Abstract
Psychostimulants are highly effective in the treatment of attention deficit hyperactivity disorder (ADHD). The clinical efficacy of these drugs is strongly linked to their ability to improve cognition dependent on the prefrontal cortex (PFC) and extended frontostriatal circuit. The procognitive actions of psychostimulants are only associated with low doses. Surprisingly, despite nearly 80 years of clinical use, the neurobiology of the procognitive actions of psychostimulants has only recently been systematically investigated. Findings from this research unambiguously demonstrate that the cognition-enhancing effects of psychostimulants involve the preferential elevation of catecholamines in the PFC and the subsequent activation of norepinephrine α2- and dopamine D1 receptors. In contrast, while the striatum is a critical participant in ‘PFC-dependent’ cognition, where examined, psychostimulant action within the striatum is not sufficient to enhance cognition. At doses that moderately exceed the clinical range, psychostimulants appear to improve PFC-dependent attentional processes at the expense of other PFC-dependent processes (e.g. working memory, response inhibition). This differential modulation of PFC-dependent processes across dose appears to be associated with the differential involvement of noradrenergic α2 vs. α1 receptors. Collectively, this evidence indicates that at low, clinically-relevant doses, psychostimulants are devoid of the behavioral and neurochemical actions that define this class of drugs and instead act largely as cognitive enhancers (improving PFC-dependent function). This information has potentially important clinical implications as well as relevance for public health policy regarding the widespread clinical use of psychostimulants and for the development of novel pharmacological treatments for ADHD and other conditions associated with PFC dysregulation.
Keywords: ADHD, Prefrontal Cortex, Cognition, Norepinephrine, Dopamine, Stimulants
Psychostimulants elicit potent arousing, behaviorally-activating and reinforcing actions that are associated with significant potential for abuse (1–6). Nonetheless, these drugs are highly effective in treating hyperactivity, impulsivity and inattention associated with attention deficit hyperactivity disorder (ADHD; 7–10). Given these apparently contradictory actions, it was initially proposed that psychostimulants acted ‘paradoxically’ in individuals with ADHD: calming, rather than activating behavior. This ‘paradoxical hypothesis’ strongly influenced clinical and basic science research into the neurobiology and pharmacology of ADHD.
A major breakthrough in our understanding of psychostimulant action was the demonstration in 1980 that the cognition-enhancing and behavioral-calming actions of psychostimulants are not unique to ADHD, with similar effects seen in healthy human subjects (11). This and subsequent studies unambiguously demonstrate that when used at low and clinically-relevant doses, psychostimulants improve prefrontal cortex (PFC)-dependent behavioral/cognitive processes in human subjects with and without ADHD (11–15). This ability of psychostimulants to act as cognitive enhancers drives the recent growth in use of these drugs by the general population to improve academic and work-related performance (16–18). Low-dose psychostimulant improvement in PFC-dependent function is consistent with evidence indicating ADHD involves dysregulation of the PFC and extended frontostriatal circuitry (19–22). Moreover, the procognitive actions of psychostimulants are also observed in ‘normal’ animal subjects (see Figure 1; 5,6,23,24), indicating an animal model of ADHD is not required to study the neural mechanisms underlying the cognition-enhancing/therapeutic effects of psychostimulants. This is an important advantage given there are no animal models known to definitively mimic the neurobiology of ADHD.
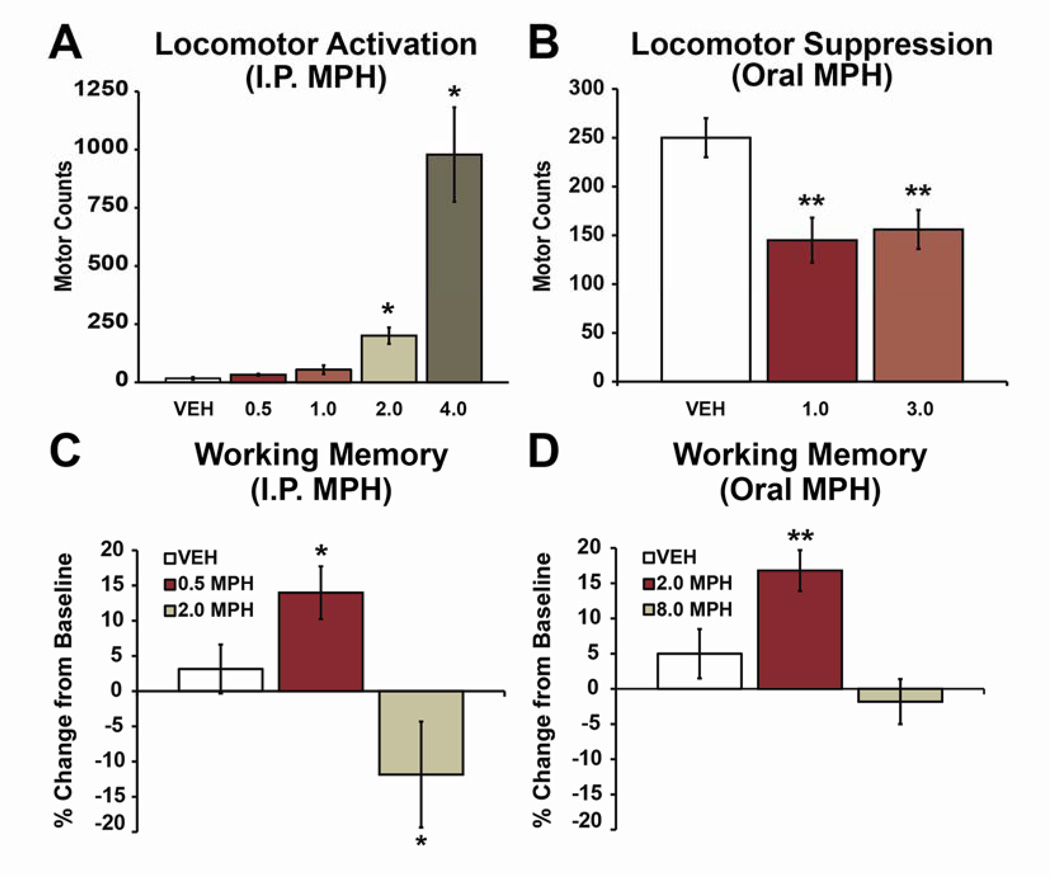
Fig 1. Behavioral-calming and cognition-enhancing actions of clinically-relevant doses of methylphenidate (MPH) in rats.
A: Lack of locomotor activation. Bars indicate motor counts (quadrant entries + rears) in the 90-minute period following subcutaneous treatment with vehicle (VEH) or varying doses of MPH (0.5, 1.0, 2.0 and 4.0 mg/kg) during the light (inactive) phase of the circadian cycle in rats (i.e. under low arousal conditions). Doses that produce clinically-relevant plasma concentrations (0.5, 1.0 mg/kg) do not elicit significant locomotor activation. Higher doses elicit dose-dependent increases in locomotor activity. The 4.0 mg/kg dose was peri-threshold for eliciting mild stereotypy. B: Motoric calming. When tested under conditions associated with elevated motor activity (during the dark/active phase) clinically relevant doses of oral MPH (1.0, 3.0 mg/kg) suppressed motor activity, similar to that seen in ADHD patients. C, D: Cognition enhancement. When administered in doses that elicit clinically relevant plasma concentrations, intraperitoneally (C) or orally (D) administered MPH improves working memory performance as measured by percent change from baseline performance in a delayed-response test of working memory (T-maze). 4-fold higher doses impair or do not improve performance. All bars indicate mean ± SEM. *P<0.05, **P <0.01 compared with vehicle-treated animals. Modified from (6,24,33).
Collectively these observations indicate that the neural mechanisms responsible for the therapeutic/cognition-enhancing effects of low-dose psychostimulants cannot be extrapolated from actions of higher doses that exert opposing behavioral and cognitive effects. Over the past 10 years, correlative evidence has suggested the hypothesis that the PFC is a key site in the cognition-enhancing actions of psychostimulants (for review, 25,26). More recent work provides critical, causal evidence for a role of the PFC in the cognition-enhancing effects of psychostimulants. In the following sections we review the collective body of research on the neurobiology of clinically-relevant and procognitive doses of psychostimulants.
Clinically-Relevant Doses of Psychostimulants Elevate Catecholamine Signaling Preferentially in the PFC
The two most commonly used psychostimulants in the treatment of ADHD are methylphenidate (MPH; e.g. Ritalin®) and amphetamine (e.g. Adderall®). At behaviorally-activating doses, these drugs potently increase extracellular levels of norepinephrine (NE) and dopamine (DA) throughout the brain, largely by blocking NE and DA reuptake (27,28). Some psychostimulants, particularly amphetamine, actively stimulate DA efflux through the DA transporter (29). Although amphetamine can also stimulate NE efflux and block serotonin reuptake, these actions only occur at high and clinically-inappropriate doses (30). In contrast, MPH acts only to block NE and DA reuptake, neither inhibiting serotonin reuptake nor stimulating NE or DA efflux (31).
Early animal studies demonstrated a central role of DA acting in the striatal subregion, the nucleus accumbens (NAcc), in the motor activating and reinforcing effects of higher doses of psychostimulants. Based on this, the early clinical literature emphasized the potential role of NAcc DA in the therapeutic effects of psychostimulants. However, given low, clinically-relevant doses of psychostimulants exert qualitatively different behavioral effects vs. higher, behaviorally-activating doses, the neurobiology of higher doses may not have strong translational relevance. However, in order to study the clinically-relevant actions of psychostimulants in animals, it is essential to identify doses that model clinical use. Pharmacokinetic studies identified doses of MPH in animals that elicit plasma concentrations associated with clinical efficacy in humans (~10–40 ng/ml; 23,24,32,33). In rats, clinically-relevant plasma levels are seen following 1.0–3.0 mg/kg MPH given orally and 0.25–1.0 mg/kg MPH administered intraperitoneally (24,32,33). At these doses, MPH is largely devoid of behaviorally-activating or arousing actions (Figure 1; 24,32,33). Moreover, under conditions associated with elevated locomotor activity, these clinically-relevant doses of MPH suppress motor activity, similar to that seen in ADHD (Figure 1; 33). Finally, these doses of MPH improve PFC-dependent cognition (working memory, sustained attention), similar to that seen in humans (Figure 1; 5,24,34). The behavioral/cognitive effects of low and clinically-relevant doses contrast with the motor activating, arousal-promoting, and cognition-impairing actions associated with abuse-related doses of psychostimulants.
