Abstract
Purpose
To demonstrate the utility and characteristics of fundus autofluorescence in late-onset retinitis pigmentosa.
Methods
Observational case series. Patients diagnosed with late-onset retinitis pigmentosa were identified retrospectively in an institutional setting. Twelve eyes of six patients were identified and medical records were reviewed.
Results
All patients presented with slowly progressive peripheral field loss and initial clinical examination revealed only subtle retinal changes. There was a notable lack of intraretinal pigment migration in all patients. Five out of six patients underwent magnetic resonance imaging of the brain to rule out intracranial processes and all were referred from another ophthalmologist for further evaluation. Fundus autofluorescence was ultimately employed in all patients and revealed more extensive retinal pathology than initially appreciated on clinical examination. Fundus autofluorescence directed the workup toward a retinal etiology in all cases and led to the eventual diagnosis of late-onset retinitis pigmentosa through electroretinogram testing.
Conclusion
Fundus autofluorescence may be a more sensitive marker for retinal pathology than stereo fundus biomicroscopy alone in late-onset retinitis pigmentosa. Early use of fundus autofluorescence imaging in the evaluation of patients with subtle retinal lesions and complaints of peripheral field loss may be an effective strategy for timely and cost-efficient diagnosis.
Keywords: Fundus autofluorescence, late-onset retinitis pigmentosa, magnetic resonance imaging
INTRODUCTION
Retinitis pigmentosa represents a heterogeneous group of retinal dystrophies characterized by photoreceptor degeneration. The disease occurs in approximately 1 in 4000 individuals and affects 1.5 million people worldwide.1–4 Rods are the predominantly affected photoreceptors and dysfunction causes night blindness and peripheral field loss beginning as early as the teenage years.4 Progression leads to central acuity loss and legal blindness in the majority of patients.1
Late-onset retinitis pigmentosa represents a relatively rare subset of the disease and is associated with slower progression of visual dysfunction. This entity can be diagnostically challenging, as symptoms may be disproportionate to clinical findings. Dilated funduscopic examination may reveal only subtle peripheral changes or even an absence of classical findings typically associated with retinitis pigmentosa such as perivascular bony spicule pigmentation, attenuated arterioles, and waxy optic disc pallor.5–7
In this case series, we demonstrate that fundus autofluorescence may be a more sensitive marker of retinal pathology than stereo fundus biomicroscopy examination alone in late-onset retinitis pigmentosa. We suggest that early use of autofluorescence in the evaluation of patients with subtle retinal changes and complaints of peripheral field loss may improve diagnostic clarity in a rapid and cost-efficient manner.
METHODS
Patients diagnosed with late-onset retinitis pigmentosa were identified retrospectively from March 2006 to August 2012 at the Edward S. Harkness Eye Institute of Columbia University Medical Center (New York, NY) and Vitreous, Retina, Macula Consultants of New York (New York, NY). Seven patients were identified and medical records were reviewed. Data evaluated included patient demographics, ocular history, and best-corrected Snellen visual acuity. Color photographs, red-free photographs, electroretinogram (ERG), and fundus autofluorescence studies were reviewed. Genetic testing was conducted using the autosomal recessive retinitis pigmentosa genotyping microarray (Asper Ophthalmics, Tartu, Estonia) to screen for 585 mutations in CERKL, CNGA1, CNGB1, MERTK, PDE6A, PDE6B, PNR, RDH12, RGR, RLBP1, SAG, TULP1, CRB, RPE65, USH2A, USH3A, LRAT, PROML1 genes.
The study adhered to the tenets of the Declaration of Helsinki and received approval by the Institutional Review Board (Protocol AAAE1564) of New York-Presbyterian Hospital at Columbia University Medical Center (New York, NY). Medical charts were reviewed in compliance with the Health Insurance Portability and Accountability Act.
FAF imaging was performed with a confocal scanning laser ophthalmoscope (cSLO, Heidelberg Retina Angiograph 2; Heidelberg Engineering, Dossenheim, Germany) after pupil dilation with topical tropicamide and phenylephrine. FAF imaging was performed using a 30° field of view at a resolution of 1536 × 1536 pixels. An optically pumped solid-state laser (488 nm) was used for excitation and a 500 nm barrier filter was used to modulate the blue argon excitation light. Standard procedure was followed for the acquisition of FAF images, including focus of the retinal image in the infrared reflection mode at 820 nm, sensitivity adjustment at 488 nm, and acquisition of nine single 30° × 30° FAF images encompassing the entire macular area with at least a portion of the optic disc. The nine single images were computationally averaged to produce a single frame with improved signal-to-noise ratio.
