cellular proliferation, reducing tumor vascularization, and increasing cellular apoptosis, with negligible toxicity. Thus, a unique clinical viable nanoparticle based drug has been developed containing leelamine for the treatment of melanoma that acts by inhibiting the activity of major signaling pathways regulating the development of this disease.
Keywords: Melanoma, Leelamine, PEGylated liposomes, Akt, Stat3, Multi-target inhibitor
INTRODUCTION
Malignant melanoma is one of the most difficult cancers to treat due to the development of recurrent resistant disease (1). Although encouraging recent studies showed promising results with Zelboraf, a selective V600EB-Raf kinase inhibitor, development of drug resistance remains a problem (2). To address this concern, one approach has been to develop liposomes containing novel agents specifically targeting multiple key pathways or signaling cascades leading to recurrent resistance disease development (3).
Loading of drugs into nanoparticles to circumvent bioavailability, toxicity or lethality can be used to overcome limitations of standard drug delivery systems, which involve nonspecific targeting and bio distribution with low solubility at a modest therapeutic index (4, 5). Nanoparticles can be designed with optimal size and surface characteristics to increase the circulation time and biodistribution (6). One advantage of nanoparticles is the enhanced permeability and retention (EPR) effect that enables nanoparticles to accumulate in malignant tumors at much higher concentrations than in normal tissue (6). The EPR effect occurs because the vasculature of tumors is poorly developed and leaky, enabling nanoparticles and macromolecules to preferentially accumulate and concentrate in tumors (7). Natural or synthetic polymers or lipids can be used as drug delivery vectors (7). Lipid-based delivery vehicles have established track records, and have suitable biological properties, including biocompatibility, biodegradability and the ability to accommodate both hydrophilic and hydrophobic drugs (8).
Liposomes primarily consist of phospholipid amphiphiles that self assemble to form bilayer membranes. Liposomes have been extensively studied as drug-delivery systems and are used as biocompatible carriers of drugs, enzymes, peptides, vaccines, imaging agents and/or genetic material (9). Depending on the properties of the drugs, they can be loaded into the aqueous or lipid compartments of liposomes and can be effective for delivering the therapeutic cargo into tumors in animals (9). In addition, liposomes can be used as models for artificial cells that are able to mimic cell membrane trafficking and studying drug movement (10). Currently, nanoliposomal based therapeutics are used clinically and many are at various stages of clinical development (3, 11).
Recently, a cell-based screen of a natural product library was undertaken to identify a pharmacological agent that can decrease melanoma development by targeting the PI3K, STA3 and MAPK signaling cascade, which is detailed in the article by Gowda et al (12). Leelamine-mediated inhibition of these cascades was attributed to the inhibition of receptor-mediated endocytosis of receptor tyrosine kinases causing aberrant accumulation of these proteins in the perinuclear region of cells, which is detailed in the article by Kuzu OF et al (13). Using a combination of protein arrays and systems biology followed by validation studies, leelamine was found to inhibit the PI3K/Akt, STAT3 and MAPK pathways by disrupting cancer cell cholesterol transport. These are key driver pathways in melanoma cells constitutively activated in 50 to 70% of melanomas, functioning to reduce cellular apoptosis, increase proliferation and aid the invasive processes promoting melanoma progression (14-16).
Leelamine dissolved in DMSO and administered by intraperitoneal injection, inhibited the growth of preexisting xenografted melanoma tumors by 60% without significant toxicity. However, due to poor bioavailability, toxicity and lethality of this agent when administered intravenously, a stable PEGylated nanoliposomal delivery system has been developed called Nanolipolee-007. The nanoparticle has an average size of 80 nm and is stable in saline for 1 year at 4°C. Nanolipolee-007 was 5.69-fold more effective at killing melanoma than normal cells, decreased cellular proliferation and triggered apoptosis through a G0/G1 block resulting in fewer cells in S-phase. Intravenously administered Nanolipolee-007 had negligible toxicity and retarded existing xenograft melanoma tumor growth by up to 60% without affecting animal weight or organ function by decreasing melanoma cell proliferation, increasing apoptosis and decreasing vascular development. Therefore, Nanolipolee-007 is a potentially clinically viable intravenous nanoparticle formulation that can be used to treat melanoma in mice and has potential for use in humans.
MATERIALS AND METHODS
Cell lines and culture conditions
Normal human primary melanocytes FOM103 and wild type B-Raf containing SbCl2 (provided by Dr. Herlyn between 2003-2005; Wistar Institute, Philadelphia, PA) were cultured as described (14). Human fibroblast FF2441 cells (Provided by Dr. Craig Myers lab between 2005-2006, Penn State College of Medicine, Hershey, PA). Mutant type B-Raf melanoma cell lines UACC 903 (from Mark Nelson provided between 1995-1999), University of Arizona, Tucson, AZ) and 1205 Lu (provided by Dr. Herlyn between 2005-2006; Wistar Institute, Philadelphia, PA). Wild type B-Raf melanoma cell line containing C8161.Cl9 provided by Dr. Danny Welch (2003), University of Kansas, Kansas City, KS and MelJuSo provided by Dr. Judith Johnson (between 1995-1999), Institute for Immunology, Germany). Cell lines were maintained in a 37°C humidified 5% CO2 atmosphere incubator and periodically monitored for genotypic characteristics, phenotypic behaviour, and tumorigenic potential to confirm the cell line indentity (14).
Intravenous administration of free leelamine and liposomal leelamine
Four to six weeks old female Athymic-Foxn1nu nude mice (Harlan Sprague Dawley, IN) were injected intravenously with 30 mg/Kg body weight leelamine dissolved in DMSO or Nanolipolee-007. Vehicle control animals were treated with DMSO or empty liposome. Animal mortatlity or hemolysis and coagulation was recoreded after 1 hour (n=3).
Generation of Nanolipolee-007
Leelamine hydrochloride (Tocris Biosciences, Ellisville, MO) was encapsulated into a nanoliposome (called Nanolipolee-007) prepared by combining egg L-α-Phosphatidylcholine (ePC) and 1,2-Dipalmitoyl-sn-Glycero-3-Phosphoethanolamine-N-[Methoxy(Polyethylene glycol)-2000] ammonium salt (DPPE-PEG-2000) in chloroform at 80:20 mol% and a final lipid concentration of 25 mg/mL in buffer solution (Avanti Polar Lipids Inc- Alabaster, AL). The same lipid formulation was found to load Abietic acid (Sigma Chemical Co. St. Louis, MO) and an empty control nanoliposome was also made. 7.5 mg of leelamine hydrochloride and/or abietic acid (in methanol) was added to 1.0 mL of nanoliposome solution resulting in a 22.6 mM concentration. Mixture was then dried under nitrogen gas and resuspended in 0.9% saline at 60°C. Following rehydration, the mixture was sonicated at 60°C for 30 minutes followed by extrusion at 60°C through a 100-nm polycarbonate membrane using Avanti Mini Extruder (Avanti Polar Lipids Inc- Alabaster, AL). The particle size and charge characteristics were determined using a Malvern Zetasizer (Malvern Instruments, UK)
Characterization of Nanolipolee-007
Loading efficacy
Amount of leelamine encapsulated into the nanoliposome was measured by calculating the amount of tritium labeled leelamine incorporated into the nanoparticle. Tritium labeled leelamine was synthesized by bromination, followed by replacing the bromine atom with tritium with a specific activity of 25 Ci/mmol (American Radio Chemicals Inc, St. Louis, MO), (http://www.arc-inc.com/index.php). Dialysis and size exclusion chromatography were used to calculate tritium labeled leelamine loading efficacy (17, 18). One milliliter of Nanolipolee-007 made with tritium labeled leelamine was placed into a 1×5 cm long dialysis membrane bag (Molecular weight cut off: 25 kDa; Spectra Por, Los Angeles, CA). The dialysis bag was suspended in 1 L of 0.9% saline with constant stirring (300 rpm) for 12 hours and the amount of tritium labeled leelamine remaining in the dialysis bag was measured by liquid scintillation counter (LS-6500- Beckman Coulter, Fullerton, CA). Sepharose CL-4B (wet bead size, 45-165 μm) (Sigma Chemical Co. St. Louis, MO) was loaded into a column (0.6×60 cm) equilibrated in 0.9% saline, pH 7.0. Next, 200 μL of 1×106 cpm units was loaded onto the column and eluted with 0.9% saline at room temperature. Eluted fractions were collected at a flow rate of 10 mL/hours and the amount of tritium in each fraction measured. Peak fractions were pooled and the amount of tritium labeled leelamine encapsulated in Nanolipolee-007 was measured. The percentage incorporation of leelamine was determined using the following formula: Amount of radioactivity in peak-I (liposomal encapsulated) / Amount of radioactivity in peak-I + Amount of radioactivity in peak-II (free leelamine)×100.
