Key Clinical Message
We report a case of an elderly man who developed myositis of the deltoid muscle 8 days after influenza vaccination. Adverse reactions to influenza vaccine are generally immediate reactions. However, delayed-type hypersensitivity reactions, although rare, can occur after routine influenza vaccination.
Keywords: Adverse reactions, delayed-type hypersensitivity reactions, influenza vaccine, myositis
Introduction
The incidence of influenza infection reaches epidemic proportions each winter in Japan. Influenza is potentially fatal in the elderly, as it can be complicated by pneumonia and other serious diseases in this subpopulation. In several studies, influenza vaccination in the elderly has been reported to reduce influenza-associated mortality by 39–54% and pneumonia-associated hospitalization by 27–73% 1–3.
The commonly encountered adverse reactions to seasonal influenza vaccine are redness, pain and/or swelling at the injection site, which are reported to occur in 20% of all vaccine recipients. These adverse reactions are generally mild, the majority occurring within 48 h of vaccination and resolving within 2 or 3 days 4,5. Although rare systemic adverse reactions, such as Guillain–Barre syndrome and acute disseminated encephalomyelitis, are known to occur a few days to a few weeks after vaccination, the incidence rates of these reactions are extremely low. Given that influenza-associated mortality is 30–150 per 100,000 persons each year, it has been concluded that the benefits of the vaccine exceed the risks 6,7.
Case Report
An 80-year-old man received influenza vaccination in the deltoid muscle area of the left upper arm. On the evening of day seven after the vaccination, he noticed trembling of his left hand for ∽10 min. At that time, he was not aware of either fever or pain at the vaccination site. He then went to bed as usual. In the morning on day eight after the vaccination, his family discovered that he had urinated in his bed and vomited in his sleep. Because he did not respond to their calls, he was brought to our hospital by ambulance. The physical findings on arrival at the hospital were as follows: Glasgow coma scale E4V3M6, blood pressure 116/79 mmHg, pulse rate 79/min (regular), peripheral arterial oxygen saturation by pulse oximetry 97% (room air), respiratory rate 16/min, and body temperature 37.3°C. The hematological findings were as follows: white blood cell (WBC) count 10,400/μL (neutrophils: 79.8%), serum creatine kinase (CK) 1272 IU/L, and serum C-reactive protein (CRP) 2.39 mg/dL. Head computed tomography (CT) revealed no abnormal findings that could explain the impaired consciousness. By the time the patient arrived at the hospital, the patient had become conscious again, and the general condition was favorable. The patient was therefore placed under observation. After admission, there was no relapse of the impaired consciousness, and neither head magnetic resonance imaging (MRI) nor electroencephalography revealed any abnormalities. On day two of hospitalization, the patient was found to have a fever (∽38°C). Hematological examination revealed an increase in the serum CK and CRP levels (1975 IU/L and 3.95 mg/dL, respectively), and redness and swelling were observed in the left shoulder region. On day three of hospitalization, the fever and the redness of the left shoulder resolved, and the hematological findings revealed a decreasing trend of the WBC count and serum CK. However, because the swelling in the left shoulder was almost unchanged, CT and MRI were performed. Contrast-enhanced CT of the left shoulder joint (Fig.1) revealed swelling and an increase in area of the surrounding subcutaneous adipose tissue in the left deltoid muscle, and MRI of the left shoulder joint (Fig.2) revealed muscle swelling and inflammatory changes around the left deltoid muscle. These findings suggested the development of myositis after influenza vaccination. Although there seemed to be a tendency toward improvement of the general condition and hematological findings, oral administration of nonsteroidal anti-inflammatory drugs and intravenous infusion of sulbactam/ampicillin were started on day four of hospitalization, considering the possibility of a complicating bacterial infection at the inflamed site. Subsequently, the redness and swelling of the left shoulder resolved. The patient was discharged on day 11 after admission.
Figure 1.
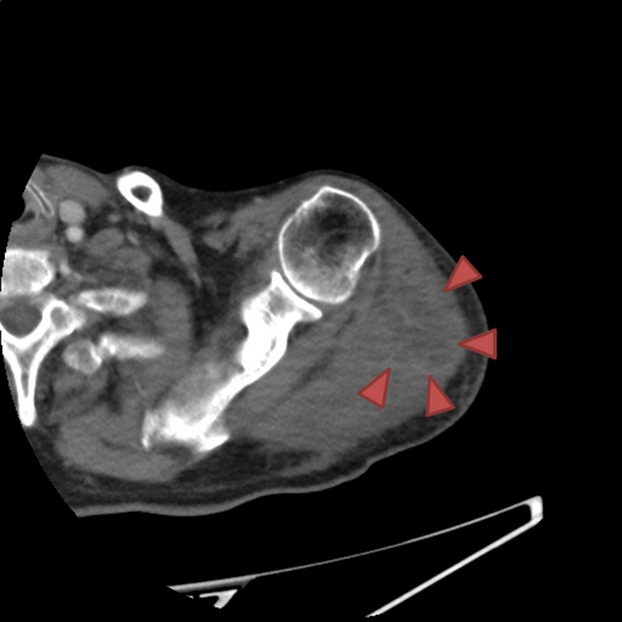
Axial contrast-enhanced computed tomography (CT) of the shoulder showing the low density area, in the left deltoid muscle, with slightly enhanced the marginal region (arrow).
