Abstract
Introduction
Retinitis pigmentosa (RP) encompasses many different hereditary retinal degenerations that are caused by a vast array of different gene mutations and have highly variable disease presentations and severities. This heterogeneity poses a significant therapeutic challenge, although an answer may eventually be found through two recent innovations: induced pluripotent stem cells (iPSCs) and clustered regularly interspaced short palindromic repeats (CRISPR)/Cas genome editing.
Areas covered
This review discusses the wide-ranging applications of iPSCs and CRISPR–including disease modelling, diagnostics and therapeutics – with an ultimate view towards understanding how these two technologies can come together to address disease heterogeneity and orphan genes in a novel personalized medicine platform. An extensive literature search was conducted in PubMed and Google Scholar, with a particular focus on high-impact research published within the last 1 – 2 years and centered broadly on the subjects of retinal gene therapy, iPSC-derived outer retina cells, stem cell transplantation and CRISPR/Cas gene editing.
Expert opinion
For the retinal pigment epithelium, autologous transplantation of gene-corrected grafts derived from iPSCs may well be technically feasible in the near future. Photoreceptor transplantation faces more significant unresolved technical challenges but remains an achievable, if more distant, goal given the rapid pace of advancements in the field.
Keywords: clustered regularly interspaced short palindromic repeats, gene therapy, induced pluripotent stem cells, retinal degenerations
1. Introduction
Blindness and vision problems are among the most common disabilities affecting adults in the USA and carry both significant quantitative economic costs as well as qualitative impairments in independence, social interactions and family life [1,2]. Retinitis pigmentosa (RP) describes a genetically and phenotypically heterogenous group of inherited retinal degenerations that are characterized by initial loss of night vision followed by constriction of visual fields and progressive blindness. Vision loss in RP is notable because, unlike the more common causes of blindness – cataracts, glaucoma, age-related macular degeneration (AMD) and diabetic eye disease – that generally affect older adults, some forms of RP present in adolescence or even infancy and can therefore impact vision at much earlier ages. Thus, blindness in the context of RP leads to significantly higher direct costs and a greater number of lost disability-adjusted life years for the patient.
Moreover, the vast heterogeneity in RP poses a challenge both to the development of therapeutic strategies and to our understanding of the underlying mechanisms and pathophysiology of the disease [3]. Currently, over 3000 different disease alleles spread across > 60 different genes and with variable inheritance patterns are associated with classical forms of RP limited to photoreceptor degenerations only; another 40 genes are associated with various forms of syndromic RP, where vision loss is accompanied by extraocular symptoms, and still others harbor mutations that cause degeneration of the retinal pigment epithelium (RPE) [4,5]. Whether this great genetic diversity in RP ultimately converges on one or several shared mechanistic pathways leading to retinal cell death is poorly understood at this time, and this lack of knowledge consequently impedes efforts to elucidate ‘universal’ therapeutic strategies that uniformly treat RP in all of its many different forms. To date, such mutation-independent treatment approaches in humans have been limited to vitamin supplementation, neurotrophic factor-secreting intraocular implants or electronic retinal prostheses, although in the absence of mechanism-based therapies that address the underlying molecular defect, these treatments are only minimally effective at best in slowing disease progression or rescuing vision [6–10].
Inversely, gene supplementation therapy targets RP at the level of the gene mutation and has achieved notable successes. Landmark Phase I/II human clinical trials of RPE65 gene therapy for RPE65-related early onset retinal dystrophy, a form of RP, successfully rescued visual function and improved pupillary light reflex and full-field sensitivity in a small group of pediatric patients [11–14]. A more recent trial involving replacement of the REP1 gene for choroideremia similarly found improved visual acuity and retinal sensitivity [15]. For RPE65, the functional rescue effect was sustained in follow-up studies for at least 3 years after the initial treatment, but unfortunately these studies also suggested at the same time that the rate of structural and cellular degeneration, as extrapolated from retinal thickness data in optical coherence tomography (OCT) scans, was unmodified and continued unabated [16]. This finding will require further validation with more robust sample sizes and longer-term follow-up and, even if true, in no way undermines the immense achievement of restoring years of stable, functional vision to affected children [17]. Nevertheless, this potential pitfall presents the possibility that the window of efficacy for gene therapy is limited by treatment magnitude and/or timeliness, where insufficient or late addition of the normal gene copy to retinal cells will fail to halt or modify the disease course. And although future developments and innovation may expand the therapeutic capacity of gene therapy, in its current implementation gene supplementation therapy can only be expected to slow or halt, but not reverse, the progression of symptomatic disease. This makes it unsuitable for late-stage RP when vision has already been lost. Even among recessive disease alleles most amenable to gene supplementation, some genes, such as USH2A in Usher syndrome and dystrophin (DMD) in Duchenne muscular dystrophy-associated retinopathy, are too large to fit within the adeno-associated virus (AAV) vectors used for gene therapy; addressing these genes would also require alternative approaches, such as the use of aminoglycoside compounds to promote read-through of nonsense mutations, currently being investigated in retinal explant cultures and preclinical animal models [18,19].
More recently, gene editing tools using the bacterial clustered regularly interspaced short palindromic repeats/ CRISPR-associated protein (CRISPR/Cas) system has opened up new therapeutic avenues that can address dominant as well as recessive forms of RP. Coupled with the ability to generate induced pluripotent stem cells (iPSCs) from mature cells and to differentiate them into retinal cells, CRISPR brings within reach the possibility of autologous iPSC-derived retinal cell transplantation, which might not only halt, but also potentially reverse progressive vision loss in RP. At the same time, efficient gene editing would also allow the rapid generation of cell culture and animal disease models tailored for the study of particular disease mutations in individual patients. Here in this review, we will examine these two relatively new technologies, generation of iPSCs and CRISPR gene editing, and discuss how their intersection holds exciting promises of new tools for the study and treatment of RP that are at once broadly applicable across the vast heterogeneity of the disease while remaining adaptable to the specific mutations in each individual patient.
2. New disease models and drug development
2.1 Induced pluripotent stem cell applications
In 2006, Takahashi and Yamanaka demonstrated that viral transduction of just four transcription factors into mature mouse dermal fibroblasts could cause de-differentiation of the cells back to a pluripotent stem cell capable of generating all three germ layers [20]. Shortly thereafter, the same principles were successfully applied to human dermal fibroblasts to generate human iPSCs, and a number of different modifications and protocols for the generation of human and mouse iPSCs have since been published [21–25]. Although much attention has been justifiably focused on the direct therapeutic applications of iPSCs, equally important is the potential for iPSCs and iPSC-derived retinal cells to serve as manipulable disease models that can accurately recreate the biochemical and structural phenotypes of inherited retinal degenerations. A number of similar ‘disease-in-a-dish’ iPSC models have been engineered for a diverse set of conditions including mutations causing choroideremia, Rett syndrome, long QT syndrome, familial Alzheimer’s and LEOPARD syndrome [26–30]. These and other models in turn can serve as the basis for high-throughput drug screening or gene therapy optimization, and they could thereby revolutionize a therapeutic development process that is currently hampered by major financial and logistical challenges.
Two recent success stories for gene therapy in fact illustrate the difficulties of the current process. For RPE65-related early-onset retinal dystrophy, the first preclinical gene therapy proof of principle was published in 2001, describing rescue of vision in a naturally occurring canine model of the disease; more than a decade later in 2014, the same therapy – consistently and demonstrably effective – is now in Phase III trials but has yet to reach market [31]. Alipogene tiparvovec (Glybera®) is another gene therapy, for the treatment of lipoprotein lipase deficiency, which became the first gene theraphy ever approved therapy for use in the Western world. However this approval, by the European Commission in 2012, came only some 15 years after the first experimental treatments in mice. The timeline of Glybera’s development in particular highlights not only the lengthiness of the approval process, but also the immense cost to both developers and patients. For Amsterdam Molecular Therapeutics, the original developers of Glybera, the multiple rounds of reassessments and associated costs ultimately overwhelmed the company and necessitated their acquisition by a competitor. In turn, the projected cost to patients for Glybera gene therapy is expected to be US$1.6 million for a one-time, lifelong treatment [32–34]. The time and expense of approving each new gene therapy is clearly unsuitable for addressing all of the 100+ RP-associated disease genes, and drug companies will find that many of the rare RP genes affecting relatively few patients worldwide will be financially complicated or non-viable in the convoluted context of the regulatory approval process [35].