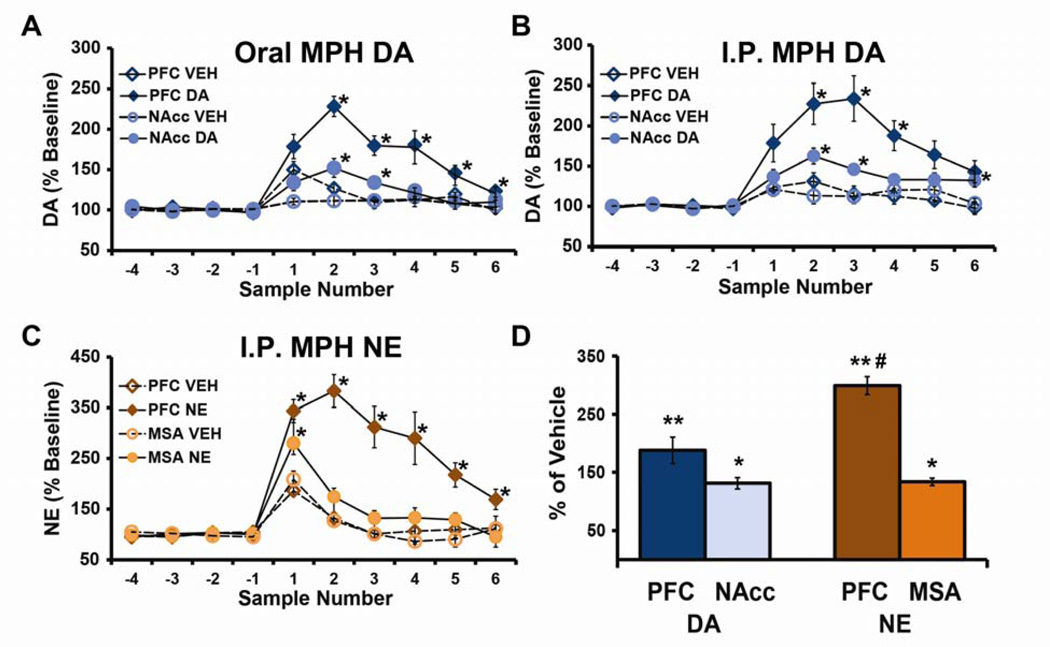
Microdialysis studies demonstrate that clinically relevant doses of MPH elicit prominent increases in extracellular NE and DA within the PFC (100–250%; Figure 2). In contrast, substantially smaller effects are observed on DA levels in the NAcc and NE levels in the medial septum, regions associated with psychostimulant-induced motor activation and arousal, respectively (see Figure 2; 24,32,33,35–39). As with the cognitive effects of MPH (6,24), the preferential targeting of PFC catecholamines is not dependent on route of administration, as long as dose is adjusted to yield similar plasma concentrations (24). These doses of MPH increase hippocampal and somatosensory cortical NE levels similar to that seen in the medial septal area (32,33,40), indicating the greater sensitivity of PFC catecholamines reflects a relatively unique aspect of PFC physiology (32,33,40).
Fig 2. Clinically relevant doses of methylphenidate (MPH) preferentially increase extracellular catecholamines within the prefrontal cortex (PFC).
A–C: Shown are the mean ± SEM catecholamine levels expressed as a percentage of baseline (±SEM) values in 16-minute microdialysis samples collected before (negative numbers) and after (positive numbers) injection of vehicle (VEH) or MPH. Clinically-relevant doses of MPH administered orally (A; 2.0 mg/kg) or intraperitoneally (B; 0.5 mg/kg) elicited large increases in extracellular dopamine (DA) within the PFC relative that that seen in the nucleus accumbens (NAcc). (C) Similarly, for norepinephrine (NE), intraperitoneal administration of 0.5 mg/kg MPH elicited a large increase in extracellular NE in the PFC with little change in the medial septal area (MSA). For both DA and NE, although clinically relevant doses of MPH elicit smaller effects outside the PFC, these effects are nonetheless statistically significant relative to both baseline and vehicle-treated animals. D: Bars represent effects of 0.5 mg/kg MPH intraperitoneally expressed as a % change from vehicle-treated animals (mean ±SEM) for DA and NE over a 30-minute period beginning at 15-minutes post-treatment. When measured in this manner, although MPH increases NE and DA outside the PFC by ~30% this effect was not significantly significant. *P<0.05, **P <0.01 compared with vehicle-treated animals. #P<0.01 relative to PFC DA. From (24).
The modest actions of low-dose psychostimulants on extracellular catecholamines in subcortical regions associated with motor activation (e.g. accumbens) and arousal (e.g. medial septum) appears to explain why, clinically, psychostimulants are devoid of the prototypical behavioral-activating and arousal-enhancing effects of psychostimulants. Moreover, the fact that low-dose psychostimulants do not increase, and may reduce, drug abuse in ADHD patients (41,42) is consistent with a relatively modest impact of clinically-relevant doses on neural circuitry posited to underlie drug abuse (e.g. NAcc DA; 39).
Potential Mechanisms Underlying the Preferential Sensitivity of PFC Catecholamines
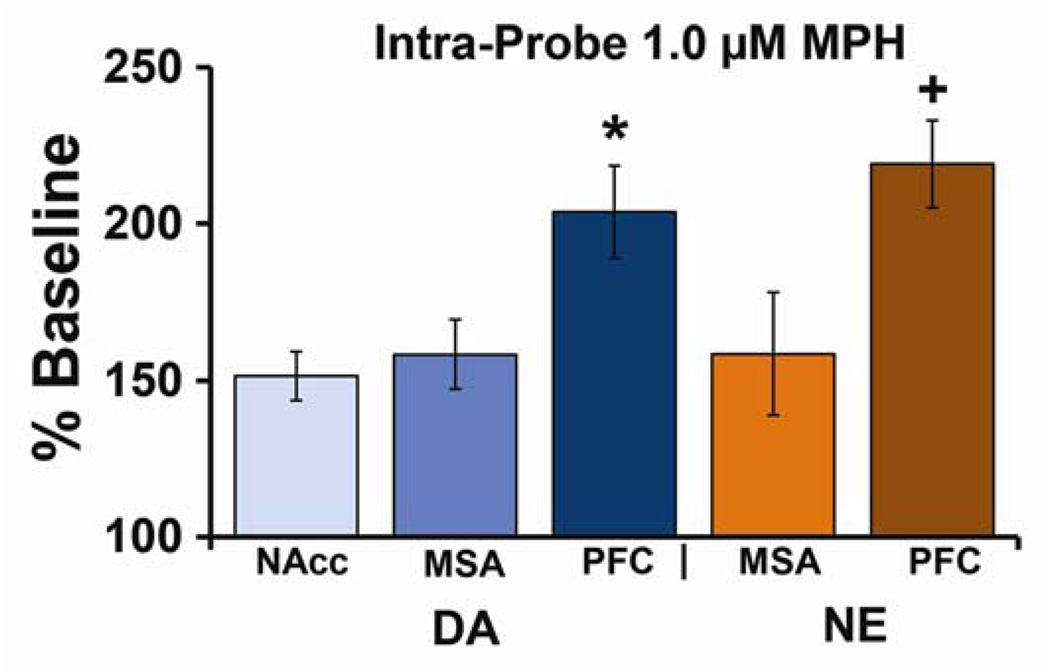
The above-described studies fail to identify the circuit mechanisms associated with the preferential sensitivity of PFC catecholamines. Recent studies using reverse microdialysis demonstrate that when infused in low concentrations, MPH elicits significantly larger increases in extracellular NE and DA in the PFC relative to the medial septum or NAcc (Figure 3; 43). This selectivity for the PFC disappeared with higher concentrations of MPH, similar to that seen with systemic administration (24,31). These observations demonstrate that the enhanced sensitivity of PFC catecholamines reflects, at least in part, an intrinsic property of the PFC.
Fig 3. Preferential sensitivity of PFC catecholamines involves local mechanisms.
Locally administered methylphenidate (MPH) increases dopamine (DA) and norepinephrine (NE) preferentially within the prefrontal cortex (PFC). Shown are the effects of local application of 1.0 µM MPH on extracellular levels of DA and NE within the PFC, nucleus accumbens (NAcc), and medial septal area (MSA). Data are an average (±SEM) of three 30-min samples following MPH application and are expressed as percent above baseline. At this concentration, MPH produced only modest increases in DA and NE levels (~50–60%) in subcortical regions (NAcc and MSA), while eliciting significantly larger increases in DA and NE levels (~100–120%) within the PFC. A similar pattern of effects is seen with systemic administration of clinically-relevant doses of MPH (see Figure 2). *P<0.05 relative to NAcc DA and +P<0.05 relative to MSA NE. From 43.