Figure 2.
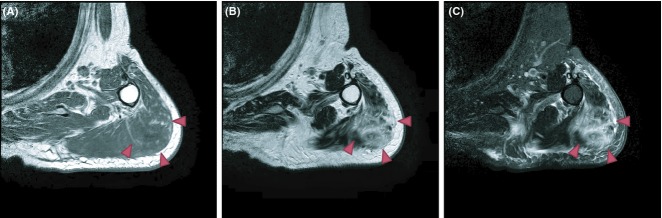
(A) Axial T1-weighted MR image, (B) Axial T2-weighted MR image, (C) Axial T2-weighted fat-saturated MR image of the shoulder showing increased T2-weighted signal in left deltoid muscle and subcutaneous fat tissue (arrow), which might suggest focal inflammatory changes.
Discussion
In Japan, in the 1970s, it was reported that intramuscular injection of antipyretics and antimicrobial drugs caused quadriceps contracture in as many as ∽3600 patients. Since then, there has been a tendency to avoid administration of drugs by intramuscular injection. Thus, almost all vaccines, including influenza vaccine, with the exception of human papillomavirus vaccine, are administered by subcutaneous injection. As compared to intramuscular injection, subcutaneous injection is considered to be more likely to cause local adverse reactions (pain, erythema, induration, etc.) 8. Thus, even adjuvant-containing vaccines that contain a substance to enhance the immune responses to the administered antigens are not used in Japan, with the objective of preventing adverse reactions.
According to reports on the adverse reactions after influenza vaccination by the Japanese Ministry of Health, Labour and Welfare, the majority of cases of local reactions are reported within 2 days of vaccination. A summary report based on surveys conducted in 13,386 vaccine recipients over a period of 12 years shows that there were only eight vaccine recipients who developed local reactions 1 week or more after vaccination. Unlike our patient, however, none of the eight vaccine recipients had any systemic reaction 9. On the other hand, inactivated vaccines are administered by intramuscular injection abroad. According to several studies, local reactions to intramuscular injection are considered to be mild, in general, and to mainly occur within 2 days of vaccination, similar to the case in Japan 10–13.
Adverse reactions after vaccination are different from the adverse effects of drugs. The adverse reactions include events caused by errors in vaccination procedures and events occurring by coincidence, in addition to allergic reactions to vaccine preparations. Thus, it is considered to be extremely difficult to prove a causal relationship between a serious adverse reaction and a vaccine preparation. In our patient, a causal relationship with the vaccination was strongly suspected based on the temporal course and muscle swelling corresponding to the vaccination site. In most cases, allergic reactions to vaccine preparations are immediate hypersensitivity reactions mediated by immunoglobulin E antibody, that occur within a few minutes to a few hours after the vaccination 14. However, allergic reactions may be caused not only by the vaccine antigen, but also by residual egg protein, preservative, buffer and other components, and depending on the components responsible, delayed-type hypersensitivity reactions may also occur. In general, hypersensitivity reactions such as contact dermatitis due to thimerosal, which is a preservative contained in vaccines, are reported to be delayed-type hypersensitivity reactions occurring 2–10 days after vaccination 15. While our patient was receiving influenza vaccination every year, in the current year, it was possible that the vaccine was administered by intramuscular injection. Given that the myositis occurred 1 week after the influenza vaccination, we assumed that thimerosal contained in the vaccine administered by intramuscular injection might have been responsible for the delayed-type hypersensitivity reaction. However, it has also been reported that currently, there are no differences in safety among thimerosal-free or thimerosal-containing vaccine 16.
Events associated with the vaccination procedure include infection with viruses or bacteria at the puncture site. Our patient developed fever and transient impairment of consciousness. It was assumed that the impaired consciousness in this patient was not caused by encephalitis, but was a result of a symptomatic seizure triggered by fever, based on the following reasons: the short duration of the impaired consciousness, absence of abnormal findings on head CT, head MRI and electroencephalography, and presence of a history of intracranial hemorrhage. In regard to bacterial infection, a case of necrotizing fasciitis at the vaccination site after influenza vaccination has been reported previously 17. In our patient, the blood culture result was negative, and defervescence and a trend toward improvement in the hematological findings were observed even prior to the administration of the antibiotic therapy. Thus, bacterial infection was assumed to be unlikely.
There are no vaccines yet that are without any adverse reactions. However, vaccination should still be recommended, in view of their clinical benefits and the very small incidence of serious adverse reactions. Meanwhile, we consider that adverse reactions which occur should be examined to identify their causes and be properly classified in order to promote the safe use of vaccines.
Conflict of Interest
None declared.
