Abstract
Mature amygdala-prefrontal circuitry regulates affect in adulthood, but shows protracted development. In (semi-)altricial species, caregivers provide potent affect regulation when mature neurocircuitry is absent. This investigation examined a potential mechanism through which caregivers provide regulatory influences in childhood. Children, but not adolescents, showed evidence of maternal buffering, such that maternal stimuli suppressed amygdala reactivity. In the absence of maternal stimuli, children exhibited immature amygdala-prefrontal connectivity. However, in the presence of maternal stimuli, children displayed mature-like connectivity that resembled adolescents’ connectivity. Children showed improved affect-related regulation in the presence of their mother. Individual differences emerged, with maternal influence on amygdalaprefrontal circuitry associated with stronger mother-child relationships and maternal modulation of behavioral regulation. These findings suggest a neural mechanism through which caregivers modulate children's regulatory behavior by inducing mature-like connectivity and buffering against heightened reactivity. Maternal buffering in childhood, but not adolescence, suggests that childhood may be a sensitive period for amygdala-prefrontal development.
Keywords: amygdala, prefrontal cortex, development, fMRI, functional connectivity, emotion regulation, anxiety, mother–child relationship, sensitive period
Introduction
Adults rely on intrinsic sources of regulation to manage their emotions and effectively navigate social interactions and the environment (Gross, 1998). At the neural systems level, emotion regulation is supported by mature amygdala–prefrontal circuitry in healthy adults (Banks et al., 2007; Hariri et al., 2003; Kim et al., 2003). However, children have yet to develop these internal mechanisms of regulation, as amygdala–prefrontal circuitry undergoes protracted development through adolescence (Gee, Humphreys, et al., 2013; Guyer et al., 2008; Hare et al., 2008; Monk et al., 2003). Because of this relative immaturity, external sources (primarily caregivers) of regulation play a critical role in children's emotion regulation (Hofer, 1994; McCoy & Masters, 1985).
Early in life, the caregiver, often the mother, is one of the most potent stimuli for (semi-) altricial species and plays an essential role in typical behavioral and neural development (Tottenham, 2012). The mother* provides widespread regulatory influences in childhood that include physiological, thermal, and nutritional regulation (Hofer, 1994). The affective mother-child bond is intense and learned, resulting in mother preference, stranger wariness/avoidance in infants, and greater approach-related behavior (Zarbatany & Lamb, 1985), greater exploration (Ainsworth & Bell, 1970), and less fear in children (Campos, Emde, Gaensbauer, & Henderson, 1975). Taken together, these studies suggest that the mother is uniquely relevant as an external source of regulation when the child approaches and engages with the environment.
In both non-human animals and humans, the maternal stimulus is distinguished from nonmaternal stimuli at the neural level as well (Caldji et al., 1998; Hostinar, Sullivan, & Gunnar, 2014; Moriceau & Sullivan, 2006; Plotsky et al., 2005; Todd, Evans, Morris, Lewis, & Taylor, 2011; Tottenham, Shapiro, Telzer, & Humphreys, 2012). In rodents, maternal presence reduces amygdala activity in the pup, thus promoting attachment behaviors and reducing fear (Moriceau & Sullivan, 2006). Given the functional homologies between the rat and human early in life, it is possible that the mother exerts a similar influence on amygdala circuitry in the human, who remains dependent on maternal care for a much longer period. Thus, the present study focused on neural mechanisms underlying the mother's external regulation of emotion in human development.
Recently, we characterized developmental changes in the amygdala–prefrontal circuit that supports emotion regulation in adulthood, with evidence suggesting a shift from an immature to a mature phenotype of amygdala-prefrontal circuitry around the transition between childhood and adolescence. Specifically, children exhibit positive connectivity between the amygdala and medial prefrontal cortex (mPFC) and higher amygdala reactivity, compared with negative amygdala–mPFC connectivity and lower amygdala reactivity in adolescence and adulthood (Gee, Humphreys, et al., 2013). For children, the normative state is having access to their caregiver who can provide external regulation of developmentally normative heightened amygdala reactivity. We hypothesized that maternal stimuli might induce phasic modulation of amygdala–mPFC circuitry to promote emotion regulation in childhood by modulating amygdala–mPFC connectivity (instantiating mature-like connectivity) and reducing amygdala and affective reactivity. We anticipated that this effect would be absent in adolescence, a time of increasing independence from the mother. In order to examine the potential mechanism through which the mother provides affect regulation, the present study used a functional magnetic resonance imaging (fMRI) task with maternal and non-maternal stimuli, and an affect-related regulation task, to examine whether the mother effectively modulates amygdala–prefrontal connectivity and associated regulatory behavior.
*We use “mother” for simplicity, although other adults can serve as the primary caregiver.
Method
fMRI Session: Does the Maternal Stimulus Alter Amygdala–mPFC Circuitry?
Participants
Participants were 23 children (ages 4-10 years old, mean age (S.D.) = 7.34 (2.14); 14 females, 9 males) and 30 adolescents (ages 11-17 years old, mean age (S.D.) = 14.21 (1.87); 18 females, 12 males). Sample size was determined through an a priori targeted enrollment of 60 participants, which accounted for expected data loss due to excessive motion during fMRI scanning. We aimed to obtain at least 50 participants with usable scanning data to ensure sufficient sampling across the age range of 4-17. Data collection was stopped following completion of the targeted enrollment. Seven participants were excluded due to excessive motion in fMRI data. All participants were physically and psychiatrically healthy (no medical or psychiatric disorders), as confirmed by a telephone screening prior to participation. The Child Behavior Checklist (CBCL) (Achenbach, 1991) was used to assess clinical symptoms, and all participants fell within the normal range on the CBCL Total Problems, Internalizing Problems, and Externalizing Problems scales. Participants were from European American (37.7%; 34.8% of children, 40% of adolescents), African American (32.1%; 26.1% of children, 36.7% of adolescents), Asian American (15.1%; 26.1% of children, 6.7% of adolescents), American Indian (5.7%; 4.3% of children, 6.7% of adolescents), and other (9.4%; 8.7% of children, 10% of adolescents) backgrounds. Among participants, 22.6% identified as Latino (21.7% of children, 23.3% of adolescents). Cognitive ability was assessed using the Wechsler Abbreviated Scale of Intelligence for participants ages 6-17 (assessments were conducted with 48 participants). The average full-scale intelligence quotient of the sample was 110 (S.D.=16). Data on household income was obtained regarding the families of 49 participants, with a modal income range of $55,001-70,000 and no difference between the child and adolescent groups. Data on primary caregiver's education was obtained for families of 52 participants, with a modal education of a four-year college degree for both the child and adolescent groups. All participants were right-handed. The protocol was approved by the Institutional Review Board at the University of California, Los Angeles. Participants provided informed consent or assent (parental informed consent for minors).
