Abstract
Alzheimer’s disease (AD), the most common form of dementia, is characterized histopathologically by the deposition of β-amyloid (Aβ) plaques and neurofibrillary tangles-containing hyperphosphorylated tau protein in the brain. Parkinson’s disease (PD), the most common movement disorder, is characterized by the aggregation of α-synuclein protein in Lewy body inclusions and the death of dopaminergic neurons in the substantia nigra. Based on their pathological signatures, AD and PD can be considered as two different disease entities. However, a subpopulation of PD patients also exhibit Aβ plaques, and AD patients exhibit α-synuclein aggregates. This overlap between PD and AD suggests that common pathological pathways exist for the two diseases. Identification of factors and cellular mechanisms by which these factors can trigger pathological hallmarks for AD/PD overlap may help in designing disease-modifying therapies that can reverse or stop the progression of AD and PD.
For the last decade, work in our laboratory has shown that fluctuations in the levels of cholesterol oxidation products (oxysterols) may correlate with the onset of AD and PD. In this review, we will provide results from our laboratory and data from literature that converge to strongly suggest the involvement of cholesterol and cholesterol oxidation products in the pathogenesis of AD and PD. We will specifically delineate the role of and the underlying mechanisms by which increased levels of the oxysterol 27-hydroxycholesterol contribute to the pathogenesis of AD, PD, and AD/PD overlap.
Introduction
Alzheimer’s disease (AD) is a complex and heterogeneous disorder that presently affects more than 4 million citizens in the U.S., and is projected to affect more than 14 million people within the next 50 years if no cure is found. Present ‘treatment approaches’ are, at best, modestly helpful in stabilizing clinical symptoms for a limited duration. Because of the devastating nature, the increasing prevalence and the subsequent social and economic burden of AD, research into the pathogenesis of this disorder is a major health priority. The research effort deployed for the last decades has led to the identification of genetic defects related to the familial forms of AD. Subsequently to the identification of these genes, various cellular and animal models were designed. These models have helped in understanding aspects of the pathophysiology of the early-onset familial forms of AD. However, the causes of the sporadic late-onset forms of AD (LOAD), which represent the vast majority of AD cases, remain to be identified.
Parkinson’s disease (PD) is the most common movement disorder and the second common progressive neurological disorder after AD. PD affects people over the age of 50 and, in most cases, PD patients become severely disabled. It is estimated that about 1 million people in the United States have PD. In addition to the disabling effects and the emotional toll related to PD, management of this disease is costly to society. The total cost is estimated to exceed $6 billion annually and this cost is anticipated to increase as the population gets older. Currently, there is no cure for PD, and most of the available drugs can at best manage the symptoms associated with the disease. Unfortunately, the long-term use of these symptomatic treatments is associated with adverse effects including motor fluctuations and cognitive symptoms. The search for efficacious therapies that can prevent, reverse or stop the progression of PD would benefit from a better understanding of the pathogenesis of this disease.
Accumulation of β-amyloid (Aβ) peptide and hyper-phosphorylation of tau protein are the two major pathological hallmarks of AD. Cellular pathways that control Aβ and phosphorylated tau levels are of particular interest for the search of agents that reduce Aβ and phosphorylated tau accumulation and ultimately reduce the progression of AD. On the other hand, PD is characterized by the death of dopaminergic neurons in the substantia nigra and the aggregation of α-synuclein protein in Lewy body inclusions in the brain. As such, AD and PD can be seen as two distinct pathologies. However, a large number of AD patients also have α-synuclein deposition and a subset of PD population accumulates Aβ in the brain. The distribution of Lewy bodies in AD occurs mostly in the amygdala, where Lewy bodies are observed in approximately 60% of both sporadic and familial AD (Kotzbauer et al., 2001; Choi et al., 2012). This overlap strongly suggest that specific pathological pathways converge to trigger common hallmarks of both AD and PD in same brain. In addition to the histopathological post mortem overlap between AD and PD, both AD patients and PD patients develop similar disorders including severe depression, hallucination, and psychosis at the advanced stages of the disease. Thus, it may be possible that specific triggers act on cellular pathways that converge to induce abnormalities that are common to AD and PD. In this review, we will attempt to demonstrate that cholesterol metabolism dyshomeostasis may be a common pathological event that leads to both AD and PD progression as well as potential AD and PD overlap. Work by others and from our laboratory for the last ten years points to the cholesterol oxidation product (oxysterol) 27-hydroxycholesterol (27-OHC) as a potential functional link between dysregulation in cholesterol metabolism and AD/PD pathology.