For those RP patients with orphaned or novel gene mutations, iPSC-derived in vitro models are powerful tools for several reasons. First, in certain cases where the pathogenic mutation itself is unknown or in doubt, iPSC models can be used to confirm a genetic diagnosis. Although Polymorphism Phenotyping v2 (PolyPhen-2) and similar software applied to whole-exome sequencing can provide relatively insightful predictions of pathogenic genetic variations [36], carefully controlled experiments using isogenic iPSCs can more definitively demonstrate mutation pathogenicity. Second, the lower cost and easier manipulations of iPSCs, as compared with animal experiments, make it more feasible to pursue focused, mutation-specific drug discovery and/or gene therapy optimization in the laboratory for commercially nonviable orphan genes. While any clinical products arising from such basic laboratory research are still subject to regulatory oversight and its attendant costs, pursuit of Orphan Drug Designation and Phase I/II clinical trials for novel experimental therapies may be the fastest, cheapest or only way for some patients with rare disease genes to receive effective treatment. An illustrative example of this strategy is the treatment of Wiskott-Aldrich syndrome and metachromatic leukodystrophy using autologous transplantation of gene-corrected hematopoietic stem cells: small pediatric cohorts with severe disease and no conventional therapeutic options received a safe experimental treatment that produced both clinical improvement for patients and scientific discovery for researchers [37,38].
Recent studies underscore the fact that iPSCs can be instrumental in confirming and studying a patient’s specific disease-causing mutations [39–44]. Tucker et al. used iPSCs from an RP patient to confirm a splice site mutation that was not detected by exome sequencing. Analysis of mRNA in retinal cells differentiated from patient-derived iPSCs revealed that the transcript of the USH2A gene contained an unspliced intronic sequence resulting in a premature termination codon [39]. Yoshida et al. more recently illustrate this same point in using iPSCs derived from an RP patient to confirm the pathogenicity of a suspected rhodopsin disease mutation [40]. They directly demonstrated the pathogenicity of the patient’s E181K rhodopsin mutation by rescuing patient-derived cells using HDAdV-mediated gene correction and precipitating rod photoreceptor death by introducing the mutation into wild-type control iPSCs. Furthermore, they then used their iPSC lines to assay ER-stress modifying drugs for efficacy in reducing in vitro rod photoreceptor death and for effects on various apoptosis and autophagy markers. Li et al. similarly used iPSCs as a model for therapeutic testing, showing that viral-mediated gene supplementation therapy could potentially be effective for RP patients with recessive Membrane Frizzled-related Protein (MFRP) mutations because it could successfully rescue the disease phenotype in human iPSC-derived RPE cells with the mutation. This study also used the iPSC-derived RPE as a representative disease model in order to study a few of the signaling interactions and molecular associations of MFRP that may contribute to the pathophysiology behind its mutation [41].
As with any in vitro model, iPSCs lack the biochemical context and microenvironment that in vivo retinal cells have in animal models. Nevertheless, they uniquely allow for exquisitely controlled comparisons between mutant and normal photoreceptor cells, thereby detailing disease initiation phenotypes, drug efficacy, biochemical processes and bio-markers during degeneration and rescue of photoreceptors at a level that cannot easily be achieved in any other animal model or human patient.
2.2 CRISPR/Cas9 genome editing
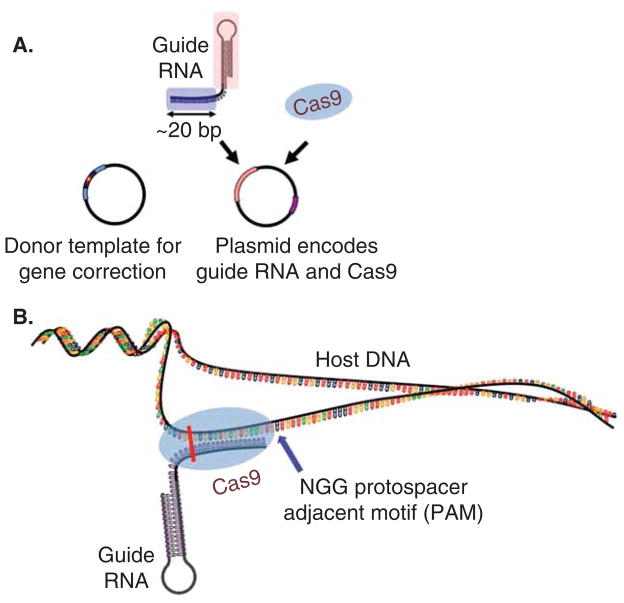
Although the ability to perform in vivo gene editing was earlier demonstrated using zinc-finger nucleases (ZFNs) [45] and transcription activator-like effector nucleases (TALENs) [46,47], the bacterial CRISPR/Cas system provides a radically simpler gene editing strategy by using an RNA-rather than protein-based approach to sequence recognition and nuclease targeting. Originally discovered in Streptococcus species as a form of bacterial adaptive immunity against phages, CRISPR has since been classified into three types, of which the type II system requires only a single Cas protein, Cas9, for both RNase and endonuclease activity. Using a programmable short (17 – 24 nucleotide) guide RNA (gRNA), Cas9 can then be directed to essentially any target site in the genome containing a 2 – 4 nucleotide protospacer-adjacent motif sequence and induce double-strand breaks (DSBs) in the DNA (Figure 1) [48–50]. Repair of DSBs occurs through either non-homologous end joining (NHEJ), which allows for missense or nonsense insertion-deletion (indel) mutations to be introduced into the target gene, or homology-directed repair (HDR), which can use a homologous template to carry out editing or correction on the target gene.
Figure 1. Schematic of clustered regularly interspaced short palindromic repeats/Cas9 gene editing.
(A) A plasmid encoding the guide RNA (gRNA) and Cas9 protein can be introduced into host cells via direct injection or a viral vector and is sufficient to induce targeted cutting of the host genome. Co-injection of a donor template containing the desired editing sequence will allow point mutations or corrections to be made via homology direct repair. (B) Once expressed within the cell, the 17 – 24 nucleotide gRNA will pair with the complementary sequence in the host gene, provided that the host sequence is upstream of a NGG trinucleotide motif, and direct Cas9 endonuclease to induce a DSB at that site (vertical bar).
A major benefit of CRISPR/Cas9 is the relative ease with which new gRNA can be synthesized for a given locus of interest, compared with the time-intensive process of protein engineering required for ZFNs and TALENs. Comparisons of mutagenesis using TALENs and CRISPR in human pluripotent stem cells also showed that CRISPR can introduce NHEJ indels and HDR knock-in mutations in a highly allele-specific manner and at much higher efficiencies than can TALENs at the same loci [51,52]. Furthermore, use of multiple different gRNA within a single CRISPR/Cas9 construct, each targeted to different loci, allows for efficient, multiplex genome editing [53]. In zebrafish, such a multiplex CRISPR/ Cas system has been shown to induce efficient biallelic mutagenesis in up to five different loci simultaneously [54]. In turn, these distinct advantages of CRISPR/Cas – cost, flexibility, efficiency, specificity and multiplex ability – unlock new, higher throughput means of studying the specific and often rare RP mutations in individual patients.
