Key Clinical Message
We describe a neonate with abdominal distension, massive hepatomegaly, and high serum neuron-specific enolase level suggestive of congenital neuroblastoma. The patient died of pulmonary hemorrhage after therapy. Autopsy revealed that the tumor cells in the liver indicated acute megakaryocytic leukemia with the RBM15-MKL1 fusion gene.
Keywords: Acute megakaryoblastic leukemia, neuroblastoma, neuron-specific enolase, RBM15-MKL1
Introduction
Acute megakaryoblastic leukemia (AMKL) with t(1;22)(p13;q13) represents <1% of all acute myeloid leukemias (AML), and is associated with the RBM15 (OTT)-MKL1 (MAL) fusion gene. It occurs at higher incidence in the pediatric population and accounts for approximately 70% of infants with AMKL 1,2. In contrast to infants with 11q23-associated leukemia who frequently exhibit hyperleukocytosis, those with RBM15-MKL1 fusion-positive AMKL usually present with decreased leukocyte count, severe anemia, and thrombocytopenia. The pathological findings show fibrosis along with the leukemic cells in the bone marrow and lymph node 3. AMKL with t(1;22)(p13;q13) is a rare disease that is often associated with massive organomegaly. Therefore, it may be misdiagnosed as solid organ tumors including hepatoblastoma and neuroblastoma. Neuron-specific enolase (NSE), the γ-subunit of enolase, is found not only in neuroendocrine cells but also in other cells including erythrocytes and subsets of lymphocytes 4,5. Increased levels of serum NSE were reported in several patients with acute lymphoblastic leukemia 6. Here, we report on a neonate who presented with massive hepatomegaly and increased NSE levels and was misdiagnosed with congenital neuroblastoma. Autopsy revealed that the patient was finally diagnosed with RBM15-MKL1 fusion-positive AMKL.
Material and Methods
Case report
The patient was the second daughter of healthy and non-consanguineous Japanese parents, born at 38 weeks of gestation following an uncomplicated pregnancy, and her body weight was 3164 g. At birth, she was found to have abdominal distension. Physical examination revealed hepatosplenomegaly and petechiae in the skin. Peripheral blood analysis demonstrated thrombocytopenia with a platelet count of 32 × 109/L. White blood cell count was 17.8 × 109/L without blastic cells. Laboratory investigations revealed the following: total bilirubin of 8.8 mg/dL, direct bilirubin of 0.7 mg/dL, lactate dehydrogenase of 2942 IU/L, aspartate aminotransferase of 248 IU/L, alanine aminotransferase of 66 IU/L, γ-glutamyl transpeptidase of 222 IU/L, blood urea nitrogen of 4 mg/dL and creatinine of 0.3 mg/dL. Serum NSE level was extremely high (1050 ng/mL; normal value <25 ng/mL) and α-fetoprotein was 59,540 ng/mL (within the normal range of age-matched controls). Urinary homovanillic acid (HVA) and vanillomandelic acid (VMA) were normal. Bone marrow aspiration showed 4.8% blastic cells (Fig.1). Immunophenotyping and karyotyping of the bone marrow sample could not be examined because of the very small size of the sample.
Figure 1.
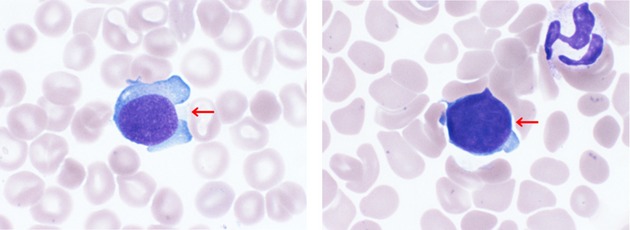
Bone marrow aspiration smear in the patient. Arrows indicate blast cells with large nuclei and basophilic cytoplasm (May–Giemsa staining, original magnification ×1000).
Abdominal MRI showed multiple nodular lesions in the liver (Fig.2). An initial diagnosis was made of neuroblastoma stage 4S with an unknown primary lesion, although urine VMA and HVA levels were normal. She subsequently presented with massive hepatomegaly and respiratory distress. We started chemotherapy and irradiation with a diagnosis of oncologic emergency. However, she died of pulmonary hemorrhage 1 day after therapy.
Figure 2.