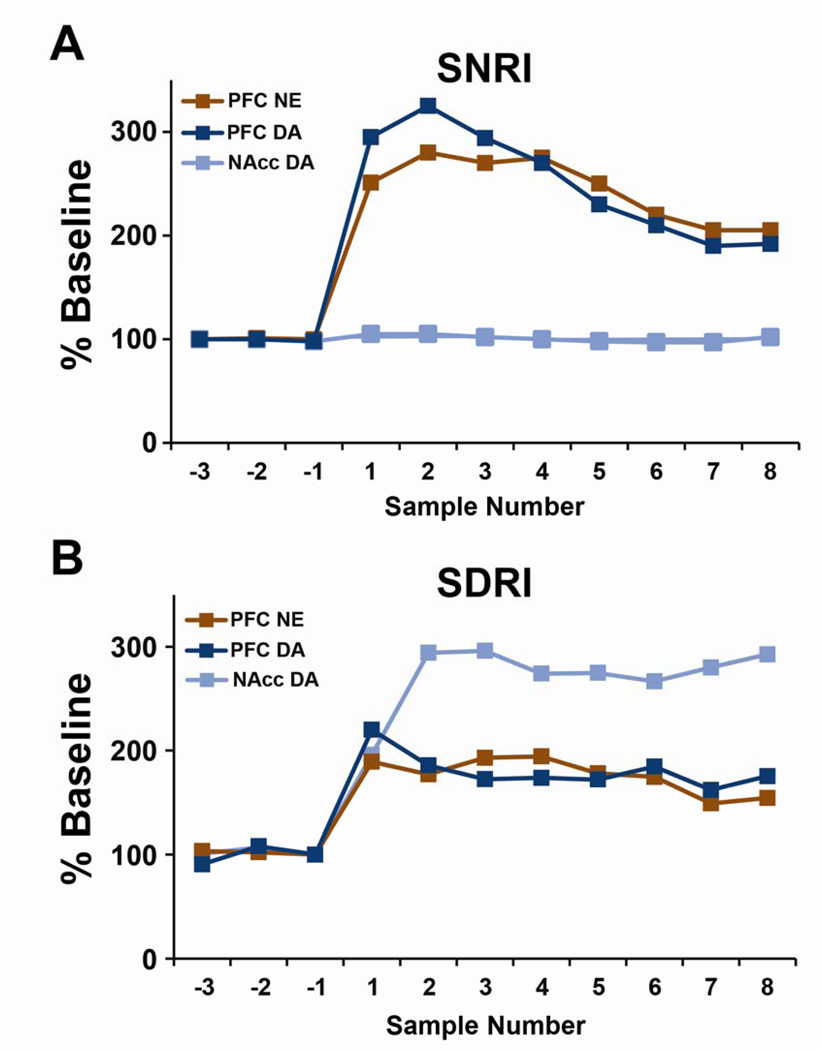
The mechanisms within the PFC responsible for the increased sensitivity of PFC catecholamines are less clear. However, this may involve the fact that the density of DA transporters (DAT) is sparse in the PFC relative to the striatum (44,45), largely consistent with the reduced density of DA innervation of the PFC relative to the striatum (except see, 45). Moreover, the NE transporter (NET) displays a high affinity for DA and plays a prominent role in PFC DA clearance (46–48). Consistent with this, selective NET inhibitors effective in ADHD increase both NE and DA in the PFC without altering striatal DA (Figure 4; 49,50). It is important to note that ‘limited’ DAT density in the PFC (relative to the striatum) does not indicate an absence of functional DAT. Indeed, evidence demonstrates measurable DAT in the medial PFC of rats (44,45). Further, DAT inhibitors are known to elevate extracellular DA levels in this region (43,51,52). Moreover, recent studies demonstrate that when administered at a cognition-enhancing dose, a selective DAT inhibitor increases both extracellular DA and NE in the PFC similar to cognition-enhancing doses of psychostimulants (Figure 4; 53). The elevation in NE is posited to result indirectly from drug-induced increases in extracellular DA that compete with NE at the NET, resulting in an increase in extracellular NE.
Fig 4. Similar neurochemical actions in the prefrontal cortex (PFC) of a selective norepinephrine reuptake inhibitor (SNRI) and a selective dopamine reuptake inhibitor (SDRI).
Shown are the mean (±SEM) change in extracellular norepinephrine (NE) in the PFC and extracellular dopamine (DA) in the PFC and nucleus accumbens (NAcc) expressed as a percent change from baseline. Data are shown for 30-min samples collected prior to (negative numbers) and following (positive numbers) systemic treatment with either (A) the selective NE inhibitor (SNRI), atomoxetine (3 mg/kg) or (B) the selective DA inhibitor (SDRI), AHN 2005 (10 m/kg). At these doses, these drugs exert cognition-enhancing effects. A: The SNRI elicited similar increases in extracellular levels of both NE and DA (~150% above baseline) while having no effect on DA within the NAcc. B: The SDRI also elicited significant increases in both extracellular DA and NE in the PFC that were comparable in magnitude (~100% above baseline). However, in contrast to the SNRI, the SDRI elicited a relatively large increase in extracellular DA levels in the NAcc (~200% above baseline). Panel A adapted from 49. Panel B adapted from 53. Error bars removed for clarity. *P< 0.05, compared to vehicle-treated animals.
Collectively, these observations suggest that, in the PFC, NE and DA competitively bind to the NET, with elevations in one transmitter increasing extracellular levels of the other. We posit that this ‘feed-forward’ mechanism contributes to the preferential sensitivity of PFC catecholamines to clinically-relevant doses of psychostimulants. These observations further indicate a close relationship between drug-induced increases in PFC catecholamines and the cognition-enhancing effects of a variety of drug classes, including psychostimulants, NET inhibitors and DAT inhibitors. Specifically, at cognition-enhancing doses all of these drugs elicit moderate elevations in PFC NE and DA of ~100%–250%. In contrast, the effects of these drugs on striatal DA fail to predict their cognition-enhancing effects (a range of 0%–200%; see Figures 2 and 4).
Do the Cognition-Enhancing Effects of Psychostimulants Involve Direct Action Within the PFC?
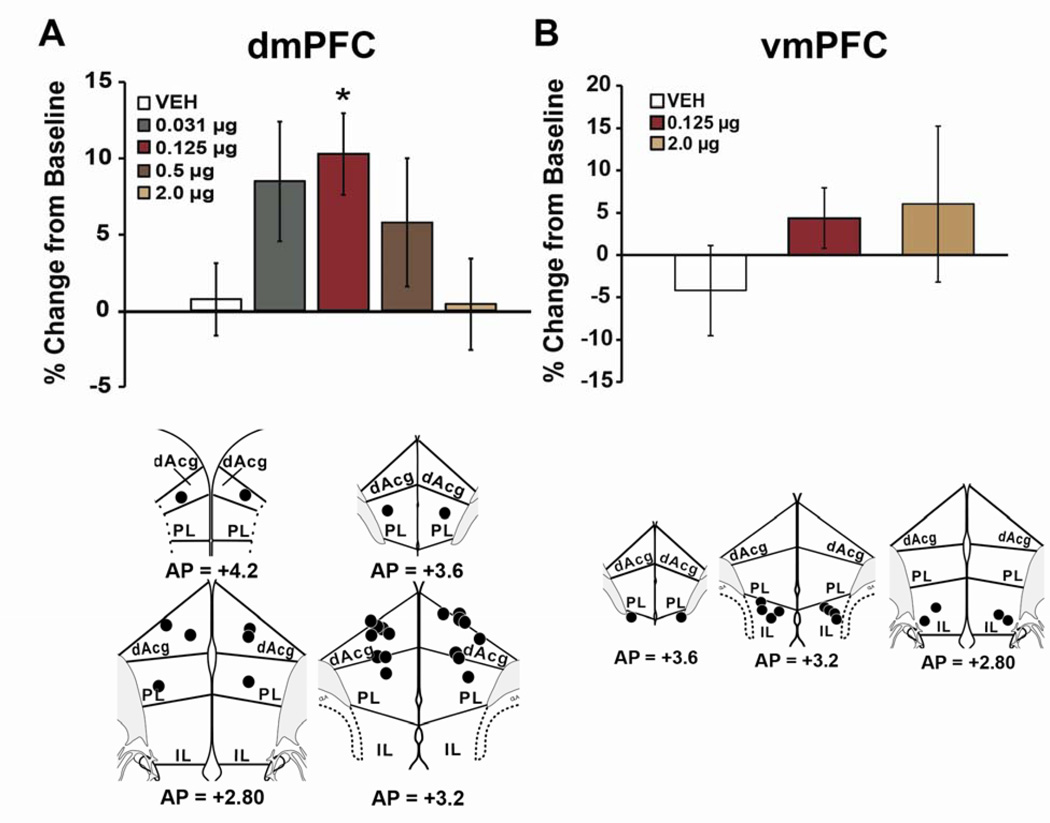
Though correlative in nature, the neurochemical effects of low-dose psychostimulants reviewed above suggest the hypothesis that the PFC is an important site of action in the cognition-enhancing properties of these drugs. As in primates, the rat medial PFC is functionally and anatomically heterogeneous, with the dorsomedial PFC (dmPFC; i.e. dorsal anterior cingulate, dorsal prelimbic PFC) associated with flexible cognitive function and the ventromedial PFC (vmPFC; i.e. infralimbic PFC, ventral prelimbic PFC) associated with affective/motivationrelated processes (54–57). Recent studies demonstrate that when infused directly into the rat dmPFC, but not vmPFC, MPH facilitates working memory in an inverted-U shaped manner, comparable to systemic administration (Figure 5; 58). Thus, MPH action in the dmPFC is sufficient to elicit cognitive improvement.
Fig 5. Cognition-enhancing effects of methylphenidate (MPH) involve actions within the prefrontal cortex (PFC).