One exciting application of CRISPR is the new ability to perform precise and multiplex genetic manipulations in fertilized oocytes that in turn allows for the rapid generation of transgenic animals in a single step. Wang et al. showed that mouse zygotes co-injected with Cas9 mRNA and one or more gRNA targeted to disrupt different loci could then be implanted into surrogate mothers to produce viable, biallelic, single- and double-transgenic offspring with high efficiency. Specific point mutations via HDR could also be introduced into two genes simultaneously by co-injecting mouse zygotes with template oligos alongside the Cas9 mRNA and gRNAs [55]. Use of CRISPR/Cas9 in these experiments did not produce any observable off-target effects or embryo toxicity and notably shortened the timeframe for generating single-and double-transgenic mice to within one gestational period, or < 1 month. Conventional techniques to generate single-and double-mutant mice require several generations and 6 months to a year or longer, and are moreover ill suited to use in higher order animals. Only recently in 2009 were the first transgenic dogs created expressing a red-fluorescent protein construct [56], and as such, many studies of eye disease in dog models, including RPE65 LCA and achromatopsia, have focused on naturally occurring mutations instead [31,57,58]. The use of CRISPR/Cas9 for generating transgenic animals therefore overcomes significant barriers that limit the number of different mutations that can be modelled and the range of species that can be used.
While CRISPR-based technology remains economically infeasible for use at the personalized level to test and create therapies for patients individually, the ability to generate timely new transgenic mice could work synergistically with new iPSC models of RP to develop and test experimental therapies for those ‘orphan’ mutations that are insufficiently prevalent to be commercially viable [3,59]. For example, promising drug candidates or optimal gene therapy dosages identified in initial iPSC-derived in vitro screens can then be translated into preclinical animal studies to further assess toxicity and efficacy before limited experimental ‘compassionate use’ is implemented in human patients. The significantly contracted timeline and much lower costs of this process, as compared with multi-phase human clinical trials, makes it more feasible to uncover new drugs or to scale gene therapy technology for more and rarer RP mutations and patients. However, even with higher throughput means of developing these new treatments, it remains unlikely for gene therapy or a small molecule drug to target all RP mutations, and almost impossible for them to reverse the widespread damage that is present in late-stage disease retinas.
3. Potential regenerative therapies for retinal degenerations
Cell transplantation has the ability to reverse degeneration and vision loss more so than any other treatment available to the damaged retina and has been a focus of research for at least three decades [60–63]. Over the years, diverse transplant approaches, with varying degrees of success, have used different graft techniques and cell types, including dissociated hippocampal-derived neural stem cells [64,65], embryonic retinal progenitors [66,67] and postnatal photoreceptor precursor cells [68–70]. Of these, postnatal, postmitotic photoreceptor precursors were first shown by MacLaren et al. to be most efficient in migrating to the appropriate location in the outer nuclear layer (ONL), forming outer segments (OS), establishing synaptic connections with surviving host cells, and rescuing light-sensitive vision [68]. Perhaps counterintuitively, earlier retinal progenitors that had not yet committed to a photoreceptor fate, as well as fully matured adult photoreceptors, were not as successful for transplantation and did not recapitulate the photoreceptor OS, migrate to the ONL or integrate within the host retina [66,67,71].
However, photoreceptor precursor cells that have undergone final mitosis are by their definition and nature a non-expandable and limited population. Moreover, rod development occurs fairly late in human life and continues postnatally, with only slightly more than half of all rod precursors generated at the beginning of the third trimester [72]. These characteristics make precursor cells both an unethical and impractical source for retinal cell transplantation. Instead, pluripotent stem cells offer a source for unlimited number of cells and, especially in the case of iPSCs, one that is generally free from ethical constraints. Studies of embryonic stem cells (ESCs) can inform our expectations for potential iPSC-based therapies in the future, although earlier studies are contradictory in their conclusions regarding the efficacy of ESC-derived photoreceptor precursor cell transplantation: among others, Lamba et al. showed that human ESC-derived precursor cells could mature and integrate in mouse host retina and restore some light responses, whereas West et al. reported that mouse ESCs in a similar set of experiments did not integrate or mature [73,74]. More recently, and more promising, a novel ESC differentiation protocol has been used to produce in vitro neuroretina that forms optic cups and is better able to recreate the embryonic environment in which photoreceptor precursor cells are born and matured [75]. Photoreceptor precursors derived from these cultured retinas were transplanted into mouse retinas and were able to integrate and mature into cells that morphologically and biochemically resembled normal in vivo photoreceptors [76,77].
Results from human trials using ESC-derived cell replacement are much more limited. Only three trials have ever been initiated, of which one – Geron’s spinal cord injury trial – was terminated early for financial reasons and with no data published. Perhaps encouraging for the treatment of RP, however, the other two stem cell studies – Advanced Cell Technology’s treatment trials for Stargardt macular dystrophy and AMD – recently reported encouraging findings from short- and medium-term follow-ups [78,79]. Although the improvement in visual acuity for treated eyes was modest and potentially subject to patient and observer bias, it is also possible that the late-stage severity of the patients at the time of treatment (all with visual acuity of 20/200 or worse) mitigated any protective effects that might have resulted from earlier replacement of the supportive RPE cell layer. Regardless, the lack of serious adverse effects (no tumors, ectopic growth or transplant rejection) and demonstration of some efficacy for these small Phase I trials will likely encourage further study of stem cell-based transplantation therapies.
Theoretically, iPSCs offer an autologous alternative to ESC transplantation that would likely not require lifelong immunosuppressive therapy since the graft would contain the patient’s own cells expressing self-antigens and would therefore largely preclude the possibility of rejection. This point is relevant to cell transplantation therapies for RP, despite the immunoprivileged space established by the blood–retinal barrier (BRB), because previous studies have found that degenerative conditions and surgical trauma can compromise the BRB and lead to loss of immune privilege, microglial infiltration and destruction of allogenic retinal grafts [61,80].
Although currently there are no ongoing human treatment trials involving iPSCs, transplantation of human iPSC-derived RPE cells into a mouse retinal degeneration model rescued electroretinogram function and could be detected by histology [81]. Likewise, preclinical studies of iPSC-derived photoreceptor precursors reproduce many of the findings first seen in studies of ESC-based transplants, including synaptic integration between graft and host, maturation of outer segments and some restoration of functional vision [82–84]. More recently, the three-dimensional culture protocol by Eiraku et al. discussed previously for ESC-derived retina was applied to the culture of mouse iPSCs to generate optic vesicle-like structures. These iPSC-derived retinal cultures were then used for retinal sheet transplantation into the retinas of 6- and 8-week rd1 mice modelling late-stage RP. The en bloc grafts formed outer segments, retained normal ONL structure, were not obstructed by gliosis and most importantly were able to synapse and integrate with host bipolar cells [85]. Overall, these and other studies of iPSC-based retinal transplantation indicate that iPSCs possess many of the same capabilities, characteristics and therapeutic promises of ESCs.
In some cases even without correction of the underlying mutation, iPSCs can be therapeutically useful to RP patients if the degeneration timeline is sufficiently protracted, as it is for many autosomal dominant, and some autosomal recessive, conditions. Tucker et al. noted that the course of retinal degeneration in their particular Usher syndrome patient was fairly slow and that photoreceptor function was normal until the third decade of life. As such, this patient at later stages of retinal degeneration and vision loss could benefit from autologous transplantation of new photoreceptors, generated from his or her own dermal fibroblast- or keratinocyte-derived iPSCs [39]. Although unaltered transplant cells would also eventually degenerate, resetting the degeneration timeline with new cells, at least in cases of slowly progressive disease, may be sufficient to preserve useful vision for the remainder of a patient’s life.