Self- and Parent-Report Measures
Anxiety was measured using the Revised Children's Anxiety and Depression Scale – parent report (RCADS-P) (Chorpita, Moffitt, & Gray, 2005). The current study was primarily interested in the subscale for separation anxiety. Attachment-related behaviors were measured using the Security Scale (Kerns, Klepac, & Cole, 1996). Attachment security is known to be difficult to measure after infancy (during which measurement relies on the Ainsworth Strange Situation task); however, the Security Scale assesses children's perceptions of security in parent-child relationships in middle childhood and early adolescence. The instrument provides scores for three subscales: 1) children's belief that their attachment figure is responsive and available; 2) children's reliance on the attachment figure in times of stress; and 3) children's ease and interest in communicating with the attachment figure.
Procedure
Participants came to the laboratory for two sessions. In the first session, behavioral measures were collected and children performed an affect-related regulation task in the presence of their mother and separately in the presence of a stranger (experimenter). Children were also acclimated to the scanner environment with an MRI replica. During the second visit, which occurred on a separate day, the mother/stranger task was administered in the MRI scanner. Participants completed the fMRI session an average of 3.81 months (S.D.=4.34, range=0-20) following the behavioral session.
Mother/Stranger fMRI Task
Participants completed a block design mother/stranger task while in the MRI scanner. Based on rodent studies that present olfactory cues associated with the mother to the developing rodent (reviewed in Moriceau, Roth, & Sullivan, 2010), the present task used visual cues (the mother's face) representative of the mother. Participants viewed pictures of their mother and an ethnicitymatched unfamiliar individual, who was the mother of another child (stranger), in alternating blocks of 28 seconds each. Both mother and stranger stimuli posed happy and neutral expressions, where all models wore white material around their necks; thus there were two images of mother stimuli and two images of strangers. These images were obtained within the laboratory and standardized for size and luminance. The face images were in full color with a vertical visual angle of approximately 15°. Participants were instructed to respond quickly for the happy facial expression (regardless of model), which was presented 50% of the time with a fixed random order. There were 4 blocks of mother (M), 4 blocks of stranger (S), and 3 blocks of fixation, which were presented in alternating blocks of mother and stranger (+MSMS+SMSM+) and counterbalanced across subjects. Each block contained 18 stimuli of either mother (happy and neutral) or stranger (happy and neutral), resulting in a total of 144 stimulus presentations -- 72 mother trials and 72 stranger trials. Each face stimulus was presented for 500 milliseconds followed by 1000 ms of fixation. Thus, participants were allowed approximately 1500 ms to respond by pressing a button with their index finger. Participants viewed images through video goggles (Resonance Technology, Inc., model: VisuaStim Digital, software version 8). A response box (Current Designs, Inc., model: 932 fORP, with custom MacStim 1–9 no 5 setting) was used for recording behavioral responses. The entire task lasted 4:54 minutes. Prior to scanning, participants were given the opportunity to practice to ensure that they understood and could perform the task.
Scanning Parameters
Participants were scanned with a Siemens Trio 3.0-Tesla fMRI scanner. During data collection, an air vacuum pillow (Siemens Comfort Pack) was used to pad and secure the child's head in a comfortable, steady position. A whole brain, high resolution, T1-weighted anatomic scan (MP-RAGE; 192 × 192 inplane resolution, 250 mm field of view [FOV]; 176 mm × 1 mm sagittal slices) was acquired for each subject for transformation and localization of functional data into Talairach space (Talairach et al., 1988). For the functional run of the mother/stranger task, we collected 143 T2*-weighted echoplanar images (34 slices, slice thickness 4 mm (skip 0), TR=2000 ms, TE=30 ms, flip angle =90 degrees, matrix 64 × 64) at an oblique angle of approximately 30 degrees.
fMRI Preprocessing
Functional imaging data were preprocessed and analyzed with the Analysis of Functional NeuroImages (AFNI) software package. All included data were free of movement greater than 2.5 mm in any direction. After slice time correction, images were registered to the first image volume after the high-resolution anatomical dataset with rigid body transformations and smoothed with anisotropic 6-mm Gaussian kernel. Time series were normalized to percent signal change to allow comparisons across runs and individuals by dividing signal intensity at each time point by the mean intensity for that voxel and multiplying the result by 100. The model included regressors for each of the two variable types (two stimulus types; mother and stranger) by convolving the stimulus timing files with canonical hemodynamic response function. Six motion parameters were included as separate regressors for a total of 8 regressors. General linear modeling (GLM; random effects) was performed to fit the percent signal change time courses to each regressor. Linear and quadratic trends were modeled in each voxel time course to control for correlated drift. Group-level analyses were conducted on the regression coefficients from the individual analysis after transformation into the standard coordinate space of Talairach and Tournoux with parameters obtained from the transformation of each subject's high- resolution anatomical scan. Talairached transformed images had a resampled resolution of 3 mm3.
Task-Dependent Functional Connectivity
A whole-brain psychophysiological interaction (PPI) analysis was conducted to examine whether the mother versus stranger context modulated functional connectivity with the amygdala. The PPI analysis tested the extent to which the amygdala covaried with other brain regions more during the viewing of mother faces, compared with the viewing of stranger faces. A GLM analysis was performed in AFNI for each participant with regressors for task, seed region timeseries, interaction of task and timeseries, accuracy, and six motion regressors. Two psychological (task) regressors modeled whether a given trial consisted of viewing the mother's face or the stranger's face. The physiological (seed region timeseries) regressor comprised the timeseries for the right anatomically defined amygdala. Two interaction regressors modeled the interaction of the psychological regressors and the physiological regressor, such that each interaction regressor identified regions whose timeseries correlated in a task-dependent manner with the amygdala timeseries. The GLM analyses fit the percent signal change timeseries to each regressor, and linear and quadratic trends were modeled for each voxel's timeseries to control for correlated drift. The individual-level regression coefficients were then submitted to random-effects, group level analyses.