Shown are the effects of intra-PFC infusion of MPH on performance in a test of working memory expressed as percent change from baseline (mean ± SEM). Top Panels: Infusion of methylphenidate into the dmPFC (A) but not vmPFC (B) improved working memory in an inverted-U dose-dependent manner, with 0.125 µg/hemisphere producing maximal improvement. The magnitude of MPH-induced improvement in performance was comparable to that seen with systemic administration of clinically relevant doses (see Figure 1). Note that doses 4 and 16-fold higher than an optimal dose infused into the dmPFC do not impair performance as seen with systemic administration. Bottom Panels: Schematic diagram indicating all 0.125 µg infusion sites into the dmPFC (A) and vmPFC (B). Numbers represent anterior-posterior (AP) level (data from [Swanson]). *P<0.05 relative to vehicle treatment. dAcg, dorsal anterior cingulate; IL, infralimbic; PL, prelimbic.
In contrast to the cognition-enhancing effects of systemically administered clinically-relevant doses of MPH, 4–8-fold higher doses impair working memory (Figure 1). Thus, it is surprising that intra-PFC MPH fails to impair performance, even at concentrations 16-fold and 32-fold higher than a performance-improving concentration (Figure 5; 58). These observations indicate: 1) there is a mechanism within the PFC that drives the descending limb of the inverted-U curve (to zero) and 2) the cognition-impairing actions of systemically administered psychostimulants involve drug action outside the PFC. A possible candidate system for the cognition-impairing effects of psychostimulants is the hypothalamo-pituitary-adrenal (HPA) axis. Thus, behaviorally-activating doses of psychostimulants activate the HPA axis, elevating circulating glucocorticoids (59) and impair spatial working memory through glucocorticoid receptor activation within the PFC (60). Clinically, it would likely be beneficial to extend the effective dose range of psychostimulants, particularly in patients that do not respond optimally to lower doses. Understanding the neural mechanisms responsible for the cognition-impairing actions of psychostimulants is a translationally important area for future research.
Psychostimulant Action Outside the PFC: Striatum
The above reviewed information unambiguously demonstrates the PFC is a key region for the cognition-enhancing/therapeutic effects of psychostimulants. Nonetheless, the PFC does not act in isolation to support higher cognitive function, representing one node in a broader corticothalamocortical circuit. As part of this, the PFC extends topographically organized projections to the striatum (61,62), a region that plays a prominent role in cognitive processes historically associated with the PFC and dysregulation of frontostriatal circuitry is implicated in ADHD (20). Thus, it is of interest that clinically-relevant doses of MPH increase striatal DA signaling in both humans and animals (24,63,64), albeit to a lesser degree than in the PFC (Figure 2; 24). Furthermore, in humans, cognition-enhancing actions of MPH are associated with changes in DA signaling in both the dorsal and ventral striatum (63,65). However, an important caveat to these imaging studies is that catecholamine signaling in the PFC was not measured. Based on the observations reviewed above (Figure 2) it is likely that a similar, if not stronger, relationship exists between drug-induced changes in cognition and catecholamine signaling in the PFC. Nonetheless, collectively, these observations suggest that psychostimulant-induced increases in DA in the striatum could contribute to the cognition-enhancing effects of psychostimulants.
In rats, the dorsomedial and ventromedial striatum receive direct projections from the dmPFC (57,58,66), a region associated with the cognition-enhancing actions of MPH (see above). Inactivation or lesion of either the dorsomedial or ventromedial striatum significantly impairs working memory (Figure S1; 58,67). Nonetheless, MPH infusion into either of these striatal regions (Figure S1; 58) has no effect on working memory performance. Thus, unlike the dmPFC, psychostimulant action within the striatum is not sufficient for the working memory enhancing effects of MPH. Nonetheless, drug-induced alterations in PFC neuronal function are likely relayed to downstream targets, altering the overall function/activity of frontostriatal-thalamocortical networks. Additionally, it remains possible that although action in the striatum is not sufficient for the procognitive effects of psychostimulants, combined actions in the PFC and striatum may contribute to the maximal therapeutic effects of these drugs.
Clinically-Relevant Doses of Psychostimulants Strengthen Neuronal Signaling in the PFC
A number of imaging studies indicate that cognition-enhancing doses of psychostimulants normalize ADHD-related hypoactivity within frontostriatal circuitry (12,68–70). However, additional evidence indicates that clinically-relevant doses of psychostimulants exert a more complex pattern of actions on frontostriatal activity that are task, region, and hemisphere-dependent (13,71–74). Currently, there exists only limited information regarding the electrophysiological mechanisms that underlie the cognition-enhancing effects of psychostimulants. In unanesthetized animals, cognition-enhancing doses of MPH increase the responsiveness of PFC neurons to afferent signals while exerting minimal effects on spontaneous discharge rates (Figure S2; 6). Similar to that seen neurochemically (see Figure 2), these doses of MPH do not alter neuronal discharge properties of cortical neurons outside the PFC (e.g. somatosensory cortex; 6,40). A similar improvement in PFC signal processing is observed with cognition-enhancing doses of the NET inhibitor, atomoxetine (34).
Combined, these observations suggest that the procognitive/therapeutic effects of psychostimulants involve strengthening of signal processing within the PFC. This action may bias neuronal activation to salient, task-related information while simultaneously reducing responding to less-salient or irrelevant stimuli. Conversely, the cognition-impairing effects of higher, motorically activating doses of psychostimulants likely involve suppression of neuronal signal processing in the PFC. A similar pattern of low and high dose effects on PFC reactivity has been observed in human imaging studies (75–77). Finally, as reviewed above, psychostimulant action in the PFC is expected to alter signal processing broadly within corticostriatal-thalamocortical loops. Further studies will need to explore how MPH alters signal processing within this broader circuitry involving flexible goal-directed behavior.
Receptor Mechanisms in the PFC: NE α2 and DA D1 receptors
DA and NE exert an inverted-U shaped facilitation of both PFC dependent working memory and PFC neuronal signaling. In the case of DA, these actions involve inverted-U shaped modulatory actions of D1 receptors (78). For NE, high-affinity postsynaptic α2 receptors improve, whereas lower affinity α1 receptors, engaged at higher rates of NE release, impair working memory and PFC neuronal signaling (79). Importantly, α2-agonists are efficacious in the treatment of ADHD and improve PFC-dependent function in healthy animal and human subjects (80–84), similar to that seen with psychostimulants and selective NE reuptake blockers used in ADHD. Moreover, similar to MPH, α2-agonist infusion into the PFC improves working memory performance and PFC neuronal signaling (85).
This evidence suggests that PFC α2 and/or D1 receptors play a prominent role in the cognition-enhancing actions of low-dose psychostimulants. Consistent with this, the working memory effects of both low-dose MPH and the selective NET inhibitor, atomoxetine, are prevented with systemically administered α2 and D1 antagonists (Figure S3; 5,34). Moreover, we recently observed that the cognition-enhancing effects of intra-PFC infusion of MPH are blocked by co-infusion of α2 and D1 antagonists (86).
The neurophysiological actions of clinically-relevant doses of psychostimulants described above likely involve catecholamine-dependent modulation of amino acid signaling. Thus, catecholamines exert receptor-dependent modulation of excitatory and inhibitory amino acid signaling in a variety of brain regions (87–89), including the PFC (90,91). In the PFC, NMDA-receptor mediated excitatory postsynaptic currents (EPSCs) are potentiated by a cognition-enhancing dose of MPH, an action that involves D1 receptors (89–91). Moreover, at least a subset of catecholamine actions in the PFC is linked to the modulation of hyperpolarizationactivated cyclic nucleotide (HCN) channels (92). Of particular relevance to the current discussion, stimulation of cognition-enhancing postsynaptic α2 receptors in the PFC inhibit HCN channels, enhancing neuronal responsiveness to behaviorally salient signals (92). Thus, this mechanism may contribute to the psychostimulant-induced strengthening of PFC neuronal responding. In contrast, suppressive effects of high dose psychostimulant on evoked discharge of PFC neurons (reduced by ~80%; Figure S2; 6) involve D1-mediated activation of HCN channels and/or α1-mediated activation of SK channels (92). Additionally, psychostimulant-driven reductions in evoked discharge may involve suppression of glutamatergic signaling, as high doses of MPH (8.0 mg/kg) suppress both NMDA and AMPA-receptor mediated EPSCs (89).
Combined, the available evidence indicates that the procognitive and enhanced neuronal signal processing actions of psychostimulants (and selective NE-reuptake blockers and α2 agonists) involve moderate increases in NE α2 and/or DA D1 receptor activation in the PFC. Conversely, the cognition-impairing actions of higher doses of psychostimulants likely involve suppression of signal processing in the PFC via high rates of α1 and D1 receptor activation.
Differing Dose-Response Curves Across Cognitive Tasks Reflect Differing Noradrenergic Receptor Action
In 1977 Sprague and Sleator reported that in ADHD children, MPH elicited a narrow inverted-U shaped facilitation of performance in a ‘cognition/learning’ task, while classroom behavior was improved across a wider dose range (93). However, subsequent studies generally failed to observe differential sensitivity to MPH dose across a range of cognitive tasks vs. overt behavior in ADHD patients (94–96). Importantly, Sprague and Sleator used a memory task that involved short delays (seconds), typical of PFC-dependent working memory tasks, while subsequent studies examined other forms of cognition (93,94). MPH was also observed to produce a narrow inverted-U shaped improvement in a PFC-dependent test of response inhibition in ADHD patients while eliciting a generally linear dose-dependent improvement in behavioral/motor activity (96).