For those cases with more rapid degenerations or where a definitive cure is desired, the CRISPR/Cas system can be tailored to precisely and specifically target the patient’s particular mutation. Use of CRISPR/Cas in this treatment scheme can occur either in conjunction with or independent of iPSC transplantation. AAV vectors used in gene supplementation therapy are also optimal vehicles for genome editing machinery and can deliver components directly to the organ or cells of interest. For example, systemic injection of an AAV vector carrying a zinc-finger nuclease and donor template construct was able to correct mutant transgenic clotting Factor IX in mice and reconstitute low but clinically detectable levels of circulating protein [86]. AAV-mediated delivery of CRISPR/ Cas components was also successful in vivo, achieving low but not insignificant editing efficiency (0.5%) in mouse hepatocytes following tail vein injection [87]. Given the successes of subretinal injection in gene supplementation therapy, it seems reasonable to extrapolate that AAV-CRISPR can also be delivered directly and locally to treat the diseased retina. This hypothetical, novel viral vector treatment would potentially have broader applications than conventional gene supplementation therapy for several reasons. First, it can address dominant mutations via the same gene correction mechanism that is used for recessive mutations. Second, it is not limited by size constraints with regards to the gene of interest. Whereas full-length DMD will likely never be delivered via viral vectors, the size of CRISPR components remain constant and are independent of the size of the target gene; a template oligo used to correct a DMD mutation would not necessarily need to be larger than that used for any other gene mutation. Finally, CRISPR editing can maintain the endogenous gene expression stoichiometry within a cell as long as it does not introduce mutations to knockout the gene. Contrarily, gene supplementation results in variable increases in gene copy number and expression that can be deleterious to the treated cell. For some genes whose expression patterns fall within a narrow physiologic window, the excess production of functional protein may be just as pathologic as underproduction [88–90].
These and other advantages of CRISPR/Cas editing give it a wide range of possible clinical applications. Recently, a multiplex approach targeting different enhancers and promoters within the long terminal repeats of HIV-1 was able to excise latent infection as well as sterilely vaccinate against new infection for myeloid cells in vitro [91,92]. For RP, the precision with which CRISPR/Cas can correct mutations without leaving a genetic footprint, combined with the regenerative capacities of autologous iPSC-derived cell transplantation, could realize the first platform for personalized medicine – one that is adaptable to any patient’s mutation and effective at any stage of the disease. In this treatment model, a patient’s keratinocytes or dermal fibroblasts would be transformed back into iPSCs, which would then be differentiated into photoreceptor precursor cells (Figure 2). CRISPR editing of the disease mutation could occur either on the iPSCs prior to retinal differentiation or on the precursor cells prior to transplantation. And because the graft would contain the patient’s own cells expressing self-antigens, immunosuppression theoretically would not be needed following transplantation.
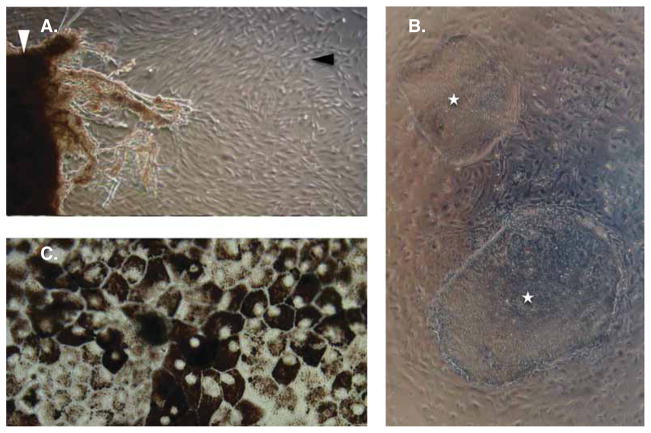
Figure 2. Photographic sequence of the steps involved in autologous retinal transplantation.
(A) Skin punch biopsy (white arrowhead) is cultured in DMEM, 10% FBS, 1× nucleosides, 1× L-glutamine and 1× non-essential amino acids to yield dermal fibroblasts (black arrowhead) which are then reprogrammed into (B) colonies of iPSCs (white stars) in knockout DMEM, 15% knockout serum replacement, 1× L-glutamine, 1× non-essential amino acid, 1× penicillin-streptomycin, 0.1 mM β-mercaptoethanol and 10 ng/ml basic fibroblast growth factor media. iPSCs can then be differentiated into many different cell fates. For the treatment of retinitis pigmentosa, differentiation into (C) retinal pigment epithelium or photoreceptor precursor cells (not shown) will be most common.
iPSC: Induced pluripotent stem cell.
4. Challenges and future directions
Ideally the patient, after transplant in the process described above, would experience rescue of photoreceptor integrity and visual acuity. In reality, a number of substantial barriers currently stand in the way of bringing this combined CRISPR-iPSC treatment modality to human patients. One such obstacle is the need to optimize the efficiency of precursor cell transplantation. For current methods in animal models, < 0.5% of transplanted cells ultimately survive and integrate into the host retina, and the absolute number of integrated cells is usually < 1000 in a mouse retina that normally has 6.4 million rods [68,77,93]. Although a precise therapeutic threshold has not been delineated and may vary for different genes and mutations, this small transplant population is likely insufficient for functional visual correction. For example, it is much less than the ~ 10% rod population threshold below which secondary loss of cones (and therefore visual acuity) begins to occur [94]. By comparison, gene therapy in mid-stage RP mouse models, administered at a time point analogous to when symptomatic human patients generally present to the clinic, can rescue 30% of photoreceptors, prevent secondary cone death and preserve electroretinographic function [95].
Additionally, questions remain as to how effectively grafted photoreceptors can make correct and meaningful synaptic connections with ON and OFF host bipolar cells, and whether inappropriate graft-host synapses would ultimately affect vision rescue. CRISPR also currently has limitations, especially with regards to off-target Cas9 effects and unintended mutations introduced into normal parts of the genome. Although a 17 – 24 nucleotide complementarity between host DNA and CRISPR gRNA should be sufficient to describe a unique location in the human genome, early studies show that the latter half of the gRNA is especially permissive for mismatches and can sometimes result in off-target mutations [49,53]. The recent creation of mutated Cas9 converted to a single-strand ‘nickase’ partially alleviates this problem of specificity and off-target DNA damage, but does so at a cost of reduced endonuclease efficiency in producing DSBs [96,97].
Finally, regulatory considerations and partnerships between academic research and industry will need to be considered as these therapies progress towards commercialization. A major question regarding clinical approval is whether an iPSC-based therapy can be approved as a single method or process covering all future patient cell lines, or whether each patient’s cells will need to be approved as separate products. This is a salient issue because cell phenotypes differ in many ways between individuals, and the precise treatments and procedures applied to patient cell lines will change for different cases or diseases [98]. Most likely, for a CRISPR/iPSC-based personalized treatment to be approved at the commercial level would require a major institutional paradigm shift from the current process of evaluating static products and devices, to one that is capable of granting broad approval to the general technologies of iPSCs and CRISPR while preserving their essential flexibility in engineering different cell types and genetic cargoes for different variants of disease. In the event that personalized, patient-derived iPSCs do not receive blanket approval for clinical use, another option would be to pursue comprehensive iPSC banking such that there can be an extensive but finite set of approved cell lines for transplantation into a variety of different HLA genotypes. New developments that allow iPSCs to be extracted from a finger-prick volume of blood would greatly aid such an effort [99].
5. Conclusion
Recent times have been particularly exciting for the field of retinal degenerations, as a number of promising and innovative therapies have begun to emerge both in preclinical models and for human patients. Gene therapy for the treatment of an eye disease appears on track to reach the commercial market as Phase III trials involving RPE65-related early onset retinal dystrophy conclude in the next few years. However, some questions remain regarding the ultimate long-term efficacy of gene therapy, and the wide diversity and heterogeneity in RP mutations continue to pose a challenge for scaling up the treatment to reach more patients with rarer mutations. To that end, iPSCs and CRISPR/Cas genome editing are two fairly new innovations that hold great promise both for the further study and understanding of RP as well as for the development of additional effective therapies. Most importantly, although there remain considerable obstacles that must first be resolved, the combination of autologous iPSC-derived cell transplantation with CRISPR-mediated gene correction presents the possibility of personalized, precision-medicine that can be designed for a specific mutation and achieve a reliable and persistent therapy.