Stability of Nanolipolee-007
Nanolipolee-007 stored in sterile saline at 4°C was measured at 1, 3, 6, 9 and 12 months by comparing size, charge and efficacy for killing UACC 903 melanoma cells. During this interval, the particle size and charge characteristics were determined by Malvern Zetasizer Nano, (Malvern Instruments, UK). Efficacy of UACC 903 was measured using the MTS assay (Promega, Madison, WI). Briefly, 5×103 UACC 903 cells per well in 100 μL of media were plated and grown in a 96-well plate for 48 hours and treated with 0.62 to 20 μmol/L of nanoliposome alone and Nanolipolee-007 for 24 hours. IC50 values for each treatment agent in μmol/L for respective cell lines were calcutated from two independent experiments using GraphPad Prism version 4.01 (GraphPad Software, La Jolla, CA).
In vitro drug release kinetics
The in vitro release of leelamine from the nanoparticle was measured at room temperature by dialysis through a molecular weight cut off 25 kDa membrane (Spectra Por, Los Angeles, CA). Briefly, 1 mL of tritium labeled Nanolipolee-007 was placed into a dialysis membrane and suspended in 1 L of 0.9% saline (or 10 mM GSH medium) with constant stirring at 300 rpm for 12 hours. Free unbound leelamine was separated, and the dialysis bag containing the encapsulated tritiated leelamine was immersed in 500 mL of releasing medium containing 0.9% saline (or 10 mM reduced glutathione). A 1 mL sample from the releasing medium was taken at 1, 2, 4, 8, 12, 24, 36 and 48 hours, and radioactivity associated with the tritiated leelamine measured using a liquid scintillation counter (LS-6500- Beckman Coulter, Fullerton, CA).
Hemolytic activity
Hemolytic activity assay was followed as described by Perumal et al. (19). In brief, fresh human blood was drawn and placed into an EDTA test tube. Erythrocytes were separated from plasma by centrifugation at 1500 rpm for 10 minutes at 4 °C using phosphate buffer saline (PBS). Erythrocytes pellet was diluted with PBS to a give a 5% v/v solution and 50 μL added into microcentrifuge tubes and treated with leelamine (100 μmol/L), nanolipolee-007 (100 μmol/L), DMSO or empty liposome or 1 % Triton X-100 (positive control). Samples were incubated at 37 °C for 60 minutes and then centrifuged at 12,000 rpm for 10 minutes. Next, supernatants were transferred to a 96-well plate and absorption measured at 540 nm. Amount of hemoglobin released in the presence of 1% Trition X-100 was set as 100% lysis and % hemolysis was calculated as: (absorbance of the samples at 540nm/absorbance of the positive control)*100.
Pharmacokinetics
Leelamine contained in Nanolipolee-007 was extracted from serum as reported previously (20). Swiss Webster (n = 5) mice were intravenously injected with 30 mg/kg body weight of Nanolipolee-007, animals were euthanized and blood drawn by cardiac puncture at various time periods. Samples were kept at room temperature for 30 minutes followed by serum separation by centrifugation for 5 minutes at 5000 rpm. Next, 20 μL of the collected serum was added to 80 μL of acetonitrile along with 5 μL of propranolol (RS)-1-(1-methylethylamino)-3-(1-naphthyloxy)propan-2-ol), an internal standard (transition of m/z 259.9 to 116.0). Solution was vortexed for 30 seconds and then centrifuged at 10,000 rpm for 10 minutes. Supernatant was transferred to the autosampler vials and subject to liquid chromatography-mass spectrometry using LC-MS/MS 2010 EV system (Shimadzu, Tokyo, Japan).
Cell viability, proliferation, apoptosis and cell cycle analysis
Viability and IC50 of normal human melanocytes, fibroblast and melanoma cells (UACC 903 and 1205 Lu) following treatment was measured by MTS assay (Promega, Madison, WI) (21). Briefly, 5×103 melanoma or fibroblast (FF2441) or 20×103 melanocytes (FOM103) cells per well in 100 μL of media were plated and grown in a well of a 96-well plate for 48 or 72 hours and treated with 0.62 to 100 μmol/L of empty nanoliposome, abietic acid control nanoliposome or Nanolipolee-007 for 24 hours.
Cellular proliferation and apoptosis rates
5×103 UACC 903 and 1205 Lu melanoma cells were seeded in 96-well plates, followed by treatment with 0.62 to 100 μmol/L of empty control or abietic acid nanoliposomes or Nanolipolee-007 for 24 hours. Percentage proliferating or apoptotic cells were quantified by a colorimetric cell proliferation ELISA BrdU kit (Roche Applied Sciences, Indianapolis, IN) or fluorimetric Apo-ONE Homogenous caspase-3/7 assay kit as previously reported (Promega, Madison, WI) (21).
Cell cycle analysis
Cells in each phase of the cell cycle were calculated by growing UACC 903 or 1205 Lu melanoma cells in 100-mm culture dishes followed by treatment with empty control nanoliposome or Nanolipolee-007 (2-3 μmol/L) for 24 hours. Total floating and adherent cells were collected following trypsinization and stained with a 1 mL propidium iodide solution containing 100 μg/mL propidium iodide; (Sigma, St Louis, MO), 20 μg/mL Ribonuclease A (Roche diagnostics, Indianapolis, IN) and 3 μg/mL Triton X-100 dissolved in 0.1% (W/V) sodium citrate for 30 minutes at 4°C. Stained cells were analyzed using the FACScan analyzer (Becton Dickinson, Franklin lakes, NJ) and data processed utilizing ModFit LT software (Verity Software House, Topsham, ME) (21).
Western blot analysis
Cell lysates treated with empty control nanoliposome or Nanolipolee-007 (3-5 μmol/L) for 3 to 24 hours were harvested in RIPA lysis buffer containing protease and phosphatase inhibitors (Pierce Biotechnology, Rockford, IL) (21). Blots were probed with antibodies according to each supplier’s recommendations: antibodies to total Akt, phospho-Akt (Ser473), phospho-PRAS40 (Thr246), total Bad, pBad (Ser 112), total Erk1/2, phospho-Erk1/2 (Thr202/Tyr 204), total CDK2, phospho-CDK2 (Thr160), phospho-Rb (Ser807), total Stat, phospho-Stat3 (Tyr705), caspase-3 and cleaved PARP from Cell Signaling Technology (Danvers, MA); total PRAS40 from Invitrogen (Carlsbad, CA); cyclin D1, α-enolase and secondary antibodies conjugated with horseradish peroxidase from Santa Cruz Biotechnology (Santa Cruz, CA).