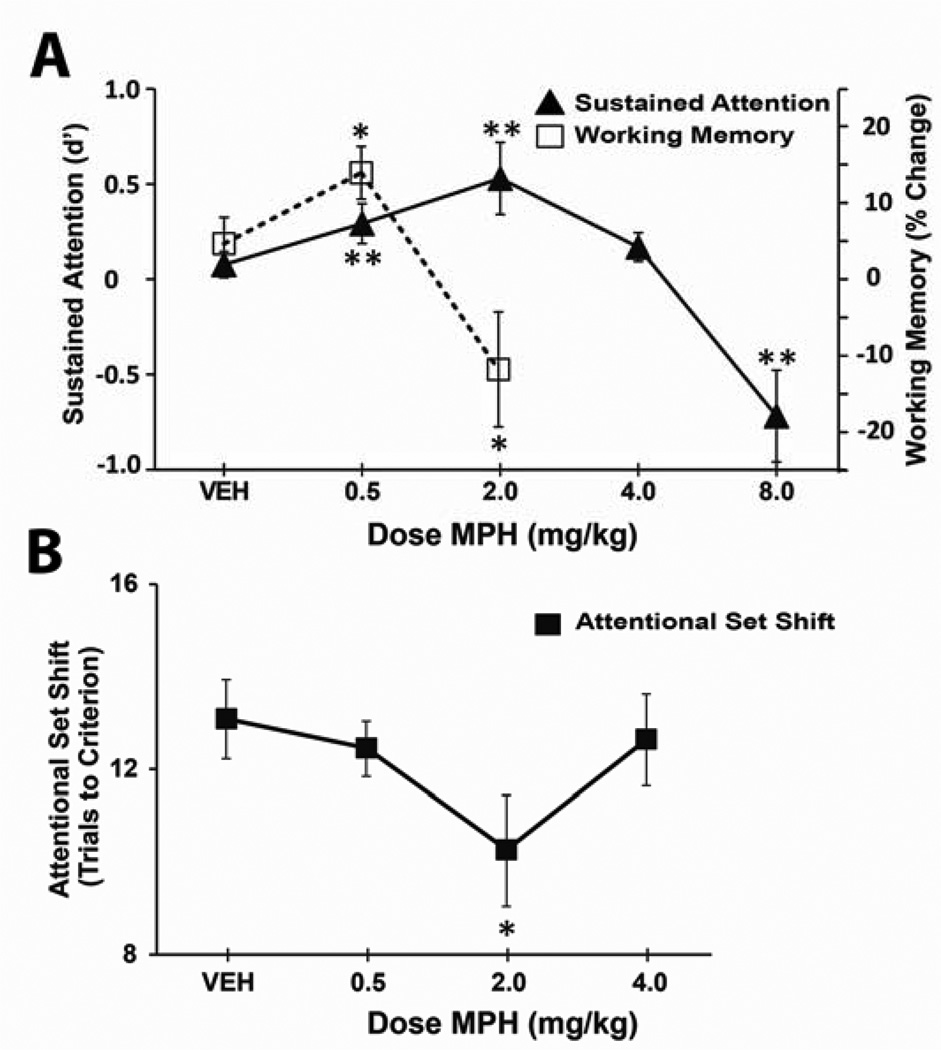
These observations could suggest that psychostimulants exert differential dose-dependent effects on PFC-dependent vs. PFC-independent processes. However, when the dose-dependent actions of MPH were compared across working memory and sustained attention tasks, two PFC-dependent tasks (Figure S2; 97), a narrower, left-shifted inverted-U curve was observed for working memory vs. sustained attention (Figure 6; 98). A similar shift in dose response curves across these tasks occurs with MPH infusion directly into the dmPFC (99). Prior studies have suggested that PFC NE may differentially regulate flexible vs. focused attention (100,101). Sustained attention requires little cognitive flexibility, relative to working memory. However, when examined in a PFC-dependent attentional set shifting task typically used to measure flexible attention (102,103), MPH elicited dose-dependent improvements in performance identical to sustained attention (Figure 6; 98). Collectively, these observations indicate that differing PFC-dependent cognitive processes display differing dose sensitivity to MPH that are not predicted by the degree to which they are associated with cognitive flexibility.
Fig 6. Different PFC-dependent cognitive tasks show differential sensitivity to methylphenidate.
Methylphenidate (MPH) exerts a relatively narrow inverted-U shaped facilitation of working memory performance (A), with maximal improvement seen following 0.5 mg/kg I.P. In contrast, MPH exerts a right shifted dose-dependent facilitation of sustained attention (A) and attentional set shifting (B). Importantly, doses that improve sustained attention and attentional set shifting impair performance in working memory (2.0 mg/kg). **P <0.01 compared to vehicle treatment. Data from (98).
As reviewed above, noradrenergic α2-receptors improve, while α1-receptors impair, working memory (104). In contrast, α1-receptors improve attentional set shifting while α2-receptors have little impact in this task (100). These observations suggest the hypothesis that the narrow vs. broad inverted-U shaped actions of psychostimulants described above involve differential activation of α2- vs. α1-receptors, respectively. Consistent with this, MPH-induced improvement in working memory is dependent on α2 receptors (see above; 104) while MPH-induced improvement in sustained attention is dependent on α1 receptors (98).
Implications for Divergent Dose-Response Curves Across PFC-Dependent Processes
Preclinically, the differential dose sensitivity of PFC-dependent tasks to psychostimulants suggests that not all PFC-dependent tasks are well suited for ADHD-focused drug discovery programs. Extensive evidence demonstrates that the pharmacology of working memory mirrors the pharmacology of ADHD: all approved ADHD-related drugs, including α2 agonists (105), low-dose psychostimulants (5,25) and selective NE reuptake blockers (e.g. atomoxetine; 34) improve working memory. For psychostimulants, their beneficial effects on working memory only occur across a narrow range of doses that produce clinically-relevant plasma concentrations (32,33,37,105,106). Thus, delayed response tests of working memory are a well-suited preclinical tool for identifying potentially efficacious compounds for use in ADHD and possibly other clinical conditions associated with PFC dysfunction.
In contrast, in sustained attention, attentional set shifting, and 5-choice serial reaction time tests, MPH exerts maximal beneficial actions at higher, behaviorally-activating and impulsivity-promoting doses that result in blood concentrations that exceed the clinically-relevant range (Figure 1; 33,37,107,108). Moreover, limited observations indicate that α2-agonists lack beneficial effects on attentional set shifting (100) as well as sustained attention (unpublished observations, JL Berkowitz, BD Waterhouse, JS Shumsky). Thus, the potential utility of these tests in an ADHD-focused preclinical program is less clear.
Clinically, as suggested by Sprague and Sleator (93) and Tannock and colleagues (96), differential actions of psychostimulants across cognitive processes raise the question of whether doses that are optimal for controlling classroom behavior impair, or no longer improve, cognitive/behavioral processes important for other functional domains. For example, largely anecdotal evidence suggests that cognitive-constriction or over-focusing can be a side effect of psychostimulant treatment (96). This action may be related to the ability of higher doses of MPH to improve attentional processes while impairing certain forms of cognitive flexibility evinced in tests of working memory and response inhibition (96,98,107). If so, the results reviewed above could suggest the use of α1-antagonists as an adjunct treatment for ADHD. Given α1 receptors in the PFC are necessary for the motor-activating and reinforcing effects of psychostimulants, α1 antagonists may also lessen certain adverse actions of psychostimulants, including abuse liability. Further studies are needed to better understand the degree to which the dose-dependent actions of psychostimulants across PFC-dependent processes relate to clinical, social, and academic outcomes and role of α1-receptors in these actions.
Summary and Implications
Low-dose psychostimulants are the first-line treatment for ADHD. At clinically-relevant doses these drugs improve frontostriatal cognitive function in ADHD patients and healthy individuals. The procognitive and behavioral calming actions of psychostimulants are in stark contrast to the behaviorally-activating and cognition-impairing effects seen with higher doses. For much of the history of psychostimulant treatment of ADHD there has been an emphasis on the possible involvement of striatal DA signaling in the therapeutic actions of these drugs. Much of this view was driven by the known neurobiological actions of high and behaviorally-activating doses. However, research over the past ten years has provided significant insight into the neural mechanisms that support the procognitive/therapeutic effects of psychostimulants. In particular, evidence demonstrates that the cognition-enhancing effects of psychostimulants involve an elevation in catecholamine signaling at α2 and D1 receptors preferentially within the PFC. The regionally-selective action of low-dose psychostimulants contrasts with the widespread and uniform actions of higher doses of these drugs. This regional selectivity appears to explain why the clinical use of psychostimulants is not associated with the pronounced arousal, motor activation and abuse liability of higher doses. Evidence also indicates PFC D1 and/or α2 receptors contribute to the the therapeutic actions of the two other FDA approved treatments: selective NE reuptake inhibitors and α2 agonists. Collectively, this indicates a prominent role of PFC catecholamines in the pharmacology of ADHD. In identifying the PFC as a key site of action in the procognitive effects of psychostimulants, this research provides important guiding information for future drug discovery research focused on ADHD. It is hoped further understanding of the neurobiology of the PFC will lead to the identification of novel, non-catecholamine targets for ADHD and other disorders of frontostriatal function.
From a public policy perspective, there has been significant concern about the widespread use of ‘psychostimulants’ in the treatment of ADHD. In the discussion of this issue, the term ‘psychostimulant’ often carries strong negative connotations that are associated with the arousing, activating and abuse liability of these drugs. However, information reviewed above demonstrates that at low and clinically relevant doses, psychostimulants do not exert behavioral and neurochemical actions that define this class of drugs. Instead at these lower doses, these drugs act as ‘cognitive enhancers’ that improve frontostriatal cognitive function while exerting only modest effects in neural circuits associated with psychostimulant-related arousal, motor activation and abuse. While this does not indicate these drugs are devoid of risk, it is important information for policy-based discussions regarding the appropriateness of the clinical use of ‘psychostimulants’. One important caveat to this discussion is that the vast majority of studies on the neural and behavioral actions of clinically relevant doses of psychostimulants in both humans and animals have largely involved males. Extending this work to females is an important area for future research.