6. Expert opinion
Retinal cell transplantation holds promise for the treatment of retinitis pigmentosa. RPE cell replacement is likely to reach patients earlier than photoreceptor cell replacement for several reasons, including the fact that early transplantation trials for Stargardt disease and AMD showed encouraging results. The most important contribution of these trials is to show that transplantation with ESC-derived material is safe and does not lead to ectopic growths or tumor formation over the long-term. Improvements in visual acuity were also reported, although significant rescue of visual function would not necessarily have been expected in these patients with late-stage eye disease. Indeed, the reported improvements in vision were modest and potentially confounded by a lack of blinding either the investigators or the patients to the intervention. Nevertheless, we would expect stem cell transplantation to have great therapeutic efficacy for patients with particular forms of RP as well as those with Stargardt disease or AMD. RPE cells are ideal for transplant because they are fairly resilient and amenable to in vitro treatments such as gene addition therapy or gene-editing prior to surgery. Moreover, they do not make synaptic connections with other neurons in the retina, meaning that they will simply have to survive and migrate to the proper location in order to carry out their supportive and nourishing functions. In the future, if iPSC-RPE can be used for autologous transplant, adverse effects of this treatment will decrease further as patients will no longer need lifelong immunosuppressive therapy.
Photoreceptor transplantation remains an important goal of research in RP therapies, especially since the majority of RP mutations involve photoreceptor genes and cause primary death of photoreceptor cells. However, there remain a number of hurdles that may delay or derail this effort. First, photoreceptor cells exhibit a delicate metabolic balance that is vulnerable to disruption intrinsically via genetic mutations or extrinsically via inhospitable environmental conditions or other factors. This partially explains why so many diverse mutations cause photoreceptor death in the first place, and also why transplantation strategies in preclinical models have so far failed to graft large populations of cells into the host retina. Integration of photoreceptor grafts within host retinal tissue is also an ongoing problem despite evidence that transplanted photoreceptors do make connections with host cells. The normal retina is a highly organized, hierarchical system of signal transduction, and it remains to be seen whether specific, directed connections between individual transplanted photoreceptor cells and host bipolar cells are either necessary or even possible. If the ultimate goal of advanced transplantation therapy is to restore useful vision and not merely light and motion perception (which are already achievable via the electronic retinal implant), then it may be problematic if the seemingly indiscriminant connections that are established in current transplantation studies disrupts normal retinal signaling or retinotopic visual processing. Finally, photoreceptor transplantation carries additional risks for iatrogenic malignancy or ectopic growth due to the need to transplant precursor cells rather than mature photoreceptors. Because these cells are not fully mature, the population of transplanted precursor cells would likely also include a proportion of multipotent cells of early stages that would have greater malignant potential.
In short, iPSCs and CRISPR genome editing are remarkable innovations that have many applications to the study and potential treatment of RP. The goal of combining these tools to produce a personalized therapy is possibly within reach for degenerations affecting the RPE layer. For RP patients with primary defects in photoreceptors, realizing this therapy may prove more difficult, although it is important to emphasize that these are still very nascent technologies that in a short time have continually yielded remarkable and unexpected advancements.
Article highlights.
Gene therapy can be effective in the treatment of retinitis pigmentosa but has some technical and logistical limitations.
Induced pluripotent stem cells can be used for disease modelling, drug screening and retinal transplantation.
Clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 is a simple, novel and manipulable system for gene editing with both research and therapeutic applications.
Transplantation of photoreceptors with integration into host retina is possible but still in early stages.
Combination of induced pluripotent stem cells with CRISPR gene editing could provide a personalized therapy where a patient’s own cells are corrected and used to replace their degenerating retina.
This box summarizes key points contained in the article.
Footnotes
Declaration of interest
The Bernard & Shirlee Brown Glaucoma Laboratory and Barbara & Donald Jonas Stem Cell Laboratory are supported by NIH core grants 5P30CA013696 and 5P30EY019007 and unrestricted funds from Research to Prevent Blindness (New York, NY, USA). SH Tsang is a member of the RD-CURE consortium and is supported by the Tistou and Charlotte Kerstan Foundation. This work is also supported by NIH R01EY018213, the Research to Prevent Blindness Physician-Scientist Award, the Nancy and Kobi Karp Foundation, the Schneeweiss Stem Cell Fund, New York State (M09G-302), the Foundation Fighting Blindness New York Regional Research Center Grant (C-NY-0705-0312), the Joel Hoffman Fund, Charles Culpeper Scholarship, the Irma T. Hirschl Charitable Trust, the Professor Gertrude Rothschild Stem Cell Foundation and Gebroe Family Foundation. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Bibliography
Papers of special note have been highlighted as either of interest
(•) or of considerable interest
(••) to readers.
- 1.CDC. Prevalence of disabilities and associated health conditions among adults–United States, 1999. MMWR Morb Mortal Wkly Rep. 2001;50(7):120–5. [PubMed] [Google Scholar]
- 2.Ellwein LB, Friedlin V, McBean AM, Lee PP. Use of eye care services among the 1991 medicare population. Ophthalmology. 1996;103(11):1732–43. doi: 10.1016/s0161-6420(96)30433-8. [DOI] [PubMed] [Google Scholar]
- 3.Wiley LA, Burnight ER, Songstad AE, et al. Patient-specific induced pluripotent stem cells (iPSCs) for the study and treatment of retinal degenerative diseases. Prog Retin Eye Res. 2014;44:15–35. doi: 10.1016/j.preteyeres.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Daiger SP, Sullivan LS, Bowne SJ. Genes and mutations causing retinitis pigmentosa. Clin Genet. 2013;84(2):132–41. doi: 10.1111/cge.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daiger SP. RetNet. The Retinal Information Network; [Accessed 05 January 2015]. Summaries of genes and loci causing retinal diseases. Updated 08 December 2014. Available from: www.sph.uth.tmc.edu/RetNet/ [Google Scholar]
- 6.Berson EL, Rosner B, Sandberg MA, et al. A randomized trial of vitamin a and vitamin e supplementation for retinitis pigmentosa retinitis. Arch Ophthalmol. 1993;111:761–72. doi: 10.1001/archopht.1993.01090060049022. [DOI] [PubMed] [Google Scholar]
- 7.Berson EL, Rosner B, Sandberg MA, et al. Clinical trial of lutein in patients with retinitis pigmentosa receiving vitamin A. Arch Ophthalmol. 2010;128(4):403–11. doi: 10.1001/archophthalmol.2010.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zein WM, Jeffrey BG, Wiley HE, et al. CNGB3-achromatopsia clinical trial with CNTF: diminished rod pathway responses with no evidence of improvement in cone function. Invest Ophthalmol Vis Sci. 2014;55(10):6301–8. doi: 10.1167/iovs.14-14860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahuja AK, Dorn JD, Caspi A, et al. Blind subjects implanted with the Argus II retinal prosthesis are able to improve performance in a spatial-motor task. Br J Ophthalmol. 2011;95(4):539–43. doi: 10.1136/bjo.2010.179622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dorn JD, Ahuja AK, Caspi A, et al. The Detection of Motion by Blind Subjects With the Epiretinal 60-Electrode (Argus II) Retinal Prosthesis. JAMA Ophthalmol. 2013;131(2):183–9. doi: 10.1001/2013.jamaophthalmol.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11••.Bainbridge JW, Smith AJ, Barker SS, et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med. 2008;358(21):2231–9. doi: 10.1056/NEJMoa0802268. The three references noted here were published within 1 month of each other and together constitute the first reports of safe and effective gene addition therapy in human patients. [DOI] [PubMed] [Google Scholar]