Oxysterol homeostasis in the brain
Cholesterol is an essential molecule in that it regulates a wide variety of functions including a role in the plasma membrane architecture. The brain makes the cholesterol it needs independently of the peripheral cholesterol pool. In the brain, cholesterol is removed by conversion to the oxysterol 24-hydroxycholesterol (24-OHC) via the cytochrome P450 CYP enzyme (CYP46A1) that is expressed exclusively in the brain, primarily in neurons and some astrocytes. On the other hand, the oxysterol 27-hydroxycholesterol (27-OHC) is the major cholesterol metabolite in the circulation and is synthesized by almost all cells from cholesterol by the CYP27A1 enzyme. Circulating 27-OHC levels are 0.15-0.73 μM, and these concentrations can be in the millimolar range in some pathological situations such as atherosclerosis (Brown et al., 1999). 27-OHC is also made in the brain as the CYP27A1 gene is expressed in the brain in neurons, astrocytes and oligodendrocytes, but at very low concentrations (Brown et al., 2004). Conversely to cholesterol that does not cross the blood brain barrier (BBB), both 27-OHC and 24-OHC have the ability to cross into and out of the brain (Lutjohann et al. 1996). Currently, the role of oxysterols and the extent to which these cholesterol oxidation products are important to the pathogenesis of AD/PD are not defined. Oxysterol homeostasis in the brain is tightly regulated with specific levels maintained in various brain regions. For example, the 27-OHC: 24-OHC ratio is of ~1:8 in the frontal cortex, 1:5 in the occipital cortex, and 1:10 in the basal ganglia (Heverin et al., 2004). While there exists a normal physiological need for conversion of cholesterol to 27-OHC and 24-OHC as a route of elimination of cholesterol, increased levels of 27-OHC in the brain may have widespread pathological ramifications. Indeed, excessive formation of 27-OHC occurs following hypercholesterolemia and also oxidative stress, a condition found to accelerate conversion of even normal basal levels of cholesterol to oxysterols (Vaya et al. 2007; Infante et al. 2010). Thus, under oxidative stress conditions, normal levels of cholesterol, either in the peripheral system or in the brain, can be converted to 27-OHC, that when accumulated in the brain alters the 27-OHC: 24-OHC ratio. Alterations in this ratio may have deleterious consequences that increase the risk for AD/PD.
AD and cholesterol metabolism
The finding by Corder et al. in 1993 that the ε4 allele of the apolipoprotein E (ApoE) gene is associated with a high incidence of late-onset AD suggests that disturbances in cholesterol homeostasis are a potential risk for AD. Indeed, ApoE is a major transporter of both peripheral and brain cholesterol, and carrying of ApoE ε4 in humans is associated with high plasma cholesterol incidence (Eto et al., 1988). The discovery by Larry Sparks (1994) that rabbits fed with high cholesterol diets exhibit cerebral β-amyloid (Aβ) plaques reinforced the cholesterol-AD link hypothesis (Sparks et al., 1994). Hypercholesterolemia accelerates the Alzheimer’s amyloid pathology in a transgenic mouse model (Refolo et al., 2000). Additionally, processing of the amyloid-β precursor protein (AβPP) by BACE1 yielding Aβ is shown to be enhanced by a cholesterol-rich environment (Raffai and Weisgraber, 2003). A report from an epidemiological study on a large cohort of women and men has demonstrated that high cholesterol levels during mid-life are associated with a 66% increase in AD risk late in life (Solomon et al., 2009). These findings suggest that the plasma cholesterol level in our 40s determines our degree of risk for AD. At later age, when AD progresses, plasma cholesterol levels do not appear to correlate with progression of dementia-with patients having either normal, low or high plasma cholesterol levels. In addition to cholesterol, altered levels of sphingolipidome, ceramides and several phospholipids levels may be involved in AD. There is also increasing evidence that saturated free fatty acids (FFA) increase the risk of AD (Bazan et al., 2011). More recently, a set of lipids from peripheral blood was discovered to predict conversion to AD with over 90% accuracy (Mapstone et al., 2014). All together, these studies suggest that lipids, including cholesterol, can influence the pathogenesis of AD. However, the question as to how cholesterol contributes to AD remains to be answered. The difficulty in understanding the role of high plasma cholesterol in AD pathogenesis emanates from the facts that (i) the brain makes its own cholesterol and (ii) little or no plasma cholesterol enters into the brain to induce AD pathology because of the impermeability of the blood brain barrier (BBB).
Several hypotheses can be advanced to explain how high levels of cholesterol in plasma can trigger neurodegeneration in the brain. First, the BBB integrity in AD is very likely to be compromised and we have shown that a diet rich in cholesterol makes the BBB leaky (Chen et al., 2008). Second, fluctuations in levels of low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C), rather than cholesterol per se, may correlate better with the progression of AD. Indeed, high LDL-C and low HDL-C levels were found to be associated with cerebral amyloidosis in humans (Reed et al., 2014), and high HDL-C levels have also been shown to correlate with a lower risk of late onset AD in elderly individuals (Reitz et al., 2010). Third, it may also be possible that cholesterol oxidized products (oxysterols) may represent the link between peripheral cholesterol and the neuropathology of AD in the brain. This review will provide data from our laboratory demonstrating a potential link between oxysterols and AD-like pathology in animal models.
We have been studying the potential association between cholesterol metabolism and the development of Alzheimer’s disease (AD)-related pathology in cell cultures, in rabbit brains and in organotypic slices from adult rabbit brains for the last ten years. The following are results we obtained that (a) strongly suggest that cholesterol is linked to AD-like pathology, (b) shed light on oxysterols as the link between plasma cholesterol and AD-like pathology, and (c) identify cellular mechanisms involved in cholesterol and oxysterol-induced AD-like pathology.
a. Cholesterol-enriched diets increase plasma cholesterol, induce AD-like pathology but does not affect brain cholesterol levels
The cholesterol-fed rabbit was initially used as a model for experimental atherosclerosis by Nikolaj Anitschkow in 1913 and was later found by Larry Sparks (1994) to exhibit Aβ plaques. We have also demonstrated that, in addition to increasing Aβ, cholesterol-enriched diets increase tau phosphorylation and oxidative stress in rabbits (Ghribi et al., 2006 a,b). Additionally, the eye blink classical conditioning that is impaired in AD patients is also impaired in the rabbit model (Woodruff-Pak et al., 2007. Schreurs et al., 2012). Furthermore, rabbits have a phylogeny closer to humans than rodents (Graur et al., 1996), and their Aβ sequence, unlike that of rodents, is similar to the Aβ sequence of the human (Johnstone et al., 1991). All these findings suggest that the cholesterol-fed rabbit is a relevant model for sporadic AD studies, as it is a unique model system that exhibits the major pathological hallmarks of AD without genetic manipulations. Thus, this rabbit model provides a complementary system to the current mouse models which are formulated around the autosomal dominant forms of AD. We have measured cholesterol levels in the plasma and the brain of cholesterol-fed rabbits and have found that although blood cholesterol levels are dramatically increased, brain cholesterol concentrations are unchanged (Ghribi et al., 2006a,b). The unchanged brain cholesterol levels suggest that plasma cholesterol itself does not place the brain at risk for AD.