Ganzfeld full-field ERGs (Diagnosys LLC, Lowell, USA) were recorded from both eyes with DTL electrodes according to the International Society for Clinical Electrophysiology of Vision (ISCEV) standard (see www.iscev.org), in both scotopic and photopic states to assess retinal function. Following 10 min light adaptation the photopic 30 Hz flicker and transient photopic ERGs were recorded. Pupils were dilated before full-field ERG testing using tropicamide (1%) and phenylephrine hydrochloride (2.5%).
RESULTS
Case 1
A 79-year-old female noted slowly decreased visual acuity and peripheral field in both eyes over the previous two months. Family history was significant for glaucoma in both her father and sister. The patient underwent visual field testing with her comprehensive ophthalmologist, which revealed non-specific bitemporal field loss unresponsive to intraocular pressure lowering agents (see Supplementary Figure 1 – online only). She was referred to a glaucoma specialist, who ruled out a diagnosis of glaucoma and referred the patient to neuro-ophthalmology. She underwent an MRI of the brain, which was unrevealing, and was subsequently referred to the retina service for further evaluation of peripheral pigmentary changes noted on initial examination.
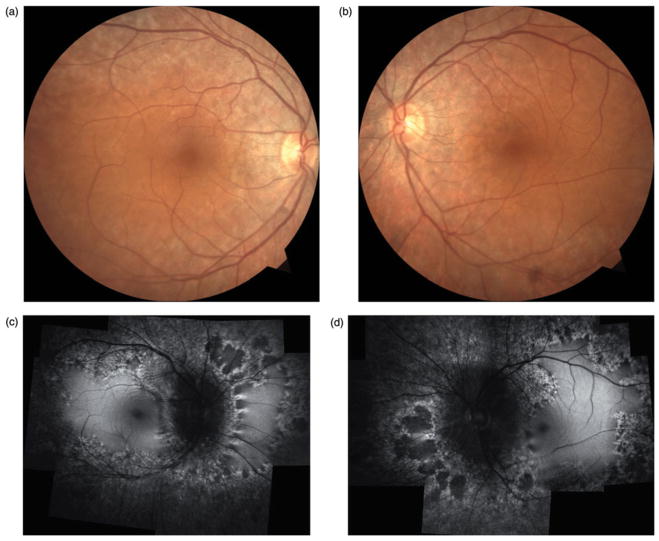
Best-corrected visual acuity measured 20/20 in the right eye and 20/25 in the left eye with mild astigmatic correction in both eyes. Anterior segment was notable only for well-placed posterior chamber intraocular lens and intraocular pressure measured 14 mmHg in both eyes. Dilated funduscopic examination (Figure 1a and b) revealed peripapillary retinal pigment epithelium mottling extending along the arcades and mid-peripheral regions. No intraretinal pigment migration was noted.
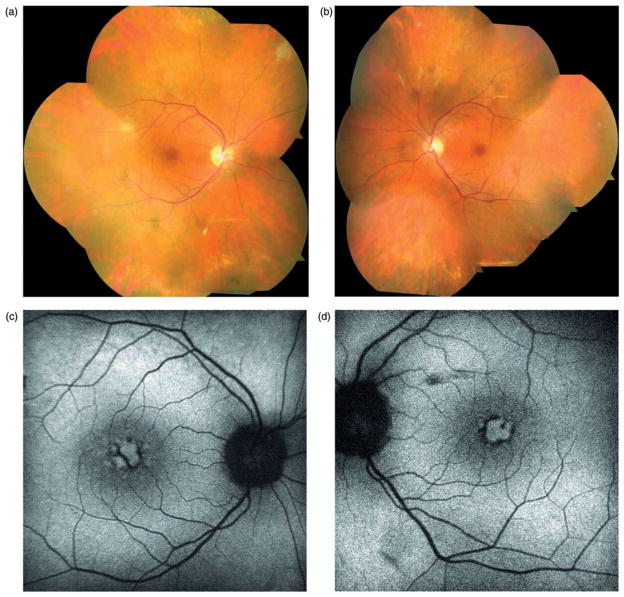
FIGURE 1.