Reference
- Nichol KL, Nordin JD, Nelson DB, Mullooly JP. Hak E. Effectiveness of influenza vaccine in the community-dwelling elderly. N. Engl. J. Med. 2007;357:1373–1381. doi: 10.1056/NEJMoa070844. [DOI] [PubMed] [Google Scholar]
- Nichol KL, Margolis KL, Wuorenma J. Von Sternberg T. The efficacy and cost effectiveness of vaccination against influenza among elderly persons living in the community. N. Engl. J. Med. 1994;331:778–784. doi: 10.1056/NEJM199409223311206. [DOI] [PubMed] [Google Scholar]
- Jefferson T, Rivetti D, Rivetti A, Rudin M, Pietrantonj CD. Demicheli V. Efficacy and effectiveness of influenza vaccines in elderly people: a systematic review. Lancet. 2005;366:1165–1174. doi: 10.1016/S0140-6736(05)67339-4. [DOI] [PubMed] [Google Scholar]
- Falsey AR, Treanor JJ, Tornieporth N, Capellan J. Gorse GJ. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J. Infect. Dis. 2009;200:172–180. doi: 10.1086/599790. [DOI] [PubMed] [Google Scholar]
- Govaert TME, Dinant GJ, Aretz K, Masurel N, Sprenger MJW. Knottnerus JA. Adverse reactions to influenza vaccine in elderly people: randomised double blind placebo controlled trial. BMJ. 1993;307:988–990. doi: 10.1136/bmj.307.6910.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K. Idehara R. Acute disseminated encephalomyelitis following 2009 H1N1 influenza vaccination. Intern. Med. 2012;51:1931–1933. doi: 10.2169/internalmedicine.51.7487. [DOI] [PubMed] [Google Scholar]
- Salmon DA, Proschan M, Forshee R, Gargiullo M, Bleser W, Burwen DR, et al. Association between Guillain-Barré syndrome and influenza A (H1N1) 2009 monovalent inactivated vaccines in the USA: a meta-analysis. Lancet. 2013;381:1461–1468. doi: 10.1016/S0140-6736(12)62189-8. [DOI] [PubMed] [Google Scholar]
- Cook IF, Barr I, Hartel G, Pond D. Hampson AW. Reactogenicity and immunogenicity of an inactivated influenza vaccine administered by intramuscular or subcutaneous injection in elderly adults. Vaccine. 2006;24:2395–2402. doi: 10.1016/j.vaccine.2005.11.057. [DOI] [PubMed] [Google Scholar]
- The Japanese Ministry of Health, Labour and Welfare. Survey group on adverse reactions and health status after vaccination. The 2011 summary report of surveys on the health status after vaccination. Available from: http://www.mhlw.go.jp/stf/shingi/2r9852000002qfxs-att/2r9852000002qfz9.pdf (accessed 27 February 2014)
- Gałaj A, Grześk G, Kuziemski A, Szadujkis-Szadurski L. Sinjab T. Adverse reactions after vaccination against influenza in chronically ill people. Pol. Merkur. Lekarski. 2002;12:496–499. [PubMed] [Google Scholar]
- Mayet A, Haus-Cheymol R, Bouaiti EA, Decam C, Simon F, Merens A, et al. Adverse events following vaccination in the French armed forces: an overview of surveillance conducted from 2002 to 2010. Euro. Surveill. 2012;17:1–8. [PubMed] [Google Scholar]
- Nichol KL, Margolis KL, Lind A, Murdoch M, McFadden R, Hauge M, et al. Side effects associated with influenza vaccination in healthy working adults. A randomized, placebo-controlled trial. Arch. Intern. Med. 1996;156:1546–1550. [PubMed] [Google Scholar]
- Vellozzi C, Burwen DR, Dobardzic A, Ball R, Walton K. Haber P. Safety of trivalent inactivated influenza vaccines in adults: background for pandemic influenza vaccine safety monitoring. Vaccine. 2009;27:2114–2120. doi: 10.1016/j.vaccine.2009.01.125. [DOI] [PubMed] [Google Scholar]
- Wood RA, Berger M, Dreskin SC, Setse R, Engler RJM, Dekker CL, et al. An algorithm for treatment of patients with hypersensitivity reactions after vaccines. Pediatrics. 2008;122:771–777. doi: 10.1542/peds.2008-1002. [DOI] [PubMed] [Google Scholar]
- Patrizi A, Rizzoli L, Vincenzi C, Trevisi P. Tosti A. Sensitization to thimerosal in atopic children. Contact Dermatitis. 1999;40:94–97. doi: 10.1111/j.1600-0536.1999.tb05998.x. [DOI] [PubMed] [Google Scholar]
- McMahon AW, Iskander JK, Haber P, Braun MM. Ball R. Inactivated influenza vaccine (IIV) in children <2 years of age: examination of selected adverse events reported to the Vaccine Adverse Event Reporting System (VAERS) after thimerosal-free or thimerosal-containing vaccine. Vaccine. 2008;26:427–429. doi: 10.1016/j.vaccine.2007.10.071. [DOI] [PubMed] [Google Scholar]
- Pitta GBB, Dantas JM, Pitta MR, Raposo RC, Seganfredo IB. Gomes MM. Necrotizing fasciitis after influenza vaccine: case report. J. Vasc. Bras. 2011;10:185–188. [Google Scholar]