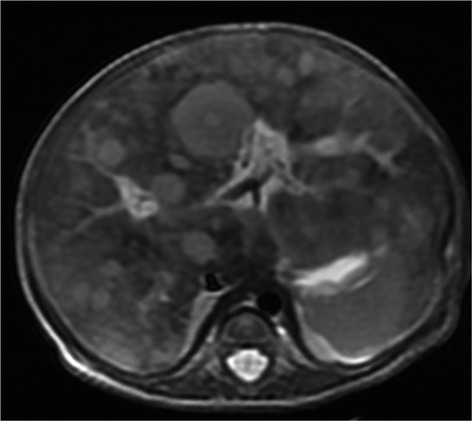
Abdominal MRI imaging. Multiple nodular lesions in the liver were demonstrated by fat-suppression MRI.
Molecular analysis
Reverse transcriptase-polymerase chain reaction (RT-PCR) assay for the detection of RBM15-MKL1 fusion transcripts was performed in a not bone marrow, but liver sample with forward primer RBM15-S (5′-TTCCTCAGCTGCATCAGACA-3′) located in RBM15 exon 1 (GenBank accession no. NM_022768.4) and reverse primers MKL1-AS (5′-TCATTGAGGTCATCGGCTAG-3′) and MKL1-AS2 (5′-TCATTGAGGTCATCGGCTAG-3′) located in MKL1 exon 7 (GenBank accession no. NM_020831.3). The expression of β-actin was used as a control. PCR amplifications were performed using the Qiagen One-Step RT-PCR Kit (Qiagen GmbH, Hilden, Germany), in accordance with a previously published protocol 7. Sequence analysis was directly performed on the amplified RT-PCR product by using BigDye Terminator Cycle Sequencing Chemistry (Applied Biosystems, Foster City, CA) on an automated sequencer ABI Prism 310 Genetic Analyser (Applied Biosystems), in accordance with the manufacturer's instructions.
Results and Discussion
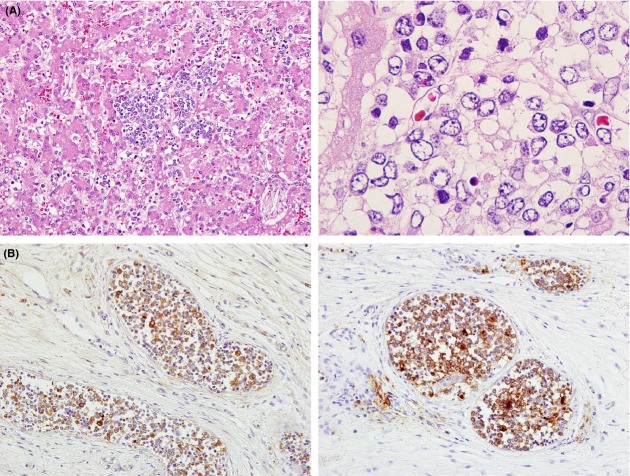
After obtaining informed consent, autopsy was performed. At the autopsy, there were multiple nodules composed of diffuse proliferation of small round cells in the liver (Fig.3A). Multinucleated cells with eosinophilic cytoplasm were occasionally observed. Immunohistochemically, the proliferating cells were positive for CD41, CD42b, CD43, and NSE, but negative for myeloperoxidase, TdT, CD7, CD68, CD163, PGP9.5, and tyrosine hydroxylase (Fig.3B). The findings indicated the liver involvement of AMKL. Tumor cells were seen in the vessels of the adrenal gland and lung. Tumor cell involvement was observed in the gastric lymph node. Bone marrow showed marked myelofibrosis with scattered megakaryoblasts. Fluorescence in situ hybridization revealed chromosome 21 disomy, indicating that the patient did not have Down syndrome. Genetic analysis disclosed the RBM15-MKL1 fusion gene specific for AMKL (Fig.4).
Figure 3.
Histopathological findings of the liver. (A) Microscopic findings. Atypical cells are observed in the liver (hematoxylin and eosin staining – left panel: original magnification ×200; right panel: original magnification ×1000). (B) Immunohistochemical findings. The lesion is positive for NSE (left panel) and CD41 (right panel) (original magnification ×200).
Figure 4.