b. Oxysterol levels are disturbed as a result of increased plasma cholesterol
Cholesterol homeostasis in the brain is regulated through de novo synthesis, with no or very poor transfer from the peripheral circulation, due to the impermeability of the BBB to lipoproteins that carry cholesterol (Lange et al., 1999). Conversely to plasma cholesterol, some of the cholesterol oxidized metabolites (oxysterols) have the ability to cross lipophilic membranes into and out of the brain (Heverin et al., 2005). Disturbances in the levels of these oxysterols in the brain may trigger neurodegeneration. 24-hydroxycholesterol (24S-OHC) and 27-hydroxycholesterol (27-OHC) are prominent members of the oxysterol family group. While 24-OHC originates primarily from the brain, 27-OHC originates mostly from the circulation (Bjorkhem et al., 2002; Lutjohann et al., 1996). Brain levels of 27-OHC have been shown to dramatically increase in AD brains (Heverin et al., 2004). In contrast to 27-OHC, 24-OHC levels decrease in brain and increase in plasma as well as cerebrospinal fluid in patients with AD (Lutjohann et al., 2000). We have also measured the concentrations of 27-OHC and 24-OHC in cholesterol-fed rabbits and found that levels of both 24-OHC and 27-OHC markedly increase in the plasma. In the brain, the 24-OHC/27-OHC ratio decreases. Taken together, the above data suggest that increased entrance of 27-OHC into the brain and/or increased exit of 24-OHC out of the brain may represent the link by which high cholesterol levels in plasma induce pathological features in the brain suggestive of AD. As the concentrations of oxysterols are tightly regulated in the brain, the shift in the 24-OHC/27-OHC ratio may have severe consequences that place the brain at risk of degeneration.
In order to determine the extent to which oxysterols cause pathological hallmarks of AD, we have developed an organotypic slice system from adult animals (12-18 month-old mice, Schrag et al., 2008 and 1-3 year-old rabbits, Sharma et al., 2008). The organotypic slice system has many advantages over other in vitro systems including that the connectivity between neurons, interneurons and glia is maintained. We have incubated organotypic slices and human neuroblastoma SH-SY5Y cells with 27-OHC and 24-OHC and found that while 27-OHC increases AβPP, Aβ and phosphorylated tau levels (Marwarha et al., 2010; Prasanthi et al., 2009), 24-OHC facilitates the cleavage of AβPP to the non-amyloidogenic pathway (Prasanthi et al., 2009). More importantly, co-treatment with 24-OHC opposes the 27-OHC-induced increase in levels of AβPP, Aβ and phosphorylated tau. Our data suggest that increased levels of 27-OHC are toxic to the brain while supplementation with 24-OHC can reverse the deleterious effects of 27-OHC. Preventing dysregulation in the 24-OHC/27-OHC ratio in the brain may therefore preclude the formation of AD-like pathology.
c. Cellular mechanisms involved in 27-OHC-induced AD-like pathology
Epidemiological studies have suggested an inverse relationship between the adipocytokine leptin and the onset of Alzheimer’s disease (AD), and leptin supplementation decreases amyloid-β (Aβ) production and tau phosphorylation (p-tau), two major biochemical events that play a key role in the pathogenesis of AD. We have shown that 27-OHC inhibits leptin expression, an effect that correlated with increased levels of Aβ and p-tau (Marwarha et al., 2010). We have also shown that 27-OHC induces endoplasmic reticulum (ER) stress, a cellular response that is implicated in AD and confers leptin resistance (Marwarha et al., 2012). However the extent to which ER stress is involved in 27-OHC-induced attenuation in leptin expression has not been determined. We found that 27-OHC-induced ER stress attenuates leptin expression by activating C/EBP Homologous Protein (CHOP) which negatively regulates C/EBPα, a transcription factor required for leptin expression. The molecular chaperone 4-phenylbutyric acid (4-PBA) precludes 27-OHC-evoked ER stress and down-regulation of leptin. Furthermore, we demonstrate that the activation of the transcription factor CHOP in response to ER stress is pivotal in the attenuation of leptin expression as knocking-down CHOP alleviates the attenuation in leptin expression (Marwarha et al., 2012). Our study implicates ER stress as the mechanistic link in the 27-OHC-induced negative regulation of leptin, a hormone that has potential therapeutic effects in AD by reducing Aβ and phosphorylated tau accumulation. We silenced CHOP gene with siRNA and reduced 27-OHC-induced Aβ production. These data suggest that ER stress-mediated CHOP activation plays a central role in the triggering of AD pathological hallmarks. We also demonstrated that CHOP and the transcription factor NF-κB work in concert to regulate the transcription of BACE1, the enzyme that initiates the cleavage of the amyloid precursor protein to yield Aβ (Marwarha et al., 2013). We further showed that leptin attenuates the activation and transcriptional activity of NF-κB by reducing the acetylation of the p65 subunit in a SIRT1-dependent manner, thus inhibiting NF-κB-mediated transcription of BACE1 and consequently reducing Amyloid-β genesis. Agents that inhibit CHOP activation and subsequent interaction with NF-κB might be promising targets to reduce BACE1 and Aβ overproduction and may ultimately serve as disease-modifying treatments for AD.