Tumorigenicity assessments
Tumor kinetics were measured by subcutaneous injection of 1.5×106 UACC 903 or 1205 Lu cells in 0.2 mL of DMEM supplemented with 10% FBS. Cells were injected above both left and right rib cages of 3 to 4 week-old female Athymic-Foxn1nu nude mice (Harlan Sprague Dawley). Six days later, when a fully vascularized 50-75 mm3 tumor had formed, mice were randomly divided into vehicle control or experimental groups (5 mice/group; 2 tumors/mouse) and treated intravenously everyday for 3-4 weeks with 30-mg/kg body weight of Nanolipolee-007 or control abietic acid or empty nanoliposome. Body weight in grams and dimensions of developing tumors in mm3 were measured on alternate days (21).
Size and time match tumors for analysis of tumorigenic processes regulating tumor development
Mechanism by which Nanolipolee-007 delayed tumor growth was determined by comparing size and time matched xenografted melanoma tumors treated with Nanolipolee-007 or empty control nanoliposome. 2.5×106 UACC 903 cells were injected s.c. into nude mice, generating tumors of the same size developing at parallel time points. Six days later, mice were treated i.v. with empty nanoliposome or Nanolipolee-007 (30 mg/kg body weight) daily upto day 15. Tumors were harvested at 11, 13 and 15 days for comparison of rates of cellular proliferation, apoptosis and vessel density by immunohistochemistry (22, 23).
Toxicity assessments
Four to six weeks old female Athymic-Foxn1nu nude mice (Harlan Sprague Dawley, IN) were treated intravenously either empty or abietic acid control nanoliposomes or Nanolipolee-007 (n = 5). Animals were weighed daily to ascertain toxicity leading to changes in body weight. At the end of treatment, blood was collected from each euthanized animal in a serum separator tube with lithium heparin (BD Microtainer) following cardiac puncture and analyzed for blood makers of major organ function indicative of toxicity (24). A portion of liver, heart, kidney, pancreas, and spleen tissue from each animal was formalin-fixed and paraffin-embedded to examine changes in cell morphology and tissue organization following H&E staining (24).
Statistical Analysis
Statistical analysis was performed using Prism 4.0 GraphPad Software. One-way or Two-way Analysis Of Variance (ANOVA) was used for group wise comparisons (24). Results represent at least three to four independent experiments and are shown as averages ± S.E.M. Number of asterisks in the figures indicates the level of statistical significance as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
RESULTS
Development of Nanolipolee-007 and its efficacy for killing melanoma cells
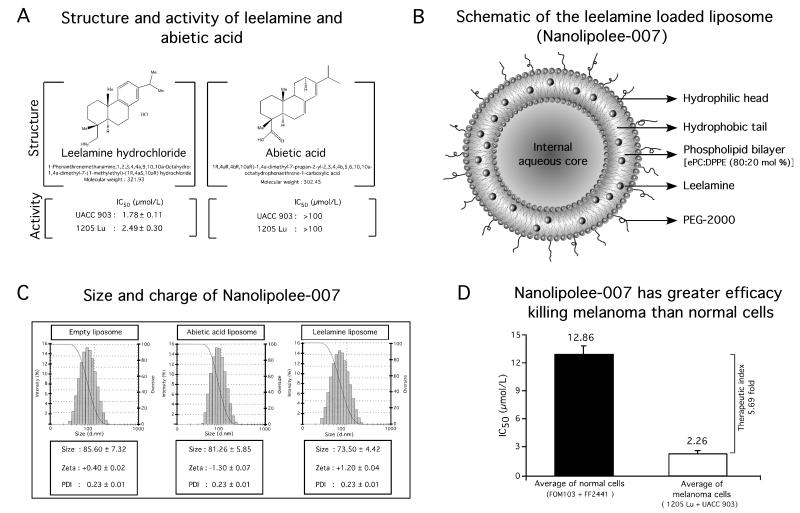
Natural Product Library (NPL-480) consisting of 480 compounds derived from plants, animal, bacteria and fungal sources was used to identify the agents effective at killing melanoma cells, which is detailed in the manuscript by Gowda et al (12). Leelamine was found to be the most potent of the agents identified. Leelamine simultaneously inhibits the PI3K/Akt, STAT3 and MAPK cascades to inhibit melanoma development by disrupting cholesterol transport and endosomal trafficking, which are detailed in the article by Kuzu OF et al (13). Leelamine and a structurally similar inactive control compound called abietic acid are shown in Fig. 1A. The IC50 of leelamine dissolved in DMSO for killing UACC 903 or 1205 Lu melanoma cells are 1.78 and 2.49 μmol/L, respectively (Fig. 1A). In contrast, control abietic acid had no effect on cell viability at concentrations >100 μmol/L. Therefore, abietic acid that shares structural similarity with leelamine was used as a control (Fig. 1A).
Figure 1. Development of Nanolipolee-007 and comparison of its killing efficacy on normal as well as on melanoma cells.
1A. To identify a novel drug targeting multiple key pathways in melanoma, a cell-based screen was undertaken of a natural product library, identifying leelamine (1A, left panel). Abietic acid, structurally similar to leelamine had no effect on melanoma cell viability (1A, right panel). 1B. Schematic of Nanolipolee-007. 1C. Size and charge of Nanolipolee-007 was 70-80 nm in size with a neutral surface charge in saline. The particle size and charge characteristics were established using a Malvern Zetasizer. 1D. Efficacy of Nanolipolee-007 for killing normal and melanoma cells. Nanolipolee-007 was 5.69-fold more effective at killing metastatic melanoma than normal cells, suggesting potential cancer therapeutic utility at concentrations <2.26 μmol/L (P < 0.01, one-way analysis of variance). Data represents an average of at least 3 independent experiments.
Use of Leelamine in animals or humans is limited by its poor bioavailability and insolubility in saline (Supplemental Figure S1). It had previously been dissolved in DMSO for use in animals, which is not useful for clinical applications requiring intravenous administration (25, 26). Intravenously administrated DMSO is lethal to mice necessitating the development of a clinically viable formulation (27). Toxicity limiting the potential clinical utility of free leelamine in DMSO, but not liposomal leelamine, was corroborated in animal studies (Supplemental Table S1). Free leelamine at 30 mg/Kg body weight led to death of all animals within 1 hour of treatment, where as liposomal leelamine at this concentration exhibited no mortality. Due to the toxicity associated with leelamine, a clinical viable delivery system was developed. A novel PEGylated neutral leelamine loaded liposomal system (80:20 mol % of ePC: DPPE PEG-2000) called Nanolipolee-007 was developed (Fig. 1B), which was based on the size, zeta potential, efficacy, stability and loading efficiency leelamine (Supplemental Table S2). The same lipid formulation also loaded abietic acid. An empty nanoliposome served as a vehicle control.
Nanolipolee-007, the control abietic acid nanoliposome, and the empty nanoliposome were 70-80 nm in size with a surface charge close to zero in saline (Fig. 1C). Nanolipolee-007 retained the growth inhibitory activity of leelamine dissolved in DMSO as evidenced by decreased viability of melanoma cells in culture, killing these cells 5.69-fold more effectively than that normal cells (Fig. 1D). The average IC50 of Nanolipolee-007 for killing normal FOM103 human melanocytes and FF2441 human fibroblast cells was 12.86 μmol/L compared to 2.26 μmol/L for melanoma cells. This was a concentration similar to the killing efficacy previously found for the leelamine dissolved in DMSO reported in the article by Gowda et al (12). Furthermore, the efficacy of Nanolipolee-007 for wild type B-RAF containing melanoma cell lines SbCl2, C8161.Cl9 and MelJuSo was examined and found to range from 7-8 μmol/L compared to cell lines containing V600EB-RAF (SK-Mel-24, 1205 Lu, UACC 903), having 2-2.3 μmol/L (Supplemental Table S3).