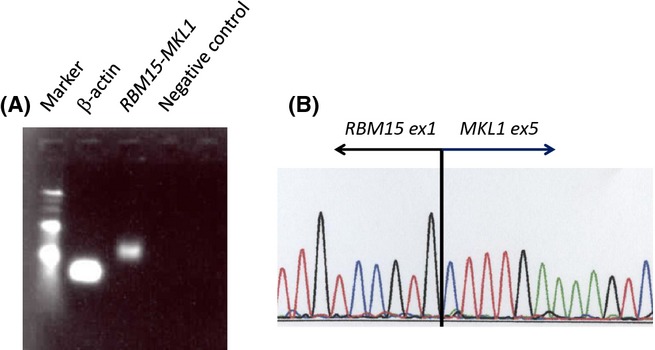
Molecular characterization of the RBM15-MKL1 fusion gene. (A) RT-PCR analysis showing the presence of the RBM15-MKL1 transcript. Lanes 1, 2 and 4 indicate a 100-bp molecular marker, an internal control, and a negative control, respectively. (B) Sequence analysis of the amplified RT-PCR product revealed an in-frame fusion between RBM15 exon 1 and MKL1 exon 5.
Leukemia occurs much less frequently in the perinatal period than in later childhood. Although not as common as neuroblastoma, leukemia is the leading cause of death due to neoplastic disease in neonates 8. Many cases of congenital neoplasms do not present with overwhelming signs and symptoms, and rapid and accurate diagnosis may be difficult. AMKL is a rare variant of AML, and the leukemic blasts show characteristic morphologic and phenotypic features, indicating megakaryocytoid differentiation. A distinct entity characterized by t(1; 22)(p13;q13), resulting in the RBM15-MKL1 fusion oncogene, has recently been identified. Of the recognized subtypes of AMKL, the disease associated with t(1;22) has clinicopathologic features that present in neonates and infants and is frequently associated with abdominal organomegaly 3,9. The liver was involved as the primary site in some patients with congenital leukemia 9. Neonates with Down syndrome sometimes present transient myeloproliferative disorder mimicking AMKL 10; however, this patient was not diagnosed with Down syndrome.
At the initial evaluation, the patient was diagnosed with neuroblastoma stage 4S. The diagnosis of AMKL was obtained upon postmortem examination. In the present case, the elevation of serum NSE level and a few and absent blastic cells in the bone marrow and the peripheral blood might have led to a clinical misdiagnosis. NSE is usually synthesized by neurons and neuroendocrine cells, and it is a useful marker for the diagnosis and monitoring of patients with neuroendocrine tumors such as neuroblastoma and small cell lung cancer 11. However, NSE is found not only in neuroendocrine cells but also in other cells including erythrocytes and subsets of lymphocytes 4,5. Some of the hematopoietic cell lines including T-cell leukemia and Epstein–Barr virus-immortalized B lymphoblastoid cell lines produce NSE. Thus, NSE is not exclusively expressed in neuroendocrine tumor cells 12.
In the present case, the most remarkable symptom was progressive hepatomegaly. Myelofibrosis is a well-recognized characteristic of AMKL. The fibrosis might have been caused by the production and secretion of transforming growth factor beta from the megakaryoblasts, which stimulates collagen synthesis in bone marrow fibroblasts 13. There are very few documented cases of congenital leukemia in which the primary site of involvement was shown to be the liver, without initial bone marrow findings. A case of congenital anerythremic erythroleukemia was reported to present with hepatic failure and have a fetal outcome of multivisceral involvement 14. An infant with AMKL and a complex translocation demonstrated only 5–20% blasts on the initial bone marrow aspirate and core biopsy. Therefore, myelodysplastic syndrome was initially diagnosed; however, only later did blasts become apparent in the peripheral blood 15. In the present case, AMKL might have occurred in the fetal period because hematopoietic cells are mainly produced in the fetal liver.
Here, we report on a neonate who presented with massive hepatomegaly, increased serum NSE levels, and was misdiagnosed with congenital neuroblastoma. Autopsy revealed a final diagnosis of RBM15-MKL1 fusion-positive AMKL. Rapid diagnosis and early treatment are required for patients with neonatal leukemia. A neonate with massive hepatomegaly would be suggestive of RBM15-MKL1 fusion-positive AMKL.
Acknowledgments
We thank Tomoko Iehara, Hajime Hosoi, Etsuro Ito, and Seiji Kojima for critical discussions.