Case 1. Fundus photographs (a & b) reveal retinal pigment epithelium atrophy along the arcades and peripapillary regions in both eyes without suggestions of intraretinal pigment migration. Fundus autofluorescence (c & d) shows extensive hypofluorescent regions along the arcades and mid-periphery. Regions of scalloped atrophy are noted nasally in both eyes.
Fundus autofluorescence (Figure 1c and d) demonstrated a marked annular ring of hypofluorescence loss around the arcades and mid-periphery. Peripapillary hypofluorescence and scalloped regions of atrophy were noted nasally in both eyes. ERG testing was subsequently pursued, which demonstrated generalized retinal dysfunction affecting both the rod and cone systems (see Supplementary Figure 2 – online only). Genetic testing did not yield a specific genetic etiology and anti-retinal antibodies were not present.
Case 2
A 66-year-old male presented with complaints of decreased visual acuity and peripheral vision for 6 months. He was previously evaluated by a community ophthalmologist and underwent visual field testing, which revealed a ring scotoma in both eyes. He was evaluated for diagnoses of melanoma- and cancer-associated retinopathy with serologic testing, complete dermatologic workup, and computed tomography scans of the chest and abdomen. The patient also underwent MRI of the brain to rule out an intracranial mass. The results of all imaging, serologic, and clinical evaluations were unremarkable.
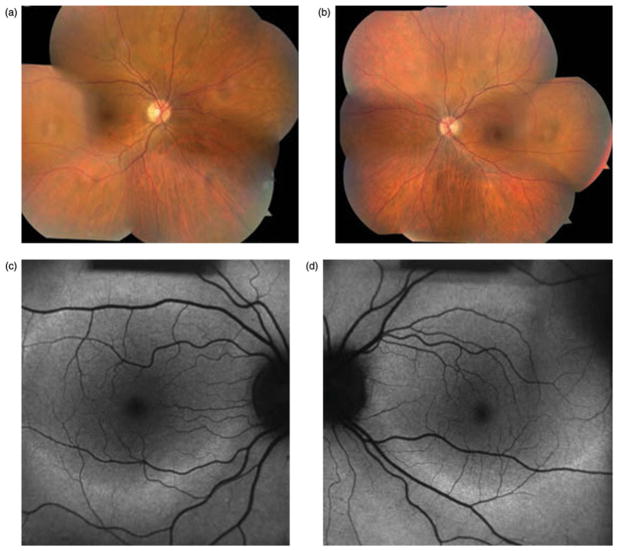
On examination, best-corrected visual acuity was 20/20 in both eyes. Anterior segment examination was unremarkable aside from posterior intraocular lenses in both eyes. Applanation tonometry measured 16 mmHg in both eyes. Dilated funduscopic examination (Figure 2a and b) was unremarkable in the right eye. Findings in the left eye were significant for congenital hypertrophy of the retinal pigment epithelium inferonasally and a large chorioretinal scar superonasally. The optic nerves and arterioles were unremarkable and no intraretinal pigment migration was noted.
FIGURE 2.
Case 2. Fundus photographs (a & b) reveal peripapillary atrophy in both eyes and a large chorioretinal scar superonasally and congenital hypertrophy of the retinal pigment epithelium inferonasally in the right eye. No optic nerve pallor, arteriolar attenuation, or intraretinal pigment migration is noted. Fundus autofluorescence (c & d) shows hypofluorescence in the peripapillary regions and along the arcades of both eyes.
Fundus autofluorescence (Figure 2c and d) revealed more extensive retinal pathology than stereo biomicroscopy alone, highlighting hyperfluorescence along the peripapillary and arcades of both eyes. The patient subsequently underwent electroretinogram testing, which revealed generalized retinal dysfunction affecting the rod more than the cone system. Genetic testing did not yield a specific genetic etiology.
Case 3
A 56-year-old man presented with complaints of progressive loss of visual acuity and peripheral field for the past 6 months. He was initially evaluated by a community retinal specialist who noted rare pigmentary changes in the periphery and subsequently referred the patient for neuro-ophthalmologic consultation. He subsequently underwent an MRI of the brain that did not demonstrate any significant findings. The patient then sought a second opinion and presented to the retina service.