- 12••.Hauswirth WW, Aleman TS, Kaushal S, et al. Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum Gene Ther. 2008;19(10):979–90. doi: 10.1089/hum.2008.107. The three references noted here were published within 1 month of each other and together constitute the first reports of safe and effective gene addition therapy in human patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13••.Pugh EN, Mingozzi F, Bennicelli J, et al. Safety and efficacy of gene transfer for leber’s congenital amaurosis. N Engl J Med. 2008;358:2240–8. doi: 10.1056/NEJMoa0802315. The three references noted here were published within 1 month of each other and together constitute the first reports of safe and effective gene addition therapy in human patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maguire AM, High KA, Auricchio A, et al. Age-dependent effects of RPE65 gene therapy for Leber’s congenital amaurosis: a phase 1 dose-escalation trial. Lancet. 2009;374(9701):1597–605. doi: 10.1016/S0140-6736(09)61836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacLaren RE, Groppe M, Barnard AR, et al. Retinal gene therapy in patients with choroideremia: initial findings from a phase 1/2 clinical trial. Lancet. 2014;383(9923):1129–37. doi: 10.1016/S0140-6736(13)62117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16•.Cideciyan AV, Jacobson SG, Beltran WA, et al. Human retinal gene therapy for Leber congenital amaurosis shows advancing retinal degeneration despite enduring visual improvement. Proc Natl Acad Sci USA. 2013;110(6):E517–25. doi: 10.1073/pnas.1218933110. Follow-up studies to the landmark reports on RPE65 gene therapy have been more equivocal. This paper and the next present opposing perspectives on the long-term therapeutic effects and expectations of this treatment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17•.Wojno AP, Pierce EA, Bennett J. Seeing the light. Sci Transl Med. 2013;5(175):175fs8. doi: 10.1126/scitranslmed.3005798. Follow-up studies to the landmark reports on RPE65 gene therapy have been more equivocal. This paper and the previous paper present opposing perspectives on the long-term therapeutic effects and expectations of this treatment. [DOI] [PubMed] [Google Scholar]
- 18.Goldmann T, Rebibo-Sabbah A, Overlack N, et al. Beneficial read-through of a USH1C nonsense mutation by designed aminoglycoside NB30 in the retina. Invest Ophthalmol Vis Sci. 2010;51(12):6671–80. doi: 10.1167/iovs.10-5741. [DOI] [PubMed] [Google Scholar]
- 19.Goldmann T, Overlack N, Wolfrum U, Nagel-Wolfrum K. PTC124-mediated translational readthrough of a nonsense mutation causing Usher syndrome type 1C. Hum Gene Ther. 2011;22(5):537–47. doi: 10.1089/hum.2010.067. [DOI] [PubMed] [Google Scholar]
- 20••.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76. doi: 10.1016/j.cell.2006.07.024. This landmark paper details the groundbreaking discovery that mature, terminally differentiated somatic cells in mice could be reprogrammed to a pluripotent state by the application of only four transcription factors. [DOI] [PubMed] [Google Scholar]
- 21••.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–72. doi: 10.1016/j.cell.2007.11.019. Replication of the iPSC reprogramming phenomenon using human cells, as reported in this paper, opened a new field of research into clinical applications of iPSCs for the treatment of disease. [DOI] [PubMed] [Google Scholar]
- 22.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448(7151):313–17. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 23.Nakagawa M, Koyanagi M, Tanabe K, et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26(1):101–6. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- 24.Kim D, Kim C, Moon J, et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009;4(6):472–6. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi Y, Do JT, Desponts C, et al. A combined chemical and genetic approach for the generation of induced pluripotent stem cells. Cell Stem Cell. 2008;2(6):525–8. doi: 10.1016/j.stem.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 26.Vasireddy V, Mills JA, Gaddameedi R, et al. AAV-mediated gene therapy for choroideremia: preclinical studies in personalized models. PLoS One. 2013;8(5):e61396. doi: 10.1371/journal.pone.0061396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ananiev G, Williams EC, Li H, Chang Q. Isogenic pairs of wild type and mutant induced pluripotent stem cell (iPSC) lines from Rett syndrome patients as in vitro disease model. PLoS One. 2011;6(9):e25255. doi: 10.1371/journal.pone.0025255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Itzhaki I, Maizels L, Huber I, et al. Modelling the long QT syndrome with induced pluripotent stem cells. Nature. 2011;471(7337):225–9. doi: 10.1038/nature09747. [DOI] [PubMed] [Google Scholar]
- 29.Yagi T, Ito D, Okada Y, et al. Modeling familial Alzheimer’s disease with induced pluripotent stem cells. Hum Mol Genet. 2011;20(23):4530–9. doi: 10.1093/hmg/ddr394. [DOI] [PubMed] [Google Scholar]
- 30.Carvajal-Vergara X, Sevilla A, D’Souza SL, et al. Patient-specific induced pluripotent stem-cell-derived models of LEOPARD syndrome. Nature. 2010;465(7299):808–12. doi: 10.1038/nature09005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Acland GM, Aguirre GD, Ray J, et al. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet. 2001;28(1):92–5. doi: 10.1038/ng0501-92. [DOI] [PubMed] [Google Scholar]
- 32.Excoffon KJ, Liu G, Miao L, et al. Correction of hypertriglyceridemia and impaired fat tolerance in lipoprotein lipase–deficient mice by adenovirus-mediated expression of human lipoprotein lipase. Arterioscler Thromb Vasc Biol. 1997;17:2532–9. doi: 10.1161/01.atv.17.11.2532. [DOI] [PubMed] [Google Scholar]
- 33.Libby AE, Wang H. An update on gene therapy for the treatment of lipoprotein lipase deficiency. Orphan Drugs Res Rev. 2014;4:47–54. [Google Scholar]
- 34.Bryant LM, Christopher DM, Giles AR, et al. Lessons learned from the clinical development and market authorization of Glybera. Hum Gene Ther Clin Dev. 2013;24(2):55–64. doi: 10.1089/humc.2013.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brennan TA, Wilson JM. The special case of gene therapy pricing. Nat Biotechnol. 2014;32(9):874–6. doi: 10.1038/nbt.3003. [DOI] [PubMed] [Google Scholar]
- 36.Adzhubei I, Jordan DM, Sunyaev SR. Predicting functional effect of human missense mutations using PolyPhen-2. Curr Protoc Hum Genet. 2013;Chapter 7(Unit 7.20) doi: 10.1002/0471142905.hg0720s76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Biffi A, Montini E, Lorioli L, et al. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science. 2013;341(6148):1233158. doi: 10.1126/science.1233158. [DOI] [PubMed] [Google Scholar]