Conflict of Interest
None declared.
References
- Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- Orazi A. Histopathology in the diagnosis and classification of acute myeloid leukemia, myelodysplastic syndromes, and myelodysplastic/myeloproliferative diseases. Pathobiology. 2007;74:97–114. doi: 10.1159/000101709. [DOI] [PubMed] [Google Scholar]
- Chan WC, Carroll A, Alvarado CS, Phillips S, Gonzalez-Crussi F, Kurczynski E, et al. Acute megakaryoblastic leukemia in infants with t(1;22)(p13;q13) abnormality. Am. J. Clin. Pathol. 1992;98:214–221. doi: 10.1093/ajcp/98.2.214. [DOI] [PubMed] [Google Scholar]
- Brown KW, Kynoch PA. Thompson RJ. Immunoreactive nervous system of specific enolase (14-3-2 protein) in human serum and cerebrospinal fluid. Clin. Chim. Acta. 1980;101:257–264. doi: 10.1016/0009-8981(80)90251-x. [DOI] [PubMed] [Google Scholar]
- Muroi K, Tarumoto T, Akioka T, Kirito K, Nagai T, Izumi T, et al. Sialyl-Tn- and neuron-specific enolase-positive minimally differentiated erythroleukemia. Intern. Med. 2000;39:843–846. doi: 10.2169/internalmedicine.39.843. [DOI] [PubMed] [Google Scholar]
- Cooper EH, Pritchard J, Bailey CC. Ninane J. Serum neuron-specific enolase in children's cancer. Br. J. Cancer. 1987;56:65–67. doi: 10.1038/bjc.1987.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs H., Jr Fetal and neonatal leukemia. J. Pediatr. Hematol. Oncol. 2003;25:348–361. doi: 10.1097/00043426-200305000-00002. [DOI] [PubMed] [Google Scholar]
- Torres L, Lisboa S, Vieira J, Cerveira N, Santos J, Pinheiro M, et al. Acute megakaryoblastic leukemia with a four-way variant translocation originating the RBM15-MKL1 fusion gene. Pediatr. Blood Cancer. 2011;56:846–849. doi: 10.1002/pbc.22765. [DOI] [PubMed] [Google Scholar]
- Bernstein J, Dastugue N, Haas OA, Harbott J, Heerema NA, Huret JL, et al. Nineteen cases of the t(1;22)(p13;q13) acute megakaryblastic leukaemia of infants/children and a review of 39 cases: report from a t(1;22) study group. Leukemia. 2000;14:216–218. doi: 10.1038/sj.leu.2401639. [DOI] [PubMed] [Google Scholar]
- Gamis AS. Smith FO. Transient myeloproliferative disorder in children with Down syndrome: clarity to this enigmatic disorder. Br. J. Haematol. 2012;159:277–287. doi: 10.1111/bjh.12041. [DOI] [PubMed] [Google Scholar]
- Odagiri E. Neuron specific enolase (in Japanese) Nihon Rinsho. 2005;63(Suppl. 8):717–719. [PubMed] [Google Scholar]
- Påhlman S, Esscher T. Nilsson K. Expression of gamma-subunit of enolase, neuron-specific enolase, in human non-neuroendocrine tumors and derived cell lines. Lab. Invest. 1986;54:554–560. [PubMed] [Google Scholar]
- Terui T, Niitsu Y, Mahara K, Fujisaki Y, Urushizaki Y, Mogi Y, et al. The production of transforming growth factor-beta in acute megakaryoblastic leukemia and its possible implications in myelofibrosis. Blood. 1990;75:1540–1548. [PubMed] [Google Scholar]
- Lazure T, Beauchamp A, Croisille L, Ferlicot S, Feneux D. Fabre M. Congenital anerythremic erythroleukemia presenting as hepatic failure. Arch. Pathol. Lab. Med. 2003;127:1362–1365. doi: 10.5858/2003-127-1362-CAEPAH. [DOI] [PubMed] [Google Scholar]
- Toretsky JA, Everly EM, Padilla-Nash HM, Chen A, Abruzzo LV, Eskenazi AE, et al. Novel translocation in acute megakaryoblastic leukemia (AML-M7) J. Pediatr. Hematol. Oncol. 2003;25:396–402. doi: 10.1097/00043426-200305000-00009. [DOI] [PubMed] [Google Scholar]