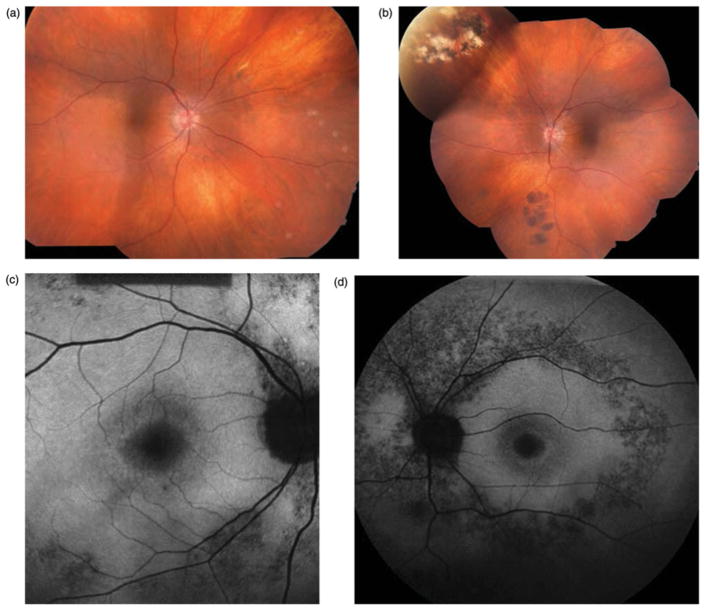
On examination, best-corrected visual acuity measured 20/30 in both eyes with minimal myopic correction. Anterior segment examination was unremarkable and intraocular pressures were 15 mmHg in the right eye and 12 mmHg in the left eye. Dilated fundus examination (Figure 3a and b) showed optic nerves with good rims but mild pallor. The central macula featured mild atrophic changes in both eyes. Rare intraretinal pigment migration was observed in the peripheral retina with lacunar cobblestone degeneration.
FIGURE 3.
Case 3. Fundus photographs (a & b) demonstrate mild optic nerve pallor and minimal pigmentary changes in the macula of both eyes. In the peripheral retina (not shown) there was a rare intraretinal pigment migration with lacunar cobblestone degeneration at the periphery. Fundus autofluorescence (c & d) shows hypofluorescence in the peripapillary region extending along the arcades.
Fundus autofluorescence (Figure 3c and d) revealed more extensive retinal involvement than stereo fundus biomicroscopy alone and demonstrated mottled hypofluorescence in the mid-periphery and central macular regions. ERG testing was subsequently pursued and results were consistent with a diagnosis of retinitis pigmentosa. Genetic testing using a microarray for autosomal recessive retinitis pigmentosa did not identify a specific causative gene.
Case 4
A 59-year-old male presented with complaints of loss of peripheral vision, most notable when playing tennis evaluated by community ophthalmologist and referred for neuro-ophthalmic evaluation. The patient underwent an MRI of the brain, which did not yield any significant findings. He presented for further evaluation.
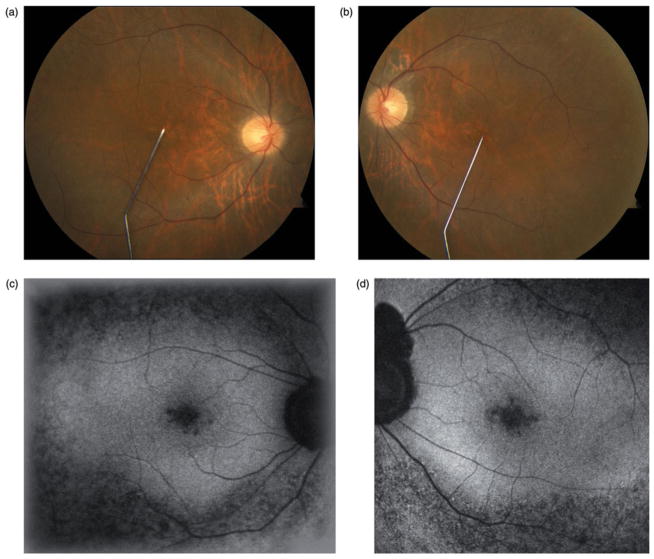
On examination, best-corrected visual acuity was 20/30 in the right eye and 20/20 in the left eye. Anterior segment examination was unremarkable aside from posterior intraocular lens implants in both eyes and intraocular pressures measured 14 mmHg in both eyes. Dilated funduscopic examination (Figure 4a and b) was unremarkable with healthy-appearing optic nerves, normal arterioles, and an absence of intraretinal pigment migration.