PD and lipid metabolism
The causes of PD are likely multi-factorial with several factors including environmental agents and genetic susceptibility participating in the pathogenesis of the disease. For the last few years, lipid dyshomeostasis has been under scrutiny as a risk factor for PD. A standardized food frequency questionnaire has shown an association of PD with high intake of total fat, saturated fats, and cholesterol (Johnson et al. 1999). A large prospective study has also suggested that total serum cholesterol at baseline is associated with an increased risk of PD (Hu et al. 2008; 2010). In other studies however, elevated serum levels of cholesterol were either not associated with PD risk (de Lau et al. 2005) or were related to a decreased PD risk (Powers et al. 2009). More recently, secondary analysis of the DATATOP (Deprenyl and Tocopherol Antioxidative Therapy of Parkinsonism) trial indicated that higher total serum cholesterol concentrations may be associated with a modest slower clinical progression of PD (Huang et al., 2011). The discrepancies in all of the above studies may be due to fluctuations in oxysterols, but not in cholesterol per se, that correlate better with the onset of PD. In line with our hypothesis is a recent study showing increased levels of several oxysterols, including 27-OHC in cortex of PD brains (Cheng et al., 2011). Oxysterols were also shown to be elevated in the cerebral cortex of individuals with Lewy body dementia where they are suggested to accelerate α-synuclein aggregation (Bosco et al. 2006). However, whether the accumulation of 27-OHC in PD brains contributes to the accumulation of α-synuclein remains to be determined. We initiated a series of experiments to examine the extent to which cholesterol metabolism affects levels of α-synuclein.
a. Cholesterol-enriched diets increase α-synuclein
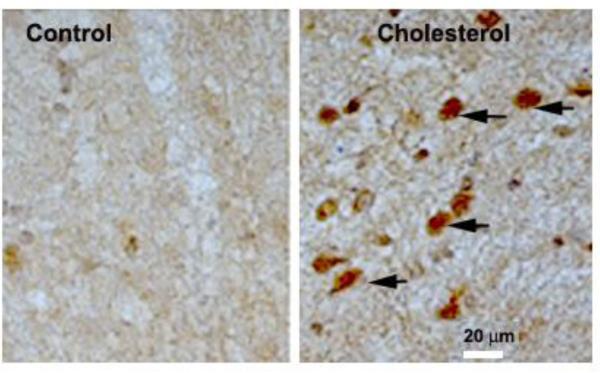
We have fed rabbits a 2% cholesterol-enriched diet for 12 weeks and examined the extent to which this diet affects α-synuclein levels in the substantia nigra (SN) of these animals. We have found that immunoreactivity for α-synuclein increases in SN from cholesterol-fed rabbits (Fig.1). These results show that long-term dietary intake of cholesterol-enriched food causes pathological hallmarks of relevance to PD. One major drawback of the rabbit model is that, although visual examination shows that these animals exhibit pronounced muscle rigidity and impaired movement, this model is unsuitable for behavioral evaluation with the conventional tests available for mice.
Fig. 1.
Photomicrographs of substantia nigra of a control rabbit and a rabbit fed a 2% cholesterol-enriched diet for 12 weeks stained for α-synuclein. Note the substantial increase in α-synuclein immunoreactivity in the cholesterol-fed rabbit.
It is logical to speculate that cholesterol-enriched diet trigger PD-like pathology in rabbits by increasing cholesterol content in the brain. We measured cholesterol levels in brains of cholesterol-fed rabbits and found no increase in these levels despite an increase in plasma cholesterol. We then incubated human SH-SY5Y neuroblastoma cells, or slices from SN of rabbits with various concentration of cholesterol (water soluble, Sigma) and found no changes in α-synuclein expression levels. These results suggest that cholesterol does not directly affect α-synuclein expression levels, raising the question as to how high plasma cholesterol causes α-synuclein accumulation in the brain.
b. 27-OHC increases α-synuclein levels
As the cholesterol metabolite 27-OHC has been reported to accumulate in synucleinopathies, we treated SH-SY5Y cells with this oxysterol and found a marked increase both in soluble and insoluble α-synuclein (Rantham Prabhakara et al. 2008). Conversely, treatment with the oxysterol 24-OHC reduces levels of α-synuclein levels in human neuroblastoma SH-SY5Y cells. Co-treatment with 27-OHC and 24-OHC reduces the changes induced by 27-OHC on α-synuclein levels. These results demonstrate that oxysterols can regulate levels of a protein that is central to PD, and that oxysterols can have differential effects on the regulation of these protein levels. The modulatory effects of these oxysterols can either have destructive consequences, with 27-OHC increasing α-synuclein levels, or have a protective potential, with 24-OHC reducing α-synuclein levels. This regulation of PD-related proteins levels is novel and worthy of further investigation.
c. What are the cellular targets for 27-OHC effects?
Binding to and activating liver X receptors LXR is one of the major mechanisms by which 27-OHC and other oxysterols exert their major functions (Janowski et al. 1996). LXRs bind to promoters of specific genes to recruit co-activators or co-repressors, thus impacting expression of target genes. 27-OHC has been shown for the first time by us to upregulate α-synuclein expression levels (Rantham Prabhakara et al. 2008). Further evidence that 27-OHC increases α-synuclein levels through activation of LXRs (Cheng et al. 2008) was provided shortly after the publication of our data. We conducted an additional study to demonstrate the extent to which increased α-synuclein expression by 27-OHC can be enhanced by the LXR agonist GW3965 or reduced by the specific LXR antagonist 5α-6α-epoxycholesterol-3-sulfate (ECHS). Our results show that 27-OHC and the LXR agonist GW3965 increase α-synuclein protein (monomer and dimer) and mRNA expression levels. Treatment with the LXR antagonist ECHS prevents the 27-OHC effects. These results demonstrate that the LXR pathway regulates the basal as well as the 27-OHC-induced increase in α-synuclein expression at the level of transcription.