Statistical and Behavioral Data Analysis
fMRI
Participants were grouped by age (4-10 years versus 11-17 years), based on prior studies demonstrating a valence switch in amygdala–mPFC coupling around age 10 (Gabard-Durnam et al., 2014; Gee, Gabard-Durnam, et al., 2013). We performed a 2x2 repeated-measures ANOVA to test for effects of age group (children/adolescents) and condition (mother/stranger) on beta weights for amygdala reactivity and functional connectivity. Individual differences in anxiety and attachment were examined using univariate ANOVAs with factors for age group and amygdala–mPFC connectivity valence (positive or negative to mother, relative to implicit baseline).
The right anatomical amygdala, as defined by the Talairach & Tournoux Atlas in AFNI, was selected as a region-of-interest (ROI). We were particularly interested in the right amygdala given past literature on hemispheric asymmetry suggesting that the left hemisphere is more involved in positive emotion whereas the right hemisphere is more threat-sensitive and involved in negative emotion (Canli, Desmond, Zhao, Glover, & Gabrieli, 1998; Compton, Heller, Banich, Palmieri, & Miller, 2000), even in infants (Davidson & Fox, 1982). Thus, we hypothesized that maternal buffering would occur via the right amygdala, which would normally mediate the monitoring of threat presence or absence. In addition, we have previously shown that the mother potentiates activation in the left amygdala (Tottenham et al., 2012). Results for the left amygdala (also defined anatomically using the Talairach & Tournoux Atlas in AFNI) are presented in the Supplemental Results. The mPFC ROI consisted of z=10 to z=20 of the anatomically defined anterior cingulate cortex in the Talairach & Tournoux Atlas in AFNI. This particular region was selected based on prior work demonstrating its role in affective tasks (Bush, Luu, & Posner, 2000), as well as changes in its connectivity with the amygdala across typical development (Gabard-Durnam et al., 2014; Gee, Humphreys, et al., 2013); beta weights were extracted from the PPI analysis for this ROI.
Behavior in the Scanner
For each participant, we calculated accuracy and mean reaction time (RT) for each condition (mother, stranger). We calculated accuracy as the difference between the total number of trials (72) and total errors. Total errors equaled the sum of false alarms to neutral faces (errors of commission; i.e., pressing to a neutral face) and misses to happy faces (errors of omission; i.e., not pressing to a happy face). We calculated the mean RT for correct hits to happy faces and for false alarms to neutral faces.
Motion Correction
Systematic procedures were implemented to reduce motion, particularly in younger participants, and to ensure that children remained still throughout the duration of the task. Before the MRI scanning session, children participated in a mock scanning session to help them to acclimate to the scanning environment and to feel comfortable with the scanning procedures. In addition, this step provided an opportunity for children to practice and receive feedback on lying still in order to optimize children's ability to remain still during actual data collection. During data collection, an air vacuum pillow (Siemens Comfort Pack) was used to pad and secure the child's head in a comfortable, steady position. Additional padding was placed around the child's head. In addition, all participants were provided with feedback and reminders regarding motion throughout the scanning session.
Multiple steps were taken to correct for motion. All analyzed data were free of motion greater than 2.5mm in any direction. Volumes with motion greater than 2.5mm in any direction were excluded (via censoring), and all participants had fewer than 27% of total volumes censored (mean % of censored volumes=1.4%; mode=0%). Preprocessing included standard spatial realignment to correct for motion. Motion regressors were included in our imaging analyses (at the subject level, motion in all six directions at the trial by trial level). In addition, multiple analyses were conducted to rule out potential effects of motion. Average motion (across all six directions) did not correlate with age, amygdala reactivity, or amygdala–mPFC functional connectivity and did not differ between the two age groups (all ps>.05). Given recent advances in methods for controlling for motion, we also conducted a secondary analysis in which we re-analyzed our functional connectivity data controlling for different motion levels across participants (Van Dijk, Sabuncu, & Buckner, 2012). The mean absolute displacement value was calculated for each participant and was entered as a covariate into the repeated measures ANOVA examining functional connectivity to mother versus functional connectivity to stranger as a function of age group. Results of this secondary analysis replicated our original finding of the condition x age group interaction (F(1,49)=3.98, p=.052). Moreover, mean displacement value was not associated with age, amygdala reactivity, or amygdala–mPFC functional connectivity and did not differ between the two age groups (ps>.05).
Behavioral Session: Does Maternal Presence Alter Regulation in Affective Contexts?
Of the original 53 participants, 47 also completed the emotional go/no-go task, which aims to measure regulatory behavior in an affective context. The 47 participants had a mean age of 11.44 (S.D.=3.86, range=4-17). Of the 6 participants who did not complete the behavioral session, 3 were children and 3 were adolescents.
Affect-Related Regulation Task
Participants completed an affect-related regulation task (emotional face go/nogo) (Hare et al., 2008; Tottenham, Hare, & Casey, 2011) outside of the scanner, during which they viewed facial expressions of emotion. Face stimuli consisted of color images of four female faces from the Karolinska Directed Emotional Faces database, each posing happy, sad, and neutral faces (Lundqvist, Flykt, & Ohman, 1998). The face images were in full color with a vertical visual angle of approximately 12°. The three task blocks consisted of the following: happy as “go” expression in context of sad “no-go” expression, happy as “go” expression in context of neutral “no-go” expression, and neutral as “go” expression in context of happy “no-go” expression. Participants were instructed to press quickly for the “go” face and were not told which expression would be the “no-go” stimulus. Blocks were counterbalanced across participants. The “go” facial expression was presented 66.6% of the time to create a prepotent tendency for responding. Thus, the task required pressing a button when the target facial expression (“go”) appeared, and inhibiting this behavioral response when a distracter facial expression (“no-go”) appeared. Stimuli were presented in a fixed random order. Each face stimulus was presented for 500 milliseconds (ms) followed by 1000 ms of fixation to ensure enough time for responding.
Maternal Presence Manipulation
The task was administered twice, once in the presence of the participant's mother and once in the presence of a stranger (a research assistant). During each administration, the mother or stranger sat next to the participant while he/she completed the computerized task. Participants were instructed to focus on the task in both conditions, and mothers were instructed not to speak with the participant during the task, but instead to occupy themselves with paperwork. The order of administration (mother or stranger) was counterbalanced.