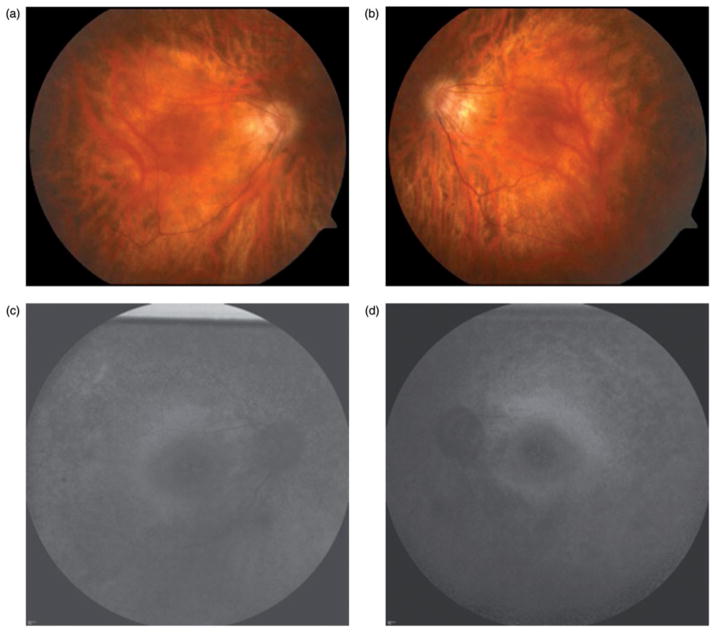
FIGURE 4.
Case 4. Color photographs (a & b) demonstrate a normal funduscopic examination and a notable absence of the typical clinical signs of retinitis pigmentosa such as waxy optic nerve pallor, attenuated arterioles, and intraretinal pigment migration. Fundus autofluorescence (c & d) reveals a hyperfluorescent ring commonly associated with retinitis pigmentosa.
Fundus autofluorescence (Figure 4c and d) revealed a hyperfluorescent ring in both eyes, characteristic of retinitis pigmentosa. Optical coherence tomography demonstrated loss of photoreceptors outside of the hyperfluorescent ring. The patient underwent electroretinogram testing which revealed generalized rod greater than cone loss, consistent with retinitis pigmentosa. The patient underwent genetic testing, which did not identify a causative gene.
Case 5
A 67-year old woman complained of poor visual acuity in both eyes for the previous 5 years, which did not improve following cataract extraction 2 years prior. She also noted slowly progressive peripheral field loss. She was evaluated by neuro-ophthalmology and underwent an MRI of the brain, which did not yield any significant findings. She was then referred to a uveitis specialist for further evaluation of rare peripheral retinal pigmentary changes. A uveitic etiology was determined to be unlikely and the patient was subsequently referred to the retina service for further evaluation of rare perivascular pigmentation noted on initial examination. The patient’s ophthalmic history was significant for strabismus and amblyopia of the left eye. She denied any family history of severe visual loss.
Best-corrected visual acuity was 20/40 in the right eye and 20/80 in the left eye with mild astigmatic correction. A 30 prism diopter exotropia was present. Anterior segment exam was unremarkable aside from posterior chamber intraocular lens and there were no signs of previous or active ocular inflammation. Applanation tonometry measured 21 mmHg in the right eye and 19 mmHg in the left eye. Dilated fundus examination (Figure 5a and b) showed optic nerves with intact rims and color. Cystoid macular edema was present in both eyes and rare perivascular pigment was noted inferiorly in both eyes. There were numerous drusen in the periphery. No vitreous cells were noted.
FIGURE 5.
Case 5. Fundus photographs (a & b) reveal rare intraretinal pigment inferiorly and peripheral drusen in both eyes. Fundus autofluorescence (c & d) shows high-density macular fluorescence rings and displacement of macular pigment in the foveal region consistent with chronic cystoid macular edema.