We hypothesized that 27-OHC might increase α-synuclein expression by activating LXR and increasing the binding of LXR to the LXR response element (LXRE) in the α-synuclein promoter. To this end, we first performed an EMSA to determine the LXR interaction with an exogenous consensus sequence corresponding to the LXRE in the α-synuclein promoter region. We found increased binding of LXR to this oligomeric probe comprising of the LXRE in the α-synuclein promoter. LXRs are composed of LXRα and LXRβ; both are highly homologous transcription factors that belong to the nuclear receptor family. 27-OHC is characterized as an LXRα/β agonist. We performed a ChIP assay to further characterize the involvement of LXR and elucidate the subtype of LXR (α and β) that mediates 27-OHC-induced increase in α-synuclein expression. We found that LXRβ, but not LXRα, is involved in the regulation of basal expression of α-synuclein as well as 27-OHC-induced increase in α-synuclein expression.
Overall, we show that 27-OHC and the synthetic LXR agonist GW3965 increase the binding of LXRβ, not LXRα, to the α-synuclein promoter (Marwarha et al., 2011). This may be primarily attributed to the fact that α-synuclein is an LXRβ-regulated gene rather than LXRβ being more abundantly expressed in SH-SY5Y cells. Our results suggest that the effects of 24-OHC and 27-OHC on α-synuclein are mediated through LXR activation. While 27-OHC acts as an agonist of LXR and increases α-synuclein levels, 24-OHC may reduce α-synuclein levels by acting as an inverse agonist of LXR.
Conclusions
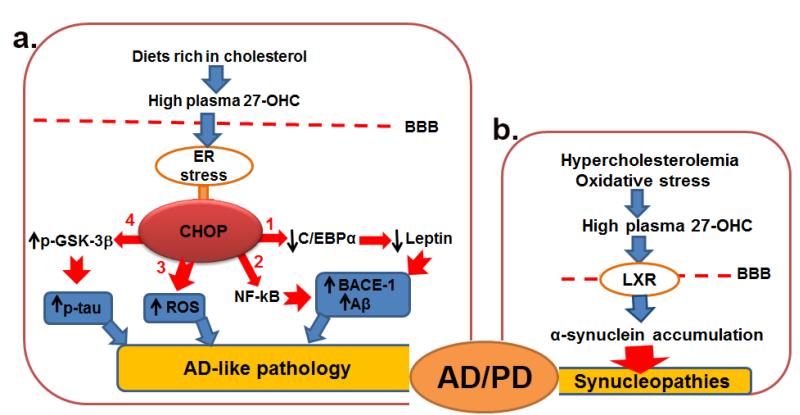
Our published data show that 27-OHC increases Aβ production, tau phosphorylation, α-synuclein accumulation and also oxidative stress, which are all hallmarks of AD and PD. Plasma levels of 27-OHC can increase by three distinct ways. First, intake of diets rich in cholesterol gives raise of plasma cholesterol that in turn with increase 27-OHC formation. Second, even in condition of normal plasma cholesterol levels, oxidative stress can accelerate the conversion of cholesterol to 27-OHC, increasing levels of this oxysterol. Third, dietary products that contain cholesterol and are improperly stored either exposed to sunlight or inadequate temperatures can be oxidized and large part of the oxidation products will be in the form of 27-OHC. Therefore, individuals with hypercholesterolemia, subjected to oxidative stress, or consuming improperly stored products can develop high plasma concentration of 27-OHC. Excess plasma 27-OHC can cross the BBB to the brain where it can activate CHOP, NF-κB, and LXR, causing Aβ overproduction, tau hyperphosphorylation, α-synuclein accumulation, oxidative stress and inflammation, which are all hallmarks of AD and PD (Fig. 2). Oxysterols are bioactive ligands that are known to act on various nuclear receptors such as liver X receptors and estrogen receptors and are involved in several functions including bile acid and sex hormone synthesis, inflammation, proliferation, glucose metabolism, and cell death. It may therefore be possible that high levels of 27-OHC can triggers in some individuals either AD, PD or overlapping AD and PD. It has been shown that high plasma levels of cholesterol at mid age correlates with AD progression and that both AD and PD victims have high levels of 27-OHC in the brain. Increased plasma levels of 27-OHC can thus be a predictor and may be used as a biomarker for developing of AD/PD. Further animal and human studies however are necessary to examine the role of the oxidized cholesterol metabolites and their potential involvement in neurodegenerative diseases.
Fig. 2.