Statistical and Behavioral Data Analysis
For each participant, we calculated accuracy as the difference between the number of correct hits to “go” faces and the number of false alarms (i.e., pressing to a “no-go” face). We calculated the mean reaction time for correct hits to neutral faces in the context of happy and sad faces. Repeated-measures ANOVAs assessed differences in behavioral performance when in the presence of the mother versus the stranger. A repeated-measures ANOVA tested the effects of amygdala– mPFC connectivity valence (between-subjects factor) and mother/stranger presence (within-subjects factor) on behavior. In order to assess whether amygdala reactivity related to how children performed in the presence of their mother or stranger, we tested whether children with greater suppression of the amygdala to mother (versus stranger) differed from children with less amygdala suppression to mother on false alarm rate when in the presence of their mother or a stranger.
Results
fMRI Session: Does the Maternal Stimulus Alter Amygdala–mPFC Circuitry?
To examine the effect of the maternal stimulus on amygdala–mPFC circuitry, participants were scanned while viewing alternating blocks of the faces of their mother and a stranger (the mother of another child), each posing happy and neutral faces (Tottenham et al., 2012). Participants were asked to make a button press when viewing the happy expressions, which were presented 50% of the time. Participants were grouped by age (4-10 years old; 11-17 years old) based on prior work demonstrating a shift in amygdala–mPFC functional connectivity around age 10 (Gabard-Durnam et al., 2014; Gee, Gabard-Durnam, et al., 2013).
Effect of Mother on Amygdala Reactivity
Beta weights corresponding to BOLD signal were extracted from the right amygdala, which was anatomically defined by the Talairach & Tournoux Atlas in AFNI (1988). The right amygdala was selected based on prior research on hemispheric asymmetry suggesting that maternal buffering would occur via the right hemisphere, which is more sensitive to threat than the left (Compton et al., 2000). A mother/stranger condition x age interaction demonstrated that differential activation to the mother versus stranger stimuli depended on age (F(1,53)=4.065, p=.049; η2 = .071, medium effect size (Cohen, 1988)). Specifically, right amygdala reactivity in children, which is typically high (Gee, Humphreys, et al., 2013), was suppressed when viewing their mother, relative to the stranger condition (Figure 1). By contrast, adolescents did not show differential amygdala reactivity to mother versus stranger. Supplemental Figure 1 shows amygdala reactivity to mother and stranger reactivity versus implicit baseline for children and adolescents.
Figure 1.
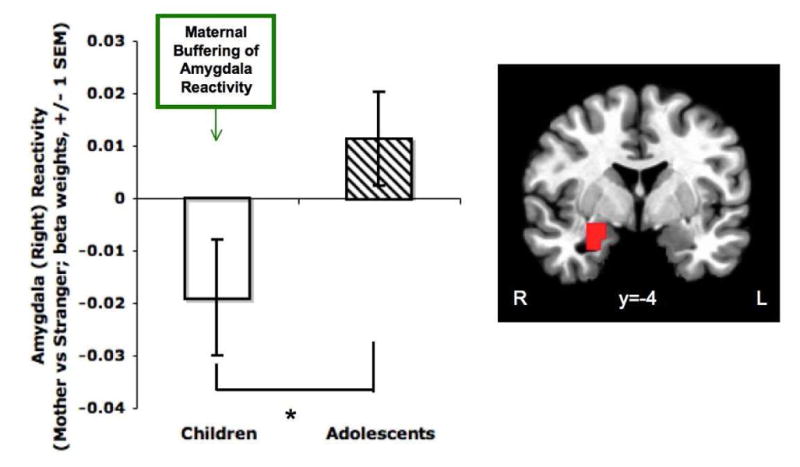
Maternal buffering of amygdala reactivity in childhood. Presence of the maternal stimulus phasically buffered right amygdala reactivity in children but not adolescents (p=.049). Specifically, children showed lower activation of the right amygdala to their mother compared with a stranger (i.e., the mother of another youth). The right amygdala was defined using the Talairach & Tournoux Atlas in AFNI.
Effect of Mother on Functional Connectivity
A voxelwise psychophysiological interaction (PPI) analysis, with the seed region of the right anatomically defined amygdala, was conducted to examine how the mother and stranger task contexts modulated functional connectivity between the amygdala and mPFC. Results demonstrated a mother/stranger condition x age group interaction for amygdala–mPFC functional connectivity (F(1,51)=4.737, p=.034; η2 = .085, medium effect size). Whereas adolescents showed negative amygdala–mPFC functional connectivity relative to baseline that did not differ between the mother and stranger conditions (t(28)=.139, p=.89), children showed differential connectivity to the mother and stranger conditions (t(23)=2.496, p=.02; η2 = .213, large effect size; Figure 2). Amygdala–mPFC functional connectivity in children was negative relative to implicit baseline (inversely correlated) only for the mother (t(23)=2.52, p=.019; d = 0.51, medium effect size) but was not significantly different than zero for the stranger relative to implicit baseline (t(23)=.70, p=.494). Connectivity for the mother did not differ between children and adolescents (t(51)=.48, p=.634).
Figure 2.
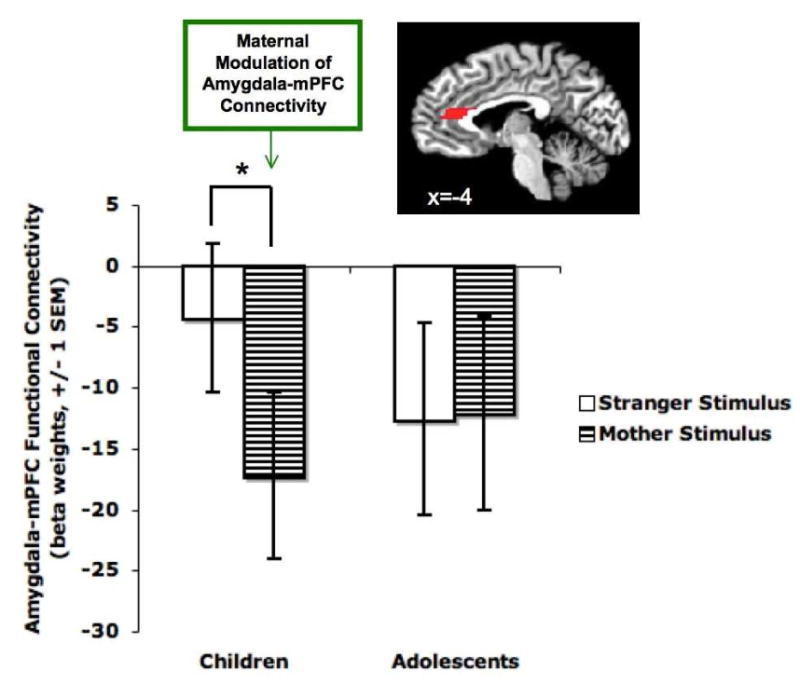
Mature-like neural connectivity patterns in response to maternal stimuli in children. The PPI analysis of amygdala–mPFC functional connectivity revealed an interaction between age group and the maternal stimulus manipulation (p=.034). Specifically, adolescents showed a mature (negative relative to implicit baseline) pattern of amygdala–mPFC functional connectivity to both their mother and the stranger (i.e., the mother of another youth). In contrast, children exhibited a mature-like (negative relative to implicit baseline) pattern of functional connectivity to their mother (p=.019). However, functional connectivity to the stranger did not differ from implicit baseline in children, suggesting that the phasic presence of the maternal stimulus may induce a more maturelike pattern of amygdala–prefrontal interaction in childhood.