Fundus autofluorescence (Figure 5c and d) imaging revealed pigmentary changes in the foveal region consistent with chronic macular edema. ERG was subsequently performed and revealed generalized retinal dysfunction affecting the rod system more than the cone system. Optical coherence tomography confirmed cystoid macular edema in both eyes. Genetic testing did not identify a causative gene.
Case 6
A 62-year old male presented with a 5-year history of progressively worsening nyctalopia and poor peripheral vision. He had routinely seen a retina specialist since the age of 53 when he was treated with laser surgery for a retinal tear. Upon presentation, the patient complained of difficulty reading and worsening peripheral vision. He was not taking any ocular or systemic medications and denied any family history of severe visual problems, including retinitis pigmentosa, in his family of Italian and Albanian descent.
Best-corrected visual acuity on examination was 20/60 in the right eye and 20/40 in the left eye with correction for myopic astigmatism. There were severely constricted confrontational visual fields, confirmed on Humphrey 10-2 testing, and measured to be limited to 6° in both eyes on microperimetry MP1 testing. Motility, intraocular pressure, pupils, and anterior segment exam were all within normal limits. On dilated fundus exam (Figure 6a and b), the patient had laser scars near an adjacent atrophic hole in both eyes. Both eyes had rare intraretinal pigment but the right eye had a bull’s eye maculopathy. Hypofluorescence of the midperiphery on fundus autofluorescence imaging confirmed this finding, and pointed towards a diagnosis of retinitis pigmentosa (Figure 6c and d). Subsequent ERG revealed generalized rod greater than cone loss, confirming the diagnosis of retinitis pigmentosa. Genetic testing did not identify a causative gene.
FIGURE 6.
Case 6. Fundus photographs (a & b) reveals rare intraretinal pigment and bull’s eye maculopathy. Hypofluorescence of the midperiphery is seen on fundus autofluorescence imaging (c & d), consistent with retinitis pigmentosa.
DISCUSSION
We present a series of retinitis pigmentosa patients who presented with symptoms after 55 years of age. Initial clinical examination revealed only subtle retinal changes with an absence of the classic funduscopic findings associated with retinitis pigmentosa. Five out of the six patients underwent MRI of the brain to rule out an intracranial process and all patients were referred to various subspecialties including neuro-ophthalmology, glaucoma, and uveitis for evaluation prior to presentation. Visual field defects were variable, and results were often misleading (Supplementary Figure 1). Fundus autofluorescence was ultimately employed in every patient and revealed more extensive retinal pathology than initially appreciated on clinical examination. Fundus autofluorescence directed the workup toward a retinal etiology and led to the eventual diagnosis of late-onset retinitis pigmentosa through ERG testing.
Our case series suggests that early use of fundus autofluorescence in the evaluation of patients with subtle peripheral retinal lesions and complaints of peripheral field loss may be an effective strategy for rapid and cost-efficient diagnosis. Fundus autofluorescence may be a more sensitive marker for retinal pathology than stereo fundus biomicroscopy examination alone and may be critical in identifying the outer retina as the source of dysfunction. Early use of fundus autofluorescence may eliminate the need for costly MRI studies and unnecessary subspecialist evaluation, allowing for a more cost-efficient and timely route to diagnosis.
Fundus autofluorescence has become a commonly available non-invasive imaging modality in evaluating the photoreceptor/RPE-complex.8–9 Fundus autofluorescence has been useful in illustrating characteristic abnormalities in retinitis pigmentosa10–12 in addition to other diseases including vitelliform macular dystrophy, central serous chorioretinopathy, chorioretinal inflammatory disorders, and pseudoxanthoma elasticum.13 Fundus autofluorescence relies on the stimulated emission of light from molecules, chiefly lipofuscin, in the RPE and provides indirect information on the level of metabolic activity of the RPE.14 Hypoautofluorescence is thought to correspond to areas of decreased metabolism resulting from photoreceptor and/or RPE atrophy and has been used as a marker for the integrity of the RPE/photoreceptor complex.15–17 In each of the cases presented, autofluorescence revealed disproportionately more extensive areas of degeneration than funduscopy alone and directed our workup toward a retinal etiology. While the histopathology of late-onset retinitis pigmentosa remains unclear, it appears that autofluorescence imaging in such cases may allow for earlier diagnosis and obviate the need for MRI studies and additional referrals.