(a) Excess 27-OHC in plasma, as a result of diets rich in cholesterol, crosses the blood brain barrier (BBB) into the brain where it induces endoplasmic reticulum (ER) stress and activates CHOP. CHOP (1) negatively regulates C/EBPα, a transcription factor required for leptin expression. Reduced leptin expression increases processing of amyloid precursor protein by BACE1 enzyme, leading to increased Aβ production; (2) increases the expression of NF-κB, a transcription factor that increases BACE1 levels and Aβ production; (3) increases glutathione depletion and triggers the generation of reactive oxygen species (ROS), an event that exacerbates oxidative stress; and (4) increases the phosphorylation of GSK-3β (p-GSK-3β), an effect that enhances tau phosphorylation (p-tau). Inhibiting CHOP activation would potentially preclude generation of AD hallmarks and slow or stop the progression of AD. (b) Hypercholesterolemia and oxidative stress can accelerate conversion of cholesterol to 27-OHC. High levels of 27-OHC in plasma can activate LXR and increase the transcription of α-synuclein that accumulates in the brain. Chronic over-production of α-synuclein leads to aggregation of this protein, ultimately increasing the risk for synucleinopathies. Agents that reduce 27-OHC levels or inhibit LXRs signaling would reduce α-synuclein accumulation and may protect against synucleinopathies. Individuals with high levels of 27-OHC can therefore exhibit AD, PD or AD/PD pathology.
Highlights.
Alzheimer’s and Parkinson’s diseases share common hallmarks
Lipid dyshomeostasis may be involved in the pathophysiology of these diseases
27-hydroxycholesterol may link dyslipidemia to Alzheimer’s and Parkinson’s diseases
Endoplasmic reticulum stress may underlie the effects of 27-hydroxycholesterol
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bazan NG, Molina MF, Gordon WC. Docosahexaenoic Acid signalolipidomics in nutrition: significance in aging, neuroinflammation, macular degeneration, Alzheimer’s, and other neurodegenerative diseases. Annu Rev Nutr. 2011;31:321–51. doi: 10.1146/annurev.nutr.012809.104635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkhem I, Meaney S, Diczfalusy U. Oxysterols in human circulation: which role do they have? CurrOpinLipidol. 2002;13:247–253. doi: 10.1097/00041433-200206000-00003. [DOI] [PubMed] [Google Scholar]
- Bosco DA, Fowler DM, Zhang Q, Nieva J, Powers ET, Wentworth P, Lerner RA, Kelly JW. Elevated levels of oxidized cholesterol metabolites in Lewy body disease brains accelerate alpha-synuclein fibrilization. Nat. Chem. Biol. 2006;5:249–53. doi: 10.1038/nchembio782. [DOI] [PubMed] [Google Scholar]
- Brown J, III, Theisler C, Silberman S, Magnuson D, Gottardi-Littell N, Lee JM, Yager D, Crowley J, Sambamurti K, Rahman MM, Reiss AB, Eckman CB, Wolozin B. Differential expression of cholesterol hydroxylases in Alzheimer’s disease. J Biol Chem. 2004;279:34674–34681. doi: 10.1074/jbc.M402324200. [DOI] [PubMed] [Google Scholar]
- Brown AJ, Jessup W. Oxysterols and atherosclerosis. Atherosclerosis. 1999;142:1–28. doi: 10.1016/s0021-9150(98)00196-8. [DOI] [PubMed] [Google Scholar]
- Chen X, Gawryluk JW, Wagener JF, Ghribi O, Geiger JD. Caffeine blocks disruption of blood brain barrier in a rabbit model of Alzheimer’s disease. J Neuroinflammation. 2008 Apr 5;:12. doi: 10.1186/1742-2094-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng D, Jenner AM, Shui G, Cheong WF, Mitchell TW, Nealon JR, Kim WS, McCann H, Wenk MR, Halliday GM, Garner B. Lipid pathway alterations in Parkinson’s disease primary visual cortex. PLoS. One. 2011;6:e17299. doi: 10.1371/journal.pone.0017299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng D, Kim WS, Garner B. Regulation of alpha-synuclein expression by liver X receptor ligands in vitro. Neuroreport. 2008;19:1685–1689. doi: 10.1097/WNR.0b013e32831578b2. [DOI] [PubMed] [Google Scholar]
- Choi SH, Olabarrieta M, Lopez OL, Maruca V, Dekosky ST, Hamilton RL, Becker JT. Gray matter atrophy associated with extrapyramidal signs in the Lewy bodyvariant of Alzheimer’s disease. J Alzheimers Dis. 2012;32(4):1043–9. doi: 10.3233/JAD-2012-121108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- de Lau LM, Bornebroek M, Witteman JC, Hofman A, Koudstaal PJ, Breteler MM. Dietary fatty acids and the risk of Parkinson disease: the Rotterdam study. Neurology. 2005;64:2040–2045. doi: 10.1212/01.WNL.0000166038.67153.9F. [DOI] [PubMed] [Google Scholar]
- Eto M, Watanabe K, Chonan N, Ishii K. Familial hypercholesterolemia and apolipoprotein E4. Atherosclerosis. 1988 Aug;(2-3):123–8. doi: 10.1016/0021-9150(88)90072-x. [DOI] [PubMed] [Google Scholar]
- Ghribi O, Golovko MY, Larsen B, Schrag M, Murphy EJ. Deposition of iron and beta-amyloid plaques is associated with cortical cellular damage in rabbits fed with long-term cholesterol-enriched diets. J Neurochem. 2006a;99(2):438–49. doi: 10.1111/j.1471-4159.2006.04079.x. [DOI] [PubMed] [Google Scholar]