Amygdala–mPFC Connectivity and Individual Differences
In order to understand the relationship between maternally-induced neural phenotype and individual differences, participants were grouped into those showing positive connectivity relative to implicit baseline (n=17; indicative of child-like neural response) and those showing negative connectivity relative to implicit baseline (n=32; indicative of adult-like neural response). Participants who demonstrated negative amygdala–mPFC functional connectivity to mother (versus implicit baseline) had lower separation anxiety than those who exhibited positive connectivity to mother (F(1,48)=6.12, p=.021; η2 = .21, large effect size; Figure 3a). As anticipated, adolescents had lower separation anxiety than children (F(14,48)=3.11, p=.008; η2 = .65, large effect size). There was no interaction between age group and connectivity valence on separation anxiety (F(1,48)=1.11, p=.394). Participants with negative amygdala–mPFC functional connectivity to mother (versus implicit baseline) also had higher attachment security, as measured by the Security Scale's subscale for reliance on attachment figure in times of stress (Kerns et al., 1996) (F(1,41)=4.20, p=.047; η2 = .11, medium-large effect size; Figure 3b). There was no main effect of age group (F(1,41)=.15, p=.70) or interaction effect between age group and connectivity valence on reported attachment (F(1,41)=2.17, p=.149).
Figure 3.
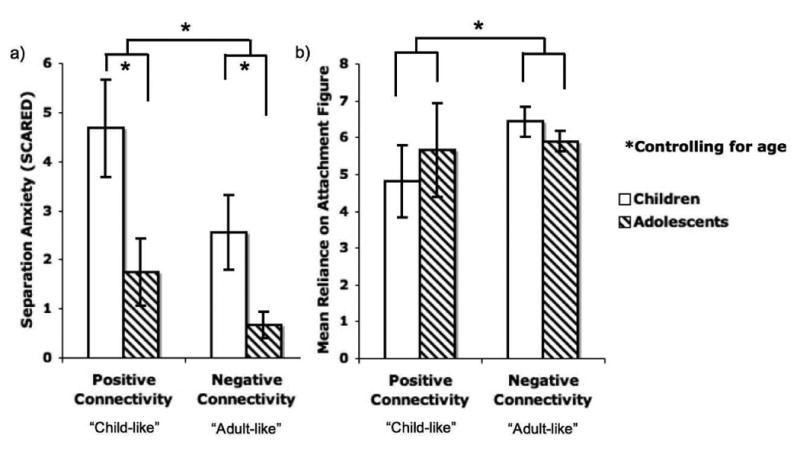
Individual differences in separation anxiety and attachment. Participants who displayed the mature-like, negative pattern of amygdala–mPFC functional connectivity to the maternal stimulus differed on anxiety and attachment from participants who showed positive connectivity to the maternal stimulus. Specifically, children for whom the mother was an effective modulator of amygdala–mPFC circuitry exhibited a) lower separation anxiety (p=.021) and b) more secure attachment (p=.008). Consistent with a normative decline in separation anxiety across development, children also displayed higher separation anxiety than adolescents.
Behavioral Session: Does Maternal Presence Alter Regulation in Affective Contexts?
To investigate whether maternal presence modulated affect-related regulatory behavior, participants completed an affect-related regulation task (an emotional face go/nogo) (Hare et al., 2008; Tottenham et al., 2011) twice – once seated next to their mother, and once seated next to a stranger (i.e., experimenter) (with the order of administration counterbalanced). The affect-related regulation task involves viewing two facial expressions of emotion during any given block, and participants are instructed to respond to the “go” expression (presented 66.6% of the time to create a prepotent tendency for responding) and inhibit response to the “no-go” expression (see Materials and Methods for additional details). The task thus provides a measure of regulatory behavior in an affective context.
Effect of Mother on Behavioral Performance
Analyses of behavioral performance were conducted using Bonferroni-adjusted alpha levels of .0167 (.05/3) per test to control for tests of false alarm rate, miss rate, and reaction time. There was a mother/stranger condition x age group interaction (F(1,46)=6.35, p=.015; η2 = .12, medium-large effect size) for false alarm rate, such that children (but not adolescents) exhibited fewer false alarms in the presence of the mother compared with the stranger (t(21)=2.24, p=.037; d = .35, small effect size; Figure 4). There were no main effects of or interactions with the mother/stranger condition on miss rate or reaction time. This laboratory-based regulatory behavior was associated with maternal buffering of the amygdala during childhood (see Supplemental Results).
Figure 4.