ERG testing has been accepted as the standard for diagnosis of retinitis pigmentosa and can detect photoreceptor abnormalities years before manifestation of visual symptoms or fundus changes.18 Testing is particularly helpful in late-onset retinitis pigmentosa, since this slowly progressive retinal dystrophy may lack appreciable funduscopic findings5–7 typically associated with retinitis pigmentosa such as midperipheral bony spicule pigmentation, attenuated arterioles, vitreous cells, waxy optic disc pallor. Testing can also differentiate retinitis pigmentosa from other forms of retinal disease that may be difficult to distinguish in the early stages. ERG testing typically demonstrates moderate to severe abnormalities in retinitis pigmentosa with reduced a- and b-wave amplitudes and prolonged implicit times.1 ERG amplitudes may become undetectable late in the disease course.
Baseline ERG testing can provide an estimation of long-term visual prognosis. Results of a single ERG test can be correlated with an ERG actuarial table,19 which assumes loss of ERG cone function of about 10% per year. Thus a 40-year-old patient with a cone amplitude of 3.5 mV would be expected to retain some useful vision (30-Hz cone ERG amplitude ≥0.05 mV) until age 80.19 More precise prognostic estimates require serial ERG testing.
In summary, fundus autofluorescence imaging may be a more sensitive marker of retinal pathology than stereo fundus biomicroscopy examination alone and may be a cost-efficient adjunct test. In patients presenting with peripheral field loss and subtle retinal changes, fundus autofluorescence may identify more extensive regions of retinal pathology and direct the workup toward a retinal etiology. Earlier use of fundus autofluorescence in such cases may reduce the need for costly MRI studies and unnecessary subspecialist consultations while reducing the time to diagnosis. Late-onset retinitis pigmentosa is a relatively rare disease associated with only subtle peripheral retinal changes or even an absence of classical signs typically associated with retinitis pigmentosa. The lack of intraretinal pigment migration is consistent with the defective genes in late-onset RP which may impair normal function in retinal pigment epithelium (RPE). The use of fundus autofluorescence can identify or confirm abnormalities of the RPE and focus a timely and cost-efficient diagnosis of late-onset retinitis pigmentosa through ERG testing.
Supplementary Material
Acknowledgments
Funding and support was received from the NIH (Grant nos.: R01EY018213, DOD-W81XWH-09-1-0575), and the Foundation Fighting Blindness. SHT is a Fellow of the Burroughs-Wellcome Program in Biomedical Sciences, and has been supported by the Bernard Becker-Association of University Professors in Ophthalmology-Research to Prevent Blindness Award and Foundation Fighting Blindness, Dennis W. Jahnigen Award of the American Geriatrics Society, Joel Hoffman Fund, Gale and Richard Siegel Stem Cell Fund, Charles Culpeper Scholarship, Schneeweiss Stem Cell Fund, Irma T. Hirschl Charitable Trust, and Bernard and Anne Spitzer Stem Cell Fund, Barbara & Donald Jonas Family Fund, Eye Surgery Fund and Professor Gertrude Rothschild Stem Cell Foundation. CSL is the Homer Rees scholar. NKW is supported by the Taiwan National Science Council NSC-096-2917-I-002-105 & 98-2314-B-182A-078- and the Chang Gung Memorial Hospital CMRPG360571 & 360572.