Nanolipolee-007 stably loaded leelamine
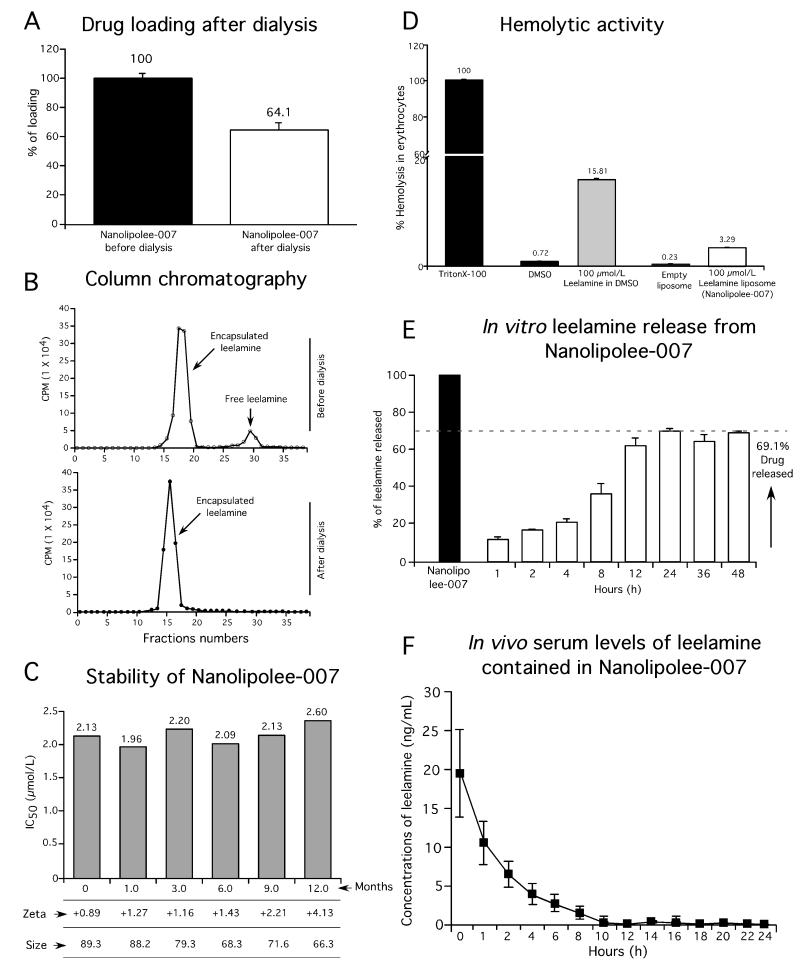
Parameters to examine the stability of Nanolipolee-007 such as size, charge, and melanoma cell killing efficacy were analyzed for multiple batches (28). Loading of leelamine into the nanoliposomal formulation was assessed using tritium labeled drug during the manufacture of Nanolipolee-007, followed by dialysis to remove free compound. Tritium labeled leelamine was synthesized by bromination, followed by replacing the bromine atom with tritium with a specific activity of 25 Ci/mmol and purity of the compound was determined by HPLC (Supplemental Figure S2A). Biological efficacy of tritiated leelamine for killing melanoma cells was compared to leelamine by MTS assay (Supplemental Figure S2B). Size exclusion chromatography was used to measure removal of loosely bound leelamine from that contained more tightly in the nanoliposome. Leelamine loading after dialysis showed that 64.1% of the drug was incorporated into the nanoliposomal formulation (Fig. 2A). Column chromatography of samples prior to and after dialysis, showed the disappearance of the loosely bound leelamine after dialysis (Fig. 2B). Loading efficiency was also measured by UV-visible spectrophotometry following centrifugation using 10 kDa Centricon filters to remove free drugs from the nanoparticle. The loading efficiency of leelamine was found to be 70.6% (Supplemental Figure S3). Stability of Nanolipolee-007 stored in sterile saline at 4°C was measured at 1, 3, 6, 9 and 12 months, and size, charge as well as efficacy for killing UACC 903 melanoma cells compared (Fig. 2C). During this period, the nanoliposomes retained similar size and charge distributions as well as efficacy for killing UACC 903 melanoma cells with an IC50 ranging from 1.96-2.60 μmol/L (Fig. 2C). No aggregation or precipitation of the nanoparticles occurred during this period.
Figure 2. Physicochemical characterization of Nanolipolee-007.
2A. Leelamine loading into Nanolipolee-007. Loading was measured using tritium labeled leelamine when making Nanolipolee-007, showing 64.1% loading. Data represents an average of at least 3 independent experiments. 2B. Characterization of loosely and tightly bound leelamine in Nanolipolee-007. Both dialysis and size exclusion chromatography based approaches were used to measure drug loading efficiency. The amount of tritium labeled leelamine remaining in Nanolipolee-007 was measured. Data represents an average of at least 3 independent experiments. 2C. Long-term stability of Nanolipolee-007. Nanolipolee-007 was stable in saline stored at 4°C for a year; maintaining its activity for killing UACC 903 cells with an IC50 of 1.96-2.60 μmol/L. 2D. Hemolytic activity of Nanolipolee-007 compared to leelamine. Free leelamine in DMSO induced significant hemolysis (15.81%) compared to liposomal leelamine (3.29%). Hemoglobin released in the presence of Trition X-100 served as a control for 100% lysis. Percent of hemolysis was calculated as: absorbance of the samples at 540nm/absorbance of the positive control×100. Data represents an average of at least 3 independent experiments. 2E. Leelamine release from Nanolipolee-007. The in vitro release of tritium labeled leelamine from Nanolipolee-007 was carried out at room temperature using molecular weight cut off 25 kDa dialysis membrane. 69.1% leelamine was released from Nanolipolee-007 within 24 hours. Data represents an average of at least 3 independent experiments. 2F. Leelamine presence in the serum of mice following intravenous administration. Leelamine present in Nanolipolee-007 remains in the circulation >10 hours after intravenous administration. Data represents an average of at least 3 independent experiments.
Nanolipolee-007 decreased the hemolytic activity of leelamine
Liposomal formulations of a hydrophobic drug can overcome solubility and hemolysis occurring with the free drug (29). A hemolytic assay was performed to examine whether Nanolipolee-007 caused the same level of red blood cell lysis occurring with leelamine. Leelamine in DMSO induced 15.81% hemolysis, which is significant compared to 3.29% occurring with Nanolipolee-007 (29).
Leelamine released from Nanolipollee-007 was present in the serum of animals
Leelamine release from Nanolipolee-007 was measured in vitro by dialysis in 0.9% saline for 24 hours, which showed that approximately 69.1% of leelamine was released. Release initially occurred slowly for the first four hours and reached a maximum level 12 hours later (Fig. 2E). Leelamine release was also examined with 10 mM glutathione, leading to similar results, suggesting that unencapsulated drug is not observed at the concentrations released from the individual nanoliposomes (Supplemental Figure 4). Next, presence of leelamine in the serum of mice was measured following intravenous injection of 30 mg/kg body weight of Nanolipolee-007 and serum analyzed for the presence of the drug over a 24 hour period by LC-MS/MS. Leelamine contained in Nanolipolee-007 was present in the serum of mice for >10 hours after intravenous administration (Fig. 2F).