- Ghribi O, Larsen B, Schrag M, Herman MM. High cholesterol content in neurons increases BACE, beta-amyloid, and phosphorylated tau levels in rabbit hippocampus. Exp Neurol. 2006b;200(2):460–7. doi: 10.1016/j.expneurol.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Graur D, Duret L, Gouy M. Phylogenetic position of the order Lagomorpha (rabbits, hares and allies) Nature. 1999;379:333–335. doi: 10.1038/379333a0. [DOI] [PubMed] [Google Scholar]
- Heverin M, Meaney S, Lutjohann D, Diczfalusy U, Wahren J, Bjorkhem I. Crossing the barrier: Net flux of 27-hydroxycholesterol into the human brain. J. Lipid Res. 2005;46(5):1047–52. doi: 10.1194/jlr.M500024-JLR200. [DOI] [PubMed] [Google Scholar]
- Heverin M, Bogdanovic N, Lütjohann D, Bayer T, Pikuleva I, Bretillon L, Diczfalusy U, Winblad B, Björkhem I. Changes in the levels of cerebral and extracerebral sterols in the brain of patients with Alzheimer’s disease. J Lipid Res. 2004;445(1):186–93. doi: 10.1194/jlr.M300320-JLR200. [DOI] [PubMed] [Google Scholar]
- Hu G. Total cholesterol and the risk of Parkinson’s disease: a review for some new findings. Parkinsons Dis. 2010;2010:836962. doi: 10.4061/2010/836962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G, Antikainen R, Jousilahti P, Kivipelto M, Tuomilehto J. Total cholesterol and the risk of Parkinson disease. Neurology. 2008 May 20;70(21):1972–9. doi: 10.1212/01.wnl.0000312511.62699.a8. [DOI] [PubMed] [Google Scholar]
- Huang X, Auinger P, Eberly S, Oakes D, Schwarzschild M, Ascherio A, Mailman R, Chen H. Parkinson Study Group DATATOP Investigators. Serum cholesterol and the progression of Parkinson’s disease: results from DATATOP. PLoS One. 2011;6(8):e22854. doi: 10.1371/journal.pone.0022854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infante J, Rodríguez-Rodríguez E, Mateo I, Llorca J, Vázquez-Higuera JL, Berciano J, Combarros O. Gene-gene interaction between heme oxygenase-1 and liver X receptor-beta and Alzheimer’s disease risk. Neurobiol Aging. 2010 Apr;31(4):710–4. doi: 10.1016/j.neurobiolaging.2008.05.025. [DOI] [PubMed] [Google Scholar]
- Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature. 1996;383:728–731. doi: 10.1038/383728a0. [DOI] [PubMed] [Google Scholar]
- Johnson CC, Gorell JM, Rybicki BA, Sanders K, Peterson EL. Adult nutrient intake as a risk factor for Parkinson’s disease. Int. J. Epidemiol. 1999;28:1102–1109. doi: 10.1093/ije/28.6.1102. [DOI] [PubMed] [Google Scholar]
- Johnstone EM, Chaney MO, Norris FH, Pascual R, Little SP. Conservation of the sequence of the Alzheimer’s disease amyloid peptide in dog, polar bear and five other mammals by cross-species polymerase chain reaction analysis. Brain Res Mol Brain Res. 1999;10:299–305. doi: 10.1016/0169-328x(91)90088-f. [DOI] [PubMed] [Google Scholar]
- Kotzbauer PT, Trojanowsk JQ, Lee VM. Lewy body pathology in Alzheimer’s disease. J MolNeurosci. 2001 Oct;17(2):225–32. doi: 10.1385/jmn:17:2:225. [DOI] [PubMed] [Google Scholar]
- Lange Y, Ye J, Rigney M, Steck TL. Regulation of endoplasmic reticulum cholesterol by plasma membrane cholesterol. J Lipid Res. 1999;40:2264–2270. [PubMed] [Google Scholar]
- Lutjohann D, Breuer O, Ahlborg G, Nennesmo I, Siden A, Diczfalusy U, Bjorkhem I. Cholesterol homeostasis in human brain: evidence for an age-dependent flux of 24S-hydroxycholesterol from the brain into the circulation. ProcNatlAcadSci U S A. 1996;93:9799–9804. doi: 10.1073/pnas.93.18.9799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutjohann D, Papassotiropoulos A, Bjorkhem I, Locatelli S, Bagli M, Oehring RD, Schlegel U, Jessen F, Rao ML, von BK, Heun R. Plasma 24S-hydroxycholesterol (cerebrosterol) is increased in Alzheimer and vascular demented patients. J Lipid Res. 2000;41:195–198. [PubMed] [Google Scholar]
- Marwarha G, Raza S, Meiers C, Ghribi O. Leptin attenuates BACE1 expression and amyloid-β genesis via the activation of SIRT1 signaling pathway. BiochimBiophysActa. 2014 Sep;1842(9):1587–95. doi: 10.1016/j.bbadis.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marwarha G, Raza S, Prasanthi JR, Ghribi O. Gadd153 and NF-κB crosstalk regulates 27-hydroxycholesterol-induced increase in BACE1 and β-amyloid production in human neuroblastoma SH-SY5Y cells. PLoS One. 2013 Aug 9;8(8):e70773. doi: 10.1371/journal.pone.0070773. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Marwarha G, Dasari B, Ghribi O. Endoplasmic reticulum stress-induced CHOP activation mediates the down-regulation of leptin in human neuroblastoma SH-SY5Y cells treated with the oxysterol 27-hydroxycholesterol. Cell Signal. 2012 Feb;24(2):484–92. doi: 10.1016/j.cellsig.2011.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marwarha G, Dasari B, Prasanthi JRP, Schommer J, Ghribi O. Leptin is involved in accumulation of amyloid-beta and tau phosphorylation induced by 27-hydroxycholesterol in organotypic slices from adult rabbit hippocampus. J Alzheimers Dis. 2010;19(3):1007–19. doi: 10.3233/JAD-2010-1298. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marwarha G, Rhen T, Schommer T, Ghribi O. The oxysterol 27-hydroxycholesterol regulates α-synuclein and tyrosine hydroxylase expression levels in human neuroblastoma cells through modulation of liver X receptors and estrogen receptors--relevance to Parkinson’s disease. J Neurochem. 2011 Dec;119(5):1119–36. doi: 10.1111/j.1471-4159.2011.07497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapstone M, Cheema AK, Fiandaca MS, Zhong X, Mhyre TR, MacArthur LH, Hall WJ, Fisher SG, Peterson DR, Haley JM, Nazar MD, Rich SA, Berlau DJ, Peltz CB, Tan MT, Kawas CH, Federoff HJ. Plasma phospholipids identify antecedent memory impairment in older adults. Nat Med. 2014 Apr;20(4):415–8. doi: 10.1038/nm.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers KM, Weller T, Franklin GM, Longstreth WT, Jr., Swanson PD, Checkoway H. Dietary fats, cholesterol and iron as risk factors for Parkinson’s disease. Parkinsonism. RelatDisord. 2009;15:47–52. doi: 10.1016/j.parkreldis.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasanthi JR, Larson T, Schommer J, Ghribi O. Silencing GADD153/CHOP gene expression protects against Alzheimer’s disease-like pathology induced by 27-hydroxycholesterol in rabbit hippocampus. PLoS One. 2011;6(10):e26420. doi: 10.1371/journal.pone.0026420. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Prasanthi JRP, Huls A, Thomasson S, Thompson A, Schommer E, Ghribi O. Differential effects of 24-hydroxycholesterol and 27-hydroxycholesterol on beta-amyloid precursor protein levels and processing in human neuroblastoma SH-SY5Y cells. Mol Neurodegener. 2009;4:1. doi: 10.1186/1750-1326-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffai RL, Weisgraber KH. Cholesterol: from heart attacks to Alzheimer’s disease. J Lipid Res. 2003 Aug;44(8):1423–30. doi: 10.1194/jlr.R300007-JLR200. [DOI] [PubMed] [Google Scholar]
- Rantham Prabhakara JP, Feist G, Thomasson S, Thompson A, Schommer E, Ghribi O. Differential effects of 24-hydroxycholesterol and 27-hydroxycholesterol on tyrosine hydroxylase and alpha-synuclein in human neuroblastoma SH-SY5Y cells. J. Neurochem. 2008;107:1722–1729. doi: 10.1111/j.1471-4159.2008.05736.x. [DOI] [PubMed] [Google Scholar]
- Reed B, Villeneuve S, Mack W, DeCarli C, Chui HC, Jagust W. Associations between serum cholesterol levels and cerebral amyloidosis. JAMA Neurol. 2014 Feb;71(2):195–200. doi: 10.1001/jamaneurol.2013.5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refolo LM, Malester B, LaFrancois J, Bryant-Thomas T, Wang R, Tint GS, Sambamurti K, Duff K, Pappolla MA. Hypercholesterolemia accelerates the Alzheimer’s amyloid pathology in a transgenic mouse model. Neurobiol Dis. 2000 Aug;7(4):321–31. doi: 10.1006/nbdi.2000.0304. [DOI] [PubMed] [Google Scholar]
- Reitz C, Tang MX, Schupf N, Manly JJ, Mayeux R, Luchsinger JA. Association of higher levels of high-density lipoprotein cholesterol in elderly individuals and lower risk of late-onset Alzheimer disease. Arch Neurol. 2010 Dec;67(12):1491–7. doi: 10.1001/archneurol.2010.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreurs BG, Wang D, Smith-Bell CA, Burhans LB, Bell R, Gonzalez-Joekes J. Dietary Cholesterol Concentration and Duration Degrade Long-Term Memory of Classical Conditioning of the Rabbit’s Nictitating Membrane Response. Int J Alzheimers Dis. 2012;2012:732634. doi: 10.1155/2012/732634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrag M, Sharma S, Brown-Borg H, Ghribi O. Hippocampus of Ames dwarf mice is resistant to beta-amyloid-induced tau hyperphosphorylation and changes in apoptosis-regulatory protein levels. Hippocampus. 2008;18(3):239–44. doi: 10.1002/hipo.20387. [DOI] [PubMed] [Google Scholar]
- Sharma S, Prasanthi RPJ, Schommer E, Feist G, Ghribi O. Hypercholesterolemia-induced Abeta accumulation in rabbit brain is associated with alteration in IGF-1 signaling. Neurobiol Dis. 2008 Dec;32(3):426–32. doi: 10.1016/j.nbd.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon A, Kivipelto M, Wolozin B, Zhou J, Whitmer RA. Midlife serum cholesterol and increased risk of Alzheimer’s and vascular dementia three decades later. Dement Geriatr Cogn Disord. 2009;28:75–80. doi: 10.1159/000231980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks DL, Scheff SW, Hunsaker JC, III, Liu H, Landers T, Gross DR. Induction of Alzheimer-like beta-amyloid immunoreactivity in the brains of rabbits with dietary cholesterol. Exp Neurol. 1994;126:88–94. doi: 10.1006/exnr.1994.1044. [DOI] [PubMed] [Google Scholar]
- Vaya J, Song W, Khatib S, Geng G, Schipper HM. Effects of heme oxygenase-1 expression on sterol homeostasis in rat astroglia. Free RadicBiol Med. 2007 Mar 15;42(6):864–71. doi: 10.1016/j.freeradbiomed.2006.12.022. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Seta SE, Roker LA, Lehr MA. Effects of paradigm and inter-stimulus interval on age differences in eyeblink classical conditioning in rabbits. Learn Mem. 2007 Apr 6;14(4):287–94. doi: 10.1101/lm.504107. [DOI] [PMC free article] [PubMed] [Google Scholar]