Footnotes
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Berson EL. Retinitis pigmentosa. The Friedenwald Lecture. Invest Ophthalmol Vis Sci. 1993;34:1659–1676. [PubMed] [Google Scholar]
- 2.Haim M, Holm NV, Rosenberg T. Prevalence of retinitis pigmentosa and allied disorders in Denmark. I. Main results. Acta Ophthalmol (Copenh) 1992;70:178–186. doi: 10.1111/j.1755-3768.1992.tb04121.x. [DOI] [PubMed] [Google Scholar]
- 3.Dryja TP, Li T. Molecular genetics of retinitis pigmentosa. Hum Mol Genet. 1995;4:1739–1743. doi: 10.1093/hmg/4.suppl_1.1739. [DOI] [PubMed] [Google Scholar]
- 4.Haim M. Prevalence of retinitis pigmentosa and allied disorders in Denmark. II. Systemic involvement and age at onset. Acta Ophthalmol (Copenh) 1992;70:417–426. doi: 10.1111/j.1755-3768.1992.tb02109.x. [DOI] [PubMed] [Google Scholar]
- 5.Phelan JK, Bok D. A brief review of retinitis pigmentosa and the identified retinitis pigmentosa genes. Mol Vis. 2000;6:116–124. [PubMed] [Google Scholar]
- 6.Wang Q, Chen Q, Zhao K, et al. Update on the molecular genetics of retinitis pigmentosa. Ophthalmic Genet. 2001;22:133–154. doi: 10.1076/opge.22.3.133.2224. [DOI] [PubMed] [Google Scholar]
- 7.Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368:1795–1809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- 8.Han M, Giese G, Schmitz-Valckenberg S, et al. Age-related structural abnormalities in the human retina-choroid complex revealed by two-photon excited autofluorescence imaging. J Biomed Opt. 2007;12:024012. doi: 10.1117/1.2717522. [DOI] [PubMed] [Google Scholar]
- 9.Lima LH, Cella W, Greenstein VC, et al. Structural assessment of hyperautofluorescent ring in patients with retinitis pigmentosa. Retina. 2009;29:1025–1031. doi: 10.1097/IAE.0b013e3181ac2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kellner U, Kellner S, Weber BH, et al. Lipofuscin- and melanin-related fundus autofluorescence visualize different retinal pigment epithelial alterations in patients with retinitis pigmentosa. Eye. 2009;23:1349–1359. doi: 10.1038/eye.2008.280. [DOI] [PubMed] [Google Scholar]
- 11.Popovic P, Jarc-Vidmar M, Hawlina M. Abnormal fundus autofluorescence in relation to retinal function in patients with retinitis pigmentosa. Graefes Arch Clin Exp Ophthalmol. 2005;243:1018–1027. doi: 10.1007/s00417-005-1186-x. [DOI] [PubMed] [Google Scholar]
- 12.Wegscheider E, Preising MN, Lorenz B. Fundus autofluorescence in carriers of X-linked recessive retinitis pigmentosa associated with mutations in RPGR, and correlation with electrophysiological and psychophysical data. Graefes Arch Clin Exp Ophthalmol. 2004;24:501–511. doi: 10.1007/s00417-004-0891-1. [DOI] [PubMed] [Google Scholar]
- 13.Schmitz-Valckenberg S, Holz FG, Bird AC, Spaide RF. Fundus autofluorescence imaging: review and perspectives. Retina. 2008;28:385–409. doi: 10.1097/IAE.0b013e318164a907. [DOI] [PubMed] [Google Scholar]
- 14.Delori FC, Dorey CK, Staurenghi G, et al. In vivo fluorescence of the ocular fundus exhibits retinal pigment epithelium lipofuscin characteristics. Invest Ophthalmol Vis Sci. 1995;36:718–729. [PubMed] [Google Scholar]
- 15.Robson AG, Michaelides M, Saihan Z, et al. Functional characteristics of patients with retinal dystrophy that manifest abnormal parafoveal annuli of high density fundus autofluorescence; a review and update. Doc Ophthalmol. 2008;116:79–89. doi: 10.1007/s10633-007-9087-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsui I, Chou CL, Palmer N, et al. Phenotype-genotype correlations in autosomal dominant retinitis pigmentosa caused by RHO, D190N. Curr Eye Res. 2008;33:1014–1022. doi: 10.1080/02713680802484645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsui I, Fuchs BS, Chou CL, et al. Non-vascular vision loss in pseudoxanthoma elasticum. Doc Ophthalmol. 2008;117:65–67. doi: 10.1007/s10633-007-9100-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berson EL, Gouras P, Gunkel RD. Rod responses in retinitis pigmentosa, dominantly inherited. Arch Ophthalmol. 1968;80:58–67. doi: 10.1001/archopht.1968.00980050060009. [DOI] [PubMed] [Google Scholar]
- 19.Berson EL. Long-term visual prognoses in patients with retinitis pigmentosa: the Ludwig von Sallmann lecture. Exp Eye Res. 2007;85:7–14. doi: 10.1016/j.exer.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.