- 38.Aiuti A, Biasco L, Scaramuzza S, et al. Lentiviral hematopoietic stem cell gene therapy in patients with Wiskott-Aldrich syndrome. Science. 2013;341(6148):1233151. doi: 10.1126/science.1233151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tucker BA, Mullins RF, Streb LM, et al. Patient-specific iPSC-derived photoreceptor precursor cells as a means to investigate retinitis pigmentosa. Elife. 2013;2:e00824. doi: 10.7554/eLife.00824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshida T, Ozawa Y, Suzuki K, et al. The use of induced pluripotent stem cells to reveal pathogenic gene mutations and explore treatments for retinitis pigmentosa. Mol Brain. 2014;7(1):45. doi: 10.1186/1756-6606-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41•.Li Y, Wu W-H, Hsu C-W, et al. Gene therapy in patient-specific stem cell lines and a preclinical model of retinitis pigmentosa with membrane frizzled-related protein defects. Mol Ther. 2014;22(9):1688–97. doi: 10.1038/mt.2014.100. This paper demonstrates the utility of iPSCs in research involving both new therapies as well as disease mechanisms. It is the first to show that genetic defects in patient-specific, iPSC-derived retinal cells can be successfully corrected in vitro with gene therapy, a crucial step in a potential autologous transplantation treatment scheme. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tucker BA, Scheetz TE, Mullins RF, et al. Exome sequencing and analysis of induced pluripotent stem cells identify the cilia-related gene male germ cell-associated kinase (MAK) as a cause of retinitis pigmentosa. Proc Natl Acad Sci USA. 2011;108(34):E569–76. doi: 10.1073/pnas.1108918108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jin Z-B, Okamoto S, Osakada F, et al. Modeling retinal degeneration using patient-specific induced pluripotent stem cells. PLoS One. 2011;6(2):e17084. doi: 10.1371/journal.pone.0017084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwarz N, Carr A-J, Lane A, et al. Translational read-through of the RP2 Arg120 stop mutation in patient iPSC-derived retinal pigment epithelium cells. Hum Mol Genet. 2014 doi: 10.1093/hmg/ddu509. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Urnov FD, Miller JC, Lee Y-L, et al. Highly efficient endogenous human gene correction using designed zinc-finger nucleases. Nature. 2005;435(7042):646–51. doi: 10.1038/nature03556. [DOI] [PubMed] [Google Scholar]
- 46.Miller JC, Tan S, Qiao G, et al. A TALE nuclease architecture for efficient genome editing. Nat Biotechnol. 2011;29(2):143–8. doi: 10.1038/nbt.1755. [DOI] [PubMed] [Google Scholar]
- 47.Bedell VM, Wang Y, Campbell JM, et al. In vivo genome editing using a high-efficiency TALEN system. Nature. 2012;491(7422):114–18. doi: 10.1038/nature11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48••.Gasiunas G, Barrangou R, Horvath P, Siksnys V. Cas9-crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proc Natl Acad Sci USA. 2012;109(39):E2579–86. doi: 10.1073/pnas.1208507109. This and the next reference outline the basic framework and components of the CRISPR/Cas system, as well as the first adaptations that were made to apply it as a tool for genome editing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49••.Jinek M, Chylinski K, Fonfara I, et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337(6096):816–21. doi: 10.1126/science.1225829. This and the previous reference outline the basic framework and components of the CRISPR/Cas system, as well as the first adaptations that were made to apply it as a tool for genome editing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fu Y, Sander JD, Reyon D, et al. Improving CRISPR-Cas nuclease specificity using truncated guide RNAs. Nat Biotechnol. 2014;32(3):279–84. doi: 10.1038/nbt.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith C, Abalde-Atristain L, He C, et al. Efficient and allele-specific genome editing of disease loci in human iPSCs. Mol Ther. 2014 doi: 10.1038/mt.2014.226. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ding Q, Regan SN, Xia Y, et al. Enhanced efficiency of human pluripotent stem cell genome editing through replacing TALENs with CRISPRs. Cell Stem Cell. 2013;12(4):393–4. doi: 10.1016/j.stem.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cong L, Ran FA, Cox D, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339(6121):819–23. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jao L-E, Wente SR, Chen W. Efficient multiplex biallelic zebrafish genome editing using a CRISPR nuclease system. Proc Natl Acad Sci USA. 2013;110(34):13904–9. doi: 10.1073/pnas.1308335110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang H, Yang H, Shivalila CS, et al. One-step generation of mice carrying mutations in multiple genes by CRISPR/ Cas-mediated genome engineering. Cell. 2013;153(4):910–18. doi: 10.1016/j.cell.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hong SG, Kim MK, Jang G, et al. Generation of red fluorescent protein transgenic dogs. Genesis. 2009;47(5):314–22. doi: 10.1002/dvg.20504. [DOI] [PubMed] [Google Scholar]
- 57.Komáromy AM, Alexander JJ, Rowlan JS, Garcia MM. Gene therapy rescues cone function in congenital achromatopsia. Hum Mol Genet. 2010;19(13):2581–93. doi: 10.1093/hmg/ddq136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Narfstrom K. Functional and structural recovery of the retina after gene therapy in the RPE65 null mutation dog. Invest Ophthalmol Vis Sci. 2003;44(4):1663–72. doi: 10.1167/iovs.02-0595. [DOI] [PubMed] [Google Scholar]
- 59.Tucker BA, Mullins RF, Stone EM. Stem cells for investigation and treatment of inherited retinal disease. Hum Mol Genet. 2014;23(R1):R9–R16. doi: 10.1093/hmg/ddu124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gouras P, Flood MT, Kjeldbye H. Transplantation of cultured human retinal cells to monkey retina. An Acad Bras Cienc. 1984;56(4):431–43. [PubMed] [Google Scholar]
- 61.Algvere PV, Berglin L, Gouras P, et al. Transplantation of RPE in age-related macular degeneration: observations in disciform lesions and dry RPE atrophy. Graefe’s Arch Clin Exp Ophthalmol. 1997;235(3):149–58. doi: 10.1007/BF00941722. [DOI] [PubMed] [Google Scholar]
- 62.Gouras P. New Developments in retinal cell transplantation and the impact of stem cells. In: Tsang SH, editor. Stem cell biology and regenerative medicine in opthamology. New York: Humana Press; 2013. pp. 121–38. [Google Scholar]
- 63.Wright LS, Phillips MJ, Pinilla I, et al. Induced pluripotent stem cells as custom therapeutics for retinal repair: progress and rationale. Exp Eye Res. 2014;123:161–72. doi: 10.1016/j.exer.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Young MJ, Ray J, Whiteley SJ, et al. Neuronal differentiation and morphological integration of hippocampal progenitor cells transplanted to the retina of immature and mature dystrophic rats. Mol Cell Neurosci. 2000;16(3):197–205. doi: 10.1006/mcne.2000.0869. [DOI] [PubMed] [Google Scholar]
- 65.Akita J, Takahashi M, Hojo M, et al. Neuronal differentiation of adult rat hippocampus-derived neural stem cells transplanted into embryonic rat explanted retinas with retinoic acid pretreatment. Brain Res. 2002;954(2):286–93. doi: 10.1016/s0006-8993(02)03356-5. [DOI] [PubMed] [Google Scholar]
- 66.Klassen HJ, Ng TF, Kurimoto Y, et al. Multipotent retinal progenitors express developmental markers, differentiate into retinal neurons, and preserve light-mediated behavior. Invest Ophthalmol Vis Sci. 2004;45(11):4167–73. doi: 10.1167/iovs.04-0511. [DOI] [PubMed] [Google Scholar]
- 67.Qiu G, Seiler MJ, Mui C, et al. Photoreceptor differentiation and integration of retinal progenitor cells transplanted into transgenic rats. Exp Eye Res. 2005;80(4):515–25. doi: 10.1016/j.exer.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 68••.MacLaren RE, Pearson RA, MacNeil A, et al. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444(7116):203–7. doi: 10.1038/nature05161. This paper was the first to define and demonstrate the developmental stage at which rod photoreceptor precursors are most amenable to successful transplantation. As such, it represents a milestone advancement towards realizing a retinal disease treatment involving photoreceptor transplantation. [DOI] [PubMed] [Google Scholar]