Finally, it is now clear that psychostimulants do not exert a uniform facilitation of PFC-dependent processes. In particular, in both animals and humans, lower doses maximally improve performance in tests of working memory whereas maximal suppression of overt behavior and facilitation of attentional processes occurs at higher doses. These differing sensitivities of PFC-dependent processes appear to depend on differential involvement of α2 vs. α1 receptors. These observations raise a number of clinical and preclinical questions regarding the degree to which higher doses that maximally control classroom behavior may exert detrimental actions in other functional domains via activation of α1 receptors.
Supplementary Material
Acknowledgements
This work was supported by PHS grants, MH098631, MH081843, DA000389, the National Science Foundation (NSF 0918555), the Wisconsin Institutes of Discovery, and the University of Wisconsin Graduate School.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: Mr. Spencer and Dr. Berridge report no biomedical financial interests or potential conflicts of interest. Dr. Devilbiss is the founder of NexStep Biomarkers, LLC. NexStep Biomarkers had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. NexStep Biomarkers, does not employ anyone who worked on this project, hold patents related to this project, sell products related to this project, or provide consultation on this project. This manuscript provides no financial gain for NexStep Biomarkers.
References
- 1.Segal DS. Behavioral and neurochemical correlates of repeated d-amphetamine administration. Adv Biochem Psychopharmacol. 1975;13:247–262. [PubMed] [Google Scholar]
- 2.Rebec GV, Bashore TR. Critical issues in assessing the behavioral effects of amphetamine. Neurosci Biobehav Rev. 1984;8:153–159. doi: 10.1016/0149-7634(84)90030-7. [DOI] [PubMed] [Google Scholar]
- 3.McGaughy J, Sarter M. Behavioral vigilance in rats: task validation and effects of age, amphetamine, and benzodiazepine receptor ligands. Psychopharmacology (Berl) 1995;117:340–357. doi: 10.1007/BF02246109. [DOI] [PubMed] [Google Scholar]
- 4.Berridge CW, Stalnaker TA. Relationship between low-dose amphetamine-induced arousal and extracellular norepinephrine and dopamine levels within prefrontal cortex. Synapse. 2002;46:140–149. doi: 10.1002/syn.10131. [DOI] [PubMed] [Google Scholar]
- 5.Arnsten AF, Dudley AG. Methylphenidate improves prefrontal cortical cognitive function through alpha2 adrenoceptor and dopamine D1 receptor actions: Relevance to therapeutic effects in Attention Deficit Hyperactivity Disorder. Behav Brain Funct. 2005;1:2. doi: 10.1186/1744-9081-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devilbiss DM, Berridge CW. Cognition-enhancing doses of methylphenidate preferentially increase prefrontal cortex neuronal responsiveness. Biol Psychiatry. 2008;64:626–635. doi: 10.1016/j.biopsych.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradley C. The Behavior of Children Receiving Benzadrine. Am J Psychiatry. 1937;94:577–585. [Google Scholar]
- 8.Greenhill LL. Clinical effects of stimulant medication in ADHD. In: Solanto MV, Arnsten AFT, Castellanos FX, editors. Stimulant Drugs and ADHD: Basic and Clinical Neuroscience. New York: Oxford University Press; 2001. pp. 31–71. [Google Scholar]
- 9.Hechtman L, Abikoff H, Klein RG, Weiss G, Respitz C, Kouri J, et al. Academic achievement and emotional status of children with ADHD treated with long-term methylphenidate and multimodal psychosocial treatment. J Am Acad Child Adolesc Psychiatry. 2004;43:812–819. doi: 10.1097/01.chi.0000128796.84202.eb. [DOI] [PubMed] [Google Scholar]
- 10.Scheffler RM, Brown TT, Fulton BD, Hinshaw SP, Levine P, Stone S. Positive association between attention-deficit/ hyperactivity disorder medication use and academic achievement during elementary school. Pediatrics. 2009;123:1273–1279. doi: 10.1542/peds.2008-1597. [DOI] [PubMed] [Google Scholar]
- 11.Rapoport JL, Buchsbaum MS, Weingartner H, Zahn TP, Ludlow C, Mikkelsen EJ. Dextroamphetamine - Its Cognitive and Behavioral-Effects in Normal and Hyperactive Boys and Normal Men. Arch Gen Psychiatry. 1980;37:933–943. doi: 10.1001/archpsyc.1980.01780210091010. [DOI] [PubMed] [Google Scholar]
- 12.Vaidya CJ, Austin G, Kirkorian G, Ridlehuber HW, Desmond JE, Glover GH, et al. Selective effects of methylphenidate in attention deficit hyperactivity disorder: A functional magnetic resonance study. Proc Natl Acad Sci. 1998;95:14494–14499. doi: 10.1073/pnas.95.24.14494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehta MA, Owen AM, Sahakian BJ, Mavaddat N, Pickard JD, Robbins TW. Methylphenidate enhances working memory by modulating discrete frontal and parietal lobe regions in the human brain. J Neurosci. 2000;20:RC65. doi: 10.1523/JNEUROSCI.20-06-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rapoport JL, Inoff-Germain G. Responses to methylphenidate in Attention-Deficit/Hyperactivity Disorder and normal children: update 2002. J Atten Disord. 2002;6:S57–S60. doi: 10.1177/070674370200601s07. [DOI] [PubMed] [Google Scholar]
- 15.Elliott R, Sahakian BJ, Matthews K, Bannerjea A, Rimmer J, Robbins TW. Effects of methylphenidate on spatial working memory and planning in healthy young adults. Psychopharmacology (Berl) 1997;131:196–206. doi: 10.1007/s002130050284. [DOI] [PubMed] [Google Scholar]
- 16.Setlik J, Bond GR, Ho M. Adolescent Prescription ADHD Medication Abuse Is Rising Along With Prescriptions for These Medications. Pediatrics. 2009;124:875–880. doi: 10.1542/peds.2008-0931. [DOI] [PubMed] [Google Scholar]
- 17.McCabe SE, Knight JR, Teter CJ, Wechsler H. Non-medical use of prescription stimulants among US college students: prevalence and correlates from a national survey. Addiction. 2005;100:96–106. doi: 10.1111/j.1360-0443.2005.00944.x. [DOI] [PubMed] [Google Scholar]
- 18.Maher B. Poll results: look who's doping. Nature. 2008;452:674–675. doi: 10.1038/452674a. [DOI] [PubMed] [Google Scholar]
- 19.Barkley RA. Attention-deficit/hyperactivity disorder, self-regulation, and time: toward a more comprehensive theory. J Dev Behav Pediatr. 1997;18:271–279. [PubMed] [Google Scholar]
- 20.Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: The search for endophenotypes. Nat Rev Neurosci. 2002;3:617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- 21.Casey BJ, Epstein JN, Buhle J, Liston C, Davidson MC, Tonev ST, et al. Frontostriatal connectivity and its role in cognitive control in parent-child dyads with ADHD. Am J Psychiatry. 2007;164:1729–1736. doi: 10.1176/appi.ajp.2007.06101754. [DOI] [PubMed] [Google Scholar]
- 22.Bush G, Valera EM, Seidman LJ. Functional neuroimaging of attention-deficit/hyperactivity disorder: a review and suggested future directions. Biol Psychiatry. 2005;57:1273–1284. doi: 10.1016/j.biopsych.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 23.Swanson J, Volkow N. Pharmacokinetic and Pharmacodynamic Properties of Methylphenidate in Humans. In: Solanto MV, Arnsten AFT, Castellanos FX, editors. Stimulant Drugs and ADHD: Basic and Clinical Neuroscience. New York: Oxford University Press; New York, New York: Oxford University Press; 2001. [Google Scholar]
- 24.Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AF, Kelley AE, Schmeichel B, et al. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry. 2006;60:1111–1120. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 25.Berridge CW, Devilbiss DM. Psychostimulants as Cognitive Enhancers: The Prefrontal Cortex, Catecholamines, and Attention-Deficit/Hyperactivity Disorder. Bioll Psychiatry. 2011;69:e101–e111. doi: 10.1016/j.biopsych.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arnsten AF. Toward a new understanding of attention-deficit hyperactivity disorder pathophysiology: an important role for prefrontal cortex dysfunction. CNS Drugs. 2009;23(Suppl 1):33–41. doi: 10.2165/00023210-200923000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Kuczenski R, Segal DS. Regional norepinephrine response to amphetamine using dialysis: comparison with caudate dopamine. Synapse. 1992;11:164–169. doi: 10.1002/syn.890110210. [DOI] [PubMed] [Google Scholar]
- 28.Kuczenski R, Segal DS, Cho AK, Melega W. Hippocampus norepinephrine, caudate dopamine and serotonin, and behavioral responses to the stereoisomers of amphetamine and methamphetamine. J Neurosci. 1995;15:1308–1317. doi: 10.1523/JNEUROSCI.15-02-01308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuczenski R, Segal DS. Neurochemistry of amphetamine. In: Cho AK, Segal DS, editors. Amphetamine and its analogues: psychopharmacology, toxicology and abuse. San Diego: Academic Press; 1994. pp. 81–113. [Google Scholar]
- 30.Florin SM, Kuczenski R, Segal DS. Regional extracellular norepinephrine responses to amphetamine and cocaine and effects of clonidine pretreatment. Brain Res. 1994;654:53–62. doi: 10.1016/0006-8993(94)91570-9. [DOI] [PubMed] [Google Scholar]
- 31.Kuczenski R, Segal DS. Effects of methylphenidate on extracellular dopamine, serotonin, and norepinephrine: comparison with amphetamine. J Neurochem. 1997;68:2032–2037. doi: 10.1046/j.1471-4159.1997.68052032.x. [DOI] [PubMed] [Google Scholar]
- 32.Kuczenski R, Segal DS. Locomotor effects of acute and repeated threshold doses of amphetamine and methylphenidate: relative roles of dopamine and norepinephrine. J Pharmacol Exp Ther. 2001;296:876–883. [PubMed] [Google Scholar]