Nanolipolee-007 inhibited melanoma tumor development with negligible major organ related toxicity
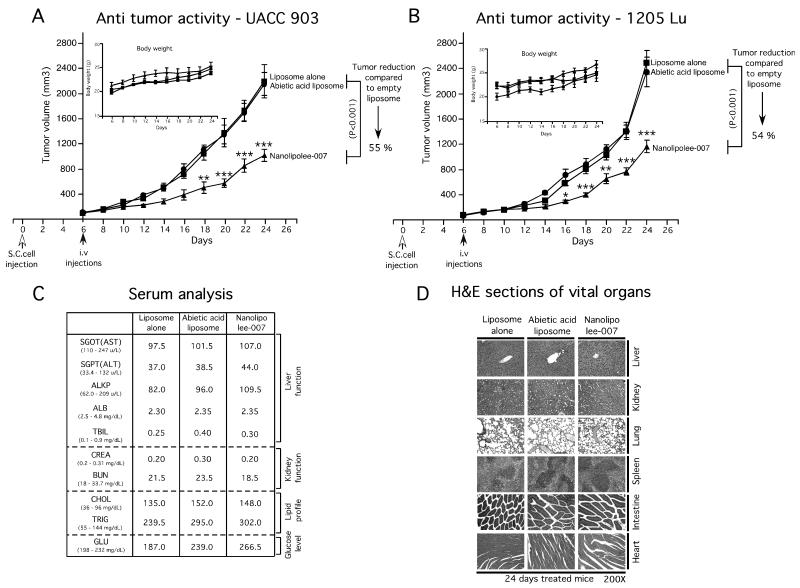
The study by Gowda et al (12), showed that intraperitoneal injection of leelamine dissolved in DMSO at 7.5 mg/kg body weight retarded existing xenograft melanoma tumor growth by up to 60%. To determine whether intravenously administered Nanolipolee-007 would function in a similar manner, mice were injected subcutaneously with 1.5 million UACC 903 or 1205 Lu melanoma cells and tumors let develop for 6-days at which time fully vascularized tumors had formed. Animals were then treated by daily intravenous injections of 30 mg/kg body weight of Nanolipolee-007 and compared to controls. Nanolipolee-007 decreased tumor volume by approximately 55% for UACC 903 (Fig. 3A) and 1205 Lu (Fig. 3B) cells compared to controls. Nanolipolee-007 treated animals did not show any significant changes in body weight, suggesting negligible toxicity was observed (Figs. 3A & 3B; inset). No noticeable changes in the serum parameters indicative of vital organ toxicity was observed (Fig. 3C), but an increase in cholesterol and triglyceride levels was seem following nanoliposomal treatment after 24 days. This is expected following daily administration of lipid-based nanoparticles. However, analysis of H&E stained liver from vehicle or Nanolipolee-007 treated mice showed no changes in the morphology or histological architecture of the organ (Fig. 3D). Furthermore, no changes were detected in histological sections from heart, lung, kidney or spleen (Fig. 3D). These data demonstrated that Nanolipolee-007 effectively inhibited xenografted melanoma tumor development leading to tumor regression without significant organ related toxicity other than increased lipid levels that could be controlled pharmacologically or by administering the agent on alternate days.
Figure 3. Nanolipolee-007 inhibits melanoma tumor development.
3A & 3B. Effect of Nanolipolee-007 on melanoma tumor development. Animals with pre-existing tumors were treated with i.v. injections of Nanolipolee-007 at a daily dose of 30 mg/Kg body weight. Empty or abietic acid nanoliposomes were used as controls. Nanolipolee-007 significantly decreased tumor volume by 55% and 54% for UACC 903 (3A) and 1205 Lu (3B) cells respectively, compared to vehicle treated animals. Nanolipolee-007 treated animals did not show any significant changes in body weight, suggesting negligible toxicity (3A & 3B; inset). 3C. Analysis of blood parameters showed no indications of organ related toxicity following systemic administration of Nanolipolee-007 following 24 days of treatment. Increased lipid levels were observed consistent with administration of a nanoliposomal based drug. 3D. Nanolipolee-007 treatment did not alter the histological structure of liver, kidney, lung, spleen or heart.
In vivo mechanistic study of Nanolipolee-007 in size and time match xenograft tumors
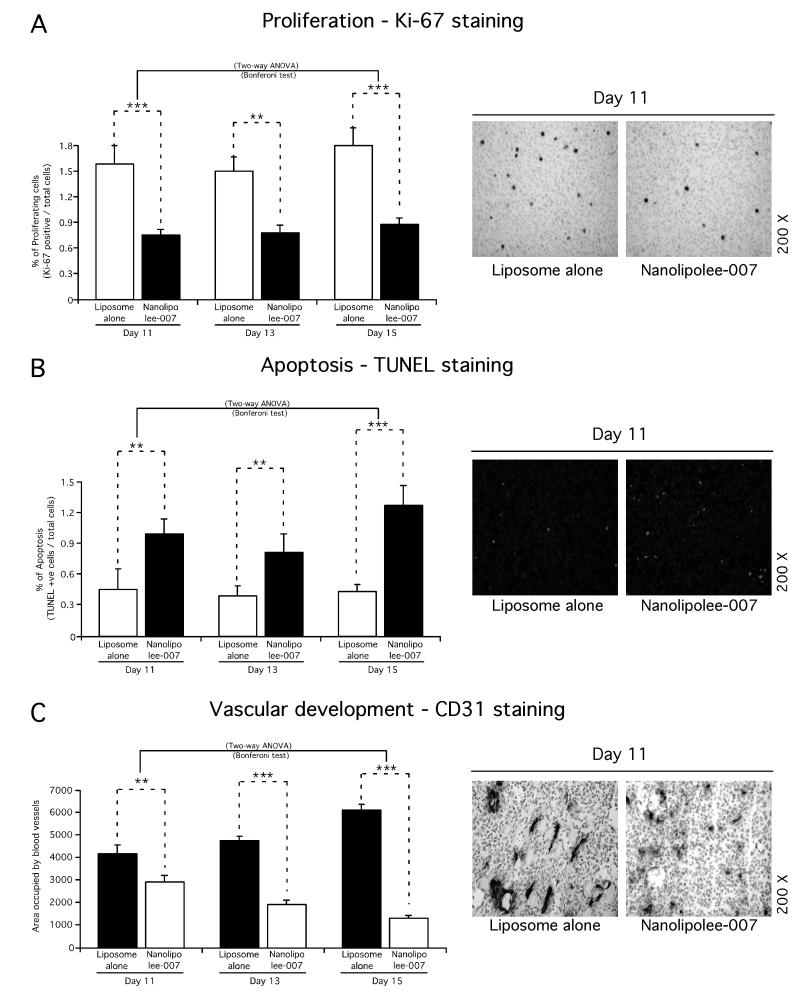
To investigate the mechanism by which Nanolipolee-007 delayed tumor growth, the rates of cell proliferation, apoptosis and tumor angiogenesis occurring in time and size matched xenograft tumors treated with Nanolipolee-007 were compared to empty control nanoliposome treated animals (22, 23). Size and time matched tumors at days 11, 13 and 15 were compared to identify statistically quantifiable differences in cell proliferation, apoptosis or vascular development affected by Nanolipolee-007 treatment. At day 11, a statistically significant 60% reduction in proliferating cells was observed as well as an number of cells undergoing apoptosis compared with control treated animals (Figs. 4A and 4B). Furthermore, differences in vascular development were also detected in all tumors compared with vehicle controls. Thus, intravenously developed Nanolipolee-007 at 30 mg/kg inhibited melanoma tumor development by decreasing proliferation, triggering apoptosis and decreasing vascular development (Fig. 4A, 4B and 4C).
Figure 4. Mechanistic basis for tumor inhibition mediated by Nanolipolee-007.
Formalin-fixed paraffin-embedded size and time matched tumors sections were subjected to Ki-67 staining for cell proliferation (4A), TUNEL staining for apoptosis (4B), CD31 staining for tumor angiogenesis (4C), and compared to empty nanoliposome treated animals. From day 11, a statistically significant decrease in proliferating tumor cells, increase in number of cells undergoing apoptosis and reduction in vascular development were identified compared with vehicle treated animals (4A, 4B and 4C).