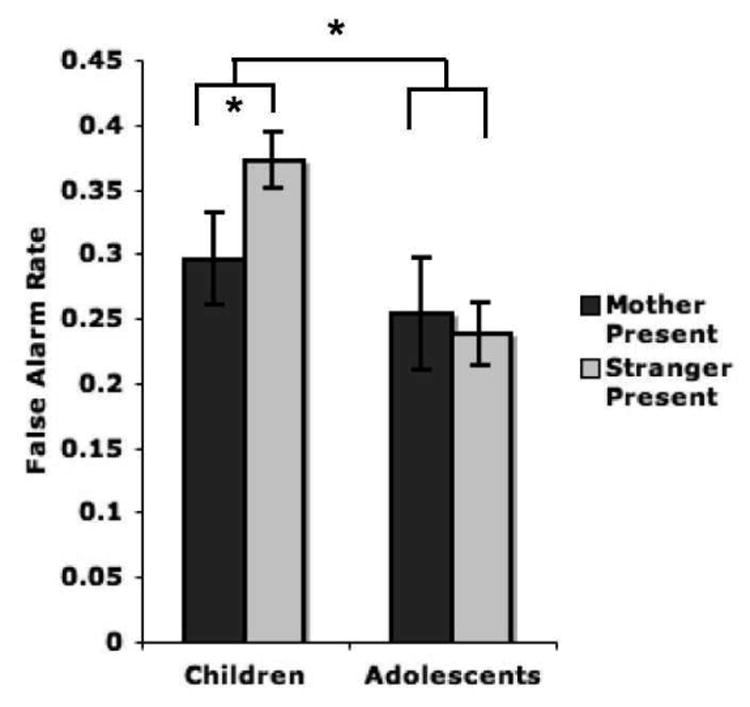
Maternal presence and behavioral regulation in childhood. During the affect-related regulation task, children performed better when seated next to their mother than when seated next to a stranger, whereas adolescents’ performance did not differ by condition (p=.015). Specifically, maternal presence appeared to improve affect-related regulatory behavior among children, as false alarm rate was lower but overall miss rate was not lower when children were with their mothers. There was also a main effect of age group with adolescents performing with fewer false alarms than children.
Discussion
The present study examined the effects of the mother (visual presentation of her image or her physical presence) on amygdala–prefrontal circuitry and regulatory behavior in children and adolescents. The maternal stimulus induced the mature-like pattern of negative amygdala–prefrontal coupling and suppressed amygdala reactivity in children, whereas adolescents showed the expected pattern of mature connectivity to both the maternal and control conditions and did not show reduced amygdala reactivity to their mother. Moreover, children, but not adolescents, performed with better regulatory behavior in the presence of their mother, whereas adolescents’ performance was unaffected by maternal presence. These findings demonstrate that the maternal stimulus can modulate amygdala–mPFC circuitry and affect behavior, supporting the hypothesis that the mother serves as an external source of regulation through buffering effects on amygdala–prefrontal circuitry in childhood.
The finding that maternal stimuli buffer amygdala reactivity among children provides a neurobiological basis for the regulatory effects of caregiver presence early in life. Suppressed amygdala reactivity to the mother in this study parallels prior findings of maternal suppression of amygdala reactivity in rodents (Moriceau & Sullivan, 2006). Evidence shows that maternal presence also reduces corticosterone in rodents (Levine, 2001) and cortisol in non-human primates (Bayart, Hayashi, Faull, Barchas, & Levine, 1990) and humans (Gunnar & Donzella, 2002). Thus, the mother may buffer against stress through direct effects on the hypothalamic-pituitary-adrenal (HPA) axis, which acts on the developing amygdala. This potential mechanism has been explored early in life, with evidence that social regulation through maternal buffering serves a regulatory function in humans (Gunnar & Donzella, 2002). Unlike the regulatory effects of the mother on children in the present study, adolescents did not exhibit maternal neural modulation, possibly reflecting decreased behavioral relevance of caregivers as independence increases. Adults do not show differential amygdala activation to the face of their mother compared a stranger (Taylor et al., 2009), suggesting that childhood is a unique time during which the mother can modulate neurocircuitry. A similar period in rats has been identified as a “conditional sensitive period” for the amygdala (Moriceau & Sullivan, 2006). Though understanding the function of maternal amygdala suppression requires further investigation in humans, participants with reduced amygdala reactivity to their mother made fewer false alarms in the presence of their mother, compared to participants without maternal amygdala suppression. Thus, maternal presence may act on amygdala circuitry to enhance regulatory behavior.
Adolescents exhibited negative amygdala–mPFC connectivity (versus baseline) regardless of condition, consistent with evidence for a switch from positive to negative amygdala–mPFC connectivity around the transition to adolescence (Gee, Gabard-Durnam, et al., 2013; Gee, Humphreys, et al., 2013). In contrast to connectivity that did not differ from baseline to the stranger's face, children showed connectivity to their mother that was negative relative to baseline. Thus, children's connectivity resembled the pattern of connectivity in adolescents only for the maternal condition. Given the known regulatory role of the mPFC in adults (Hariri et al., 2003; Kim et al., 2003), the observation in children of negative amygdala–mPFC connectivity with reduced amygdala reactivity to their mothers suggests that the mother might buffer against amygdala reactivity through modulation of mPFC coupling with the amygdala. Though PPI analyses can compare how two tasks modulate functional connectivity, it is important to understand how each task modulates connectivity relative to implicit baseline. For adolescents, the mother and stranger conditions modulated amygdala-mPFC connectivity in similar ways; by contrast, children showed modulation by the mother (more negative than baseline) but not by the stranger context. Thus, the modulation of amygdala-mPFC functional connectivity by maternal or stranger context changed with age. It is important to note the phasic nature of maternal neural modulation here, compared with prior evidence that prolonged maternal separation accelerates amygdala-prefrontal development (Callaghan & Richardson, 2011; Gee, Gabard-Durnam, et al., 2013). Thus, while extended maternal deprivation may induce early development of mature connections with deleterious consequences, strong parent—child attachment may promote regulation through phasic amygdala-prefrontal modulation in the mother's presence.
The finding that maternal buffering was present in children, but absent in adolescents, suggests that childhood may be a sensitive period for amygdala-prefrontal development. Identifying sensitive periods, during which the environment has particularly strong influences on neurodevelopment and thus lasting effects on behavior, is critical to understanding the developmental timing of neuroplasticity and how adult phenotypes are constructed. Sensitive periods have been shown in animal models of affective neurodevelopment (Callaghan & Richardson, 2011; Moriceau & Sullivan, 2006; Yang, Lin, & Hensch, 2012) but have been elusive in humans. The present study provides initial evidence for a sensitive period in human amygdalamPFC development, during which the mother can modulate the system.
Physical maternal presence also affected performance on the affect-related regulation task, which has been used previously to measure behavioral regulation in an emotional context (Hare et al., 2008). Specifically, children made fewer false alarms in their mother's presence, which may reflect the mother enabling better behavioral regulation. Notably, children's false alarm rate, which indexes a failure to regulate a response to an emotional stimulus, but not miss rate, was modulated by maternal presence. These findings suggest that maternal presence does not improve general performance but specifically enhances affect-related behavioral regulation. Consistent with the increased behavioral relevance of the mother in childhood and our finding that access to maternal stimuli affected amygdala–prefrontal circuitry in children but not adolescents, only children performed differently in the maternal context.