- 33.Kuczenski R, Segal DS. Exposure of adolescent rats to oral methylphenidate: preferential effects on extracellular norepinephrine and absence of sensitization and cross-sensitization to methamphetamine. J Neurosci. 2002;22:7264–7271. doi: 10.1523/JNEUROSCI.22-16-07264.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gamo NJ, Wang M, Arnsten AF. Methylphenidate and atomoxetine enhance prefrontal function through alpha2-adrenergic and dopamine D1 receptors. J Am Acad Child Adolesc Psychiatry. 2010;49:1011–1023. doi: 10.1016/j.jaac.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arnsten AF, Pliszka SR. Catecholamine influences on prefrontal cortical function: relevance to treatment of attention deficit/hyperactivity disorder and related disorders. Pharm Biochem Behav. 2011;99:211–216. doi: 10.1016/j.pbb.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Delfs JM, Schreiber L, Kelley AE. Microinjection of cocaine into the nucleus accumbens elicits locomotor activation in the rat. J Neurosci. 1990;10:303–310. doi: 10.1523/JNEUROSCI.10-01-00303.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berridge CW. Neural substrates of psychostimulant-induced arousal. Neuropsychopharmacology. 2006;31:2332–2340. doi: 10.1038/sj.npp.1301159. [DOI] [PubMed] [Google Scholar]
- 38.Kelly PH, Seviour PW, Iversen SD. Amphetamine and apomorphine responses in the rat following 6-OHDA lesions of the nucleus accumbens septi and corpus striatum. Brain Res. 1975;94:507–522. doi: 10.1016/0006-8993(75)90233-4. [DOI] [PubMed] [Google Scholar]
- 39.Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- 40.Drouin C, Page M, Waterhouse B. Methylphenidate enhances noradrenergic transmission and suppresses mid- and long-latency sensory responses in the primary somatosensory cortex of awake rats. J Neurophysiol. 2006;96:622–632. doi: 10.1152/jn.01310.2005. [DOI] [PubMed] [Google Scholar]
- 41.Biederman J. Pharmacotherapy for attention-deficit/hyperactivity disorder (ADHD) decreases the risk for substance abuse: findings from a longitudinal follow-up of youths with and without ADHD. JClin Psychiatry. 2003;64(Suppl 11):3–8. [PubMed] [Google Scholar]
- 42.Wilens TE, Faraone SV, Biederman J, Gunawardene S. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse? A meta-analytic review of the literature. Pediatrics. 2003;111:179–185. doi: 10.1542/peds.111.1.179. [DOI] [PubMed] [Google Scholar]
- 43.Schmeichel BE, Berridge CW. Neurocircuitry Underlying the Preferential Sensitivity of Prefrontal Catecholamines to Low-Dose Psychostimulants. Neuropsychopharmacology. 2013;38:1078–1084. doi: 10.1038/npp.2013.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meyers B, Kritzer MF. In vitro binding assays using (3)H nisoxetine and (3)H WIN 35,428 reveal selective effects of gonadectomy and hormone replacement in adult male rats on norepinephrine but not dopamine transporter sites in the cerebral cortex. Neuroscience. 2009;159:271–282. doi: 10.1016/j.neuroscience.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI. Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J Neurosci. 1998;18:2697–2708. doi: 10.1523/JNEUROSCI.18-07-02697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giros B, Wang YM, Suter S, McLeskey SB, Pifl C, Caron MG. Delineation of discrete domains for substrate, cocaine, and tricyclic antidepressant interactions using chimeric dopamine-norepinephrine transporters. J Biol Chem. 1994;269:15985–15988. [PubMed] [Google Scholar]
- 47.Gu H, Wall SC, Rudnick G. Stable expression of biogenic amine transporters reveals differences in inhibitor sensitivity, kinetics, and ion dependence. J Biol Chem. 1994;269:7124–7130. [PubMed] [Google Scholar]
- 48.Moron JA, Brockington A, Wise RA, Rocha BA, Hope BT. Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: evidence from knock-out mouse lines. J Neurosci. 2002;22:389–395. doi: 10.1523/JNEUROSCI.22-02-00389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: A potential mechanism for efficacy in Attention Deficit/Hyperactivity Disorder. Neuropsychopharmacology. 2002;27:699–711. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]
- 50.Carboni E, Tanda GL, Frau R, Di CG. Blockade of the noradrenaline carrier increases extracellular dopamine concentrations in the prefrontal cortex: evidence that dopamine is taken up in vivo by noradrenergic terminals. J Neurochem. 1990;55:1067–1070. doi: 10.1111/j.1471-4159.1990.tb04599.x. [DOI] [PubMed] [Google Scholar]
- 51.Carboni E, Silvagni A, Vacca C, Di CG. Cumulative effect of norepinephrine and dopamine carrier blockade on extracellular dopamine increase in the nucleus accumbens shell, bed nucleus of stria terminalis and prefrontal cortex. J Neurochem. 2006;96:473–481. doi: 10.1111/j.1471-4159.2005.03556.x. [DOI] [PubMed] [Google Scholar]
- 52.Cass WA, Gerhardt GA. In vivo assessment of dopamine uptake in rat medial prefrontal cortex: comparison with dorsal striatum and nucleus accumbens. J Neurochem. 1995;65:201–207. doi: 10.1046/j.1471-4159.1995.65010201.x. [DOI] [PubMed] [Google Scholar]
- 53.Schmeichel B, Zemlan F, Berridge CW. A selective dopamine reuptake inhibitor imporves prefrontal cortex-dependent cognitive function: potential relevant to attention deficit hyperactivity disorder. Neuropharmacology. 2012;64:321–328. doi: 10.1016/j.neuropharm.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kesner RP. Subregional analysis of mnemonic functions of the prefrontal cortex in the rat. Psychobiology. 2000;28:219–228. [Google Scholar]
- 55.Mehta MA, Goodyer IM, Sahakian BJ. Methylphenidate improves working memory and set-shifting in AD/HD: relationships to baseline memory capacity. J Child Psychol Psychiatry. 2004;45:293–305. doi: 10.1111/j.1469-7610.2004.00221.x. [DOI] [PubMed] [Google Scholar]
- 56.Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 57.Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- 58.Spencer RC, Klein RM, Berridge CW. Psychostimulants act within the prefrontal cortex to improve cognitive function. Biol Psychiatry. 2012;72:221–227. doi: 10.1016/j.biopsych.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Armario A. Activation of the hypothalamic-pituitary-adrenal axis by addictive drugs: different pathways, common outcome. Trends Pharm Sci. 2010;31:318–325. doi: 10.1016/j.tips.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 60.Barsegyan A, Mackenzie SM, Kurose BD, McGaugh JL, Roozendaal B. Glucocorticoids in the prefrontal cortex enhance memory consolidation and impair working memory by a common neural mechanism. Proc Natl Acad Sci. 2010;107:16655–16660. doi: 10.1073/pnas.1011975107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J Neurosci. 2007;27:8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Balleine BW, O'Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2010;35:48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Clatworthy PL, Lewis SJ, Brichard L, Hong YT, Izquierdo D, Clark L, et al. Dopamine release in dissociable striatal subregions predicts the different effects of oral methylphenidate on reversal learning and spatial working memory. J Neurosci. 2009;29:4690–4696. doi: 10.1523/JNEUROSCI.3266-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Volkow ND, Wang GJ, Fowler JS, Logan J, Franceschi D, Maynard L, et al. Relationship between blockade of dopamine transporters by oral methylphenidate and the increases in extracellular dopamine: therapeutic implications. Synapse. 2002;43:181–187. doi: 10.1002/syn.10038. [DOI] [PubMed] [Google Scholar]
- 65.Volkow ND, Wang GJ, Tomasi D, Kollins SH, Wigal TL, Newcorn JH, et al. Methylphenidate-elicited dopamine increases in ventral striatum are associated with long-term symptom improvement in adults with attention deficit hyperactivity disorder. J Neurosci. 2012;32:841–849. doi: 10.1523/JNEUROSCI.4461-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol. 2005;492:145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- 67.Floresco SB, Braaksma DN, Phillips AG. Thalamic-cortical-striatal circuitry subserves working memory during delayed responding on a radial arm maze. J Neurosci. 1999;19:11061–11071. doi: 10.1523/JNEUROSCI.19-24-11061.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smith A, Cubillo A, Barrett N, Giampietro V, Simmons A, Brammer M, et al. Neurofunctional effects of methylphenidate and atomoxetine in boys with attention-deficit/hyperactivity disorder during time discrimination. Biol Psychiatry. 2013;74:615–622. doi: 10.1016/j.biopsych.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 69.Rubia K, Halari R, Cubillo A, Mohammad AM, Brammer M, Taylor E. Methylphenidate normalises activation and functional connectivity deficits in attention and motivation networks in medication-naive children with ADHD during a rewarded continuous performance task. Neuropharmacology. 2009;57:640–652. doi: 10.1016/j.neuropharm.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 70.Rubia K, Halari R, Cubillo A, Smith AB, Mohammad AM, Brammer M, et al. Methylphenidate normalizes fronto-striatal underactivation during interference inhibition in medication-naive boys with attention-deficit hyperactivity disorder. Neuropsychopharmacology. 2011;36:1575–1586. doi: 10.1038/npp.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marquand AF, O'Daly OG, De Simoni S, Alsop DC, Maguire RP, Williams SC, et al. Dissociable effects of methylphenidate, atomoxetine and placebo on regional cerebral blood flow in healthy volunteers at rest: a multi-class pattern recognition approach. Neuroimage. 2012;60:1015–1024. doi: 10.1016/j.neuroimage.2012.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marquand AF, De Simoni S, O'Daly OG, Williams SC, Mourao-Miranda J, Mehta MA. Pattern classification of working memory networks reveals differential effects of methylphenidate, atomoxetine, and placebo in healthy volunteers. Neuropsychopharmacology. 2011;36:1237–1247. doi: 10.1038/npp.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pauls AM, O'Daly OG, Rubia K, Riedel WJ, Williams SC, Mehta MA. Methylphenidate effects on prefrontal functioning during attentional-capture and response inhibition. Biol Psychiatry. 2012;72:142–149. doi: 10.1016/j.biopsych.2012.03.028. [DOI] [PubMed] [Google Scholar]