Nanolipolee-007 decreased cellular proliferation, triggered apoptosis and arrested melanoma cells in the G0/G1 phase of the cell cycle
To further unravel the mechanisms leading to cell growth inhibition after treatment of mice with Nanolipolee-007, the rates of cellular proliferation, apoptosis, and the percentage of cells in the various phases of the cell cycle were measured. Increasing concentrations of Nanolipolee-007 from 0.62 to 10 μmol/L decreased the cellular proliferative potential as measured by BrdU incorporation (Fig. 5A) and increased cellular apoptosis measured by caspase-3/7 activity (Fig. 5B). Cell cycle analysis of propidium iodide stained UACC 903 and 1205 Lu cells following 24 hours Nanolipolee-007 treatment showed increases in the sub-G0/G1 and G0/G1 cell populations, with a corresponding decrease in the S-phase population (Fig. 5C). Thus, Nanolipolee-007 reduced melanoma cell survival by decreasing proliferation and triggering apoptosis mediated through a G0/G1 block resulting in fewer cells in the S-phase population of the cell cycle.
Figure 5. Nanolipolee-007 decreased cellular proliferation and increased apoptosis by arresting melanoma cells in the G0/G1 phase of the cell cycle.
5A & 5B. Cell Proliferation and apoptosis rates following treatment of cultured cells with Nanolipolee-007. Cellular proliferation and apoptosis rates were measured for UACC 903 and 1205 Lu melanoma cells following treatment with 0.62 to 100 μmol/L of nanoliposome alone or abietic acid nanoliposome or Nanolipolee-007 for 24 hours. Data represent averages of at least 3 independent experiments; bars; S.E.M. 5C. Cell cycle analysis of cultured cells treated with Nanolipolee-007. Cell cycle distribution was measured by analyzing propidium iodide stained UACC 903 and 1205 Lu cells. Nanolipolee-007 treatment elevated the sub-G0/G1 and G0/G1 cell populations. Data represents an average of at least 2 independent experiments.
Nanolipolee-007 inhibited the activity of three driver pathways promoting melanoma development
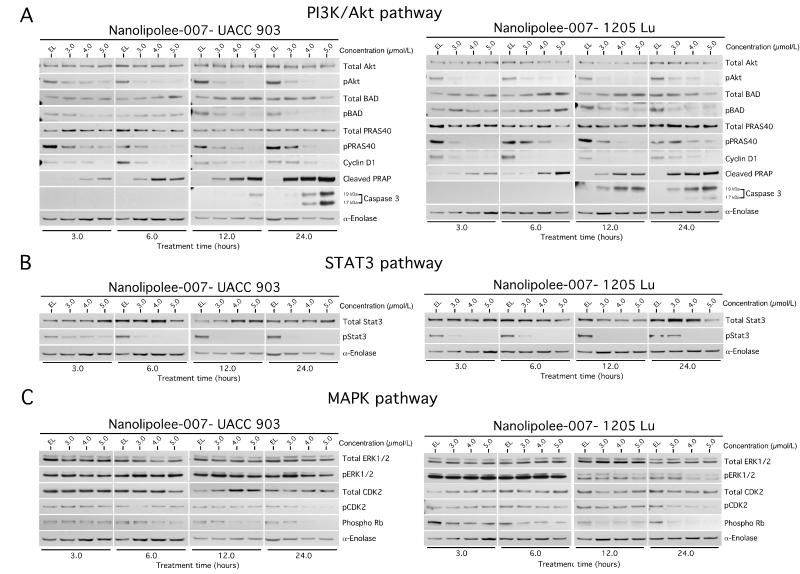
Pathways targeted by leelamine in melanoma cells were previously identified and detailed in the article by Gowda et al & Kuzu OF et al, using a Kinexus antibody microarray and Ingenuity Pathway Analysis (IPA) followed by validation using Western blotting (12, 13). Nanolipolee-007 decreased the activity of the PI3K/Akt, STAT3 and MAPK pathways, which are major signaling cascades promoting melanoma development (14-16). Decreased signaling through both UACC 903 and 1205 Lu melanoma cells following treatment with 3 to 6 μmol/L of Nanolipolee-007 for 3 to 24 hours is shown for PI3K/Akt (Fig. 6A) and STAT3 (Fig. 6B) pathways. Nanolipolee-007 significantly inhibited Akt phosphorylation without affecting total Akt protein levels in a dose and time dependent manner (Fig. 6A). In addition, Nanolipolee-007 inhibited the phosphorylation of other downstream signaling components of the cascade regulating cell survival. It also decreased expression of cyclin D1 and increased cleaved caspase-3 and PARP protein levels at later time points (Fig. 6A). STAT3 is also constitutively activated in melanomas and contributes to tumor cell growth, proliferation, metastasis and angiogenesis in various cancers (30, 31). Nanolipolee-007 inhibited phosphorylation of STAT3 without affecting total STAT3 protein levels (Fig. 6B). Nanolipolee-007 also had a minor effect on the MAP kinase pathway (Fig. 6C), which was likely due to its unique mechanism of action and is detailed in the article by Kuzu OF et al (13), which describes the molecular mechanism leading to this effect.
Figure 6. Nanolipolee-007 inhibits key signaling pathways regulating melanoma development.
6A, 6B & 6C. Western blot analysis of cultured cells treated with Nanolipolee-007. UACC 903 and 1205 Lu melanoma cells were treated with 3 to 6 μmol/L of Nanolipolee-007 for 3 to 24 hours and cell lysates analyzed to determine the expression as well as activity of PI3 kinase (6A), STAT3 (6B) and MAP kinase pathways (6C). Alpha-enolase served as a control for equal protein loading.
DISCUSSION
Melanoma is the most deadly type of skin cancer diagnosed in the United States (32). Incidence and mortality rates continue to increase annually and it remains one of the most invasive as well as drug resistant cancer types (33). Although Zelboraf has been approved by the FDA for the treatment of malignant melanomas harboring a V600EB-Raf mutation, development of drug resistance are raising concerns regarding the utility of Zelboraf as a single agent therapy for malignant melanoma (34). Moreover, Zelboraf is effective only in ~50% of melanoma patients having mutant V600EB-Raf, leaving the other 50% in need of other treatment modalities (34). Therefore, agents targeting multiple driver pathways in melanoma patients leading to lower rates of resistance development are needed as well as drugs for treating those patients lacking the V600EB-Raf mutation (35).
Natural products have played an important role in the development of promising new anticancer drugs and currently constitute over 60% of cancer therapeutics in the clinic (36, 37). Leelamine was identified from a natural product library screen, for inhibiting melanoma cells growth but intravenous administration was limited by hemolysis and animal death. Liposomal formulation of drugs such as leelamine can be used to moderate these concerns (29). Leelamine in DMSO induced 15.81% hemolysis, which is considered high, compared to 3.29% observed with Nanolipolee-007. The difference might be due to the rigidity of the liposome and electrostatic repulsion of anionic RBCs (29). Rigid molecules such as DPPE PEG-2000 are less prone to attach to the RBC membrane compared to flexible molecules (29).
Nanotechnology can be used to improve solubility, pharmacokinetics and reduce side effects associated with various drugs (38, 39). Among different nanoparticles, liposomes are well-studied colloidal particles delivering drugs to tumors and increasing the solubility of amphiphilic agents (40, 41). Liposomes less than 100 nm can enter tumors due to the leaky vasculature, which does not occur in the normal vasculature due to the EPR effect (4). Nanolipolee-007 fulfills all these criteria having an average size of 70-80 nm and a neutral charge. The most common surface modification of nanoparticles is PEGylation, in which polyethylene glycol is covalently linked through lipids to the surface of the liposome (42). PEGylated liposomes tend to be stable, have enhanced circulation time, avoid clearance by the reticulo-endothelial system, and have minimal toxicity (43). In this report, leelamine was loaded into a PEGylated neutral liposomal formulation that was stable at 4°C for a year and had an increased circulating half-life promoting accumulation at the tumor site.