Within the children, individual differences in maternal amygdala-prefrontal modulation related to behavior. Those children who benefited from maternal buffering against amygdala reactivity were able to better regulate their affect-related regulatory behavior than children whose mothers did not reduce amygdala reactivity. Moreover, the adolescent-like neural phenotype elicited by maternal stimuli may reflect positive aspects of the parent—child relationship. Specifically, children who displayed negative connectivity to their mother (relative to implicit baseline) reported lower separation anxiety and more secure attachment than children who displayed positive connectivity to their mother. It may be that the nature of a secure attachment relationship results in the mother's ability to regulate amygdala–prefrontal circuitry in children.
Though the fMRI task lacked an explicit demand for emotion regulation, it provides a unique opportunity to test the hypothesis that the mother buffers against amygdala reactivity in childhood. Prior work suggests normatively heightened amygdala reactivity in childhood (Decety, Michalska, & Kinzler, 2012; Gee, Humphreys, et al., 2013; Swartz, Carrasco, Wiggins, Thomason, & Monk, 2014; Vink, Derks, Hoogendam, Hillegers, & Kahn, 2014). In novel situations (e.g., fMRI scanner), young children can typically access their caregiver, who would buffer against developmentally normative high amygdala reactivity. Employing threat-related stimuli or acute stressors will be important to further test maternal buffering. Though mother/stranger physical presence was manipulated in the behavioral task, visual images were manipulated in the fMRI task. It may be that the mother's physical presence would produce even stronger neural effects of maternal buffering. A limitation of our fMRI task is the lack of stimuli normed by independent raters for valence, arousal, or perceived age. Moreover, because we purposefully compared amygdala reactivity to mothers versus strangers, we cannot rule out the possible influence of facial familiarity. This possibility seems less likely, however, because the effect was absent in adolescents (who should have even greater familiarity with their mothers). Future research would benefit from the inclusion of a familiar non-maternal control condition. Finally, because the present study had a wide age range, future studies that focus on a group of similarly-aged participants in different stages of puberty (e.g., Forbes et al., 2011) would help to disentangle influences of age and puberty.
Caregivers are one of the most salient stimuli early in life and provide many types of regulation for developing youth, including emotion regulation. Our findings provide a neuromechanistic framework for how caregivers regulate children's emotional reactivity. Specifically, maternal presence appears to buffer against amygdala reactivity and induce phasic modulation of amygdala–mPFC connectivity, reflecting positive attachment and reduced anxiety. Moreover, for children who experience neural regulatory effects of their mother, maternal presence enables enhanced regulatory behavior. Thus, caregivers may serve an external regulatory function while circuitry supporting emotion regulation develops in childhood. With age, this maternal effect may become internalized, allowing for independent regulation via mature neural systems. The present findings provide evidence for how emotion regulation is supported in humans prior to mature central nervous system development.
Supplementary Material
Acknowledgments
The project described was supported by the National Institute of Mental Health [R01MH091864 (NT) & P50MH078105], the Dana Foundation (NT), and the National Science Foundation [Graduate Research Fellowship awards (DGG, LGD)].
Footnotes
Author Contributions: N. Tottenham developed the study concept. All authors contributed to data collection. D.G. Gee, L. Gabard-Durnam, and N. Tottenham analyzed and interpreted the data. D.G. Gee, L. GabardDurnam, and N. Tottenham drafted the manuscript, and all authors provided critical revisions. All authors approved the final version of the manuscript for submission.
Declaration of Conflicting Interests: The authors declare no competing financial interests.
References
- Achenbach TM. Manual for Child Behavior Checklist 4-18, 1991 Profile. Univ Vermont/Dept Psychiatry; 1991. [Google Scholar]
- Ainsworth MDS, Bell SM. Attachment, Exploration, and Separation: Illustrated by the Behavior of One-Year-Olds in a Strange Situation. Child Development. 1970;41(1):49. doi: 10.2307/1127388. [DOI] [PubMed] [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala–frontal connectivity during emotion regulation. Social Cognitive and Affective Neuroscience. 2007;2(4):303–312. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayart F, Hayashi KT, Faull KF, Barchas JD, Levine S. Influence of maternal proximity on behavioral and physiological responses to separation in infant rhesus monkeys (Macaca mulatta) Behavioral Neuroscience. 1990;104(1):98–107. [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4(6):215–222. doi: 10.1016/S1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(9):5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan BL, Richardson R. Maternal separation results in early emergence of adultlike fear and extinction learning in infant rats. Behavioral Neuroscience. 2011;125(1):20–28. doi: 10.1037/a0022008. [DOI] [PubMed] [Google Scholar]
- Campos JJ, Emde RN, Gaensbauer T, Henderson C. Cardiac and behavioral interrelationships in the reactions of infants to strangers. Developmental Psychology. 1975;11(5):589–601. doi: 10.1037/0012-1649.11.5.589. [DOI] [Google Scholar]
- Canli T, Desmond JE, Zhao Z, Glover G, Gabrieli JD. Hemispheric asymmetry for emotional stimuli detected with fMRI. Neuroreport. 1998;9(14):3233–3239. doi: 10.1097/00001756-199810050-00019. [DOI] [PubMed] [Google Scholar]
- Chorpita BF, Moffitt CE, Gray J. Psychometric properties of the Revised Child Anxiety and Depression Scale in a clinical sample. Behaviour Research and Therapy. 2005;43(3):309–322. doi: 10.1016/j.brat.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. L. Erlbaum Associates; 1988. [Google Scholar]
- Compton RJ, Heller W, Banich MT, Palmieri PA, Miller GA. Responding to threat: hemispheric asymmetries and interhemispheric division of input. Neuropsychology. 2000;14(2):254–264. doi: 10.1037//0894-4105.14.2.254. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Fox NA. Science. 4578. Vol. 218. New York, N.Y.: 1982. Asymmetrical brain activity discriminates between positive and negative affective stimuli in human infants; pp. 1235–1237. [DOI] [PubMed] [Google Scholar]