- 74.Cubillo A, Smith AB, Barrett N, Giampietro V, Brammer M, Simmons A, et al. Drug-specific laterality effects on frontal lobe activation of atomoxetine and methylphenidate in attention deficit hyperactivity disorder boys during working memory. Psychol Med. 2014;44:633–646. doi: 10.1017/S0033291713000676. [DOI] [PubMed] [Google Scholar]
- 75.Mehta MA, Sahakian BJ, Robbins TW. Comparative psycholpharmacology of methylphenidate and related drugs in human volunteers, patients with ADHD, and experimental animals. In: Solanto MV, Arnsten AFT, Castellanos FX, editors. Stimulant Drugs and ADHD: Basic and Clinical Neuroscience. New York: Oxford University Press; 2001. pp. 303–331. [Google Scholar]
- 76.Clark L, Blackwell AD, Aron AR, Turner DC, Dowson J, Robbins TW, et al. Association between response inhibition and working memory in adult ADHD: a link to right frontal cortex pathology? Biol Psychiatry. 2007;61:1395–1401. doi: 10.1016/j.biopsych.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 77.Lundqvist T. Imaging Cognitive Deficits in Drug Abuse. Berlin: Springer-Verlag; 2009. [DOI] [PubMed] [Google Scholar]
- 78.Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- 79.Arnsten AF. Through the looking glass: differential noradenergic modulation of prefrontal cortical function. Neural Plast. 2000;7:133–146. doi: 10.1155/NP.2000.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hunt RD, Minderaa RB, Cohen DJ. Clonidine benefits children with attention deficit disorder and hyperactivity: report of a double-blind placebo-crossover therapeutic trial. J Am Acad Child Psychiatry. 1985;24:617–629. doi: 10.1016/s0002-7138(09)60065-0. [DOI] [PubMed] [Google Scholar]
- 81.Scahill L, Chappell PB, Kim YS, Schultz RT, Katsovich L, Shepherd E, et al. A placebo-controlled study of guanfacine in the treatment of children with tic disorders and attention deficit hyperactivity disorder. Am J Psychiatry. 2001;158:1067–1074. doi: 10.1176/appi.ajp.158.7.1067. [DOI] [PubMed] [Google Scholar]
- 82.Biederman J, Melmed RD, Patel A, McBurnett K, Konow J, Lyne A, et al. A randomized, double-blind, placebo-controlled study of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics. 2008;121:e73–e84. doi: 10.1542/peds.2006-3695. [DOI] [PubMed] [Google Scholar]
- 83.Franowicz JS, Arnsten AF. The alpha-2a noradrenergic agonist, guanfacine, improves delayed response performance in young adult rhesus monkeys. Psychopharmacology (Berl) 1998;136:8–14. doi: 10.1007/s002130050533. [DOI] [PubMed] [Google Scholar]
- 84.Berridge CW, Arnsten AF, Foote SL. Noradrenergic modulation of cognitive function: clinical implications of anatomical, electrophysiological and behavioural studies in animal models [editorial] Psychol Med. 1993;23:557–564. doi: 10.1017/s0033291700025332. [DOI] [PubMed] [Google Scholar]
- 85.Tanila H, Rama P, Carlson S. The effects of prefrontal intracortical microinjections of an alpha-2 agonist, alpha-2 antagonist and lidocaine on the delayed alternation performance of aged rats. Brain Res Bull. 1996;40:117–119. doi: 10.1016/0361-9230(96)00026-3. [DOI] [PubMed] [Google Scholar]
- 86.Spencer RCBC. Receptor and frontostriatal circuit mechanisms underlying the cognition-enhancing actions of psychostimulants. Soc Neurosci Abst. 2013 288.16:KKK14. [Google Scholar]
- 87.Wang Y, Liu J, Gui ZH, Ali U, Fan LL, Hou C, et al. alpha2-Adrenoceptor regulates the spontaneous and the GABA/glutamate modulated firing activity of the rat medial prefrontal cortex pyramidal neurons. Neuroscience. 2011;182:193–202. doi: 10.1016/j.neuroscience.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 88.Salgado H, Garcia-Oscos F, Patel A, Martinolich L, Nichols JA, Dinh L, et al. Layer-specific noradrenergic modulation of inhibition in cortical layer II/III. Cereb Cortex. 2011;21:212–221. doi: 10.1093/cercor/bhq081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cheng J, Xiong Z, Duffney LJ, Wei J, Liu A, Liu S, et al. Methylphenidate Exerts Dose-Dependent Effects on Glutamate Receptors and Behaviors. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Seamans JK, Gorelova N, Durstewitz D, Yang CR. Bidirectional dopamine modulation of GABAergic inhibition in prefrontal cortical pyramidal neurons. J Neurosci. 2001;21:3628–3638. doi: 10.1523/JNEUROSCI.21-10-03628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Seamans JK, Durstewitz D, Christie BR, Stevens CF, Sejnowski TJ. Dopamine D1/D5 receptor modulation of excitatory synaptic inputs to layer V prefrontal cortex neurons. Proc Natl Acad Sci U S A. 2001;98:301–306. doi: 10.1073/pnas.011518798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Arnsten AF. Catecholamine and second messenger influences on prefrontal cortical networks of "representational knowledge": a rational bridge between genetics and the symptoms of mental illness. Cereb Cortex. 2007;17(Suppl 1):i6–i15. doi: 10.1093/cercor/bhm033. [DOI] [PubMed] [Google Scholar]
- 93.Sprague RL, Sleator EK. Methylphenidate in hyperkinetic children: differences in dose effects on learning and social behavior. Science. 1977;198:1274–1276. doi: 10.1126/science.337493. [DOI] [PubMed] [Google Scholar]
- 94.Solanto MV. Neuropsychopharmacological mechanisms of stimulant drug action in attention-deficit hyperactivity disorder: a review and integration. Behav Brain Res. 1998;94:127–152. doi: 10.1016/s0166-4328(97)00175-7. [DOI] [PubMed] [Google Scholar]
- 95.Rapport MD, Kelly KL. Psychostimulant effects on learning and cognitive function: findings and implications for children with attention deficit hyperactivity disorder. Clin Psych Rev. 1991;11:61–92. [Google Scholar]
- 96.Tannock R, Schachar R, Logan G. Methylphenidate and cognitive flexibility: dissociated dose effects in hyperactive children. J Abnorm Child Psychol. 1995;23:235–266. doi: 10.1007/BF01447091. [DOI] [PubMed] [Google Scholar]
- 97.Granon S, Passetti F, Thomas KL, Dalley JW, Everitt BJ, Robbins TW. Enhanced and impaired attentional performance after infusion of D1 dopaminergic receptor agents into rat prefrontal cortex. J Neurosci. 2000;20:1208–1215. doi: 10.1523/JNEUROSCI.20-03-01208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Berridge C, Shumsky JS, Andrzejewski ME, McGaughy J, Spencer RC, Devilbiss D, et al. Differential sensitivity to psychostimulants across prefrontal cognitive tasks: differential involvement of Noradrenergic α1- vs. α2-Receptors. Biol Psychiatry. 2012;71:467–473. doi: 10.1016/j.biopsych.2011.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Spencer RCSJ, Waterhouse BD, Berridge CW. Neurocircuitry and receptor mechanisms underlying the differential sensitivity of prefrontal cognitive processes to psychostimulants. Soc Neurosci Abst. 2014 in press. [Google Scholar]
- 100.Lapiz MD, Morilak DA. Noradrenergic modulation of cognitive function in rat medial prefrontal cortex as measured by attentional set shifting capability. Neuroscience. 2006;137:1039–1049. doi: 10.1016/j.neuroscience.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 101.Aston-Jones G, Rajkowski J, Cohen J. Locus coeruleus and regulation of behavioral flexibility and attention. Prog Brain Res. 2000;126:165–182. doi: 10.1016/S0079-6123(00)26013-5. [DOI] [PubMed] [Google Scholar]
- 102.Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Owen AM, Roberts AC, Polkey CE, Sahakian BJ, Robbins TW. Extra-dimensional versus intra-dimensional set shifting performance following frontal lobe excisions, temporal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia. 1991;29:993–1006. doi: 10.1016/0028-3932(91)90063-e. [DOI] [PubMed] [Google Scholar]
- 104.Arnsten AF, Li BM. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol Psychiatry. 2005;57:1377–1384. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 105.Arnsten AF. The use of α-2A adrenergic agonists for the treatment of attention-deficit/hyperactivity disorder. Expert Rev Neurother. 2010;10:1595–1605. doi: 10.1586/ern.10.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Doerge DR, Fogle CM, Paule MG, McCullagh M, Bajic S. Analysis of methylphenidate and its metabolite ritalinic acid in monkey plasma by liquid chromatography/electrospray ionization mass spectrometry. Rapid Comm Mass Spectrometry. 2000;14:619–623. doi: 10.1002/(SICI)1097-0231(20000430)14:8<619::AID-RCM916>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 107.Navarra R, Graf R, Huang Y, Logue S, Comery T, Hughes Z, et al. Effects of atomoxetine and methylphenidate on attention and impulsivity in the 5-choice serial reaction time test. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:34–41. doi: 10.1016/j.pnpbp.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 108.Milstein JA, Dalley JW, Robbins TW. Methylphenidate-induced impulsivity: pharmacological antagonism by beta-adrenoreceptor blockade. J. Psychopharmacology. 2010;24:309–321. doi: 10.1177/0269881108098146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.