In clinical studies, liposomes have improved the pharmacokinetics and bio-distribution properties of therapeutic agents as well as an ability to reduce toxicity by accumulation in tumors due to the EPR effect (44). Currently, there are twelve liposome-based drugs approved for clinical use and others are in various stages of clinical development (3, 5, 11). For example, PEGylated liposomal formulations of doxorubicin such as Doxil and Lipo-dox are approved for intravenous application with minor dose-limiting toxicity compared to doxorubicin (45, 46). Nab-paclitaxel, a nanoparticle formulation of paclitaxel, has also demonstrated higher therapeutic efficacy against breast cancer than paclitaxel and several other nab-based chemotherapeutics are currently under clinical evaluation (47, 48).
Mechanism of leelamine mediated cell death has been investigated and reported in the manuscript by Kuzu OF et al (13). Briefly, leelamine is a lysosomotropic compound accumulating inside acidic cell compartments such as lysosomes and endosomes. Accumulation leads to disruption of intracellular cholesterol homeostasis and interferes with autophagic flux as well as receptor-mediated endocytosis. Inhibition of receptor-mediated endocytosis shut downs receptor tyrosine kinase (RTK) signaling and inhibits the activation of downstream PI3K/Akt, STAT3 and MAPK signaling cascades. B-Raf mutation is not able to trigger melanoma development alone and requires cooperation with other cellular alterations in RTK signaling such as the Akt pathway (49) and leelamine has the potential to target these pathways as well.
Nanolipolee-007 inhibited the phosphorylation of Akt without affecting total Akt protein levels in a dose and time dependent manner. This in turn decreased downstream levels of active PRAS40 and BAD proteins. In addition, decreased the expression of proliferation marker cyclin D1 as well as increasing levels of apoptosis markers cleaved caspase-3 and PARP proteins (14). Furthermore, Nanolipolee-007 inhibited Stat3 signaling in melanomas. Targeted inhibition of Stat3 is known to retard melanoma development and others studies also demonstrated that targeting Stat3 in conjunction with Akt synergistically inhibits melanomas (16, 50).
In conclusion, Nanolipolee-007 retained the tumor inhibitory activity of leelamine dissolved in DMSO and improved the solubility of the drug with negligible toxicity in mice, suggesting its potential as a therapeutic agent for the treatment of melanoma or other cancers in which the PI3/Akt kinase, and STAT3, and to a lesser extent MAP kinase pathways are deregulated.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Katie Huber for technical assistance.
Grant support. NIH grants R01 CA-136667-02, RO1 CA-1138634-02, RO1 CA-127892-01A (GP, Robertson), The Foreman Foundation for Melanoma Research (GP Robertson) and H.G. Barsumian, M.D. Memorial Fund (A Sharma).
Footnotes
Conflict of interest: G. Robertson, R. Gowda, O. Kuzu, and S. Madhunapantula on behalf of Penn State, have patent protected this discovery, Nanolipolee-007, patent number 8,785,502, which has subsequently been licensed to Melanovus Oncology. Melanovus Oncology is partly owned by Penn State University and Gavin P. Robertson, who is also CSO of the company.
REFERENCES
- 1.Bagrodia S, Smeal T, Abraham RT. Mechanisms of intrinsic and acquired resistance to kinase-targeted therapies. Pigment cell & melanoma research. 2012;25:819–31. doi: 10.1111/pcmr.12007. [DOI] [PubMed] [Google Scholar]
- 2.Solit DB, Rosen N. Resistance to BRAF inhibition in melanomas. N Engl J Med. 2011;364:772–4. doi: 10.1056/NEJMcibr1013704. [DOI] [PubMed] [Google Scholar]
- 3.Schroeder A, Heller DA, Winslow MM, Dahlman JE, Pratt GW, Langer R, et al. Treating metastatic cancer with nanotechnology. Nat Rev Cancer. 2011;12:39–50. doi: 10.1038/nrc3180. [DOI] [PubMed] [Google Scholar]
- 4.Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov. 2010;9:615–27. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 5.Thierry B. Drug nanocarriers and functional nanoparticles: applications in cancer therapy. Curr Drug Deliv. 2009;6:391–403. doi: 10.2174/156720109789000474. [DOI] [PubMed] [Google Scholar]
- 6.Drummond DC, Meyer O, Hong K, Kirpotin DB, Papahadjopoulos D. Optimizing liposomes for delivery of chemotherapeutic agents to solid tumors. Pharmacological reviews. 1999;51:691–743. [PubMed] [Google Scholar]
- 7.Maeda H, Nakamura H, Fang J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Advanced drug delivery reviews. 2013;65:71–9. doi: 10.1016/j.addr.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–60. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 9.Immordino ML, Dosio F, Cattel L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomedicine. 2006;1:297–315. [PMC free article] [PubMed] [Google Scholar]
- 10.Szostak JW, Bartel DP, Luisi PL. Synthesizing life. Nature. 2001;409:387–90. doi: 10.1038/35053176. [DOI] [PubMed] [Google Scholar]
- 11.Zhao W, Karp JM, Ferrari M, Serda R. Bioengineering nanotechnology: towards the clinic. Nanotechnology. 2012;22:490201. doi: 10.1088/0957-4484/22/49/490201. [DOI] [PubMed] [Google Scholar]
- 12.Gowda R, Madhanupantula SVD, Kuzu OF, Sharma A, Robertson GPD. Targeting Multiple Key Signaling Pathways in Melanoma using Leelamine. Mol Cancer Ther. 2014 doi: 10.1158/1535-7163.MCT-13-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuzu OF, Gowda R, Sharma A, Robertson GPD. Leelamine mediates cancer cell death through inhibition of intracellular cholesterol transport. Mol Cancer Ther. 2014 doi: 10.1158/1535-7163.MCT-13-0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gowda R, Madhunapantula SV, Desai D, Amin S, Robertson GP. Simultaneous targeting of COX-2 and AKT using selenocoxib-1-GSH to inhibit melanoma. Mol Cancer Ther. 2012;12:3–15. doi: 10.1158/1535-7163.MCT-12-0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madhunapantula SV, Robertson GP. Is B-Raf a good therapeutic target for melanoma and other malignancies? Cancer Res. 2008;68:5–8. doi: 10.1158/0008-5472.CAN-07-2038. [DOI] [PubMed] [Google Scholar]
- 16.Kortylewski M, Jove R, Yu H. Targeting STAT3 affects melanoma on multiple fronts. Cancer Metastasis Rev. 2005;24:315–27. doi: 10.1007/s10555-005-1580-1. [DOI] [PubMed] [Google Scholar]
- 17.Ruysschaert T, Marque A, Duteyrat JL, Lesieur S, Winterhalter M, Fournier D. Liposome retention in size exclusion chromatography. BMC Biotechnol. 2005;5:11. doi: 10.1186/1472-6750-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng S, Chang S, Lu J, Chen Z, Xie L, Nie Y, et al. Characterization of 9-nitrocamptothecin liposomes: anticancer properties and mechanisms on hepatocellular carcinoma in vitro and in vivo. PloS one. 2011;6:e21064. doi: 10.1371/journal.pone.0021064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nie Y, Ji L, Ding H, Xie L, Li L, He B, et al. Cholesterol derivatives based charged liposomes for doxorubicin delivery: preparation, in vitro and in vivo characterization. Theranostics. 2012;2:1092–103. doi: 10.7150/thno.4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song M, Lee D, Lee T, Lee S. Determination of leelamine in mouse plasma by LC-MS/MS and its pharmacokinetics. Journal of chromatography B, Analytical technologies in the biomedical and life sciences. 2013;931:170–3. doi: 10.1016/j.jchromb.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 21.Madhunapantula SV, Hengst J, Gowda R, Fox TE, Yun JK, Robertson GP. Targeting sphingosine kinase-1 to inhibit melanoma. Pigment cell & melanoma research. 2013;25:259–74. doi: 10.1111/j.1755-148X.2012.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma A, Tran MA, Liang S, Sharma AK, Amin S, Smith CD, et al. Targeting mitogen-activated protein kinase/extracellular signal-regulated kinase kinase in the mutant (V600E) B-Raf signaling cascade effectively inhibits melanoma lung metastases. Cancer Res. 2006;66:8200–9. doi: 10.1158/0008-5472.CAN-06-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stahl JM, Cheung M, Sharma A, Trivedi NR, Shanmugam S, Robertson GP. Loss of PTEN promotes tumor development in malignant melanoma. Cancer Res. 2003;63:2881–90. [PubMed] [Google Scholar]