- 69.Pearson RA, Barber AC, Rizzi M, et al. Restoration of vision after transplantation of photoreceptors. Nature. 2012;485(7396):99–103. doi: 10.1038/nature10997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barber AC, Hippert C, Duran Y, et al. Repair of the degenerate retina by photoreceptor transplantation. Proc Natl Acad Sci USA. 2013;110(1):354–9. doi: 10.1073/pnas.1212677110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Singh MS, Charbel Issa P, Butler R, et al. Reversal of end-stage retinal degeneration and restoration of visual function by photoreceptor transplantation. Proc Natl Acad Sci USA. 2013;110(3):1101–6. doi: 10.1073/pnas.1119416110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Swaroop A, Kim D, Forrest D. Transcriptional regulation of photoreceptor development and homeostasis in the mammalian retina. Nat Rev Neurosci. 2010;11(8):563–76. doi: 10.1038/nrn2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lamba DA, Gust J, Reh TA. Transplantation of human embryonic stem cell-derived photoreceptors restores some visual function in Crx-deficient mice. Cell Stem Cell. 2009;4(1):73–9. doi: 10.1016/j.stem.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.West EL, Gonzalez-Cordero A, Hippert C, et al. Defining the integration capacity of embryonic stem cell-derived photoreceptor precursors. Stem Cells. 2012;30(7):1424–35. doi: 10.1002/stem.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75•.Eiraku M, Takata N, Ishibashi H, et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 2011;472(7341):51–6. doi: 10.1038/nature09941. This is an interesting report on the spontaneous formation of optic cup-like structures, in three-dimensional mouse stem cell cultures, containing stratified tissue layers resembling in vivo retinal layers. This work has significant potential applications for retinal sheet transplantation, an alternative to the transplantation of dissociated retinal cells. [DOI] [PubMed] [Google Scholar]
- 76.Busskamp V, Krol J, Nelidova D, et al. miRNAs 182 and 183 are necessary to maintain adult cone photoreceptor outer segments and visual function. Neuron. 2014;83(3):586–600. doi: 10.1016/j.neuron.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 77.Gonzalez-Cordero A, West EL, Pearson RA, et al. Photoreceptor precursors derived from three-dimensional embryonic stem cell cultures integrate and mature within adult degenerate retina. Nat Biotechnol. 2013;31(8):741–7. doi: 10.1038/nbt.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78••.Schwartz SD, Regillo CD, Lam BL, et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet. 2014;6736(14):1–8. doi: 10.1016/S0140-6736(14)61376-3. This reference summarizes the medium-term follow-up for the first successful Phase I/II clinical trials of stem cell-derived cell transplantation therapy. It reports that transplantation was generally safe, without risk for tumor formation or immune rejection, and possibly therapeutic even in patients with late-stage AMD and Stargardt. [DOI] [PubMed] [Google Scholar]
- 79.Schwartz SD, Hubschman J-P, Heilwell G, et al. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 2012;379(9817):713–20. doi: 10.1016/S0140-6736(12)60028-2. [DOI] [PubMed] [Google Scholar]
- 80.Hambright D, Park K, Brooks M, et al. Long-term survival and differentiation of retinal neurons derived from human embryonic stem cell lines in un-immunosuppressed mouse retina. Mol Vis. 2012;18:920–36. [PMC free article] [PubMed] [Google Scholar]
- 81.Li Y, Tsai Y-T, Hsu C-W, et al. Long-term safety and efficacy of human-induced pluripotent stem cell (iPS) grafts in a preclinical model of retinitis pigmentosa. Mol Med. 2012;18:1312–19. doi: 10.2119/molmed.2012.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lamba DA, McUsic A, Hirata RK, et al. Generation, purification and transplantation of photoreceptors derived from human induced pluripotent stem cells. PLoS One. 2010;5(1):e8763. doi: 10.1371/journal.pone.0008763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhou L, Wang W, Liu Y, et al. Differentiation of induced pluripotent stem cells of swine into rod photoreceptors and their integration into the retina. Stem Cells. 2011;29(6):972–80. doi: 10.1002/stem.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tucker BA, Park I-H, Qi SD, et al. Transplantation of adult mouse iPS cell-derived photoreceptor precursors restores retinal structure and function in degenerative mice. PLoS One. 2011;6(4):e18992. doi: 10.1371/journal.pone.0018992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Assawachananont J, Mandai M, Okamoto S, et al. Transplantation of embryonic and induced pluripotent stem cell-derived 3D retinal sheets into retinal degenerative mice. Stem cell reports. 2014;2(5):662–74. doi: 10.1016/j.stemcr.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li H, Haurigot V, Doyon Y, et al. In vivo genome editing restores haemostasis in a mouse model of haemophilia. Nature. 2011;475(7355):217–21. doi: 10.1038/nature10177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Senís E, Fatouros C, Große S, et al. CRISPR/Cas9-mediated genome engineering: an adeno-associated viral (AAV) vector toolbox. Biotechnol J. 2014;9(11):1402–12. doi: 10.1002/biot.201400046. [DOI] [PubMed] [Google Scholar]
- 88.Olsson JE, Gordon JW, Pawlyk BS, et al. Transgenic mice with a rhodopsin mutation (pro23his): a mouse model of autosomal dominant retinitis pigmentosa. 1992;9:815–30. doi: 10.1016/0896-6273(92)90236-7. [DOI] [PubMed] [Google Scholar]
- 89.Seo S, Mullins RF, Dumitrescu AV, et al. Subretinal gene therapy of mice with Bardet-Biedl syndrome type 1. Invest Ophthalmol Vis Sci. 2013;54(9):6118–32. doi: 10.1167/iovs.13-11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Burnight ER, Wiley LA, Drack AV, et al. CEP290 gene transfer rescues Leber congenital amaurosis cellular phenotype. Gene Ther. 2014;21(7):662–72. doi: 10.1038/gt.2014.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91•.Ebina H, Misawa N, Kanemura Y, Koyanagi Y. Harnessing the CRISPR/ Cas9 system to disrupt latent HIV-1 provirus. Sci Rep. 2013;3:2510. doi: 10.1038/srep02510. Although unrelated to the treatment of retinal disease, this and the next reference describe the most recent of several nascent clinical applications for CRISPR/Cas genome editing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92•.Hu W, Kaminski R, Yang F, et al. RNA-directed gene editing specifically eradicates latent and prevents new HIV-1 infection. Proc Natl Acad Sci USA. 2014;111(31):11461–6. doi: 10.1073/pnas.1405186111. Although unrelated to the treatment of retinal disease, this reference and the one above describe the most recent of several nascent clinical applications for CRISPR/Cas genome editing. In this report, researchers are able to eradicate HIV reservoirs in infected cells and achieve sterile immunization against infection in healthy cells, two findings that may form the conceptual basis for eventual treatments and vaccines in humans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jeon C, Strettoi E, Masland RH. The major cell populations of the mouse retina. J Neurosci. 1998;18(21):8936–46. doi: 10.1523/JNEUROSCI.18-21-08936.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Punzo C, Kornacker K, Cepko CL. Stimulation of the insulin/mTOR pathway delays cone death in a mouse model of retinitis pigmentosa. Nat Neurosci. 2009;12(1):44–52. doi: 10.1038/nn.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wert KJ, Sancho-Pelluz J, Tsang SH. Mid-stage intervention achieves similar efficacy as conventional early-stage treatment using gene therapy in a pre-clinical model of retinitis pigmentosa. Hum Mol Genet. 2014;23(2):514–23. doi: 10.1093/hmg/ddt452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ran FA, Hsu PD, Lin C-Y, et al. Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell. 2013;154(6):1380–9. doi: 10.1016/j.cell.2013.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mali P, Aach J, Stranges PB, et al. CAS9 transcriptional activators for target specificity screening and paired nickases for cooperative genome engineering. Nat Biotechnol. 2013;31(9):833–8. doi: 10.1038/nbt.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zarzeczny A, Scott C, Hyun I, et al. iPS cells: mapping the policy issues. Cell. 2009;139(6):1032–7. doi: 10.1016/j.cell.2009.11.039. [DOI] [PubMed] [Google Scholar]
- 99.Tan H-K, Toh C-XD, Ma D, et al. Human finger-prick induced pluripotent stem cells facilitate the development of stem cell banking. Stem Cells Transl Med. 2014;3(5):586–98. doi: 10.5966/sctm.2013-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]