- Decety J, Michalska KJ, Kinzler KD. Cerebral Cortex. 1. Vol. 22. New York, N.Y.: 2012. The contribution of emotion and cognition to moral sensitivity: a neurodevelopmental study; pp. 209–220. 1991. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Phillips ML, Silk JS, Ryan ND, Dahl RE. Neural systems of threat processing in adolescents: role of pubertal maturation and relation to measures of negative affect. Developmental Neuropsychology. 2011;36(4):429–452. doi: 10.1080/87565641.2010.550178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabard-Durnam LJ, Flannery J, Goff B, Gee DG, Humphreys KL, Telzer E, Tottenham N. The development of human amygdala functional connectivity at rest from 4 to 23years: A cross-sectional study. NeuroImage. 2014 doi: 10.1016/j.neuroimage.2014.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, Tottenham N. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(39):15638–15643. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, Tottenham N. A developmental shift from positive to negative connectivity in human amygdalaprefrontal circuitry. The Journal of Neuroscience. 2013;33(10):4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ. The emerging field of emotion regulation: An integrative review. Review of General Psychology. 1998;2(3):271–299. doi: 10.1037/1089-2680.2.3.271. [DOI] [Google Scholar]
- Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27(1–2):199–220. doi: 10.1016/S0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- Guyer AE, Monk CS, McClure-Tone EB, Nelson EE, Roberson-Nay R, Adler AD, Ernst M. A Developmental Examination of Amygdala Response to Facial Expressions. Journal of Cognitive Neuroscience. 2008;20(9):1565–1582. doi: 10.1162/jocn.2008.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological Substrates of Emotional Reactivity and Regulation in Adolescence During an Emotional Go-Nogo Task. Biological Psychiatry. 2008;63(10):927–934. doi: 10.1016/j.biopsych.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR. Neocortical modulation of the amygdala response to fearful stimuli. Biological Psychiatry. 2003;53(6):494–501. doi: 10.1016/S0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- Hofer MA. Early relationships as regulators of infant physiology and behavior. Acta Pædiatrica. 1994;83:9–18. doi: 10.1111/j.1651-2227.1994.tb13260.x. [DOI] [PubMed] [Google Scholar]
- Hostinar CE, Sullivan RM, Gunnar MR. Psychobiological mechanisms underlying the social buffering of the hypothalamic-pituitary-adrenocortical axis: a review of animal models and human studies across development. Psychological Bulletin. 2014;140(1):256–282. doi: 10.1037/a0032671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns KA, Klepac L, Cole A. Peer relationships and preadolescents’ perceptions of security in the child-mother relationship. Developmental Psychology. 1996;32(3):457–466. doi: 10.1037/0012-1649.32.3.457. [DOI] [Google Scholar]
- Kim H, Somerville LH, Johnstone T, Alexander AL, Whalen PJ. Inverse amygdala and medial prefrontal cortex responses to surprised faces. Neuroreport. 2003;14(18):2317–2322. doi: 10.1097/01.wnr.0000101520.44335.20. [DOI] [PubMed] [Google Scholar]
- Levine S. Primary social relationships influence the development of the hypothalamic– pituitary–adrenal axis in the rat. Physiology & Behavior. 2001;73(3):255–260. doi: 10.1016/S0031-9384(01)00496-6. [DOI] [PubMed] [Google Scholar]
- Lundqvist D, Flykt A, Ohman A. Karolinska Directed Emotional Faces. Psychology Section, Department of Clinical Neuroscience, Karolinska Institute; Stockholm: 1998. [Google Scholar]
- McCoy CL, Masters JC. The Development of Children's Strategies for the Social Control of Emotion. Child Development. 1985;56(5):1214–1222. doi: 10.2307/1130236. [DOI] [PubMed] [Google Scholar]
- Monk CS, McClure EB, Nelson EE, Zarahn E, Bilder RM, Leibenluft E, Pine DS. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. NeuroImage. 2003;20(1):420–428. doi: 10.1016/S1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- Moriceau S, Roth TL, Sullivan RM. Rodent model of infant attachment learning and stress. Developmental Psychobiology. 2010;52(7):651–660. doi: 10.1002/dev.20482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Maternal presence serves as a switch between learning fear and attraction in infancy. Nature Neuroscience. 2006;9(8):1004–1006. doi: 10.1038/nn1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17(2):117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, Sharma S, Meaney MJ. Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2005;30(12):2192–2204. doi: 10.1038/sj.npp.1300769. doi:10.1038/sj.npp. 1300769. [DOI] [PubMed] [Google Scholar]
- Swartz JR, Carrasco M, Wiggins JL, Thomason ME, Monk CS. Age-related changes in the structure and function of prefrontal cortex-amygdala circuitry in children and adolescents: a multi-modal imaging approach. NeuroImage. 2014;86:212–220. doi: 10.1016/j.neuroimage.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system: an approach to cerebral imaging. Thieme; 1988. [Google Scholar]
- Taylor MJ, Arsalidou M, Bayless SJ, Morris D, Evans JW, Barbeau EJ. Neural correlates of personally familiar faces: parents, partner and own faces. Human Brain Mapping. 2009;30(7):2008–2020. doi: 10.1002/hbm.20646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd RM, Evans JW, Morris D, Lewis MD, Taylor MJ. The changing face of emotion: age-related patterns of amygdala activation to salient faces. Social Cognitive and Affective Neuroscience. 2011;6(1):12–23. doi: 10.1093/scan/nsq007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N. Human amygdala development in the absence of species-expected caregiving. Developmental Psychobiology. 2012;54(6):598–611. doi: 10.1002/dev.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Casey BJ. Behavioral assessment of emotion discrimination, emotion regulation, and cognitive control in childhood, adolescence, and adulthood. Frontiers in Developmental Psychology. 2011;2:39. doi: 10.3389/fpsyg.2011.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Shapiro M, Telzer EH, Humphreys KL. Amygdala response to mother. Developmental Science. 2012;15(3):307–319. doi: 10.1111/j.1467-7687.2011.01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KRA, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. NeuroImage. 2012;59(1):431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink M, Derks JM, Hoogendam JM, Hillegers M, Kahn RS. Functional differences in emotion processing during adolescence and early adulthood. NeuroImage. 2014;91:70–76. doi: 10.1016/j.neuroimage.2014.01.035. [DOI] [PubMed] [Google Scholar]
- Yang EJ, Lin EW, Hensch TK. Critical period for acoustic preference in mice. Proceedings of the National Academy of Sciences of the United States of America. 2012;109 Suppl 2:17213–17220. doi: 10.1073/pnas.1200705109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarbatany L, Lamb ME. Social referencing as a function of information source: Mothers versus strangers. Infant Behavior and Development. 1985;8(1):25–33. doi: 10.1016/S0163-6383(85)80014-X. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