- 24.Sharma A, Sharma AK, Madhunapantula SV, Desai D, Huh SJ, Mosca P, et al. Targeting Akt3 signaling in malignant melanoma using isoselenocyanates. Clin Cancer Res. 2009;15:1674–85. doi: 10.1158/1078-0432.CCR-08-2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montaguti P, Melloni E, Cavalletti E. Acute intravenous toxicity of dimethyl sulfoxide, polyethylene glycol 400, dimethylformamide, absolute ethanol, and benzyl alcohol in inbred mouse strains. Arzneimittel-Forschung. 1994;44:566–70. [PubMed] [Google Scholar]
- 26.Sharma A, Mayhew E, Bolcsak L, Cavanaugh C, Harmon P, Janoff A, et al. Activity of paclitaxel liposome formulations against human ovarian tumor xenografts. International journal of cancer Journal international du cancer. 1997;71:103–7. doi: 10.1002/(sici)1097-0215(19970328)71:1<103::aid-ijc17>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 27.DiStefano V, Klahn JJ. Observations on the pharmacology and hemolytic activity of dimethyl sulfoxide. Toxicology and applied pharmacology. 1965;7:660–6. doi: 10.1016/0041-008x(65)90122-5. [DOI] [PubMed] [Google Scholar]
- 28.Tran MA, Gowda R, Sharma A, Park EJ, Adair J, Kester M, et al. Targeting V600EB-Raf and Akt3 using nanoliposomal-small interfering RNA inhibits cutaneous melanocytic lesion development. Cancer Res. 2008;68:7638–49. doi: 10.1158/0008-5472.CAN-07-6614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mourtas S, Michanetzis GP, Missirlis YF, Antimisiaris SG. Haemolytic activity of liposomes: effect of vesicle size, lipid concentration and polyethylene glycol-lipid or arsonolipid incorporation. Journal of biomedical nanotechnology. 2009;5:409–15. doi: 10.1166/jbn.2009.1050. [DOI] [PubMed] [Google Scholar]
- 30.Jackson DP, Watling D, Rogers NC, Banks RE, Kerr IM, Selby PJ, et al. The JAK/STAT pathway is not sufficient to sustain the antiproliferative response in an interferon-resistant human melanoma cell line. Melanoma Res. 2003;13:219–29. doi: 10.1097/00008390-200306000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Liu F, Cao J, Wu J, Sullivan K, Shen J, Ryu B, et al. Stat3-Targeted Therapies Overcome the Acquired Resistance to Vemurafenib in Melanomas. J Invest Dermatol. 2013 doi: 10.1038/jid.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roth BJ, Krilov L, Adams S, Aghajanian CA, Bach P, Braiteh F, et al. Clinical cancer advances 2012: annual report on progress against cancer from the american society of clinical oncology. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2013;31:131–61. doi: 10.1200/JCO.2012.47.1938. [DOI] [PubMed] [Google Scholar]
- 33.Villanueva J, Vultur A, Herlyn M. Resistance to BRAF inhibitors: unraveling mechanisms and future treatment options. Cancer Res. 2011;71:7137–40. doi: 10.1158/0008-5472.CAN-11-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sullivan RJ, Flaherty KT. Resistance to BRAF-targeted therapy in melanoma. European journal of cancer. 2013;49:1297–304. doi: 10.1016/j.ejca.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 35.Tsao H, Chin L, Garraway LA, Fisher DE. Melanoma: from mutations to medicine. Genes & development. 2012;26:1131–55. doi: 10.1101/gad.191999.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007;70:461–77. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 37.Altmann KH, Gertsch J. Anticancer drugs from nature--natural products as a unique source of new microtubule-stabilizing agents. Nat Prod Rep. 2007;24:327–57. doi: 10.1039/b515619j. [DOI] [PubMed] [Google Scholar]
- 38.Cho K, Wang X, Nie S, Chen ZG, Shin DM. Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res. 2008;14:1310–6. doi: 10.1158/1078-0432.CCR-07-1441. [DOI] [PubMed] [Google Scholar]
- 39.Wang J, Sui M, Fan W. Nanoparticles for tumor targeted therapies and their pharmacokinetics. Curr Drug Metab. 2010;11:129–41. doi: 10.2174/138920010791110827. [DOI] [PubMed] [Google Scholar]
- 40.Yezhelyev MV, Gao X, Xing Y, Al-Hajj A, Nie S, O’Regan RM. Emerging use of nanoparticles in diagnosis and treatment of breast cancer. Lancet Oncol. 2006;7:657–67. doi: 10.1016/S1470-2045(06)70793-8. [DOI] [PubMed] [Google Scholar]
- 41.van Vlerken LE, Amiji MM. Multi-functional polymeric nanoparticles for tumour-targeted drug delivery. Expert Opin Drug Deliv. 2006;3:205–16. doi: 10.1517/17425247.3.2.205. [DOI] [PubMed] [Google Scholar]
- 42.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. Journal of controlled release: official journal of the Controlled Release Society. 2000;65:271–84. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 43.Jain A, Jain SK. PEGylation: an approach for drug delivery. A review. Crit Rev Ther Drug Carrier Syst. 2008;25:403–47. doi: 10.1615/critrevtherdrugcarriersyst.v25.i5.10. [DOI] [PubMed] [Google Scholar]
- 44.Tran MA, Watts RJ, Robertson GP. Use of liposomes as drug delivery vehicles for treatment of melanoma. Pigment cell & melanoma research. 2009;22:388–99. doi: 10.1111/j.1755-148X.2009.00581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poh SB, Bai LY, Chen PM. Pegylated liposomal doxorubicin-based combination chemotherapy as salvage treatment in patients with advanced hepatocellular carcinoma. Am J Clin Oncol. 2005;28:540–6. doi: 10.1097/01.coc.0000177911.36579.3d. [DOI] [PubMed] [Google Scholar]
- 46.Green AE, Rose PG. Pegylated liposomal doxorubicin in ovarian cancer. Int J Nanomedicine. 2006;1:229–39. [PMC free article] [PubMed] [Google Scholar]
- 47.Montero AJ, Adams B, Diaz-Montero CM, Gluck S. Nab-paclitaxel in the treatment of metastatic breast cancer: a comprehensive review. Expert Rev Clin Pharmacol. 2011;4:329–34. doi: 10.1586/ecp.11.7. [DOI] [PubMed] [Google Scholar]
- 48.Gradishar WJ. Albumin-bound paclitaxel: a next-generation taxane. Expert Opin Pharmacother. 2006;7:1041–53. doi: 10.1517/14656566.7.8.1041. [DOI] [PubMed] [Google Scholar]
- 49.Haluska F, Pemberton T, Ibrahim N, Kalinsky K. The RTK/RAS/BRAF/PI3K pathways in melanoma: biology, small molecule inhibitors, and potential applications. Seminars in oncology. 2007;34:546–54. doi: 10.1053/j.seminoncol.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 50.Krasilnikov M, Ivanov VN, Dong J, Ronai Z. ERK and PI3K negatively regulate STAT-transcriptional activities in human melanoma cells: implications towards sensitization to apoptosis. Oncogene. 2003;22:4092–101. doi: 10.1038/sj.onc.1206598. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.